HAL Id: tel-01927140
https://tel.archives-ouvertes.fr/tel-01927140
Submitted on 19 Nov 2018HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Multiple regulators mediate the transcriptional activities
of ERRalpha and its capacity to promote cell invasion
Ling Zhang
To cite this version:
Ling Zhang. Multiple regulators mediate the transcriptional activities of ERRalpha and its capac-ity to promote cell invasion. Molecular biology. Université de Lyon; East China normal universcapac-ity (Shanghai), 2018. English. �NNT : 2018LYSEN057�. �tel-01927140�
Numéro National de Thèse : 2018LYSEN057
THESE DE DOCTORAT DE L’UNIVERSITE DE LYON
opérée par
l’Ecole Normale Supérieure de Lyon
en co-tutelle avec
l’East China Normal University
Ecole Doctorale N° 340
Biologie Moléculaire, Intégrative et Cellulaire Discipline : Biologie
Soutenue publiquement le 05/09/2018, par :
Madame Ling ZHANG
Multiple regulators mediate the transcriptional activities of ERRalpha and its capacity to promote cell invasion
Régulation de l'activité transcriptionnelle de ERRα et de sa capacité à favoriser l'invasion cellulaire par différents complexes
____________________________________________________
Devant le jury composé de :
Céline GUIGON, CR INSERM, Univ. Paris Diderot Rapporteure
Baojie LI, Pr. Univ Jiao Tong, Shanghai Rapporteur
Jean-Marc VANACKER, DR CNRS, ENS de Lyon Directeur de thèse
Jiemin WONG, Pr. ECNU, Shanghai Co-tuteur de thèse
1
Acknowledgements
First of all, I would like to thank jury members for agreeing to evaluate my thesis.I
look forward to your opinion on the results obtained, the questions raised and suggestions given for my projects.
As times fly, I still remembered clearly the day that I boarded in France, it was also
my first time to go abroad, new, unfamiliar, afraid, e ited, u ious… all i ed togethe , a d
I started my PhD life in Lyon. I would like to thank my supervisor Pro. Jean-Marc Vanacker. Thank you for you to accept me in your lab, your broad knowledge, rigorous attitude deeply influence me, academically guidance, meticulous care in life, and never giving up in diffi ulties, e lighte ed i o fused……all of hi h ake e ake i p o e e t, little little, day by day, from a youth knowing little in science to gradually thinking, performing experiment, and analyzing results independently. For me, the upcoming to an end of PhD study is another start, anyway, here is always my cornerstone. I also would like to thank Pro. Wong jiemin, my first supervisor introduced me into the biology world. Diligent, work hard,
serious……let e to ealize that if ou ha e a d ea , the ou ust go afte it. You igo ous
academic attitude let me know that knowledge should be serious, your inculcation, tireless guidance let me benefit a lot. Only way to express my thanks is that I wish both of you everything goes well.
I also would like to thank every member in JMV team. Thank Catherine for your ki dl help i d e pe i e t guida e, a d ou e ou age e t Bo ou age e e night/ every Friday warmed me a lot during writing my thesis, our daily talking in French life
is o e of p e ious e o ies i life. Tha k Ch istelle, fo ou ki dl help i et
experiment guidance, the suggestion you gave me in my each presentation help me a lot, without you, it will more difficult for me to perform the experiment. Thank Severine, I remembered clearly that we joined the lab almost at the same time, and we leave here almost at the same time, I am precious four years accompany, you spend time to practice French with me, and you correct every fault with super patience, merci beaucoup! Thank Violai e, ou la a age sa e us lots of ti e, a d I a g ateful fo ou to spe d ti e to care my safety during my manipulation. Thank Bruno, your humor and hilarious gave me e e su shi e da , ou always remember to take me and send me back home in our every lab dinner, all these warm things touched me in my heart. Thank Julie, for your kindly greetings and scientific advices. I will also thank you every intership student, because of you, I always have my right neighbor. I would also like to thank Pro. Li jiwen, thank your kindly care and help in our daily life. Thank Wang zhenxing, Wang zhiqiang, for your help in my ad i ist ati e stuff i ECNU, tha k e e e e i P o. Wo g s la . I ish ea h of you everything is well.
I would like to thank Elise on my zebrafish experiment guidance, your patience and serious on how to inject, how to take picture, how to analysis data, etc benefited me a lot in
2
my project. Thank Karine, for your kindly care in life and study; thank Florence and Fred for creating us a better and better scientific environment. Thank Fabienne and Martine for the administrative coordination. Thank Madam Halima and Madam Myriam for your patience and nice on my long titre de sejour issue and inscription each year. Also thank frank and kind guys Christian and Laurent, for providing us the convenience to do experiment, thank the gu s i se u it , B ahi , Ke i ……fo ou ki d e ou age e t e e da .
For experimental students, most of time is in lab, so the moment to stay with friends is very precious. The fixed lunch time at carteen seems our meeting time, we support, share, help with each other. I missed the time we spent together for every Chinese holiday, Dawei, Zhengzheng, and Wu nannan always have great cookings! The gentel and elegant girl Lu i a al a s p a ti e the lo e life a d lo e t a el , )hu i is atu e, espo si ilit , a d has wide interests, giving me another point view to look at the world. Fu xiaopeng is generous and kind, appreciation for your inclusive and precious advice. Jingyun let me know more about the life in French outside science. Everyone works hard and achieve their own dream in their own way. I am happy to see new face each time, and it is also sad to farewell each friend. Thank ENS-Chinese group, with you, my life in Lyon is colorful and happiness. I would like to thank wenyue, for your help and care when I arrived Lyon, your performance in science and your positive attitude towards life inspired me a lot. Thank qingyi, a lovely, thoughtful and intimate girl took me so much comfort, thank you to bring me lots of smile and sweet, thank Xu hao, for your frank and patience, a friend of life, thank the sweet couple dandan and Zhang zhi, you always give me family feeling, thank tingting, a lovely and sportive girl always has good ideas and refresh me, thank jiang, haixiu, yunlong, mengzhong, chongyang, cong, for your generous, care and help in life or study. I would also like to thank Fang lan, like friend and like teacher, you proved to me what is devotion to science by your actual actions, and I am benefited a lot from you. Thank my friends changmei and Li bo, even though we are kilometers distance and hours jetlag, but it does not influence our friendship, I am grateful for both of your encouragement and help in my last periods PhD study. Thank you each of you, because of you, I am not lonely, and I can travel around Europe, and my memory in France must have you! And I wish every of you can arrive you dream in future, do what you do and love what you love!
Thank Madam Qian, for your organization JORISS program which provides us the opportunity to continue the PhD training in France. Thank Madam Liu xiaoling and Liu xiaoyan, never stop endenvor to make the JORISS program better and better. Thank China Scholarship Coucil (CSC) for supporting us to study in France without economic worries.
Almost finishing 23 years study, there are lots of teachers and friends I met enlightened and encouraged me, thank each of you for your care and instillation to keep me positive towards life and study!
3
I want to thank my parents and family most, for your understanding, patience,
supports and encouragement, ithout ou, I a t fi ish stud a d pu sue d ea .
Thank for you to give me enough time to let me grow up and be independent.
The beautiful scenery has been deeply imprinted in my mind; the true feelings of the past have also been treasured in my heart.
Finally, thank you again for everyone in my life, and I wish each of you has a bright future!
