© Jivan Khlghatyan, 2020
Regulation of glutamatergic neurotransmission,
synaptic plasticity, sleep and behavior by
D2-GSK3B-FXR1
Thèse
Jivan Khlghatyan
Doctorat en neurobiologie
Philosophiæ doctor (Ph. D.)
Québec, Canada
Regulation of glutamatergic neurotransmission, synaptic plasticity,
sleep and behavior by D2-GSK3β-FXR1
Thése
Jivan Khlghatyan
Sous la direction de:
Katalin Toth, directrice de recherche Jean-Martin Beaulieu, co-directeur de recherche
iii
Résumé
Les études GWAS associent les variantes du gène Fxr1 à la schizophrénie, les maladies bipolaires, l’insomnie et la durée du sommeil. Gsk3β peut directement
phosphoryler et ainsi réguler négativement Fxr1. De plus, les interactions fonctionnelles entre Gsk3β et Fxr1 sont associées avec la stabilité émotionnelle chez les humains. Comment Gsk3β-Fxr1 régule l’activité neuronale, la plasticité et le comportement reste inconnu. Gsk3β peut être activé en aval des récepteurs D2 de dopamine. L’activité de Gsk3β peut être modulée par les stabilisateurs d’humeur, les antipsychotiques et les antidépresseurs en régulant des comportements. Néanmoins, les corrélations
neuroanatomiques de Gsk3β en aval des récepteurs D2 restent inexplorées. Nous avons étudié, en premier lieu, les relations de Gsk3β-Fxr1 avec l’activité neuronale et les comportements. Nous avons découvert que Fxr1 et son régulateur négatif Gsk3β affectent les comportements liés à l’anxiété ainsi que la neurotransmission
glutamatergique via la régulation des récepteurs AMPA synaptiques. Deuxièmement, nous avons exploré l’Implication de Gsk3β-Fxr1 dans la plasticité synaptique et le sommeil. Nous avons constaté que Fxr1 est le régulateur central («maître») de la mise à l’échelle synaptique homéostatique. D’ailleurs, il est aussi engage dans l’homéostasie du sommeil et module la force synaptique en régulant les transcripts impliqués dans la synthèse locale des protéines et la structure synaptique. Troisièmement, dans le but de comprendre les corrélations neuroanatomiques nous avons généré une carte des neurones exprimant des récepteurs D2 de tout le cortex et leurs projections. En quatrième lieu, nous avons visé d’investiguer les fonctions de Gsk3β en aval des récepteurs D2 dépendamment de leur emplacement anatomique. L’invalidation
(knockout) intersectoriel de Gsk3β dans les neurones D2 du cortex préfrontal murin par CRISPR/Cas9 nous a permis de révéler sa contribution dans la régulation des
comportements cognitifs, sociaux et de ceux associés à l’humeur. En résumé, cette thèse de doctorat élucide les fonctions de Fxr1 dans le cerveau tout en démontrant l’utilité du CRISPR/Cas9 dans le ciblage génétique ayant pour but d’explorer les fonctions des gènes spécifiquement dans un circuit donné.
iv
Abstract
Variants in Fxr1 gene are GWAS-associated to schizophrenia, bipolar disorders,
insomnia, and sleep duration. Gsk3β can directly phosphorylate and negatively regulate Fxr1. Moreover, functional interaction between Gsk3β and Fxr1 is associated with emotional stability in humans. How Gsk3β-Fxr1 regulates neuronal activity, plasticity and behaviors remains unclear. Gsk3β can be activated downstream of dopamine D2 receptors. Gsk3β activity can be modulated by mood stabilizers, antipsychotics and antidepressants to regulate behaviors. Nevertheless, neuroanatomical correlates of Gsk3β functions downstream of D2 receptors remain elusive. First, we investigated the relationship of Gsk3β-Fxr1 to neuronal activity and behaviors. We discovered that Fxr1 and its negative regulator Gsk3β affect anxiety-related behaviors and glutamatergic neurotransmission via regulation of synaptic AMPA receptors. Second, we addressed the involvement of Gsk3β-Fxr1 in synaptic plasticity and sleep. We discovered that Fxr1 is a master regulator of homeostatic synaptic scaling. Moreover, it is engaged during sleep homeostasis to modulate synaptic strength via regulation of transcripts involved in local protein synthesis and synaptic structure. Third, to understand neuroanatomical correlates of D2 receptor signaling we generated a cortex-wide map of D2 expressing neurons and their projection targets. Fourth, we aimed to understand anatomically defined functions of Gsk3β downstream of D2 receptors. CRISPR/Cas9 mediated intersectional knockout of Gsk3β in D2 neurons of mPFC elucidated its contribution to the regulation of cognitive, social and mood-related behaviors. Overall, this thesis sheds light on brain functions of a GWAS-identified risk gene Fxr1 and shows the utility of intersectional CRISPR/Cas9 mediated genetic targeting for the interrogation of circuit-specific functions of genes.
v Table of contents Résumé ... iii Abstract ... iv List of figures ... xi List of abbreviations ... xv Acknowledgments ... xix Avant-propos ... xx Introduction ... 1 Dopaminergic system... 1
Anatomical correlates of D2 receptors brain function ... 1
D2Rs in SN and VTA ... 2
D1Rs and D2Rs in the striatum ... 2
D1Rs and D2Rs in nucleus accumbens ... 3
D2Rs in cortical areas ... 3
D2Rs in the amygdala ... 4
D2Rs in the thalamus ... 5
D2Rs in the hippocampus ... 5
D2Rs in colliculus ... 6
Gprotein-mediated signaling downstream of D2 receptors ... 6
β-Arrestin-2-AKT-Gsk3β signaling downstream of D2 receptor ... 10
Association of D2-βARR2-GSK3β pathway to mental illnesses and action of drugs ... 11
Regulation of behaviors by GSK3 ... 14
Substrates of GSK3 ... 16
Circadian rhythms ... 16
β-Catenin ... 18
Cytoskeleton ... 18
GSK3 regulates AMPA, NMDA receptors and synaptic plasticity ... 19
Dynamin I ... 20
FXR1 ... 20
FXR1 and other members of the FXR family ... 21
vi
Regulation of synaptic plasticity ... 23
Regulation of homeostatic plasticity ... 27
Homeostatic synaptic scaling ... 27
FMR1 in synaptic scaling ... 29
Regulation of behaviors ... 29
Disease association ... 31
Thesis objectives ... 34
Chapter 1. Results. Mental illnesses associated Fxr1 and its negative regulator Gsk3β are modulators of anxiety and glutamatergic neurotransmission ... 36
1.1. Résumé... 37
1.1. ABSTRACT ... 39
1.2. INTRODUCTION ... 40
1.3. MATERIALS AND METHODS ... 42
Experimental animals ... 42
DNA constructs ... 42
Cell line culture and transfection. ... 43
Genomic DNA extraction and SURVEYOR assay. ... 44
AAV viral particle preparation... 45
Stereotaxic injections ... 45
Acute slice preparation ... 46
Electrophysiology ... 46
Drugs ... 47
Immunofluorescent staining ... 47
Tissue dissection ... 49
Synaptosome isolation and western blot ... 49
Chemogenetic inhibition ... 51
Behavioral tests ... 51
Quantification and statistical analysis ... 52
1.4. RESULTS... 54
1.4.1. CRISPR/Cas9 mediates efficient somatic knockout of Gsk3b ... 54
