HAL Id: tel-03204740
https://tel.archives-ouvertes.fr/tel-03204740
Submitted on 21 Apr 2021HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
The reorganization of human brain networks in the early
stages of multiple sclerosis
Ismail Koubiyr
To cite this version:
Ismail Koubiyr. The reorganization of human brain networks in the early stages of multiple sclerosis. Neurons and Cognition [q-bio.NC]. Université de Bordeaux, 2019. English. �NNT : 2019BORD0152�. �tel-03204740�
THÈSE PRÉSENTÉE POUR OBTENIR LE GRADE DE
DOCTEUR DE
L’UNIVERSITÉ DE BORDEAUX
École doctorale des sciences de la vie et de la santé Spécialité Neurosciences
Par Ismail KOUBIYR
THE REORGANIZATION OF HUMAN BRAIN NETWORKS IN
THE EARLY STAGES OF MULTIPLE SCLEROSIS
Sous la direction de Monsieur le Professeur Bruno Brochet
Soutenue publiquement le 17 Septembre 2019
Membres du jury :
Madame la Professeure Iris-Katharina Penner, Heinrich Heine University, Düsseldorf. Rapporteur, Présidente Monsieur le Docteur Menno M. Schoonheim, VU University Medical Center, Amsterdam. Rapporteur
Monsieur le Professeur Charles R.G. Guttmann, Harvard University, Boston. Examinateur Madame le Docteur Céline Louapre, Université Paris-Sorbonne. Examinatrice Madame le Docteur Gwenaëlle Catheline, Université de Bordeaux. Examinatrice
Titre : Réorganisation des réseaux cérébraux dans les stades précoces de la
sclérose en plaques
Résumé : Les troubles cognitifs sont fréquents dans la sclérose en plaques (SEP) mais leurs mécanismes sous-jacents sont encore mal connus. Les techniques d’IRM ont été indispensables pour essayer de mieux comprendre les substrats biologiques des processus cognitifs. L’objectif de cette thèse est de mieux comprendre les mécanismes physiopathologiques du fonctionnement cognitif dans les stades précoces de la SEP. Pour cela, nous avons étudié une cohorte de patients atteints de syndrome cliniquement isolé (SCI) pendant un an, en réalisant une batterie de tests neuropsychologiques ainsi qu’un examen IRM. Nous avons tout d’abord démontré une atteinte précoce de la substance grise, en particulier au niveau de l’hippocampe, se propageant vers le cortex après un an d’évolution. L’atteinte microstructurale précoce de l’hippocampe était capable de prédire sa perte de volume. Ensuite, nous nous sommes intéressés à la réorganisation des réseaux cérébraux fonctionnels à ce stade précoce de la maladie. En utilisant l’IRM fonctionnelle de repos, nous avons démontré une réorganisation cérébrale fonctionnelle précoce impliquant plusieurs régions cérébrales. Cette réorganisation était encore plus prononcée après un an d’évolution. Au même moment, nos patients présentaient un fonctionnement cognitif normal qui était associé au niveau de réorganisation cérébrale présente. Ces résultats suggèrent un mécanisme de compensation aux stades précoces de la pathologie. La relation entre ces modifications fonctionnelles et l’anatomie sous-jacente est inconnue dans la SEP. Nous avons ainsi décidé de combiner l’IRM fonctionnelle de repos et l’imagerie par tenseurs de diffusion pour étudier à la fois la connectivité fonctionnelle et la connectivité structurelle. En utilisant le paramètre de couplage structurel-fonctionnel, nous avons démontré un découplage, un an après l’apparition de la maladie, au niveau de trois réseaux cérébraux du repos (salience, visuel et somato-moteur). Ce découplage était observé alors même que les performances cognitives de nos patients étaient préservées et que la réorganisation fonctionnelle était présente. Ces résultats suggèrent que cette réorganisation fonctionnelle à ce stade, agissant comme un mécanisme de compensation, se produit à travers des connections anatomiques indirectes. Afin de confirmer ces résultats et de suivre l’évolution des réseaux cérébraux et leur impact sur la cognition, nous avons recontacté nos patients SCI pour un suivi à 5 ans.
Mots clés : Sclérose en plaques, IRM, syndrome cliniquement isolé, connectivité, IRM fonctionnelle, imagerie par tenseur de diffusion.
Title: The reorganization of human brain networks in the early stages of
multiple sclerosis
Abstract: Cognitive impairment is frequent in multiple sclerosis (MS) but its underlying mechanisms are still poorly understood. MRI techniques have been a valuable tool to investigate the biological substrates of cognitive processes. The objective of this thesis was to better understand the pathophysiological mechanisms of cognitive functioning at the early stage of MS. We followed clinically isolated syndrome (CIS) patients for one year, using neuropsychological tests, conventional and more advanced MRI techniques. We first demonstrated a differential gray matter vulnerability at the beginning of MS with a pathological spread from the hippocampus towards the cortex. We showed that the first microstructural alterations taking place within the hippocampus were able to predict its future volume loss. After that, we were interested in the potential brain functional reorganization at this stage of the disease. Using resting-state functional MRI, we were able to demonstrate very early regional brain functional reorganization starting from the disease onset and becoming more pronounced after one year of evolution. We also noticed a preservation of cognitive performances in CIS patients, which we found was associated to more functional reorganization. These results suggested then a compensation mechanism at the first year after a CIS. However, the relationship between these functional changes and the underlying anatomy was still missing. Thus, we combined resting-state functional MRI and diffusion tensor imaging to represent both functional and structural connectivity. Using the structural-functional coupling parameter, representing the association between structural and functional connections, we showed a decoupling one year after the disease onset in three major networks (salience, visual and somatomotor networks). This decoupling was noticed while cognitive performances were preserved and functional reorganization present. These last results led us to suggest that the functional reorganization at this stage, acting as a compensation mechanism, occurs along indirect anatomical pathways. In order to confirm these results and further follow-up brain networks topology and its impact on cognition, we are currently calling back our CIS patients for their 5-year visit.
Keywords: Multiple sclerosis, MRI, clinically isolated syndrome, connectivity, functional MRI, diffusion tensor imaging.
Unité de recherche
Acknowledgements
Au Professeur Bruno Brochet,
Je vous remercie de m’avoir fait l’honneur de diriger ce travail. Je tiens à vous remercier pour votre disponibilité, votre soutien et votre confiance au cours de ces trois années. Merci à vous pour votre patience à toutes épreuves lors de la relecture (mais aussi la soumission) de mes papiers. Merci pour toutes les connaissances que vous m’avez apportées et pour votre encadrement qui ont permis d’aboutir à ce résultat. J’aimerais vous témoigner ici de ma
gratitude, mon profond respect et mon admiration pour votre carrière et votre implication dans la recherche.
To Professor Iris-Katharina Penner and to Doctor Menno Schoonheim,
Thank you very much for accepting to judge this work. Thank you for taking the time to make the journey to Bordeaux. It is a great honor to present my results in front of international experts in the field of multiple sclerosis. Your professional careers are a model to me.
Au Professeur Charles Guttmann,
Je tiens d’abord à te remercier d’avoir accepté d’être présent au sein de mon jury de thèse. Merci de m’avoir transmis ta passion pour la recherche et plus particulièrement pour la sclérose en plaques. Merci de m’avoir accueilli au sein de ton équipe. J’ai énormément appris lors de ce stage. Merci de m’avoir judicieusement dirigé vers Bordeaux afin de réaliser cette thèse. Enfin je tiens à te remercier pour tous tes conseils et ton soutien lors de ces dernières années.
Au Docteur Gwenaëlle Catheline et au Docteur Céline Louapre,
Vous me faites l’honneur de juger mon travail au regard de vos expertises respectives. Je vous remercie de votre présence aujourd’hui.
To Doctor Lucina Uddin,
Thank you very much for making the journey to join us in Bordeaux and for accepting to judge this thesis work. It is a great honor to present my work in front of an international expert of brain imaging. I have the utmost admiration for your work and your career.
Au Docteur Aurélie Ruet,
Je te remercie pour ton aide, ton soutien infaillible et ta participation à ce travail de thèse. J’ai une profonde admiration pour tes connaissances et ton éthique de travail. Merci aussi de m’avoir fait confiance pour continuer à travailler sur tes cohortes, je suis heureux de pouvoir poursuivre mon travail à tes côtés pendant encore deux années.