4
Contents
Abstract ... 7 List of Figures ... 10 Abbreviations ... 12 Introduction ... 17Chapter I --Nuclear Receptors: general concepts: ... 17
1.1 Basic structure and molecular functions of nuclear receptors ... 19
1.2 Mechanisms of activation ... 20
1.3 NRs coregulators ... 24
1.4 NRs and disease ... 25
Chapter II--E‘‘α: Ge e al i t odu tio ... 28
2.1 Structural and expression pattern ... 28
. ‘elatio ships et ee E‘‘α a d E‘α ... 29
2.3 Agonists/Antagonists ... 30 2.4 Action mechanism ... 31 2.4.1 PGC-1 coactivators ... 31 2.4.2 Repressors ... 32 2.5 Post-translational modifications ... 33 2.6 Physiological functions ... 33 . . E‘‘α a d o e fate ... 33
. . E‘‘α a d Meta oli effe ts ... 34
. . E‘‘α a d Ca e ... 34
Chapter III--Epigenetics ... 39
3.1 The role of Epigenetics ... 39
3.2 Histone modification ... 39
3.3 Histone modification mechanism in transcription ... 41
3.4 Histone methylation ... 41
3.5 Methylation and disease ... 44
Chapter IV--Histone lysine demethylation ... 45
4.1 Histone lysine specific demethylase 1 ( LSD1)... 45
4.2 LSD1 represses gene expression ... 47
4.2.1 With BHC and CoREST ... 47
5
4.3 LSD1 activates gene expression... 48
4.4 LSD1 demethylates more than histone ... 49
4.5 Biological effects of LSD1... 51
4.6 LSD1 and metabolism ... 51
Chapter V--Nuclear Respiratory Factor 1 (NRF1) ... 53
5.1 General introduction ... 53
5.2 NRF1 and disease ... 54
Chapter VI--Histone methyltransferases (HTMs) ... 55
6.1 Identification of SET7 and its structural analysis ... 55
6.2 SET7 mediates H3K4 mono-methylation ... 56
6.3 Physiological role of H3K4 methylation ... 57
6.4 Non-histone lysine methylation ... 57
6.5 Examples of SET7 mediated non-histone methylation and biological effects ... 59
6.5.1 SET7-mediated lysine methylation and protein stability ... 59
6.5.2 SET7-mediated lysine methylation and transcriptional activity ... 59
6.5.3 SET7-mediated lysine methylation and cellular location ... 60
6.6 SET7 involvement in disease ... 60
Chapter VII— ETS family ... 62
7.1 General introduction of ETS family and structure ... 62
7.2 Biological roles ... 62
7.3 ETS1 ... 62
7.3.1 Structure of human ETS1 ... 62
7.3.2 Transcriptional regulation ... 63
7.3.3 Physiological role of ETS1 ... 64
7.4 Conclusion ... 66
Results ... 67
General introduction of article 1 ... 67
General introduction of article 2 ... 69
General Introduction of project 3 ... 71
Discussion and perspective ... 89
Common aspects between both two complexes. ... 91
Othe o egulato s of E‘‘α... 94
Othe s…… ... 94
6
RNA-seq information and Oligonucleotides ... 96
Table 1. SET7-modulated genes ... 96
Table 2. Genes modulated by siSET7 and siERR• ... 109
Table 3. Genes modulated by siSET7, but not by siERR•. ... 110
Table 4. Genes modulated by siERR•, but not by siSET7. ... 114
Table 5. Oligonucleotides used in this study ... 116
References ... 118
ANNEXE 1... 138
Publication list ... 138
ANNEXE 2... 142
Articles published as first author... 142
7
Abstract
ERR is a nuclear receptor whose activity mainly depends on its interaction with transcription co-regulators. High levels of ERR are found in various cancer types and
correlate with poor prognosis. However, the mechanisms linking E‘‘α to cancer cell
migration as well as the coregulators involved are unclear. In our study, we found two
histone-modifying enzymes, LSD1 and SET7, acting as positive regulators of ERRα.
I. ERR impacts the biochemical activities of the LSD1 demethylase. Activation of
ERR-LSD1 targets (identified by RNA-Seq) requires the recruitment of this complex at Transcriptional Start Sites (TSSs), which is achieved by the NRF1 transcription factor. In our
study, we have shown several points: NRF1, but not ERR, is involved in positioning LSD1 to
TSS, whereas ERR, but not NRF1, regulates LSD1 enzymatic activities towards
demethylating H3K9me2.
II. A distinct group of ERR target genes (identified by RNA-Seq) is under the control
of the histone methyltransferase SET7 which mono-methylates H3K4. Appropriate recruitment of SET7 at TSSs is controlled by the ETS1 transcription factor, promoting the
interaction between SET7-ERR, leading to target gene expression.
Gene Ontology analysis revealed that ERR-LSD1 targets, as well as ERR-SET7
co-targets, are enriched in terms of cell invasion. Consistently, depletion of each of these factors, as well as depletion of NRF1 or ETS1, leads to reduced cell invasion capacities as observed in transwell assays or in vivo, using xenotransplantation in the zebrafish embryo.
Altogether, our results show two regulatory networks involving histone modifications induced by nuclear receptors, leading to increased cell invasion.
8
Résumé
ERR est u epteu u l ai e do t l a ti it est o t ol e pa des o-régulateurs
transcriptionnels. La forte expression de ERR dans les cancers est corrélée à un mauvais
pronostic. Les mécanismes par lesquels E‘‘α régule la migration des cellules cancéreuses
sont mal compris, tout comme les co-régulateurs impliqués. Nous avons identifié deux e z es odifi at i es d histo e, L“D et SET7, agissant comme régulateurs positifs de ERRα.
I. ERR modifie les activités biochimiques de la déméthylase LSD1 vers la
déméthylation (activatrice) de H3K9me2. L activation des cibles de ERR-LSD1 (identifiées
par RNA-Seq) requiert le recrutement de e o ple e au sites d i itiatio de la
transcription (TSSs), réalisé par le facteur de transcription NRF1 qui, lui, ne régule pas l a ti it e z ati ue de L“D .
II. Un autre groupe de cibles de ERR (identifié par RNA-Seq) est sous le contrôle de
l histone méthyltransférase SET7 qui mono-méthyle H3K4. Le recrutement de SET7 aux TSSs est contrôlé par le facteur de transcription ETS1, qui promeut les interactions entre SET7 et
ERR, o duisa t à l a ti atio de l e p essio des g es e a al.
Des analyses par Gene Ontology ont montré que les cibles communes de ERR/LSD1
et de ERR/“ET so t fo te e t e i hies e te es d i asio ellulai e. De a i e
cohérente, la déplétion individuelle de chacun de ces facteurs (et également celle de NRF1
ou ET“ duit les apa it s d i asio , o se e e tests i it o t a s ell ou i i o pa
xénogreffe sur embryons de poisson-zèbre.
En résumé, nos résultats montrent deux réseaux de régulation impliquant des odifi atio s d histo e i duites pa ERR, o duisa t à l i asio ellulai e.
