© Mao Li, 2019
Iron(III) catalyzed asymmetric Diels-Alder reaction –
Iron(II) catalyzed thia-Michael addition and aldehyde
allylation reactions
Thèse
Mao Li
Doctorat en chimie
Philosophiæ doctor (Ph. D.)
Québec, Canada
Iron(III) catalyzed asymmetric Diels-Alder
reaction – Iron(II) catalyzed thia-Michael
addition and aldehyde allylation reactions
Thèse
Mao Li
Sous la direction de:
III
Résumé
En raison de leur grande performance, leur prix peu élevé, et leur abondance sur la terre, les catalyseurs de fer ont été choisis pour être testés dans trois différentes transformations de la chimie organique. Le premier projet concerne les réactions asymétriques de Diels-Alder catalysées par Fe+III et le ligand bipyridine chiral à des dérivés α,β-insaturés de l’oxazolidin-2-one. Dans un premier temps, nous avons testé différents solvants, diverses quantités en catalyseur, temps de réaction variés et divers sels de fer tels que Fe(ClO4)3·6H2O, Fe(ClO4)2·6H2O, Fe(OTf)3, Fe(OTf)2, FeCl2, FeCl3, FeBr3 et FeI3. Nous avons constaté que 2 mol% de Fe(ClO4)3·6H2O, 2.4 mol% de ligand bipyridine chiral utilisés à –30 oC dans CH3CN, conduisait à un très bon rendement (99%) et à un bon excès énantiomérique (98%) pour la réaction entre le cyclopentadiène et la 3-alcénoyl-1,3-oxazolidin-2-one. Ensuite, un grand nombre de diénophiles et de diènes moins réactifs ont été testés. Globalement, moins de 10 mol% de catalyseur a été utilisé. L’avantage de ce projet est de pouvoir réaliser la réaction à une température modérée, utiliser de très faibles quantités de catalyseur, obtenir de très bons rendements et d’excellentes énantiosélectivités, et avec une large gamme de substrats. Par la suite, les catalyseurs de fer ont été appliqués aux additions de
thia-Michael par deux approches différentes. La première consiste en additions de thia-Michael catalysées par Fe(OTf)2 dans l’éthanol à température ambiante. Cette méthode permet aux additions de thia-Michael d'être catalysées par un sel de fer vert et beaucoup plus écologique, en quantité catalytique (5 mol% de Fe(OTf)2), dans un solvant couramment utilisé, EtOH, à température douce, et à atmosphère ambiante. L’avantage de cette réaction a été démontré en l’appliquant à différents accepteurs de Michael et à des thiols aliphatiques et aromatiques. La deuxième méthode consiste en des additions de thia-Michael, catalysées par Fe(OTf)2 dans le 2-Me-THF, qui est en accord avec les principes de chimie verte en utilisant un sel de vert, Fe(OTf)2, et un solvant vert 2-Me-THF à température ambiante ou à 50 oC sous air. Le dernier projet est l'allylation asymétrique catalysée par le Fe(OTf)2 portant un ligand chiral. Avec l'étude d'une variété de ligands chiraux, nous avons sélectionné 5 mol% de Fe(OTf)2 et 6 mol% de ligand Pybox qui ont catalysé la réaction avec un bon rendement (70%) et 32% d'excès énantiomérique. 20 mol% de TMSCl se sont avérés essentiels pour l'efficacité de la réaction.
IV
Abstract
Iron catalysts are employed in three different organic transformations owing to their advantages: environmental friendliness, being less expensive and abundant on the Earth. The first project deals with asymmetric Diels-Alder reactions of α,β-unsaturated oxazolidin-2-one derivatives catalyzed by FeIII and a chiral bipyridine ligand. In order to obtain the optimized reaction conditions, we screened different solvents, catalyst loading, various reaction times and a variety of iron salts such as Fe(ClO4)3·6H2O, Fe(ClO4)2·6H2O, Fe(OTf)3, Fe(OTf)2, FeCl2, FeCl3, FeBr3 and FeI3. As a result, the reaction between cyclopentadiene and 3-alkenoyl-1,3-oxazolidin-2-one was carried out at –30 oC in CH3CN in 1.5 h, with Fe(ClO4)3·6H2O (2 mol%) complexed with the chiral bipyridine ligand (2.4 mol%) as catalyst, providing an excellent yield (99%) and an excellent enantiomeric excess (98%). Decreased enantioselectivities were observed for less-reactive dienes. Overall, less than 10 mol% of catalyst loading was employed. The great advantages of this project are the mild reaction temperature, very low catalyst loading, excellent yields and enantioselectivities and the applicability to a wide scope of substrates. Meanwhile, iron catalysts were used in thia-Michael additions by two different approaches. The first one is about thia-Michael additions catalyzed by Fe(OTf)2 in EtOH at room temperature. This green method allows the thia-Michael additions to be catalyzed by a green iron salt (5 mol% of Fe(OTf)2), a green and commonly used solvent EtOH at room temperature under ambient atmosphere. The generality of this reaction was demonstrated by applying it to different Michael acceptors, and to aromatic and aliphatic thiols. The second method is about
thia-Michael additions catalyzed by Fe(OTf)2 in 2-Me-THF, which is in agreement with the green chemistry principles by using a green Fe(OTf)2 and a green solvent 2-Me-THF at room temperature or 50 oC under air atmosphere. The last project is about asymmetric allylation reactions catalyzed by Fe(OTf)2 using a chiral ligand. With the study of a variety of chiral ligands, we selected 5 mol% of Fe(OTf)2 and 6 mol% of Pybox ligand which catalyzed the reaction in good yield (70%) and 32% of ee. The utilization of 20 mol% of TMSCl is essential for the effectiveness of the reaction.
V
Contents
Résumé ... III Abstract ... IV List of schemes ... VIII List of tables ... XII List of figures ... XIV List of abbreviations ... XV Acknowledgements ... XVIII
Introduction ... 1
Properties of iron ... 1
Reactions catalyzed by iron catalysts ... 1
Representative examples of typical reactions in the presence of iron catalysts ... 2
Introduction to Diels-Alder reactions ... 8
General introduction to Diels-Alder reactions ... 8
Enantioselective Diels-Alder reactions catalyzed by chiral metal complexes ... 9
Enantioselective Diels-Alder reactions catalyzed by other chiral catalysts ... 19
Research objectives about enantioselective Diels-Alder reactions catalyzed by iron complexes ... 24
Chapter I Development of enantioselective Diels-Alder reactions catalyzed by iron catalysts ... 27
1.1 Synthesis of the 2,2’-bipyridyl ligand... 27
1.2 Preliminary feasibility studies to asymmetric Diels-Alder reaction catalyzed by iron catalysts ... 28
1.3 Results for the asymmetric Diels-Alder reactions ... 33
1.3.1 Solvent effects on the asymmetric Diels-Alder reactions ... 33
1.3.2 Temperature effects on asymmetric Diels-Alder reactions ... 35
1.3.3 Effects of the catalyst loading on asymmetric Diels-Alder reactions ... 37
1.3.4 Effects of iron salts on asymmetric Diels-Alder reactions ... 38
1.3.5 Influence of the different structures of the dienophiles and dienes on asymmetric Diels-Alder reactions ... 40
VI
1.3.6 Research on other influential parameters for the studied asymmetric
Diels-Alder reaction... 50
1.3.7 Proposed mechanism of enantioselective Diels-Alder reactions ... 61
1.4 Conclusions ... 64
Chapter II Iron catalyzed thia-Michael additions to ,-unsaturated oxazolidin-2-ones ... 66
2.1 Introduction to thia-Michael additions ... 66
2.1.1 Thia-Michael additions with catalysts-free or water-mediated approaches ... 66
2.1.2 Thia-Michael additions catalyzed by different catalysts ... 66
2.1.3 Thia-Michael additions catalyzed by various metals ... 68
2.2 Green chemistry principle ... 71
2.3 Research objectives ... 73
2.4 Development of thia-Michael additions ... 73
2.4.1 Thia-Michael additions catalyzed by Fe(OTf)2 in EtOH ... 73
2.4.2 Thia-Michael additions catalyzed by Fe(OTf)2 in 2-Me-THF ... 84
2.5 Conclusions ... 92
Chapter III Iron catalyzed asymmetric allylation of ,-unsaturated carbonyl compounds ... 94
3.1 Introduction to allylation reactions ... 94
3.1.1 Allylation reactions of aldehydes ... 94
3.1.2 Allylation reactions of ketones ... 96
3.1.3 Allylation reactions of imines ... 98
3.1.4 Asymmetric allylation reactions of carbonyl derivatives ... 100
3.2 Research objectives ... 104
3.3 Development of asymmetric allylation reactions of carbonyl compounds ... 104
