OATAO is an open access repository that collects the work of Toulouse
researchers and makes it freely available over the web where possible
Any correspondence concerning this service should be sent
to the repository administrator:
tech-oatao@listes-diff.inp-toulouse.fr
This is an author’s version published in:
http://oatao.univ-toulouse.fr/27443
To cite this version:
Bacchin, Patrice
and Brutin, David and Davaille, Anne and Di Giuseppe,
Erika and Dong Chen, Xiao and Gergianakis, Ioannis
and
Giorgiutti-Dauphiné, Frédérique and Goehring, Lucas and Hallez, Yannick
and Heyd,
Rodolphe and Jeantet, Romain and Le Floc-Fouéré, Cécile and Meireles,
Martine
and Mittelstaedt, Eric and Nicloux, Céline and Ludovic, Pauchard
and Saboungi, Marie-Louise Drying colloidal systems: Laboratory models for
a wide range of applications. (2018) European Physical Journal E, 41 (94).
1-34. ISSN 1292-8941
Drying colloidal systems: Laboratory models for a wide range of
applications
Patrice Bacchin1, David Brutin2, Anne Davaille3, Erika Di Giuseppe4, Xiao Dong Chen5, Ioannis Gergianakis, Fr´ed´erique Giorgiutti-Dauphin´e3, Lucas Goehring6, Yannick Hallez1, Rodolphe Heyd7, Romain Jeantet8, C´ecile Le Floch-Fou´er´e8, Martine Meireles1, Eric Mittelstaedt9, C´eline Nicloux10, Ludovic Pauchard3,a, and Marie-Louise Saboungi11
1
Laboratoire de G´enie Chimique, Universit´e de Toulouse, CNRS, INPT, UPS, Toulouse, France 2
Aix-Marseille University, IUSTI UMR CNRS 7343, Marseille, France 3
Laboratoire FAST, UMR 7608 CNRS - Univ. Paris-Sud, Universit´e Paris-Saclay, 91405, Orsay, France 4
MINES ParisTech, PLS Research University, CEMEF - Centre de mise en forme des mat´eriaux, UMR CNRS 7635, CS 10207, 06904 Sophia Antipolis Cedex, France
5
Suzhou Key Lab of Green Chemical Engineering, School of Chemical and Environmental Engineering, College of Chemistry, Chemical Engineering and Material Science, Soochow University, Suzhou, China
6
School of Science and Technology, Nottingham Trent University, Clifton Lane, Nottingham NG11 8NS, UK 7
LAMPA, Arts et M´etiers ParisTech, 2, Boulevard du Ronceray, BP 93525, cedex 01, F-49035 Angers, France 8
Agrocampus Ouest, INRA, UMR STLO, Rennes, France 9
Department of Geological Sciences, University of Idaho, Moscow, ID, USA 10
Institut de Recherche Criminelle de la Gendarmerie Nationale, 5, Boulevard de l’Hautil, Pontoise, France 11
Institut de Min´eralogie de Physique des Mat´eriaux et de Cosmochimie (IMPMC); CNRS UMR7590 - Universit´e Pierre et Marie Curie, Case 115, 4, place Jussieu, 75005 Paris, France
Abstract. The drying of complex fluids provides a powerful insight into phenomena that take place on time and length scales not normally accessible. An important feature of complex fluids, colloidal dispersions and polymer solutions is their high sensitivity to weak external actions. Thus, the drying of complex fluids involves a large number of physical and chemical processes. The scope of this review is the capacity to tune such systems to reproduce and explore specific properties in a physics laboratory. A wide variety of systems are presented, ranging from functional coatings, food science, cosmetology, medical diagnostics and forensics to geophysics and art.
1 Introduction
The drying of complex fluids, colloidal dispersions and polymer solutions, involves a large number of physical and chemical processes. The complex spatial and tempo-ral evolutions of these systems during this process depend in particular on solvent diffusion, mass transfer at the vapour-medium interface and phase transitions. Drying is frequently observed over a wide variety of scientifically and technologically important systems: it is involved, for example, in the entire process of coating formation and significantly affects the quality of the final films. Further-more, due to the number and nature of the components involved, the drying of complex fluids offers a way to
high-a
e-mail: ludovic.pauchard@u-psud.fr
light and analyze processes that take place on time and length scales not normally accessible. The capacity to re-produce specific phenomena in such systems in a physics laboratory is the scope of this review. Several phenom-ena in a wide variety of systems at different time and length scales can be probed with analogous techniques as depicted in fig. 1.
The tunability of these systems emerges from the fol-lowing properties. Polymer solutions and colloidal disper-sions represent heterogeneous systems since from a micro-scopic point of view the components are organized into well-defined structures at the supramolecular scale. An important feature of the resulting structures is high sen-sitivity to weak external actions caused by mechanical stresses as well as by chemical processes. Among complex fluids, colloids are stable dispersions of minute particles
Fig. 1. (a) TEM image of a dried boehmite nanorod suspension [1]. (b) Casein micelles isolate. (c) Blisters in a cosmetic thin film. (d) Drying pool of blood. (e) Various crack patterns in Mona Lisa (reproduced from [2], with the permission of AIP Publishing). (f) Well-ordered permafrost in Beacon Valley, Antarctica. (g) Columns in lava near Fingal’s Cave. (h) Convection in the solid mantle involving creation of a plate that will subduct back in the mantle.
in a continuous phase. A particular property of colloids, both organic and inorganic, is the extremely small parti-cle size, typically ranging from 10 nm to 1 µm, exposing a large external surface per unit mass. Hence, the surface forces that dominate on colloidal lengthscales become a key factor in the stabilization or destabilization of a col-loidal dispersion. In the absence of evaporation, if parti-cles repel each other the dispersion remains stable, keep-ing the particles in suspension without sedimentation. De-spite the complexity of the suspension due to the number and nature of its components, a colloidal dispersion can be described by effective potentials, most prominently for electrostatic repulsion and van der Waals attraction, the former serving to stabilize the dispersions and the latter being the primary cause for particle aggregation. The bal-ance of these forces is addressed by the DLVO theory [3]. In addition, the stability of the colloidal dispersion can be strongly affected by altering the surface chemistry of the particles. For example, flocculation and aggregation of charged particles can be caused by increasing the ionic strength or lowering the surface charge. The control of the interparticle potential is one of the most important parameters for exploring different characteristics of the system and tuning its physical properties. Among the pos-sible bonds between particles are depletion interactions, hydrogen bonds and hydrophobic effects at high concen-trations. In polymer-colloid mixtures steric repulsion due to the presence of polymer chains or ligands serves to sta-bilize neighbouring particles. In the case of solvent flow, induced for example by evaporation, hydrodynamic forces between the dispersed particles can be entrained within the flow fields; liquid convection can be induced by many different causes including evaporation or temperature gra-dients, e.g. Marangoni flow, forced filtration or pressure-driven flow.
