Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Research Paper (National Research Council of Canada. Division of Building
Research); no. DBR-RP-562, 1973-04-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=77be8a75-ba23-4b3d-a057-c212b0a03420 https://publications-cnrc.canada.ca/fra/voir/objet/?id=77be8a75-ba23-4b3d-a057-c212b0a03420
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/40001663
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Thermal properties of selected masonry unit concretes
TITLE
NO.
70-15
Thermal Properties
of
Selected
Masonry Unit Concretes
By T. Z. HARMATHY and L. W. ALLEN
Authorized r e p r i n t from c o p y r i g h t e d JOURNAL OF
THE
AMERICAN CONCRETE INSTITUTETITLE
NO.
70-15
Thermal Properties of Selected
Masonry Unit Concretes
By T. Z. HARMATHY and
L.
W. ALLENThe thermal characteristics of 16 concretes have been studied a t elevated temperatures with the aid of thermogravimetry, dilatometry, and "thermal property tests." A n analysis of the results is given from the point of view of material behavior under fire exposure.
Keywords: concretes; fire resistance: fire tests: high tem- perature; lightweight concretes; masonry: specific heat: ther- mal conductivity; thermal diffusivity; thermal properties.
DURING THE PAST FEW YEARS a comprehensive fire test program was performed in the National Research Council of Canada, Division of Building Research (NRCIDBR) laboratories, with the aim of obtaining information on the thermal perform- ance of concrete masonry walls in fire. This test program included 71 fire endurance tests, and hundreds of complementary tests concerned with the thermal properties of the concretes at room temperature and at elevated temperatures.
The findings of the fire endurance tests were reported earlier,l and were analyzed more recently in two separate papers.*.2 In the present paper the information obtained by the complementary tests will be summarized and interpreted.
PREVIOUS WORK
The interest in the thermal properties of con- cretes dates back to the early years of the cen- tury. In 1912 Norton3 reported some information on the thermal expansion, specific heat, and thermal conductivity of concretes of several dif- ferent mixes, at room temperature, and at ele- vated temperatures. Dozens of papers have since been published, related directly or indirectly to this subject area. A comprehensive bibliography
of these papers can be found in an excellent paper by Zoldners,4 which gives a wealth of experi- mental information regarding the thermal prop- erties of concretes and aggregates. In a more recent study, Harmathy" presented a discussion of primarily theoretical nature, on methods of estimating the thermal properties of concretes at elevated temperatures.
MATERIALS USED
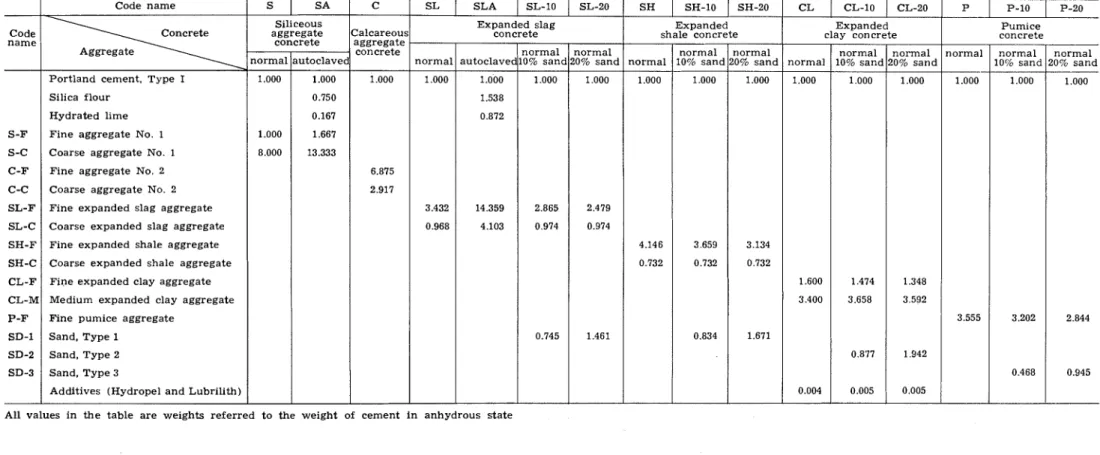
Sixteen different concretes were studied in the test series reported here. Of these, three can be classified as normal weight concretes, and 13 as lightweight concretes. Information concerning the mixes is presented in Table 1. For convenience, the various concretes and aggregates will often be identified by code names, which are also given in the table.