3.3.1 Preliminary studies of different chiral ligands for the asymmetric allylation of benzaldehyde ... 104
3.3.2 Influence of different iron salts on asymmetric allylation of benzaldehyde 116 3.3.3 Influence of different solvents on asymmetric allylation of benzaldehyde . 117 3.3.4 Influence of other different chiral ligands on the asymmetric allylation of benzaldehyde ... 118
VII
3.4 Conclusions ... 120
General conclusions and perspectives ... 122
Experimental section ... 128
Experimental procedure and characterization of iron catalyzed asymmetric Diels-Alder Reactions ... 128
General remarks ... 128
Characterization of dienophiles ... 129
General procedure for asymmetric Diels-Alder reactions ... 132
A representative procedure for asymmetric Diels-Alder reaction ... 133
Characterization of cycloadducts ... 134
Experimental procedure and characterization of iron catalyzed thia-Michael addition .... 145
Experimental procedure for thia-Michael additions catalyzed by Fe(OTf)2 in EtOH ... 145
General method for thia-Michael addition in EtOH ... 148
Synthesis and characterization of products ... 149
Experimental procedure and characterization for thia-Michael additions in 2-Me-THF ... 158
Experimental procedure and characterization of iron catalyzed asymmetric allylation reactions ... 160
General method for synthesizing allylation products ... 160
A representative procedure for the allylation reaction ... 160
Characterization of the allylation product and ligands and ligand precursors ... 161
References ... 168
Copies of NMR spectra and chiral HPLC chromatograms for chapter I ... 178
Copies of NMR spectra for chapter II ... 209
VIII
List of schemes
Scheme 0.1 Asymmetric epoxidation reaction catalyzed by Fe-porphyrin ... 2 Scheme 0.2 Asymmetric hydroxylation of a benzylic compound catalyzed by Fe-porphyrin
... 4 Scheme 0.3 cis-Dihydroxylation of olefins with H2O2 catalyzed by iron complex 3 ... 4
Scheme 0.4 Enantioselective sulfide oxidation catalyzed by a chiral Fe/6,6’-bis(4-isopropyloxazolin-2-yl)-2,2’-bipyridine (bipybox-iPr) complex... 5 Scheme 0.5 Asymmetric sulfide oxidation catalyzed by Fe(salen) immobilized on F-m-MCF-50 ... 6 Scheme 0.6 Proposed catalytic mechanism of the sulfoxidation with PhIO catalyzed by Fe(salen) ... 6 Scheme 0.7 Enantioselective thia-Michael conjugate addition catalyzed by Fe(salen) 6 .... 7 Scheme 0.8 Application of thia-Michael route to (R)-montelukast using Fe(salen) 6 ... 7 Scheme 0.9 Asymmetric Diels-Alder reaction catalyzed by Mg(OTf)2 and a chiral
bis(oxazoline) ligand ... 10 Scheme 0.10 Zinc and a chiral N,N’-dioxide ligand catalyzed asymmetric Diels-Alder reaction ... 10 Scheme 0.11 Asymmetric Diels-Alder reaction catalyzed by iridium with an amide linker
... 11 Scheme 0.12 Cu(OTf)2 and a bis(oxazoline) ligand catalyzed asymmetric Diels-Alder
reaction ... 11 Scheme 0.13 Cu(OTf)2 and a chiral ligand 8 catalyzed asymmetric Diels-Alder reaction . 12
Scheme 0.14 Asymmetric Diels-Alder reaction catalyzed by a chiral PyOx ligand complexed with Cu(OTf)2 ... 12
Scheme 0.15 A chiral aluminum complex catalyzed asymmetric Diels-Alder reaction... 13 Scheme 0.16 Asymmetric Diels-Alder reaction catalyzed by TiCl2(OiPr)2 and a chiral
TADDOL ligand ... 13 Scheme 0.17 TADDOL-promoted enantioselective intramolecular Diels-Alder reaction . 14 Scheme 0.18 Asymmetric Diels-Alder reaction catalyzed by a chiral titanium-based diol catalyst ... 14 Scheme 0.19 Asymmetric Diels-Alder reaction catalyzed by a boron ligand combination with Tf2NH-TiCl4 ... 15
Scheme 0.20 Asymmetric Diels-Alder reaction catalyzed by Sc(OTf)3 complex ... 15
Scheme 0.21 Ni(ClO4)2·6H2O and a chiral binaphthalene diimine ligand catalyzed
asymmetric Diels-Alder reaction ... 16 Scheme 0.22 Asymmetric Diels-Alder reaction catalyzed by Ni(OTf)2 and chiral ligand 8
IX
Scheme 0.23 Ytterbium-catalyzed Asymmetric Diels-Alder reaction of Danishefsky’s
diene... 17
Scheme 0.24 Asymmetric Diels-Alder reaction catalyzed by the (R,R)-4,6-dibenzofurandiyl-2,2’-bis(4-phenyloxazoline)·Fe(ClO4)2 complex ... 17
Scheme 0.25 L·FeCl2I (L=2,2-bis[2-[4(S)-phenyl-l,3-oxazolinyl]]propane) complex catalyzed the Diels-Alder reaction ... 18
Scheme 0.26 Asymmetric Diels-Alder reaction catalyzed by a bis-sulfoxide ligand and FeI3 ... 18
Scheme 0.27 Asymmetric Diels-Alder reaction catalyzed by Kündig’s cationic iron(II) complex ... 19
Scheme 0.28 Asymmetric Diels-Alder reaction catalyzed by a chiral amine-thiourea organocatalyst ... 20
Scheme 0.29 Asymmetric Diels-Alder reaction catalyzed by a chiral binaphthyl silica catalyst ... 20
Scheme 0.30 Enantioselective Diels-Alder reaction catalyzed by a chiral amino alcohol . 21 Scheme 0.31 A polyester with chiral imidazolidinone for the asymmetric Diels-Alder reaction ... 21
Scheme 0.32 Chiral organocatalysts for asymmetric Diels-Alder reactions ... 22
Scheme 0.33 Chiral ligands for enantioselective Diels-Alder reactions ... 23
Scheme 0.34 Biomolecular catalysts for enantioselective Diels-Alder reactions ... 23
Scheme 0.35 Enantioselective Mukaiyama aldol reaction catalyzed by a Fe(II)-2,2’-bipyridyl chiral complex ... 25
Scheme 0.36 Opening reaction of aromatic meso-epoxides with indoles catalyzed by a Fe(II)-2,2’-bipyridyl chiral complex ... 25
Scheme 0.37 Enantioselective meso-epoxide-opening with anilines catalyzed by a Fe(II)-2,2’-bipyridyl chiral complex ... 25
Scheme 0.38 Enantioselective Michael addition catalyzed by a Fe(II)-2,2’-bipyridyl chiral complex ... 26
Scheme 1.1 Synthesis of the 2,2’-bipyridyl ligand ... 27
Scheme 2.1 Thia-Michael addition catalyzed by VO(OTf)2 ... 68
Scheme 2.2 Thia-Michael addition catalyzed by a nano cubic LiF ... 68
Scheme 2.3 Thia-Michael addition catalyzed by LiOH... 69
Scheme 2.4 Thia-Michael addition catalyzed by InCl3 ... 69
Scheme 2.5 Thia-Michael addition catalyzed by Yb(OTf)3 ... 70
Scheme 2.6 Thia-Michael addition catalyzed by a Ni complex ... 70
Scheme 2.7 Thia-Michael addition catalyzed by Cu(BF4)·xH2O ... 71
X
Scheme 2.9 The scope of Michael acceptors reacted with thiophenol ... 81
Scheme 2.10 (E)-3-(But-2-enoyl) oxazolidin-2-one reacted with different thiols ... 83
Scheme 2.11 Different Michael acceptors reacted with thiophenol ... 87
Scheme 2.12 (E)-3-(But-2-enoyl) oxazolidin-2-one reacted with different thiols at room temperature using 2 mol% of Fe(OTf)2 ... 89
Scheme 2.13 (E)-3-(But-2-enoyl) oxazolidin-2-one reacted with different thiols at room temperature using 5 mol% of Fe(OTf)2 ... 90
Scheme 2.14 (E)-3-(But-2-enoyl) oxazolidin-2-one reacted with different thiols ... 91
Scheme 3.1 Hosomi-Sakurai reaction catalyzed by TiCl4 ... 94
Scheme 3.2 Iron(III)-catalyzed allylation of aldehydes ... 94
Scheme 3.3 Allylation of aldehyde using allyltrimethoxysilane and a CuCl-TBAT catalyst ... 95
Scheme 3.4 Allylation reaction catalyzed by CdF2 using allyltrimethoxysilane esterification of the functionalized aldehyde ... 95
Scheme 3.5 Aldehyde allylation using allylstannanes and catalyst GdCl3·6H2O ... 96
Scheme 3.6 Sc(OTf)3-catalyzed allylation of aldehyde with tetraallyltin ... 96
Scheme 3.7 Allylation of cyclododecanone using tetra-allylstannane and the catalyst Cu(OTf)2 ... 96
Scheme 3.8 CdF2-catalyzed allylation of 4’–nitroacetophenone with allylmethoxysilane . 97 Scheme 3.9 GdCl3·6H2O–catalyzed allylation of ketone with allylstannane ... 97
Scheme 3.10 Sc(OTf)3-catalyzed ketone allylation using tetraallyltin in aqueous media .. 97
Scheme 3.11 Allylation of acetophenone using Zn(OTf)2–pyridine ... 98
Scheme 3.12 FeCl3·6H2O catalyzed allylation of imine using allyltributylstannane ... 98
Scheme 3.13 CuCl-TBAT catalyzed imine allylation using allylsilane ... 99
Scheme 3.14 Imine allylation of allyltrimethylsilane with benzaldehyde using TsNH2, NCS and SnCl2 ... 99
Scheme 3.15 In(OTf)3-catalyzed aldimine using tetraallyltin ... 99
Scheme 3.16 One-pot, three-component reaction of an aldehyde using allyl trifluoroacetate ... 100
Scheme 3.17 Allylation of benzaldehyde with trimethoxysilyl compound catalyzed by BINAP/AgOTf/KF/18-Crown-6 ... 100