1.4.2. Medial prefrontal cortex specific overexpression of Fxr1 or Gsk3b somatic knockout result in reduced anxiety-related behaviors ... 56
vii
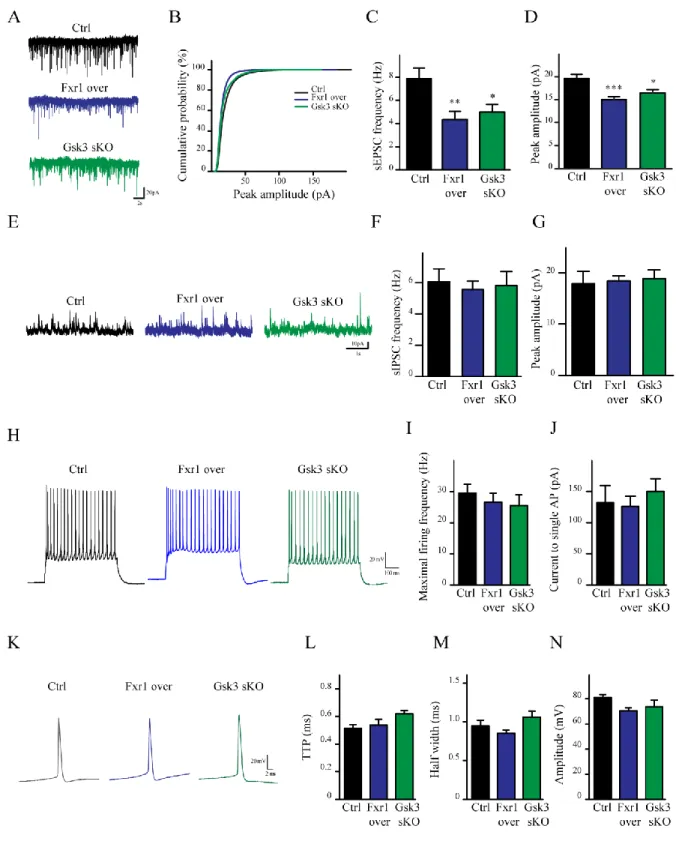
1.4.3. Prefrontal overexpression of Fxr1 or Gsk3b sKO reduce excitatory synaptic currents ... 57
1.4.4. KORD mediated silencing of mPFC pyramidal neurons reduce anxiety-related behaviors ... 57
1.4.5. Prefrontal overexpression of Fxr1 and Gsk3b sKO does not affect spine density ... 58
1.4.6. Prefrontal overexpression of Fxr1 or Gsk3b sKO alters AMPA receptor mediated currents ... 59
1.4.7. Prefrontal overexpression of Fxr1 or Gsk3b sKO affect components of the glutamatergic synapse ... 60
1.5. DISCUSSION ... 62
1.6. Figures ... 73
1.7. Supplementary Material ... 87
Chapter 2. Results. Fxr1 regulates sleep and synaptic homeostasis ... 90
2.1. Résumé... 91
2.1. Abstract ... 92
2.2. Main Text ... 93
2.3. References and Notes: ... 100
2.4. Figures ... 103
2.5. Supplementary Materials... 111
2.5.1 Materials and Methods ... 112
Experimental model and subject details ... 112
Experimental animals ... 112
Primary cultures ... 112
DNA constructs ... 113
AAV viral particle preparation... 114
Cell line culture and transfection ... 114
Genomic DNA extraction and SURVEYOR assay ... 114
Primary cortical culture transfection and infection ... 115
Stereotaxic injections ... 116
Surgeries for EEG recordings ... 116
EEG recording and analyses ... 117
Statistical analyses for EEG recordings ... 118
Electrophysiology recordings and drugs ... 119
Acute slice preparation ... 119
viii
Drugs ... 120
Analysis of electrophysiology recordings ... 120
Immunofluorescent staining ... 121
Western blot ... 122
Sleep deprivation ... 123
Immunoprecipitation of polyribosomes and RNA isolation ... 124
RT-PCR qPCR ... 125
RNAseq analysis ... 125
Quality control ... 125
Processing Pipeline ... 125
Differential Expression Analysis ... 126
Gen set enrichment analysis ... 127
Quantification and statistical analysis ... 128
2.5.2. Supplementary Text ... 128
2.5.3. Supplementary Figures ... 129
Chapter 3. Results. High sensitivity mapping of cortical dopamine D2 receptor expressing neurons ... 143
3.1. Résumé... 144
3.1. Abstract ... 145
3.2. Introduction ... 146
3.3. Material and Methods ... 149
Animals ... 149
AAV viruses ... 149
Mouse stereotaxic surgery... 149
Tissue dissection ... 150
Immunoprecipitation of polyribosomes and RNA isolation ... 150
cDNA synthesis and quantitative RT-PCR ... 151
Affymetrix Mouse Gene 2.0 ST array ... 152
Immunohistochemistry ... 153
RNAscope in situ hybridization ... 154
Statistical analysis ... 154
3.4. Results ... 155
ix
3.4.2. Expression of Cre recombinase in Drd2 cells during adulthood ... 156
3.4.3. Drd2 mRNA is expressed in multiple cortical regions ... 157
3.4.4. Molecular heterogeneity of Drd2 cells across different cortical regions. ... 159
3.4.5. Cellular heterogeneity of Drd2 clusters in different cortical regions ... 160
3.4.6. The dopaminergic input of Drd2 cells from different cortical regions ... 161
3.4.7. Projection targets of Drd2 cells from different cortical regions ... 162
3.5. Discussion ... 165
3.6. References ... 171
3.7. Figures ... 177
3.8. Supplementary Figures ... 191
Chapter 4. Results. CRISPR/Cas9 mediated intersectional knockout of GSK3β in D2 receptor expressing mPFC neurons reveals contributions to emotional regulation ... 199
4.1. Résumé... 200
4.1. Abstract ... 202
4.2. Introduction ... 203
4.3. Materials and Methods ... 206
Animals ... 206
Mouse stereotaxic surgery and AAV viruses... 206
Immunohistochemistry ... 207
Drugs ... 208
Data analysis and statistics ... 208
4.4. Results ... 210
4.4.1. Efficient knockout of GSK3β in mPFC D2 neurons of adult mice ... 210
4.4.2. Gsk3sKO in mPFC D2 neurons alters gene expression ... 211
4.4.3. Gsk3sKO in mPFC D2 neurons exerts an anxiolytic-like effect on mouse behavior ... 213
4.4.4. Gsk3β in mPFC D2 neurons is implicated in cognitive and social behaviors ... 214
4.4.5. Gsk3β in mPFC D2 neurons contributes to resilience ... 214
4.5. Discussion ... 217
4.6. References ... 223
4.7. Figures ... 229
4.8. Supplementary Material ... 239
x
Cell line culture, transfection and western blot ... 239
Behavioral tests ... 240
Open field test (OFT) ... 240
Dark-light emergence test (DLET) ... 240
Elevated plus maze (EPM) ... 240
Behavioral Z scoring ... 241
Novel object recognition (NOR) ... 241
Preference for social novelty ... 241
Free social interaction ... 242
Tail suspension test (TST) ... 242
Unpredictable chronic mild stress (UCMS) ... 243
Tissue dissection, Immunoprecipitation of polyribosomes and RNA isolation ... 243
cDNA synthesis and quantitative RT-PCR ... 244
4.8.2. Supplementary tables ... 245
4.8.3. Supplementary figures ... 250
4.8.4.Supplementary references... 254
Discussion... 255
Future perspectives ... 256
Functional studies of genetic risk factors for mental disorders in mice ... 256
Homeostatic synaptic scaling ... 258
Studying circuit specific role of genes ... 259
Conclusion ... 261
xi
List of figures
Introduction
Figure 1 Signaling downstream of dopamine D2 receptor 7
Figure 2 Gαi/o and Gβγ mediated signaling downstream of dopamine D2
receptor
8
Figure 3 The action of drugs on D2 receptor signaling 13
Figure 4 GSK3 targets and their potential contribution to neuronal functions 16
Figure 5 Integration of dopamine receptor D2 mediated signaling and glutamatergic neurotransmission by FXR1
23
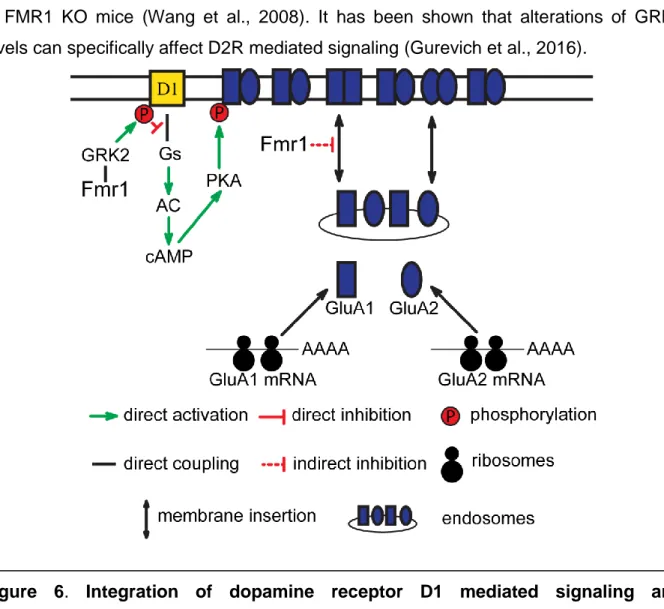
Figure 6 Integration of dopamine receptor D1 mediated signaling and glutamatergic neurotransmission by FMR1
25
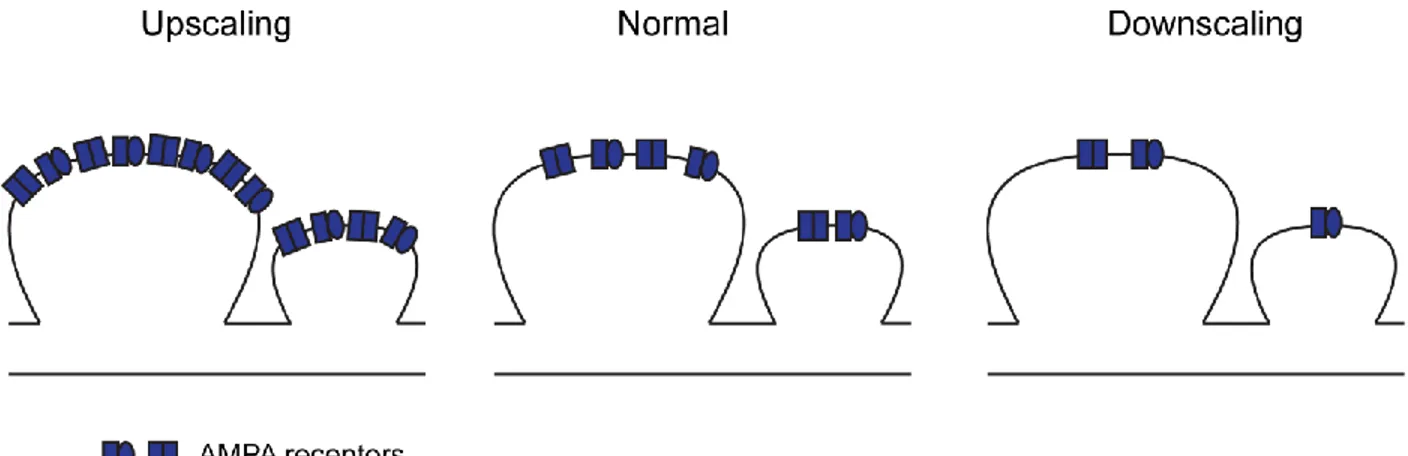
Figure 7 Homeostatic synaptic scaling of the excitatory postsynaptic compartment
27
Chapter 1
Figure 1 CRISPR/Cas9 mediated somatic knockout (sKO) of Gsk3b in medial prefrontal cortex (mPFC)
72
Figure 2 Prefrontal CRISPR/Cas9 mediated Gsk3b sKO or Fxr1 overexpression modulate mood related behaviors
74
Figure 3 Prefrontal CRISPR/Cas9 mediated Gsk3b sKO or Fxr1 overexpression modulate the spontaneous neuronal activity
76
Figure 4 KORD mediated silencing of mPFC pyramidal neurons modulate anxiety related behavior
78
Figure 5 Prefrontal CRISPR/Cas9 mediated Gsk3b sKO or Fxr1 overexpression have no effect on spine density
79
Figure 6 Prefrontal CRISPR/Cas9 mediated Gsk3b sKO or Fxr1 overexpression affect AMPA current
81
Figure 7 Prefrontal CRISPR/Cas9 mediated Gsk3b sKO or Fxr1
overexpression affect pre- and postsynaptic components of the
xii glutamatergic synapse
Figure S1 Knockout of Gsk3b by CRISPR/Cas9 86
Figure S2 Cas9 expression does not alter anxiety-related behaviors 87
Chapter 2
Fig. 1 Fxr1 is a central component of homeostatic synaptic upscaling 102
