© Mitra Zarifi Khosroshahi, 2019
Study of the hepatic stability and the therapeutic
potential of novel antimitotic prodrugs selective for
CYP1A1-expressing breast cancer cells
Mémoire
Mitra Zarifi Khosroshahi
Maîtrise en sciences pharmaceutiques - avec mémoire
Maître ès sciences (M. Sc.)
Study of the hepatic stability and the therapeutic
potential of novel antimitotic prodrugs selective for
CYP1A1-expressing breast cancer cells
Mémoire
Mitra Zarifi Khosroshahi
Sous la direction de :
Dr Sébastien Fortin, directeur de recherche
Dr Stéphane Gobeil, codirecteur de recherche
iii
Résumé
Nous avons récemment découvert et étudié une nouvelle classe d'agents antimicrotubules appelés phényl 4-(2-oxo-3-alkylimidazolidin-1-yl)benzènesulfonates (PAIB-SOs) hautement actifs et sélectifs pour les cellules cancéreuses du sein. Leur mécanisme de sélectivité est basé sur la métabolisation des PAIB-SOs par le CYP1A1 en agents antimitotiques puissants appelés phényl 4-(2-oxo-3-imidazolidin-1-yl)benzènesulfonates (PIB-SOs). L'objectif principal de ma recherche était d'évaluer la période nécessaire à l’induction d’une activité cytotoxique significative de quelques-uns de ces PAIB-SOs prometteurs sur les cellules cancéreuses du sein. Dans un second volet, nous désirions étudier la stabilité métabolique et la cinétique de bioactivation par le CYP1A1 de 8 PAIB-SOs phare en vue d’en sélectionner 4 pour des essais sur des animaux. Premièrement, nos études ont montré que l'activité antiproliférative des PAIB-SOs est concentration et temps dépendante. Un temps de contact des PAIB-SOs étudiés avec les cellules cancéreuses du sein variant de 24 à 36 h est nécessaire pour observer une activité antiproliférative significative. Deuxièmement, nous avons confirmé que tous les PAIB-SOs évalués sont rapidement biotransformés en présence de CYP1A1 en PIB-SOs. Troisièmement, les expériences de stabilité hépatique des PAIB-SOs incubés avec des microsomes soit humains, de souris ou de rats ont montré que leur demi-vie et leur clairance intrinsèque varient selon la structure du PAIB-SO et l’espèce animale étudiée. La stabilité des PAIB-SOs incubés avec des microsomes de souris est plus faible que lorsqu’incubé avec des microsomes humains ou de rats. De plus, la stabilité métabolique de ces PAIB-SOs avec les microsomes humains ou de rats est similaire. Le criblage de notre chimiothèque a permis d’identifier les CEU-829, -938, -934 et -913 comme étant les PAIB-SOs métaboliquement les plus stables et générant les plus basses concentrations de PIB-SOs avec des demi-vies respectives de 22, 55, 31 et 41 minutes dans les microsomes humains, de 43, 52, 23 et 44 minutes dans les microsomes de rat et de 3,7, 20, 12 et 1,6 minutes dans les microsomes de souris. Nos études ont également mis en évidence les CEU-934 et -938 comme des molécules candidates prometteuses pour des études de pharmacocinétique et de pharmacodynamie chez la souris et les CEU-829 et -913 chez les rats.
iv
Abstract
We recently found and studied a new class of antimicrotubule agents named phenyl 4-(2-oxo-3-alkylimidazolidin-1-yl)benzenesulfonates (PAIB-SOs) that are highly active and selective to breast cancer cells. PAIB-SOs are the first antimicrotuble CYP1A1-dependent prodrugs and their mechanism of selectivity is based on the metabolization in human breast cancer cells of PAIB-SOs by CYP1A1 into potent antimitotics named phenyl 4-(2-oxoimidazolidin-1-yl)benzenesulfonates (PIB-SOs). The main objective of my research was to evaluate the period necessary to trigger an efficient antiproliferative activity on breast cancer cells and to study the metabolic stability and the kinetics of activation by CYP1A1 of 8 promising PAIB-SOs exhibiting high antiproliferative activity and selectivity toward breast cancer cells aiming to select 4 PAIB-SOs for animal studies. We first found that the antiproliferative activities of PAIB-SOs are concentration and time-dependent. A contact time of the selected PAIB-SOs with breast cancer cells varying from 24 to 36 h is required to observe a significant antiproliferative activity. We also found that all PAIB-SOs are rapidly metabolized in the presence of CYP1A1 into PIB-PAIB-SOs. Finally, the hepatic stability experiments of PAIB-SOs using human, mouse or rat microsomes showed that the half-life and the intrinsic clearance depend on the structure of the PAIB-SO and the animal species studied. The stability of PAIB-SOs in mouse microsomes was weaker than in rat or human microsomes which were equivalent. Our screening program identified CEU829, 938, 934 and -913 as our most stable PAIB-SOs and generating the lowest quantity of PIB-SOs when incubated with human and rodent microsomes. In addition, they respectively exhibited half-lives of 22, 55, 31 and 41 minutes in human microsomes, 43, 52, 23 and 44 minutes in rat microsomes and 3.7, 20, 12 and 1.6 minutes in mouse microsomes. Altogether, our studies identified CEU934 and -938 as suitable candidates for further pharmacokinetic and pharmacodynamic evaluation in a mouse model and CEU-829 and -913 in a rat model.
v
Table of contents
Résumé ... iii
Abstract ... iv
Table of contents ... v
List of figures ... viii
List of tables ... xi
List of abbreviations ... xii
Acknowledgments... xiii
Foreword ... xiv
Introduction ... 1
1.1 Cancer and generality ... 1
1.2 Cancer statistics ... 3
1.3 Breast cancer ... 4
1.4 History of breast cancer and treatment ... 5
1.5 Risk factors and symptoms of breast cancer ... 6
1.6 Types and classification of breast cancers ... 7
1.6.1 Breast cancer types ... 7
1.6.2 Breast cancer subtypes ... 8
1.6.3 Breast cancer stages and grades ... 9
1.7 Breast cancer detection methods ... 10
1.8 Common therapies for breast cancer treatment ... 10
1.9 Hormonal therapy ... 11
1.10 Classification of chemotherapy used in breast cancer treatment ... 12
1.11 Chemotherapies used in breast cancer treatment ... 13
1.11.1 Alkylating agents ... 13
1.11.2 Antimetabolites ... 14
1.11.3 Natural products ... 15
1.12 Targeted therapy used in breast cancer treatment ... 18
1.12.1 HER2 inhibitors ... 19
1.12.2 CDK inhibitors ... 20
1.12.3 mTOR inhibitors ... 21
1.12.4 PARP inhibitors ... 22
1.13 Challenges for the development of new drugs for breast cancer treatment ... 23
1.13.1 Chemoresistance ... 23
vi
1.13.3 Cost of the new drugs ... 24
1.14 Cytochromes P450 ... 25
1.14.1 CYP1 subfamily and CYP1A1 ... 26
1.14.2 Relationship between CYP1A1 and breast cancer cells ... 26
1.15 CYP1A1-dependent prodrugs for the treatment of breast cancers ... 27
1.16 Phenyl 4-(2-Oxo-3-alkylimidazolidin-1-yl)benzenesulfonates... 29
1.17 Hypothesis and purpose underlying our study ... 31
Chapter 2 Evaluation of the time-dependent antiproliferative activity and liver microsome stability of 3 phenyl 4‑(2-oxo-3-alkylimidazolidin-1-yl)benzenesulfonates as promising CYP1A1-dependent antimicrotubule prodrugs... 32
2.1 Résumé ... 32
2.2 Abstract ... 33
2.3 Cover Page ... 34
2.4 Introduction ... 35
2.5 Materials and Methods ... 37
2.5.1 Chemicals and reagents ... 37
2.5.2 In vitro antiproliferative activity assay ... 38
2.5.3 Biotransformation of PAIB-SOs into PIB-SOs by CYP1A1 and in vitro hepatic stability ... 39
2.6 Results ... 40
2.6.1 The antiproliferative activity of CEU-818, -820 and -913 is time and concentration-dependent ... 40
2.6.2 Biotransformation of CEU-818, -820 and -913 by CYP1A1 into their PIB-SO counterparts ... 41
2.6.3 In vitro hepatic stability of PAIB-SOs in human, mouse and rat pooled liver microsomes ... 44
2.6.4 Production of antimicrotubule PIB-SOs in pooled liver microsomes ... 45
2.7 Discussion ... 45
2.8 Conclusion ... 48
2.9 Declarations ... 48
2.9.1 Conflict of interest ... 48
2.9.2 Acknowledgments ... 48
Chapter 3 Phenyl 4-(2-oxo-3-pentylimidazolidin-1-yl)benzenesulfonates as new potent and selective PAIB-SO derivatives. Antiproliferative activity and in vitro hepatic stability studies .. 50
3.1 Introduction ... 50
3.2 Materials and methods ... 51
vii
3.3.1 Antiproliferative activity of CEU-826, -829, -835, -934 and -938 on sensitive MCF-7
and insensitive MDA-MB-231 cell lines ... 52
3.3.3 In vitro hepatic stability of CEU-826, -829, -835, -934 and -938 in human, mouse and rat pooled liver microsomes ... 54
3.3.4 Production of antimicrotubule PIB-SOs in pooled liver microsomes ... 59
General discussion and conclusion ... 61
viii
List of figures
Figure 1.1. Schematic illustration of angiogenesis and the invasion of cancer into the blood and
lymphatic systems to enable invasion and metastasis adapted from cancer research UK uploader [7, 8]. ... 2
Figure 1.2. Schematic female breast anatomy and lobular carcinoma adapted from Terese
Winslow image (National Cancer Institute) [17]. ... 5
Figure 1.3 Classification and receptor status characterization of the 5 main intrinsic breast cancer
subtypes [35]. ... 8
Figure 1.4 Molecular structures of (a) tamoxifen and (b) fulvestrant (c), exemestane, (d) letrozole
and (e) anastrozole [59]. ... 12
Figure 1.5 Molecular structures of (a) cyclophosphamide, (b) cisplatin and (c) carboplatin [66].