Scheme 3.18 Enantioselective addition of allyltributylstannane to furaldehyde catalyzed by Ti(OiPr)4/(R)-BINOL 181 ... 100
Scheme 3.19 Bismuth triflate catalyzed enantioselective allylation of aromatic aldehyde ... 101
XI
Scheme 3.21 Hg(OTf)2 catalyzed asymmetric allylation of isatin... 102
Scheme 3.22 Ti(OiPr)4/BINOL-catalyzed ketone allylation utilizing tetraallylstannane ... 102
Scheme 3.23 SnCl4-catalyzed ketone allylation using tetraallyltin ... 102
Scheme 3.24 In(III)-catalyzed ketoester allylation using tetraallyltin ... 103
Scheme 3.25 ZnF2-catalyzed hydrazone using allylmethoxysilane ... 103
Scheme 3.26 Asymmetric allylation reactions at –20 °C (L1–L6) ... 105
Scheme 3.27 Influence of the ratio of reactants on asymmetric allyaltion reactions (L1) ... 110
Scheme 4.1 Asymmetric Diels-Alder reaction according to our protocol ... 123
Scheme 4.2 Application of the Diels-Alder reaction cycloadduct-I ... 124
Scheme 4.3 Application of the Diels-Alder reaction cycloadduct-II ... 125
XII
List of tables
Table 1.1 Effects of the iron salts on the reaction between 3-alkenoyl -1,3-oxazolidin-2-one (II-2a) and cyclopentadiene (II-1a) in CH2Cl2a ... 29
Table 1.2 Effects of the temperature on the reaction between 3-alkenoyl -1,3-oxazolidin-2-one (II-2a) and cyclopentadiene (II-1a) in CH2Cl2
a
... 30 Table 1.3 Effects of the iron salts on the reaction between
(E)-4-oxo-4-(2-oxooxazolidin-3-yl)but-2-en-1-ylium (II-2b) and cyclopentadiene (II-1a) in CH2Cl2
a
... 32 Table 1.4 Solvent effects on the reaction between 3-alkenoyl-1,3-oxazolidin-2-one (II-2a)
and cyclopentadiene (II-1a)a ... 34 Table 1.5 Effects of the temperature on the reaction of 3-alkenoyl-1,3-oxazolidin-2-one
(II-2a) and cyclopentadiene (II-1a) in CH3CN
a
... 36 Table 1.6 Effects of the catalyst loading on the reaction of 3-alkenoyl-1,3-oxazolidin-2-
one (II-2a) and cyclopentadiene (II-1a) in CH3CN
a
... 37 Table 1.7 Effects of the iron salts on the reaction of 3-alkenoyl-1,3-oxazolidin-2-one
(II-2a) and cyclopentadiene (II-1a) in CH3CNa ... 39
Table 1.8 Influence of precomplexation time on the asymmetric Diels-Alder reaction (II-3a)a ... 50 Table 1.9 Influence of the temperature on the asymmetric Diels-Alder reactions (II-3b)a 52 Table 1.10 Influence of the temperature on the asymmetric Diels-Alder reaction (II-3c)a 53 Table 1.11 Influence of the temperature on the asymmetric Diels-Alder reaction (II-3d)a
... 54 Table 1.12 Effects of the temperature on the asymmetric Diels-Alder reaction (II-3f)a .... 55 Table 1.13 Influence of the temperature on the asymmetric Diels-Alder reaction (II-3g)a ...
... 55 Table 1.14 Influence of the temperature on the asymmetric Diels-Alder reaction (II-3h)a ...
... 56 Table 1.15 Influence of the temperature on the asymmetric Diels-Alder reaction (II-3i)a ....
... 57 Table 1.16 Influence of the temperature on the asymmetric Diels-Alder reaction (II-3j)a ....
... 58 Table 1.17 Influence of the temperature on the asymmetric Diels-Alder reaction (II-3k)a ...
... 59 Table 1.18 Influence of the temperature on the asymmetric Diels-Alder reaction (II-3l)a ....
... 60 Table 1.19 Influence of the temperature on the asymmetric Diels-Alder reaction (II-3n)a ...
... 61 Table 2.1 Influence of different metal salts on the thia-Michael addition in DMC ... 74 Table 2.2 Effects of solvents on the thia-Michael addition using Fe(ClO4)3·6H2O ... 76
XIII
Table 2.3 Effects of metal salts on the thia-Michael addition in EtOH ... 77
Table 2.4 Influence of the iron salts on the thia-Michael addition in DMC ... 85
Table 2.5 Effects of solvents on the thia-Michael addition using Fe(OTf)2 ... 86
Table 3.1 Asymmetric allylation reactions at room temperature (L3, L7) ... 106
Table 3.2 Asymmetric allylation reactions at room temperature (L1) ... 108
Table 3.3 Effects of TMSCl on the asymmetric allylation reaction (L1) ... 111
Table 3.4 Influence of the iron salts on the asymmetric allylation reaction (L8) ... 112
Table 3.5 Effects of the iron salts on the asymmetric allylation reaction (L2,L7-L8) ... 113
Table 3.6 Influence of different chiral ligands on the asymmetric allylation reaction (L1– L2, L7–L8) ... 114
Table 3.7 Effects of various iron salts on the asymmetric allylation reaction using L8 ... 116
Table 3.8 Effects of different solvents on the asymmetric allylation reaction using L8 ... 117
Table 3.9 Influence of other different chiral NHC–ligand precursors on the asymmetric allylation (L9L17) ... 119
Table 4.1 Comparison of literature precedents with our protocol for the asymmetric Diels-Alder benchmark reaction ... 124
XIV
List of figures
Figure 0.1 Proposed catalytic mechanism for the asymmetric epoxidation catalyzed by
Fe-porphyrin ... 3
Figure 0.2 HOMO, LUMO, secondary orbital overlap and endo transition state in the Diels-Alder reaction... 8
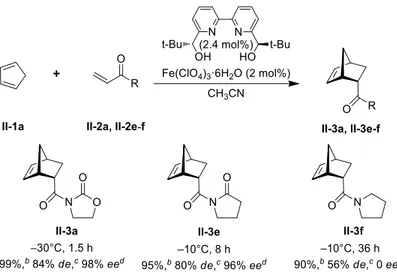
Figure 1.1 Asymmetric Diels-Alder reactions between 3-alkenoyl-1,3-oxazolidin-2-one dienophiles (II-2b-d) and cyclopentadiene (II-1a)a ... 43
Figure 1.2 Asymmetric Diels-Alder reaction of 3-alkenoyl-1,3-oxazolidin-2-one and analogues as dienophilesa ... 44
Figure 1.3 Asymmetric Diels-Alder reactions between electron-poor dienophiles and cyclopentadienea ... 47
Figure 1.4 Asymmetric Diels-Alder reactions with other representative dienesa ... 49
Figure 1.5 Proposed catalytic cycle of dienophile II-2a reacted with cyclopentadiene II-1a catalyzed by Fe(III)-L* ... 63
Figure 1.6 Proposed enantioselective outcome for Diels-Alder reaction catalyzed by Fe(ClO4)3•6H2O and the chiral bipyridine ligand L1 ... 64
Figure 2.1 The plot of Mulliken positive charges of different metal ions against the conversions of Michael addition ... 78
Figure 2.2 HOMO of III-1a and LUMO of Fe(OTf)2 ... 79
Figure 2.3 HOMO of III-1a and LUMO of Ca(OTf)2 ... 80
Figure 2.4 Comparison of LUMOs of Ca(OTf)2, Ga(OTf)2 and Bi(OTf)3·4H2O ... 80
Figure 2.5 A plausible cyclization mechanism of Fe(OTf)2 as a catalyst for thia-Michael additions ... 84
XV
List of abbreviations
Ǻ Angström
Ac Acetyl
Bipy 2,2’-Bipyridine
BLA Brønsted acid assisted chiral Lewis acid
Boc tert-Butyloxycarbonyl Box Bis-oxazoline br s Broad singlet Bu Butyl o C Celsius degree
CPME Cyclopentyl methyl ether D-A Diels-Alder
DCE 1,2-Dichloroethane DMC Dimethyl carbonate DME Dimethyl ether DMF Dimethylformamide DMSO Dimethyl sulfoxide
de Diastereomeric excess DS Dodecyl sulfate ee Enantiomeric excess er Enantiomeric ratio GO Graphene oxide Hex Hexane
HOMO Highest occupied molecular orbital HPLC High-performance liquid chromatography
XVI
IR Infrared
J Coupling constant
L Liter
LUMO Lowest unoccupied molecular orbital
m Multiplet
Me Methyl
mg Milligram
MHz Megahertz
m-MCF Modified mesocellular foam mmol Millimole MS Molecular sieve MW Microwave Napht Naphthyl NCS N-Chlorosuccinimide NHC N-Heterocyclic carbene
NMR Nuclear magnetic resonance
p Para
PA Poly{(–)-(S)-4-tert-butyl-2-[5-(4-tert-butylphenyl)-3-vinylpyridin-2-yl]-ox azoline}
P.E. Petroleum ether
Ph Phenyl
ppm Parts per million
Pybox Pyridine-2,6-bis-oxazoline PyOx Pyridine oxazoline
s Singlet
Salen N,N'-bis(salicylidene)ethylenediamine
XVII SDS Sodium dodecyl sulfate
SFAA Squaramide-fused amino alcohol
t Triplet
TBAT Tetrabutylammonium difluorotriphenylsilicate TADDOL α,α,α,α-Tetraaryl-1,3-dioxolane-4,5-dimethanol TBAT Tetrabutylammonium difluorotriphenylsilicate temp. Temperature TMS Trimethyl silyl t-Bu tert-Butyl THF Tetrahydrofuran tt Triplet of triplet UV Ultra violet
XVIII
Acknowledgements
During my entire PhD study period, I received so much help and support from numerous brilliant people. Firstly, I would like to express my deep and sincere appreciation to my supervisor, Professor Thierry Ollevier. Without his great help, I could not start and finish my studies. I am really grateful for his knowledge, support and creative thinking about all my projects and in my whole study period. Meanwhile, I would like to sincerely thank China Scholarship Council for the financial support.
