Analysis of Patent Data for Flame-Retardant Plastics Additives
by
Ciara Mulcahy
Submitted to the
Department of Materials Science and Engineering
In partial fulfillment of the requirements for the degree of
Bachelor of Science
at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
May 2020
© 2020 Mulcahy All rights reserved.
The author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part in any medium now known or
hereafter created.
Signature of Author……… Department of Materials Science and Engineering May 1, 2020
Certified by……… Elsa Olivetti Atlantic Richfield Associate Professor of Energy Studies Thesis Supervisor
Accepted by………... Juejun Hu Associate Professor of Materials Science and Engineering Chairman, DMSE Undergraduate Thesis Committee
Acknowledgements
I am grateful to the Olivetti Group, which provided me a wonderful community in which to conduct research. First and foremost, I would like to recognize the help of Zachary Jensen, who performed all the computational work described in this paper. Working with him taught me how to communicate across the materials science and computer science disciplines. His patience with me through this iterative design and learning process cannot be overstated. My discussions with Basuhi Ravi motivated the industrial focus on plastics in this project. Her recommendations also greatly bolstered the literature review portion of this paper.
My advisor, Elsa Olivetti, demonstrates unfettered pursuit of helping industries become more sustainable. Furthermore, her unequivocal encouragement of all those around her to explore their interests and feel fulfilled in their work is absolutely inspiring. I deeply appreciate the support and encouragement of Prof. Olivetti in this project and throughout my undergraduate education.
The Department of Materials Science and Engineering at MIT has a supportive and intellectually-engaging culture, for which I am extremely grateful. In particular, I would like to thank the laboratory technicians and technical instructors who so graciously facilitate our education and experimentation.
Abstract:
Plastics are commercially produced by selecting a polymer resin and incorporating chemical additives to affect specific mechanical, chemical or aesthetic properties of the plastic products. The number of possible combinations of polymers and additives yields an enormous engineering space to meet the design requirements of the many applications of plastic materials. However, the broad scope of plastics science hinders both the invention of new plastics
formulations and efforts to investigate potentially harmful polymer resins and plastic additives. In this thesis, a method of representing and analyzing the claims section of patents is presented and applied to a set of patents that refer to flame retardants. The claims section of a patent is presented as a graph, with individual claims as points and references between claims as lines connecting those points. The chemical terms mentioned in the text of each of the claims were split into individual words or short sequences of words, called “tokens”, by an existing materials tokenizer that had been trained on scientific journal articles. The term frequency - inverse document frequency (tf-idf) statistic for each token within each claim was computed, using the entire claims section of the individual patent to calculate the document frequency. Each claim was attributed the tokens that had tf-idf scores greater than the highest-scoring term shared with a claim to which that claim referred. By researcher inspection, this method served to extract relevant chemical terms, while omitting words that did not contribute to the chemical relevance of the claim or patent as a whole. A visualization of these labelled graphs of the claims was generated. This reduced, graphical representation of materials patents could be implemented to aid in researcher review or computational tasks to survey for chemical components or resin-additive compatibilities. Such a representation of patent data could make the prioritization and review of commercial chemicals a more tractable task.
Introduction:
Advanced computational techniques, including machine learning or natural language processing, have recently been applied to extract information from unstructured data, such as scientific journal articles. These computational systems are appropriate for tasks such as
predicting academic trends within a field (Kim, Edward, et al., 2020). However, patents present a more structured and formulaic source of text data than academic journal articles. As the scales of industrial production of materials are much greater than those of research-focused production, patent information likely better indicates the producers and uses of chemical species.
Flame-retardant additives to plastics have a history of having evidenced significant harm to human health, while remaining unregulated or, in some instances, mandated (Blum et al. 2019). Although several chemical within this class of materials has been phased-out in recent years, a systematic representation of the technology space is required to anticipate what waste streams may contain known toxic flame retardants and identify in what applications less harmful chemicals might be substituted.
Background:
The following overview of the different classes of plastics additives serves to contextualizes flame retardants as one of many classes of additives that are in commercial plastics. The “History of Flame Retardants in the United States,” subsection explains how flame retardant plastics additives were developed and adopted broadly. Descriptions of interest groups and regulatory organizations that publish information about flame retardants are included in the history subsection, so that a reader may evaluate what responsibilities or biases might influence information they process about flame retardants. Contradictory information from credible sources was encountered during a review of the flame retardant literature, so this disclosure of advocacy and interest groups was deemed necessary.
Flame retardant describes the function of a set of plastics additives which can be further divided into classes based on their chemistry. The “Classes of Flame Retardants” section familiarizes the reader with chemical terms that may be included in the claims of patents for flame retardant plastics. To return to the industrial scale, a brief description of the waste management and environmental concerns about flame retardants is included. The final subsection introduces the structure of patents which is relevant to this analysis.
Plastics Additives
Plastics are prevalent due to the processability and broad range of mechanical performance that they offer. To meet the engineering requirements specific to a use case, polymeric resins are combined with plastics additives during the synthesis of plastic products. Polymeric resins are made of repeating units or “-mers” that consist of a sequence of atoms. Due to their repeating pattern and flexibility of arrangement, the term “polymer chains” is often used to describe the microstructure of plastics. Plastics additives are usually compounds with far lower molecular weights than those of the polymer chains, so the additives must be compatible with the spaces along or between polymer chains.
The intrinsic properties of polymer resins can be generally gauged by examining the components of the chemical structure of the constituent polymer chains that are not linear sequences of covalently-bound carbon and hydrogen atoms. These “functional groups” are sites where the polymer is more apt to react with environmental chemicals or conditions. The
presence of more complex structures, such as rings or double bonds or halogens, as is the case of polyvinyl chloride (PVC)), along the polymer chain contribute to the distinct physical behavior of the polymer resin (Zweifel & Amos & Stephen, 2001).
Absent the functional groups, the remainder of the polymer chains are hydrocarbons, so many plastic materials are combustible. This property has resulted in the inclusion of flame-retardant additives to delay the propagation of fires through an otherwise readily flammable plastic item (EBFRIP Publications, n.d.). The mechanisms of flame retardancy and the history of the adoption of flame retardants are expounded upon in subsequent sections of this document.
Often the physical properties that are desirable during the manufacturing stage of the plastic product are much less desirable during the use stage of the product. Thus, plastics additives are introduced to the polymer chains to make the chemical reactivity or physical behavior of the material more distinct between the manufacturing and use stages of the product. For instance, the chemical reactivity of polymer precursors is advantageous during
polymerization, the reactions to form polymer chains. However, if a material continues to have reactive sites during its application and use, it may react with substances in its environment, such as oxygen, or have its bonds cleaved through exposure to ultraviolet light. Call to mind plastic objects that have become brittle or crumbled after being outside or in the sunlight for relatively long periods of time. Polymer resins that are known to degrade upon exposure to oxygen or light
often have “antioxidant” or “light stabilizer” plastics additives embedded in them during the synthesis of plastic products (Pritchard, 1998).