Fig. 2 Fxr1 modulates sleep duration and recovery after sleep deprivation 104
Fig. 3 Fxr1 regulates synaptic strength during sleep deprivation 106
Fig. 4 Neuronal translatome regulation by Fxr1 during sleep deprivation 108
Fig. S1 Expression of Fxr2, Fmr1 and GluA2 during synaptic scaling 128
Fig. S2 Fxr1 positively regulates expression of GluA1 130
Fig. S3 CRISPR/Cas9 mediated knockout of Gsk3b and Fxr1 in primary neurons
131
Fig. S4 The decrease in Fxr1 expression induces multiplicative upscaling 133
Fig. S5 Wakefulness and sleep duration and time course of EEG spectral activity
135
Fig. S6 Expression of Fxr1, Fxr2, Fmr1 and synaptic activity changes during sleep deprivation
136
Fig. S7 Pathway enrichment analysis of transcripts that are affected by sleep deprivation and Fxr1
138
Chapter 3
Figure 1 Distribution of Drd2 cell clusters in multiple cortical regions 176
Figure 2 Cortical clusters of HA+ cells express Cre during adulthood 177
xiii
Figure 4 Transcriptomic heterogeneity of Drd2 cells from different cortical regions
181
Figure 5 Drd2 expressing cells throughout the cortex 183
Figure 6 Dopaminergic projections in cortical Drd2 clusters 185
Figure 7 Projections of mPFC Drd2 neurons 187
Figure 8 Schematic representation of projections of Drd2 expressing cortical neurons
189
Suppl. Figure 1
Drd2 cells in the cortex revealed by D2GFP reporter mice 190
Suppl. Figure 2
Immunofluorescent staining for HA and different interneuron markers 191
Suppl. Figure 3
Projections of Drd2 neurons of Claustrum/Insula anterior 192
Suppl. Figure 4
Projections of Drd2 neurons of Claustrum/Insula medial 193
Suppl. Figure 5
Projections of Drd2 neurons of Claustrum/Insula posterior 194
Suppl. Figure 6
Projections of Drd2 neurons of Claustrum/Insula somatosensory cortex
195
Suppl. Figure 7
Projections of Drd2 neurons of Claustrum/Insula auditory cortex 196
Suppl. Figure 8
Projections of Drd2 neurons of Claustrum/Insula visual cortex 197
Chapter 4
Fig. 1 Knockout of GSK3β in D2 neurons of mPFC 228
Fig. 2 GSK3β of mPFC D2 neurons regulates gene expression 230
xiv
Fig. 4 GSK3β of mPFC D2 neurons contributes to cognitive and social
behaviors
234
Fig. 5 GSK3β knockout in mPFC D2 neurons abolishes UCMS induced
hyperactivity and depressive-like behavior
236
Suppl. Figure 1
Cas9 expression in mPFC D2 neurons 249
Suppl. Figure 2
Enrichment of common genes of Gsk3 DKO vs translatome of mPFC D2 in pathways and behavioral phenotypes
250
Suppl. Figure 3
Enrichments of transcripts in major cell types of frontal cortex. 251
Suppl. Figure 4
xv
List of abbreviations
DA Dopamine
D1 to D5 Dopamine receptor D1 to D5
SN Substantia nigra
VTA Ventral tegmental area
MSN Medium spiny neuron
NAc Nucleus accumbens
mPFC Medial prefrontal cortex
BLA Basolateral amygdala
CeA Central amygdala
PVN Paraventricular nucleus
fMRI Functional magnetic resonance imaging
PET Positron emission tomography
GABA Gamma-aminobutyric acid
GPCR G-protein coupled receptor
cAMP Cyclic adenosinemonophosphate
GRK G-protein coupled receptor kinase
PKA Protein kinase A
CREB cAMP response element-bindingprotein
AMPA Alpha-Amino-3-Hydroxy-5-Methyl-4-Isoxazole Propionic Acid
NMDA N-Methyl-D-aspartic acid
DARPP32 Dopamine- and cAMP-regulated phosphoprotein
AC Adenylate cyclase
PP2A Protein phosphatase subunit 2A
GSK3 Glycogene synthase kinase 3
βARR2 Β Arrestin 2
PP2B Protein phosphatase subunit 2B
CDK5 Cycline dependent kinase 5
CK1, 2 Casein kinase 1, 2
GIRK G-protein-gated inwardly rectifying potassium channel
PLC Phospholipase C
DAT Dopamine transporter
GWAS Genome wide association study
SZ Schizophrenia
BD Bipolar disorder
BRET Bioluminescence resonance energy transfer
SSRI Selective serotonin reuptake inhibitors
HET Heterozygous
KO Knockout
KI Knockin
AAV Adeno-associated virus
5HT 5-hydroxytriptamine
SCN Suprachiasmatic nucleus
TCF T cell factor/lymphoid enhancer factor
xvi
MAP Microtubule associated protein
CRMP Collapsin response mediator proteins
LTD Long-term depression
LTP Long-term potentiation
GluA1 AMPA receptor subunit 1
GluA2 AMPA receptor subunit 2
NR1 NMDA receptor subunit 1
NR2B NMDA receptor subunit 2B
ADBE Activity-dependent bulk endocytosis
FXR1 Fragile X mental retardation syndrome-related protein 1
FXR2 Fragile X mental retardation syndrome-related protein 2
FMR1 Fragile X mental retardation 1
FXG Fragile X granules
UTR Untranslated region of mRNA
mGluR Metabotropic glutamate receptor
TTX Tetrodotoxin
PTX Picrotoxin
BIC Bicuculline
SNP Single nucleotide polymorphism
sQTL Splicing quantitative trait loci
DLPFC Dorsolateral prefrontal cortex
Mir MicroRNA
CRISPR Clustered regularly interspaced short palindromic repeats
vGlut Vesicular glutamate transporter
PCR Polimerase chain reaction
SYN Synapsin promoter
GFP Green fluorescent protein
ACSF Artificial cerebrospinal fluid
PFA Paraformaldehyde
PBS Phosphate buffer saline
DREADD Designer receptors exclusively activated by designer drugs
KORD κ-opioid derived DREADD
OFT Open field test
DLET Dark-light emergence test
EPM Elevated plus maze test
NOR Novel object recognition test
TST Tail suspension test
UCMS Unpredictable chronic mild stress
sgRNA Single guide RNA
EPSC Excitatory postsynaptic current
IPSC Inhibitory postsynaptic current
SalB Salvinorin B
CNO Clozapine-N-oxide
EEG Electroencephalogram
EMG Electromyogram
xvii CDS Coding sequence SD Sleep deprivation S Sleep BL Baseline REC Recovery WAKE Wakefulness
SWS Slow wave sleep
PS Paradoxical sleep
DET Differentially expressed transcripts
GO Gene ontology
BP Biological pathways
CC Cellular components
N2A Neuroblastoma 2A cells
DIV Days in vitro
RT-qPCR Reverse transcription quantitative PCR
FDR False discovery rate
STR Striatum
PV Parvalbumin
BAC Bacterial artificial chromosome
CL Claustrum
Ant Anterior
Med Medial
Post Posterior
SS Somatosensory cortex
AUD Auditory cortex
VIS Visual cortex
HA Hemagglutinin THAL Thalamus HIP Hippocampus SPT Septum HYP Hypothalamus EP Endipyriform Ent Entorinal SST Somatostatin Calb Calbindin NPY Neuropeptide Y Cck Cholecystokinin
Vip Vasoactive intestinal polypeptide
TH Tyrosone hydroxylase
Pir Piriform cortex
AMY Amygdala
DEn Dorsal endopiriform nucleus
VEn Ventral endopiriform nucleus
Ect Ectorhinal cortex
xviii
xix
Acknowledgments
I first thank my supervisor Dr. Jean-Martin Beaulieu for his support scientifically and financially. I enjoyed every moment of my PhD, our interactions and discussions. Thanks for exposing me to the scientific world at large and sharing your personal experience with me. You have contributed tremendously to the development of my independence and confidence as a scientist. Thanks Martin for making my PhD so exciting.