... 14
Figure 1.6 Molecular structures of (a) cytosine, (b) thymine, (c) 5-fluorouracil, (d) capecitabine
and (e) gemcitabine [70]. ... 15
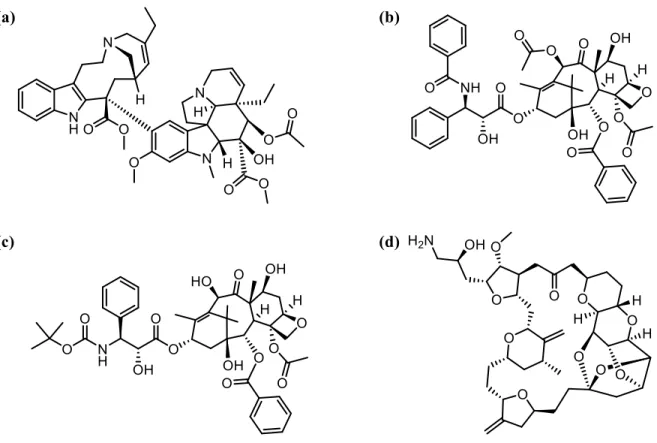
Figure 1.7 Molecular structures of (a) doxorubicin and (b) epirubicin. ... 15 Figure 1.8 Schematic representation of the mitotic spindle as well as α, β-tubulin heterodimers,
tubulin polymerization and depolymerization. ... 16
Figure 1.9 Schematic representation of microtubule structure and the (a) vincristine-, (b)
colchicine- and (c) paclitaxel-binding sites[86]. ... 17
Figure 1.10 Molecular structures of (a) vinorelbine, (b) paclitaxel (c) docetaxel and (d) eribulin.
... 18
Figure 1.11 Molecular structure of the monoclonal antibody trastuzumab [95]. ... 20 Figure 1.12. Molecular structures of (a) lapatinib and (b) neratinib [95]. ... 20 Figure 1.13 Molecular structures of selective CDK4/6 inhibitors (a) palbociclib, (b) ribociclib and (c) abemaciclib [100]. ... 21 Figure 1.14 Molecular structure of everolimus. ... 22 Figure 1.15 Molecular structure of olaparib [105]. ... 22 Figure 1.16 Molecular structures of the (a) duocarmycin precursor ICT2700, (b) benzothiazole
Phortress and (c) aminoflavone AFP464. ... 28
ix
(PIB-SOs) and (b) phenyl 4-(2-oxo-3-alkylimidazolidin-1-yl)benzenesulfonates (PAIB-SOs). . 30
Figure 2.1 (a) Molecular structures of Phortress and AFP464. (b) Bioactivation of phenyl
4-(2-oxo-3-alkylimidazolidin-1-yl)benzenesulfonates (PAIB-SOs) into potent antimicrotubule phenyl 4-(2-oxoimidazolidin-1-yl)benzenesulfonates (PIB-SOs) by CYP1A1. (c) Molecular structures of PAIB-SOs (CEU-818, -820 and -913) and their PIB-SOs counterparts (CEU-602, -699 and -868). ... 36
Figure 2.2 Dose-response of the antiproliferative activity of (a) 818, (b) 820, (c)
CEU-913 and (d) CEU-602 at 24, 36 and 48 h. ... 40
Figure 2.3 (a) Standard curves of CEU-818, -820 and -913. Typical chromatograms of CEU-818,
-820 and -913 obtained in the presence of (b) CYP1A1 Supersomes™ (3 min) as well as (c) human (10 min), (d) mouse (1 min) and (e) rat (20 min) pooled liver microsomes. Color code: Blue: PAIB-SOs, Red: PIB-SOs. ... 42
Figure 2.4 Metabolic stability, half-life (t1/2) and intrinsic clearance (CLint) of CEU-818, -820 and
-913 in the presence of (a) CYP1A1 Supersomes™ as well as (b) human, (c) mouse and (d) rat pooled liver microsomes. ... 43
Figure 2.5 Generation of antimicrotubule PIB-SOs (CEU-602, -699 and -868) in the presence of (a) CYP1A1 Supersomes™ as well as (b) human, (c) mouse and (d) rat liver microsomes. ... 44 Figure 3.1 Molecular structures of (a) first generation of PAIB-SOs, (b) PAIB-SOs with decreased
activity and (c) second generation of PAIB-SOs. (d) Molecular structures of selected 4-(2-oxo-3-pentylimidazolidin-1-yl)benzenesulfonates (CEU-826, -829, -835, -934 and -938) and their PIB-SOs counterparts (CEU-602, -699, 722, 733 and -700). ... 51
Figure 3.2 Dose-response for the antiproliferative activity of (a) CEU-826, (b) CEU-829, (c)
CEU-835, (d) CEU-934, (e) CEU-938 and (f) CEU-602 at 24, 36 and 48 h on sensitive MCF-7 cell line. ... 53
Figure 3.3 Dose-response of the antiproliferative activity of (a) 826, (b) 829, (c)
CEU-835, (d) CEU-934, (e) CEU-938 and (f) CEU-602 at 24, 36 and 48 h on insensitive MDA-MB-231 breast cancer cell line. ... 54
Figure 3.4 (a) Standard curves of CEU-826, -829 and -835. HPLC chromatograms of CEU-826,
-829 and -835 obtained in the presence of (b) human (20 min), (c) mouse (3 min) and (d) rat (20 min) pooled liver microsomes. Color coding: Blue: PAIB-SOs, Red: PIB-SOs. ... 55
-x
938 obtained in the presence of (b) human (20 min), (c) mouse (3 min) and (d) rat (20 min) pooled liver microsomes. Color coding: Blue: PAIB-SOs, Red: PIB-SOs. ... 56
Figure 3.6 Metabolic stability, half-life (t1/2) and intrinsic clearance (CLint) of CEU826, 829,
-835, -934 and -938 in the presence of (a) human, (b) rat and (c) mouse pooled liver microsomes. ... 58
Figure 3.7 Generation of antimicrotubule PIBSO metabolites (CEU602, 699, 722, 733 and
xi
List of tables
Table 1.1. Estimated number of new cancer cases and deaths by sex in 2017 in the United States
adapted from Siegel et al. [13]. ... 4
Table 3.1. Half-life (t1/2) and PIB-SOs generation after incubation of CEU-818, -820, -913, -826,
xii
List of abbreviations
AhR Aryl hydrocarbon receptor
AUC Area under curve
CDK Cyclin-dependent kinases
CYPs Cytochromes P450
DMSO Dimethyl sulfoxide
ER Estrogen receptor
FDA Food and Drug Administration
HER2 Human epidermal growth factor receptor
HLM Human liver microsomes
HPLC High performance liquid chromatography
HT-1080 Human fibrosarcoma cells
IC50 Half maximal inhibitory concentration
M21 Human skin melanoma cells
MCF-7 Human estrogen receptor-positive breast adenocarcinoma cells
MDA-MB-231 Triple-negative human breast carcinoma cells
MeOH Methanol
MTOR Mammalian target of rapamycin inhibitor
NCI/NIH National Cancer Institute/National Institutes of Health
PAIB-SOs Phenyl 4-(2-oxo-3-alkylimidazolidin-1-yl)benzensulfonates
PARP Poly (ADP-ribose) polymerase
PIB-SOs Phenyl 4-(2-oxoimidazolidin-1-yl)benzenesulfonates
PR Progesterone receptor
SRB Sulforhodamine B
t1/2 Half-life
TNBC Triple-negative breast cancer
xiii
Acknowledgments
This work was carried out at the laboratory of medicinal chemistry at CHU de Québec-Université Laval Research Center (Saint-Francois d'Assise Hospital) in Quebec City, under the guidance of Dr. Sébastien Fortin (director) and Dr. Stéphane Gobeil (co-director).