9
摘要
E‘‘α 雌激素 体相关 体) 属于细胞 激素 体超家族中的 孤儿 体, 于 没有天然的配体,研究发现,E‘‘α 的转录活性 决于 直接或者间接相互作用的调控 蛋白。另外,ERR在 乳腺癌,前列腺癌等癌症中是高表达的,但是 体的机制以及 之相互作用的 调控蛋白 是未知的。在 我的博士 课题中,发现 并鉴定了 两个组蛋白修饰 酶 LSD1 和 SET7,均可作为 E‘‘α 的激活调控蛋白,两个复合体通过 同的机制,共同 影响癌细胞的侵染性。1. ERRα 调控LSD1 的去 基酶活性。通过RNA 测序的方法鉴定出ERRα-LSD1共
同调控的靶基因,以激活基因为例,利用生化实验的方法,我们发现 ERRα-LSD1 复合
体 富 集 在 靶 基 因 的 启 子 区 域 , 同 时 ,LSD1 定 位 在 启 子 区 域 需 要 第 三 个 转 录 因 子
NRF1 的协 。即:NRF1 负责招募 LSD1 到靶基因的启 子区域,而 ERRα 可通过
LSD1 的 相 互 作 用 改 去 基 化 酶 的 方 向 , 去 基 化 H3K4me2 转 为 去 基 化
H3K9me2,进而激活下游靶基因的表达。
2. 利用 Luciferase 的方法发现组蛋白H3K4 单 基化酶SET7 可作为ERR的另一
个激活调控蛋白质,RNA 测序的方法鉴定出另外一组 同的 ERR-SET7 调控的靶基因。
依然以激活基因为例,研究 ERR-SET7 如何激活靶基因的表达。我们发现同 存在第
三个转录因子 ETS1,负责将 SET7 招募到靶基因的启 子区域,促进 ERR的相互作
用,发生H3K4me1的修饰,激活下游靶基因的表达。
Gene Ontology的方法发现在ERR-LSD1 靶基因以及ERR-SET7靶基因的功能中,
癌症相关的是细胞侵染。通过体外 transwell 的方法以及体内 xenotransplantation 的
方法,分别下敲 ERRˎLSD1ˎSET7ˎNRF1 以及 ETS1 后,细胞侵染的能力均有明显的下降。
结论,我们的结果发现 ERR通过 LSD1 或者 SET7 的相互作用,调控 组蛋白
10
List of Figures
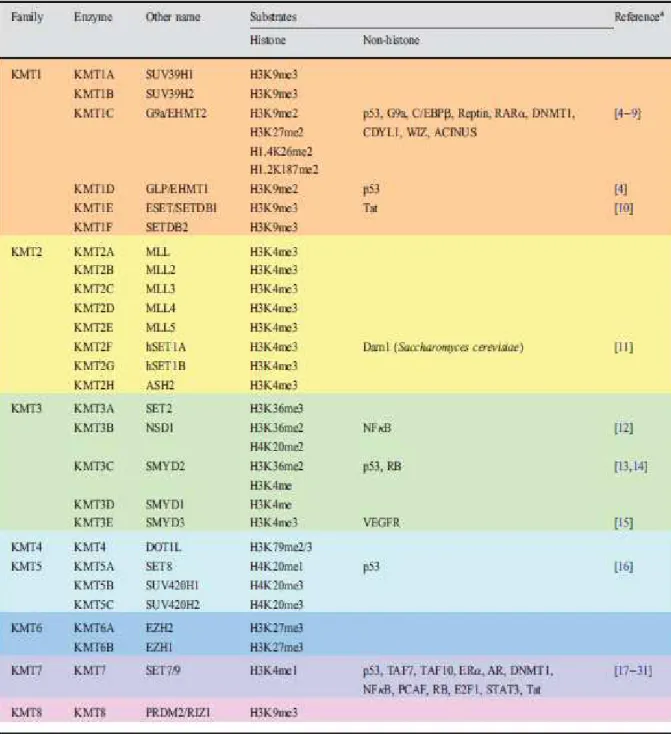
Figure 1. Phylogenetic Tree Connecting the 65 Known NR Genes in Vertebrates, Arthropods and Nematodes
Figure 2. Structural organization of the NRs
Figure 3. Action of steroid and non-steroid ligand receptors
Figure 4. Positive regulation of NR transcriptional activity through phosphorylation Figure 5. Inhibition of NR transcriptional activity through phosphorylation
Figure 6. Positive (red arrows) and negative (green arrows) effects of NR phosphorylation on the transactivation of target genes
Figure 7. Level of sequence identity between domains of ERR isoforms Figure 8. Distributio of ‘NA e p essio fo E‘‘α, E‘‘β, E‘‘γ
Figure 9. ERs and ERRs bind different DNA sequences and display strict binding specificity , and both receptors share binding sequence which EREs are integrated within ERREs Figu e . Model of E‘‘α mediated gene regulation
Figu e . E‘‘α transcriptional pathway in regulation of cellular metabolism
Figure . Opposite i flue e of E‘‘α a d E‘‘γ i eta oli status of east a se ells Figure 13. Effe t of E‘‘α in cell migration
Figu e . “u a of the effe ts of E‘‘α o a e Figure 15. General chromatin organization
Figure 16. Histone lysine methylases and demethylases
Figure 17. The overall structure of the epigenome in normal human cells Figure 18. Global changes in histone methylation in various types of cancers Figure 19. Diagram of LSD1-like amine oxidase family members in different species Figure 20. Domain distribution of LSD1
Figure 21. Crystal structure of LSD1
Figure 22. Histone demethylases complexes and their activities Figure 23. LSD1 mediates gene activation
11
Figure 24. Effect of LSD1 on non-histone protein Figure 25. NRF1 domain distribution
Figure 26. NRF1 target genes implicated in mitochondrial biogenesis and function Figure 27. Structure of the SET7/9 ternary complex
Figure 28. Histone methyltransferases and their histone and non-histone substrates Figure 29. Set7 methylates lysine on non-histone proteins
Figure 30. SET7 transcription network in mESC differentiation
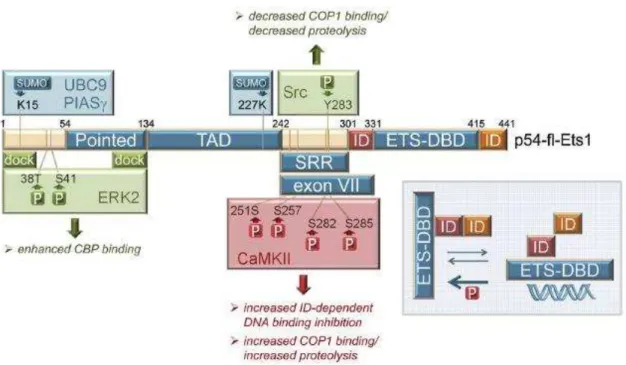
Figure 31. Structure of full length ETS1 protein and its transcriptional regulation Figure 32. ETS1 regulates the expression of genes associated with cancer progression
12
Abbreviations
-UTR Three prime Untranslated Transcribed Region
4-OHT 4-hydroxytamoxifen
53BP1 Tumor suppressor p53-binding protein 1
AF-1/2 Activation Function 1/2
AMPK AMP-activated protein kinase
ang2 Angiopoietin-2
AOL Amine oxidase like
AR Androgen Receptor
AREs AR response elements
ASH1 Absent small or homeotic 1
Ash2L ASH2 Like Histone Lysine Methyltransferase Complex Subunit
BHC BRAF-histone deacetylase complex
CaMKII Calcium-dependent phosphorylation kinase II
cAMP/PKA Cyclic AMP/ Protein kinase A
CARM1 Coactivator-associated arginine methyltransferase 1
CDK2 Cyclin-dependent kinase 2
CDKs Cyclin-dependent kinases
CHD1 Chromodomain Helicase DNA Binding Protein 1
ChIP-DSL Chromatin Immunoprecipitation-DNA Selection and Ligation
COP1 Constitutively photomorphogenic 1
CoREST REST corepressor 1
CRPC Castration resistant prostate cancer
CtBP C-terminal-binding protein 1
CTEs C-Terminal Extensions
DBD DNA Binding Domain
DES Diethylstilbestrol
DNMT1 DNA Methyltransferase 1
E2 β-estradiol
E2F1 E2F transcription factor 1
E6-AP E6-associated protein
EMT Epithelial to mesenchymal transition
ER Estrogen Receptor
13
ERRE ERR response element
E‘‘α/β/γ Estrogen receptor related receptor α/β/γ
ESET ERG-associated protein with SET domain
ETS E26-transformation-specific or E-twenty-six specific sequence
EZH2 Enhancer of zeste homolog 2
FAD Flavin adenine dinucleotide
FAK Focal adhesion kinase
FRET Fluorescence resonance energy transfer assays
GPS2 G protein pathway suppressor 2
GR Glucocorticoid Receptor
GSK3 Glycogen synthase kinase 3
H1, H2A, H2B, H3, H4 Histone 1/2A/2B/3/4
H12 Helix 12
H2BK5me1 Histone 2B lysine 5 monomethylation
H3K27 Histone 3 lysine 27
H3K27me1/me3 Histone 3 lysine 27 mono-/tri-methylation
H3K36 Histone 3 lysine 36
H3K36me3 Histone 3 lysine 36 trimethylation
H3K4 Histone 3 lysine 4
H3K4me1/me2/me3 Histone 3 lysine 4 mono-/di-/tri-methylation
H3K79me2/3 Histone 3 lysine 79 di-/tri-methylation
H3K9 Histone 3 lysine 9
H3K9me1/me2/me3 Histone 3 lysine 9 mono-/di-/tri-methylation
H3S10 Histone 3 serine 10
H3T6 Histone 3 threonine 6
H4K20 Histone 4 lysine 20
H4K5acK12ac Histone 4 lysine 5 acetylated lysine12 acetylated
H4K79 Histone 4 lysine 79
H4K8 Histone 4 lysine 8
HDAC3 Histone deacetylase 3
HIF Hypoxia inducible factor
HIFα Hypoxia-inducible factor alpha
HMTs Histone methyltransferases
14
IFN-γ Interferon-γ
IncRNAs long non-coding RNAs
ING1 Inhibitor of growth protein 1
JMJD2C Jumonji domain 2C
JNKs C-Jun N-terminal kinases
KDMs Histone lysine demethylases
KTMs Histone lysine methyltransferases
LBD Ligand Binding Domain
LSD1 Lysine specific demethylase 1
LXRs Liver X Receptors
MAO-A/B Monoamine oxidase A and B
MAPK Mitogen-Activated Proteins Kinases
MBD3 Methyl-CpG-binding domain protein 3
MLL Mixed-lineage leukemia protein
MMP1/7/9 Matrix metalloproteinase 1/7/9
MRP Mitochondria RNA processing
MTA1/2/3 Metastasis-associated protein 1/ 2/ 3
MtDNA Mitochondrial DNA
MYPT1 Myosin phosphatase target subunit 1
NCoR Nuclear receptor co-repressor
NFE2L2 Nuclear factor (erythroid-derived 2)-like 2
NF-Κ Nuclear factor kappa-light-chain-enhancer of activated B cells
NLS Nuclear localization signal
NRF1 Nuclear respiratory factor1
NRs Nuclear Receptors
NuRD Nucleosome Remodeling Deacetylase
OXPHOS Oxidative phosphorylation
P/CAF P300/CREB-binding protein-associated protein (P/CAF)
P300/CBP CREB-binding protein and its homologue p300
p53K370me2 p53 lysine 370 dimethylation
PAI-1 Plasminogen activator inhibitor-1
PcG Polycomb Group
PELP1 Proline glutamic acid and leucine-rich protein1
15
PGC- α/β Peroxisome Proliferator-activated receptor gamma coactivatior 1
PHD The plant homeodomain
PI3K Phosphatidylinositol-3-kinases