I also thank my co-supervisor Dr. Katalin Toth for her support at the most difficult stages of my PhD. For her help during transition period of my PhD and for making sure I get to have a second chance. Thank you for inspiring comments after my meetings with the evaluation committee. Thank you for everything Katalin.
I also thank members of my PhD evaluation committee Dr. Paul De Koninck, Dr. Jean-Pierre Julien and Dr. Keith Murai for valuable comments.
I thank students who contributed to my research, Clementine Quintana, Alesya Evstratova, Simon Chamberland, Lusine Bozoyan, Alexandra Marakhovskaia, Tiago Soares Silva, Arash Bahremand. I also thank members of our lab, Ghazal Fakhfouri, Camille Latapy, Pavel Pawlowski, Abygael St-Pierre and Lakshmi Rajakrishnan.
I thank my friends that I met in Quebec, Filippo, Sofia, Vincent, Lynda,
Charalampos, Helia, Alex, Yanina, Delphine, Archana, Karen, Vahe, Anush and Sona. I thank my friends that I met in Toronto, Kasia, Dave and Kurt.
I thank my family in Armenia for emotional support. My parents Suren and Seda, my grandmother Larisa and my sister Arpine.
And finally I thank my family in Canada. My wife Lusine, she has been my biggest supporter and my biggest critic. No doubt, without her my PhD journey would have been impossible. I also thank our kids for giving another meaning to my life and making the time out of the lab so much fun.
xx
Avant-propos
The first part of the results of this thesis is published in the journal Frontiers in
Molecular Neuroscience in April 2018. Several students were involved in this study and
I am the first author. I performed design and testing of CRISPR/Cas9 in vitro and in
vivo, stereotaxic injections, brain dissections and synaptosome preparations, protein
expression analysis in vitro and in vivo, mouse behaviors, spine counting and data
analysis. Alesya Evstratova and Simon Chamberland performed whole cell patch clamp
recordings and data analysis. Alexandra Marakhovskaia performed CRISPR/Cas9 KO
experiments with puromycin selection followed by detection of Gsk3β and Fxr1 expression. Arash Bahremand performed behaviors of KORD injected mice.
The second part of the results of this thesis is submitted and is currently under
revision. Several students were involved in this study and I am the first author. I
performed design and testing of CRISPR/Cas9 in vitro and in vivo, stereotaxic
injections, protein expression analysis in vitro and in vivo, primary neuronal culture
preparation, drug treatment and receptor surface staining and quantification, sleep
deprivation, RiboTag IP and RNA extraction and RNAseq data analysis. Alesya
Evstratova and Simon Chamberland performed whole cell patch clamp recordings and
data analysis. Alexandra Marakhovskaia performed CRISPR/Cas9 KO experiments with
puromycin selection followed by detection of Fxr1 expression. Lusine Bozoyan
performed cloning and luciferase assay. Valerie Mongrain performed EEG recordings
and analysis. Alexandra Marakhovskaia and Tiago Soares Silva participated in mouse
xxi
The third part of the results of this thesis is published in the journal Cerebral
Cortex in October 2018. I and Clementine Quintana are co-first authors of the paper and
we have equal contributions to the paper.
The fourth and last part of the results of this thesis is submitted and is currently
1
Introduction
Dopaminergic system
Dopamine (DA) is one of the major monoamine neurotransmitters in the brain. Dopamine is produced in dopamine neurons located in certain nuclei of the brain named A8, A9, and A10 (Beaulieu and Gainetdinov, 2011). Dopamine neurons send projections to multiple brain regions. There are four major dopaminergic pathways in the mammalian brain: nigrostriatal, mesolimbic, mesocortical and tuberoinfundibular. Physiological actions of dopamine are mediated via dopamine receptors. On the basis of structural, pharmacological, and biochemical properties, dopamine receptors are divided into two classes, D1-class (D1 and D5) and D2-class (D2, D3, and D4). It is commonly accepted that the D1-class dopamine receptors activate the Gαs/olf family of G proteins to stimulate cAMP production by adenylate cyclase (AC), while the D2-class dopamine receptors couple to the Gαi/o family of G proteins and thus induce inhibition of AC (Kebabian and Calne, 1979; Kebabian and Greengard, 1971; Ohara et al., 1988). Dopamine receptors have a wide expression pattern in the brain and thus regulate various functions based on their anatomical and cellular localization (Beaulieu and Gainetdinov, 2011).
Anatomical correlates of D2 receptors brain function
D2 dopamine receptors have been shown to be expressed in the striatum, nucleus accumbens, olfactory tubercle, substantia nigra (SN), ventral tegmental area (VTA), hypothalamus, cortical areas, septum, amygdala, and hippocampus (Gaspar et al., 1995; Gerfen, 2000; Missale et al., 1998; Santana et al., 2009; Tritsch and Sabatini, 2012; Vincent et al., 1993). D2 receptors are involved in a variety of central nervous system functions including movement, feeding, affect, reward, sleep, attention, decision making, working memory, and learning (Beaulieu and Gainetdinov, 2011). Regulation of brain function by D2 receptors is most likely based on their brain regions specific and cell type-specific expression as well as subcellular localization. Thus, precise knowledge of expression in different brain regions and cell types is crucial for the understanding of D2Rs function.
2
D2Rs in SN and VTA
D2Rs are expressed in somas and dendrites of dopamine neurons in VTA and SN as well as in their axonal terminals in projection areas (Beaulieu and Gainetdinov, 2011; Missale et al., 1998). D2Rs are autoreceptors in dopamine neurons and play a feedback-regulatory role. D2 autoreceptors can modulate the activity of dopamine neurons through the activation of a potassium conductance and through control of the expression of tyrosine hydroxylase and the dopamine transporter. Activation of these receptors decreases both the excitability of dopamine neurons and the release of dopamine (Ford, 2014). Transgenic mice with specific ablation of D2 autoreceptors show hyperactivity and increased sensitivity to cocaine (Anzalone et al., 2012; Bello et al., 2011). Specific knockdown of D2 autoreceptors in adult rats indicated the involvement of these receptors in incentive motivation (de Jong et al., 2015). In line, human studies indicate that altered autoreceptor functions correlate with changes in impulsivity and novelty seeking behaviors (Buckholtz et al., 2010; Zald et al., 2008). Overall, these and other studies highlight that activation of D2 autoreceptors decrease locomotion and alters reinforcing properties of drugs of abuse (Ford, 2014).
D1Rs and D2Rs in the striatum
Dopamine receptors in striatum are expressed on medium spiny neurons (MSN). Using transgenic mice it has been shown that D2R and D1R expressing medium spiny neurons represent two distinct populations of neurons and can be defined by expression markers and projection sites. D1R expressing neurons express dynorphin and project to medial globus pallidus and the substantia nigra pars reticulata, so-called direct pathway. This pathway is called direct, since its excitation has the net effect of exciting thalamic neurons (which in turn make excitatory connections onto cortical neurons) and fascilitating motor programs. D2R expressing neurons express enkephaline and projects to lateral globus pallidus and subthalamic nucleus, so-called indirect pathway. Excitation of the indirect pathway has the net effect of inhibiting thalamic neurons (rendering them unable to excite motor cortex neurons) thus inhibiting execution of motor program (Beaulieu and Gainetdinov, 2011). There are also MSNs that express
3
both D2Rs and D1Rs. Their proportions are low in the dorsal striatum (5%) but may reach to 20-25% in nucleus accumbens (Beaulieu and Gainetdinov, 2011). The projection targets of these D1R/D2R MSNs are not investigated.