At the end of my studies, I would like to express my gratitude toward all those who contributed to the completion of this work. First, I would like to express my appreciation to my research director, Dr. Sébastien Fortin for providing me the opportunity to pursue my master’s degree in his lab. He has allowed me to pursue extremely interesting and rewarding graduate studies. He has always been available to supervise my research. I want to thank him especially for his confidence to me, his valuable suggestions, his scientific skills with unlimited supports, his patience and for his good humor throughout my studies. I would also like to warmly thank Dr. Stéphane Gobeil and Dr. René C-Gaudreault for their precious recommendations and advices.
I am grateful to my friends and colleagues Stéphanie, Melis, Letecia, Syrine, Hend, Richard, Martin, Marie-France, Mathieu, Daphné, Sébastien, Corinne and Chahrazed. Thank you for all the good times and friendship we shared in the laboratory or outside.
I would also like to thank my family from deep in my heart and a special mention for my son Iman. Thank you for your emotional support, your understanding and your patience throughout my life. Finally, I would like to thank the donation from Merck Sharpe & Dohme Corp to the Faculty of medicine, Université Laval and studentships from the Fonds d'enseignement et de recherche (FER) of the Faculty of Pharmacy of Université Laval for their financial support.
xiv
Foreword
Hereby I would like to give you a perspective of an international student with a different culture, language and educational background who had the unique opportunity to learn French, Canadian culture as well as science and technology according to North American standards. During my program at the Faculty of Pharmacy of Laval University, I shared my endeavor with my colleagues and professors. I enjoyed working with my colleagues as a team and deepened my knowledge in the field of oncology and medicinal chemistry.
My master thesis was written by articles insertion. It begins with an introduction followed by 2 Chapters, a discussion and a general conclusion. The introduction is a review of the literature introducing various aspects relating to the understanding of my master thesis. The chapter 2 is the manuscript submitted to the Editor of the Journal of Pharmacy and Pharmacology and describing the biological study of CEU-818, -820 and -913. The title of this manuscript is: “Evaluation of the
time-dependent antiproliferative activity and liver microsome stability of 3 phenyl 4-(2-oxo-3-alkylimidazolidin-1-yl)benzenesulfonates as promising CYP1A1-dependent antimicrotubule prodrugs”. The chapter 3 is the second part of the work realized during my master studies. This
Chapter deals with the biological evaluation of CEU-826, CEU-829, CEU-835, CEU-934 and CEU-938. The work done in that Chapter will be the subject of a second manuscript to be submitted within the next year to the Editor of a high impact factor journal.
1
Introduction
1.1 Cancer and generality
Cancer is an unlimited cell proliferation in a part of the body that can arise from all cell types and organs [1]. Cancer is not just one disease but it is a cluster of related diseases [2]. When normal cells become old or damaged, they are replaced by new ones which are growing within normal processes and speed. In the case of cancer cells, this process goes wrong and cells growth rate is much higher than usual. The cell division process is uncontrollable and cancer cells resist to cell death. They also have the ability to invade other tissues through the blood and lymphatic systems. Cancers developed in a tissue or organ are called primary cancers while dissemination of cancer cells from primary tumors to other organs, and subsequent seeding of new tumor colonies in distant tissues is known as the invasion metastasis cascade [3]. Cancer that stays solid in a defined area of an organ and that does not invade other organs is called “localized cancer” while cancer that create tumors in new distant site is called a “secondary or a metastatic cancer”. Cancer cells have higher energy and nutrient requirements (e.g. oxygen, glucose) than normal cells because of their rapid cell divisions [4, 5]. Therefore, they must reprogram their metabolic pathways and their energy metabolism to support their rapid cell divisions. Cancer cells are also characterized by their ability to: 1) escape the immune system, 2) eliminate growth suppressants, 3) support proliferative signals, 4) enable replicative immortality, 5) promote inflammation and 6) induce vascularization [4-6]. The process of developing new blood systems is called angiogenesis. Figure 1.1 illustrates the invasion of tumor cells in blood vessels. Tumor cells in the periphery of blood and lymphatic vessels have the ability to change their cellular adhesion and their extracellular matrix to penetrate into the blood and lymphatic system to enable invasion and metastasis.
2
Figure 1.1. Schematic illustration of angiogenesis and the invasion of cancer into the blood
and lymphatic systems to enable invasion and metastasis adapted from cancer research UK uploader [7, 8].
There are different approaches to classify cancers. One of them is to classify them based on their origin and types of cancer. For examples, carcinomas are cancers that are initiated in the skin or in the cells that coat and cover internal organs. Sarcomas origin in bones, cartilages, fat, muscle, blood vessels or other connective and supportive tissues. Cancers that start in blood-forming tissues such as bone marrow producing erythrocytes, leucocytes and platelets are designated as leukemia. Lymphomas and myelomas begin in the cells of the immune system. Melanomas are cancers that begin in cells producing melanin such as skin or eye [2].
Genetic origin and lifestyle play an important role in the development of cancers. The number of cancer patients is increasing every year due to the aging of the population, smoking, obesity, physical inactivity as well as the development of civilization and industrial life [1, 9]. Frequencies of incidence and mortality of cancers vary by type of cancer, region and country [10]. Even if there are lots of advances and achievements in medicinal and drug sciences, we cannot cure all cancers with the currently available treatments [11]. In this context, new and more effective anticancer treatments must be developed to improve both the life expectancy and the quality of life of cancer patients.
3
1.2 Cancer statistics
Every year, the number of people dying due to cancer and the total number of new cancer cases increase worldwide. Frequency of incidence and mortality of cancer vary by type of cancer, region, geographic variations and country. Lung cancer has the highest cancer mortality rate among men and women around the world. Based on data issued by the International Agency for Research on Cancer, there were 14.1 million new cancer cases diagnosed worldwide in 2012 and the number of total cancer deaths were 8.2 million [12]. Herein, a comprehensive report on statistics 2017 in the United States is provided [13]. According to the American Cancer Society, it is estimated that 1 688 780 of new cancer cases occurred for both sexes in 2017 in the United States population corresponding to 836 150 cases for males and 852 630 cases for females [13]. The estimated deaths of all cancer cases are 600 920 cases for both genders corresponding to 318 420 cases for males and 282 500 cases for females. Breast cancer is the second-deadliest form of cancer in the female population [10]. The number of estimated new breast cancer cases was 255 180 cases for both genders (2 470 cases for males and 252 710 cases for females) and total number of deaths was 41 070 cases (460 cases for males and 40 610 cases for females). It means about 99 % of breast cancer patients population among estimated deaths data, are women. Among entire female patients with new cancers in 2017, 30% was related to breast cancers. Table 1.1 shows the estimated number of new cancer cases and deaths by sex in the United States in 2017.