PKA/PKC Protein Kinase A/C
PKCβ Protein ki ase C β1
PNT Pointed domain
PPARs Peroxisome Proliferator Activated Receptors
PR Progesterone Receptor
PREs PcG response element
PRK1 Protein kinase C (PKC)-related kinase 1
PRMT-1 Protein arginine N-methyltransferase 1
PTMs Post-translational modifications
RA Retinoic Acid
RARs Retinoic Acid Receptors
RC4 Rat cytochrome c
RIP140 Receptor interacting protein of 140 kDa
ROS Reactive oxygen species
RXRs Retinoid X Receptors
SAGA Spt-Ada-Gcn5-acetyltransferase complex
SAM S-adenosylmethionine
SET Suppressor SU (VAR)39- Enhancer of zeste- Trithorax-group protein
SIRT1 NAD-dependent deacetylase sirtuin-1
SMRT Silencing mediator for retinoid or thyroid-hormone receptors
SMYD2/3 SET and MYND Domain Containing 2/3
SRA Steroid receptor RNA activator
Src Proto-oncogene tyrosine-protein kinase
SRC-1/NCoA-1 Steroid receptor coactivator-3/ Nuclear receptor coactivator
SRC-2/GRIP-1/TIF2 Steroid receptor coactivator-2/ Glucocorticoid receptor-interacting
protein 1 /Transcriptional intermediate factor 2
SRC-3/CIP/RAC3 Steroid receptor coactivator-3/ CBP/co-integrator-associated
protein /Receptor associated coactivator 3
Suv39a Suppressor of variegation 3–9 homolog 1
SWI/SNF Switch/Sucrose Non-Fermentable
SWIRM Swi3p, Rsc8p, Moira
16
TAF10 TATA box-binding protein-associated factor 10
TBL1 Transducing beta-like factors
TBLR1 Transducing Beta Like 1 X-Linked Receptor 1
TCA Tricarboxylic acid cycle
TERRA Telomere repeat-containing RNA
Tfam Mitochondrial transcription factor A,
TFB1M/ TFB2M Transcription Factor B1/2, Mitochondrial
TFIIH Transcription Factor II Human
TGFβ Transforming Growth Factor-beta
Tip60 Tat interactive protein 60KDa
TrXs Trithorax-group protein
TSS Transcriptional start site
VDR Vitamin D Receptor
VEGF Vascular endothelial growth factor
YAP Yes-associated protein
17
Introduction
Chapter I --Nuclear Receptors: general concepts:
Nuclear receptors (NRs) form a superfamily of transcription regulators expressed in all animals, from the simplest organisms (such as sponges) to the complex mammals (Tecalco-Cruz, 2018). There are 48 members identified in human, they play central roles in controlling the expression of genes linked to cellular processes such as proliferation, differentiation, survival and apoptosis, in response to a wide range of developmental, physiological and environmental cues. This family can be subdivided in two groups: the ligand-regulated receptors and orphan nuclear receptors (Jin and Li, 2010; Lonard and
O Malle , (Figure 1).
The ligand-dependent receptors for steroid hormones include the estrogen receptor(ER), the androgen receptor (AR), the progesterone receptor (PR) and the glucocorticoid receptor (GR); for non-steroid hormones comprise the vitamin D receptor (VDR), the retinoic acid receptors (RARs), the retinoid X receptors (RXRs), the peroxisome proliferator activated receptors (PPARs), as well as thyroid hormone receptors and liver X receptors (LXRs) (Rochette-Egly, 2003). Their expression level are tissue and cell context dependent, moreover, their distribution (cytoplasm, nucleus, or membrane-associated) are also based on the type of NRs and their cellular context. Most often, steroid receptors are held in the cytoplasm, while non-steroid ones are preferentially located in the nucleus
18
Figure 1. Phylogenetic Tree Connecting the 65 Known NR Genes in Vertebrates, Arthropods and Nematodes (Nuclear Receptors Nomenclature Committee, 1999)
19
1.1 Basic structure and molecular functions of nuclear receptors
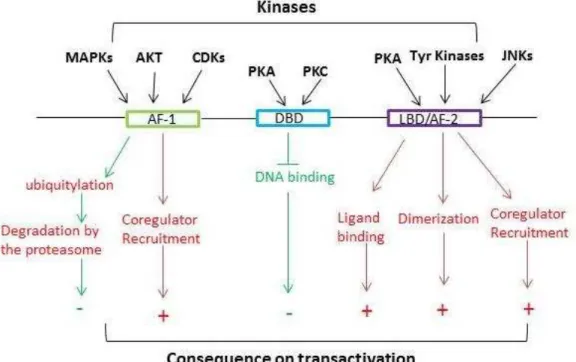
Nuclear receptors share similarity structural organization. The N-terminal A/B region (also known as modulator domain) is highly variable in terms of sequence and length. Attributed to alternative splicing, different promoters and transcriptional start sites, a single gene can produce multiple A/B domains (Giguère, 1999). Within this region, there is a transcriptional activation domain called AF-1 whose activity is ligand-independent and can be enhanced through phosphorylation by multiple kinases, such as cyclin-dependent kinases (CDKs) and MAP kinases. Studies have shown that the A/B region contains many consensus phosphorylation sites which can be targeted by multiple kinases activated in response to growth factors, stress, cytokines or other signals, that are important for cell survival and proliferation (Rochette-Egly, 2003). For example, AKT mediated phosphorylation of AR at Ser210 inhibits the interaction between AR and AR coregulators (ARA70), resulting in suppression of AR transactivation and blocking androgen/AR mediated apoptosis (Lin et al.,
2001); on the other hand, AKT increases the activity of AF-1 of ERthrough phosphorylation
of Ser167, leading to up-regulation of ERtarget gene, and inhibition cell apoptosis (Campbell et al., 2001).
The DNA binding domain (DBD) is the most conserved one and harbors two zinc finger modules, encoded by about 66-70 amino acid residues. This domain can be further divided into P-box and D-box, P-box is involved in the specific recognition of hormone response element (generally comprising AGGTCA or a close variant with A/T-rich sequence) located in close proximity to the transcriptional start site or enhancer of target genes; while D-box is responsible for the dimerization determinants (Giguère, 1999). Most often, steroid nuclear receptors bind DNA as homodimers, while for the non-steroid receptors bind DNA as heterodimers with RXRs (Rochette-Egly, 2003). The hinge region is the bridge between DBD and LBD, this region is also poorly conserved and highly variable in both length and sequence, it is flexible and needed for receptor dimerization and DNA binding; besides, it also contains a DNA minor groove recognition structure (known as C-terminal extensions (CTEs)) which facilitates the interaction with coregulators (Giguère, 1999; Robinson-Rechavi et al., 2003).
The ligand binding domain (LBD) contains 12 alpha-helices whose arrangement results in the formation of a hydrophobic pocket. Helices 3,7 and 10 shape the pocket, helices 9 and 10 form the dimerization interface, and the most important helix 12 (H12) determines the activity of ligand-dependent transactivation function, also known as AF-2. This motif is conserved among NRs and is located at the end of LBD. It is multifunctional and mediates ligand binding, dimerization, interaction with coregulators (Giguère, 1999; Huang et al., 2010). Generally, ligand binding induces a conformational change in the LBD, leading to creating a new surface to recruit coactivators, such as members of PGC-1 family, p160 family (SRC-1, SRC-2, SRC-3) or p300/CBP. These co-activators can further recruit large complexes, such as chromatin remodelers, histone-modifying enzymes in order to
20
decondense the chromatin to promote transcriptional activation (Rochette-Egly, 2003) (Figure 2).