Au Professeur Thomas Tourdias,
Je te remercie pour ton aide constante lors de ces trois dernières années, ta bienveillance, la qualité de tes conseils et pour ta disponibilité indéfectible à relire mes papiers et à m’orienter lors de mes travaux. J’ai une admiration pour ton enthousiasme pour la recherche, ta passion pour l’imagerie et ton parcours professionnel.
Au Professeur Vincent Dousset,
Merci pour nos discussions, pour votre soutien, et pour votre accueil au sein de la plateforme de bio-imagerie. Soyez assuré de mon respect et ma gratitude.
Au Docteur Mathilde Deloire,
Merci infiniment pour ton soutien et ton aide dès mon arrivée dans l’équipe. J’aimerais te
remercier pour ta disponibilité, ton implication à toute épreuve et tes idées qui m’ont guidées lors de ces travaux de thèse. Enfin, merci pour ta gentillesse et ton humour tout au long de ces
années.
Au Docteur Cécile Dulau,
Merci pour ta gentillesse, ta disponibilité, ton soutien et ta bonne humeur. J’ai hâte qu’on puisse avancer ensemble sur SO-COG.
Au Docteur Delphine Lamargue-Hamel,
Merci pour ton soutien, ta disponibilité et ton aide au cours de ces trois années. Nos discussions et tes conseils m’ont beaucoup aidé lors de mon parcours. Enfin, merci d’avoir fait le tour de Carreire avec moi pour trouver une salle de thèse.
Au Docteur Amandine Moroso et au Docteur Vincent Planche,
Merci pour votre soutien, votre gentillesse et votre disponibilité. J’ai énormément appris à partir de vos travaux de thèse respectifs. J’espère qu’on aura l’occasion de collaborer à nouveau. Au Docteur Pierrick Coupé,
Merci pour ta gentillesse et ta disponibilité pour m’aider et me guider lors de mes travaux de thèse. J’ai une grande admiration pour ta maitrise de l’imagerie cérébrale et ton parcours professionnel.
Au Docteur Aude Panatier et au Docteur Stéphane Oliet,
Merci infiniment de m’avoir accueilli et intégré à votre équipe. Merci pour votre disponibilité, votre gentillesse, votre soutien et votre regard critique sur mon travail. J’ai une fascination pour les travaux que vous menez chez l’animal et j’apprends énormément à chaque réunion de labo.
Merci aussi à tous les membres de l’équipe pour leur sympathie et pour les moments passés autour de bons repas.
A l’équipe du CRMBM de Marseille,
Au Professeur Jean Pelletier, au Professeur Jean-Philippe Ranjeva, au Professeur Bertrand Audoin, au Docteur Pierre Besson pour nous avoir accueilli au sein de leur équipe et nous avoir transmis toutes leurs connaissances de l’IRM fonctionnelle. Merci plus particulièrement à Pierre pour ton aide précieuse et pour ta participation à ces travaux, en espérant que cette collaboration puisse continuer.
A toute l’équipe du service de Neurologie du CHU de Bordeaux,
A Katy, Dieynaba, Timothé, Julie, Aurore, Emeline, Nath, pour m’avoir si bien accueilli dans l’équipe. Merci pour votre gentillesse, votre bonne humeur et tous ces bons moments qui ont fait que ces trois années ont filé à toute vitesse.
A ma sœur, mes amis, et en particulier Moncef, pour les moments passés ensembles et leur soutien tout au long de ces années.
A mes parents,
Pour leur amour, leur générosité, leur soutien inconditionnel, leur éducation et les valeurs qu’ils m’ont inculquées. Je vous dois ce que je suis aujourd’hui et je continuerai à faire de mon mieux pour vous rendre fier. Il n’y a pas assez de mots pour vous exprimer ma gratitude, ma
reconnaissance et mon amour. A ma femme Zineb,
Pour ton amour, ta tendresse, ton soutien et ta patience durant ces dernières années de thèse pas toujours faciles. Tu étais la bouffée d’oxygène qui me ressourçait dans les moments les plus difficiles. Sans toi, cette thèse ne serait pas. Je n’ai pas de mot pour pouvoir te dire ce que tu représentes, ce que tu m’as apporté, et le bonheur d’être à tes côtés.
Enfin, j’aimerais dédier ce travail à la mémoire de ma grand-mère. Une femme unique. Pour son amour inconditionnel, sa générosité sans limite, sa bonté et ses encouragements. J’ai eu la chance de grandir à tes côtés. Tu es toujours dans mon cœur et aurais été très fière de me voir arriver jusque-là.
Table of contents
Presentation of the candidate 13
Glossary 15
Chapter 1: General introduction 17
MS pathology 18
Clinical phenotypes 18
Clinically isolated syndrome 19
Treatment 20
Cognitive impairment in MS 20
Visualizing brain abnormalities in MS 21
o White/gray matter lesions 21
o Brain atrophy 24
o Advanced MRI techniques 24
o Other techniques 25
o Connectomics: Mapping the human brain 28
Aims of the thesis 30
Thesis outline 32
Chapter 2: Differential gray matter vulnerability in the early course of MS 37
Summary page 39
Article: Differential gray matter vulnerability in the one year following
a clinically isolated syndrome 41
Chapter 3: Longitudinal functional brain network reorganization in early MS 61
Summary page 63
Article: Longitudinal study of functional brain network reorganization
in clinically isolated syndrome 65
Chapter 4: Dynamic structural-functional coupling alterations in early MS 91
Summary page 93
Article: Dynamic modular-level alterations of structural–functional
coupling in clinically isolated syndrome 95
Chapter 5: General discussion 127
Main findings 129
Mechanisms of cognitive impairment in MS 130
Limitations 133
Perspectives 134
o 5-year follow-up of the CIS-COG study 134
o The GM-COG study 134
o Hippocampal subfields in the early stage of MS 134
o Thalamic subfields 136
Annex 143
Article: Regional hippocampal vulnerability in early multiple sclerosis:
Presentation of the candidate
Ismail Koubiyr
54 Rue El-Alamein, 33000 Bordeaux ismail.koubiyr@gmail.com
+33(0)7.61.51.36.01 Education
INSERM U1215, Neurocentre Magendie – Bordeaux, France October 2016 – September 2019 PhD Candidate in Neuroscience - Neuroimaging
The reorganization of human brain networks in the early stages of multiple sclerosis
Supervisor: Pr.Bruno Brochet
Institut National des Sciences Appliquées (INSA) & Université Claude Bernard Lyon I – Lyon, France Graduation: September 2016 Master of Research: Electronics, Power Electronics, Automatism and processes (EEAP)
- Specialized in Digital signal and image processing.
Institut National des Sciences Appliquées (INSA) - Lyon, France Graduation: September 2016 Electrical Engineer
- Specialized in Digital signal and image processing.
- Accomplished the preparatory cycle under the SCAN program (English learning). - Completed team-management training.
Korean Advanced Institute of Science and Technology (KAIST) - Daejeon, South Korea September 2014- June 2015 - One-year exchange program.
- Specialized in Digital Image processing (Image processing, Image analysis). Experience
Research Trainee at Center for Neurological Imaging, Brigham and Women’s Hospital, Harvard Medical School – Boston, USA - Transversal characterization of abnormally diffused white matter in multiple sclerosis. March 2016 - September 2016 - Development of new tools to analyze structural MRI (T1,T2,PD,Diffusion).
Tutor at INSA – Lyon, France September 2011 – June 2014 - Offered Mathematics and Physics support to both freshmen at my school and to middle/high school students.