Specific optogenetic stimulation of D2 MSNs resulted in freezing and decreased locomotion and conversely, D1 MSN stimulation led to increased locomotion (Kravitz et al., 2010). It is important to note that direct and indirect pathways can interact to modulate behavior. It has been shown that D1R MSN activation causes reinforcement and D2R MSN activation induces punishment (Kravitz et al., 2012). Moreover, the direct pathway is shown to be involved in reward learning and sensitization, whereas the indirect pathway is implicated in aversive behavior (Hikida et al., 2010). Overall, findings suggest the role of D2R of the striatum in the regulation of movement, reward, punishment, and behavioral sensitization.
D1Rs and D2Rs in nucleus accumbens
Nucleus acumbens (NAc) mostly (95% of cells) consists of D1R and D2R expressing MSNs. It consists of NAc core and shell, receives dopaminergic input from VTA and sends projections to ventral pallidum, VTA and SN. D2Rs of NAc are involved in the regulation of several brain functions. Optogenetic stimulation of D2R expressing MSNs in NAc is shown to increase motivation by decreasing inhibitory transmission in the ventral pallidum (Gallo et al., 2018; Soares-Cunha et al., 2016; Soares-Cunha et al., 2018). Pharmacological activation of dopamine receptors revealed that activation of both D2 and D1 receptors in NAc have a cooperative effect on reward (Ikemoto et al., 1997). Suppression of D2R MSN activity in NAc impaired reversal learning (Macpherson et al., 2016). Overall, the major role of D2Rs in NAc includes regulation of motivation and reward.
D2Rs in cortical areas
Early studies using radioactive labeling showed a distribution of dopamine D2 receptors throughout the cerebral cortex (Lidow et al., 1989). D2Rs have been shown to be expressed in the medial prefrontal cortex (Vincent et al., 1993) and several studies also indicated expression in auditory cortex (Kudoh and Shibuki, 2006) and visual
4
cortex (Papadopoulos et al., 1989; Phillipson et al., 1987). D2Rs have been shown to be expressed in pyramidal neurons and interneurons (Gaspar et al., 1995; Santana et al., 2009; Tritsch and Sabatini, 2012). D2 receptors can be expressed in postsynaptic compartments of pyramidal neurons as well as on their axonal terminals in the site of projections, such as striatum (Wang and Pickel, 2002) and amygdala (Pinto and Sesack, 2008). mPFC D2R neurons receive dopaminergic input from VTA (Carr et al., 1999). It has been shown that mPFC is also innervated by locus coeruleus projections that co-release noradrenalin and dopamine (Devoto et al., 2005a, b). Dopaminergic projections are also found in auditory and visual cortex in lamina V, VI and I of rats and humans (Kudoh and Shibuki, 2006; Papadopoulos et al., 1989; Phillipson et al., 1987).
mPFC D2Rs can regulate the activity of pyramidal neurons (Gee et al., 2012; Wang and Goldman-Rakic, 2004) as well as interneurons (Tseng and O'Donnell, 2004). D2R antagonist infusion into mPFC abolished auditory fear memory extinction. Thus indicating D2R activity in mPFC may be involved in the regulation of fear memory (Dadkhah et al., 2018). Prefrontal D2R stimulation improved working memory-related neuronal circuit dynamics in monkeys (Ott and Nieder, 2017). Infusion of D2R antagonist into rat mPFC impaired executive function in set shifting behavioral paradigm (Floresco et al., 2006). Modulation of D2R activity in PFC-BLA circuit revealed the involvement of this receptor in the process of decision making (Jenni et al., 2017). Overall, this shows that mPFC D2R contribute to memory-related tasks, cognitive flexibility, and decision making.
The role of D2R in other cortical regions is not well characterized. Using dopamine projection lesion and pharmacological inhibition it has been shown that D2Rs in auditory cortex are important for sound sequence discrimination learning in rats (Kudoh and Shibuki, 2006).
D2Rs in the amygdala
Pharmacological stimulation or blockade of D2Rs in the basolateral amygdala (BLA) attenuated physiological and behavioral responses to stress in rats. D2R agonist quinpirole infusion in BLA facilitated fear extinction, conversely infusion of D2R antagonist sulpride impaired fear extinction (Shi et al., 2017). Bidirectional modulation of
5
D2R signaling in extended amygdala regulates consolidation of fear responses. Blockade of D2R induces generalized threat responses, while its stimulation facilitates discriminative learning between stimuli representing safety or threat (De Bundel et al., 2016). D2R expression in neurons of the central amygdala (CeA) is involved in the regulation of impulsive behaviors. Using optogenetic manipulation of the activity of CeA D2R expressing neurons it was found that specific input to bed nucleus of the stria terminalis and not to VTA is important in the regulation of impulsivity (Kim et al., 2018). Overall, studies indicate the role of amygdala D2Rs in the regulation of fear, impulsive behaviors, and stress response.
D2Rs in the thalamus
Studies of D2-like receptors (D2R, D3R and D4R) in human thalamus by autoradiographic techniques and positron emission tomography revealed the heterogeneous distribution of the receptor. Relatively high densities of D2-like receptor was revealed in intralaminar and midline thalamic nuclei, including the paraventricular, parataenial, paracentral, centrolateral, and centromedian/parafascicular nuclei (Rieck et al., 2004). Mouse studies also showed expression of D2Rs in the thalamus, particularly enriched within the paraventricular nucleus (PVN) (Clark et al., 2017). Manipulation of D2R expression specifically in D2R expressing neurons of PVN uncovered their specific role in the regulation of locomotor sensitization to cocaine, without affecting anxiety, fear and sensimotor gating (Clark et al., 2017). In a mouse model of schizophrenia-associated 22q11 deletion syndrome, the aberrant elevation of D2R expression was detected in thalamus-auditory cortex glutamatergic projections neurons. Altered expression of D2Rs in this projection neurons caused deficient acoustic startle response similar to what is observed in schizophrenia patients. Moreover, the increase of D2R expression renders thalamocortical projection neurons sensitive to antipsychotic treatment that may explain the effect of drugs on auditory function related symptoms in schizophrenia (Chun et al., 2014).
6
Using transgenic mouse models and immunolabeling it has been shown that D2Rs are expressed in glutamatergic hilar mossy cells as well as in different interneuron subtypes of the hippocampus (Puighermanal et al., 2015). The specific role of hippocampal D2Rs is not well characterized. One study using fMRI and PET imaging in healthy human subjects uncovered the relation between hippocampal D2R availability and episodic memory (Nyberg et al., 2016). D2Rs in the hippocampus are also shown to be involved in the acquisition of morphine-induced place preference and that the antagonism of D2Rs in the hippocampus can reduce the rewarding properties of morphine (Assar et al., 2016; Katebi et al., 2018).
D2Rs in colliculus
Using RNAseq analysis high expression of D2Rs and D1Rs were identified in the superior colliculus of rats. Interestingly, these dopamine receptor expressions were segregated to distinct locations, D1Rs were enriched in superficial layers, while D2Rs were enriched in the intermediate multimodal/motor superior colliculus. Cell type expression was also different. 65% of D2R expressing cells were glutamatergic neurons and 35% were GABAergic interneurons. This was different from D1R expressing cells, where the majority (70%) of cells was GABAergic. Surprisingly, major dopamine cell groups (in area A8, A9, and A10) did not project to the superior colliculus. Instead, using retrograde labeling it was identified that A13 cell group in zona inerta provides dopamine input to the superior colliculus. Electrophysiological recordings revealed the inhibitory role of dopamine on the activity of D2R expressing cells (Bolton et al., 2015). In line with this finding, it has been shown that pharmacological modulation of D2R receptors activity in superior and inferior colliculus can impact unconditioned (innate) fear in rodents (de Oliveira et al., 2014; Muthuraju et al., 2016).