4
Table 1.1. Estimated number of new cancer cases and deaths by sex in 2017 in the United
States adapted from Siegel et al. [13].
Estimated new cases Estimated deaths
Both sexes Male Female Both sexes Male Female
All Sites 1 688 780 836 150 852 630 600 920 318 420 282 500
Oral cavity & pharynx 49 670 35 720 13 950 9 700 7 000 2 700
Digestive system 310 440 175 650 134 790 157 700 92 350 65 350
Respiratory system 243 170 133 050 110 120 160 420 88 100 72 320
Bones & joints 3 260 1 820 1 440 1 550 890 660
Soft tissue (including
heart) 12 390 6 890 5 500 4 990 2 670 2 320
Skin (excluding basal
& squamous) 95 360 57 140 38 220 13 590 9 250 4 340
Breast 255 180 2 470 252 710 41 070 460 40 610
Genital system 279 800 172 330 107 470 59 100 27 500 31 600
Urinary system 146 650 103 480 43 170 32 190 22 260 9 930
Eye & orbit 3 130 1 800 1 330 330 180 150
Brain & other nervous
system 23 800 13 450 10 350 16 700 9 620 7 080
Endocrine system 59 250 15 610 43 640 3 010 1 440 1 570
Lymphoma 80 500 44 730 35 770 21 210 12 080 9 130
Myeloma 30 280 17 490 12 790 12 590 6 660 5 930
Leukemia 62 130 36 290 25 840 24 500 14 300 10 200
Other & unspecified
primary sites 33 770 18 230 15 540 42 270 23 660 18 610
1.3 Breast cancer
Breast cancers is complex disease related to organs which are involved in female beauty, sexuality organs and production of the milk feeding babies (figure 1.2). We can find breast cancers in both genders, but it is a hundred times more prevalent in women population than in men [14]. Breast cancer emerges following growth of malignant cells within the mammary tissues [15, 16]. The main causes of breast cancerogenesis are not fully understood. Nowadays, it is quite clear that the term of breast cancer encompasses a heterogeneous collection of malign diseases with a wide variety of clinical behaviors. Due to the high mortality rate of breast cancer, its treatment and prevention continue to be an important issue of public health in our industrial societies.
5
Figure 1.2. Schematic female breast anatomy and lobular carcinoma adapted from Terese
Winslow image (National Cancer Institute) [17].
1.4 History of breast cancer and treatment
It is not surprising that the evidence and recorded documents of breast cancer date back to antiquity. Papyrus written on breast cancers between 3500 and 2500 before Christ have been found in Egyptian pyramids [16, 18]. Ancient Egyptians presented the earliest descriptions of breast tumors around 3500 before Christ and treatments were based on the use of opium and castor oil. Descriptions of breast cancer treatments were also found in Indian civilization between 3500 and 400 before Christ. The treatments of breast cancer at that time were surgical excision, cautery and arsenic compounds [16]. A major breakthrough for the treatment of breast cancer was the development of modern and aseptic surgical methods for the excision of breast cancers in the mid-18th century [16]. Another major breakthrough was the introduction of modern chemotherapy in the 20th century with the development of
6
nitrogen mustards that were discovered after the observation, analysis and evaluation of these chemical war agents [19]. In the 50s and 60s, several chemotherapeutic agents have emerged notably cyclophosphamide, 5-fluorouracil and carboplatin that are still in use to treat breast cancer. The approval of tamoxifen as antiestrogen for treatment of estrogen receptor (ER)-positive breast cancer and of trastuzumab (Herceptin®) for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer in the 80s and 90s, respectively have also been important innovations for the treatment of these diseases. The discovery of radiotherapy and the refinement of surgical methods in the 20th century also benefits to breast cancer treatment. In addition, state of the art imaging techniques and screening programs also made breast cancers more diagnosed at an earlier stage which are all factors that have contributed to improving the prognosis of breast cancer patients. Traditionally, most treatments were commercialized for all patients affected with the same type of cancer, not considering that it was proved that tumors with similar histological behavior, show different clinical responses to treatment. Therefore, with remarkable advances, traditional treatments have moved forward to target therapy and personalized medicine in the 21st century. Today, in addition to the traditional treatments, targeted therapies increase the armamentarium of anticancer agents and bring more personalized approaches to treat breast cancer. In the next few years, the development of new targeted therapies as well as the personalization of the treatments will continue to grow. Therefore, it is important to pay attention to new approaches to improve the treatment of breast cancer [20].
1.5 Risk factors and symptoms of breast cancer
Risk factors differ according to the type of breast cancer. However, several conditions are identified as general risk factors including environmental factors, nutritional conditions, alcohol, tobacco, genetics, radiation exposure, family antecedent, menstrual history, obesity, hormone replacement therapy, immune system state and age [21, 22]. About 5–10% of breast cancers are attributed to family antecedent and related to an inherited mutation in the BRCA1 or BRCA2 gene [23]. Mutated BRCA1 or BRCA2 is a strong risk factor for diagnosis of breast cancer at a younger age in women [24]. Nonetheless, among all risk factors of breast cancer in women, age, particularly >50 years, is known as the most important one. Moreover, incidence rate increases proportionally with age. Breast cancer is therefore considered as an
7
age-related disease at early stages. Breast cancer may be asymptomatic. However, the symptoms appear as it develops, and that the tumor occupies a vast region of breast tissue. At this stage, the common symptoms consist of asymmetry and breast size differences, modification of the shape or the nipple, skin lesions, a lump in the armpit, discharge of fluid or blood of the nipple, bone pain, weight loss, nausea, loss of appetite, shortness of breath, cough, headache, double vision and muscle weakness [25].
1.6 Types and classification of breast cancers
1.6.1 Breast cancer types
There are many types of breast cancer and their classification is based on the specific cells affected in different areas of the breast tissues. Breast carcinomas are the most common type of breast cancer, and they are involving the epithelial cells. A subtype form of carcinomas is designated as adenocarcinoma and its origins from the lactiferous ducts or the lobules. Sarcomas, phyllodes, angiosarcomas, Paget disease, papillary carcinoma and inflammatory are other types of breast cancer that are however rare [23]. Less than 1% of primary breast cancers are sarcomas, arising from the stromal component of the breast which includes myofibroblasts and blood vessel cells. Phyllodes tumor occurs in the stromal cells of the breast and is mostly found in 30 and 40 years old women [26]. Angiosarcomas are other types of uncommon breast cancer that originate from epithelial cells of blood or lymph vessels. Angiosarcoma can involve the breast tissue itself or the skin surrounding it. Another rare form of breast cancer is Paget disease (≈ 3%) which originates from breast ducts, invades the skin of the nipple and then expands to the areola [27]. Papillary carcinoma cancer cells are very small, and in most cases, are invasive. Inflammatory breast cancer is a rare type of very invasive breast cancer. It mostly occurs in younger women and its symptoms are characterized by inflammation-like breast swelling, purple or red color of the skin, and pitting or thickening of the skin of the breast, all of which are likely caused by cancer cells blocking lymph vessels in the skin.
8
1.6.2 Breast cancer subtypes
Breast cancers are also classified according to different criteria based on: histological appearance, grade, stage, type of receptors involved and gene expression status [14, 28]. The traditional classification of breast cancers is based on their hormone and receptor status. This classification includes estrogen receptors (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) [29]. The rate of their expression plays an important role in the initial diagnosis of breast cancers [30]. Figure 1.3 shows the classification of the 5 main intrinsic breast cancer subtypes namely: luminal A-like, luminal B-like, HER2-like, triple-negative breast cancer (TNBC or basal-like) and normal-like breast cancer subtypes [23, 31-35].
Figure 1.3 Classification and receptor status characterization of the 5 main intrinsic breast
cancer subtypes [35].