Improving thus, their grades and overall performance by implementing effective and adequate programs. Teaching Experience
Teaching assistant at the Université de Bordeaux. Academic year 2018/2019
• Image processing and analysis (L3) 14h
• Introduction to medical imaging (L1) 6h
Academic year 2017/2018
• Pathology and MRI: exploring cognitive dysfunction in multiple sclerosis (M2) 4h
• Image processing and analysis (L3) 12h
• Introduction to medical imaging (L1) 6h
Publications
1. Koubiyr I, Besson P, Deloire M, Charré-Morin J, Saubusse A, Tourdias T, Brochet B and Ruet A (2019) Dynamic modular-level alterations of structural-functional coupling in clinically isolated syndrome. Brain. doi: 10.1093/brain/awz270
2. Koubiyr I, Deloire M, Besson P, Coupé P, Dulau C, Pelletier J, Tourdias T, Brochet B, Ranjeva JP and Ruet A (2018) Longitudinal Study of Functional Brain Network Reorganization in Clinically Isolated Syndrome. Mult Scler. 1–13. doi: 10.1177/1352458518813108
3. Koubiyr I, Deloire M, Coupé P, Dulau C, Besson P, Moroso A, Planche V, Tourdias T, Brochet B and Ruet A (2018) Differential Gray Matter Vulnerability in the 1 Year Following a Clinically Isolated Syndrome. Front. Neurol. 9:824. doi: 10.3389/fneur.2018.00824
4. Planche V, Koubiyr I, Romero J.E, Manjon J.V, Coupé P, Deloire M, Dousset V, Brochet B, Ruet A and Tourdias T (2018) Regional hippocampal vulnerability in early multiple sclerosis: dynamic pathological spreading from dentate gyrus to CA1. Human Brain Mapping. doi: 10.1002/hbm.23970
Talks and posters
1. Koubiyr I, Deloire M, Charre-Morin J, Saubusse A, Coupé P, Dulau C, Tourdias T, Besson P, Ranjeva JP, Pelletier J, Audoin B, Brochet B, Ruet A. Longitudinal study of functional brain networks in clinically isolated syndrome. European Charcot Foundation (ECF), Baveno, Italy, November 15th-17th 2018
2. Koubiyr I, Palotai M, Deloire M, Charre-Morin J, Saubusse A, Tourdias T, Guttmann CRG, Brochet B, Ruet A. Microstructural damage in cortico-subcortical white matter tracts in patients with clinically isolated syndrome: prediction of cognitive functioning and follow-up of its change for 1 year. European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Berlin, Germany, October 10th-12th 2018
3. Koubiyr I, Deloire M, Ruet A, Charre-Morin J, Saubusse A, Brochet B, Dulau C. Changes in select resting-state brain functional networks and preservation of social cognitive performances in multiple sclerosis. European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Berlin, Germany, October 10th-12th
2018
4. Palotai M, Koubiyr I, Morales Pinzon A, Makris N, Healy B.C, Glanz B, Weiner H.L, Chitnis T, Guttmann CRG. Microstructural damage to associative cortico-thalamic tracts play a role in the pathophysiology of fatigue in multiple sclerosis. European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Berlin, Germany, October 10th-12th 2018
5. Koubiyr I, Deloire M, Besson P, Coupé P, Dulau C, Tourdias T, Pelletier J, Audoin B, Brochet B, Ranjeva JP, Ruet A. Reorganization of functional brain network topology in clinically isolated syndrome: A 1-year longitudinal study. Organization for Human Brain Mapping (OHBM), Singapore, 17th-21st 2018
6. Koubiyr I, Deloire M, Besson P, Coupé P, Dulau C, Tourdias T, Pelletier J, Audoin B, Brochet B, Ranjeva JP, Ruet A. Reorganization of functional brain network topology in clinically isolated syndrome: A 1-year longitudinal study. ARSEP Multiple Sclerosis meeting, June 1st 2018
7. Koubiyr I, Deloire M, Charre-Morin J, Saubusse A, Coupé P, Dulau C, Tourdias T, Besson P, Ranjeva JP, Pelletier J, Audoin B, Brochet B, Ruet A. Relationships between reorganization of functional brain network topology and cognition in Clinically Isolated Syndrome: A 1 year Resting state fMRI longitudinal study. European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), October 25th-28th 2017
8. Koubiyr I, Deloire M, Charre-Morin J, Saubusse A, Coupé P, Dulau C, Tourdias T, Besson P, Ranjeva JP, Pelletier J, Audoin B, Brochet B, Ruet A. Microstructural alterations precede deep grey matter volume loss in patients with clinically isolated syndrome. European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), October 25th-28th 2017
Grants and awards
• 2-year post-doctoral grant from LabEx TRAIL, Bordeaux, France (100 000€)
• Best poster presentation award from the European Charcot Foundation (ECF), Baveno, Italy, 2018 (4000€) • European Charcot Foundation (ECF) Travel grant, Baveno, Italy, 2018
• European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) Travel grant, Berlin, Germany, 2018
• European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) Travel grant, Paris, France, 2017
Glossary
BDI = Beck Depression Inventory
BOLD = Blood Oxygen Level-Dependent CIS = Clinically Isolated Syndrome CSF = Cerebrospinal Fluid
Cth = Cortical thickness DGM = Deep gray matter
DIR = Double Inversion Recovery DTI = Diffusion Tensor Imaging
EDSS = Expanded Disability Status Scale FA = Fractional Anisotropy
FC = Functional Connectivity FDR = False Discovery Rate
FLAIR = Fluid-Attenuated Inversion Recovery HC = Healthy Controls
HLA = Human Leukocyte Antigen IPS = Information Processing Speed MD = Mean Diffusivity
MRI = Magnetic Resonance Imaging MS = Multiple Sclerosis
MSFC = Multiple Sclerosis Functional Composite NABT = Normal Appearing Brain Tissue
PPMS = Primary Progressive Multiple Sclerosis PwCIS = Patients with Clinically Isolated Syndrome RRMS = Relapsing-Remitting Multiple Sclerosis SC = Structural Connectivity
SPMS = Secondary Progressive Multiple Sclerosis TIV = Total Intracranial Volume
Chapter 1
General introduction
MS pathology
Multiple sclerosis (MS) is an inflammatory, demyelinating and neurodegenerative disease of the central nervous system.1 It is one of the most common neurological disorders among young adults
and women are more prone to develop the disease (2.3/1 female-to-male ratio).2 This chronic
disease affects more than 2.3 million people worldwide with approximately 100000 patients in France.3 Although its exact etiology is currently unknown, the disease is thought to be
multifactorial as some genetic and environmental risk factors have been identified, such as Epstein-Barr viral infection, HLA genotype, vitamin D levels (sun exposure), salty diet and smoking.1
It is usually accepted that in MS, an autoimmune reaction is initiated in the periphery, where macrophages and leukocytes (e.g. T-cells, B-cells) migrate to the brain due to the leakage of the blood brain barrier. As these lymphocytes and macrophages accumulate, pro-inflammatory cytokines increase the immune response by recruiting microglia. Then, a part of infiltrating T-lymphocytes recognizes myelin, leading to acute demyelination, notoriously known as lesions.1 These lesions can be partly remyelinated by oligodendrocytes in the early stage of the disease, resulting in the preservation of axons and neurons to a certain degree.4 Axonal damage becomes more pronounced as the disease progresses, which is thought to be mediated by mitochondrial dysfunction with the production of reactive oxygen species.5 At this stage, both white and grey matter (cortex and deep grey matter structures) atrophy is observed.6
Clinical phenotypes
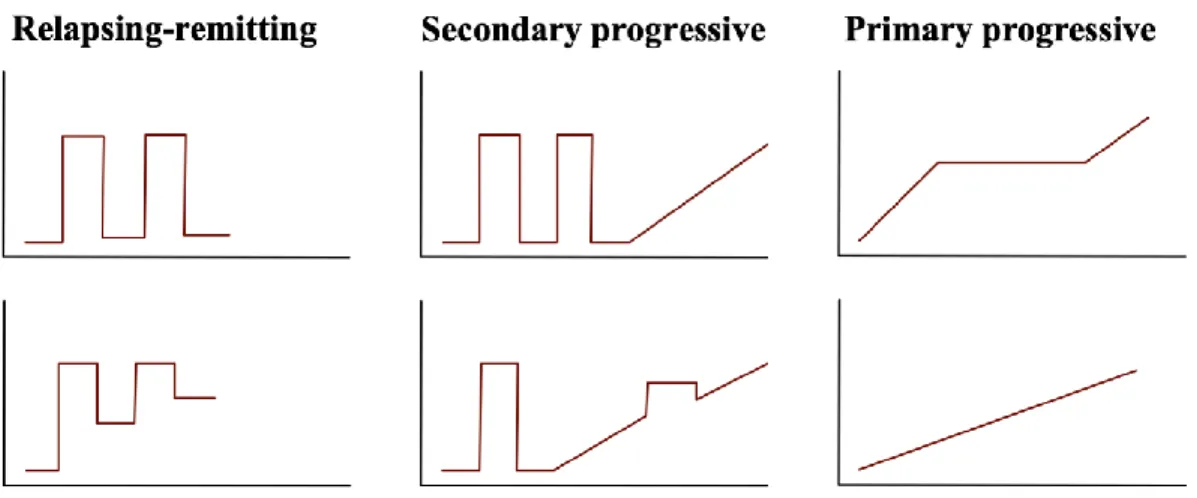
Multiple sclerosis in characterized by an unpredictable clinical course, as some patients progress rapidly, while others remain relatively stable over the course of the disease. The early phase of MS usually presents acute symptom worsening (also called attacks or exacerbations), followed by partial or complete recovery (relapsing-remitting MS, RRMS) due to demyelination and remyelination processes. With time, recovery from each episode is incomplete and persistent symptoms accumulate. In some patients, symptoms will progressively worsen without relapses or partial recovery, this phase is called secondary progressive MS (SPMS). In some other cases, patients may not present relapse-onset MS, as they show steady worsening of neurologic functioning without any distinct relapses or periods of remission (primary-progressive MS, PPMS) (Figure 1).1
Figure 1. Multiple sclerosis phenotypes
Depending on the location of lesions, RRMS patients may present symptoms including acute unilateral optic neuritis, partial myelitis or a brainstem syndrome,7 while progressive-onset patients’ symptoms tend to progress slowly over the course of months or even years, and include symptoms such as paraparesis, hemiparesis or cerebellar ataxia.7 Other MS physical symptoms include tingling, body weakness, numbness, imbalance, spasms, gait impairment and vision loss.1 According to the consensual McDonald criteria based on clinical and/or MRI criteria, MS is diagnosed by the dissemination in time and space of the demyelinating lesions.8 More recently, the presence of CSF-specific oligoclonal bands may now substitute for dissemination in time even if baseline MRI findings do not meet dissemination in time criteria, and the presence of cortical lesions may now fulfill MRI criteria for dissemination in space (in addition to juxtacortical lesions).9
Clinically isolated syndrome
Clinically isolated syndrome (CIS) describes the first monophasic episode of acute or sub-acute onset suggestive of MS.10 Patients presenting at first with a CIS, may be diagnosed with MS if fulfilling the dissemination in time criteria. Additionally, the long-term risk for clinically definite MS is 60–80% in CIS patients with demyelinating lesions on their first MRI scan.10 This very early stage of MS is then the perfect window to study the first brain alterations present in MS to obtain a better understanding of the evolving pathogenesis of CIS. This explains our choice to study the evolution of such population in the remainder of the manuscript.