Gprotein-mediated signaling downstream of D2 receptors
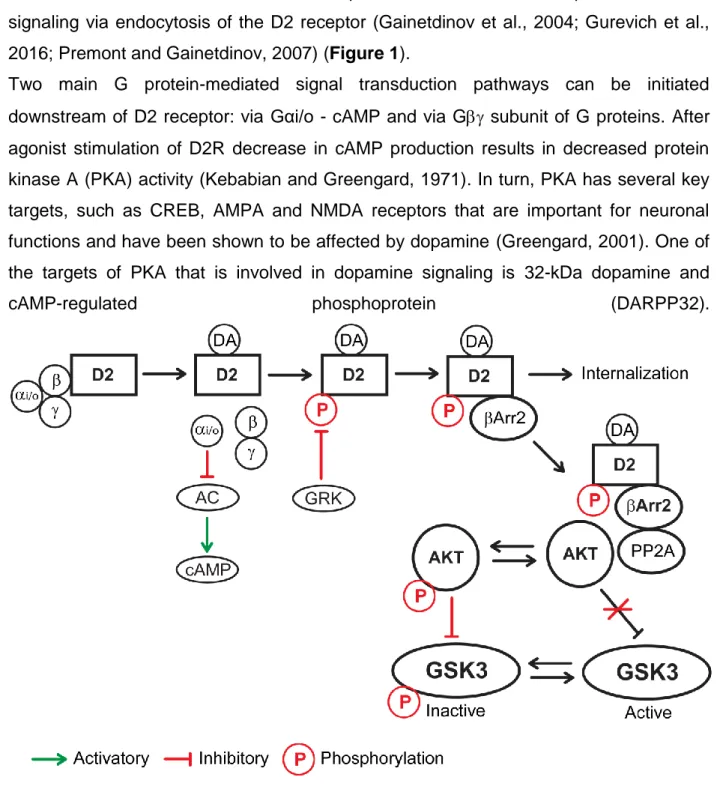
The D2 receptor is a G-protein coupled receptor (GPCR). Upon stimulation, D2 receptors uncouple from αi/o, and subunit of G proteins, this results to inactivation of AC by Gαi/o and decrease of cAMP production (Kebabian and Calne, 1979; Kebabian and Greengard, 1971). Activated D2 receptors can be phosphorylated by G
protein-7
coupled receptor kinases (GRKs). This phosphorylation makes the D2 receptor a target for the recruitment of the multifunctional adaptor proteins, termed arrestins. Recruited arrestins then can bind to clathrin and promote inactivation of G protein-mediated signaling via endocytosis of the D2 receptor (Gainetdinov et al., 2004; Gurevich et al., 2016; Premont and Gainetdinov, 2007) (Figure 1).
Two main G protein-mediated signal transduction pathways can be initiated downstream of D2 receptor: via Gαi/o - cAMP and via G subunit of G proteins. After agonist stimulation of D2R decrease in cAMP production results in decreased protein kinase A (PKA) activity (Kebabian and Greengard, 1971). In turn, PKA has several key targets, such as CREB, AMPA and NMDA receptors that are important for neuronal functions and have been shown to be affected by dopamine (Greengard, 2001). One of the targets of PKA that is involved in dopamine signaling is 32-kDa dopamine and
cAMP-regulated phosphoprotein (DARPP32).
8
Dopamine receptor D2 can signal via G protein-mediated or Arr2 mediated mechanisms. During G protein-mediated signaling stimulation of D2 results to inactivation of AC and decrease of cAMP. During Arr2 mediated signaling a complex consisting of Arr2, AKT, and PP2A form, which results in dephosphorylation and inactivation of AKT. This, in turn, lifts inhibitory phosphorylation of GSK3 by AKT, resulting in an increase of GSK3 activity.
It has been shown that stimulation of D2Rs results in a reduction of phosphorylation of DARPP32 at threonine 34. It can be a result of reduction of PKA activity (Bateup et al., 2008) or dephosphorylation of DARPP32 by calmodulin-dependent protein phosphatase 2B (PP2B) that is activated after stimulation of D2Rs (Nishi et al., 1997). It is important to mention that phospho-34 DARPP32 has protein phosphatase 1 (PP1) inhibitory function. DARPP32 can be phosphorylated in different positions by cyclin-dependent kinase 5 (CDK5), casein kinase 1 and 2 (CK1 and CK2) and can be dephosphorylated by PP2A (Beaulieu and Gainetdinov, 2011) (Figure 2). Thus, DARPP32 can serve as an integrator of several upstream signaling pathways, since those kinases and phosphatases are activated by different neurotransmitters and hormones.
9
G protein signaling downstream of dopamine receptor D2 can be mediated via Gαi/o and Gβγ. Gαi/o regulates the activity of PKA and its targets such as CREB, AMPA, NMDA, and DARPP32. Gβγ can regulate PLC and intracellular calcium, L, N-type Calcium channels and GIRK channels.
Using DARPP32 knockout mice or transgenic mice lacking threonine-34 it has been shown that DARPP32 is one of the players involved in the regulation of dopamine-dependent behaviors (Beaulieu and Gainetdinov, 2011).
After agonist stimulation of D2R G subunit dissociates from Gαi/o subunit and can impact several downstream events. G subunits have been shown to activate phospholipase C (PLC) and increase intracellular calcium (Hernandez-Lopez et al., 2000). G also reduces the activity of L-type and N-type calcium channels (Yan et al., 1997). Gcan also regulate G protein-coupled inwardly rectifying potassium channels (GIRKs) (Kuzhikandathil et al., 1998; Lavine et al., 2002) (Figure 2). Activation of D2 receptors results in activation of GIRKs that have an inhibitory role for neuronal activity (Beaulieu and Gainetdinov, 2011). Overall, D2R stimulation can affect neuronal functions via G-protein mediated downstream events that involve kinases, calcium and ion channels.
Several lines of research focused on investigating whether abnormalities in G protein-mediated signaling downstream of D2 receptors can be involved in neuropsychiatric disorders. It has been reported that protein and mRNA levels of DARPP-32 are decreased in the dorsolateral prefrontal cortex and leukocytes of patients with schizophrenia or bipolar disorder (Albert et al., 2002; Ishikawa et al., 2007; Torres et al., 2009). Genetic evidence implicates DARPP-32 in human frontostriatal structure, function, and cognition (Meyer-Lindenberg et al., 2007). Early studies indicated a possible genetic association of PP2B to schizophrenia, bipolar disorder, and drugs of abuse (Beaulieu and Gainetdinov, 2011). However, PP2B, DARPP32 and other genes in this signaling pathway have not been identified to be associated to neuropsychiatric disorders in genome-wide association studies. Further preclinical and clinical investigations are needed to study the involvement of this pathway in response to medication.
10
β-Arrestin-2-AKT-GSK3β signaling downstream of D2 receptor
Several studies indicated that apart from G protein-mediated signaling stimulation of D2 receptors result in modulation of AKT and GSK3 activity (Beaulieu et al., 2009). AKT is a major serine-threonine kinase which is involved in the regulation of synaptic plasticity, monoamine transporter trafficking, neuron morphology, and fate. Activated AKT phosphorylates other proteins including GSK3. The inhibitory phosphorylation of GSK3 occurs at N-terminal serine residues, GSK3α at Ser21 and GSK3 at ser9 by AKT (Cross et al., 1995). GSK3 is a highly conserved serine-threonine kinase, which exists under 2 isoforms GSK3α and which are encoded by distinct gene loci. In contrast to several other kinases, GSK3 is constitutively active and can be negatively regulated through phosphorylation and complex formation. The first evidence for regulation of AKT and GSK3 by dopamine came from a pioneering study on DA transporter knockout (DAT-KO) mice. These animals exhibit impaired DA reuptake leading to a ~5 fold elevation of extracellular DA levels in the striatum (Gainetdinov et al., 1999; Giros et al., 1996). This state of hyperdopaminergia results, among other consequences, in a reduction of AKT phosphorylation/activity and activation of GSK3α and GSK3β (Beaulieu et al., 2004). The reverse effect was observed when DA was depleted in the striatum following administration of an inhibitor of DA synthesis (Beaulieu et al., 2004; Bychkov et al., 2007). Administration of DA receptor agonist amphetamine, methamphetamine or apomorphine to wild type mice also decreased AKT activity and resulted in increased GSK3α and GSK3β activity (Beaulieu et al., 2004; Bychkov et al., 2007; Chen et al., 2007b).
Further studies involving the use of DA receptor antagonists and receptor knockout mice revealed a central contribution of D2 receptors in the regulation of AKT and GSK3 by dopamine (Beaulieu et al., 2007). Regulation of AKT by D2 receptor is not dependent on cAMP and canonical D2 signaling pathway. It is instead mediated by the scaffolding protein β-Arrestin-2 (ARR2) (Beaulieu et al., 2005). When DA receptor agonists were administered to mice lacking ARR2 (ARR2-KO) inhibition of AKT was not observed anymore (Beaulieu et al., 2005). Further characterization of how ARR2 regulates AKT in response to D2 activation showed that stimulation of DA receptor facilitates the formation of protein complex comprised of ARR2, AKT, and PP2A, which
11
causes the dephosphorylation/inactivation of AKT by PP2A (Beaulieu et al., 2005) (Figure 1). Consequently, diminished activity of AKT results in dephosphorylation and activation of GSK3. Finally, investigations involving gain or loss of function in mice revealed that GSK3 contributes to the regulation of this modality of signaling by facilitating the formation of the ARR2/PP2A/AKT complex (O'Brien et al., 2011; Urs et al., 2012).
The βARR2-AKT-GSK3 signaling pathway is also important for the regulation of behaviors by D2 receptors. Indeed, βARR2-KO mice are less active than wild type littermates and have decreased responses to DA receptor agonists (Beaulieu et al., 2005). In addition, DAT-KO mice, which also lack βARR2 display a reduction of the hyperactive phenotype (Beaulieu et al., 2005). Furthermore, GSK3 inhibitors can reduce hyperactivity in DAT-KO mice as well as in normal mice treated with amphetamine (Beaulieu et al., 2004). Finally, mice that are haploinsufficient for the GSK3b gene or mice lacking GSK3β expression specifically in neurons that express D2R also show a decreased locomotor response to amphetamine treatment (Beaulieu et al., 2004; Urs et al., 2012).