I wish to express my gratitude to Dr. Hoda Keipour who helped me in life and shared all the valuable discussions during my study, Dr. Angela Jalba and Dr. Martin Pichette Drapeau for their help in the lab. Deep thanks are given to Mathieu Lafantaisie who helped me settle down in Quebec City and in my study, Professor Jamil Kraïem for his abundant lab experience and optimistic spirit about research. I would like to thank Dr. Di Meng for his countless assistance in life and study and accompaniment for endeavours in the lab, Dazhi Li for his experienced lab work, patient knowledge sharing and support from life, Wan Xu and Dandan Miao for their support in life. Thank Virgine Carreras and Samuel Lauzon for their assistance in manuscript corrections, and Samuel Cashman Kadri, Nour Tanbouza and Margaux Jacquet for their help in the group.
Deep thanks are given to Mr. Pierre Audet for his useful guidance on instrument operations. A sincere thank would be given to the departmental staff: Professor Anna Ritcey, Professor Jean-François Morin, Denyse Michaud, Mélanie Tremblay, Christian Côté, Magali Goulet, Marie Tremblay and Jean Laferrière. I really appreciate Professor John Boukouvalas, Prof. Jean-François Morin and Prof. Denis Giguère’s groups for the generous sharing of chemicals. Many thanks are given to Ramesh Muddala with whom I always have the backup for the chemical search. Also, I would like to give my sincere thanks to Dr. Wenhua Bi and Dr. Huan Liang for their help in life and emotional support during my study, Professor Rong Shi for his inspiration for my study. Last but not least, I would like to thank my family and friends in life for their unconditional love, support and encouragement during my PhD study.
1
Introduction
Properties of iron
Iron compounds as catalysts have received much attention due to several reasons. At first, iron is the most abundant metal in the Earth’s crust after aluminum and hence is inexpensive compared to the precious metals and can be employed as a cheap alternative in many reactions.1-2 Secondly, it is environmentally benign and leads to many applications in biological systems, such as many metabolic processes including safe disposal pathways. Meanwhile, it is employed in the food industry, the pharmaceutical industry and cosmetics. Iron possesses formal oxidation states ranging from −II to +V (rarely +VI) which is a property for its usefulness in redox reactions. Iron cations can be coordinated to lots of N- or O-based ligands as well as to
N-heterocyclic carbenes, which avoids using phosphines and consequently saves costs
of labor force and is in favor of the environment.2-3
Reactions catalyzed by iron catalysts
It should be noted that the present literature suggests that iron catalysis potentially involves almost all the areas in organic synthesis,1-3 doubtlessly encompassing asymmetric transformations. It has already been applied in asymmetric reactions including Friedel−Crafts-type reaction,4 Nazarov cyclizations,4 C−H bond functionalization of indoles,4-5 O−H bond insertion reactions,4-5 nitrene transfer/iron-nitrene species,4-5 sulfide oxidations,4,6,15a,17,20 Diels-Alder reaction,4-5,7-8 hydrosilylation of ketones,4-5,9,15a cyclopropanation,4-5,15a cross-dehydrogenative coupling of 2-naphthols,10 epoxidation (porphyrin-based and non-porphyrin-based catalysts),4-5,11,15a,19,20 oxidative coupling of 2-naphthols,4,12 ring opening of meso- and terminal epoxides with aromatic and cyclic amines,13,18 aminohydroxylation and aminofluorination of olefins,5,14,15a Michael additions,4,15a,16 hydroxylation at benzylic
2
carbon,4 cis-dihydroxylation,4 transfer hydrogenation of ketones,4,15a,15b hydrogenation and transfer hydrogenation of imines,4 1,3-dipolar cycloaddition,4,15a Mukaiyama−Aldol reaction,4,20 Mannich-type reaction,4,15a carbanion transfer/organoiron species,4 oxidative kinetic resolution of secondary alcohols,4 azidation of β-keto esters and oxindoles,5,15a,20 sulfimidation,5,15a kinetic resolution of N-sulfonyl oxaziridines,5 cycloisomerization reactions,5,15a Conia-ene carbocyclization16 and oxyamination of olefins.4-5,15a Given the broad scope of this field, herein, it is limited to some typical examples which disclose asymmetric reactions using iron catalysts.
Representative examples of typical reactions in the presence of iron catalysts
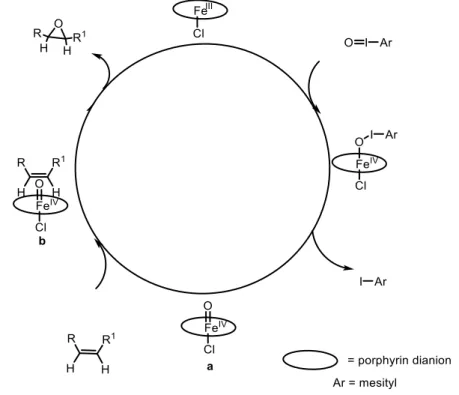
The first example of asymmetric epoxidation of olefins catalyzed by a Fe-porphyrin complex was disclosed in 1983. For the reaction between p-cholorostyrene and iodosylmesitylene catalyzed by a FeIII catalyst 1, the highest yield and enantioselectivity were 63% and 51%, respectively (Scheme 0.1). The proposed mechanism of this epoxidation is described in Figure 0.1. It involves the formation of a reactive iron-oxo intermediate a from the iron-porphyrin complex and iodosylarene. Styrene approaches the reactive oxoiron group from the side and in a parallel way to the plane of the
3
porphyrin ring as indicated in b, and an R styrene epoxide is formed as shown in Figure 0.1. 4a,4b,15a
Figure 0.1 Proposed catalytic mechanism for the asymmetric epoxidation catalyzed by Fe-porphyrin
The iron porphyrin complex does not only catalyze asymmetric epoxidation reactions but has also been applied in hydroxylation reactions of benzylic compounds, which is shown in Scheme 0.2.
From Scheme 0.2, one can see that a good enantiomeric excess (72%) and a moderate yield (47%) were obtained when the asymmetric hydroxylation reaction was carried out with tetrahydronaphthalene, using only 1 mol% of iron complex 2 (a vaulted binaphthyl porphyrin) as catalyst, which is the first reported catalytic asymmetric hydroxylation of simple hydrocarbons.4a,4c,15a
4
Scheme 0.2Asymmetric hydroxylation of a benzylic compound catalyzed by Fe-porphyrin In 2008, an iron-catalyzed asymmetric cis-dihydroxylation of olefins was investigated. The reaction of a trans-2-heptene with H2O2 in the presence of a catalytic amount of preformed iron complex 3, [FeII(6-Me2-BPBP)], was reported (Scheme 0.3).