A large diversity of regimes are encountered during the drying process as a result of strong modifications of the chemical and physical properties of the systems. The
0.22 - 0.30 0.32 0.34 0.36 - 0.47
solid volume fraction
Fig. 2.Sketch of the increase of the particle concentration dur-ing the solvent removal of a colloidal dispersion. Aggregation or repulsive interaction result in different particles network de-pending on both the system and the evaporation rate. Adapted from Di Giuseppe et al. [4].
nanoscopic structure of the film evolves and crosses the liquid-solid boundary as the medium follows an out-of-equilibrium route governed mainly by thermodynamics and transport phenomena (fig. 2). At the macroscopic scale, the rheological properties of the medium, which change from a fluid phase into a viscoelastic, plastic, or brittle solid, result in hydrodynamic or mechanical insta-bilities.
Thus, the adaptability of the system in question opens up a multitude of possibilities of tuning it to exhibit specific properties. Based on laboratory experiments, an overview of the following problems will be presented:
– Fabrication of functional coatings exhibiting anti-corrosion properties: in particular, when such homo-geneous coatings are formed.
– Heterogeneities in cosmetic thin films by phase separa-tion induced by evaporasepara-tion: experimental modelling aims to highlight and understand the formation of such heterogeneities.
– Problems related to food science: the aim is to enhance the understanding of dairy colloids and processes in-volved in concentration ranges similar to those encoun-tered in drying.
– Problems related to medicine and/or forensics: exper-imental modelling aims to study dried blood that can be used for medical diagnostics, and/or determine the time and conditions in a homicide investigation. – Problems related to art: experimental modelling aims
to reproduce craquelures in paintings to determine the methods used by the artists, judge authenticity and determine their stability under environmental condi-tions.
– Experimental modelling provides a means to under-stand morphologies observed in nature such as colum-nar jointing in a fracture pattern.
– Problems related to surface phenomena at the geo-physical scale: experimental modelling can provide quantitative access to phenomena in the earth or other planets over time and length scales inaccessible to hu-mans.
2 Functional coatings through colloidal
dispersion evaporation
Functional coatings are films deposited on surfaces to confer specific properties upon them, as chemical protec-tion (resistance to oxidaprotec-tion), mechanical strength, opti-cal properties (plasmonic effects for Raman spectroscopy), electrical properties, etc. Common examples of functional coatings are paints, anti-corrosion films for steel or ceramic membranes for fuel cells.
Such coatings are usually obtained by drying complex fluids or colloidal dispersions. The role of colloids is gen-erally to provide their intrinsic properties to the film (op-tical response, conduction, . . . ) and/or to introduce an important specific surface area useful for heterogeneous reactions, catalysis, or mechanical strength. The success of the coating procedure depends on the homogeneity of the dry film obtained in the end. This homogeneity has been ensured for years by using Volatile Organic Compounds (VOC) as solvents because these liquids provide notably strong colloidal stability by enhancing electrostatic repul-sions between nanoparticles and by solvating polymers ef-ficiently. The new legislation for sustainable development urged industry to use environmentally friendly coatings only. This has motivated the shift from the 100% VOC dis-persions towards zero-VOC waterborne coatings. The re-duced stability associated to this change of solvent makes the fine control of the drying process all the more impor-tant to obtain an homogeneous film strongly attached to the substrate. Some examples of functional coatings with industrial relevance are briefly presented below.
The new legislation has motivated the shift from the 100% VOC paints used in the automobile industry to-wards waterborne coatings. These new systems are mainly suspensions of hard polymer particles, which yield hard top-coats upon drying. With such suspensions of hard nanoparticles, it is a challenge to produce flat, smooth, shiny, crack-free and durable films. So far industry still uses up to 15% VOC to soften the particles in order to achieve crack-free films. Though there is overall progress,
Fig. 3.Ball bearing parts reinforced by ceramic coatings [5,6].
the remaining VOC are still harmful for the environment and particularly for the Ozone layer preservation. Thus avoiding cracks during drying of hard particles suspen-sions would actually help to preserve the environment!
Ceramic nanocomposites have been introduced in Solid Oxide Fuel Cells. The membranes carrying the oxygen ions are now made with yttria-doped zirconia. Currently these ceramics are made through processing and sintering of micrometer-sized dispersions of oxide particles. A ma-jor goal is the reduction of particle sizes down to about 100 nm. This would allow sintering at lower temperatures and to produce thinner films, with higher conductance and better performance. However, the control of the deposition and drying stages for nanometric dispersions has proved to be difficult, due to premature aggregation of the par-ticles. Similar problems occur in ceramic thermal barriers for aircraft engines.
Nano-ceramics functional coatings have also been de-signed to create tougher materials. Using nanoparticles is one route to improve the performance of ceramic materi-als that are submitted to high temperatures and intense wear as the ball bearing parts shown in fig. 3. Work along these lines has a large impact on the development of parts for car or aircraft engines. Here as well, it is expected that the use of particles in the 100 nm size range would make it possible to sinter at lower temperatures and to produce materials that are more ductile and less fragile than cur-rent ceramics. However, progress has been limited so far, because ceramics made from nanometric dispersions tend to crack upon drying. In the next section, we discuss in more details another type of functional coating used in aeronautics: anti-corrosion protections.
2.1 Anti-corrosion coatings for aeronautics
Specific anti-corrosion coatings are developed for the aero-nautics industry as the integrity of the fuselage and wings metallic parts is affected by the external action of the envi-ronment. Coatings designed for corrosion protection must offer an effective physical barrier, impeding the access of aggressive species to the metallic interface, as well as a chemical one to provide a self-healing capacity in case of breaching. For many years, efficient anti-corrosion paints
Fig. 4. Nano-container–based anti-corrosion coating: if the protecting functional film schematized on the right part is breached, the local change in ionic composition triggers a local desorption of corrosion inhibitors that will react with aggres-sive species to form a protective layer before the material is damaged.
were based on the use of chromium VI, a strong corrosion inhibitor. It has however been classified as carcinogenic, mutagenic or toxic for reproduction by the European reg-ulation REACH and must be replaced. This last example is developed in more details in what follows.
A promising route to design new anti-corrosion coat-ings is to use cerium (Ce) as corrosion inhibitor. The large quantity of cerium salt to be used for an efficient protec-tion is however not compatible with the mechanical stabil-ity of the coating. To overcome this limitation, cerium can be trapped on colloidal particles embedded in the coating. The latter are thus often referred to as “nano-reservoirs”. cerium salt can be trapped between layers of particles, in-side hollow capsules, or on the external surface of colloids. If the coating is breached, the physico-chemical environ-ment near particles close to the breach changes, which triggers the local release of adsorbed cerium salt as de-scribed in fig. 4.
