Bacterial oxidases of the cytochrome bd family: redox enzymes of unique structure, function, and utility as drug targets
Texte intégral
Figure
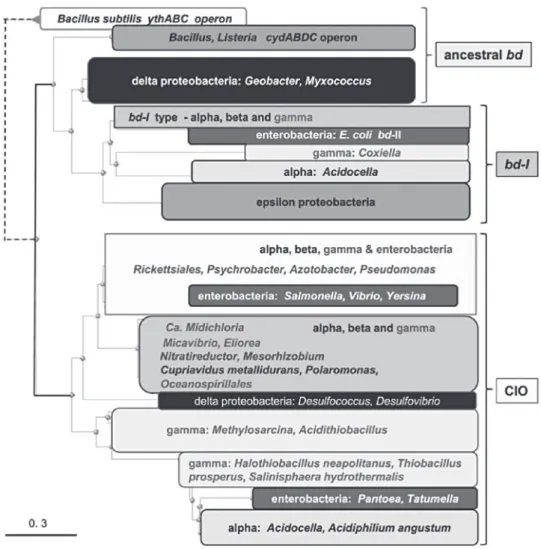



Documents relatifs
En effet, dans ce lieu, vous trouverez tout ce qu’il vous faut pour être en forme toute l’année: des produits naturels et bio tels que des compléments alimen- taires, des cristaux
Pour plus de précision concernant le mode de transmission de cette maladie héréditaire, on a déterminé par une technique appropriée le nombre d’allèles du
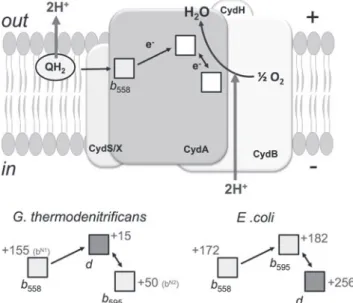
In Mycobacterium tuberculosis, a transient upregulation of cyto- chrome bd was observed in vivo during the transition from acute to chronic infection of mouse lungs, as was
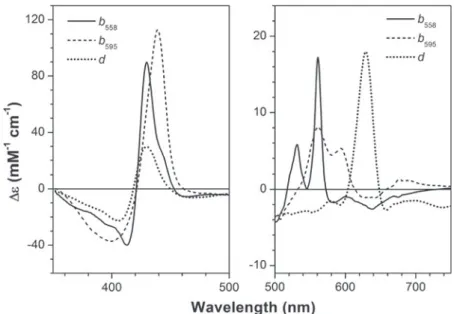
During turnover, the reduction level of the CcOX redox sites, and particularly of the metals in the active site, depends on (i) the actual concentration of reduced cytochrome c and O
Spectral deconvolution analysis was carried out after subtracting the spectrum of the fully reduced enzyme from the experimental set of time-resolved spectra, acquired after stopped-
In these monohemic c-type cytochromes, the iron atom is linked to methionine and histidine residues at neutral pH ; when pH is raised, a transition between
constants for the oxidation of longer chain primary aliphatic alcohols by CgrAlcOx and CglAlcOx are of the order of 10 4 to 10 5 M ‒1 s ‒1 , some 10–100 times higher than that for
To explain the full CV, we propose the following kinetic steps: (i) complex formation between the oxidized forms of the protein, (ii) heterogeneous reduction of Cyt c 4 Heme H