Scheme 0.3cis-Dihydroxylation of olefins with H2O2 catalyzed by iron complex 3
The asymmetric induction obtained from iron complex 3 was excellent, resulting from the rigid bipyrrolidine backbone relative to the 1,2-diaminocyclohexane backbone and the cis-α ligand topology. A very high enantioselectivity (97%) was obtained, which is the first example of a synthetic nonheme iron catalyst, affording the high enantioselectivity found in cis-dihydroxylating enzymatic systems.4a,4d,15a
5
Scheme 0.4 shows that using an in situ generated, chiral and bioinspired non-haem (N4) FeII catalyst with aqueous H2O2, the enantioselective sulfoxidation (up to 98.5:1.5 er) took place, leading to a moderate yield of sulfoxide when the mono-oxidation of the sulfide was performed in combination with the kinetic resolution of the sulfoxide to the sulfone.4e
Scheme 0.4 Enantioselective sulfide oxidation catalyzed by a chiral Fe/6,6’-bis(4-isopropyloxazolin-2-yl)-2,2’-bipyridine (bipybox-iPr) complex The asymmetric oxidation of sulfides was catalyzed by a “free” Fe(salen) inside the nanocages of SBA-16 and of m-MCF. These solid catalysts which are heterogeneous at the macroscopic scale but homogeneous at the microscopic level were prepared by the encapsulation of Fe(salen) by a “ship in a bottle” approach. Chiral Fe(salen) trapped in m-MCF materials showed a higher activity than the complex immobilized on SBA-16. Scheme 0.5 shows that a 92% conversion was obtained for the reaction catalyzed by Fe(salen) immobilized on m-MCF, which was higher than that catalyzed by Fe(salen) trapped in SBA-16 (67% convesion). In general, the structure of m-MCF materials provides a higher loading of Fe(salen) compared to SBA-16, probably due to their larger cage sizes. The activity and enantioselectivity of the catalysts based on m-MCF were almost the same as those obtained with the homogeneous Fe(salen) solution in MeOH. For the catalytic performance, enantioselectivity of sulfoxidation was observed only when PhIO was used as an oxidant; no enantioselectity was obtained using oxidants H2O2 or NaOCl, no matter the solvents used CH3CN, CH2Cl2 or MeOH, as
6
reported in the literature. A mechanism is described in Scheme 0.6.6,15a During recyclability tests, the immobilized complex showed deactivation, namely, no enantioselectivity after the second cycle, which is related to the decomposition of the encapsulated Fe(salen) and the formation of iron oxide particles. Nevertheless, compared to the homogenous counterpart with which reutilization was not possible, it was obviously superior.17
Scheme 0.5Asymmetric sulfide oxidation catalyzed by Fe(salen) immobilized on F-m-MCF-50
Scheme 0.6Proposed catalytic mechanism of the sulfoxidation with PhIO catalyzed by Fe(salen)
7
Moreover, in addition to a Fe(salen) catalyzing a Michael addition reported in ref. 15a, a salen ligand based on a chiral cis-2,5-diaminobicyclo[2.2.2]octane scaffold was recently developed. Scheme 0.7 shows that the highest enantioselectivity (98%) and an excellent yield (96%) were obtained for the thia-Michael addition catalyzed by Fe(salen)
6 at –5 oC in two days. The use of the chiral Fe(salen) 6 as catalyst provided a new method for conducting the reaction under environmentally sustainable conditions. In terms of the application of the reaction catalyzed by 6, Scheme 0.8 shows the synthesis of (R)-montelukast (b) from an achiral conjugated ketone (a). The sodium salt of (b) is an orally active drug Singulair (MK-0476), which is widely used for treatment of asthma and other respiratory conditions. It was found that employing catalyst 6, the sulfur functionality in (R)-montelukast (b) can be inserted directly with excellent enantioselectivity into the achiral conjugated ketone (a).16
Scheme 0.7 Enantioselective thia-Michael conjugate addition catalyzed by Fe(salen) 6
8
Introduction to Diels-Alder reactions
General introduction to Diels-Alder reactions
The Diels-Alder reaction is one of the most straightforward and atom economical methods. It constructs chiral six-membered carbocyclic compounds in organic chemistry, which constitutes a versatile method for the synthesis of cyclohexene-containing building blocks of great interest for the total synthesis of bioactive natural products.21-22 Four contiguous asymmetric centers are generated in principle. A cyclic transition state is formed, which allows relative stereochemistry to be well defined, with endo approach.23-24 The reactivity, regiochemistry and stereochemistry of Diels-Alder reactions can be explained by the frontier molecular orbital theory (FMO). It is believed that the overlap between the highest occupied molecular orbital (HOMO) of one component and the lowest unoccupied molecular orbital (LUMO) of another component controls the Diels-Alder reactions. These orbitals are close in energy, as shown in Figure 0.2. Specifically, in Figure 0.2, the dominant interaction is between LUMO of dienophile and HOMO of diene (right part of the Figure 0.2). The reactivity of a Diels-Alder reaction relies on the HOMO-LUMO energy separation of these two components.
Figure 0.2 HOMO, LUMO, secondary orbital overlap and endo transition state in the Diels-Alder reaction
9
HOMO diene-controlled Diels-Alder reactions are accelerated by electron-donating substituents in the dienes and electron-withdrawing substituents in the dienophiles (normal electron-demand Diels-Alder reaction). LUMO diene-controlled Diels-Alder reactions are influenced by electronic effects of the substituents in the opposite way (inverse electron-demand Diels-Alder reaction). The neutral electron-demand Diels-Alder reactions are HOMO-LUMO-diene controlled and are insensitive to substituents in either the dienophile or the diene. In Figure 0.2, the electron withdrawing carbonyl group of the dienophile lowers the LUMO energy which decreases the transition state energy. Secondary orbital interactions (the left part of Figure 0.2) satisfy Alder’s notion of “maximum accumulation of unsaturations”, and this “tighter” transition state leads to enhanced endo diastereoselection.25-26
Enantioselective Diels-Alder reactions catalyzed by chiral metal complexes
Enantioselective Diels-Alder reactions can be achieved by different approaches with achiral substrates, such as catalysis by chiral metal complexes and by other chiral catalysts (organocatalysis, chiral ligands, biomolecular assistance).
Here, at first, we present enantioselective Diels-Alder reactions catalyzed by chiral metal complexes. This area has been extensively developed with various metals, such as magnesium,27-29 zinc,30 iridium,31 copper,32-49 aluminum,40-42 titanium,43-47 scandium,48 nickel,49-50 ytterbium,51 tin52 and iron.50,53-62 Since numerous articles reported
asymmetric Diels-Alder reactions catalyzed by chiral metal complexes, we only introduce some detailed examples showing the catalytic results, which are classified according to different metal salts.
Scheme 0.9 shows that cyclopentadiene and acryloyl oxazolidinone reacted at –50 °C in CH2Cl2 for 12 h. Two equivalents of water as an additive, 10 mol% of Mg(OTf)2 and a bis(oxazoline) ligand were used, leading to a quantitative yield, 88% de and 95% ee.28
10
Scheme 0.9 Asymmetric Diels-Alder reaction catalyzed by Mg(OTf)2 and a chiral
bis(oxazoline) ligand
Scheme 0.10 Zinc and a chiral N,N’-dioxide ligand catalyzed asymmetric Diels-Alder reaction
An efficient catalyst (N,N’-dioxide ligand/Zn(NTf2)2 complex) 8 has been developed for the highly enantioselective Diels-Alder reaction of cyclopentadiene with alkynones, which is shown in Scheme 0.10. Various 2-acyl norbornadiene derivatives were obtained in moderate to high yields. 1-(4-(Trifluoromethyl)phenyl)prop-2-yn-1-one reacted with cyclopentadiene to give the acylnorbonadiene in 95% of yield and 95% of enantiomeric excess.30
An enantioselective Diels-Alder reaction of an α,β-unsaturated 2-acyl imidazole with 2,3-dihydrofuran was successfully catalyzed by a chiral-at-metal iridium Lewis acid catalyst on a polystyrene resin through an amide linker. Moreover, the catalyst is very robust and can be recycled multiple times without affecting enantioselectivity of the