During manufacturing, the physical properties of plastics change broadly, yet predictably, with temperature changes or the removal of solvents. Such changes in physical properties
facilitates the production of plastic products with extreme or complex geometries through extrusion, blow molding, or injection molding. The processability of polymer resins can be enhanced by plasticizer additives that enable procedures, such as molding or heat-sealing, to be conducted at lower or broader ranges of temperatures. If the malleable behavior of a polymer resin is not desirable during the use of the plastic product, rigid fillers or impact stabilizers are introduced into the formulation of the final plastic product (Zweifel & Amos & Stephen, 2001).
Additives, such as dyes, fillers, and colorants, affect the appearance of objects made of plastics. Rather than painting or coating a color onto consumer products, which present the challenge of incompatible coefficients of thermal expansion and interfacial incompatibility generally, impregnating the color of plastic products into the polymer resin-additive mixes themselves is common industry practice.
In recent decades, combinations of plastics additives that provide a corresponding set of properties to plastics, called “master batches” have dominated the plastics additives market. The formulation of masterbatches allows plastics producers to order a single additive product, rather than several and having to adjust the ratios of constituent plastics additives (Hahladakis et al., 2018; Zweifel & Amos & Stephen, 2001).
History of Flame Retardants in the United States
In 1821, the French chemist Joseph Louis Gay-Lussac discovered that the addition of either ammonium phosphates or borax made textiles more flame retardant (Evolution of Flame-Resistant Clothing -- Occupational Health & Safety, 2016). Over a century later, the industrial adoption of halogenated flame retardants utilized the procurement and production infrastructure developed for the leaded gasoline industry (Kitman, 2018).
In 1923, General Motors and Standard Oil of New Jersey formed a joint venture called the Ethyl Corporation to market leaded gasoline, which increased the octane level and therefore power output of engines. However, the lead deposited within the engines, causing engine failure within a couple thousand miles of driving. This technical failure initially led to the more frequent
sale of new vehicles, but representatives of the military threatened to discontinue their leaded gasoline contracts, because leaded gasoline was destroying their airplane engines. In response, ethylene dibromide (EDB), manufactured by Dow Chemical engineers, was added to fuels. With the new formulation, combustion caused the formation of lead bromide, which volatilized in the exhaust, rather than building up in the engine as lead additive alone had. In 1934, Ethyl
Corporation opened a plant in North Carolina to extract bromine from seawater, in which bromine has a concentration of 67ppm. By 1941, worldwide production of bromine was 40,000 tons per year, 90 percent of this product was for use in leaded gasoline. By 1970, worldwide production of bromine was 320,000 tons. However, as the lead content of leaded gasoline was anticipated to face environmental regulations, the catalytic converter was being developed by General Motors (Kitman, 2018).
In 1962, GM and Standard Oil of New Jersey sold the Ethyl Corporation to the
Albemarle Corporation, which was 13 times smaller than Ethyl Corporation itself. Using a $200 million loan from Chase Bank and several insurance companies, Albemarle bought Ethyl for a price equivalent to 100 times Albemarle’s annual profits. When the Clean Air Act of 1970 stimulated the phaseout of leaded gasoline, the EDB product of Ethyl under Albemarle lost its original market for bromine. Albemarle Corporation readily marketed EDB as a fumigant to kill microbiota in soils. However, EDB persisted in the environment and was detected in food goods. By 1981, the EPA concluded that EDB was a “potent mutagen,” based on evidence of exposure causing damage to the liver, stomach, adrenal glands, and reproductive systems (Kitman, 2018).
The Occupational Safety and Health Act of 1970 was signed into law by President Richard Nixon on December 29, 1970. In response, high risk industries, such as petroleum refining and chemical processing, adopted flame retardant uniforms to prevent workers from injuries caused by flash fires igniting their clothing (Evolution of Flame-Resistant Clothing -- Occupational Health & Safety, 2016). In subsequent years, the use of flame-retardants in textiles transitioned from personal protective equipment to consumer products, such as children’s
sleepwear (Giraud, 2016).
Industrial Safety and Housefires Generated a Demand for Flame Retardants:
Brominated flame retardants had been in limited use since the 1950s. However, during the 1970’s, tobacco companies adopted the use of additives in cigarettes that were intended
either enhance addiction or reduce the detectability of environmental tobacco smoke (Rabinoff, 2007). However, some of these additives had the side-effect of keeping cigarettes burning for longer. Thus, housefires caused by cigarettes became more common, and legislative pressure for the development of self-extinguishing cigarettes was anticipated. A coalition of large tobacco companies conducted a campaign, assisted by the public-relations firm, Burson-Marsteller, to shift the public blame to the flammability of consumer products and structural materials, rather than the cigarettes which ignited those housefires. This approach to the issue of housefires generated an industrial focus on flame retardants (Kitman, 2018). When the U.S. Consumer Product Safety Commission (CPSC) was founded in 1973, the regulation of cigarettes was omitted from its Congressional mandate, but significant emphasis was placed on ensuring the flame retardance of fabrics. This responsibility was significantly expanded to all household products through the Federal Hazardous Substances Act (FHSA) of 1986 (Vandenberg, 2016). Californian Furniture Regulations:
In 1975, California passed California Technical Bulletin 117 (CAL 117), a regulation that set the standard for flammability of upholstered furniture. The regulation specifies that neither cover fabric nor barrier materials may openly flame, develop char more than 1.8 inches in any direction from the applied, burning non-filtered cigarette or continue to smolder more than 45 minutes after the application of the non-filtered cigarette. According to CAL 117, the filling material could not continue to smolder, intensify in smoldering, openly flame, or have more than 20% mass loss over the course of burning (TECHNICAL BULLETIN 117, 2013). These
requirements prompted the inclusion of flame retardants in the filling foams of furniture for use in California and, as result of the Californian market share, across the United States. Due to cost and efficiency of meeting the requirements of CAL 117, brominated flame retardants
predominated and continue to dominate this use case (TECHNICAL BULLETIN 117, 2013). Later, in 1991, California’s state legislature enacted Technical Bulletin 133 (CAL 133), which specified similar flame resistance standards for all seating furniture for use in public occupancies, such as jails, prisons, nursing care homes, health care facilities, auditoriums, hotels etc. This regulation remained in effect, increasing the cost of furniture and the production of flame-retardant additives, until its repeal in January 2019. (Oliver, 2019)
Biomonitoring Findings:
In the early 1970s, a bag of Firemaster FF-1, a commercial Polybrominated biphenyls (PBB) mixture, was accidentally mixed into animal feed in Michigan. The mass deaths of the livestock and poisonings of any consumers of those animals resulted in PBBs being taken off the market. Despite this early crisis, the development and commercial adoption of other brominated flame retardants proceeded (Damstra et al., 1982).
