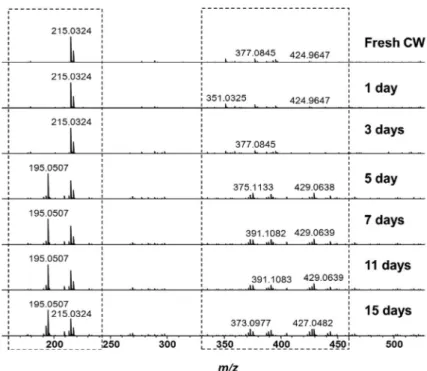
Monitoring the physicochemical degradation of coconut water using ESI-FT-ICR MS.
Texte intégral
Figure



Documents relatifs
The moderate score of the EBGO sample over the second component is mainly related to the small contribution of very aromatic neutral nitrogen compounds (Benzocarbazoles, DBE
With funding support by the Global Crop Diversity Trust (the ‘Trust’ hereafter), COGENT’s major achievements for 2012 included: an update of the data regarding global ex situ
Our results reveal the interplay of different dendritic cell subsets associated to the oral mucosa of infected mice that directly present fungal antigen to Candida-specific T cells
studied the production of Nata de coco by Acetobacter xylinum using tender coco- nut water as a medium instead of the usual mature coconut water
Quality criteria such as the water per nut ratio, Total Soluble Solids con- tent (TSS), total sugar per nut, [reducing sug- ars / total sugars] ratio, and, to a lesser extent,
The technical guidelines 18 General guidelines and recommendations for coconut in vitro transfer 19 The coconut embryo culture transfer protocol 20 Arrangements in donor
This work aimed at obtaining a commercially safe coconut water beverage by ohmic heating while looking at the kinetics of volatile compounds evolution.. Impact of
However, all models of thermal runaway in abuse conditions available at the cell level are presently based on the chemical degradation reactions considered in Kim et al.'s model