Treatment
During MS relapses, it is usual to treat the patient with high-dose methylprednisolone to immediately relieve the symptoms. However, these corticosteroid treatments have no significant impact on long-term disability and do not prevent future relapses. To this end, patients with RRMS are usually treated with disease modifying treatments with either immunomodulatory or immunosuppressive characteristics to prevent the occurrence of new inflammatory episodes and slow down disease progression. Distinction can be made between first-line therapy and second-line therapy. First-second-line therapy aims to reduce the inflammatory response by adjusting the immune response (e.g. interferon beta, glatiramer acetate, dimethyl fumarate, teriflunomide). Second-line therapies are more effective, but usually have more potential severe side effects as they interfere more importantly with the immune system (e.g. natalizumab, fingolimod).11 These current available treatments for MS all target the inflammatory component of the disease. However, new potential therapies aiming at repairing the damaged axons using a remyelination approach are currently undergoing clinical trials.12
Cognitive impairment in MS
For many years, cognitive impairment in MS used to be neglected as research and clinical attention mainly focused on the physical problems. However, it is admitted now that it is frequent in MS and appears even early during the course of the disease, from the CIS stage.13,14 This stage is
particularly interesting because it is the critical time when cognitive deficits occur and become detectable. In the years after the CIS, the frequency of these deficits increases notably (from 29% to 54% after 5 years),15 meaning that there is a strong opportunity to understand the substrate of
these deficits and to set up therapeutic strategies. Increasing evidences have shown the pejorative impact of early cognitive impairment in MS affecting quality of life and daily living with vocational impact.16,17 Cognitive impairment associated with MS concerned several cognitive domains including episodic memory, attention, working memory and executive functions.13 However, slowness of information processing speed (IPS) is the main cognitive dysfunction observed in MS even at the earlier stages and is associated with poor prognosis, significant consequences on employment status and decreased quality of life.17 Besides IPS, it is also accepted that episodic memory is frequently and early impaired. Recently an international group of MS
experts has suggested both IPS and episodic memory as the minimal cognitive assessment in patients with MS (Brief International Cognitive Assessment for MS, BICAMS).18 In addition to
BICAMS, several other well-established neuropsychological test batteries are available to assess the performance on different cognitive domains in MS (e.g. the Brief Repeatable Battery of Neuropsychological tests (BRB-N),19 the Minimal Assessment of cognitive Function in MS (MACFIMS)20).
Visualizing brain abnormalities in MS
The advent of magnetic resonance imaging (MRI) has provided a further powerful tool for the in-vivo investigation of diseases of the central nervous system. In MS, MRI has become an invaluable tool for diagnosing the disease as the diagnosis heavily relies on proof of disease dissemination in space and time.21 As such, MRI can support and substitute clinical information for MS diagnosis, enabling an early and accurate diagnosis and thus, early treatment.
During the last two decades, MRI has been extensively used in the study of MS using a variety of MRI techniques. These MRI techniques used in MS research can vary from conventional MRI sequences visualizing brain lesions or atrophy, usually used in the clinical setting, to advanced sequences quantifying the microstructural integrity of brain tissue and network characteristics. In the following, we will first introduce several of these MRI sequences. Then, we will discuss how these techniques can provide insight into the neural correlates of cognitive impairment.
White/gray matter lesions
White matter lesions represent a typical characteristic of MS and can be visualized using conventional MRI sequences such as T2-weighted or fluid attenuated inverse recovery techniques (FLAIR) (Box 1). Both lesion load and lesion counts have been intensively investigated in MS. However, studies showed poor correlation between these parameters and the actual clinical disability of patients. This is called the clinico-radiological paradox of MS.22 Indeed, some patients showing a large number of white matter lesions can be mildly affected, while others with fewer white matter lesions show a more severe disability. This led MS researchers to investigate gray matter lesions as they may be more prone to play a role in cognitive functioning. In order to do that, the double inversion recovery (DIR) sequence was used as it was shown to improve the detection of intracortical lesions which was verified in post-mortem tissues as well.23,24 Even
though the DIR sequence still lacks some sensibility in detecting all cortical lesions, active research towards a consensus DIR sequence is currently ongoing.25 These gray matter lesions were stronger
associated to cognitive deficits than white matter lesions, even though correlations were still moderate.26,27
Box 1. Conventional MRI sequences
Below, we will describe standard MRI sequences that have been mostly used in the diagnosis of MS and the patient’s follow-up.
T1- and T2-weighted imaging
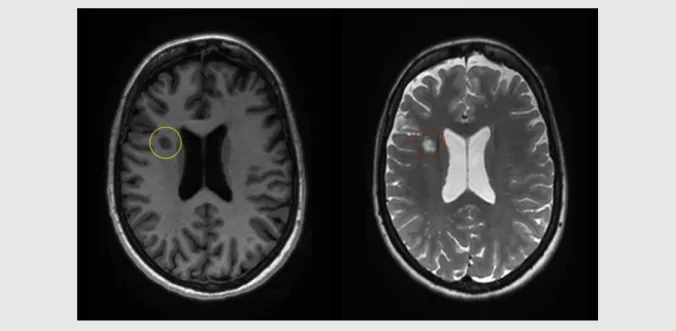
With a T1-weighted sequence, we can easily visualize the anatomy of the brain with a good contrast between white and gray matter. A subset of MS lesions can be seen as focal hypointensities (black holes) (Figure 2), indicating axonal and myelin loss. Additionally, this sequence allows the inspection of acute inflammatory processes using a contrast agent (e.g. gadolinium), that can go through the blood brain barrier if disrupted. This results in hyperintense lesions in these images. On the other hand, using a T2-weighted sequence allows to detect MS lesions as focal hyperintensities (Figure 2). With that, we can calculate the count and volume of these lesions, which represents an important parameter to monitor the evolution of the disease. Both these sequences are usually included in routine clinical practice. They are also used in research, as T2-weighted images provides the patient’s lesion load, while T1-weighted images are mostly used to segment the brain (e.g. white matter, gray matter) and calculate the corresponding volumes.