The masonry units from which the test samples were taken were supplied by several manufac- turers who used slightly different curing pro- cedures. The concretes marked as "normal" in the table were usually left to preset for 2 to 4 hr, then steamed at 74 to 82C (165 to 180F) for a period of at least 8 hr. Those marked as "auto- claved" were allowed to preset for 3 to 4 hr, then steamed in an autoclave at a temperature of 180 to 185 C (356 to 365 F) for several hours. Further details of the steam curing processes have been given in Reference 1.
Information concerning the sieve analyses of all aggregates, and the petrographic analyses
*Williams-Leir G and Allen L. W. "Prediction of Fire En- duranze of ~ o A c r e t e Masonry w a l l s from Correlations of 94 Fire Tests. T o b e published.
of the normal weight aggregates, has also been supplied in Reference 1. Aggregate S-F is char- acterized there as 100 percent calcareous; Aggre- gate S-C as 6.5 percent calcareous, 93.5 percent siliceous; Aggregate C-F as 90 percent calcareous, 10 percent siliceous; and Aggregate C-C as 100 percent calcareous. Unlike the aggregates used in structural concrete, all four of these aggregates proved chemically unstable at higher tempera- tures, as will be pointed out later when discussing some test results.
THEORETICAL CONSIDERATIONS
An adequate theoretical analysis of fire endur- ance problems is rarely feasible without a thorough knowledge of the thermal properties of the materials involved, both at room temperature and at elevated temperatures. Experimenters often find, however, that the routine techniques developed for higher temperatures are not readily applicable to building materials, because most of these become chemically unstable and undergo certain decomposition, transformation, oxidation, or other reactions on heating. Owing to these dif- ficulties, it is often necessary to supplement experimental investigations with theoretical con- siderations.
From the point of view of thermal behavior, concrete is one of the most problematic materials. The main problems are presented by the chemical changes that take place in the portland cement paste. In the case of structural concretes the aggregates rarely present particular problems, but as already mentioned, the aggregates used in normal weight masonry units may also lack chemical stability at higher temperatures. As described in an earlier paper: the cement paste undergoes an uninterrupted series of dehydration reactions as its temperature rises from 100 C
(212 F ) to about 850 C (1562 F)
.
These reactions affect the thermal properties of the cement paste in three ways; (1) by the absorption of latent heats, (2) by the changes brought about in the physicochemical characteristics of the solid matrix and, (3) by the changes in the porosity of the bulk material. The thermal conductivity depends on all three of these effects, although its depend- ence on the first is not satisfactorily understood (e. g., Reference 7 ) , and will be disregarded in this paper. The bulk density depends only on the second and third effects, and the specific heat only on the first and second.Most of the reactions that develop on the heat- ing of concrete are rather sluggish. This means that the degree of conversion of concrete into its reaction products depends, at any one tempera- ture, on the rate of heating. Consequently, the temperature dependence of various properties of
T. 2. Harrnathy is a research officer with the Fire Section, Division o f Building Research, National Research Council of Canada, Ottawa. H e obtained a degree in mechanical engineering from the Budapest University o f Technology, and a Doctor o f Engineering degree f r o m the Vienna Univer- sity o f Technology. The author o f numerous technical papers, he is a member o f A C I Committee 216, Fire Resistance and Fire Protection o f Structures.
A C I member L. W. Allen is a technical representative, Lake O n t a r i o Cement Ltd., Toronto. O n t a r i o , Canada. H e obtained a M S degree in civil engineering f r o m the Univer- sity o f Windsor. H e is a registered professional engineer in the province o f Ontario.
concrete cannot be rigorously described by unique relations. For practical reasons, however, one must assume that such unique relations exist, and that they are obtainable at rates of heating employed in some routine experimental investigations (such as those to be discussed later). It must be remembered, however, that conclusions arrived at by this assumption may be in considerable er- ror when very fast or very slowly changing pro- cesses are investigated.
From the preceding discussion, it is also appar- ent that thermal property values obtained for concrete by different experimental techniques may be in more than slight conflict. It is impor- tant, therefore, to give a rather accurate descrip- tion of the experimental procedure in any report dealing with this subject area.
Before discussing the experimental techniques used in the present studies it is necessary, how- ever, to make a few remarks in connection with the interpretation of the specific heat, which seems to be the least understood of the thermal properties of concretes.