In 1977, Arlene Blum and Bruce Ames demonstrated that the flame-retardant tris(1,3-dibromopropyl) phosphate was mutagenic and potentially carcinogenic. The publication of this research finding prompted manufacturers of children’s sleepwear to discontinue using it as an additive and the U.S. Consumer Product Safety Commission (CPSC) to ban its use in children’s clothing within the year.
Polybrominated diphenyl ethers (PBDEs) were the subject of biomonitoring research in Europe. From the 1970’s through 1999, Swedish researchers Daiva Meironyté, Koidu Norén and Åke Bergman measured the presence and concentrations of PBDEs and their metabolites in women’s breast milk. The fiftyfold increase in levels of PBDEs in women’s breast milk over this time period evidenced their bioaccumulation. This finding stimulated research into the effects of PBDEs and restrictions on the application of PentaBDE and OcataBDE by the European Union and in some states within the United States, starting in 2003 (Brown & Cordner, 2011).
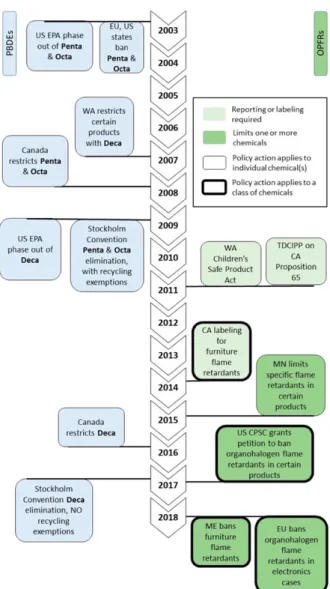
Figure 1: Timeline of regulations and industrial phaseouts of BDEs and OPFRs. Reproduced from Blum et al., 2019.
In 2009, the US EPA announced the phaseout of DecaBDE. In the same year, the
Stockholm Convention led to the elimination of PentaDBE and OctaBDE in the European Union, with the exceptions made for their inclusion in recycled materials (Blum et al., 2019).
However, the Stockholm Convention passed a stricter ban on DecaBDE in 2017, including the recycling of materials that contained DecaBDE. Within 2017, the U.S. Consumer Product Safety Commission (CPSC) granted a petition to ban organohalogen flame retardants in products that present significant exposure to children. By 2018, the EU proposed to ban the production of electronics cases than included organohalogen flame retardants, effective April 1, 2021 (Blum et al., 2019).
The U.S. Environmental Protection Agency (EPA) has the authority to regulate or publish guidelines about the inclusion of specific chemicals or classes of chemical flame retardants in production (EPA: Assessing Risks from Flame Retardants, n.d.). In 2015, the EPA published its available risk and toxicity information about four flame retardant clusters: chlorinated phosphate esters, cyclic aliphatic bromides/ hexabromocyclododecane (HBCD), tetrabromobisphenol A (TBBA), and brominated phthalates, through its Toxic Substances Control Act (TSCA) Work Plan (EPA: Assessing Risks from Flame Retardants, n.d.). Then, in 2016, Congress passed the Frank R. Lautenberg Chemical Safety in the 21st Century Act to improve upon the 1976 Toxic
Substances Control Act, which had received bipartisan criticism on account of its inadequacy. Originally, the EPA was required to weigh the economic costs of chemical regulation and adopt the “least burdensome” means to reduce chemical harms, but the 2016 reform mandated only considering economic costs “to the extent practicable.” Despite perpetuating this ambiguity of the EPA’s role, the reform improved the transparency of the chemical inventory and decision-making processes. No minimum series of tests on new chemicals was specified within the law. High-throughput screening and computational methods for decision-making were anticipated to be adopted by the EPA, given the primary responsibility to screen and test all new chemicals (Vandenberg, 2016; TSCA Chemical Substance Inventory). A major criticism of the TSCA reform was that it prohibits any state governments from regulating the use of any chemicals that the EPA has identified as safe (Kitman, 2018; Vandenberg, 2016).
In December 2019, the EPA initiated risk evaluations under TSCA of three more flame retardants: 4,4'-(1-Methylethylidene)bis[2, 6-dibromophenol] (TBBPA), Tris(2-chloroethyl) phosphate (TCEP), Phosphoric acid, triphenyl ester (TPP) (US EPA, 2019).
In 2017, the CPSC was petitioned to regulate organohalogen flame retardants (OFRs) under the Federal Hazardous Substances Act (FHSA). As the CPSC did not have the resources to produce sufficient toxicology data about all OFRs to adequately inform regulatory action, it convened a committee associated with the National Academies. The National Academies of Sciences, Engineering and Medicine published a 2019 consensus study report titled, “A Class Approach to Hazard Assessment of Organohalogen Flame Retardants.” In the report, the
National Academies noted a neglect to assess hazard or risk if there exists insufficient data about a given chemical. The three problems identified with this practice were that, “Chemicals on which data are insufficient are often deemed not hazardous,” “Untested chemicals are often
substituted for known hazardous chemicals.” and “Cumulative exposure and risk are often ignored.” The National Academies’ report proposed that hazard testing a relatively broad class of chemicals would expedite policy-making on the regulation of OFRs. The committee classified organohalogen flame retardants (OFRs) as either polyhalogenated organophosphates or polyhalogenated bisphenyl aliphatics (A Class Approach to Hazard Assessment of
Organohalogen Flame Retardants, 2019).
Advocacy and Interest Groups
Arlene Blum, previously mentioned for demonstrating the mutagenicity of tris(1,3-dibromopropyl) phosphate, founded the Green Science Policy Institute in 2008. The Green Science Policy Institute publishes research and public education content about the health and environmental risks of specific chemical additives (Flame Retardants - SixClasses.org, n.d).
In March 2011, the American Chemistry Council formed a North American Flame Retardant Alliance (NAFRA) as the “lead advocacy organization in North America for flame retardant producers and users.” NAFRA members include Albemarle Corporation, ICL Industrial Products and LANXESS. The history of Albemarle Corporation’s involvement with brominated flame retardants is detailed above. Israel Chemicals Limited Industrial Products (ICL-IP)
produces a broad range of fertilizers and chemical products, including over 33% of global demand for elemental bromine. Additionally, ICL-IP produces nitrogen, magnesium, and
phosphorous-based flame-retardant compounds. ICL offers a flame-retardant selection heuristic, called Systematic Assessment for Flame Retardants (SAFR). SAFR factors in the hazard a chemical flame retardant poses ecologically and to human health, exposure potential via contact, migration, leaching or volatilization (SAFR, Systematic Assessment for Flame Retardants, n.d.). LANXESS produces phosphorous- and bromine-based flame retardants, which include trade names such as Disflamoll, Reofos, Levagard, and Emerald Innovation (Flame Retardants Overview, n.d; LANXESS, n.d.). The European Flame Retardants Association (EFRA) and the European Flame Retardant Industry Panel (EBFRIP) are subsidiaries of the European Chemical Industry Council (Cefic), which publish similar literature about the benefits of flame retardants to that of NAFRA (EBFRIP Publications, n.d.).