As an example, we report here some work on an anti-corrosion coating based on the use of boehmite nanorods as cerium reservoirs. The protecting barrier is a film ob-tained through evaporation of a colloidal suspension con-taining both nanoparticles and ionic species. The colloidal suspension is initially stable due to repulsive particle-particle electrostatic interactions. At early times, physi-cal drying concentrates particles, but also ionic species. Therefore, depending on initial physico-chemical condi-tions and hydrodynamics, the suspension can be destabi-lized before the film assembly is completed. In this case, particle aggregates can form in the fluid or a skin can ap-pear at the free surface, leading to strongly heterogeneous or fragile coatings. In these unfavorable conditions, chem-ical drying also contributes to the assembly process, and in a unfavorable manner.
In the next two sections, we highlighted two important sources of film heterogeneity, namely the appearance of chemical drying and the flow-driven assembly.
2.2 Thermodynamic view of the drying of a salty colloidal suspension
Establishing the phase diagram of boehmite suspensions containing an added cerium salt (fig. 5) allows to estimate
Cs
φ (φi,Cs1)
(φi,Cs2)
Fig. 5. Schematic phase diagram of a boehmite suspension with added cerium nitrate Ce(NO3)3 inspired from the exper-iments detailed in [1]. Colored lines are trajectories followed during drying for a unique initial colloid volume fraction φi and various initial salt concentrations csi.
if colloids can be brought to close contact before being destabilized in the bulk suspension. First focus on the di-agram itself (black lines). For low added salt content (re-gion I), colloids experience strong electrostatic repulsions that make the suspension stable and dispersed from dilute regimes up to close packing. Depending on the colloid vol-ume fraction, the suspension can be liquid-like, glassy, or crystalline. Increasing the added salt content reduces the electrostatic repulsions, which leads to particle aggrega-tion. At low colloid volume fraction isolated aggregates are formed and have time to sediment (region II). At high colloid volume fraction, the aggregation can take the form of percolation and a colloidal gel is formed (region III).
Consider drying of a suspension with colloid and salt concentrations φ(t) and cs(t), respectively. Because re-moval of solvent concentrates both colloids and salt at the same rate, we have cs(t) = φ(t)csi/φiwhere φi= φ(t = 0) and csi = cs(t = 0). During drying, the trajectory of the suspension in the phase diagram is affine with slope csi/φi. Considering an initial colloid volume fraction φi, there are two critical initial salt concentrations denoted cs1 and cs2 in fig. 5. For csi< cs1 the trajectory remains in a dispersed phase until close packing is reached, which is desirable for the final film homogeneity. For csi > cs2, physical drying induces a salt concentration increase able to instantaneously destabilize the suspension. Aggregates appear and sediment. The final film will be heterogeneous. Finally for cs1< csi< cs2the trajectory reaches the per-colation region III during drying. If the gel phase can be reorganized, physical evaporation may still permit reach-ing close packreach-ing but the final film homogeneity may not be perfect.
Note that the discussion above is only an illustration of the effect of physical drying on the chemical compo-sition of the system, the latter being responsible for the onset of simultaneous chemical drying. In particular, the
boundaries in the phase diagram of fig. 5 depend on the experimental time. Indeed, the percolated gel phase was observed in boehmite/cerium nitrate suspensions after a few hours. If a suspension with cs1 < csi < cs2 is used in a drying process lasting only a few minutes, no gel phase will be produced. Indeed, the phase diagram is expected to be built at equilibrium and the discussion about trajecto-ries in the phase diagram above implicitly assumes these trajectories are traveled along in a quasi-static manner. This description neglects important kinetic effects. In the next section, the role of one type of kinetic effect, namely hydrodynamic transport, is highlighted.
2.3 The role of fluid flow
In forced unidirectional drying, evaporation and mass con-servation impose a simple uniform fluid flow that does not need modeling. In practice, drying is seldom unidirectional and often proceeds through front propagation. The initial colloidal suspension is one side of the front and a compact wet zone can be found on the other side. Evaporation on the large surface of the latter zone induces a significant lat-eral flow from the other side of the front. This flow brings colloids to the dense zone, allowing its growth and there-fore the front shift [7]. In this front region, the dense layer assembly is flow-driven in a manner similar to what is ob-served in filtration. The role of evaporation itself is mainly to generate the hydrodynamic flow. In this 2D drying pro-cess, the dense layer assembly therefore involves a compe-tition between stabilizing electrostatics and diffusion on one hand and destabilizing hydrodynamics and attractive colloidal forces on the other hand. A purely thermody-namic description is not sufficient anymore and the fluid flow needs to be modelled.
The general Eulerian description of suspension flows involves at least the colloid volume fraction field φ(x, t), if one does not keep track of local microstructure changes. This quantity depends on the hydrodynamic flow through a locally averaged velocity field. Several equivalent de-scriptions are possible, using either the volume averaged fluid phase velocity (i.e., the pure solvent velocity) or the suspension averaged velocity (i.e., the volume averaged velocity of the mixture of solid particles and fluid sol-vent). Here we use the latter, denoted u(x, t). For col-loidal flows, the particle Reynolds and Stokes numbers are always much smaller than unity so inertia/acceleration terms can be neglected safely in the Navier-Stokes and Newton equations for the fluid phase and particles, re-spectively. In this context, general continuity and mass and momentum conservation equations can be derived rig-orously for the suspension. The momentum conservation equation resembles the Stokes equation for a pure New-tonian fluid but an additional so-called “particle stress” is added to the usual stress [8]. A recent detailed analysis can be found in the work of Nott and coworkers [9]. These equations take the general form
0 = −∇P + ∇ · [2ηsS+ Σp] , (1)
0 = ∇ · u, (2)
∂φ
∂t + u∇φ = −∇ · j
p, (3)
where P is some average pressure field imposed by the in-compressibility condition (2), ηsis the solvent viscosity, S is the average rate-of-strain tensor, Σpis the contribution of particles to the suspension stress, and jp is a migration flux due to particle-particle and particle-fluid interactions. These equations still require closure relations. Here we use the classical suspension balance model [10–12] in the limit of slow variations of φ and u at the scale of particles and quasi-isotropic suspension microstructure. The latter condition is achieved if the hydrodynamic stress is dom-inated by the stresses due to Brownian motion colloidal interactions [13]. The particle stress Σp then reduces to a quasi-Newtonian contribution 2η(φ)S plus an isotropic stress absorbed into the pressure. ηs+ η(φ) is the effective suspension viscosity, where η(φ) is a correction depending in general on the collisions, interactions between particles. Still in the same conditions, the migration flux reduces to
jp ≃ −D0f (φ)Vp
kT∇Π, (4)
where D0 is the colloid diffusion coefficient in the di-lute limit, f (φ) is the sedimentation hindrance function (many-body mobility divided by the isolated colloid mo-bility), Vp is the volume of one colloid, and Π is the gen-eralized osmotic pressure, or particle pressure, contain-ing contributions both from colloidal and hydrodynamic forces. In the discussion below, we neglect the latter so Π is considered close to the classical osmotic pressure and jp ≃ −D(φ)∇φ where D(φ) = D0f (φ)kTVp∂Π∂φ. To sum-marize, model (1)-(2)-(3) solves the fluid flow in the sim-plest manner, i.e. with a Stokes equations supplemented with only a concentration-dependent viscosity. The model accounts, however, for the influence of this flow and of colloidal interactions on mass transport. To illustrate the influence of the evaporation-induced flow on the 2D drying of a “simple” hard sphere suspension, model (1)-(2)-(3) is solved in a 2D rectangular geometry corresponding to the microfluidic pervaporation system used in [14]. The bot-tom and left sides are solid impermeable walls, the right side is a suspension inlet at constant concentration and the top side is a pervaporation surface letting out a uniform and constant solvent flux due to evaporation but blocking colloids. The streamlines of the 2D flow are represented in fig. 6(a) and fig. 6(b) for P´eclet numbers P e = LVE/D0 equal to 1 and 10, respectively. Here the lengthscale L is the film height and VE is the evaporation rate. The influence of the flow on the colloid volume fraction is il-lustrated in fig. 6(c) and fig. 6(d). For P e < 1 colloids can diffuse against the driving flow so no skin is created and the concentration is mostly uniform in the cross section. For P e > 1, advective transport dominates diffusion so a skin builds up along the evaporation surface and the con-centration process subsequently remains two dimensional. When the hard spheres considered so far acquire sur-face charges Z, their mutual repulsion increases and they can resist flow-driven assembly more intensely. This is il-lustrated in fig. 7 where it may be seen that uncharged
Fig. 6. Hard sphere suspension 2D drying simulation in a pervaporator. In fig. 6(a) and (b), the blue (respectively, green) streamlines are results of computations with a constant (respectively, variable) viscosity. Figures 6(c) and (d) were obtained with a constant viscosity.