APPARENT SPECIFIC HEAT
The specific heat at constant pressure, cpJ is de- fined as:
where H=enthalpy, T = t e m p e r a t u r e , and P = pressure. If the heating of the solid is ac- companied by chemical reactions, the enthalpy is a function of the degree of conversion from the reactants into the products, (0 i l 1)
, as well
as of the temperature. Thus Eq. (1) becomes:Under these circumstances c p is usually referred to as "apparent" specific heat. The first term on the right side of Eq. (2) obviously represents the sensible heat contribution to the specific heat at a given degree of conversion. Thus, assuming that
ACB JOURNAL / FEBRUARY 1973
TABLE I -CONCRETE MIXES STUDIED
-
Code name-
S-F S-C C-F C-C SL-F SL-C SH-F SH-C CL-F CL-M P-F SD-1 SD-2 SD-3 Code name Concrete Aggregate--.
Portland cement. Type I Silica flour
Hydrated lime Fine aggregate No. 1 Coarse aggregate No. 1 Fine aggregate No. 2
Coarse aggregate No. 2 Fine expanded slag aggregate Coarse expanded slag aggregate Fine expanded shale aggregate Coarse expanded shale aggregate Fine expanded clay aggregate Medium expanded clay aggregate Fine pumice aggregate
Sand, Type 1 Sand, Type 2 Sand, Type 3
Additives (Hydropel and Lubrilith)
Siliceous aggregate concrete normal autoclave 1.000 1.000 0.750 0.167 1.000 1.667 8.000 13.333
!
Calcareou! aggregate concrete 1.000 6.875 2.917 SL SLA SL-10 SL-20 Expanded slag concreteAll values in t h e table are weights referred to the weight of cement i n anhydrous state
Expanded shale concrete
6
is a unique function of the temperature, this term can be given the symbol cp, T, i. e.,:In the second term, (aH/a[) ,, is the heat of re- action, AHp, T'
and thus Eq. (2) can be rewritten as:
where the second term can now be clearly rec- ognized as the latent heat contribution to the specific heat.
As described in detail in Reference 5, in the case of portland cement paste, the two major reactions are the dehydration of the "tobermorite gel" into p-wollastonite and P-dicalcium silicate, and the dehydration of calcium hydroxide into calcium oxide. Based on differential thermal analyses, and on information concerning the en- thalpy of tobermorite gel and calcium hydroxide, as well as their dehydration products, three cp
versus T curves were derived in Reference 5 for three somewhat idealized cement pastes prepared at various water-cement ratios. One of these curves, with some additions, is reproduced in Fig. 1. The figure clearly shows that the latent heat contribution to the specific heat is very significant in the entire dehydration range (roughly from 100 to 850 C or 212 to 1562 F ) .
For concrete, these latent heat effects are usual- ly less important, on account of the presence of aggregates. The aggregates, especially those used in structural concretes, normally exhibit higher degrees of chemical stability than the cement paste.
EXPERIMENTAL PROCEDURES
Samples from all concrete mixes were subjected to three kinds of tests: (1) thermogravimetric tests, (2) dilatometric tests and (3) a special series of tests referred to here for brevity as "thermal property tests." In addition, samples from all aggregates were also studied separately by thermogravimetry.
All tests, or series of tests, were performed on specimens dried in an oven at 105
*
0.5 C (221t e m p e r a t u r e F
t e m p e r a t u r e C
Fig. I -Specific heat of an idealized cement paste
2 0.9 F) for 24 hr, then, if necessary, kept in this During the test proper, the equilibrium was
"reference condition," in a desiccator containing disturbed by a slow rise in the temperature of the Mg (C104)2 2H20, until testing. specimen by 5 to 10 C (9 to 18 F) above the ref-
The instruments and procedures used in the thermogravimetric and dilatometric tests were described briefly in Reference 6. In these tests the temperature of the specimen was raised from room temperature (25 C or 77 F) to 1000 C (1832 F ) , at a nominal rate of 5 C/min (9 F/min). Obvious- ly, with this procedure, the reactants and reaction products are not allowed to achieve chemical equilibrium at any one temperature.
The thermal diffusivity, thermal conductivity, and specific heat values reported in this paper were determined by the thermal property tests. This testing technique was described in detail in Reference 8. In general, a single test specimen was used for the entire temperature interval of interest. The first test was always performed at room temperature. Following this, the tempera- ture was set stepwise at higher and higher tem- perature levels. At each temperature the specimen was "heat-soaked" for at least 6 hr before the test. During this time the specimen supposedly acquired an equilibrium composition correspond- ing to the given temperature.
erence level. Because of this, the specific heat values derived from these tests cannot be strictly identified with either the apparent specific heat (obtainable a t 5 C/min or 9 F/min rate of heat- ing) or the sensible heat contribution to the specific heat, although, they are only slightly higher than the latter.