Figure 2. T1- and T2-weighted image of a patient with MS
A T1-weighted image (left) in which black hole is indicated by a yellow circle, and a T2-weighted image (right) in which white matter lesion is indicated by a red circle.
Fluid attenuated inverse recovery sequence
Using a fluid attenuated inverse recovery sequence (FLAIR), we can highlight MS lesions as focal hyperintensities, while suppressing the cerebrospinal fluid’s (CSF) signal (Figure 3). In this case, lesions are better distinguished from CSF, such as in periventricular lesions for example. FLAIR images are therefore superior to T2-weighted images for detecting MS lesions, and are often used in research settings.
Figure 3. FLAIR image of a patient with MS
Brain atrophy
Brain atrophy in MS was first observed in the first half of the 19th century by Cruveilhier and Carswell when they described the presence of lesions accompanied by atrophy. Later on, with the advent of in-vivo brain imaging, ventricles enlargement was observed in the late 1970s using computerized tomography (CT), indicating central atrophy.28 Then MRI replaced CT for volumetric measurements as it allows to better distinguish brain tissue types with greater resolution. MS patients usually present a higher yearly whole-brain atrophy (with 0.7% loss per year) compared to healthy controls (0.1% to 0.3% loss per year).29 This observed whole-brain atrophy is a combination of both white and gray matter volume loss, and it believed to reflect both inflammation-induced axonal loss followed by Wallerian degeneration and post-inflammatory neurodegeneration.30 As the disease progresses, gray matter volume loss is thought to increase with a higher rate and relates strongly to physical and cognitive deficits.26,31
Advanced MRI techniques
It currently well known that macroscopic features such as white/gray matter lesions and atrophy do not provide sufficient information about the extent of tissue, and thus, lack specificity about the more destructive aspects of MS pathology, including diffuse damage in the so-called normal appearing brain tissue (NABT). These more subtle brain alterations can be observed using diffusion tensor imaging (DTI) (Box 2). This technique reveals the integrity of white matter tracts which are responsible for inter-region communication, and therefore better relates to cognitive problems.32
As the previous sequences allowed us to capture structural brain abnormalities in MS, they did not, however, inform us about brain function alterations in the disease. Functional MRI (fMRI) is therefore a powerful tool to explore activation of brain regions either during a specific task or at rest (Box 3), by mapping the change in the level of blood oxygenation.33 When performing a cognitive task, MS patients showed different levels of activation compared to healthy controls. Indeed, cognitively impaired patients displayed a pattern of decreased brain activation in some regions compared to healthy controls, while cognitively preserved patients showed increased brain activations compared to healthy controls.34,35 Available fMRI data suggest that cognitive impairment in MS might be a function not only of tissue loss, but also of the progressive failure of
the adaptive capacity of the brain with increasing tissue damage.36 However, whether these
functional changes are beneficial or indicate a maladaptive response to injury is still a matter of debate.37–39
Other techniques
Other approaches to assess brain integrity during the course of MS have been used. We propose to briefly introduce some of them, as they have not been used in this thesis.
A more direct approach to investigate myelination is magnetization transfer (MT) imaging. This technique is based on the MT phenomenon in which two or more environments (pools) with distinctly different magnetic resonance properties exchange magnetization. Let us consider two pools of protons with distinctly different MR properties; the liquid protons (i.e. protons associated with water, both intracellular and extracellular), and the macromolecular protons (protons associated with myelin, cell membranes and proteins). To detect the macromolecular protons, an off-resonance radio-frequency pulse (i.e. MT pulse) is used. This pulse preferentially excites the macromolecular protons and is added immediately prior to a conventional MRI sequence (usually a T1-weighted sequence). Adding this pulse induces the transfer of magnetization from the macromolecular protons to nearby liquid protons, resulting in an MRI with intensities that have been modulated by the presence of myelin. Thus, MT imaging is affected by myelin content in MS white matter and has been validated with post-mortem histopathology.40
Additionally, positron emission tomography (PET) is an in-vivo imaging technique, allowing to quantitatively investigate the cellular and molecular processes of the disease. PET provides an image of the tissue of interest after the administration of a positron-emitting molecule, ideally binding a selective target.41 Due to the complexity of MS, different possible radioligands are provided, which can be used in PET imaging with high selectivity. As such, PET imaging is capable of assessing inflammation, demyelination, neuronal damage and astrocyte activation in MS.42 However, its high cost, its relatively low resolution and the lack of available standardized procedures currently limit its use.
Electroencephalography (EEG) and Magnetoencephalography (MEG) are direct and non-invasive measures of brain function. EEG measures the small electrical currents resulting from postsynaptic potentials, while MEG measures the magnetic fields induced by these currents. They are an important tool to complement other imaging methods such as fMRI, as they have a high temporal
26
resolution in the range of milliseconds. However, these two techniques have very low spatial resolution and a lack of sensitivity to processes in deep brain areas. Using EEG and MEG acquisitions in MS, studies have shown changes in network topology and slowing of neuronal activity, affecting cognitive performances.43–46
Box 2. Diffusion tensor imaging
Diffusion tensor imaging (DTI) is a sequence used to determine the displacements of water molecules (protons) in the brain. This sequence allows to quantify the integrity of both white and gray matter by calculating the water’s motion in several directions. When the water’s motion is random (i.e. Brownian motion) as in the CSF, the diffusion is called isotropic. However, when water motion is constrained in one direction by neurites, membranes or cell infiltrates, the diffusion is called anisotropic. Thus, water diffusion properties are modified when the microstructural integrity of the tissue is altered. We can express the diffusivity of water molecules using different parameters, such as:
Fractional anisotropy (FA): a summary measure that quantifies the amount of anisotropy (i.e. directionality) in a voxel.
Mean diffusivity (MD): average of diffusion. Axial diffusivity (AD): diffusivity along the axon.
Radial diffusivity (RD): diffusivity perpendicular to the axon.
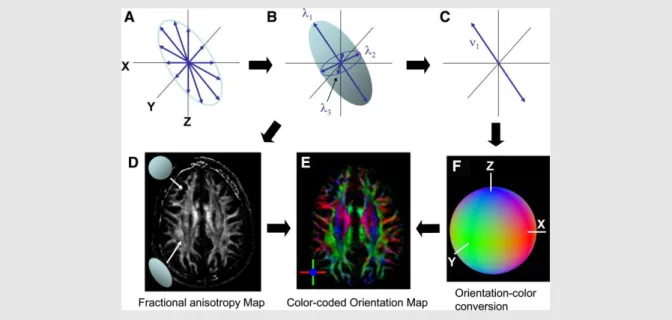
Figure 4. The Principle of DTI and Contrast Generation
From diffusion measurements along multiple axes (A), the shape and the orientation of a “diffusion ellipsoid” is estimated (B). An anisotropy map (D) can be created from the shape, in which dark regions are
isotropic (spherical) and bright regions are anisotropic (elongated). From the estimated ellipsoid (B), the orientation of the longest axis can be found (C), which is assumed to represent the local fiber orientation. This orientation information is converted to a color (F) at each pixel. By combining the intensity of the anisotropy map (D) and color (F), a color-coded orientation map is created (E). (Adapted from Mori et al.,
Neuron, 2006)
Mori S and Zhang J, Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006
Box 3. Functional magnetic resonance imaging
Functional magnetic resonance imaging (fMRI) is a sequence used to measure brain activation during a task or at rest. When a neurons population increase its activity, this region requires an increase in blood flow to deliver enough oxygen. Oxygen is bound in the blood to hemoglobin. Hemoglobin can be found in two forms: hemoglobin with bound oxygen molecules (i.e. oxygenated hemoglobin, with diamagnetic properties), and
hemoglobin without bound oxygen molecules (i.e. deoxygenated hemoglobin, with paramagnetic properties). The oxygen brought-in to the specific brain region by the increased blood flow is usually more that the consumed oxygen. This causes a decrease in deoxygenated hemoglobin which can be picked up by the MRI scanner. These changes can be assessed during:
Performance of a cognitive task (i.e. task fMRI), which depicts brain regions activated upon task demands.