Fig. 7. Volume fractions snapshots obtained in simulations with increasing colloidal surface charges.
particles tend to nucleate a dense region in the upper left corner of the evaporator while this is partly prevented at intermediate surface charge and completely avoided at the higher surface charge.
These simulations were conducted at constant inter-action range. Note that increasing the salt concentration,
either intentionally or as a consequence of evaporation, would reduce this interaction range. For high salt con-centrations and vanishing interaction ranges the solution would then be close to that of hard spheres. In a dry-ing experiment, it is thus possible to avoid skin formation at initial times thanks to charge stabilization by adjusting the pH or the salt content, but to obtain a skin later on due to the increase of the average salt concentration induced by evaporation. Accounting for these effects is possible if the full dependence of the equation of state Π(φ, cs, Z) is used in eq. (4). In this case the colloid flux is not −D(φ)∇φ anymore. It depends also on gradients of salt concentra-tion ∇cs and surface charge ∇Z. Simulations involving these terms require the additional resolution of averaged mass conservation equations for the ionic species (Nernst-Planck equations).
2.4 Conclusion
The drying of charge-stabilized colloidal suspensions is made complex by to the coupling between physico-chemistry and hydrodynamics. Indeed, the solvent evap-oration flux at the free surface induces a physical drying of both particles and solutes, in particular ionic species determining the electrostatic interaction range. Even if the details of the hydrodynamic flow are neglected, physi-cal evaporation can induce “chemiphysi-cal evaporation”. If the
hydrodynamic flow is important, it is influenced by col-loids at least at the level of the effective suspension vis-cosity, but more complex effects can arise at high P´eclet numbers. On the other hand, the colloid volume fraction field is coupled to hydrodynamics at least at the level of simple advective transport, which can induce skin forma-tion or not, but once again more complex colloid fluxes can appear for particle Peclet numbers larger than unity due to colloid-colloid hydrodynamic interactions, like for example shear-induced migration. A faithful modeling of evaporation-induced assembly of colloid-based functional coatings requires a good knowledge of the suspension rhe-ology and out-of-equilibrium thermodynamics, two active domains of research per se, and is thus a difficult fascinat-ing challenge.
3
On the interest of studying the drying
behavior
of food colloids at single droplet
scale
Drying is a well-established process to stabilize the bio-logical and physico-chemical properties of food products during long-term storage, facilitate product handling and protect encapsulated materials in carrier matrices [15,16]. It consists for food liquid materials in spraying the feed so-lution into a cloud of droplets within a flow of hot air: the water is rapidly evaporated from the droplets, thus result-ing in dried particles whose physical characteristics (eg, size distribution, shape and density) and typical aerody-namic behaviors determine their final application [17–19]. The ability of running spray drying on a large scale, with multiple possibilities to produce composite materials [20], makes it nowadays a widely spread technology in the food sector.
Nevertheless, the polydisperse feature of the flying droplets formed upon spraying comes with different dry-ing kinetics in each droplet and finally with a more or less wide particle size distribution (fig. 8), making it dif-ficult to model the transport phenomena and study the phase transition and resulting mechanical instability [21]. Moreover, the fast-drying kinetics clearly makes the ex-perimental ability to monitor and sample the process in real equipment even more complex [22].
Single droplet experiments, usually in a pendant con-figuration, have been proposed to overcome these limita-tions, as they represent an easier way to study the dry-ing of a given concentrated product in a controlled envi-ronment. The pendant configuration makes it possible to maintain a spherical shape as much as possible and avoid an inversion of the curvature of the particle, whereas the porous shell collapses under gravity and pressure gradient when water evaporates in the sessile configuration [23,24]. Moreover, the controlled boundary conditions and dry-ing timescale, which usually stands for minutes instead of seconds, make it possible to record the drying kinetics through mass and length indicators. By recording the pen-dant droplet evolution with optical or fluorescence analyt-ical techniques, rheological changes, skin formation and
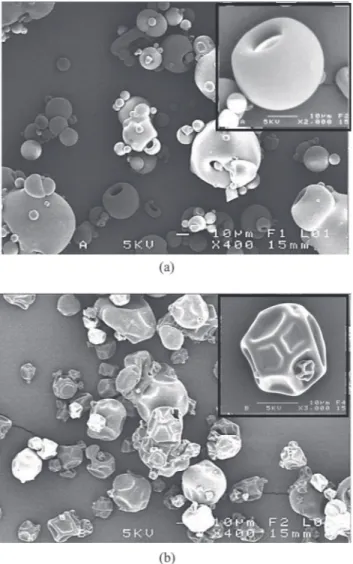
Fig. 8. Particle shapes of dairy proteins material. (a) Whey proteins isolate. (b) Casein micelles isolate.
physical deformations can be tracked. It allows a more di-rect, flexible and detailed investigation of the influence of specific ingredients on the particle characteristics. This ex-perimental concept represents therefore a complementary approach to spray drying in order to improve the under-standing of fundamental phenomena governing drying and further model these latter, that are not yet accessible on an industrial scale.
3.1 Single droplet studies of dairy particle formation During the drying of concentrated solutions of polymer and/or colloid, it has been observed first the formation of a shell at the drop free surface coming from solutes accumulation due to the evaporative flux [24]. Depend-ing on the chemical and physical properties of the solute and the external conditions, later on different surface in-stabilities occur such as buckling, invagination, delami-nation and cracks [25]. Sadek et al. [26–28] investigated on the drying dynamics of pendant droplets made of the two main milk proteins, whey proteins and casein micelles.