11
reaction. A 99% yield and 98% enantioselectivity were obtained for the reaction shown in Scheme 0.11.31c
Scheme 0.11Asymmetric Diels-Alder reaction catalyzed by iridium with an amide linker An asymmetric Diels-Alder reaction was carried out in CH2Cl2, at 0 °C in 0.3 h, with cyclopentadiene and 2-cinnamoylpyridine 1-oxide as reaction substrates, 10 mol% Cu(OTf)2 complexed to a chiral bis(oxazoline) ligand as catalyst, leading to a 98% yield, 97/2 endo/exo ratio, and 96% enantiomeric excess (Scheme 0.12).34a
Scheme 0.12 Cu(OTf)2 and a bis(oxazoline) ligand catalyzed asymmetric Diels-Alder
reaction
An efficient chiral N,N’-dioxide/Cu(OTf)2 complex system for the asymmetric nitroso Diels-Alder reactions of 2-nitrosopyridines with 1,3-diene-1-carbamates was developed. The corresponding products 3,6-dihydro-1,2-oxazines were obtained in excellent yields and enantioselectivities. A 99% yield and a 98% enantioselectivity were obtained in the example shown in Scheme 0.13.34b
12
Scheme 0.13 Cu(OTf)2 and a chiral ligand 8 catalyzed asymmetric Diels-Alder reaction
Scheme 0.14 Asymmetric Diels-Alder reaction catalyzed by a chiral PyOx ligand complexed with Cu(OTf)2
The homogeneous Diels-Alder reaction of an alkenoyl pyridine N-oxide with cyclopentadiene in tetrahydrofuran was catalyzed by a novel chiral ligand pyridine oxazoline (PyOx) complexed with Cu(OTf)2. The ligand contains a helical polymer, poly{(–)-(S)-4-tert-butyl-2-[5-(4-tert-butylphenyl)-3-vinylpyridin-2-yl]-oxazoline} (PA). This catalyst exhibited remarkably enhanced enantioselectivity and reaction rate. A 98% yield, 96% diastereoselectivity and 62.3% enantioselectivity were obtained for the example shown in Scheme 0.14.34c
A high yield (up to 92%), moderate diastereomeric excess (49%) and very high enantiomeric excess (up to 95%) were obtained for the reaction between cyclopentadiene and 3-acryloyloxazolidin-2-one catalyzed by the chiral aluminum complex 12 in CH2Cl2, at –90 °C in 10 min (Scheme 0.15).41b
13
Scheme 0.15 A chiral aluminum complex catalyzed asymmetric Diels-Alder reaction A TiCl2(OiPr)2 and chiral TADDOL ligand complex efficiently catalyzed the reaction between isoprene and (E)-methyl 4-oxo-4-(2-oxooxazolidin-3-yl)but-2-enoate in toluene-P.E., at 0 °C overnight. An excellent yield (94%) and excellent enantiomeric excess (94%) were achieved (Scheme 0.16).43a
Scheme 0.16 Asymmetric Diels-Alder reaction catalyzed by TiCl2(OiPr)2 and a chiral
TADDOL ligand
A report about stoichiometric amounts of a TiIV–TADDOL complex catalyzed asymmetric intramolecular Diels-Alder reaction was disclosed, and decent enantiomeric excesses up to 68 % were achieved. But a catalytic version of the enantioselective intramolecular Diels-Alder reactions of 1,7,9-decatrien-3-ones could not be developed. The cycloadditions gave highly trans-selective products. In Scheme 0.17, a 97:3 of
14
Scheme 0.17 TADDOL-promoted enantioselective intramolecular Diels-Alder reaction
An inexpensive and easily accessible chiral vicinal ligand (1R,2R)-1,2-bis-(2-methoxy- phenyl)-ethane-1,2-diol was complexed with a titanium salt. This chiral complex was utilized to catalyze asymmetric Diels-Alder reactions which were found to exhibit uniformly high enantioselectivity towards carboxylic esters. An 82% yield and 94% of enantiomeric excess were obtained for the reaction shown in Scheme 0.18.43c
Scheme 0.18 Asymmetric Diels-Alder reaction catalyzed by a chiral titanium-based diol catalyst
Scheme 0.19 shows that the readily available and reactive fumaryl chloride dienophile undergoes an enantioselective [4 + 2]–cycloaddition with the chiral boron catalyst/Tf2NH-TiCl4 combination. It is a very useful and practical equivalent of
15
N-protected 1,2-diaminoethylene which is inoperable and unstable. An excellent
enantioselectivity (97%) along with a moderate overall yield (58%) was achieved.43d
Scheme 0.19 Asymmetric Diels-Alder reaction catalyzed by a boron ligand combination with Tf2NH-TiCl4
Scheme 0.20 Asymmetric Diels-Alder reaction catalyzed by Sc(OTf)3 complex
Asymmetric Diels-Alder reaction was conducted between cyclopentadiene and bidentate dienophiles, for which a chiral scandium complex was used, in CH2Cl2 at 0 °C in 10 h. The very high to excellent yields, good endo/exo ratios and very good enantiomeric excesses were obtained for both Me and Ph substituents (Scheme 0.20).48
16
Scheme 0.21 Ni(ClO4)2·6H2O and a chiral binaphthalene diimine ligand catalyzed
asymmetric Diels-Alder reaction
The complex Ni(ClO4)2·6H2O with a chiral binaphthalene diimine ligand catalyzed the D-A reaction between cyclopentadiene and 2-acryloyl-4,4-dimethyl-1-(naphthalen-1-yl- methyl)pyrazolidin-3-one effectively in CH2Cl2 at -40 °C in 41 h. A 73% yield, 84% de and 94% ee were obtained using this catalytic system (Scheme 0.21).49a
Scheme 0.22 Asymmetric Diels-Alder reaction catalyzed by Ni(OTf)2 and chiral ligand 8
Another asymmetric Diels-Alder reaction catalyzed by the complex Ni(OTf)2 with chiral ligand 8 is described in Scheme 0.22. It shows that this chiral complex is efficient for the asymmetric Diels-Alder reaction of cyclopentadiene with 2,3-dioxopyrrolidines or 2-alkenoyl pyridines. High activities and high levels of stereocontrol were achieved. In this example, a 97% yield, 86% de and 96% ee were obtained for the corresponding chiral bridged compound with four adjacent stereocenters.49b
17
Scheme 0.23 Ytterbium-catalyzed Asymmetric Diels-Alder reaction of Danishefsky’s diene The ytterbium catalyst (Yb(OTf)3) with a chiral (R)-binamide ligand catalyzed Diels-Alder reactions of Danishefsky’s diene efficiently. In Scheme 0.23, only the exo diastereo-isomer was obtained. After reaction with acid, in two steps, a good yield (91%) and enantioselectivity (˃99.5%) of the compound shown were obtained.51
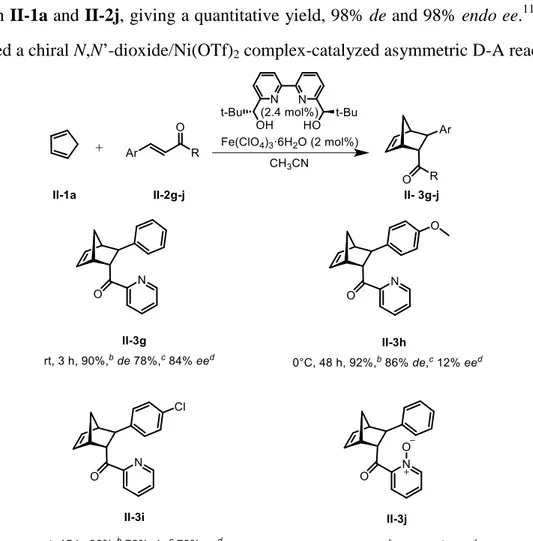
Among the iron catalyzed asymmetric Diels-Alder reactions, a chiral 2,2’-binaphthyl-diimine ligand,49a
bis-oxazolines,50,53-57 a bisulfoxide ligand58 and phosphorus ligands59-62 have been reported.
Scheme 0.24 shows that the complex of C2-symmetric trans-chelating ligand 20 ((R,R)-4,6-dibenzofurandiyl-2,2’-bis(4-phenyloxazoline)) with Fe(ClO4)2 catalyzes the
Scheme 0.24 Asymmetric Diels-Alder reaction catalyzed by the (R,R)-4,6-dibenzofurandiyl-2,2’-bis(4-phenyloxazoline)·Fe(ClO4)2 complex
18
Diels-Alder reaction of cyclopentadiene with 3-acryloyl-2-oxazolidinone, leading to the cycloadduct with a 90% yield, 98% diastereomeric excess and 98% enantiomeric excess.50
A new chiral iron(III) Lewis acid L·FeX2I (L=2,2-bis[2-[4(S)-phenyl-l,3-oxazolinyl] propane) complex catalyzed the Diels-Alder reaction between cyclopentadiene and a bidentate dienophile to afford the corresponding cycloadduct in a 85% yield, 80% ee and 97:3 endo/exo diastereoselectivity, as shown in Scheme 0.25.53
Scheme 0.25L·FeCl2I (L=2,2-bis[2-[4(S)-phenyl-l,3-oxazolinyl]]propane) complex
catalyzed the Diels-Alder reaction
The reaction between 3-acryloyl-1,3-oxazolidine-2-one and cyclopentadiene in the presence of a chiral bis(oxazoline) ligand·FeI3 complex and I2 (co-catalyst) afforded a 87% yield of the Diels-Alder adduct with a 92.5:7.5 enantioselectivity and a 95:5
endo/exo selectivity.56
19
From Scheme 0.26, we can see that a bis-sulfoxide ligand with a C2 symmetry axis in their structure is a good chiral controller in metal catalyzed asymmetric Diels-Alder reaction. The yield was 78%; the diastereoselectivity (endo/exo) was very good (de: 92%); the enantioselection was up to 56% in this example.8, 58
Kündig’s cationic iron(II) complex 22, derived from chiral trans-1,2-cyclopentane-diol, is a stable, isolable brown solid that possesses sufficient Lewis acidity to catalyze Diels-Alder reactions between unsaturated aldehydes and dienes. The highest selectivity (96%) and yield (99%) were observed using bromoacrolein as the dienophile (Scheme 0.27).62
Scheme 0.27 Asymmetric Diels-Alder reaction catalyzed by Kündig’s cationic iron(II) complex
Enantioselective Diels-Alder reactions catalyzed by other chiral catalysts
Several examples of chiral organocatalysts are presented herein.