Association of D2-βARR2-GSK3β pathway to mental illnesses and action of drugs
Genome-wide association studies (GWAS) identified that genetic variants of D2 receptors and AKT are associated to Schizophrenia (SZ) (Consortium, 2014) and genetic variants of D2 receptors are associated to depression (Howard et al., 2019; Wray et al., 2018). GSK3β and βARR2 have not been associated to mental illnesses by GWAS analysis.
Most antipsychotic drugs prescribed for the management of schizophrenia share a common ability to act as D2 receptor antagonist (Seeman et al., 1975; Seeman and Lee, 1975). Interestingly, functional genetic variants in D2 receptor are also associated to antipsychotic drug responsiveness (Rampino et al., 2018). The involvement of βARR2-AKT-GSK3 pathway in the D2 receptor signaling positions it as an attractive target for the development of novel compounds. Indeed, most existing antipsychotics have been shown to affect AKT-GSK3 signaling either directly or indirectly. Administration of the first generation antipsychotic haloperidol increases AKT and
12
GSK3β phosphorylation without affecting their expression levels in mouse brain (Emamian et al., 2004). In SH-SY5Y cells, clozapine increases Ser9 phosphorylation of GSK3β along with the accumulation of β-catenin and its migration to the nucleus. Chronic and subchronic but not acute treatment with haloperidol, risperidone, and clozapine also caused increases in β-catenin, GSK3β and pGSK3β levels in the striatum, prefrontal cortex (PFC), hippocampus and ventral midbrain of rats (Alimohamad et al., 2005a; Alimohamad et al., 2005b). Beta-catenin is a protein with dual functions in the cell, playing a role in both adhesion between cells as well as gene transcription via the canonical Wnt signalling pathway. In the brain, dysfunctions in Wnt signalling related to beta-catenin levels may also cause various pathological conditions like Alzheimer’s disease, Parkinson’s disease, and depression.
In regards to D2 receptor and βARR2 mediated mechanisms studies using bioluminescence resonance energy transfer (BRET) to quantify D2 receptor signaling processes, have shown that despite their different pharmacological properties, various antipsychotics, share the common ability to antagonize dopamine-mediated interaction of D2 receptors with βARR2. Haloperidol, clozapine, aripiprazole, chlorpromazine, quetiapine, olanzapine, risperidone, and ziprasidone all potently antagonize the βARR2 recruitment to D2 receptors induced by quinpirole (Masri et al., 2008). This observation led to the synthesis of βARR2 biased compounds that would selectively target βARR2 mediated D2 receptor signaling. On this premise, new aripiprazole derivatives UNC9975, UNC0006, and UNC9994 displayed antipsychotic-like activity in rodents (Allen et al., 2011). These three compounds have the characteristic of binding to D2 receptor without affecting cAMP signaling while acting as a partial agonist for βARR2 recruitment to D2 in the absence of a full agonist. Although this can appear as a contradiction, it is noteworthy that aripiprazole behaves as a partial agonist for βARR2 recruitment in some commercial assays when applied alone on cells (Allen et al., 2011) while acting as an antagonist of βARR2 recruitment when simultaneously applied with quinpirole (Masri et al., 2008). It is thus possible that the different UNC compounds may display different pharmacological properties when applied alone in vitro or in the context of an active DA tone in vivo. Further investigations of these compounds, including a characterization or their pharmacological profile in the presence of full D2 receptor
13
agonists, may constitute an important first step toward the development of a new class of antipsychotics targeting GSK3 mediated signaling.
In addition to antipsychotics, inhibition of GSK3β activity has been reported to occur in response to selective serotonin reuptake inhibitors (SSRIs), rapid-acting antidepressant ketamine, some anticonvulsants (mood stabilizers) and lithium (Beaulieu et al., 2009; Beaulieu et al., 2008a; Beurel et al., 2011; Del' Guidice and Beaulieu, 2015; Del'Guidice et al., 2015; Duman and Voleti, 2012). Lithium can directly inhibit GSK3 activity in vitro (Chalecka-Franasze and Chuang, 1999; Zhang et al., 2003; Beaulieu et al., 2004). However, therapeutic concentrations of lithium are not enough for direct inhibition of GSK3 in vivo. Prolonged administration of therapeutic doses of lithium is shown to indirectly inhibit GSK3 in mouse brain. This effect of lithium results from the disruption of the AKT-βARR2-PP2A complex, which mediates some of the D2 receptor’s signaling functions (Beaulieu et al., 2008a) (Figure 3).
14
All known antipsychotics are also D2 receptor antagonists. Lithium can inhibit GSK3 activity via a direct and indirect mechanism, by dissociating Arr2-PP2A-AKT complex. Green color indicates a genetic association to Schizophrenia and depression in GWAS studies.
Regulation of behaviors by GSK3
GSK3 is a serine-threonine kinase, has 2 isoforms (GSK3α and β) and is ubiquitously expressed in all the cell types of the brain. The activity of GSK3 can be regulated by a vast majority of psychoactive drugs and GSK3 is a player in several major signaling pathways, such as cellular responses to WNT, growth factors, insulin, receptor tyrosine kinases, Hedgehog pathways, and G-protein-coupled receptors
(GPCR). Thus, multiple studies focused on understanding how GSK3 is involved in the
regulation of behaviors in normal and pathological conditions.
Homozygous GSK3β knockout (KO) is lethal (Hoeflich et al., 2000), thus heterozygous (HET) GSK3β KO mice were used in assessing behaviors. GSK3β KO HET mice showed anxiolytic-like and antidepressant-like behaviors as well as reduced responsiveness to amphetamine and reduced aggression (Beaulieu et al., 2004; Beaulieu et al., 2008b). GSK3α KO mice are viable and display decreased exploratory activity, decreased depressive-like behaviors and aggression (Kaidanovich-Beilin et al., 2009). To model gain of function, knockin mice were generated that are not sensitive to inhibitory phosphorylation, since phosphorylation sites of the endogenous GSK3 alleles have been replaced by non-phosphorylatable alanine (GSK3βKI and GSK3αKI). Behavioral characterization of these mice revealed several differences. Only GSK3βKI, not GSK3αKI, displayed heightened vulnerability to the learned helplessness stress. Novel object recognition was impaired in GSK3βKI, not GSK3αKI, temporal order memory was not impaired in GSK3αKI or GSK3βKI, and coordinate spatial processing was impaired in both GSK3αKI and GSK3βKI mice. Adult hippocampal neuronal precursor proliferation was impaired only in GSK3βKI but not in GSK3αKI mice. (Pardo et al., 2016). Overall, these data indicate that brain-wide manipulation of GSK3 activity is involved in the regulation of anxiety-like, depressive-like behaviors and cognition. Moreover, GSK3β and GSK3α do not play redundant functions.
15
However, at this point, it is unclear whether there are any brain region-dependent differences in regulation of behaviors by GSK3. Several studies attempted to address this issue. The crossing of floxGSK3β transgenic mice with CamKIIaCre mouse line resulted in the specific deletion of GSK3β in forebrain pyramidal neurons. Behavioral characterization of these mice revealed anxiolytic and pro-social phenotypes (Latapy et al., 2012). Anxiolytic-like behaviors were also demonstrated after prefrontal cortex specific deletion of GSK3β via injection of AAV Cre virus into the brain of floxGSK3β transgenic mice (Del'Guidice et al., 2015). AAV mediated overexpression of GSK3β in hippocampus decreased cognitive performance in adult rats, thus revealing one of the roles of GSK3β in this region. (Hui et al., 2018). AAV mediated shRNA knockdown of GSK3β specifically in NAc shell resulted in reduced anxiety-like behavior while increasing depression-like behavior and cocaine self-administration (Crofton et al., 2017).
GSK3 can be downstream of different signaling pathways, thus its cell type-specific role has also been explored in several studies. Using pharmacological manipulations it has been shown that GSKβ activity can be modulated downstream of 5HT1A and 5HT2 serotonin receptors (Li et al., 2004). Moreover, pharmacological or genetic inhibition of GSK3β alleviates the aberrant anxiety and depressive-like behaviors produced by serotonin deficiency (Beaulieu et al., 2008b). Specific deletion of GSK3β in serotonin neurons did not result in differences in baseline behavior, however, affected locomotor and immobility behaviors in response to treatment with 5-HT1B receptor agonist anpirtoline (Zhou et al., 2012).
As described before GSK3β activity can be regulated by dopamine via D2 receptors. In one study authors crossed floxGSK3β mice with D2RCre mice to achieve specific deletion of GSK3β in D2R expressing cells. Characterization of mouse behavior after drug treatment suggested that selective deletion of GSK3β in D2 neurons mimics antipsychotic action on locomotor behavior (Urs et al., 2012).
Overall, GSK3 can affect different behavioral modalities by acting in different brain regions or cell types. However, it remains unclear what is the function of GSK3 in a given cell type in a specific brain region. One of the reasons for the lack of this
16
information is technical difficulties related with a knockout of GSK3 simultaneously in a cell type and region-specific manner.