Resting-state (resting-state fMRI), which depicts spontaneous brain activity
Connectomics: Mapping the human brain
For centuries, tracing the human brain’s connections has been an important scientific goal for neuroanatomists.47 Thanks to the advent of MRI, it is now possible to non-invasively capture the intrinsic characteristics of our brain and model connections between different brain regions. This effort to try to map the multiple connections in our brain is referred to as “connectomics”. For example, based on DTI data, we can capture the brain’s structural organization (i.e. white matter tratcs) using tractography to reveal the trajectories of white matter pathways in vivo.48 Then, in order to infer the underlying functional connectome of the human brain, we can use resting-state fMRI which identifies synchronization of activity between pairs of gray matter regions.49 Based on this functional connectome, patterns of simultaneous brain activity have been extracted using independent component analysis, leading to the identification of the so-called “resting-state networks”.50 Alterations to these networks have been shown to be involved in disability and
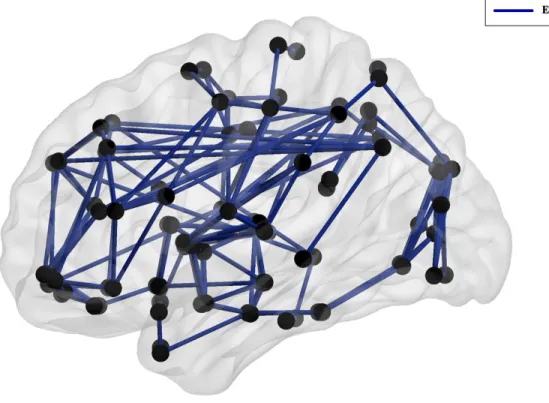
cognitive impairment in patients with MS,51 especially the default mode network known to intervene across a wide range of cognitive manipulations.52,53 Both structural and functional networks can be investigated using a mathematical modelling called “graph theory”. This model suggests that the brain is represented as a graph with nodes representing brain regions and edges representing connections between them (Figure 6).54
Figure 6. Graph theoretical representation of the brain
For functional networks, connections can be defined as the correlation coefficient between brain regions’ activity. Whereas in structural networks, these connections can represent the number of tracts connecting two gray matter regions. A variety of neurobiologically meaningful network measures can be computed to assess its characteristics, such as its efficiency, its centrality and its levels of segregation and integration.55 We briefly introduce some of the parameters used in this
thesis in Box 4. Abnormalities in both functional and structural networks have been previously reported in MS patients, showing different topological characteristics compared to HC.56 Current data on network changes in MS in relation to cognition have led to the introduction of the “network collapse” hypothesis.57 This hypothesis suggests that as the structural damage progresses, the
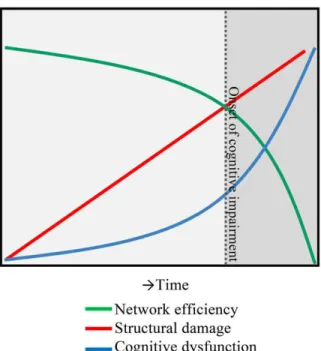
functional network’s efficiency becomes increasingly less efficient, until reaching a certain “threshold point” where the network collapses, leading to important cognitive deficits (Figure 7).57
Figure 7. A hypothesis of network collapse as a cause for developing cognitive impairment in MS.
In early stages of MS, structural damage is low, leaving network efficiency relatively high. As the structural damage accumulates over time, network efficiency levels drop, inducing a network collapse after a critical threshold (indicated by the dotted line) is exceeded. After this, the network is unable to function normally and cognitive
impairment develops. (Adapted from Schoonheim et al., Front Neurol, 2015)
Aims of the thesis
The overall aim of this thesis was to tackle some neural substrates of cognitive functioning in the early phase of MS. This was achieved using advanced MRI techniques and a comprehensive neuropsychological battery in a homogeneous cohort of CIS patients, followed over 1-year after their first episode. First, we were interested in looking into the differential gray matter vulnerability at this early stage of the disease. Then, we investigated the evolution of topological network properties (both structural and functional) in our patients related to their cognitive performances.
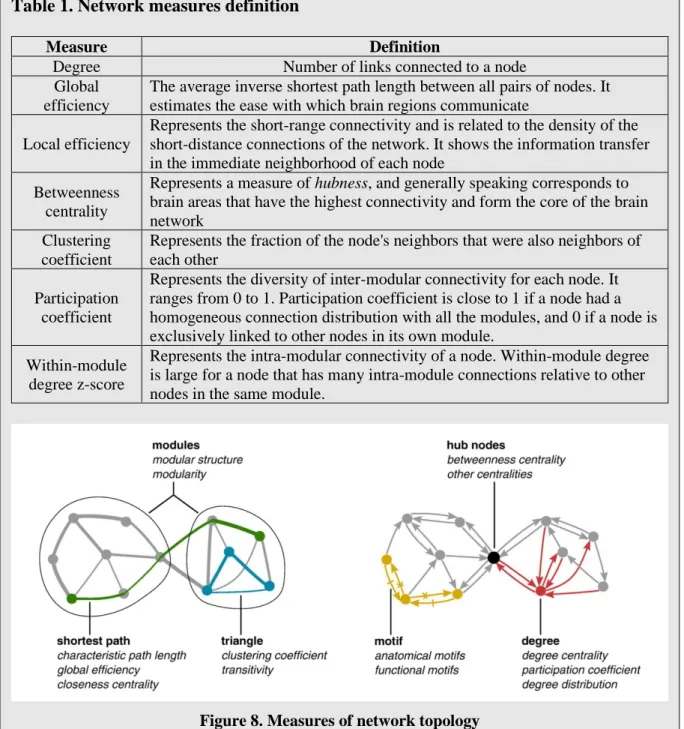
Box 4. Network measures of brain connectivity
In the following, we will briefly introduce and describe some important network measures used in our analyses. First, let us recall that a node indicates a brain region, and an edge (i.e. link) indicates the connection between a pair of brain regions.
Table 1. Network measures definition
Measure Definition
Degree Number of links connected to a node Global
efficiency
The average inverse shortest path length between all pairs of nodes. It estimates the ease with which brain regions communicate
Local efficiency
Represents the short-range connectivity and is related to the density of the short-distance connections of the network. It shows the information transfer in the immediate neighborhood of each node
Betweenness centrality
Represents a measure of hubness, and generally speaking corresponds to brain areas that have the highest connectivity and form the core of the brain network
Clustering coefficient
Represents the fraction of the node's neighbors that were also neighbors of each other
Participation coefficient
Represents the diversity of inter-modular connectivity for each node. It ranges from 0 to 1. Participation coefficient is close to 1 if a node had a homogeneous connection distribution with all the modules, and 0 if a node is exclusively linked to other nodes in its own module.
Within-module degree z-score
Represents the intra-modular connectivity of a node. Within-module degree is large for a node that has many intra-module connections relative to other nodes in the same module.
Figure 8. Measures of network topology
(Adapted from Rubinov and Sporns, NeuroImage, 2010)
Rubinov M and Sporns O, Complex network measures of brain connectivity: Uses and interpretations. NeuroImage. 2010
Thesis outline
In order to answer our previous objectives, we broke down our studies into multiple chapters. In chapter 2, we investigated whether some gray matter regions are differentially vulnerable at the early stage of MS. By quantifying deep gray matter and cortical volumes, along with their microstructural integrity using DTI metrics, we found that hippocampus was the first structure showing microstructural alterations since baseline. Then after 1-year, hippocampus volume was decreased along with damage spreading to the cortex. Hippocampus microstructural alterations at baseline were also predictive of its future volume loss.
In chapter 3, we explored the evolution of functional brain networks reorganization at rest in patients with CIS. Based on fMRI data, and using graph theoretical measures, we observed brain reorganization from the onset of the disease, by depicting a combination of underconnected and overconnected brain regions. This reorganization was even more pronounced after 1-year of evolution. Importantly, these changes were present while global brain efficiency was normal compared to HC, and correlated with the preservation of cognitive performances, suggesting a compensatory mechanism at this stage.
In chapter 4, we will analyze both structural and functional connectivity, and how they relate to each other in the early phase of MS. Structural connectivity was extracted from white matter tractography using DTI data, while functional connectivity was based on resting-state fMRI. By introducing a novel concept of structural-functional coupling in MS, we will look at brain regions directly connected and how the function evolves with the structure. Our analysis revealed that structural damage precedes functional reorganization during the first year following a clinically isolated syndrome along with normal cognitive performance, suggesting a compensatory mechanism at this stage of the disease. Importantly, structural–functional decoupling observed for the first time in MS suggests that functional reorganization occurs along indirect anatomical pathways.