With the aid of fine-tunable cinchona alkaloid derived bifunctional amine-thiourea catalysts bearing multiple hydrogen-bond donors, an unprecedented asymmetric Diels- Alder reaction of 3-hydroxy-2-pyrones with prochiral cyclopentene-1,3-diones was efficiently realized with very high stereoselective control (92% ee, Scheme 0.28). A multifunctional-bridged tricyclic lactone featuring four contiguous stereogenic centers and one remote quaternary stereogenic center was formed; a very good yield was obtained (91%).63
20
Scheme 0.28 Asymmetric Diels-Alder reaction catalyzed by a chiral amine-thiourea organocatalyst
Scheme 0.29Asymmetric Diels-Alder reaction catalyzed by a chiral binaphthyl silica catalyst
Scheme 0.29 shows that the enantioselective Diels-Alder reaction between cyclohexa-1,3-diene and an unfunctionalized chalcone derivative as the dienophile catalyzed by the chiral binaphthyl silica catalyst gives an enantiomeric excess of 59%. The dihydrosilepine-derived electron-deficient silicon cation forming an intramolecular Lewis pair is sufficiently Lewis acidic to facilitate the cycloaddition, and this new catalyst is the first one to induce considerable enantioselectivity for the reaction between chalcones and cyclohexadiene.64
The enantioselective Diels-Alder reaction between 4-bromo-2-pyridone and
N-phenylmaleimide shown in Scheme 0.30 was catalyzed efficiently by the chiral
21
Scheme 0.30 Enantioselective Diels-Alder reaction catalyzed by a chiral amino alcohol site and a hydrogen bonding site. The corresponding chiral 4-hydroxyisoquinuclidine was formed with both an excellent chemical yield and an excellent enantioselectivity (95%, 98% ee).65
Apart from the above chiral organocatalysts, there are also other chiral organocatalysts, such as polyester 26 bearing a chiral imidazolidinone salt unit as shown in Scheme 0.31,72 and, in Scheme 0.32, chiral L-pyroglutamic sulphonamide 27,66 silylated C-H acid 28,67 diphenylprolinol silyl ether 29,68 bifunctional amino-thiourea 30,69 phosphoric acid 3170 and amino alcohol 32.71 Employing these organocatalysts, good yields and good enantioselectivities are achieved for asymmetric Diels-Alder reactions.
Scheme 0.31 A polyester with chiral imidazolidinone for the asymmetric Diels-Alder reaction
22
Scheme 0.32 Chiral organocatalysts for asymmetric Diels-Alder reactions
With respect to enantioselective Diels-Alder reactions catalyzed by chiral ligands, some chiral ligands are described in Scheme 0.33. They are Brønsted acid assisted chiral Lewis acid (BLA) boron ligands 33,73 3474 and 35,75 the cationic oxazaborinane
36,76 the chiral pyridinium phosphoramide 37 (chiral Brønsted acid catalyst)77 and sulfur-stabilized silicon cations with a chiral binaphthyl backbone such as 38.78
As shown in Scheme 0.34, three biomolecular catalysts catalyze enantioselective Diels-Alder reactions. The D-A reaction between 2-azachalcone and cyclopentadiene proceeded within the cavity of NB-Pyr in the presence of CuII ion (see 39) in a very high yield (up to 94% conversion), high enantioselectivity (up to 78% ee) and very high diastereoselectivity (up to 92% de).79 DNA–silica minerals containing a Cu(ligand) complex synthesized from 40 and silica were applied in the asymmetric Diels-Alder reaction between 2-azachalcone and cyclopentadiene with an excellent conversion (up
23
Scheme 0.33 Chiral ligands for enantioselective Diels-Alder reactions
Scheme 0.34 Biomolecular catalysts for enantioselective Diels-Alder reactions
to 99%) and enantiomeric excess (up to 99%) and recycled 10 times without loss of enantioselectivity.80 The asymmetric Diels-Alder reaction between 2-azachalcone and cyclopentadiene catalyzed by a Cu(II)-coordinated helicoidal nanotube (Cu(II)-HN) (41) proceeded with up to 91% ee and 99% yield in 60 min. This single-walled nanotube was fabricated by the self-assembly of a L-glutamic acid terminated bolaamphiphile.81
24
Research objectives about enantioselective Diels-Alder reactions catalyzed by iron complexes
We would like to explore an efficient enantioselective Diels-Alder reaction catalyzed by an iron salt and a chiral 2,2’-bipyridyl ligand (L1) with a wide scope of different structures of dienophiles and dienes.
In the above section, we presented the literature precedents about enantioselective Diels-Alder reactions catalyzed by different chiral catalysts for achiral substrates. However, only a few articles on asymmetric Diels-Alder reactions catalyzed by chiral iron catalysts were reported and these were published between 1990 and 2006. There are limitations about these reports. Either a limited number of reaction substrates are disclosed, or unsatisfactory enantioselectivies are obtained. Meanwhile, the recent reports are about enantioselective Diels-Alder reactions mostly catalyzed by other catalysts such as chiral organocatalysts, chiral ligands without metal salts and biomolecular catalysts. Copper complexed with chiral ligands are the main type of chiral metal(ligand) complexes described in the recent years. In order to fill in the gaps with respect to iron catalyzed enantioselective Diels-Alder reactions, herein, we have developed this project. The reasons why we choose catalysts of iron complexed with the chiral 2,2’-bipyridyl ligand in Diels-Alder reactions are not only due to the advantages of iron catalysts but also of chiral 2,2’-bipyridyl ligands which have been often used in asymmetric reactions owing to their stability and excellent coordinating ability with a wide range of metal ions.82 Moreover, our previous work established a solid foundation for this protocol. Recently our laboratory demonstrated the effectiveness of chiral 2,2’-bipyridyl ligands for Fe(II) catalyzed reactions: the enantioselective Mukaiyama aldol reaction (Scheme 0.35),83 the enantioselective opening of diaryl meso-epoxides with indoles (Scheme 0.36),84 the enantioselective opening diaryl meso-epoxides with anilines (Scheme 0.37)85 and the enantioselective Michael addition to α,β-unsaturated oxazolidin-2-ones (Scheme 0.38).86 To expand the scope of enantioselective reactions
25
catalyzed by the chiral 2,2’-bipyridyl ligand complexed with iron salts, we present an investigation of their application to asymmetric Diels-Alder reactions, for which the experimental results are disclosed in chapter I.
Scheme 0.35 Enantioselective Mukaiyama aldol reaction catalyzed by a Fe(II)-2,2’-bipyridyl chiral complex
Scheme 0.36 Opening reaction of aromatic meso-epoxides with indoles catalyzed by a Fe(II)-2,2’-bipyridyl chiral complex
Scheme 0.37Enantioselective meso-epoxide-opening with anilines catalyzed by a Fe(II)-2,2’-bipyridyl chiral complex
26
Scheme 0.38Enantioselective Michael addition catalyzed by a Fe(II)-2,2’-bipyridyl chiral complex
27
Chapter I Development of enantioselective Diels-Alder reactions
catalyzed by iron catalysts
1.1 Synthesis of the 2,2’-bipyridyl ligand
At first, when we were considering forming a chiral environment for the reaction, we synthesized the chiral 2,2’-bipyridyl ligand L1, by following the procedure reported by Bolm and Kobayashi in the literature.87
Scheme 1.1 Synthesis of the 2,2’-bipyridyl ligand
Scheme 1.1 shows that 2,6-dibromopyridine a was reacted with n-BuLi through halogen-metal exchange at –78 °C in a dry ice-acetone bath. It was followed by reaction with pivalonitrile then hydrolysis in acidic condition, in which the ketone c was formed. After that, the most important part of this procedure was the ketone reduction with the chiral Ru catalyst, which gave the alcohol d in high yield (86% after recrystallization) and excellent enantiomeric excess ( 99%). By comparison, a 95% yield with 91% ee was achieved in ref. 87c with the same approach. According to refs 87b and 87d, the asymmetric reduction of pyridyl ketone could also be performed with (–)-(Ipc)2BCl to give (R)-bromopyridyl alcohol, in which a 61% yield with 90% ee was obtained. Also,
28
in refs 87a and 87d, (–)-B-chlorodiisopinocampheylborane and iminodiethanol were utilized for the reduction, and a 59% yield along with a 90% ee was obtained. The last step was conducted by using a homocoupling reaction with PPh3, Ni salt and Zn. The chiral (R,R)-bipyridyl ligand L1 was obtained in a 85 % yield and ˃ 99% enantiomeric excess. In the literature, a 55% yield was achieved for this last step with the same method for synthesizing the diol bipyridyl chiral ligand.87a A range of 50%–60% of yield was obtained in refs 87b and 87d. While in ref. 87c, using a catalytic amount of PdCl2(PhCN)2 and tetrakis(dimethylamino)ethylene (TDAE) as a reductant, the reaction proceeded smoothly and a 93% yield with ˃ 99.5% ee was obtained for the (S,S) enantiomer of the desired chiral diol bipyridyl ligand L1.