Fig. 9.3D confocal reconstitutions of final single pendant par-ticles shape of casein micelles ((a), (b)) and of whey proteins ((c), (d)), top and profile views, respectively.
For that purpose, the concentrated protein solution drop was hanged to a micropatterned substrate and exposed to stressful conditions (relative humidity, RH, below 5%). For both milk proteins, the drying of the single pedant droplet revealed three similar stages involving different evaporation rates and droplet dynamics, that were thus reached at different time according to the protein type. At the beginning, the droplet behaved as a pure liquid with-out a significant influence of the protein surface activity on the drying rate, that remained diffusion controlled. Next, a sol-gel transition was believed to occur at an average con-centration around 41 wt% and 15 wt% for whey proteins and casein micelles, respectively. The gelation fixed the surface area while the water was still evaporating, induc-ing the bucklinduc-ing (initial surface instability) of the droplet and revealing a concomitant hydric rather than mechan-ical equilibrium. Indeed, the surface instability was be-lieved to be mainly initiated by rheological changes here, as it did not impede at first the loss of mass (water evap-oration). The skin then progressively thickened into a ho-mogeneous protein shell, leading to the formation of an internal vacuole. Once the skin layer was formed, the sur-face underwent sursur-face instabilities such as buckling and invagination according to the protein kind, which lead to typical and reproducible particle shapes. Smooth, hemi-spherical and hollow particle was obtained for the drying of the single droplet of whey proteins, whereas deflated wrinkled particle shape resulted from the drying of the single droplet of casein micelles (fig. 9). These results sug-gest particular mechanical properties of the milk protein shell leading to a predictable and a characteristic parti-cle shape, regardless of the experimental configuration. Indeed, Bouman et al. [29] investigating the drying of a single sessile droplet of whey proteins at different drying temperatures, reported the same droplet dynamics and vacuole nucleation occurrence. On the other hand, Fu et al. [30] reported surface invagination similar to that ob-served for casein micelles during the drying of single sus-pended droplet of whole and skimmed milk that was thus mainly constituted by casein micelles.
Moreover, the mechanical properties of the final materials of proteins were characterized using micro-indentation testing by Sadek et al. [27]. It was shown that the interface of micellar casein micelles reached an earlier sol-gel transition, followed by elastic and plastic regimes in which the shell distorted and buckled to form a final wrinkled particle. On the opposite, the interface of whey proteins became elastic only half the drying time, keeping a spherical shape, which finally fractured at the
Fig. 10.Single droplet drying processes: (a) lactose solution; (b) sucrose solution ((a) shows the opacity has developed which is well known due to the lactose transformation towards crys-tallization becoming much less water soluble).
end of drying. The mechanical difference between the two plastic shells might thus be explained by the behavior of proteins in jamming conditions. Interestingly, the same authors showed that the particle shape was governed by the major protein type when drying whey protein: casein micelles droplets, mostly spherical for major whey pro-tein mix, and mostly wrinkled particles for major casein micelles mix [31]. Interestingly, these specific particle mor-phologies were similar to those observed for single pendant droplet experiments, and for powders obtained at real in-dustrial scale spray drying, regardless of the considerable differences in drying timescale and temperature.
3.2 Drying modeling based on single droplet experiment
On the other hand, single drop experiments have been widely used to establish the kinetics of drying and the parameters fed to the milk drying modeling of one-, two-and three-dimensional nature. These models, taking into account both drying kinetics and drying temperatures, have been mostly developed using a pendant configuration glass-filament method with some improvements [32] in or-der to predict the final particle characteristics including moisture content [22,33]. Figure 10 shows a single droplet hung on a glass filament tip during such drying experi-ments.
The droplet drying experiments may be conducted un-der isothermal air conditions or transiently varying tem-perature conditions as long as both the weight loss and the temperature of the droplet recorded. The following outlines the obtainment of the drying kinetics through a Reaction Engineering Approach (REA) [34,35]. The same authors [36] have summarized a wide range of the REA kinetics parameters for food materials. In particular, for several typical dairy products, drying kinetics parameters have been obtained [37–40].
The Reaction Engineering Approach (REA) is a semi-empirical model that defines evaporation as an activation energy that needs to overcome the energy obstacle in order to initiate the process of moisture removal. This approach characterizes the driving force for moisture removal at the
solid-gas interface, i.e. droplet-air interface, through vapor density difference, as expressed in the following equation:
dm
dt = −hmA(ρv,s− ρv,b) (5) where dm/dt represents the drying rate (kg · s−1), h
mthe mass transfer coefficient (m·s−1), A the surface area of the droplet (m2) and ρ
v,sand ρv,brepresent the vapor density (kg ·m−3) at the droplet surface and bulk air, respectively. Since the surface vapor density ρv,s will constantly vary throughout the drying process, it could then be expressed as
ρ(v,s)= ψρv,sat(Ts), (6) where ψ denotes a fractionality coefficient in relation to the moisture content of the droplet and ρv,sat(Ts) denotes the saturated surface vapor density and this term is a function of the surface temperature Ts (K). The distri-bution of temperature of a solid body can be captured through the Biot number. This last represents the com-petition between the thermal energy transfer fluxes inside and outside the solid body, namely the liquid droplet in our case. Previous study has shown that the drying-based Biot number was small when tested under several evapo-ration conditions, meaning that the droplet surface-centre temperature difference is negligible and thus Ts reason-ably equates to the average droplet temperature Td (K). The fractionality coefficient ψ also represents the relative humidity or water activity at the droplet-air interface. In REA modeling, the apparent activation energy of evapora-tion, represented by ∆Ev (J · mol
−1
) describes the change of ψ during moisture removal from the droplet using the subsequent correlation:
ψ = exp(−∆Ev/(RTd)) (7) where R is the universal gas constant (8.314 J · mol−1· K−1) and T
d represents the average droplet temperature. By substituting (7) into (6) and rearranging the terms, the apparent activation energy term ∆Ev can be expressed as ∆Ev = −RTsln(ρv,s/ρv,sat(Ts)). (8) Equation (8) indicates that ∆Ev is a reflection of a vapor concentration depression at the interface of the droplet. By rearranging (8) while substituting the term ρv,sinto (5), one can obtain:
∆Ev= −RTdln µµ −dm dt 1 hmA + ρv,b ¶ Á ρv,sat ¶ . (9) In order to simulate the apparent activation energy ∆Ev at a given drying condition, three parameters such as dm/dt, Td and A, need to be experimentally determined. ∆Ev can be normalized by using the equilibrium or max-imum activation energy ∆Ev,b, which yields the relative activation energy ∆Ev/∆Ev,b(value ranges between 0 and 1). When the apparent relative humidity is in equilibrium with the bulk air relative humidity, it means that the ap-parent activation energy ∆Evhas also achieved its equilib-rium ∆Ev,b. At this equilibrium point, the rate of droplet
mass loss is zero whilst the droplet temperature and mois-ture content match those of the drying medium. Hence the maximum or equilibrium activation energy ∆Ev,b can be calculated from the equation below:
∆Ev,b= −RTbln(ρv,b/ρv,sat(Tb)), (10) where Tb represents the bulk air temperature (K) and ρv,sat(Tb) represents the saturated vapor density of the bulk air (kg · m−3).