In numerical heat flow studies, the apparent specific heat versus temperature relation is the information of primary interest. This information is obtained by adding-to the specific heat values, determined by the thermal property tests, the latent heat contributions, i. e., the term AHl.d(/dT, as shown in Fig. 1. These contributions can be estimated by combining the results of some com- plementary tests (e. g., differential thermal analyses) with some theoretical considerations, similar to those described in Reference 5.
The accuracy of the thermal property tests is estimated at 2 5 percent with respect to thermal
diffusivity, and at
+
8 percent with respect to thermal conductivity. All errors in the values of the thermal diffusivity and conductivity may ac- cumulate in the calculation of the specific heat.t e m p e r a t u r e F + 5 200 400 600 800 1000 1200 1400 1600 1800 2000 I I I I I i I I I I 0 - 5
-
C:
-10 L a 0-
C .Z' -15 a 3-
D a m C-
-20 C " s l a g , s h a l e a n d c l a y c o n c r e t e s - 25----_
- 30-
35 0 100 200 300 400 500 600 700 800 9 00 1000 1100 1200 t e m p e r a t u r e C1 Fig. 2
-
Thermogravimetric curves of concreteFor reasons mentioned in Reference 8 such thermal property tests could not be performed a t temperatures higher than about 650
d
(1202 F).
RESULTS OF THERMOGRAVIMETRIC ANALYSES
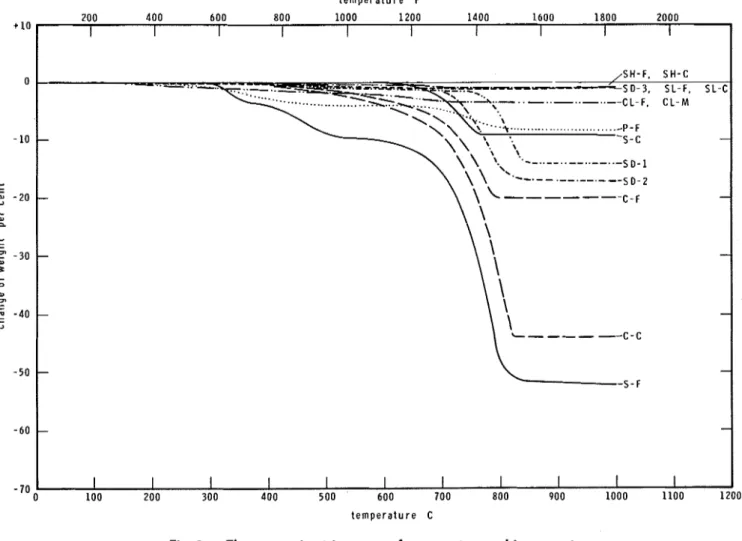
The thermogravimetric curves of the various concretes and their aggregates are shown in Fig. 2 and 3, respectively.
Such curves may prove very useful in assessing some merits or demerits of using the materials in fire resistant constructions. The loss of weight clearly indicates the progress of decomposition reactions. These reactions are accompanied by significant heat absorptions [generally of the order of 500 cal/(g loss of weight), or 900 Btu/(lb loss of weight)] which may significaritly increase the value of the apparent specific heat, see Eq. (5).
Reactions that are not accompanied by weight changes (crystalline transformations in general) are not detectable from the thermogravimetric curves. The heats absorbed or evolved in these reactions, however, are rarely of significant mag- nitude.
Fig. 3 shows that the processed lightweight aggregates, expanded slag, shale and clay, exhibit high degrees of chemical stability. The weight
loss for these aggregates is less than 4 percent a t 1000 C (1832 F). The sand used in the pumice concretes was also found to be a very stable aggre- gate.
From among the natural aggregates, the one with predominantly siliceous constituents, Ag- gregate S-C, proved more stable than the cal- careous ones. In the case of siliceous aggregates, the weight losses are most likely brought about by the dehydration of some mineral components holding water of constitution. For calcareous ag- gregates, the decomposition of the carbonates (CaC03, MgCO:,, and dolomite) is the major source of weight loss, although dehydration reactions may also be present.
From the point of view of the behavior of ma- terials under fire exposure, these decomposition reactions are usually beneficial for two reasons. First, the heat absorbed in these reactions tends to moderate the rise of temperature in the ma- terial and, second, a more porous and less con- ductive solid matrix is left behind which further helps retard the penetration of heat into the interior of the material.