First, the five intrinsic breast cancer subtypes are based on their differences of the receptor status. Each subtype is characterized by distinct rates of incidence/recurrence, prognosis and response to treatment. Moreover, the term “like” added after the subtype names indicates that immunohistochemically analysis was used to define these subtypes. Luminal A-like subtype with low-grade represents 40% of all breast cancers. It is characterized by slow growing and
9
usually treated by hormonal therapy. Indeed, it is ER-positive, PR-positive or -negative, HER2-negative and low for proliferation marker ki-67. Luminal B-like subtype is ER-positive, PR-positive or -negative, HER2-positive or -negative and high ki-67. Luminal-B accounts for < 20% of all breast cancers and cells grow slightly faster than in luminal-A. HER2-like subtype is ER-negative, PR-negative and HER2-positive. This subtype represents 10-15% of breast cancers. It is characterized by cancer cells growing faster than in luminal cancers and has a poor prognosis. The treatment of HER2-like tumors is notably the use of anti-HER2 agents. Triple-negative/basal-like subtype are ER-negative, PR-negative and HER2-negative. TNBC subtype accounts for approximately 20% of all breast cancers. TNBC exhibits a more aggressive phenotype than other breast carcinomas because it is characterized by rapid tumor growth, higher metastatic rates and higher histological grades [36]. Moreover, hormonal and anti-HER2 therapies are ineffective against this subtype of cancer and its non-surgical treatment is facing limitation of chemotherapy [37]. Normal-like subtype is ER-positive and/or PR-ER-positive, HER2-negative and has low levels of protein ki-67. This subtype is similar to luminal A disease while its prognosis is slightly worse than luminal A. Diagnosed of TNBC subtype has the worst prognosis, while luminal A subtype has the best
1.6.3 Breast cancer stages and grades
In the process of diagnosing of breast cancer, tests are available to determine the clinical stage of the disease according to the extent of breast cancer, tumor size and spread. The stages of cancer are classified according to the American Joint Committee on Cancer (AJCC) and the International Union for Cancer Control (UICC). TNM is a worldwide and common language for all doctors to describe cancer. The classic TNM staging system is based strictly on anatomic features [38]. The abbreviation TNM stands for tumor size (T), spread to regional lymph nodes (N) and metastasis (M), respectively [39].
In TNM system, the stages are divided in 5 categories including: Stage 0: ductal carcinoma in situ and non-invasive form of breast cancer [40]. Stage I: defines invasive breast cancer and is divided in two classes including stage IA and IB. Definition of these two subcategories is depending on size of tumor between 0.2 mm and 2 cm, as well as the spreading of cancer in the lymph nodes or not. Stage II includes stage IIA and IIB. In this stage, the cancer cells have the ability to spread from the ducts or lobules into the other parts of the breast and the
10
tumor size is between 2 and 5 cm. In the Stage III, breast cancer is characterized by an invasive form of cancer and measured tumor size is more than 5 cm with or without lymph nodes involvement. Spreading of tumor cells to skin of the breast is possible causing swelling or ulcers. Stage III has three subcategories including IIIA, IIIB and IIIC. Stage IV describes the most dangerous form of breast cancer with high capacity of tumor spreading to other organs of the body including, lungs, distant lymph nodes, skin, bones, liver or brain. There is another cancer terminology for breast cancer stages is grading and describing the appearance of the cancer cells and includes grade 1, 2 and 3. Cancer cells often exhibit much more variability than normal in cell size or shape. Grade 1 represents a weak difference between normal and cancer cells. Cancer cells in grade 2 do not resemble normal cells. The appearance of cancer cells in grade 3 is abnormal.
1.7 Breast cancer detection methods
One of the major problems in the treatment of breast cancer is its early diagnosis. Indeed, the appearance of cancer is mainly asymptomatic [41]. Conventional and classical methods for detection of breast cancer are not specific and look for abnormalities in the breast tissue such as masses, architectural distortions, asymmetries and microcalcifications [42].To this end, detection techniques are continually refined. For examples, sonography and mammography screening are two common technologies that are used for early detection and diagnosis as well as for the follow-up of the disease post treatment [43]. Magnetic resonance imaging is another helpful technic for diagnosis of breast cancer. The ongoing technological development of three-dimensional models and combination of two, three or even more technologies allow the identification of normal breast tissues microstructure, neoplastic tissues and proteolytic activity. The new three-dimensional approaches for early diagnosis and screening are more accurate with high sensitivity and a good signal-to-noise ratio. These approaches include notably X-ray mammography (digital breast tomosynthesis), contrast-enhanced mammography, quantifiable ultrasound techniques, acoustic radiation force impulse and diffusion-weighted magnetic resonance imaging [42, 44].
1.8 Common therapies for breast cancer treatment
11
treatments for breast cancer. Surgery (mastectomy and lumpectomy) consists of the partial or the whole ablation of the diseased breasts [45]. Radiotherapy consists of irradiating with high-energy X-rays the tumor area. This is performed after a mastectomy to improve locoregional efficiency of the treatment and to avoid local recurrences and improve survival of the patients. Hormonotherapy is usable for hormone-dependent breast cancer. Hormonotherapy can prevent the production of endogen hormones or their binding to their receptors at the cellular surface. Chemotherapy is the use of systemic cytocidal drugs to kill cancer cells. This type of treatment is widely used for advanced breast cancers, but it can also be used in different grades and stages depending on the characteristics of the disease. These drugs can be used alone or in combination [46]. Multimodal treatment decreases the risks of recurrence, improves the outcome of the treatment leading to a decline in breast cancer mortality. The patients are now living many years without recurrence beyond their initial breast cancer treatment. Since our work focuses on the study and the development of a new targeted therapy, the next paragraphs will be dedicated to the drugs currently in clinical use for the treatment of breast cancer including hormonal therapy, chemotherapy and targeted therapy.
1.9 Hormonal therapy
Estrogens and androgens are sex steroid hormones. Androgens are precursors of estrogens, as aromatization of androgens leads to synthesis of estrogens [47]. ERα and ERβ are the two main ER isoforms. ERα is expressed mainly in sex organs such as breast, uterus, ovaries, testes and epididymis while ERβ is mostly expressed in prostate and other parts of the body like skin, bone, brain, lungs, blood vessels and lymphocytes. Approximately 75% of human breast cancers are ER-positive. ER-positive tumors are responsive to hormonal therapy and generally overcome breast cancer cell proliferation [48, 49]. The antihormonal drugs used in clinics are selective ER modulators, antiestrogen, aromatase inhibitors, LHRH agonists and progestatives. In the next paragraph, we will only deal with the selective ER modulators, antiestrogen and aromatase inhibitors, as they are the most clinically used. For over 40 years, tamoxifen (figure 1.4a) has been used as the best selective ER modulator [50]. Tamoxifen is a prodrug that exhibits its anticancer activity after biotransformation by the CYP2D6 enzyme to its active metabolite (endoxifen) [51]. It can be used alone for treating of ER-positive
12
breast cancers or in combination with other therapies [41, 52]. Fulvestrant (figure 1.4b) is also an antihormonal drug used in the clinic. Fulvestrant is an antagonist of the ER and a selective ER down regulator. Fulvestrant is only used in recurrent breast cancers after tamoxifen treatment. Aromatase inhibitors are another class of hormonal therapy used in the clinic. Aromatase is a key enzyme involved in estrogen biosynthesis [53-57]. The mechanism of action of aromatase inhibitors is to block aromatase enzyme transforming androgens into estrogens via aromatization of the residual androgens produced by other tissues such as adrenal gland and adipose tissues, which are the main sites of estrogen synthesis in post-menopausal women [58]. Exemestane, letrozole, and anastrozole shown in figure 1.4c-e are well-known aromatase inhibitors used in the clinic for the treatment of postmenopausal women with hormone-sensitive breast cancer.
Figure 1.4 Molecular structures of (a) tamoxifen and (b) fulvestrant (c), exemestane, (d)
letrozole and (e) anastrozole [59].