Previous investigations have determined that the rel-ative or normalized activation energy ∆Ev/∆Ev,b can be correlated with the droplet’s moisture content X − Xb, generating a master characteristic curve of relative acti-vation energy for a given material:
∆Ev ∆Ev,b
= f (X − Xb), (11) where Xb and X are termed as the particles equilibrium moisture content (kg · kg−1
, dry basis) and moisture con-tent (kg · kg−1, dry basis), respectively. This empirical equation has been tested under many drying conditions and was found to correlate well with the drying kinetics of material with known initial solids content. In other words, this equation is the “fingerprint” of a material’s drying behavior. These kinetics data can be implemented into simulation programs home-made [41, 42] or commercially available computational fluid dynamics modeling software with specific user established operating files for scale up studies and industrial spray dryer design [43–46].
4 Thin cosmetic films structuring due to
phase separation induced by drying
The drying of complex solutions, composed of a polymer or a mixture of polymers dissolved in a mixture of solvents, is a problem of great scientific and technical interest due the diversity of physical phenomena that are implemented and to the great variety of resulting applications. We meet this process, for example, in cosmetology, where complex polymer-solvent solutions are normally used in the form of thin films, a few µm to a few hundred µm thick, spread on a support such as skin. This case involves bio-compatible polymers dissolved in water, a fundamental solvent in bio-physics. Additional additives can be used, such as preser-vatives or antibacterial agents to prevent the proliferation of microorganisms in the solutions, or additives that can restructure the film by modifying the interactions with water during drying.
There are two main aspects of the drying of thin films that interest the scientist, the industrialist and the con-sumer. The first is the kinetics of drying, which must be controlled reproducibly according to the application envis-aged, such as hydration of skin (creams), delivery of bac-tericidal and virucidal molecules (hydro-alcoholic gels) or therapeutic molecules (ointments), or controlled hydration of plants (moisturizing gels). The structuring or morphol-ogy of the dry film is another aspect of fundamental in-terest: the morphology of the film can undergo significant
changes during drying, such as the formation of blisters or craters, accumulation of matter or tearing.
These two aspects are not a priori independent. For ex-ample, rapid dehydration of a thick film may result in the formation of a skin on the free surface of the solution [47]. Under these conditions, the evaporation of solvents into the ambient atmosphere, and thus the kinetics of drying, are affected, as well as the final state of the film that will then contain a significant fraction of residual water. An ac-curate description of these two aspects requires taking into account many of the physical processes that can arise not only within the film —diffusion and convection of mate-rial and thermal energy, a glass transition and viscoelastic relaxation— but also at the interface between the film and the ambient atmosphere —evaporation, demixing, forma-tion of skin— and that between the film and its support —capillarity, wettability and pinning of the triple line.
It is therefore important to combine experimentation and modeling in order to reach a realistic physical descrip-tion of both the kinetics of drying and the structuring of thin films used in cosmetology.
Several physical phenomena related to evaporation, can lead to a structuring of thin films. In low-viscosity colloidal suspensions, pinning of the triple line can induce convective motions within the film, leading to irregular accumulation of material similar to that observed during evaporation of drops of coffee (coffee rings [48,49]). Evap-orative cooling of a solvent in the presence of a humid atmosphere can cause nucleation of fine water droplets that are structured on the surface of the film by thermo-capillary convection and then penetrate the film, giving it an ordered microstructure after complete evaporation [50]. The phenomenon of phase separation within a complex so-lution is also a potential pathway for structuring of thin films. This phenomenon is commonly used in the synthe-sis of porous polymer membranes and foams, for example. Phase separation can be induced in this case by rapidly cooling the solution (thermal-induced phase separation: TIPS), or by quenching after spreading on a support and partial evaporation of the solvent [51]. The process of air-casting is another interesting approach that consists in dissolving the polymer in a mixture of solvents of which one is very little volatile. Evaporation of the more volatile solvent then decreases the solubility of the polymer and induces structuring by phase separation [52, 53].
In each of the two cases envisaged above, the sol-vent evaporation phase plays a fundamental role in the production of membranes with anisotropic structure — highly sought after for ultra-filtration— by accumulating the polymer material inside the film. This approach is, for example, implemented during the formation of porous membranes from a binary solution of cellulose acetate and acetone [54].
It is also possible to use the process of phase sepa-ration induced by drying to structure aqueous solutions used in cosmetology. The colloidal suspensions are spread in the form of a thin film (TF) for which the evaporation of water completely determines its evolution. In this case, the aqueous solution is generally composed of a water-soluble biocompatible polymer (P) such as sodium
car-boxymethylcellulose or polyvinyl alcohol, dissolved in wa-ter (W) in the presence of a small fraction of a preservative or structuring agent (XW) very little volatile, partially sol-uble in W and incompatible with P: TF = P + W + XW. In the case of films used in cosmetics, only the solvent is notably volatile. Drying can induce interesting behavior in these ternary systems at the morphological level. The initial mass fraction w0
Wimplemented for this type of film is of the order of 95%, making it possible to obtain an initially homogeneous solution whose structuring results from separation processes during drying of the thin film.
4.1 Structuring of a cosmetic thin film
Under certain conditions of composition and drying, one can observe a progressive structuring of cosmetic aqueous films due to phase separation induced by water evapora-tion. Figure 11 shows binocular microscope figures track-ing the structurtrack-ing of a cosmetic film durtrack-ing drytrack-ing. The structuring agents XW that we have studied are phenol, phenoxyethanol and phenoxyisopropanol, common addi-tives used in cosmetology and pharmacology. Other agents were also considered —ethanol, glycerol and butanol— but these did not lead to any structuring of the film, be-cause they are either totally miscible with water (ethanol and glycerol) or too wetting to form drops within the film (butanol). We describe here the results obtained with phe-noxyethanol, whose limit of miscibility in pure water is 2.4 wt.% at 20◦
.
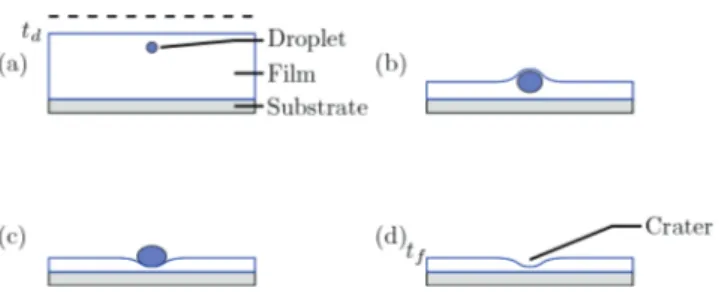
At the initial time t0, the solution is spread mechani-cally on a very clean microscope slide, which is placed in an environmental chamber allowing precise control of the drying conditions. The newly formed thin film is homoge-neous and initially has no deformation. As soon as drying begins, even blisters form within the film in the immedi-ate vicinity of the free surface (see fig. 11). The time td when all the first blisters have formed depends first on the evaporation rate, VE, i.e., the mass flow of water φm leaving the film, and secondly on the initial mass fraction of XW and its chemical nature (see previous discussion). For a relative humidity a∞
w,v = 0.42 and a typical drying temperature T∞= 20
◦
C, td varies over a few seconds for an initial mass fraction w0
X = 2.4% to a few minutes for low values of wX0 (see fig. 12).