In chapter 5, we will finally summarize and discuss the main findings of this thesis, and we will state the limitations of this work as well as the perspectives we intend to further develop.
References
1. Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372: 1502–1517.
2. Kalincik T, Vivek V, Jokubaitis V, et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain 2013; 136: 3609–3617.
3. Fromont A, Binquet C, Sauleau EA, et al. Geographic variations of multiple sclerosis in France. Brain 2010; 133: 1889–1899.
4. Popescu BFG, Lucchinetti CF. Pathology of Demyelinating Diseases. Annu Rev Pathol Mech Dis 2012; 7: 185–217.
5. Mahad DJ, Ziabreva I, Campbell G, et al. Mitochondrial changes within axons in multiple sclerosis. Brain 2009; 132: 1161–1174.
6. Lassmann H. Pathology and disease mechanisms in different stages of multiple sclerosis. J Neurol Sci 2013; 333: 1–4.
7. Brownlee WJ, Hardy TA, Fazekas F, et al. Diagnosis of multiple sclerosis: progress and challenges. Lancet 2017; 389: 1336–1346.
8. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302.
9. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173.
10. Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Epub ahead of print 2012. DOI: 10.1016/S1474-4422(11)70274-5.
11. Ciccarelli O, Thompson A. Managing the complexity of multiple sclerosis. Nat Rev Neurol 2016; 12: 70–72.
12. Plemel JR, Liu W-Q, Yong VW. Remyelination therapies: a new direction and challenge in multiple sclerosis. Nat Rev Drug Discov 2017; 16: 617–634.
13. Chiaravalloti ND, Deluca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008; 7: 1139–1151.
14. Feuillet L, Reuter F, Audoin B, et al. Early cognitive impairment in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler 2007; 13: 124–7.
15. Reuter F, Zaaraoui W, Crespy L, et al. Frequency of cognitive impairment dramatically increases during the first 5 years of multiple sclerosis. J Neurol Neurosurg Psychiatry 2011; 82: 1157–1159.
16. Morrow SA, Drake A, Zivadinov R, et al. Predicting loss of employment over three years in multiple sclerosis: Clinically meaningful cognitive decline. Clin Neuropsychol 2010; 24: 1131–1145.
17. Ruet A, Deloire M, Hamel D, et al. Cognitive impairment, health-related quality of life and vocational status at early stages of multiple sclerosis: a 7-year longitudinal study. J Neurol 2013; 260: 776–784.
18. Langdon D, Amato M, Boringa J, et al. Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Mult Scler J 2012; 18: 891–898. 19. Rao S., Society and the CFSG of the NMS. A manual for brief repeatable battery of the
neuropsychological tests in multiple sclerosis. Med Coll Wisconsin, Milwaukee, WI. 20. Benedict RHB, Fischer JS, Archibald CJ, et al. Minimal Neuropsychological Assessment
of MS Patients: A Consensus Approach. Clin Neuropsychol 2002; 16: 381–397.
21. Filippi M, Rocca MA, Ciccarelli O, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol 2016; 15: 292–303.
22. Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 2002; 15: 239–45.
23. Geurts JJG, Pouwels PJW, Uitdehaag BMJ, et al. Intracortical Lesions in Multiple Sclerosis: Improved Detection with 3D Double Inversion-Recovery MR Imaging. Radiology 2005; 236: 254–260.
24. Seewann A, Kooi EJ, Roosendaal SD, et al. Postmortem verification of MS cortical lesion detection with 3D DIR. Neurology 2012; 78: 302–308.
25. Geurts JJG, Roosendaal SD, Calabrese M, et al. Consensus recommendations for MS cortical lesion scoring using double inversion recovery MRI. Neurology 2011; 76: 418–424. 26. Geurts JJG, Calabrese M, Fisher E, et al. Measurement and clinical effect of grey matter
pathology in multiple sclerosis. The Lancet Neurology 2012; 11: 1082–1092.
27. Harrison DM, Peper JS, Dahl RE. Association of Cortical Lesion Burden on 7-T Magnetic Resonance Imaging With Cognition and Disability in Multiple Sclerosis. 2015; 22: 134– 139.
28. Cala LA, Mastaglia FL, Black JL. Computerized tomography of brain and optic nerve in multiple sclerosis. Observations in 100 patients, including serial studies in 16. J Neurol Sci 1978; 36: 411–26.
29. Vollmer T, Signorovitch J, Huynh L, et al. The natural history of brain volume loss among patients with multiple sclerosis: A systematic literature review and meta-analysis. J Neurol Sci 2015; 357: 8–18.
30. Miller DH, Barkhof F, Frank JA, et al. Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain 2002; 125: 1676– 95.
31. van Munster CEP, Jonkman LE, Weinstein HC, et al. Gray matter damage in multiple sclerosis: Impact on clinical symptoms. Neuroscience 2015; 303: 446–461.
32. Dineen RA, Vilisaar J, Hlinka J, et al. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 2009; 132: 239–249.
33. Friston KJ, Holmes AP, Poline J-B, et al. Analysis of fMRI Time-Series Revisited. Neuroimage 1995; 2: 45–53.
34. Hulst HE, Schoonheim MM, Roosendaal SD, et al. Functional adaptive changes within the hippocampal memory system of patients with multiple sclerosis. Hum Brain Mapp 2012; 33: 2268–2280.
35. Rocca MA, Valsasina P, Hulst HE, et al. Functional correlates of cognitive dysfunction in multiple sclerosis: A multicenter fMRI Study. Hum Brain Mapp 2014; 35: 5799–5814. 36. Rocca MA, De Meo E, Filippi M. Functional MRI in investigating cognitive impairment in
multiple sclerosis. Acta Neurol Scand 2016; 134: 39–46.
37. Rocca MA, Filippi M. Functional reorganization is a maladaptive response to injury – YES. Mult Scler J 2017; 23: 191–193.
38. Penner IK, Aktas O. Functional reorganization is a maladaptive response to injury - NO. Multiple Sclerosis Journal 2017; 23: 194–196.
39. Schoonheim MM. Functional reorganization is a maladaptive response to injury – Commentary. Mult Scler J 2017; 23: 194–196.
40. Schmierer K, Scaravilli F, Altmann DR, et al. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol 2004; 56: 407–415.
41. de Paula Faria D, Copray S, Buchpiguel C, et al. PET imaging in multiple sclerosis. J Neuroimmune Pharmacol 2014; 9: 468–482.
42. Moccia M, Ciccarelli O. Molecular and Metabolic Imaging in Multiple Sclerosis. Neuroimaging Clin N Am 2017; 27: 343–356.
43. Schoonheim MM, Geurts JJG, Landi D, et al. Functional connectivity changes in multiple sclerosis patients: A graph analytical study of MEG resting state data. Hum Brain Mapp 2013; 34: 52–61.
44. Tewarie P, Schoonheim MM, Schouten DI, et al. Functional brain networks: Linking thalamic atrophy to clinical disability in multiple sclerosis, a multimodal fMRI and MEG Study. Hum Brain Mapp 2015; 36: 603–618.
45. Schoonhoven DN, Fraschini M, Tewarie P, et al. Resting-state MEG measurement of functional activation as a biomarker for cognitive decline in MS. Mult Scler J 2018; 135245851881026.
46. Keune PM, Hansen S, Weber E, et al. Exploring resting-state EEG brain oscillatory activity in relation to cognitive functioning in multiple sclerosis. Clin Neurophysiol 2017; 128: 1746–1754.
47. Sporns O. The human connectome: Origins and challenges. Neuroimage 2013; 80: 53–61. 48. Maier-Hein KH, Neher PF, Houde JC, et al. The challenge of mapping the human
connectome based on diffusion tractography. Nat Commun; 8. Epub ahead of print 2017. DOI: 10.1038/s41467-017-01285-x.
49. Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci 2009; 106: 13040–13045.
50. van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 2010; 20: 519–534. 51. Rocca MA, Valsasina P, Leavitt VM, et al. Functional network connectivity abnormalities
in multiple sclerosis: Correlations with disability and cognitive impairment. Mult Scler J 2017; 135245851769987.
52. Rocca MA, Valsasina P, Absinta M, et al. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology 2010; 74: 1252–1259.