Substrates of GSK3
Despite overwhelming data on the role of GSK3 in signaling pathways and action of drugs, little is known about the mechanisms by which GSK3 activity does contribute to the regulation of behavior. There are over 100 known substrates, which can be phosphorylated and regulated in different fashions by GSK3 (Sutherland, 2011). Below are examples of GSK3 substrates that might be implicated in neurological functions and how their dysregulation may contribute to pathological conditions (Figure 4).
Circadian rhythms
Circadian rhythms occur with 24-hour periodicity and are important for organisms to adapt to environmental changes. They are orchestrated by the suprachiasmatic nucleus (SCN) located in the anterior hypothalamus. At the molecular level, regulation starts with heterodimerization of the bHLH-PAS domain proteins BMAL1 and CLOCK. This complex then activates the clock genes Per1, Per2, Cry1, and Cry2. PER and CRY accumulate in the cytosol before translocating into the nucleus where the PER–CRY complex inhibits the transcription of its own components by binding to the BMAL1– CLOCK complex.
17
Figure 4. GSK3 targets and their potential contribution to neuronal functions Summary of validated GSK3 targets indicating a potential contribution to cytoskeletal reorganization, gene expression, synaptic plasticity, and circadian rhythms.
The orphan nuclear receptor Rev-erbα also plays a role in the negative feedback loops by repressing the transcription of Bmal1 (Gachon et al., 2004). GSK3 is highly expressed in SCN. It has been shown that the non-selective GSK3 inhibitor lithium lengthens the period of circadian rhythms in vitro and in vivo (Abe et al., 2000; Iwahana et al., 2004) and that GSK3 haploinsufficiency lengthens the circadian locomotor activity in mice (Lavoie et al., 2013). GSK3 exerts its effect on circadian rhythms by regulating their components. First, GSK3 directly phosphorylates PER2 and promotes its nuclear translocation. Therefore, an alteration in the activity of GSK3 would result in phase changes due to temporal changes in the nuclear transfer of PER2 (Iitaka et al., 2005). GSK3 phosphorylates CRY2, which results in its degradation (Harada et al., 2005). GSK3 also phosphorylates and stabilizes the negative component of the circadian clock Rev-erbα. Accordingly, the rapid proteasomal degradation of Rev-erbα and the
18
activation of the gene Bmal1 were observed following GSK3 inhibition by lithium (Yin et al., 2006). Finally, GSK3 phosphorylates BMAL1 and controls the amplitude of the circadian oscillation by stabilizing this protein, which appears to be crucial to maintaining the robustness of the circadian clock (Sahar et al., 2010).
β-Catenin
One of the best described GSK3 incorporating pathways is the regulation of β-catenin downstream of Wnt signaling. During activation of the canonical Wnt pathway, ligands bind to seven transmembrane-domain protein Frizzled (Fzd) and its co-receptors the low-density lipoprotein receptor-related proteins 5 and 6 (LRP5/6) leading to the recruitment of Dishevelled (Dvl) to LRP5/6 (Sutton and Rushlow, 2011). Following activation, Dvl recruits AXIN, which is associated with GSK3, thereby facilitating GSK3 translocation to the membrane and its dissociation from -catenin. Stabilized -catenin accumulates in the cytoplasm and translocates to the nucleus where it forms a complex with co-transcription factors T cell factor/lymphoid enhancer factor (TCF/LEF) and initiates the transcription of Wnt-target genes (Wu and Pan, 2010). In addition, β-catenin interacts with cytoskeletal network and is recruited to dendritic spines following depolarization, hence it may have a role in synaptic plasticity (Murase et al., 2002). GSK3 phosphorylates β-catenin resulting in its degradation by proteosome. Therefore, it is possible that changes in β-catenin regulation by GSK3 influence gene expression and synaptic plasticity. In support of this possibility, overexpression of β-catenin mimics the effect of lithium on locomotor hyperactivity, which has been shown to be GSK3 dependant (Gould et al., 2007). However, forebrain-specific β-catenin-KO mice exhibit little behavioral phenotype, therefore, suggesting a minor role for β-catenin in the regulation of normal behavior by GSK3 (Gould et al., 2008).
Cytoskeleton
Cytoskeletal rearrangements are important during neuronal growth, axon guidance and synapse formation. Microtubules constitute a major part of the cytoskeleton and their assembly and stability are regulated by microtubule-associated proteins (MAPs). Several MAPs are substrates of GSK3. Some examples include a)
19
Tau protein is phosphorylated by GSK3 and influences the regulation of microtubule assembly (Sutherland, 2011). b) Collapsin Response Mediator Proteins (CRMP) are phosphorylated in vitro and in vivo by GSK3 and regulate axon growth, a number of axons as well as dendritic development (Alabed et al., 2010; Cole et al., 2004; Yoshimura et al., 2005). c) Microtubule-Associated Protein (MAP) 1B, a major component of the neuronal cytoskeleton, can be phosphorylated by GSK3, leading to increased stability and affinity for microtubules. GSK3 regulation of MAP1B is a crucial link between Wnt signaling and axonal remodeling (Lucas et al., 1998; Trivedi et al., 2005), d) MAP2C is another cytoskeletal component abundant in neurons. It can be phosphorylated by GSK3 and along with tau and MAP1B participates in microtubule stability (Sánchez et al., 2000). Although the exact mechanism by which GSK3-mediated phosphorylation of microtubules can give rise to behavioral changes is not clear, we speculate that by doing so, GSK3 may regulate neurodevelopment and synaptic plasticity, which can result in modulation of behavioral responses.
GSK3 regulates AMPA, NMDA receptors and synaptic plasticity
One possible scenario leading to acute modulation of behavior regulation by GSK3 is its action on ionotropic glutamate receptors AMPA and NMDA, known to be important for synaptic plasticity. Induction of long term depression (LTD) increased the activity of GSK3β in CA1 region of the hippocampus. Moreover, the application of several GSK3 inhibitors prevented the LTD (Peineau et al., 2008; Peineau et al., 2007). Following induction of long term potentiation (LTP), there is the inactivation of GSK3β in the hippocampus (Hooper et al., 2007; Peineau et al., 2007). Conversely, overexpression of GSK3β inhibits the induction of LTP (Peineau et al., 2007; Zhu et al., 2007). The opposite changes of GSK3β activity in LTP and LTD indicate that it can be one of the interaction links between these two plasticities. Indeed, it has been shown that decrease of GSK3β activity upon induction of LTP ensures inhibition of LTD, thus providing a molecular relationship between regulation of LTP and LTD (Peineau et al., 2007).
One possible mechanism of regulation of LTP and LTD by GSK3β can be regulation of AMPA and NMDA receptors or other proteins involved in synaptic
20
functions. Indeed, it has been shown that GSK3β can regulate both AMPA and NMDA receptor trafficking (Chen et al., 2007a; Wei et al., 2010; Zhu et al., 2007). In both cases inhibition of GSK3β led to decrease of AMPA or NMDA mediated currents, due to a decrease of surface GluA1 or NR2B and NR1 subunit of the receptors respectively (Chen et al., 2007a; Wei et al., 2010). This effect was not due to a direct action of GSK3β on GluA1 or NMDA, rather it was mediated via Rab5 in case of GluA1 (Wei et al., 2010) or Rab5 and PSD95 in case of NMDA (Chen et al., 2007a). GSK3β inhibition resulted to increase of Rab5 activity due to increased formation of GDP dissociation inhibitor (GDI)-Rab5 complex. This, in turn, resulted in accelerated endocytosis of AMPA receptors from the plasma membrane (Wei et al., 2010). Decrease of surface NMDA receptors during inhibition of GSK3 was blocked by dominant negative Rab5, thus indicating the same mode of regulation as in the case of GluA1. Moreover, increased internalization of NMDARs during inhibition of GSK3β was mediated because of decrease in the association of NMDA receptors with postsynaptic scaffolding protein PSD95 (Chen et al., 2007a). It is also worth mentioning that GSK3β can directly phosphorylate PSD95 (Nelson et al., 2013).
Overall, these results suggest that GSK3 can regulate AMPA and NMDA receptors via similar and distinct mechanisms and as a result impact synaptic plasticity.
Dynamin I
Large GTPase dynamin I can serve as a GSK3 substrate, the resultant phosphorylated form is then involved in activity-dependent bulk endocytosis (ADBE), but not in clathrin-mediated endocytosis. The activity of GSK3-dependent phosphorylation of dynamin I is necessary and sufficient for ADBE to take place. Thus, GSK3 may play an important role also in preparing synaptic vesicles for retrieval during elevated neuronal activity (Clayton et al., 2010).
FXR1
A recent study has shown that the fragile X mental retardation syndrome-related protein 1 (FXR1) is a GSK3β substrate (Del'Guidice et al., 2015). GSK3β can directly phosphorylate FXR1, which results in its degradation. Moreover, phosphorylation by