Figure 2. Structural organization of the NRs, the A/B domain located in the NH2-terminal part holds a trancriptional activity, and it is also a substrat for various post-translational modifications, especially phosphorylation; DNA-binding domain containing two conserved zinc finger motif is responsible for the specific DNA binding activity; the ligand-binding domain containing conserved AF-2 helix motif contributes to constitutive activity through interaction with cofactors; hinge region linking DBD and LBD, confers protein flexibility needed for receptor dimerization and DNA binding (Huss et al., 2015).
Structurally, the coregulators use an LxxLL motif to associate with H12 via a charge clamp to support the cofactors binding on the surface of LBD. Thus this is also a drug design principle: an agonist is a molecular which can enhance the interaction of LBDs with the LxxLL of cofactors, while an antagonist can compete and block the interaction with the LxxLL; besides, other antagonists can be designed to recruit corepressors via a leucine-rich motif (Huang et al., 2010; Jin and Li, 2010; Wiench et al., 2011).
1.2 Mechanisms of activation
Based on the structural characteristics of nuclear receptors, their activity can be controlled via three different mechanisms:
Firstly, the classic way is that ligands induce the recruitment of coregulators. Most often, in the absence of ligand, the steroid receptors (such as AR, GR) are located in the cytoplasm and associated with heat shock proteins; upon steroid hormone binding, receptors dissociate from the complex, dimerize, translocate into the nucleus, bind to the response elements on their target genes, and recruit specific coactivators to activate gene expression. Non-steroid receptors are in the nucleus and bound to histone deacetylases which keep the chromatin in a repressive state, ligand binding induces corepressor dissociation which facilitates interaction of the receptors with chromatin remodelers and modifiers, such as SRA, p/CAF, CBP/P300, finally leading to de-repression (Giguère, 1999; Rochette-Egly, 2003; Tecalco-Cruz, 2018) (Figure 3).
24
Figure 6. Positive (red arrows) and negative (green arrows) effects of NR phosphorylation on the transactivation of target genes. Adapted from (Rochette-Egly, 2003).
1.3 NRs coregulators
The switch ability of nuclear receptors between activation and repression in response to environmental signals is mainly ascribed to the recruitment of numerous and diverse coregulators (coactivators and corepressors). Up to now, there are about 300 coregulators
identified in human Lo a d a d O Malle , . Evidences have shown that coregulators
work as multicomponent protein complexes and are subjected to dynamic rearrangements which are required for specific transcription (Kato et al., 2011).
1.3.1 Coactivators:
As the name suggests, coactivators can be directly recruited by NRs and promote NR-target genes expression, the common structural feature of NRs coactivators is an LxxLL motif (NR box) which recognizes and binds the AF-2 domain (Kato et al., 2011). A largely shared functional property of coactivators is acetyltransferase activity which looses chromatin and facilitates the recruitment of other chromatin modifiers. The first identified coactivators were the members of the SRC/p160 family (1/NCoA-1, 2/GRIP-1/TIF2 and SRC-3/CIP/RAC3) (Hong et al., 1996; Li et al., 1997; Oñate et al., 1995). Studies have shown that SRC proteins build a complex containing approximately 6-10 stably associated proteins and a large number of less tightly bound ones. These complexes contain enzymatic proteins, such as acetyltransferase CBP/p300, ubiquitin ligases such as E6-AP, the ATP-dependent chromatin remodeling SWI/SNF complex, the RNA coactivator SRA, the protein methyltransferases CARM-1 and PRMT-1 (Chakravarti et al., 1996; Chen et al., 1999b; Lanz et
25
al., 1999; Nawaz et al., 1999; Yoshinaga et al., 1992). Mechanistically, enzymatic proteins can exert covalent modifications (including phosphorylation, acetylation, sumoylation, ubiquitination) both on the coactivators and NRs, the PTMs can enhance the coactivator enzymatic activities to promote the recruitment of other coregulators or dismantling the inhibitory ones (Kato et al., 2011). In addition, cellular signaling pathway kinases also make a contribution to the NRs or coactivators modifications. For instance, SRC-3 can be activated through a GSK3-mediated phosphorylation-mono-ubiquitination. After a transcription round, SRC-3 undergoes degradation through poly-ubiquitination (Wu et al., 2007); SRC-3 can also be targeted by other modifications (methylation and acetylation can dismantle the coregulatory complex and contribute to reorganize other complexes) to form divergent
multiprotein complexes to impact distinct functions of NRs M Ke a a d O Malle , .
In summary, ligand-specified variations in the AF-2 domain, along with covalent modifications and signaling cascade kinases can have a huge difference on the capacity of a receptor to recruit coactivators, leading to the production of a variety of genes expression
and pervasive biological consequences M Ke a a d O Malle , ; ‘ose feld et al., .
1.3.2 Corepressors
The negative counterparts of coactivators are corepressors which o tai s Co‘N‘
o . They can be recruited by NRs in the absence of ligand or in the presence of NR
synthetic antagonists such as Tamoxifen M Ke a a d O Malle , .Corepressors
include NCoR and SMRT (identified through biochemical purification) which recruit HDAC3,
GPS2 and the transducing -like factors TBL1 and TBLR1, with HDAC3 deacetylase activity
being essential for the repression (Pérez-Schindler et al., 2012;Webb et al., 2000;Yoon et al., 2003).Their function is comparable with coactivator that is they are not a bridge between the environment and NRs but instead as enzymatic tools which are necessary to the transcription. Besides, the PTMs placing on corepressors influence gene expression targeted by transcription factors (like NRs) with which the corepressor interacts (Rosenfeld et al., 2006). For instance, phosphorylation of NCoR can lead to its relocalization to the cytoplasm and reduce mRNA production in nucleus, while the sumoylation can enhance its corepressor capacity (Jonas and Privalsky, 2004; Tiefenbach et al., 2006), thus the combination among PTMs, coregulators, and NRs produce distinct transcriptional effects (Kato et al., 2011;
Lo a d a d O Malle , ;).
1.4 NRs and disease
Nuclear receptors regulate genetic network through sensing the environmental cues,
including steroids, retinoid, fatty acid, lipids… Based on the results obtained with knock-out
mice and functional mutations studies, NRs have been shown as involved in embryonic development, metabolic pathways, reproductive and cell growth. In another word, if NRs are misregulated, they may cause diverse diseases, ranging from obesity, hypertension, cardiovascular problems to cancer. A few examples will illustrate this view (Bushue and Wan, 2010; Gkikas et al., 2017; Sun and Shi, 2010; Xie, 2010).
26
The LXR subfamily comprises two sub-t pes, LX‘α a d LX‘β, a d is highly expressed
in liver, kidney and intestine. Both LXRs bind to DNA with RXRs to regulate transcription. Studies have shown that one of the LXRs direct target is ABCA1 which is required for the process of reverse cholesterol; loss of functional ABCA1 results in Tangier disease which present large accumulation of cholesterol-laden macrophages and constitutes a risk for the development of atherosclerosis (Costet et al., 2000; Hayden et al., 2000). In addition, ABCA1 defective cells cannot efflux cholesterol. Treating primary macrophages cell lines with LXR agonist can induce the expression of ABCA1 and increase cholesterol efflux, supporting the role of LXR as a regulator of ABCA1 and its inhibitory effect on the progression of atherosclerosis. This is consistent with the results obtained in Lxrα-/-/Lxrβ-/- mice, showing accumulation of cholesterol-laden macrophages and increased atherosclerosis (Wagner et al., 2003). Moreover, activation of LXRs represses the expression of genes encoding enzymes of gluconeogenesis in the liver, induces the expression of GLUT4 in adipose tissue, decreases hepatic glucose output and blood glucose levels in animal model of type II diabetes, all the activities of activation LXRs mimic the treatment with insulin, suggesting another role for LXRs as a regulator in metabolism (Cao et al., 2003; Laffitte et al., 2003; Schulman, 2010).