The Interstate Chemicals Clearinghouse (IC2) facilitates the sharing of chemicals data across the health and environmental protection departments of state, local and tribal governments [Interstate Chemicals Clearinghouse]. To disseminate the databased results of chemical hazard assessments and offer alternative chemicals, the IC2 developed Greenscreen and Quick Chemical Assessment Tool (QCAT) for use by the public (Interstate Chemicals Clearinghouse, n.d.). Classes of Flame Retardants
As of 2011, there were more than 175 chemical flame retardants (Brown & Cordner, 2011). Chemical flame retardants are used as additives in combustible materials to prevent the spread of fires. Within flame retardants, a distinction is made between additives which are mechanically mixed into the polymer and “reactives” which are chemically bound to the polymer. Being chemically-bound, reactives generally affect the physical properties of the polymer to a lesser extent and are less likely to migrate out from the plastic items (Hahladakis et al., 2018).
Flame retardants are generally classified based on whether their active compounds contain bromine, chlorine, phosphorous, nitrogen, metals, or boron (NIEHS: Flame Retardants, n.d.). Halogenated flame retardant systems often operate by “vapor phase inhibition,” in which the additive reacts with the combusting polymer vapor phase, molecularly disrupting the formation of free radicals so that combustion cannot proceed. Non-halogen flame retardant systems that contain phosphorous or nitrogen burn, forming a solid, carbonaceous char layer on the flammable material’s surface. This barrier limits the exchange of gases that might otherwise fuel combustion. Alternatively, the high heat of fire causes hydrated minerals to undergo an endothermic reaction that produces water, which evaporates removing heat energy from the system and diluting the vapor-phase combustion reactants (How Flame Retardant Systems Work In Plastics, n.d).
Brominated Flame Retardants:
As of 2000, more than 200,000 metric tons of brominated flame retardants (BFRs) were produced each year. Bromine-containing flame retardants include tetrabromobisphenol A (TBBPA), hexabromocyclododecane (HBCD) and polybrominated diphenyl ethers (PBDEs). PBDEs), which are also referred to as biphenyl oxides, include decabromodiphenyl ether
(DBDE), octabromodiphenyl ether (OBDE), and pendabromodiphenyl ether (pendaBDE). All of these brominated flame retardants are included as additives or reactives in a broad variety of polymeric and epoxy resins, but their relative consumption rates vary across regions of the world. Due to its major use as a reactive flame retardant in printed circuit boards, TBBBPA is used for 76% of the consumption of BFRs in Asia, but only 34% of the BFR consumption in the Americas. HBCD is an additive flame retardant in thermoplastic polymers, but it has also been used in textile coatings, cable, latex binders, and unsaturated polyesters. In foam applications, HBCD has substituted for PBDEs in European markets due to voluntary bans on account of ecotoxicity and risks to human health (Birnbaum & Staskal, 2004). The United States and Canada followed suit over the first decade of the twenty-first century, sequentially phasing out PBDEs and substituting them with organophosphate flame retardants. However, due to their presence in existing products and the environmental persistence of PBDEs, they continue to be researched.
The synthesis of PBDEs faced the challenge of often yielding impure mixtures of
multiple PBDE congeners, but their intended commercial uses vary categorically. DBDE, which can be produced with up to 97% purity, accounts for 80% of the worldwide PBDE production. DBDE serves as an additive flame retardant in electrical and electronic equipment and can be applied as a backcoat to textiles. OBDE is produced as a constituent of a brominated mixture and used in plastic housings and smaller components. PentaBDE is a viscous liquid, used primarily as an additive in textiles. Lower brominated congeners are more bioaccumulative and toxic than the higher cogeners. All the brominated flame retardants were the subjects of concerns about persistence, bioaccumulation, neurodevelopmental toxicity or carcinogenicity (Birnbaum & Staskal, 2004) As PBDEs became phased-out in Europe and the United States, specific organophosphate flame retardants (OPFRs) were identified as substitutes for PentaBDE in residential furniture, Octa- and DecaBDE in electronics (Blum et al., 2019).
Organophosphate Flame Retardants (OPFRs):
OPFRs are esters of phosphoric acid, in which at least one of the -OH groups is
substituted with an aromatic ring or chain of carbon and hydrogen atoms. These structures are polar and therefore far more water soluble than PBDEs. This property facilitates the leaching of OPFRs from plastic products, to which they are added to serve as both flame retardants and
plasticizers. An additional means of migration for OPFRs is volatilization, due to its higher vapor pressure than that of PBDEs. As OPFRs are often included in the plastics of electronic devices, the concentrations of OPFRs in indoor air have been tested to be an order of magnitude greater than the indoor air concentrations of PBDEs at their peak utilization in the early-to-mid 2000s.
The U.S. Food and Drug Administration approved three OPFRs: EHDPP, TPHP, and TNBP, for use in food packaging materials. The migration of those OPFRs into foods from plasticized film wrappers has been recorded. While PBDEs bioaccumulate and can be detected in human blood serum with half-lives of weeks to years after exposure, OPFRs are metabolized and excreted in human urine with half-lives of hours to days after exposure. Many of the OPFR metabolites are more difficult to attribute to specific OPFRs, but biomonitoring studies have evidenced OPFR exposure and intake throughout the human population. Although the toxicology, OPFRs have been studied less than PBDEs on account of their recent adoption, studies suggest that OPFR exposure presents similar neurotoxicity to that posed by the banned PBDEs (Blum et al., 2019).
Inorganic Flame Retardants:
Inorganic metal hydroxide FRs decompose endothermically, liberate water, and suppress smoke in the condensed phase of combustion. Metal oxide flame retardants serve only as smoke suppressants under specific combustion conditions in the vapor phase and thus are usually used in conjunction with halogenated flame retardants (Brown, 1998).
Metal hydroxides that are used in major quantities today include alumiunum trihydroxide (ATH, Al(OH)3 ) and magnesium hydroxide (Mg(OH)3 ). ATH is the product of the Bayer
process for bauxite. Most of this ATH is calcined into alumina and then electrochemically reduced to aluminum metal. However, the flame-retardant behavior of ATH, added to cotton flannelette, was first identified by Sir William Henry Perkin in the 19th century. Then, in the
1950’s, patents were published that described the addition of ATH to polyester, neoprene butyl and epoxies to prevent electronic components from arcing. From that use case, ATH was adopted for use in many thermoset and rubber products, but not in any thermoplastics, except for PVC and ‘thermoplastic’ cable sheeting. During combustion, ATH undergoes an endothermic
decomposition to aluminum oxide, absorbing 1 kJ/g of heat energy, and forming a layer of char that insulates the unburned material. Magnesium hydroxide [Mg(OH)2] is a smoke suppressor by
the same mechanism as ATH, but is more often used in thermoplastics. The Mg(OH)2 surface
absorbs atmospheric moisture and carbon dioxide, so often these additives are treated with hydrophobic coatings (Brown, 1998).