The temperature of these decomposition reac- tions is also of considerable interest to fire pro- tection engineers. Even in a fire of long duration t e m p e r a t u r e F
200 400 600 800 1000 1200 1400 1600 1800 2000
+ 10
I I I I I I I I I I
Fig. 3
-
Thermogravimetric curves of aggregates used in concreteACI JOURNAL
/
FEBRUARY
1973
0 -10
-
E-
-20 L a eL-
s .Z' -30 3-
0 0 7 C m -40 s U -50 -60-
70 0 /SH-F. S H - C -..-..-- CL-F, C L - M --
-
-
--
C - C-
--
-
I I I I I I I I I I I 100 200 300 400 500 600 700 800 900 1000 1100 1200 t e m p e r a t u r e Conly a relatively narrow surface layer of the concrete attains high temperatures, say, in excess of 500 C (932 F ) . Thus the beneficial effect of these reactions may be more or less lost if they develop only a t higher temperatures.
Only one of the aggregates examined exhibited more than 5 percent weight loss at 500 C (932 F )
,
Aggregate S-F. This aggregate was not used, how- ever, in sufficiently large quantity to noticeably affect the fire resisting characteristics of Con- crete S.While most aggregates seem to be more or less stable up to about 500 C, the dehydration of the cement paste is significant a t any temperature above 100 C (212 F ) (see Fig. 4 of Reference 5).
This fact is obvious from some previous discus- sions, and is reflected by the steady decline of the thermogravimetric curves for all concretes, as shown in Fig. 2.
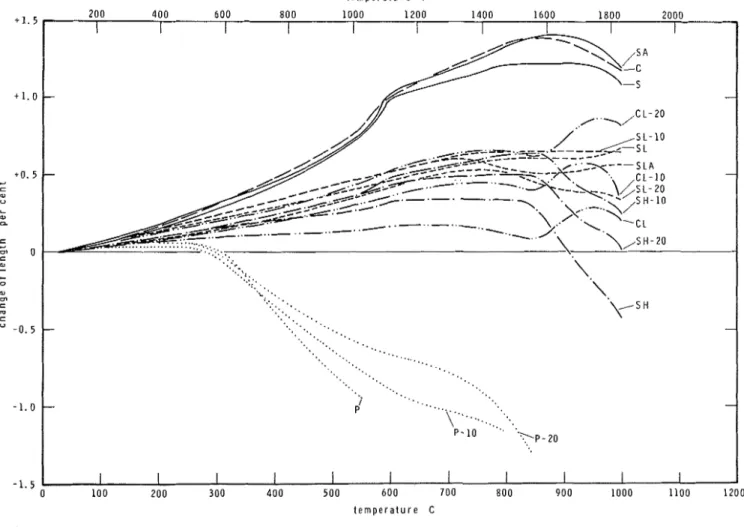
RESULTS OF DILATBMETRIC ANALYSES Because the aggregates were not available in sufficiently large sizes, dilatometric analyses were performed only on the concrete specimens. The dilatometric curves are presented in Fig. 4.
Dilatometric analyses are, in a way, comple- mentary to the thermogravimetric analyses, since
they make it possible to detect reactions which are not accompanied by change of weight, and thus are not noticeable by thermogravimetry. The best example is the a
-
p
quartz transformation that takes place a t 573 C (1063 F ) , and is indicated by significant volume expansion.For a long time it was believed that the a -
p
quartz transformation induces spalling of concrete and, therefore, the use of aggregates of significant quartz content was considered undesirable from a fire endurance point of view. The DBR/NRC fire testing laboratory, however, has never found evidence to support this belief. At higher tempera- tures the cement paste probably has sufficient plasticity to absorb most of the expansion of quartz without disruption.
An earlier suggestion that excessive moisture content is the primary cause of explosive spalling of c o n ~ r e t e , ~ has been confirmed by more recent investigations.
There are, of course, very good practical reasons to oppose the banning of quartz aggregates from fire resisting concretes. The earth's crust consists of roughly 60 percent SiO?, and most of the silica is in the form of quartz. It is rather difficult, therefore, to find natural aggregates that are en- tirely free of silica. The presence of quartz is
t e m p e r a t u r e F
Fig. 4
-
Dilatometric curves of concreteclearly detectable even in the calcareous aggregate concrete (see Fig. 4 ) , even though only the fine aggregates contained about 10 percent siliceous components.