1.10 Classification of chemotherapy used in breast cancer treatment
The National Cancer Institute defines chemotherapy as a treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. Chemotherapy involves two important classes of drugs: cytotoxic drugs and targeted therapy. Nowadays, the term chemotherapy is mostly used and known to refer to cytotoxic drugs rather than targeted therapy. Thereafter, the terms chemotherapy and targeted therapy will be used to avoid confusion. Chemotherapeutic drugs are designed to kill rapidly dividing cells.
13
Consequently, they are not specific to cancer cells and are known to affect both cancer and normal cells similarly [41]. Targeted therapy was developed to selectively kill cancer cells and is one of the most promising advances in modern oncology. Its approach is based on the targeting of biomolecule specifically expressed or overexpressed by cancer cells. In the last decades, many anticancer drugs have been discovered and developed [60]. The following sections are not intended to describe all the new approaches and new anticancer drugs in development in depth, but to provide the reader with an insight of the main options currently available for treating breast cancer. The classification of treatments currently available is based on their mechanism of action.
1.11 Chemotherapies used in breast cancer treatment
Chemotherapeutic drugs are frequently used for treating of advance breast cancers and for breast cancers that have a high risk of recurrence. These drugs are divided into 3 main classes: alkylating agents, antimetabolites and natural products.
1.11.1 Alkylating agents
Alkylating agents act mostly during resting phase of the cell cycle and show therapeutic effect by breaking of DNA strands [61]. The mechanism of action of alkylating agents is based on the substitution of a hydrogen atom of DNA by an alkyl radical to interfere with DNA synthesis and RNA transcription. This triggers mispairing of base-base derivatives, establishing an obstacle to cell division and eventually resulting in cell death [62]. Generally, alkylating agents use three different mechanisms for inhibiting of DNA function. The first mechanism leads to the formation of cross-bridges that prevent DNA strands from being separated for synthesis or transcription. In the second mechanism, mutation is generated due to incorrect pairing of the nucleotides during alkylation and in the third mechanism, an alkylating agent attaches alkyl groups to DNA bases during DNA repair. The three alkylating agents used for treating breast cancer are cyclophosphamide (figure 1.5a), a member of the nitrogen mustards family as well as carboplatin and cisplatin, two members of the platinum salts family (figure 1.5b and c) [63-65].
14
Figure 1.5 Molecular structures of (a) cyclophosphamide, (b) cisplatin and (c) carboplatin
[66].
1.11.2 Antimetabolites
The antimetabolites are anticancer agents exhibiting chemical structures similar to essential metabolites, with function during biochemical processes being completely different from that of their essential metabolite analogs [67]. An essential metabolite is defined as an agent required for key biotransformation mechanisms, such as the synthesis of DNA and RNA, while an antimetabolite is an agent that has the ability to structurally mimic the essential metabolite, preventing DNA replication and inhibiting cancer cells growth. For example, DNA synthesis requires puric and pyrimidinic DNA bases as well as folic acid. Indeed, pyrimidine DNA bases include cytosine (C) and thymine (T), (see, figure 1.6a and b) while purine nucleobases include adenine (A) and guanine (G). Antimetabolite agents are divided into three families: pyrimidine and purine analogs, and antifolate. 5-Fluorouracil, capecitabine and gemcitabine are pyrimidine analogs used for treating breast cancer (figure 1.6c-e) [68, 69]. 5-fluorouracil inhibits the enzyme thymidylate synthase via its active metabolite 5-fluorodeoxyuridine monophosphate. Capecitabine is a prodrug of 5-FU that initially is inactive, but readily actived in tumors overexpressing thymidine phosphorylase. Finally, gemcitabine is a potent inhibitor of ribonucleotide reductase, an enzyme acting to reduce the pool of deoxynucleotides that are essential for DNA synthesis.
15
Figure 1.6 Molecular structures of (a) cytosine, (b) thymine, (c) 5-fluorouracil, (d)
capecitabine and (e) gemcitabine [70].
1.11.3 Natural products
Natural products are an important class of chemotherapeutic drugs used for treating cancers. Natural products are divided into several main families of anticancer agents notably antibiotics, microtubule damaging agents, camptothecin analogs, epipodophylotoxins, enzymes and hydroxyurea [71].
Antibiotics
Anthracycline antibiotic and microtubule damaging agents are families of natural products used for the treating breast cancer. Doxorubicin and epirubicin are the two anthracycline antibiotic used for treating breast cancer (figure 1.7) [72, 73]. Anthracycline antibiotic can directly affect the synthesis of DNA by intercalating DNA, directly affecting transcription and replication or by forming the ternary complex with topoisomerase II and DNA, thus blocking the replication fork.
16
Microtubule damaging agents
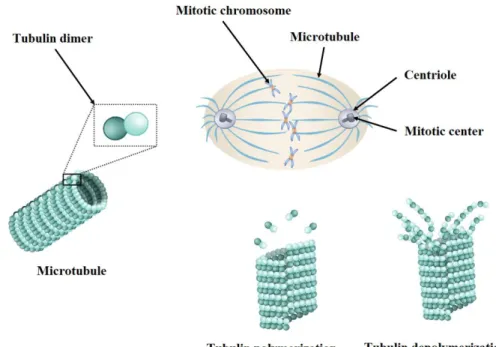
Microtubules are hollow filaments and the fundamental elements of intracellular motion. The α, β-tubulin heterodimers are the main monomers of these cytoskeleton protein polymers that assemble in a specific head-to-tail orientation to form polar microtubules with a diameter of approximately 25 nm (figure 1.8) [74, 75]. They have various dynamic responsibilities within eukaryotic cells, including cell shape maintaining, intracellular transport and movement via motor proteins, cell motility, segregation and cell division during mitosis [74, 76]. Microtubules are characterized by their dynamic instability that causes rapid shift between growth and shrinkage. Microtubule formation happens during interphase and help chromosome segregation and cell division in mitosis. Therefore, microtubule arrays start to rearrange at the onset of mitosis to guide and separate the sister chromatids toward the poles of the new daughter cells (figure 1.8).
Figure 1.8 Schematic representation of the mitotic spindle as well as α, β-tubulin
heterodimers, tubulin polymerization and depolymerization.
This process has led to the design and development of microtubule-disrupting or damaging agents known as antimitotics. Antimitotics demonstrate their activity by affecting the spindle assembly through blocking the cell division transition from metaphase to anaphase [77]. They
17
interfere with spindle formation during mitosis and arrest the cell cycle in G2-M phase and lead to mitotic catastrophe and apoptosis [78, 79]. On one hand, microtubule targeting agents are divided in two main groups based on their mechanisms of action namely, microtubule-stabilizing and microtubule-demicrotubule-stabilizing agents. Microtubule-microtubule-stabilizing agents such as paclitaxel are molecules that prevent microtubule depolymerization which increases stabilization of microtubule networks and mostly binds to the tubulin polymers (figure 1.9). In fact, these agents increase the density of cellular microtubule scaffolds by shifting the equilibrium of tubulin polymer from the soluble to the polymerized form [80, 81]. They bind notably to the paclitaxel-binding site. On the other hand, microtubule-destabilizing agents such as Vinca alkaloids and colchicine destabilize tubulin polymers and prevent the polymerization of the monomers into the tubulin polymer [74, 82-85]. They bind notably to the vincristine- or the colchicine-binding site (figure 1.9).
Figure 1.9 Schematic representation of microtubule structure and the (a) vincristine-, (b)
18
There are 3 families of antimitotics used in the clinic for treating breast cancer: Vinca
alkaloids, taxanes and eribulin. Vinca alkaloids such as vinblastine, vincristine and
vinorelbine (figure 1.10a), were isolated from the Madagascar periwinkle leaves,
Catharanthus roseus (also known as Vinca rosea) [84]. Paclitaxel and docetaxel (figure
1.10b and c) are taxoids used for breast cancer treatment. Paclitaxel was extracted from the stem bark of the Pacific yew tree (Taxus brevifolia Nutt) in 1966 while docetaxel is a semi-synthetic derivative of paclitaxel [87]. Eribulin (figure 1.10d) is the last antimitotic used in the clinic for treating breast cancer. It is a synthetic analog of halichondrin B. It is used for metastatic breast cancers that have previously been treated with at least two chemotherapeutic regimens.