Antimony trioxide [Sb2O3] is the predominant metal oxide flame retardant. It is
substantially included in PVC and serves to retard fires, even at very low loadings. During combustion, antimony trioxide can sometimes increase smoke evolution, but it converts to an antimony trihalide in the presence of halogenated polymer or halogenated flame retardants. The free radical chain reactions that sustain the heat energy required for burning are delayed to a greater extent by antimony trihalide, than the less stable non-complexed halogens (Brown, 1998).
Zinc stannate [ZS, ZnSnO3] and its precursor, zinc hydroxy stannate [ZHS, ZnSn(OH)2]
similarly are most effective at retarding fires in halogenated chemistries, but suppress both smoke and carbon monoxide. The zinc and tin content serves the flame retardancy function to a greater extent than the endothermic decomposition, in the case of ZHS. ZS and ZHS operate in both the vapor and condensed phases of combustion, which generates char and supports products passing the Underwriters Laboratory UL vertical burning test. Zinc borate has also been found to promote char formation and is usually applied to PVC and halogenated polyester (Brown, 1998).
Molybdenum trioxide [MoO3], Tin oxide [SnO2], and iron oxides have also been
identified as other metal oxide flame retardants of commercial impact, but they are limited by effectiveness, compatibility, cost, or aesthetic considerations (Brown, 1998).
End-of-Life Plastics and their Additives
When plastic or composite products are discarded, their waste can often not be
mechanically recycled. Many plastic products intrinsically contain too much contamination to be an appropriate feedstock for secondary plastics production. Some thermoplastics can undergo mechanical recycling, by which the material is sorted, cut into small uniformly-sized pieces, separated, and melted through extrusion into new precursor pellets. The plastic waste feedstock of a given resin type requires chemical treatment, so that the new precursor pellets meet
aesthetic, mechanical, and processability standards. The removal or remediation of plastics additives is a primary concern for the plastics recycling industry (Delva et al., 2018).
As an alternative to recycling, several municipalities have opted to employ waste-to-energy facilities that incinerate mixed streams of waste and sell electricity or heat back to
regional consumers. If the waste feedstock contains halogenated flame retardant molecules, they are likely to form dioxins. Dioxins are persistent organic pollutants that must be captured, contained, and stored securely (Nordic Council of Ministers, 2005).
Patent Space
Patents have well-defined fields, such as Title, Abstract, Description, and Claims. The Claims section is a list of numbered claims that specify protected attributes of the product. The cost of filing patents is charged proportionally to the number of claims included. By convention, the first claim of a patent is the broadest and the specificity of claims increases as the claim number increases. Often, subsequent claims will simply exemplify or narrow the scope of the product or attribute described by an earlier claim.
Figure 2: An example patent document with the Patent Number, Title, Abstract and Claims sections identified.
Summary
Even in the relatively short history of synthetic plastics, a huge number of both polymer resins and plastics additives, used to enhance those resins, have been generated. These feats of polymer science have resulted in an overwhelming number of potential combinations of polymer resins and additives which a waste management system is expected to process. Additionally, regulating a single chemical class within a plastics additive functionality, such as
polybrominated diphenyl ethers (PBDE) flame retardants, required a multi-decade period of evidence and industrial phaseouts, during which human and environmental exposure continued. Advancing polymer science and informing investigations into the hazards of plastics already being produced demands a better framework of representing the plastics currently in commercial production. It was hypothesized that the textual information contained in patents for plastics could be processed into useful inputs for computational methods and helpful visualizations for researchers.
Methods:
Querying the US Patent Database
Using the Patsnap service, the body of patents was searched based on inclusion and exclusion of certain terms (Patsnap, n.d.). Primarily, patents were searched for the functionality or properties that the additives were designed to give the plastic products. Some terms needed to be excluded such that irrelevant patents were omitted from the search results. For instance, patents that include “fuel” or “additive manufacturing” needed to be excluded, because many patents contained those words and the functionality terms being sought, but applied to
technologies other than plastics.
The functionalities, queries and number of patents found are presented in the table below.
Functionality Query Number of Patents
flame-retardant
flame-retardant AND additive
AND plastic 1,712
Light/UV stabilizer
(light AND (UV or ultraviolet) and stabilizer AND additive AND plastic) Not ("photoreactive" Or "LED" Or "building" or "additive manufacturing" or
Antioxidant stabilizer
Additive and (plastic OR polymer OR resin) NOT (propellant OR toner OR paint OR medical OR food) AND TA:(antioxidant OR
stabilizer) 2397
plasticizer
additive AND (plastic OR polymer OR resin) NOT (toner or fuel OR driling OR asphalt OR cosmetic OR propellant OR concrete OR "additive manufacturing" OR smoking or paint OR ink or apparatus or grout) AND
TA:(plasticizer or Additive ) 12,766
The results of searches based on these terms were downloaded in XLS format. The patent fields that were included were the Publication Number, Kind Code, Title, Abstract, Issue Date, Current Assignee, and Claims (full-text).
Subsequent procedures were performed on the set of 1,712 patent results from the flame-retardant query, using the PatSnap service. However, the methodology presented is intended to be reproducible across all types of plastics additives.
Representation of Patent Objects on a Server
These XLS files downloaded from Patsnap, based on the queries above, were processed by a Python program which read along the rows of XLS files. For each row, the elements which corresponded to the respective column headings were saved as attributes of a Patent object. The Patent class defined a dictionary object which had the headings of the patent fields (i.e.
publication number, kind code, title, abstract etc.) as keys and the content of those fields as values. The claims section of each patent was represented as a dictionary, with the claim number of each claim as the keys and the corresponding full text string as the values.
Analysis of the Claims Section of Patents
Extraction of Chemical Functionality Information:
Chemical species were identified within the claim sections using a bidirectional, recurrent sequence to sequence model implemented with a Gated Recurrent Unit neural network. The
model was trained on 300 material science synthesis paragraphs to identify import aspects of the synthesis including target material, precursors, operations, and conditions (Kim et al., 2020). In this work, all predicted material entities (targets, precursors, other materials mentioned) were used to identify chemical species in a claim.
Tokenization of Chemical Species Name:
The individual claims were tokenized, using the same sequence to sequence model as described above. This accommodated chemical species names which spanned multiple words, such as “magnesium hydrate.” Therefore, if adjacent words were identified as chemical words, they were combined into a single token.
Quantification of the Significance of Claims by tf-idf:
In order to determine which terms within claims were significant, the tf-idf statistic was computed for each token, tokenized as described above. The document frequency was the number of instances of that token in the patent’s claims section as a whole. By weighting the frequency of a token within an individual claim by the inverse of the frequency with which that token occurred in the patent’s claim as a whole, normalized by the number of words in the claim, the relevant contribution of the individual claim to the overall patent was indicated. For each claim, the tokens and their corresponding tf-idf scores were composed into a list, in descending order of tf-idf score.