The dilatometric curves of concretes reflect a rather complex superposition of the shrinkage of the cement paste matrix,"." due to dehydration on heating, and the expansion of the aggregates. The latter factor is usually predominant, so that the dilatometric curves of concretes have positive slopes, at least in the lower and intermediate tem- perature ranges. I t may be mentioned that there is some doubt as to whether the decline of the curves a t very high temperatures is an indication of actual shrinkage, or is caused by creep under the pressure of a small spring used in the instru- ment.
For most concretes, in addition to the dilato- metric curves obtained at constant rate heating, curves pertaining to the natural cooling of the specimen were also recorded. To avoid overcrowd- ing, the "cooling curves" are not shown in Fig. 4.
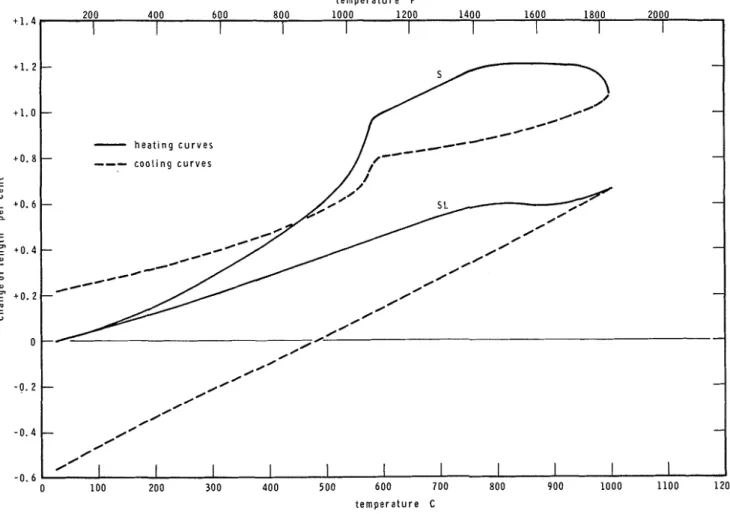
Nevertheless, it seemed desirable to reproduce two complete dilatometric curves (see Fig. 5 ) , to explain the material behavior in a complete heat- ing-cooling cycle.
The heating curves are normally quite differ- ent from the cooling curves. Most of the reactions
that take place during the heating are irreversible or not immediately reversible. Consequently the anhydrous products formed a t 1000 C (1832 F) exhibit a higher degree of chemical stability, which is reflected by the less complicated shape of the cooling curves. One could also expect that, because of the absence of decomposition reactions, the average slope of the cooling curves is higher than that of the heating curves. This expectation turns out to be correct whenever the aggregates do not undergo sudden expansion on heating, as exemplified by the dilatometric curve for the expanded slag aggregate concrete in Fig. 5. If during the heating of concrete the aggregates expand markedly a t some temperature level, microcracks will form which fail to close com- pletely when the temperature decreases. This kind of behavior is exemplified by the siliceous aggregate concrete in Fig. 5. Concretes made with such aggregates normally exhibit a residual ex- pansion following a complete heating-cooling cycle, provided that the heating extends to tem- peratures above the level of sudden expansion.