The androgen receptor (AR) plays pivotal roles in the development of the prostate gland, it is highly expressed in reproductive tissues such as the prostate, the adrenal gland and the epididymis (Keller et al., 1996). Studies showed that AR is a therapeutic target in prostate cancer. Based on the classic structure and genomic mechanism pathway, the expression of AR target gene relies on interaction with coactivators in LBD induced by androgen binding. The majority of therapeutic strategies are focused on LBD-dependent mechanism to modulate AR function (McEwan, 2004). Up to now, quite a lot of AR-binding molecules have been designed, including agonists and antagonists. AR agonists are mainly used to maintain secondary sex characteristics, and a useful agent is testosterone, but the dose should be monitored carefully, since excessive testosterone can cause erythrocytosis and other side effects, such as virilizing or feminizing; moreover, this androgen is not orally available due to metabolic instability (Bhasin et al., 2001). A great deal of attention is focused on nonsteroidal ligands, aiming to identify tissue selective, orally bioavailable AR ligands (Gao, 2010). AR antagonists are mainly used in prostate diseases, and most of antagonist mechanism is through inhibiting androgen binding to AR. However, these ligands cannot function in case of AR mutations (in which testosterone binding site is changed or in which antagonist can activate mutated AR) nor in advanced stages of disease (where AR acts in an androgen-independent manner) (Brinkmann and Trapman, 2000). The strategy is now focused on novel antagonists which block AR function in functional domain, like AF2 region, (Gao, 2010).
In summary, nuclear receptors regulate a wide range of biological processes, they are essential for normal development and are also good therapeutic targets for the treatment of cancer or metabolic diseases. However, many ligands are limited in clinical treatment due to
27
a number of side effects. Crystal structure studies revealed insights into the mechanisms of ligand binding affinity and specificity, as well as differential recruitment of coregulators. Structure-based drug design will be a promising therapeutic approach (Chen, 2010; Jin and Li, 2010).
28
Chapter II--ERRα: Ge eral i trodu tio
Since nuclear receptors share extensive homology at structure layout and nucleotide sequence, the use of cDNA encoding nuclear receptors as probes for cross-hybridization screening has led to the identification of a large number of structurally related proteins. Because there was no previously identified ligand for these newly isolated receptors, they
were na ed o pha u lea e epto s (Giguère, 1999). With research progress, some of
them were adopted by identifying natural ligands and appreciated as key transcriptional regulators of metabolic pathways, for example, oxysterols are ligands of liver X receptors; lipids are the ligands of PPARs (Hummasti and Tontonoz, 2008). However, the first identified
orphan nuclear receptor ERRαstill remains an orphan (Deblois and Giguère, 2013; Huss et al.,
2015). In 1988,Vincent Giguère et al. identified two proteins through reduced-stringency
screening of cDNA libraries with the conserved DBD of human estrogen receptor αBecause
they share structural similarities in the DBD (about 68%) and LBD (about 33%) with ERαthey
were named estrogen-related receptors, in short ERRs. Moreover, in 1998,Eudy et al discovered a third isoform, ERRγ(Eudy et al., 1998; Giguère et al., 1988; Stein and McDonnell, 2006) (Figure 7).
2.1 Structural and expression pattern
The ERRs family has structural organization features typical to classic nuclear receptors, the A/B domain located in the N-terminal part of the protein, besides, this motif is subjected to covalent modifications such as phosphorylation and sumoylation, leading to modulation of transcriptional activities. The DBD domain, which contains two conserved zinc finger motifs and is the most conserved part of the molecule, is responsible for the specific DNA-binding activity through targeting DNA sequence TCAAGGTCA (also termed as ERR response element, ERRE). The LBD domain contains an AF-2 and contributes to receptor dimerization and coregulator interaction. Since there is no ligand for ERRs, these receptors activate target gene expression in a ligand-independent manner in the presence of coactivators. The link region between DBD and LBD, can twist itself to facilitate receptor dimerization formation and DNA binding (Giguère, 1999, 2008; Huss et al., 2015).
Figure 7. Level of sequence identity between domains of ERR isoforms. The DBD and LBD show the strongest similarity among the three ERR isoforms (Huss et al., 2015).
29
During mouse development, the expression of E‘‘α can be traced back to early
embryonic stage at E8.5 in the mesodermal cells, then it continues to be expressed in mesodermal derivatives, particularly in kidney, muscles and heart, up to adult stage; E‘‘α expression can be observed in differentiating neural cells, the intermediate zone of the spinal cord at E10.5-E17.5; the cephalic vesicles at E13.5; ERRαexpression can be also detected during skin formation (epidermis, basal cell layer) and endodermal derivatives such as the epithelium of intestine, the urogenital system, liver and lung (Bonnelye et al., 1997a; Deblois and Giguère, 2013; Eichner and Giguère, 2011; Giguère, 2008).
ERRβ is expressed in the extra-embryonic ectoderm which is essential for placental
development and its expression in primordial germ cells (PGCs) is involved in the proliferation of gonadal germ cell (Eichner and Giguère, 2011; Luo et al., 1997). ERRγis highly expressed in the fetal and postnatal heart and plays an important role in the control of oxidative metabolic switch of fetal-to-adult transition (Alaynick et al., 2007; Giguère, 2008)
(Figure 8). “i e ou esea h ta get is E‘‘α hi h e ill hereafter focus on this receptor.
Figure 8. Distributio of RNA e pressio for ERRαˎ ERRβ and ERRγ. A: absent; L:low; M:medium; H:high (Giguère, 2008).
2.2 Relatio ships et ee ERRα a d ERα
E‘‘α displays high se ue e ho olog ith E‘α in the DBD and LBD, suggesting that both classes of receptors could share similarities in transcriptional and functional attributes (Tam and Giguère, 2016). Indeed, in vitro studies have shown that ERRαcan drive the expression of genes encoding lactoferrin, osteopontin, aromatase via classical estrogen response element (EREs) (Chen et al., 2001a; Vanacker et al., 1998; Zhang and Teng, 2000).
31
coactivators (Coward et al., 2001). Compound A and XCT790 are specific inverse agonists of
ERRαthat can disrupt the interaction between ERRαand the coactivators, such as PGC-1α,
they also down regulate the constitutive transcriptional activity of ERRαby inducing protein
degradation (Busch et al., 2004; Chisamore et al., 2009a, 2009b; Willy et al., 2004; Wu et al., 2009). From the attempt to identifying the ERRs ligand, we realized that the molecular basis of ERRs constitutive activity and antagonistic action of ligands is based on the LBD conformation change, in other words, that the residues located in the LBD can dictate whether or not to allow a small molecule to dock inside (Gaillard et al., 2007; Kallen et al., 2004). The existence of a potential natural ligand is however doubtful, since their LBD pocket is too small to accommodate a ligand.
2.4 Action mechanism
Crystallographic studies found that ERRα LBDis only 100 Å3, mainly because of the presence of Phe328 which is essential for the constitutive activity of ERRαIf there is a ligand, it should be very small, equivalent to 4-5 carbon atoms. The putative LBD is actually filled with amino-acid side chains, which leads to the conclusion that no useful low molecular weight agonist ligand could fit into the LBD of ERRα In fact, the transcriptional activity of ERRα is depe de t o the i te a tio ith ofa to s that pla a ole of p otei liga ds . Possible candidates are coactivators from the p160 and PGC-1 families or corepressors such as NCoR and RIP140 (Gaillard et al., 2007; Kallen et al., 2004) (Figure 10).
Figure 10. Model of ERRα mediated gene regulation. ERRs regulate target gene expression through interacting with coactivator PGC- α or corepressor RIP140 via binding to ERRE located in the promoter or at a distant site (Giguère, 2008).
2.4.1 PGC-1 coactivators
The X-ray structure of ERRαLBD together with a PGC-1αfragment indicated that E‘‘α displa s a transcriptionally active conformation in the absence of ligand, as judged by the position of H12. Mechanistically, PGC-1αacts at two levels on ERRαto regulate its transcriptional activity (Kallen et al., 2004).
Firstly, PGC-1αcan induce ERRα expression. Analyses have shown that the
ERRαmRNA level is expressed highly in tissues that have a high level of PGC-1αsuch as heart, kidney, brown adipose tissue and skeletal muscle (Schreiber et al., 2003). Besides, under physiological signals, such as cold exposure, the upregulated expression level of
PGC-32
1αis accompanied with the increased level of ERRαmRNA (Ichida et al., 2002). Secondly,
since the expression of both proteins is positive correlated, PGC-1αshould have an impact
on the expression of ERRα target genes. Evidence confirmed that ERRαhas weak
transcriptional activity which can be increased in the condition of overexpression of PGC-1α
suggesting that PGC-1αa ts as p otei liga d of ERRα (Kamei et al., 2003).