Graphical Representation of the Claims Section:
The claims section was represented by a polyforest structure of labelled, rooted trees. The node labels were the individual claim numbers. Due to the narrowing scope through the sequence in which claims sections of patents are written, the subsequent claims often referred to earlier claims. The claim to which a claim at a given vertex referred was the parent claim. This “dependency” of claims was represented by an edge list in which the first value was a claim number and the second value was the number of a claim to which that claim referred.
The labeling of the claims was based on the pair-wise comparison of the token and tf-idf score lists of the claims which shared a dependency edge. The node of each child claim was
labelled with terms that were included in that claim and had higher tf-idf scores than any tokens that were also extracted from the claim to which the child claim referred.
Summary of Methodology:
1. Convert claims section into a dictionary data structure, with the claim numbers as the keys and the full text of the corresponding claims as the entries.
2. Identify the materials or chemical terms in each claim.
3. Create a graphical representation of the claims section, by connecting the nodes of each claim that refer to another with an edge.
4. Tokenize each of the claims, using a using a recurrent neural network developed by the Olivetti group to identify chemical names.
5. Run tf-idf on all the tokens within each claim, taking the document frequency as the number of times the term occurs across all the claims of the given patent.
6. Label the node of a claim with the tokens in descending order of tf-idf scores that are greater than the first token shared by the given claim and the claim to which it refers. 7. Label any claims that do not refer to any other claims with the tokens they contain that
have the ten highest tf-idf scores.
8. Visualize each patent’s claims by displaying the graph structure, labelled as described above.
Results
Observations based on Reading a Subset of the Downloaded Patents
Although the resin-additive combination for a particular plastic product is usually a proprietary trade secret, patents held by companies that make plastic or composite products disclose the range of potential compositions for products. Patents that described a novel composition or formulation of a plastic material tended to have a first claim that lists the functional constituents of its novel formulation. The constituents could be mentioned based on functionality, such as “antioxidant,” or by broad chemical classes, such as “resin” or “metal hydrate.” Then, subsequent claims specify the chemical species or proportion of the composition for each constituent. Phrases like “selected from the group” or “kind selected from” preceded
lists of chemical species that were either the flame-retardant additive or the plastic resin which was appropriate for the given type of flame-retarding additives.
Patents for individual products that include plastic parts often describe the parts in terms of the functionality or ordering of the constituent layers. Often the product-type of patents do not specify chemical species that are included, but vaguely describe the functionality and
composition of the constituents. Claims Graphs
Representing the claims section of patents as a dictionary, with the claims numbers as keys and the corresponding text as entries facilitated the graphical representation of the claims section. Most patents reviewed in this study had a system of internal references to claims that yielded a meaningful graph structure. Often, the separate trees within the forests of claims graphs referred to separate products that utilized similar compositions.
Materials-trained Chemical Tokenization
Tokenization using basic natural language processing did not work, because chemical names that spanned multiple words or hyphens were split into separate tokens. However, the Olivetti-group tokenizer that was based on ChemDataExtractor and had been trained on scientific journal articles resolved this issue (Swain & Cole, 2016). In some instances, the chemical species were split across multiple tokens, but all the individual tokens were compound chemical words that were specific enough to have a high tf-idf score.
The tf-idf Statistic Extracted Relevant Information
Taking the document frequency as the frequency of the token within the entire claims section of the given patent seemed to produce a meaningful signal in the tf-idf statistics. As the claims sections of the patents varied in text length and subject matter, the entire set of patent claims was not taken as the corpus from which the document frequency was computed. Within a subset of patents as specific as flame retardants, the overall document frequency might have been meaningful, but that approach would have reduced the scalability of this patent survey approach. Limiting the document frequency to just the claim itself would allow for the tf-idf scores to remain consistent despite additional patents being processed and represented on the server.
The tf-idf statistic was an effective instrument to reduce the number of tokens attributed to a given claim. The patent practice of listing many chemicals that could serve as a constituent in a formulation prevented the rule-based approach of recording an arbitrary number of tf-idf tokens with the highest tf-idf scores in each patent. Instead, implementing a lower-bound tf-idf score was considered. The tf-idf scores could not be directly compared across patents, because the document frequencies from which they were computed were independent between patents. Assigning a threshold tf-idf score based on the distribution of tf-idf scores within a patent was considered, but that approach seemed circular, based on the definition of the tf-idf statistic.
However, the claims which referred to an earlier claim, often shared at least one token with the claim to which it referred. The shared token that had the highest tf-idf score in the claim that referred to the other was used as the floor tf-idf score for tokens that were significant enough to be attributed to the claim. This construct served well to include long lists of relevant chemicals, while omitting content from claims that only specified compositional ranges or vague functions. Visualizations of Claims Graphs
On the labelled claims graphs, the color, size, and word labels of nodes conveyed information about the text of the corresponding claim. The claims from which materials terms were extracted by the sequence to sequence model were colored green, whereas claims from which no materials terms were extracted were colored green. The size of a node was correlated with the number of materials terms extracted from a given claim. Nodes of claims from which no materials were extracted were the same size as the nodes from which only one materials term was extracted. In the “Claims Graph annotated with Identified Materials,” all the nodes from which materials terms were extracted were labelled with those, up to a total of 6 terms, for visualization purposes. The nodes of claims from which no materials terms were extracted were labelled with three terms that had the greatest tf-idf scores within the text of that claim. In the “Claims Graph annotated by tf-idf,” all nodes were labelled with five terms that had the greatest tf-idf scores within the text of that claim.
The edge weight between nodes corresponded to the number of words shared between the claim texts associated with the adjacent nodes. Thicker edges indicated that the claims were textually more similar. Due to inherent variability in the length and number of unique words across patents, neither the node size nor the edge thickness was normalized across patents.
Claims Graph Examples:
Patent US7153897 – “Flame-retardant seamless belt, method of manufacturing flame-retardant seamless belt, and image-forming apparatus having flame-retardant seamless belt”
Manually-annotated Claims Graph:
Figure 3: The claims graph of this patent was manually-labelled with materials information to compare with labelling by automated methods, as displayed below.
Claims Graph annotated with Identified Materials:
Many of the materials terms extracted by the sequence to sequence model were included in the lists of terms that scored highest according to the tf-idf statistic. Notably, the mention of the materials, “elastomer, “elastomer cyanurate,” and “salt” in claim 1 was extracted by the sequence to sequence model, but omitted from the list of high tf-idf-scoring terms from claim 1. This could be because those materials were mentioned in several other claims within the patent, resulting in high document frequencies of those terms. Similarly in claim 9, the term “elastomer” was included in the top five tf-idf-scoring terms, but “salt” was farther down the tf-idf-ranked list of terms for claim 9.
The tf-idf statistic served well in extracting materials terms from the subsequent claims. The top five terms according to tf-idf statistic included the relevant materials terms extracted by the sequence to sequence model. From claim 10, the term “master batch” scored highest in the tf-idf-ranked terms list. As master batches are critical to the industrial application of plastic
additives, this extraction of domain-specific materials information would be useful for the robust representation of patent information.