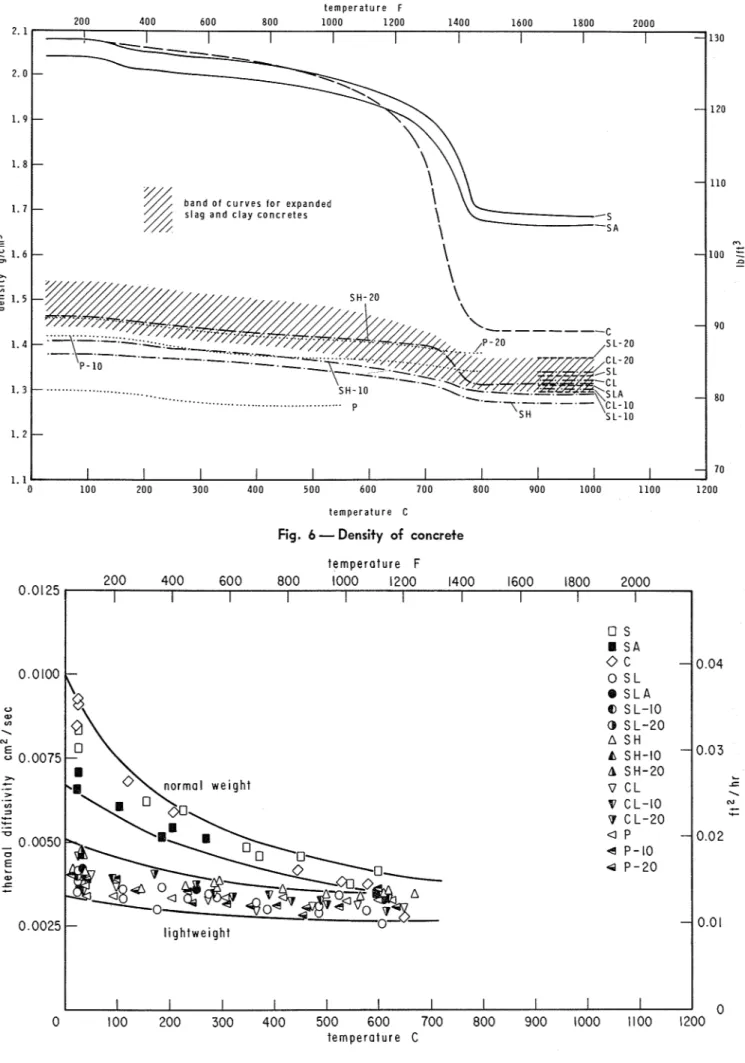
Assuming isotropy, the thermogravimetric and dilatometric curves can be used to calculate the variation of bulk density of concretes with tem- perature. The density versus temperature curves
t e m p e r a t u r e F
+1.4 200 400 600 800 1000 1200 1400 1600 1800 2000
t e m p e r a t u r e C
Fig. 5- Dilatometric curves of two selected concretes, including cooling curves
t e m p e r a t u r e F
b a n d o f c u r v e s f o r e x p a n d e d s l a g a n d c l a y c o n c r e t e s
t e m p e r a t u r e C
Fig. 6
-
Density of concretetemperature F 200 400 600 800 1000 1200 1400 1600 1800 2000 I I I I I I I I I I
1
- V lightweight 0 S I S A 0c
0 SL @ SLA 0 SL-I0 8 S L-20n
S H A SH-I0 A SH-20 D CL V CL-I0v
CL-20 a P -4 P-I0 4 P-20 I I I I I I I I I I I 100 200 300 400 500 600 700 800 900 1000 1100 1200 temperature CFig. 7
-
Thermal diffusivity of concretetemperature F 200 400 600 800 1000 1200 1400 1600 1800 2000 I S I S A
-
0c
0 SL SLA @ SL-I0-
normal weight @ SL-20-
n
S H A SH-I0 A SH-20 D CL-
V CL-I0v
C L-20 a P0
0 a P-10 I-
4 P-20-
A lightweight-
a-
0 V 100 200 300 400 500 600 700 800 900 1000 1100 1200 temperature CFig. 8
-
Thermal conductivity of concrete shown in Fig. 6 have been obtained by such cal-culations. These curves were further utilized to determine the specific heats of the concretes from the thermal property tests.
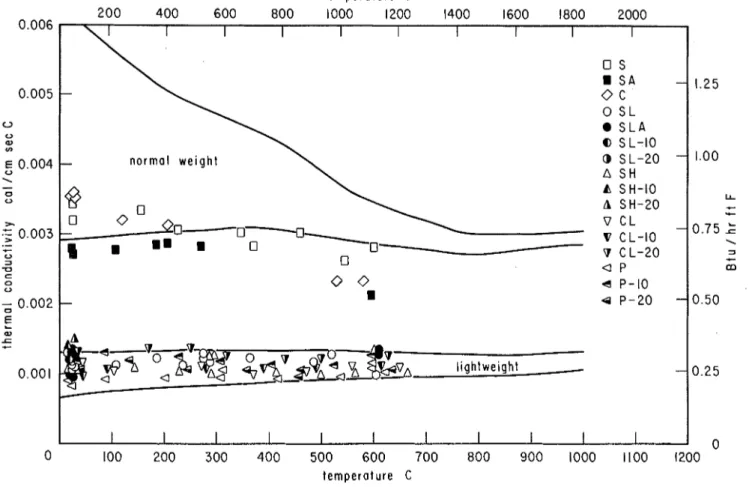
RESULTS OF THERMAL PROPERTY TESTS As mentioned earlier, the thermal diffusivity is the property that can be evaluated most ac- curately from the thermal property tests. For the sixteen concretes examined, this information is plotted in Fig. 7. It is interesting to observe that at about 600 C (1112 F ) the thermal diffusivity of all concretes studied becomes approximately equal to 0.0033 cm2/sec (0.0128 ft2/hr).