Figure 1.10 Molecular structures of (a) vinorelbine, (b) paclitaxel (c) docetaxel and (d)
eribulin.
1.12 Targeted therapy used in breast cancer treatment
Targeted therapies for treating breast cancer are mainly based on the design and development of signal transduction inhibitors. Signal transduction mainly involves chemical signals
19
between cells under the control of various kinases that regulate many functions of the cells including death, growth, and division. To this end, kinases are enzymes that transfer a phosphate group to a specific protein. Conversely phosphatases catalyse inverse reaction, removing a phosphate group from protein. This reversible reaction is the basis of transduction signals in the cells to regulate their functions [88]. Signal transduction in cancer cells is sometimes dysregulated and promotes growth and cell division. Signal transduction inhibitors selectively impede biochemical reactions under the control of specific kinases, to blocking these signals and ultimately arresting the progression of cancer [89]. Tyrosine kinase inhibitors are targeted therapies for various types of cancer and currently, they accounted for a quarter of all current drug research. A vast spectrum of kinase inhibitors are being approved or will be approved in the coming years by the Food and Drug Administration (FDA) and other regulatory agencies worldwide for clinical use. Targeted therapies used in treating breast cancer are divided into 4 main families: HER2 inhibitors, cyclin-dependent kinase (CDK) inhibitors, mammalian target of rapamycin (mTOR) inhibitors and poly (ADP-ribose) polymerase (PARP) inhibitors.
1.12.1 HER2 inhibitors
HER family comprises four homologous members named EGFR (alias HER1), HER2, HER3, and HER4, respectively [90-92]. All of HER isozymes are tyrosine kinase receptors sharing a common molecular structure, comprising an extracellular ligand-binding domain, a transmembrane domain and a cytoplasmic tyrosine kinase-containing region. Among all HER isoforms, HER2 is the one that is responsible for the major breast cancer issues. Overexpression of HER2 is found in a substantial number of human breast cancer cases (approximately 15%–20%) and is also associated with a more aggressive phenotype [90]. Trastuzumab (Figure 1.11) was the first efficacious monoclonal HER2 antibody introduced in the clinic for treating breast cancer [93] at either early or metastatic stages [92, 94].
20
Figure 1.11 Molecular structure of the monoclonal antibody trastuzumab [95].
Thereafter, pertuzumab another monoclonal antibody and the tyrosine kinase receptor inhibitor lapatinib were also marketed as anti-HER2 targeted therapy to improve survival of HER2-positive patients with advanced breast cancer [94, 96, 97]. Finally, neratinib, a tyrosine kinase inhibitor used to prevent recurrence in patients with early-stage HER2-positive breast cancer was recently introduced [88]. Figure 1.12a and b shows the molecular structures of lapatinib and neratinib.
Figure 1.12. Molecular structures of (a) lapatinib and (b) neratinib [95].
1.12.2 CDK inhibitors
CDK 4 and 6-retinoblastoma proteins have important impacts on proliferation activity [98]. In several human cancers, CDK4/6 are overactive or CDK-inhibiting proteins are inactive. CDK4/6 inhibitors target epigenetic mechanisms and impede the proliferation of cancer cells [99]. Three CDK4/6 inhibitors, namely palbociclib, ribociclib and abemaciclib are members
21
of a new generation of therapeutics that have been approved by the FDA and show advantages for treating ER-positive metastatic breast cancer (figure 1.13).
Figure 1.13 Molecular structures of selective CDK4/6 inhibitors (a) palbociclib, (b)
ribociclib and (c) abemaciclib [100].
1.12.3 mTOR inhibitors
Everolimus (figure 1.14) is a mTOR inhibitor approved by the FDA in 2012 for treating advanced-stage breast cancer in patients resistant to endocrine therapy, metastatic breast cancer and HER2-negative breast cancer in postmenopausal women who have already been treated with letrozole or anastrozole [48, 57, 101, 102]. Everolimus demonstrated efficient chemotherapeutic action through induction of apoptosis via downstream B cell lymphoma, as well as decreased phosphoinositide 3-kinase, mitogen-activated protein kinase (MAPK), protein kinase B and mTOR expression levels in breast cancer cells.
22
Figure 1.14 Molecular structure of everolimus.
1.12.4 PARP inhibitors
Several genes are essential modulators of DNA repair and replication processes, including BRCA1/2, PALB2 and RAD51 [103]. DNA repair pathway for tumor cells deficient in BRCA1/2 is mediated by PARP1 protein. PARP1 inhibitors reduce repair of both single-strand breaks and double-single-strand breaks in breast tumors. The PARP inhibitors prevent coupling of two strands, which makes subsequent double strand break and separate at replication stage, and subsequently block DNA repair system, which ultimately leads to the selective killing of tumor cells. Thus, PARP inhibitors such as Olaparib that was approved in 2014 prevent tumor’s DNA reparair and improve survival in patients with cancers involving hereditary BRCA mutations [104]. Figure 1.15 shows the molecular structure of olaparib.
23
1.13 Challenges for the development of new drugs for breast cancer
treatment
In this section, we present three impediments that must be overcome for the development of new drugs for treating breast cancer t: chemoresistance, deleterious effects and cost of new drugs.
1.13.1 Chemoresistance
Cancer chemoresistance and the survival of tumor cells in the presence of anticancer drugs make a serious impediment to chemotherapy, often leading to its failure. To summarize the different reasons for the innate and/or adaptive chemoresistance of cancer, first we focused on the absorption of drugs inside tumor cells and response of these cells to the drugs. When considering the absorption of drugs, the status of poor drug uptake inside the tumor cells causes reduced responsiveness of tumor cells [84]. Multiple hypothesis for explaining chemoresistance of cancer tumors have been described, but they typically involve activity of efflux pumps that reduce drug absorption or drug accumulation within tumor cells [106, 107]. The efflux pumps include notably P-glycoprotein (P-gp), breast cancer resistance protein (BCRP) and the bile salt exporter protein (BSEP). Moreover, several intrinsic (genetic, epigenetic, transcriptomic, proteomic properties, genomic stability, mutations, gene amplifications and deletions, etc.) and extrinsic factors (pH, hypoxia, etc.) influence the intra-tumor heterogeneity and the response of intra-tumors to the drugs [108, 109]. Several other mechanisms are known to be involved in chemoresistance of anticancer agents: 1. releasing of cytokines by the tumor microenvironment that stimulates tumor cell growth, 2. Protein- drugs interactions of altering the molecular characteristics of the drugs and ultimately inactivating them, 3. activation of detoxifying proteins such as cytochromes P450 (CYPs) modifying the phase I or II drug metabolism pathways, 4. modification of the chemotherapeutic agents target and 5. enhancement of drug-induced DNA repair and recombination repair mechanisms [106, 110]. All of these mechanisms may contribute to chemoresistance of breast cancer tumors.
24
1.13.2 Deleterious effects
The objective of chemotherapy or targeted therapy is to offer the patient the highest possible positive response rates without causing adverse effects. In addition, the treatments should significantly extend life expectancy and improve quality of life of patients. However, after initiation of therapy in most cases, toxic side effects are observed. Some of these agents exhibit so-called organ-related adverse effects that are classified according to their level of severity: 1, mild; 2, moderate; 3, severe; 4, life-threatening or disabling; 5, fatal [111]. Sometimes, long-term use of antineoplastic agents targets the immune or the nervous system. Severity of these adverse effects depends on the biological target; life-threatening complications often result from infections, inhibition of angiogenesis pathways, severe inflammatory syndromes and autoimmune disorders. Common side effects observed in the clinic are nausea, emesis, hair loss, blood test abnormalities, cardiac disease, gastrointestinal bleeding or perforation, pulmonary hypertension, exanthemata, diarrhea, neurotoxicity, liver toxicity, fever, chills, headache, asthenia, myalgia, arthralgia, back or abdominal pain, oliguria, bronchospasm, dyspnea, hypotension, confusion, urticarial reaction. Side effects depend on several parameters notably the dose administered, the duration of treatment and the drug administered. Side effects must be managed since they are the main cause of chemotherapy cessation.