Patent US20050113500A – “Flame-retardant resin composition, process for producing the same, flame-retardant-resin formed article, and process for producing flame-retardant fine particle”
Claims Graph annotated with Identified Materials:
In this patent, claims 6 and 16 specify hydrated metal compounds and claim 11 specifies hydrated metals that could be used in the flame retardant resin composition. The lists of these are abbreviated in this labelling for visualization purposes. The materials sequence by sequence model more consistently extracted materials information from this patent, but the tf-idf-based list include chemical operations that are relevant to this flame retardant resin composition. From some of the claims, tf-idf highly ranked the claim number to which that claim referred. This information is redundant in the claims graph representation, so it ought to be omitted from the tf-idf ranked terms lists.
Patent 201800099980 – “Flame-retardant engineering plastic and preparation method thereof” Claims Graph annotated with Identified Materials:
This patent contains so much chemical information that it is difficult to distinguish which method of data extraction from the claims of this patent performs better. It seems like the tf-idf ranking identified more relevant terms, but that impression is likely due to the arbitrary ordering of terms extracted using the sequence to sequence method. However, from claim 15, more chemical information was extracted by the tf-idf statistic than the single material term, “organic phosphite” identified by the sequence to sequence model. Either labelling method serves to highlight the abundance of chemical data contained within the claims of this patent.
Conclusions and Future Work
Compatibility with Machine Learning Approaches
Patent data could be a phenomenal source of information, if it can be better structured. The claims graph, with every node labelled with its most significant tokens, would require less computational resources to detect patterns across patents. Metrics on the graphical representation of the claims, such as the depth or maximum degree, would likely be correlated with whether the patent was for a chemical composition or product. Product patents tend to have less chemical information, but may be more relevant to consumers. Being able to differentiate between compositional and product patents would help resources be better allocated, based on the goals of a patent review.
Broadening Investigation to more Plastic Additives
To draw conclusion about the plastics additives industry, all the patents for plastics additives would need to be represented according to the method described above. The results of the other functionality queries, such as “antioxidant” or “plasticizer” could be uploaded and represented. Inclusion of those patents would indicate whether the claims structure of patents and chemical information extraction via tf-idf statistics are applicable to additional classes of plastics additives.
Representing Plastics Additives
Performing the tf-idf or sequence to sequence methods identifies the chemical species that are relevant within a patent. A program could traverse a claims graph and instantiate all the potential combinations of polymer resins and plastics additives protected by the patent in a
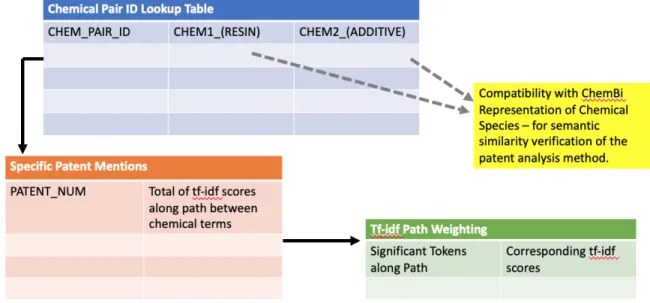
lookup table of a relational database system. The current method cannot distinguish between chemical species that are polymer resins or plastics additives, but cross -reference with databases for plastics additives or for polymer resins could supplement that metadata. These chemical names would ideally be recorded in a format that is compatible with ChemBI or another
standardized chemical representation, so that the polymer-additive compatibilities suggested by the patent claims analysis could be verified externally via other computationally-automated techniques (Ferreira & Couto, 2019). Integration of this patent data for plastics with materials informatics systems, such as the Polymer Genome platform developed by the Ramprasad Group at the Georgia Institute of Technology, is of particular interest (Huan et al., 2016; Kim et al. 2018).
In the claims graph, the shared terms and corresponding tf-idf scores along the paths between the claims containing resins and additives could suggest how significant that resin-additive pair was to the utility of the patent as a whole. By comparing those terms connecting the resin-additive pair across the body of patents, the use case or intended functionality of the
polymer-resin combination in the plastics industry could be elucidated. As the scale of the plastics industry and polymer engineering is phenomenally large, this association between resin-additive pairs and the products or functionalities that might require them would be useful to represent.
Figure 4: Proposed structure of a relational database to represent commercial plastics additives.
Applications to Waste Management, Product Design and Regulation
The association of polymer resins and plastic resins and products in which they might be used is relevant from both the product design and the waste management perspectives. Patents link the plastics additives to the resins with which they are compatible, such that managers of a recycling facility can anticipate what contaminants will be present or need to be remediated from each of their plastic resin feedstocks. Flame retardants are particularly relevant to incineration facilities as high concentrations of flame retardants, within furniture or electronics for instance, could alter the operating conditions or emissions from incineration.
On the design side, if the industrial utility of different resin-additive combinations was more transparent, commercial plastics manufacturers might more readily change their
formulations to be in accordance with health or environmental evidence. Accelerating the
innovation or substitution processes could prevent a prolonged industrial dependence on harmful chemical inputs. If the information that an additive presents less toxicity and offers the same functionality and resin compatibility, the barriers to changing industrial practices would be reduced.
Additionally, such a representation of the intellectual property of polymer science could coordinate the efforts of regulatory agencies. The U.S. Consumer Product Safety Commision (CPSC) is responsible for the products described in patents. The U.S. Environmental Protection Agency (EPA) is accountable for migration and exposure to chemical toxins. The information contained in patents links products and chemicals. Better utilization of patent data could enable the CPSC and EPA to coordinate their research efforts and better protect the American people from chemical exposure. The method presented, of extracting materials data from patents and representing it in a useful form for both computers and researchers, is intended to be the first step in establishing interagency cooperation. While “innocent until proven guilty” continues to be applied to chemicals, at the expense of the health of the populace, the need for rigorous analysis of data to expedite the review of emerging chemicals for toxicity, mutagenicity or
carcinogenicity cannot be overstated. The useful representation and analysis of patent data can help companies transition to practices by which neither their products nor their wastes pose harm to current or future generations.
References
A Class Approach to Hazard Assessment of Organohalogen Flame Retardants. (2019). National
Academies Press. https://doi.org/10.17226/25412
Birnbaum, L. S., & Staskal, D. F. (2004, January). Brominated flame retardants: Cause for concern? Environmental Health Perspectives. National Institute of Environmental Health Sciences. https://doi.org/10.1289/ehp.6559
Blum, A., Behl, M., Birnbaum, L. S., Diamond, M. L., Phillips, A., Singla, V., … Venier, M. (2019, November 12). Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers? Environmental Science and Technology
Letters. American Chemical Society. https://doi.org/10.1021/acs.estlett.9b00582
Brown, P., & Cordner, A. (2011). Lessons Learned from Flame Retardant Use and Regulation Could Enhance Future Control of Potentially Hazardous Chemicals. Health Affairs, 30(5), 906–914. https://doi.org/10.1377/hlthaff.2010.1228
Brown, S. C. (1998). Flame retardants: inorganic oxide and hydroxide systems (pp. 287–296). Springer, Dordrecht. https://doi.org/10.1007/978-94-011-5862-6_32
Bureau of Home Furnishings, Insulation & Repair. (2013). State of California Department of
Consumer Affairs TECHNICAL BULLETIN 117-2013 Requirements, Test Procedure and Apparatus for Testing the Smolder Resistance of Materials Used in Upholstered Furniture REQUIREMENTS, TEST PROCEDURE AND APPARATUS FOR TESTING THE
SMOLDER RESISTANCE OF MATERIALS USED IN UPHOLSTERED FURNITURE.