We also observe that the blisters partially coalesce and deform as the film loses solvent (see insert at t0+ 23 min, fig. 11), because of the increasing mechanical constraints exerted by the film on the blisters as drying proceeds. Once the film is dry, it exhibits a permanent structure in the form of craters with small diameters (of the order of a few µm) with a relatively homogeneous distribution. These craters occupy the places of the blisters that had appeared during drying (see inset at t0+ 50 min, fig. 11). We have established [55] that the structuring of cos-metic aqueous films could originate in the demixing of the agent XW with the water present in the film. This phase separation occurs when the mass fraction wX reaches the solubility limit of XW in the film. The modeling of the kinetics of drying that we present in the next section
con-Fig. 11. Binocular microscopy images taken at different times, typical of the temporal evolution of structuring of a cosmetic thin film by phase separation induced by evaporation. At the initial time t0, the film that has just been spread on its support, is homogeneous and shows no significant deformation. At the final time tf = t0+ 50 min, the film is completely dry and exhibits a structuring in the form of craters replacing the blisters, as shown by the insets at t0+ 18 min and t0+ 50 min.
firms to a good approximation the values of the time of appearance td of the first drops.
4.2 Modeling of the drying kinetics
Combining experimental and theoretical studies of the drying kinetics of an aqueous thin film of TF type is of paramount importance in cosmetology and pharmacol-ogy. The evolution of the morphology of the film during the drying can have dramatic consequences for the prac-tical implementation of these films. Kinetic studies can provide information on the various interactions not only between the constituents of the mixture but also between the solution and its environment (evaporation atmosphere and spreading support). Understanding the mechanisms of drying and structuring of an aqueous thin film composed of water-soluble polymers requires a preliminary study de-tailed of the kinetics of drying, combining experiments and modeling.
4.2.1 Experimental approach
From an experimental point of view, the drying kinet-ics of a solvent-polymer thin film is generally studied from a direct or indirect gravimetric monitoring of the film throughout the duration of the solvent(s) evapora-tion phase. The volatility of the polymer suspended in the film can be assumed to be negligible compared with that of the solvent(s). The polymer therefore remains present in the solution until the end of the process and gradually becomes structured to finally take the form of a thin solid film. We mainly consider here the case of thin cosmetic films with an initial thickness h06100 µm.
Direct gravimetric monitoring of the drying kinetics by weighing the film is a relatively common approach and easy to implement. However, it is essential in this case to precisely control the various environmental parameters of drying: the temperatures of the surrounding air T∞ and of the support Ts, the ambient humidity, the state of the support surface and the flow velocity v∞ of the surrounding air in the vicinity of the film. It is therefore almost indispensable to work in a controlled atmosphere, in an enclosure where the physical parameters are mea-sured continuously using various miniature sensors placed
Fig. 12.Typical drying curves of aqueous films initially hav-ing a w0
Pmass fraction of 3% for different initial mass fractions w0
X, ranging from 0.4% to 2.4% (adapted from [55]). The inset shows times td when the first drops appear in the film. The dashed line corresponds to the measurements, while the sym-bols (squares) correspond to the calculations. Three successive phases can be identified in the drying kinetics, denoted CR, IP and SP.
near and far from the film. The relative humidity a∞ w,v of the surrounding atmosphere can be imposed using various saturated saline solutions, for example. In order to min-imize the time between the physical preparation of the film (for example, by spreading on the desired support) and its positioning on the precision balance, the spreader is placed in the hermetic environmental enclosure.
Depending on the initial thickness h0, of the compo-sition {w0
i}, where wi is the mass fraction of the ith con-stituent of the mixture, and the initial shape of the film, the drying kinetics can exhibit large variations in the dy-namics (drying time), which may require special experi-mental precautions. In the case of thick samples (several cm), requiring several weeks for complete evaporation of the solvent, it is possible to manipulate the sample dur-ing drydur-ing without noticeably distortdur-ing the gravimetric monitoring [56]. It is quite different in the drying of thin films, whose dynamics can be only a few tens of minutes (see fig. 12). It is then impossible to envisage any ma-nipulation of the film during drying and furthermore it is necessary to have a sufficiently sensitive and fast weighing method to be able to monitor the evolution of the mass m(t) of the film with adequate accuracy. Several routes can be considered in this case: a high-precision gravimet-ric monitoring [55], thermogravimetgravimet-ric analysis (TGA) or differential scanning calorimetry (DSC) [57].
4.2.2 Modeling of the drying kinetics
Taking account of the measurements provided by the var-ious sensors in the environmental chamber leads to a form of inverse analysis. This consists of validating microscopic
models of water transfer based on average macroscopic quantities, such as monitoring the evolution of the mass of the sample as a function of time:
m(t) =X i Z V (t) wic(M, t)dτ, (12) where wc
i(M, t) is the mass concentration of the species (i) in the film, averaged over the entire volume V (t) of the film. It can also be interesting to access the evolution of the temperature Th(t) and the average humidity ahw,v(t) of the film surface, which usually control the evaporation of the solvent towards the atmosphere. Depending on the physical properties of the film (initial dimensions, compo-sition, homogeneity, interactions between its constituents, temperature, dynamic viscosity, elasticity, surface tension and thermal conductivity), the support (surface condition and temperature) and the surrounding atmosphere (con-vection, temperature and relative humidity) one can ob-serve a very wide variety of drying behaviors, which mani-fest mainly by very different kinetics and film structuring. Thus, a film with low viscosity spread on a hydrophobic support will tend to deform and dry like a drop, while a viscous film spread over a wetting support will tend to dry like a flat film. In the latter case, if the triple line is pinned to the support, the lateral dimensions Liof the film plane can be assumed to be constant. Moreover, if these dimen-sions are large compared with the thickness h(t) of the film, a one-dimensional modeling is generally sufficient.