1.13.3 Cost of the new drugs
Unfortunately, anticancer drugs are often costly, and the price for new drugs continues to rise beyond inflation. Thus, they are not readily available for everyone and numerous patients may die only because they cannot afford to pay for those drugs [112, 113]. Nowadays, the global anticancer drug market exceeds US$100 billions per year and it is expected to increase to $150 billions by 2020 [114]. USA Patients consume of nearly half (46%) of antineoplastics, while the price for these drugs is seemingly increasing everyday. From 2010 to 2020, the expenditure on anticancer drugs has risen over 50%. As a result, high anticancer drug prices obviously have negative consequences for patients because most of them cannot be affordable of buying these agents.
25
1.14 Cytochromes P450
Cytochromes P450 (CYPs) are an ubiquitous family of enzymes present in all living cells. CYPs are involved, through several oxidative and reductive reactions in the metabolism of numerous endogenous substrates (steroids, fatty acids, prostaglandins), pharmaceuticals, and various pollutants [115-118]. In humans, CYPs are membrane-associated proteins located in the mitochondria or in the endoplasmic reticulum of cells. It is an impressive library of enzyme isoforms exhibiting a wide variety of functions, sizes, architectures, and physicochemical properties. Indeed, there are 57 genes and more than 59 pseudogenes divided among 18 families and 43 subfamilies of genes identified sofar [119]. The general nomenclature of CYP isoforms are designed by the prefix CYP, followed by a number indicating the gene family, a capital letter indicating the subfamily, and the last numeral for the individual gene. The expression of CYP isoforms is cell type- and tissue-specific. Although CYPs are ubiquitous enzymes, they are predominantly expressed in the liver and to a lesser extent in the small intestine, lungs, placenta, and kidneys. The expression of CYP isoforms depends on several factors, notably genetic polymorphisms, epigenetics, sex, age, and disease states [120] Moreover, CYPs can be induced or inhibited by numerous endogenous and exogenous substrates and show a wide interindividual variability in the expression of CYP isoforms. Recent studies of three-dimensional structures of CYPs indicate that their active sites are highly flexible and that binding of the ligand might be related to their plasticity. Therefore, some CYP isoforms can metabolize only one substrate while others can metabolize numerous substrates. Among all CYP isoforms, CYP1A2, 2C9, 2C19, 2D6, 2E1 and 3A4 are the most abundant and they participate together in the biotransformation of 90 percent of drugs [121, 122]. Moreover, in chemotherapy, few CYP enzymes are also responsible for transformation of prodrugs into their active metabolites. For example, the prodrug cyclophosphamide is activated by CYP2B6, CYP2C9 and CYP2C19 and is inactivated by CYP3A4 and CYP3A5. In addition, the role of CYPs is increasingly recognized in tumor formation and progression as well as in tumor prevention and treatment.
26
1.14.1 CYP1 subfamily and CYP1A1
For the sake of simplicity, only CYPs that are members of the subfamily 1 will be discussed. CYP1 are important monooxygenases that were extensively studied in humans and most living species. CYP1A1, 1A2 and 1B1 are the most important in humans [123]. They have been identified for their key role in the transformation of procarcinogens into carcinogens and the transformation of carcinogens into genotoxic metabolites [118]. CYP1A1 and CYP1B1 are predominantly expressed in extrahepatic tissues such as lung, gastrointestinal tract, placenta, and skin, and the majority of these enzymes are infrequently or unexpressed in hepatic tissues in normal conditions [123-125]. They are expressed at a higher level in various cancer cells compared to normal surrounding tissues. A number of studies demonstrated that CYP1A1 and CYP1B1 in breast cancer cells are involved either in the inactivation of antitumor drugs or in the bioactivation of specifically designed prodrugs [126].
CYP1A1 is involved in the metabolism of a wide range of xenobiotics and endogenous substrates as well as in the activation of number of toxins and pollutants [127, 128]. CYP1A1 is recognized as an aryl hydrocarbon hydroxylase, due to its contribution to the metabolic activation of environmental procarcinogens such as polyhalogenated aromatic hydrocarbons and polycyclic aromatic hydrocarbons found, notably in cigarette smoke and grilled/smoked food products [123, 129]. Transcriptional activation of the CYP1A1 gene occurs when the cells are exposed to pollutants [118]. The expression of CYP1A1 is under the control of the nuclear receptor AhR. Studies of the mechanisms of action revealed that pollutants induce CYP1A1 expression by their binding to AhRs present in the cytoplasm. The complex AhR ligand migrates to the nucleus and binds to the aryl hydrocarbon nuclear translocator (ARNT) consequently stimulating the induction of CYP1A1. CYP1A1 is highly inducible when the tissues are expressing AhRs [130-133]. The rate of induction and activation depends on co-regulators and is cell type-dependent [134]. In the human body, AhRs are localized in the liver, lungs, kidneys, placenta, lymphocytes, ovaries, and breast.
1.14.2 Relationship between CYP1A1 and breast cancer cells
27
in bioactivating procarcinogens into carcinogens. Studies of CYP1A1 profile show that it is constitutively expressed in around 90% of breast tumors [135]. Surprisingly, the level of CYP1A1 expression varies among breast tumors and does not correlate with ERα levels [127]. In addition, discrepancies in the levels of CYP1A1 expression have been attributed to ethnic variability in different continents and regions. The expression of CYP1A1 correlates with increased breast cancer risk. Consequently, the level of CYP1A1 expression is considered to be a useful cancer biomarker. Studies on the expression levels of different xenobiotic-metabolizing enzymes, including CYP1A1 with 60 breast tumors from newly diagnosed patients that did not have prior treatment showed that the expression of CYP1A1 was higher in tumor cells vs. the normal surrounding tissues. The expression of CYP1A1 is also higher in premenopausal vs. postmenopausal women, and positively correlates with tumor grade [135, 136]. Due to its overexpression in breast tumors and its involvement in human mutagenicity and carcinogenicity, it worth considering its activity as a potential target for the development of a therapy using anticancer prodrug.
![Figure 1.1. Schematic illustration of angiogenesis and the invasion of cancer into the blood and lymphatic systems to enable invasion and metastasis adapted from cancer research UK uploader [7, 8]](https://thumb-eu.123doks.com/thumbv2/123doknet/3225971.92300/16.918.125.867.107.440/schematic-illustration-angiogenesis-invasion-lymphatic-metastasis-research-uploader.webp)

![Figure 1.2. Schematic female breast anatomy and lobular carcinoma adapted from Terese Winslow image (National Cancer Institute) [17]](https://thumb-eu.123doks.com/thumbv2/123doknet/3225971.92300/19.918.141.819.102.612/figure-schematic-anatomy-lobular-carcinoma-winslow-national-institute.webp)
![Figure 1.3 Classification and receptor status characterization of the 5 main intrinsic breast cancer subtypes [35]](https://thumb-eu.123doks.com/thumbv2/123doknet/3225971.92300/22.918.168.759.485.799/figure-classification-receptor-status-characterization-intrinsic-breast-subtypes.webp)
![Figure 1.4 Molecular structures of (a) tamoxifen and (b) fulvestrant (c), exemestane, (d) letrozole and (e) anastrozole [59]](https://thumb-eu.123doks.com/thumbv2/123doknet/3225971.92300/26.918.134.789.466.747/figure-molecular-structures-tamoxifen-fulvestrant-exemestane-letrozole-anastrozole.webp)
![Figure 1.5 Molecular structures of (a) cyclophosphamide, (b) cisplatin and (c) carboplatin [66]](https://thumb-eu.123doks.com/thumbv2/123doknet/3225971.92300/28.918.141.772.109.223/figure-molecular-structures-cyclophosphamide-b-cisplatin-c-carboplatin.webp)
![Figure 1.9 Schematic representation of microtubule structure and the (a) vincristine-, (b) colchicine- and (c) paclitaxel-binding sites[86]](https://thumb-eu.123doks.com/thumbv2/123doknet/3225971.92300/31.918.158.750.511.976/figure-schematic-representation-microtubule-structure-vincristine-colchicine-paclitaxel.webp)