C. Kim, A. Chandrasekaran, T. D. Huan, D. Das, R. Ramprasad. (2018). Polymer Genome: A data-powered polymer informatics platform for property predictions. J. Phys. Chem. C 122, 31, 17575-17585.
Cornez, D. M. (n.d.). State of California Office of Administrative Law In re: Bureau of Electronic
and Appliance Repair, Home Furnishings and Tl:termal Insulation AMENDED NOTICE OF APPROVAL OF REGULATORY ACTION Government Code This action by the Bureau of Electronic and Appliance Report, Home Furnishings and Thermal Insulation amends furniture flammability standards by removing requirements of Technical Bulletin 133, entitled "Flammability Test Procedure for Seating Furniture·.
Damstra, T., Jurgelski, W., Posner, H. S., Vouk, V. B., Bernheim, N. J., Guthrie, J., … Falk, H. L. (1982). Toxicity of polybrominated biphenyls (PBBs) in Domestic and laboratory animals.
Environmental Health Perspectives, 44, 175–188. https://doi.org/10.1289/ehp.8244175
Delva, L., Hubo, S., Cardon, L., & Ragaert, K. (2018, December 1). On the role of flame retardants in mechanical recycling of solid plastic waste. Waste Management. Elsevier Ltd.
EBFRIP Publications. (n.d.). Retrieved April 21, 2020, from http://www.ebfrip.org/publications.html
Ferreira, J., & Couto, F. (2019). Semantic Similarity in Cheminformatics. In Cheminformatics and
its Applications [Working Title]. IntechOpen. https://doi.org/10.5772/intechopen.89032
Flame Retardants - SixClasses.org. (n.d.). Retrieved April 21, 2020, from https://www.sixclasses.org/videos/flame-retardants
Flame Retardants Overview | Get the Facts Here. (n.d.). Retrieved April 21, 2020, from https://flameretardants.americanchemistry.com/
Flame Retardants. (n.d.). Retrieved April 21, 2020, from
https://www.niehs.nih.gov/health/topics/agents/flame_retardants/index.cfm
Giraud, S., Rault, F., Cayla, A., & Salaün, F. (n.d.). Fire retardants & textiles: past, present and
future 15-16 February, 2016 Torino-Italy HISTORY AND EVOLUTION OF FIRE RETARDANTS FOR TEXTILES.
Hahladakis, J. N., Velis, C. A., Weber, R., Iacovidou, E., & Purnell, P. (2018, February 15). An overview of chemical additives present in plastics: Migration, release, fate and
environmental impact during their use, disposal and recycling. Journal of Hazardous
Materials. Elsevier B.V. https://doi.org/10.1016/j.jhazmat.2017.10.014
High-performing phosphorus- and bromine-based Flame Retardants. (n.d.). Retrieved April 21, 2020, from https://flameretardants.lanxess.com/
How Flame Retardant Systems Work In Plastics. (n.d.). Retrieved April 21, 2020, from
https://www.rtpcompany.com/products/flame-retardant/how-flame-retardant-systems-work-in-plastics/
How to Access the TSCA Inventory | TSCA Chemical Substance Inventory | US EPA. (n.d.). Retrieved April 21, 2020, from https://www.epa.gov/tsca-inventory/how-access-tsca-inventory
IC2 - About IC2. (n.d.). Retrieved April 21, 2020, from https://www.theic2.org/about_ic2 Incineration of Plastics Containing Brominated Flame-Retardants. (2005) (pp. 19–22).
https://doi.org/10.6027/9789289336666-5-en
Kim, Edward, et al. "Inorganic Materials Synthesis Planning with Literature-Trained Neural Networks." Journal of Chemical Information and Modeling 60.3 (2020): 1194-1201. Kitman, J.C. (2018, August 15). Worse Than Lead? The Nation. Retrieved April 21, 2020, from
La Mantia, F. P. (1998). Recycled plastics: additives and their effects on properties (pp. 535–543). Springer, Dordrecht. https://doi.org/10.1007/978-94-011-5862-6_59
Nordic Council of Ministers. (2005). Emission measurements during incineration of waste
containing bromine. https://doi.org/10.6027/tn2005-529
PatSnap. (n.d.). Retrieved April 23, 2020, from https://www.patsnap.com/
Pritchard, G. (1998). Plastics Additives: An A-Z reference, I vol. Surface Science, 633. https://doi.org/10.1007/978-94-011-5862-6
Rabinoff, M., Caskey, N., Rissling, A., & Park, C. (2007). Pharmacological and chemical effects of cigarette additives. American Journal of Public Health, 97(11), 1981–1991.
https://doi.org/10.2105/AJPH.2005.078014
Swain, M. C., & Cole, J. M. "ChemDataExtractor: A Toolkit for Automated Extraction of
Chemical Information from the Scientific Literature", J. Chem. Inf. Model. 2016, 56 (10), pp 1894–1904 10.1021/acs.jcim.6b00207
T. D. Huan, A. Mannodi-Kanakkithodi, C. Kim, V. Sharma, G. Pilania, R. Ramprasad. (2016). A polymer dataset for accelerated property prediction and design. Sci. Data, 3 160012
Tf-idf: A Single-Page Tutorial - Information Retrieval and Text Mining. (n.d.). Retrieved April 23, 2020, from http://www.tfidf.com/
The Evolution of Flame-Resistant Clothing -- Occupational Health & Safety. (n.d.). Retrieved April 21, 2020, from
https://ohsonline.com/Articles/2016/09/08/The-Evolution-of-FlameResistant-Clothing.aspx?Page=1
US EPA, O. (n.d.). Chemicals Undergoing Risk Evaluation under TSCA. US EPA, O. (n.d.). Fact Sheet: Assessing Risks from Flame Retardants.
Vandenberg, L. N. (2016). Reform of the Toxic Substances Control Act (TSCA): An Endocrine Society Policy Perspective. Endocrinology, 157(12), 4514–4515.
https://doi.org/10.1210/en.2016-1712
Welcome on SAFR, The Systematic Assessment for Flame Retardants. (n.d.). Retrieved April 21, 2020, from https://safrworks.com/
Zweifel, H., Amos, S. E. & Stephen, E. (2001). Plastics additives handbook (5th ed.). Cincinnati, OH: Hanser Gardner Publications.