Mechanically, PGC-1αharbors three Leu-rich motif (L1, L2 and L3 in short), L2 containing the consensus LxxLL sequences which act as the major binding site for most
nuclear receptors, such as PPARγ, RXRαERα(Huss et al., 2002). Mutational studies showed
that L2 mutation in PGC-1α compromised for the interaction with other NRs, while still
allowed interaction with ERRα In contrast, PGC-1αmutated in L2/L3 lost interaction with
ERRα indicating that both L2 and L3 are the anchoring sites for ERRαLater evidence suggested that the L3 site harboring an LLxYL motif is a specific anchor for ERRα. Finally, this specific feature provides a tool to dissect the role of ERRαand other NRs involved in the
PGC-1αpathways (Huss et al., 2002, 2015; Scarpulla, 2011; Schreiber et al., 2003; Villena and
Kralli, 2008).
To sum up, the expression level and activity of ERRαis regulated through the
interaction with PGC-1α. The expression level of the ERRαPGC-1αcomplex is regulated by
signaling transduction pathways, including AMPK, MAP kinases, cAMP/PKA. ERRαis
responsible for the regulation of metabolic gene network to mediate the downstream effect
of PGC-1αHowever, the detailed e ha is of E‘‘α action in the PGC-1 pathways have
yet to be determined (Fritah et al., 2010; Giguère, 2008).
2.4.2 Repressors
ERRαcan act as an activator or a repressor and these specific activities are determined by the cofactors with which ERRαinteracts. For the case of repressor, the RIP140 and NCoR1 play a major role (Castet et al., 2006).
RIP140
RIP140 is a metabolic regulator highly expressed in adipose tissue, skeletal muscle, and heart. It displays coactivator and corepressors activities according to the context of
target genes and tissues. In the case of ERRα, RIP140 is mainly considered as a corepressor in
metabolic pathway. For example, RIP140 represses the expression of genes involved in glucose uptake and mitochondrial TCA and respiratory chain through the inhibition of
ERRαactivity. However, the mechanism of transcriptional repression of ERRE-dependent
activity of ERRαhas yet to be demonstrated. One hypothesis is that RIP140 contains four
inhibitory domains which can contribute to the negative effect of ERRαactivity (Castet et al., 2006; Fritah et al., 2010; Giguère, 2008).
33
NCoR
Nuclear receptor corepressor 1 (NCoR1) is a ubiquitously expressed corepressor which can interact with a number of transcriptional factors through leucine-rich domain located in its C-terminal part. NCoR1 exerts transcriptional repression through recruiting proteins displaying histone deacetylase activity, such as HDAC3, GPS2, TBL1, and HDAC3 is the core subunit (Pérez-Schindler et al., 2012 ; Webb et al., 2000 ; Yoon et al., 2003). The phenotype of NCoR1 muscle-specific knock out mice recapitulates many aspects of the phenotype of PGC-1 muscle-specific transgenic mice. For instance, high level of oxidative fibers, increased expression of mitochondrial enzymes, extended exercise endurance and
fatigue resistance have been observed in both mice types, indicating that NCoR1 and PGC-1α
are antagonistic to each other in regulating oxidative pathway (Pérez-Schindler et al., 2012).
ERRαinverse agonist XCT790 can inhibit the increased mitochondrial enzymes triggered by
NCoR1 knockdown, suggesting that ERRαis the main target gene of NCoR1 in oxidative metabolic pathway. On one hand, NCoR1 is a repressor of ERR, leading to decreased
expression of metabolic genes; on another hand, PGC-1α can activate ERRα to enhance
mitochondrial functions. Taken together, NCoR1 and PGC-1α compete with each other for
the interaction with ERRαin response to specific conditions (Giguère, 2008; Huss et al., 2015;
Pérez-Schindler et al., 2012).
2.5 Post-translational modifications
Post-translational modifications are another fundamental type of mechanisms to regulate the activities of transcription factors. Nuclear receptors are dynamically subjected to several modifications, such as methylation, acetylation, sumoylation and phosphorylation. Wilson et al found that ERRαspecifically interacts with p300 coactivator associated factor (PCAF), accompanied by acetylation at residues K129, K138, K160 and K162 in the DBD,
leading to antagonizing ERRDNA-binding activity. Conversely, this repressive effect can be
compromised by the deacetylase HDAC8 and Sir1 which interact with ERRαin the DBD,
finally potentiating its DNA-binding activity (Wilson et al., 2010). In addition, another study has shown that Ser19 and Ser22 (in A/B domain) can be phosphorylated, leading to Lys14 sumoylation, resulting in reduced interaction with the GRIP-1 coactivator, finally leading to repressive transcriptional activity of ERR(Vu et al., 2007).
2.6 Physiological functions 2.6.1 ERRα a d o e fate
ERRα mRNA and protein are expressed during endochondral and intramembranous
osteogenesis, and its expression is high in osteoblastic cell lines as well as normal human bones (Bonnelye et al., 1997; Bonnelye et al.,2001). Initially, in vitro studies have shown that
ERRαpromotes osteoblast differentiation (Bonnelye and Aubin, 2005). However, other
studies using mouse in vivo showed that ERRαknock outin female mice confers resistance
34
into osteoblasts, because of increased expression of Runx2 (the master gene for osteoblast differentiation). This leads to dysregulated expression of osteoblasts functional markers (APL, osteocalcin, osteopontin) and ultimately, increased mineralization activity (Carnesecchi and Vanacker, 2016; Gallet et al., 2013; Teyssier et al., 2009; Zhang et al., 2016a ).
2.6.2 ERRα a d Meta oli effe ts
Be ause E‘‘α is highly expressed in tissues with high energy demands such as heart, kidney, liver, muscle and fat (Bonnelye et al., 1997a; Deblois and Giguère, 2013), it was reasonable to speculate that this broad expression pattern indicates that the E‘‘α can influence a wide range of physiological processes, including mitochondrial biogenesis and function, lipid uptake and oxidation, tricarboxylic acid cycle and neoglucogenesis (Bianco et
al., 2012; Eichner and Giguère, 2011). Consistently, ERRαnull mice showed a compromised
response to stress imposed by exposure to cold, cardiac overload and bacterial infection (Huss et al., 2007; Villena et al., 2007).
E‘‘α
mice are unable to maintain their body temperature when exposed to cold,
the o adipo tes of E‘‘α-/-mice are characterized by a reduction of mitochondrial
density. A reduced expression of mitochondrial proteins (involved in fatty acid oxidation, tricarboxylic acid cycle and oxidative phosphorylation) was observed together with reduced
o idati e apa it . Ne e theless, E‘‘α-/- mice are resistant to diet-induced obesity, probably
due to the increased expression of parallel compensatory regulators in a tissue-specific manner. Indeed, PGC- α a d E‘‘γ a e up- egulated i E‘‘α-/- mice (Villena et al., 2007; Villena and Kralli, 2008).
Likewise, ERRαmice show accelerated heart failure in response to pressure overload, as well as reduced high-energy phosphate reserve and ATP synthetic capacity (Huss et al., 2007). E‘‘α is also e ui ed fo interferon-γ IFN-γ -stimulated antimicrobial
actions of macrophages. ERRα-/- macrophages are unable to increase mitochondrial
respiration and mitochondrial ROS (reactive oxygen species), and decreased a clearance of
Listeria monocytogenes in response to IFN-γ (Sonoda et al., 2007).
2.6.3 ERRα a d Ca er
E‘‘α high expression is associated with a poor prognosis in several cancers, such as ovarian, colon, endometrium, prostate and breast cancer, ERRβis weakly detected in the
above cancers. ERRγexpression is low in the endometrium and prostate cancers, but its high
expression level in ovary and breast cancers is associated with a favorable outcome which is
in contrast with ERRαThe mechanism leading to ERRs altered regulation is under exploration.
(Bianco et al., 2012; Tam and Giguère, 2016)
One of the hallmarks of cancer cells is the shift in energy production from oxidative phosphorylation to glycolysis, known as Warburg effect (Garber, 2004). Together with PGC1 coactivators, E‘‘α is an inducer of this effect (Chen et al., 2007). Apart from controlling
39
Chapter III--Epigenetics 3.1 The role of Epigenetics
The traditional concept tells us that DNA is the only carrier of genetic information (Crick, 1970).However, this rule cannot explain why genetically homogeneous genes are structurally and functionally heterogeneous. Monozygotic twins share a common genome background yet they differ in their susceptibility to various diseases (Fraga et al., 2005). Moreover, cloned animals show different phenotypes under identical genetic sequences. All this can be answered to, at least in part, by evoking epigenetics.