53. Smith V, Mitchell DJ, Duncan J. Role of the Default Mode Network in Cognitive Transitions. Cereb Cortex 2018; 28: 3685–3696.
54. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009; 10: 312–312.
55. Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010; 52: 1059–1069.
56. Fleischer V, Radetz A, Ciolac D, et al. Graph theoretical framework of brain networks in multiple sclerosis: A review of concepts. Neuroscience. Epub ahead of print 2017. DOI: 10.1016/j.neuroscience.2017.10.033.
57. Schoonheim MM, Meijer KA, Geurts JJG. Network collapse and cognitive impairment in multiple sclerosis. Front Neurol; 6. Epub ahead of print 2015. DOI: 10.3389/fneur.2015.00082.
Chapter 2
Differential gray matter vulnerability in the early course of
MS
Summary page
Rationale: Differential vulnerability of gray matter regions at the early stage of multiple sclerosis (MS) is still unknown. We aimed to investigate whether deep and cortical gray matter are differentially vulnerable after a clinically isolated syndrome (CIS) suggestive of MS.
Summary of the methods: We measured volume and diffusion tensor imaging (DTI) metrics within deep gray matter structures and the cortex in patients with CIS (PwCIS) and healthy controls (HC) at both baseline and after 1-year of follow-up.
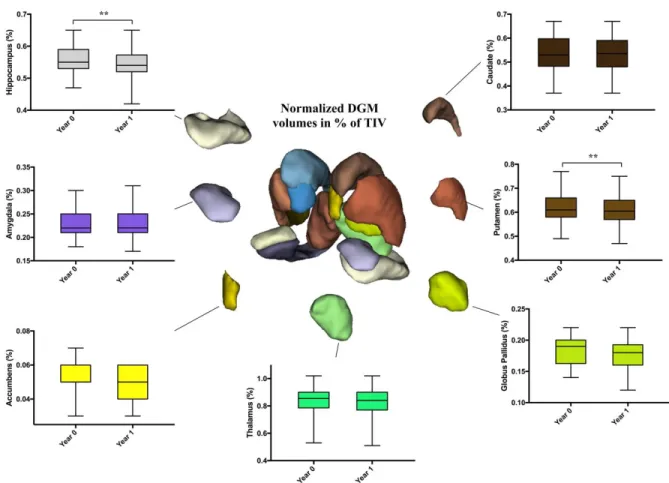
Main results: Hippocampus was the only structure altered at baseline with microstructural abnormalities measured by DTI, while no gray matter atrophy was noticed at this stage. After 1-year, gray matter damage was more widespread with putamen and hippocampus volumes decreasing in PwCIS, and cortical thinning in different parts of the cortex along with microstructural abnormalities. Furthermore, hippocampus volume loss could be predicted by its microstructural abnormalities at baseline.
Comments: This study shows a differential gray matter vulnerability at the onset of MS spreading from hippocampus to the cortex. Additionally, we could detect early structural abnormalities in the hippocampus which were predictive of its subsequent volume loss. However, gray matter abnormalities in MS could also be studied by investigating the functional networks topology in patients.
Differential gray matter vulnerability in the one year following a
clinically isolated syndrome
Ismail Koubiyr1,2; Mathilde Deloire3; Pierrick Coupé4; Cécile Dulau3; Pierre Besson5; Amandine Moroso1,3; Vincent Planche1,2,3; Thomas Tourdias1,2,3; Bruno Brochet1,2,3*; Aurélie Ruet1,2,3
1 Univ. Bordeaux, F-33000 Bordeaux, France
2 Inserm U1215 - Neurocentre Magendie, F-33000 Bordeaux, France 3 CHU deBordeaux, F-33000 Bordeaux, France
4 Laboratoire Bordelais de Recherche en Informatique, UMR CNRS 5800, PICTURA, F-33405
Talence, France
5 AixMarseille Univ, CNRS, CRMBM UMR 7339, Marseille
Abstract
Background and purpose: Whether some gray matter (GM) regions are differentially vulnerable
at the early stages of MS is still unknown. The objective of this study is to investigate whether deep and cortical GM are differentially vulnerable after a clinically isolated syndrome (CIS) suggestive of multiple sclerosis (MS).
Methods: Fifty-six patients with CIS (PwCIS) and 38 healthy controls (HC) had conventional and
diffusion tensor imaging (DTI) at baseline and 46 PwCIS and 20 HC were rescanned after one year. Deep GM (DGM) volumes, cortical thickness (CTh) and DTI metrics (FA: fractional anisotropy; MD: mean diffusivity) within these structures were calculated for each participant at each time-point and compared between PwCIS and HC. Linear regression models were used to investigate whether baseline DTI parameters could predict GM volume loss over time.
Results: At baseline, GM volumes did not differ between PwCIS and HC, but hippocampal MD
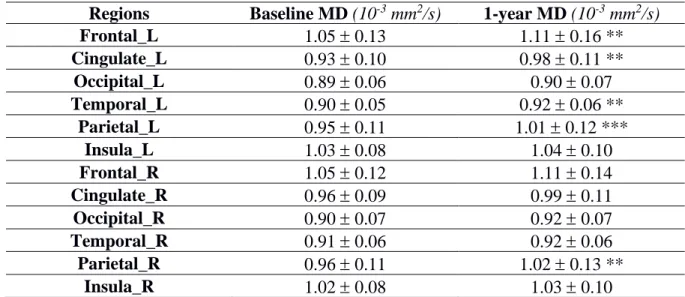
was higher in PwCIS than HC (p<0.01). Over one year, GM alterations became more widespread with putamen and hippocampus volumes decreasing in PwCIS (p<0.01), and cortical thinning in different parts of the cortex along with a significant increase of MD. Hippocampus MD at baseline could predict its volume loss (R2=0.159; p<0.05) and cortical thinning was associated to microstructural damage (Spearman’s rho ranging from -0.424 to -0.603 with p<0.003).
Conclusion: Along with MS being a diffuse inflammatory disease, GM showed a differential
vulnerability at the early stage spreading from hippocampus to the cortex. Hippocampus volume loss could be predicted by its MD at baseline.
Introduction
Multiple sclerosis (MS) is an inflammatory, demyelinating and neurodegenerative disease of the central nervous system, leading to physical deterioration and cognitive impairment. At the clinical onset of the disease, approximately 85% of patients experience a monophasic neurological episode, known as a clinically isolated syndrome (CIS).1 Gray matter (GM) atrophy has been found to occur in different phenotypes of MS associating deep GM (DGM) atrophy and cortical atrophy.2 GM atrophy has been shown to progress in the first years after the CIS,3,4 but conflicting results have been reported at the initial time of CIS, which questions whether or not the disease induces tissue loss from this very early stage.2,5–7 Especially, DGM atrophy has been inconsistently found in CIS,8–10 while cortical atrophy seems to be absent.10 The dynamics of GM vulnerability at the
early stages remain unclear, and the mechanisms leading to atrophy are not well understood. A relationship has been suggested between atrophy in some GM nuclei and lesions in the related white matter tracts through Wallerian degeneration as this has been shown for the thalamus.11
However, a direct injury of the GM by inflammation is also possible,12 as suggested by recent studies using magnetic resonance-positron emission tomography.13 Either mechanism could lead
to some differential vulnerability of the GM as some structures might be more connected than others, or some types of neurons might be more fragile than others. This selective vulnerability has been shown in Alzheimer’s disease particularly, as the pathology seems to spread from entorhinal cortex to hippocampus.14
In order to study the selective vulnerability of GM, diffusion tensor imaging (DTI) allows to explore the microstructural integrity of the structures. A few studies used this technique in CIS, showing abnormal results in the thalamus,15 hippocampus16 and the cerebellum17 suggesting microstructural changes from the early stages of MS. In the other hand, cortex has been investigated in patients with MS with fractional anisotropy (FA) and mean diffusivity (MD).18–20 Results of these studies presented some discrepancies, as FA in the normal appearing gray matter (NAGM) was found to be increased19 or decreased,18,20 while MD either increased18,20 or showed no difference compared to healthy volunteers.19 Also, the majority of these findings was cross-sectional and could not infer about the dynamics of the microstructural damage spreading. Thus, gray matter microstructural damage that may be leading to irreversible atrophy is not well assessed in MS yet, and this even more for CIS patients.