1.2 Preliminary feasibility studies to asymmetric Diels-Alder reaction catalyzed by iron catalysts
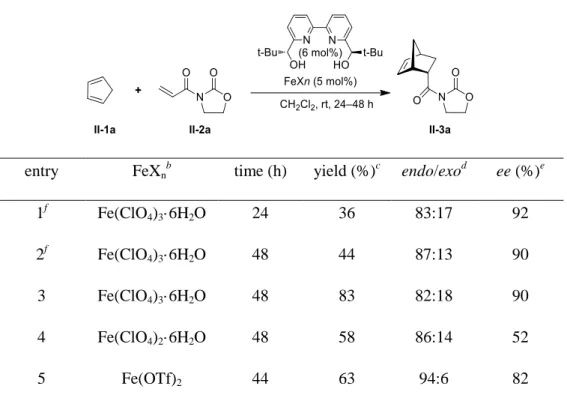
The feasibility analysis is of great significance for every project. Here, we were delighted to get some promising results using the protocol presented below. At first, after checking the existing precedents for the asymmetric Diels-Alder reactions, we selected cyclopentadiene (II-1a) and 3-alkenoyl-1,3-oxazo-lidin-2-one (II-2a) as the model substrates (the benchmark reaction for Diels-Alder reactions) to test the feasibility of this project. On one hand, compared to other dienes, cyclopentadiene is a reactive diene for Diels-Alder reactions. On the other hand, when we observe the structure of 3-alkenoyl-1,3-oxazo-lidin-2-one, we can easily estimate that, according to the frontier molecular orbital theory (FMO), with the presence of the electron-withdrawing group which lowers the energy of LUMO of the dienophile, this normal electron-demand Diels-Alder reaction would be promoted. We utilized the polar and non-coordinating dichloromethane as the solvent, different kinds of iron(II) and iron(III) salts, such as Fe(ClO4)3·6H2O, Fe(OTf)2 and Fe(ClO4)2·6H2O complexed with the chiral 2,2’-bipyridyl ligand L1 as catalysts to test the reaction. The results of the reactions are disclosed in Table 1.1.
29
Table 1.1 Effects of the iron salts on the reaction between 3-alkenoyl -1,3-oxazolidin-2-one (II-2a) and cyclopentadiene (II-1a) in CH2Cl2
a
entry FeXn
b
time (h) yield (%)c endo/exod ee (%)e
1f Fe(ClO4)3·6H2O 24 36 83:17 92 2f Fe(ClO4)3·6H2O 48 44 87:13 90 3 Fe(ClO4)3·6H2O 48 83 82:18 90 4 Fe(ClO4)2·6H2O 48 58 86:14 52 5 Fe(OTf)2 44 63 94:6 82 a
Conditions: FeXn (5 mol %), chiral diol bipyridyl ligand L1 (6 mol %), II-1a (3.5 mmol), and
II-2a (0.5 mmol) in CH2Cl2.
b
Catalysts were prepared in situ from the 2,2’-bipyridyl ligand L1
and iron salts in CH2Cl2.
c
Yields of isolated products (diastereomeric mixture) after purification by flash chromatography. dDetermined by 1H NMR analysis. eDetermined for the endo isomer by chiral HPLC analysis (Daicel Chiralpak® AD-H). fII-2a was solubilized in CH2Cl2 before
adding it to the reaction test tube by syringe.
At first, in order to strictly ensure the reaction to be conducted in the inert atmosphere, we chose to solubilize the dienophile in dichloromethane in a flame-dried test tube. It was followed by the addition of this dienophile solution to the reaction test tube dropwise by syringe. This approach led to a moderate yield for the reaction (36% yield, entry 1, Table 1.1), suggesting our ignorance of the solubility issue of the dienophile in dichloromethane. Whereas we tried to extend the reaction time from 24 h to 48 h, no significant yield gain was obtained (36% in entry 1 vs. 44% in entry 2, Table 1.1). However, changing the approach for adding II-2a (entry 3, Table 1.1) by directly
30
adding the dienophile to the reaction test tube after weighing, a very good yield (83%) was obtained, which proves that the Lewis acid Fe(ClO4)3·6H2O catalyzed this asymmetric Diels-Alder reaction effectively. Hence, we ascribe the yield gain to the better solubility for reaction reagents and catalysts in the solvent. Accordingly, a very good enantioselectivity (90% ee) was obtained for Fe(ClO4)3·6H2O catalyzed D-A reaction. While employing other iron salts such as Fe(ClO4)2·6H2O and Fe(OTf)2, good yields were achieved, with 58% and 63% in entry 4 (Table 1.1) and entry 5 (Table 1.1), respectively. A good (52% ee) and very good (82% ee) enantioselectivity was obtained for D-A reactions using Fe(ClO4)2·6H2O (entry 4, Table 1.1) and Fe(OTf)2 (entry 5, Table 1.1) as catalysts. Overall, these two FeII+ salts are not as effective as Fe(ClO4)3·6H2O in terms of the yields and enantiomeric excesses.
Table 1.2 Effects of the temperature on the reaction between 3-alkenoyl -1,3-oxazolidin-2-one (II-2a) and cyclopentadiene (II-1a) in CH2Cl2
a
entry FeXb temp (°C) time (h) yield (%)c endo/exod ee (%)e
1 Fe(ClO4)3·6H2O rt 48 83 82:18 90 2f Fe(ClO4)3·6H2O 0 32 38 90:10 40 3 Fe(OTf)2 rt 44 63 94:6 82 4 Fe(OTf)2 0 32 52 90:10 92 5 Fe(OTf)2 0 44 78 90:10 76 a
Conditions: FeXn (5 mol %), chiral diol bipyridyl ligand L1 (6 mol %), II-1a (3.5 mmol), and
II-2a (0.5 mmol) in CH2Cl2.
b
31 and iron salts in CH2Cl2.
c
Yields of isolated products (diastereomeric mixture) after purification by flash chromatography. dDetermined by 1H NMR analysis. eDetermined for the endo isomer by chiral HPLC analysis (Daicel Chiralpak® AD-H). fII-2a was solubilized in CH2Cl2 before
adding it to the reaction test tube by syringe.
Meanwhile, we tested the influence of temperature on the enantioselective Diels-Alder reactions with two different iron salts (Fe(ClO4)3·6H2O and Fe(OTf)2), as shown in Table 1.2. For Fe(ClO4)3·6H2O catalyzed reactions, not only we lowered the temperature from room temperature (entry 1, Table 1.2) to 0 oC (entry 2, Table 1.2), but also we added the dienophile differently. Specifically, in entry 2, we solubilized the dienophile in CH2Cl2 before adding it to the reaction test tube by syringe. Surprisingly, lowering the temperature from room temperature to 0 oC did not improve the enantioselectivity, but the enantiomeric excess was decreased dramatically, from 90% to 40% (entry 1 vs. entry 2, Table 1.2). It can be explained by the poor solubility of this dienophile in CH2Cl2 that leads to the drop of the enantiomeric excess as the reaction temperature is reduced. For Fe(OTf)2 catalyzed reactions, lowering the temperature from room temperature to 0 °C led to an improvement of enantioselectivity (10%) between entry 3 (Table 1.2) and entry 4 (Table 1.2). Nevertheless, there was an 11% of yield drop observed by lowering the temperature. After that, we attempted to extend the reaction time from 32 h (entry 4, Table 1.2) to 44 h (entry 5, Table 1.2), and the yield was increased from 52% to 78%. However, the enantioselectivity was decreased from 92% to 76%.
At the same time, we were interested to explore the reaction between 3-alkenoyl -1,3-oxazolidin-2-one (II-2b), in which the double bond is disubstituted, and cyclopentadiene (II-1a) catalyzed by iron salts and the 2,2’-bipyridyl ligand L1 in CH2Cl2 at room temperature. From Table 1.3, we can observe that, for this dienophile, utilizing different Lewis acids such as Fe(ClO4)3·6H2O, Fe(OTf)2 and Fe(ClO4)2·6H2O, good to very good enantiomeric excess (up to 94%) were obtained. The yields were
32
moderate to good (42%–81%). Specifically, when the reaction times were increased from 38 h to 48 h, yields gain by 39% (entry 2 vs. entry 1, Table 1.3) and by 16% (entry 4 vs. entry 3, Table 1.3) were achieved.
Table 1.3 Effects of the iron salts on the reaction between(E)-4-oxo-4-(2-oxooxazolidin-3-yl) but-2-en-1-ylium (II-2b) and cyclopentadiene (II-1a) in CH2Cl2a
Entry FeXb time (h) yield (%)c endo/exod ee (%)e
1 Fe(ClO4)3·6H2O 38 42 81:19 92 2 Fe(ClO4)3·6H2O 48 81 84:16 86 3 Fe(OTf)2 38 48 81:19 94 4 Fe(OTf)2 48 64 80:20 88 5 Fe(ClO4)2·6H2O 48 57 86:14 94 a
Conditions: FeXn (5 mol %), chiral diol bipyridyl ligand L1 (6 mol %), II-1a (3.5 mmol), and
II-2b (0.5 mmol) in CH2Cl2.
b
Catalysts were prepared in situ from the 2,2’-bipyridyl ligand L1
and iron salts in CH2Cl2.
c
Yields of isolated products (diastereomeric mixture) after purification by flash chromatography. dDetermined by 1H NMR analysis. eDetermined for the endo isomer by chiral HPLC analysis (Daicel Chiralpak® AD-H).
In short, it can be concluded that Fe(ClO4)3·6H2O and the 2,2’-bipyridyl ligand L1 catalyze the asymmetric Diels-Alder reaction in CH2Cl2 effectively in 48 h at room temperature, affording a very good yield (83%) and very good enantiomeric excess (90%) for the reaction between II-1a and II-2a with a 81% yield and 86% enantioselectivity for the reaction between II-1a and II-2b, respectively. However, we