The thermal conductivity of the concretes is plotted in Fig. 8. The curves mark two regions in which the values representing normal weight and lightweight concretes, respectively, were expected to fall, on the basis of some theoretical considera- t i o n ~ . ~
It is seen that some values representing siliceous aggregate concretes fall slightly below the theo- retical region for normal weight concretes. This finding is explainable, however,
by
the fact that this theoretical region was obtained for structural normal weight concretes for which the aggregate characteristics are quite different.It may also be noted that many of the values representing lightweight concretes lie above the
lower theoretical region. Some previous tests6 already indicated that this can be expected. The concretes in which natural sand was used as partial replacement for the fine lightweight ag- gregate tended to yield higher conductivities.
The specific heat versus temperature relation- ship for the concretes is shown in Fig. 9. The con- siderable spread of the points is not surprising in the light of some previous remarks made in connection with the interpretation of specific heat values obtained by the thermal property tests. In general, the specific heat of concrete seems to be rather insensitive to the aggregate used and to the mixing ratios.
CONCLUSIONS
Studies concerning the heat flow in concrete constructions at elevated temperatures cannot be expected to yield reasonably accurate results unless the latent heat effects associated with the process are properly taken into account. Un- fortunately, it is still difficult to obtain experi- mental information on the variation of the apparent specific heat with temperature. It is often necessary, therefore, to supplement experimental data by theoretical considerations.
In this paper the results of a comprehensive experimental study concerning sixteen concretes have been presented and analyzed. It is hoped
t e m p e r a t u r e F 200 400 600 800 1000 1200 1400 1600 1800 2000 I
I
I
I I I I I I I 0 S I S A 0c
0 SL @ SLA 0 SL-I0 @ SL-20 A S H A SH-I0 A SH-20v
CL V CL-I0 Q CL-20 a P 4 P-I0 4 P-20 100 200 300 400 500 600 700 800 900 1000 1100 1200 temperature CFig. 9
-
Specific heat of concreteI
that this information will prove useful to those concerned with the thermal performance of con- crete at elevated temperatures, in general, and to those concerned with fire endurance problems, in
particular.
It has been pointed out that the spalling of con- crete in fire is not caused by the presence of quartz in the aggregates, as often surmised, but by high moisture content.
The chemical instability of the cement paste has been shown to be beneficial from the point of view of fire protection.
ACKNOWLEDGMENT
The experiments were performed by Messrs. E. OraCheski, M. L. Fisher, and J. R. McKellar. This paper is a contribution from the Division of Building Research, National Research Council of Canada, and is published with the approval of the Director of the Division. The work which it describes was carried out as part of a cooperative research fellowship program between the National Concrete Producers' Association and the Na- tional Research Council.
REFERENCES
1. Allen, L. W., "Fire Endurance of Selected Non- Loadbearing Concrete Masonry Walls," Fire Study No. 25, NRCC 11275, Division of Building Research, National Research Council of Canada, Ottawa, 1970, 26 pp.
2. Allen, L. W., and Harmathy, T. Z., "Fire Endurance of Selected Concrete Masonry Units," ACI JOURNAL, Pro- ceedings V. 69, No. 9, Sept. 1972, pp. 562-568.
3. Norton, Charles L., "Some Thermal Properties of Concrete," Proceedings, National Association of Cement Users (ACI), V. 7, 1912, pp. 78-90.
4. Zoldners, Nickolai G., "Thermal Properties of Con- crete Under Sustained Elevated Temperatures," Tem- perature and Concrete, SP-25, American Concrete Institute, Detroit, 1971, pp. 1-31.
5. Harmathy, T. Z., "Thermal Properties of Concrete a t Elevated Temperatures," Journal of Materials, V. 5, No. 1, Mar. 1970, pp. 47-74.
6. Harmathy, T. Z., "Determining the Temperature History of Concrete Constructions Following Fire Ex- posure," ACI JOURNAL, Proceedings V. 65, No. 11, Nov. 1968, pp. 959-964.
7. Zhuze, V. P.; Novruzov, 0. N.; and Shelykh, A. I., "Thermal Conductivity Near a Continuous Phase Tran- sition," Soviet Physics-Solid State, V. 11, 1969, p. 1044. (English translation, American Institute of Physics, New York)
8. Harmathy, T. Z., "Variable-State Methods of Mea- suring the Thermal Properties of Solids," Journal of Applied Physics, Vh35, 1964, pp. 1190-1200.
9. Harmathy, T. Z., "Effect of Moisture on the Fire Endurance of Building Elements," Moisture in Materials in Relation to Fire Tests, STP-385, American Society for Testing and Materials, Phladelpha, 1965, pp. 74-94.
This p a p e r was received b y t h e Institute M a y 15, 1972.