I , the te epige ot pe as fi st i t odu ed Co ad Hal Waddi gto and
led to a new research domain, aiming to study the causal developmental mechanisms linking
the genotype and the phenotype which was later e a ed as epige eti s . The definition
of Epigenetics is persistent developmental changes in gene activities and effects that result from changing in chromosome without alteration in the DNA sequences (Berger et al., 2009; Holliday, 1994; Jablonka and Lamm, 2012).
Epigenetics plays an important role in organism development and evolution; the characteristics are heritable and reversible diversities between cell types in response to endogenous and exogenous stimuli. Based on this understanding, the different functions of genetically identical genes are due to selective expression in response to specific signals. The phenotypic diversity of monozygotic twins and cloned animals are due to epigenetic differences acquired during embryonic development or later during life, such as living in different lifestyles and being exposed to different environments (Jaenisch and Bird, 2003; Webster et al., 2013).
3.2 Histone modification
The epigenetics processes control gene expression through DNA methylation, histone modification, histone variant, chromatin remodeling and non-coding RNA.
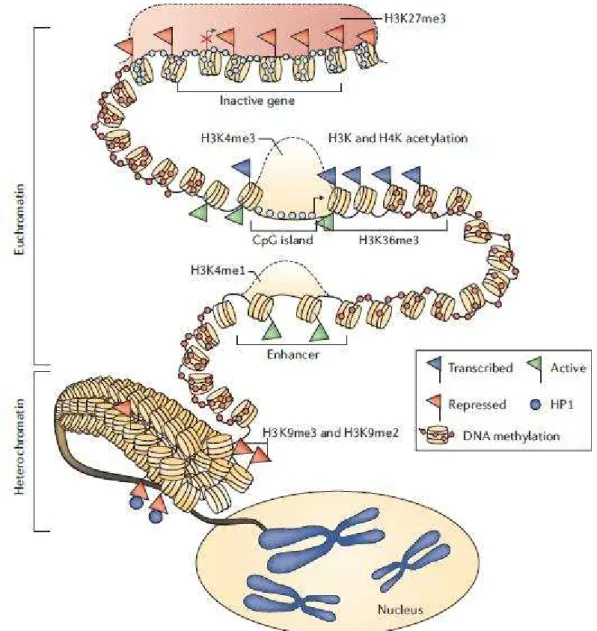
The basic unit nucleosome consists of approximately 147 bp DNA stretch which wraps around a histone octamer comprised of two copies of each core histone proteins (H2A,
H2B, H3 and H4). The u leoso e is li ked li ke DNA to fo eads-on-a-st i g
structures which are further bound by histone H1 to be compacted into high-order chromatin (Li, 2002; Strahl and Allis, 2000; Varier and Timmers, 2011) (Figure 15). Even though the chromatin is compressed tens of thousands of times, it is not a static unit, the histone tails protruding from the surface of the chromatin polymer are subjected to a variety of post-translational modifications. Up to now, the most prevalent histone post-translational modifications (PTM) are methylation, acetylation, ubiquitylation, sumoylation, phosphorylation, ADP-ribosylation and glycosylation. These modifications also impact variant histones, like H3.1, H3.3 and H2Z.1 (Berger, 2007; Varier and Timmers, 2011).
40
Figure 15. General chromatin organization (Strahl and Allis, 2000)
The attraction point which researchers spent lots of attention on epigenetics field is due to its increasing complexity as following aspects:
Firstly, gene activation or repression status is controlled by multiple histone modifications, acting sequentially or in a combinational manner on one or multiple tails,
referred to as the histo e ode (Berger, 2007; Strahl and Allis, 2000).
“e o dl , histo e odifi atio a tuall is a la guage e oded o the tails o globular domain which can be read by effector proteins to bring about distinct downstream events (Strahl and Allis, 2000; Taverna et al., 2007). For instance, The chromodomain, Tudor domain and PHD (the plant homeodomain) finger can read distinct methylation marks, leading to transcription activation/repression, interaction with other complex or enhancing other types modifications (Botuyan et al., 2006; Lachner et al., 2001; Pray-Grant et al., 2005; Shi et al., 2006; Taverna et al., 2007; Wysocka et al., 2006). Moreover, bromodomain can recognize histone lysine acetylation, such example is the interaction of bromodomain of P/CAF with acetylated H4K8 (Dhalluin et al., 1999), and the TAF1 double bromodomains target tightly to the dual acetyl lysine mark H4K5acK12ac (Jacobson et al., 2000). The bromodomain-acetyl-lysine is important for the assembly and activity of multi proteins complex in transcription activation (Jacobson et al., 2000; Taverna et al., 2007). In addition, histone modifications occur by adding chemically and structurally distinct moieties via specific enzymes (referred to as writers). PTMs are dynamic, meaning that the majority of
PTMs are removable by another kind of enzymes, referred to as e ase s , su h as histone
deacetylases (HDACs), histone demethylases (LSD1 class and Jumonji class), deubiquitinases, deiminases, and phosphatases (Chi et al., 2010). Fueled by the above writers, erasers and readers, and based on mass spectrometry technology, more than 70 distinct PTMs were reported, but the target residues are limited, including lysine (K), arginine (R), serine (S),threonine (T), tyrosine (Y), histidine (H) and glutamic acid (E) (Taverna et al., 2007). Among these, lysine is a center substrate in PTMs, including methylation, acetylation, ubiquitination and sumoylation (Berger, 2007; Sims et al., 2003). It is generally accepted that acetylation leads to activation, sumoylation generates repression, whereas the effect of methylation and ubiquitination is related to the precise residue and context. For instance,
41
methylation of H3K9, H3K27 and H4K20 can induce gene repression; while H3K4, H3K36 and H4K79 methylation linked to gene activation (Berger, 2007; Sims et al., 2003).
Thirdly, another level of complexity is associated with methylation in that activation and repression are not only dependent on the residue but also on the methylation degree. Lysine can indeed be mono-, di- or tri-methylated and each of these steps has different biological consequences. For example, genes decorated by H3K4me3 become induced, whereas genes mediated by H3K9me3 are repressed (Berger, 2007; Chi et al., 2010).
3.3 Histone modification mechanism in transcription
The mechanisms through which the histone code is deciphered by cells are under intense investigation and two conceptual models are accepted widely. In the first one, PTMs can directly alter the physical properties of chromatin so as to lose its compaction. For instance, lysine acetylation attenuates the histone-DNA or histone-histone interactions by neutralizing the positive charges on histone tails (Sims et al., 2003;Webster et al., 2013). In the second one, PTMs can serve as a binding surface for the recruitment of reader proteins to indirectly change chromatin compaction which are mentioned above (Campos and Reinberg, 2009;Taverna et al., 2007; Wolffe and Hayes, 1999).
3.4 Histone methylation
The residues undergoing methylation are arginine, lysine and histidine (Greer and Shi, 2012). Arginine can be monomethylated, symmetrically or asymmetrically dimethylated by members of the protein arginine N-methyltransferase (PRMT) family; histidine methylation is rare and little addressed (Bannister and Kouzarides, 2005; Greer and Shi, 2012). Lysine methylations are the most frequently occurring and appear in mono-,di- or tri-methylated states (Greer and Shi, 2012; Sims et al., 2003).
Lysine methylation is a process by which the methyl group from the donor S-adenosylmethionine (SAM) is transferred to acceptor by histone methyltransferases (HMTs) (Varier and Timmers, 2011). Evidence has shown that some enzymes can locate to the target residue through specific DNA response element, for instance, the enzyme PcG (Polycomb group) protein mediating H3K27me3 is recruited through PcG response element(PREs); in addition, long non-coding RNAs (IncRNAs), small non-coding RNAs as well as DNA methylation also play roles in directing histone methylation (Greer and Shi, 2012; Teperino et al., 2010). There are five lysines within H3 (K4, K9, K27, K36 and K79) and one lysine within H4 (K20) which can be modified by histone methyltransferases and histone demethylases (Figure 16). The functional outcome of lysine methylation is dependent on the residue and methylation state.