Given the high initial viscosity of the suspension (nearly 105 times that of water), because of the pres-ence of the polymer, we can completely neglect convec-tion within the film [58]. The drying kinetics shown in fig. 12 is therefore described well by an 1D isothermal (thin film, thus low Biot number and evaporative cooling neglected) evaporation-diffusion model. This model pro-vides the spatio-temporal evolution of the mass concen-tration wc
i(z, t) of each constituent (i) in the film [55] by solving the coupled equations of particle diffusion:
∂twic= ∂z(Di∂zwci) , (13) where Di is the diffusion coefficient of component (i) in the film, which depends heavily on the composition of the film at time t. These equations are accompanied by the following boundary conditions: impenetrable support at z = 0, whence
∂zwci(0, t) = 0, ∀i ∈ {P, W, XW} (14) and jump conditions (jump mass balance) at the mobile interface z = h(t) for the non-volatile species:
wic(h(t), t) ˙h = −Di∂zwci(h(t), t), ∀i ∈ {P, XW}. (15) And for the solvent [59]:
wc w(h(t), t) ˙h + Dw∂zwwc(h(t), t) = −hm¡ρhw,v− ρ ∞ w,v ¢ (16) where hm is the mass transfer coefficient; ρhw,v and ρ
∞ w,v represent the water vapor density at the free interface and
far from the film, respectively. By modeling the water va-por as a perfect gas, we can write ρz
w,v = azw,vρzw,sat where az
w,v and ρzw,sat are the water vapor activity and saturated density, respectively, for the position z > h. It remains finally to impose the conservation of the mass of the evap-orated solvent: ˙h = −vwhm¡ρhw,satahw,v− ρ ∞ w,sata ∞ w,v ¢ (17) where vw is the specific volume of the liquid water.
The differential equations (13) and (17) accompanied by the conditions (14), (15) and (16) constitute a nonlin-ear problem, whose numerical resolution can be challeng-ing if we seek to describe the totality of the drychalleng-ing kinetics represented in fig. 12. A preliminary analysis of the exper-imental drying curves, however, makes it possible to apply common laws of behavior.
Typically, we observe (see fig. 12) three distinct succes-sive phases in the drying of cosmetic aqueous films with a high initial fraction of water (w0
W > 0.9). The drying always starts with a phase with quasi-constant evapora-tion rate (CR). This type of behavior is also encountered in the drying of hydrogels, fruits and food [35, 56, 60, 61]. This first phase usually lasts about five minutes under the drying conditions that we have implemented (room tem-perature and relative humidity close to 43%). During CR, the evaporation of the solvent at the free surface of the film completely governs its loss of water. During this phase the mass fraction of water in the film remains sufficiently large that we can consider that the chemical activity of the water at the interface ah
w,v stays close to one. The polymer P is therefore practically chemically not involved in the kinetics and the film dries almost like a pure water film during this phase. The second phase (IP), which then takes place for several tens of minutes, sees the chemical activity of the solvent decrease sharply because of the in-crease in the polymer mass fraction due to evaporation. The water loss results from a competition between sur-face evaporation and mass transfers in the volume of the film. This phase is in general strongly nonlinear because of the dependence of several physical quantities (diffusion coefficients and chemical activity of water at the inter-face) with the fraction mass of water. Interactions between the different constituents of the complex aqueous solution vary greatly during IP. We then observe a final phase (SP) during which the rate of evaporation tends to zero. This phase, corresponding to solidification of the cosmetic film, can be very complex and generally presents a challenge to an evaporation-diffusion modeling.
Despite the low initial mass factions w0
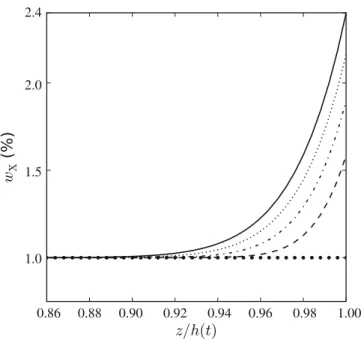
Xof the additive XW, we observe that the blisters are always formed during the first CR phase, in the immediate vicinity of the free surface of the film. This observation reflects the difficulty of diffusion of XW through the film and a trend towards accumulation at the free surface. The demixing of XW is described well by the diffusive model presented previously, with a diffusion coefficient DX which can to a good ap-proximation be assumed constant during the short-lived CR phase. The values of the diffusion coefficient DX and solvent flow φmcan be determined from the experimental
2.4
2.0
1.5
1.0
0.86 0.88 0.90 0.92 0.94 0.96 0.98 1.00
Fig. 13.Profiles of the mass fraction wXof XWin the vicinity of the film interface z/h(t) = 1 at different times (t = 0, 13, 26, 39 and 52 s). The demixing takes place at z/h(t) = 1, when wXreaches the solubility limit, here t ≈ 52 s. The initial mass fraction is taken to be w0
X= 1%. (Figure adapted from [55].)
curves of drying shown in fig. 12. This allows a numerical resolution of eq. (13) with the boundary conditions (14) and (15), for i ≡ X. We thus obtain the XW mass frac-tion profiles shown in fig. 13 and the demixing times rep-resented in the inset of fig. 12. There is a good corre-spondence between the experimental results (dashed curve in the inset) and the proposed model (square symbols), which validates the evaporation-diffusion model chosen to describe the kinetics of cosmetic aqueous films.
4.3 Crater formation
The proposed evaporation-diffusion model makes it pos-sible to predict the appearance of demixing within the cosmetic film during the drying cycle. On the other hand, it does not explain why this phase separation leads to for-mation of blisters and finally leaves craters in the dry film. Analysis of the surface of the film by micro-confocal Raman spectroscopy shows that the blisters are predom-inantly composed of water. Once the film is dry, the Ra-man spectra show that each crater contains only a small amount of XW. These observations imply that the blisters are composed of a thin film of XW, which encloses at the time of demixing a micro-drop of water. This very small amount of water is protected from evaporation by a film of XW, whose saturated vapor pressure (4 Pa in the case of phenoxyethanol) is very low compared with that of wa-ter. The viscosity of the solution greatly increases during drying, so the drop remains permanently in the imme-diate vicinity of the free surface. The water retention is maintained until the protective film breaks because of the strong mechanical constraints that develop within it in the
Fig. 14.Schematic representation of the steps leading to the crater formation. (a) Formation of drops at td, in the immedi-ate neighborhood of the free surface; the dashed line indicimmedi-ates the initial level of the free surface; (b) growth of the drop by coalescence; (c) resurgence and destruction of the drop on the film surface; (d) formation of a persistent crater in the dry film. (Figure extracted from [55].)
last moments of drying. The schematic representation of fig. 14 summarizes the successive stages that lead to the formation of craters in the dry film.
The use of XW agents that are partially miscible in water but have a surface tension a factor of about two lower than phenoxyethanol, phenoxyisopropanol and phe-nol does not lead to any structuring of the film despite the demixing. Thus, it takes a particular combination of physico-chemical properties of the XW agent to produce the formation of permanent craters in the dry film.
4.4 Conclusions
The demixing caused by the evaporation of water is an interesting way of structuring aqueous thin films used in cosmetology. Depending on the nature of the constituents of the initial aqueous suspension, it is possible to form craters of relatively homogeneous size that persist in the dry film, without noticeably changing its final elastic prop-erties. This structuring phenomenon could be put to use to design cosmetic films of a given porosity, making it possible, for example, to control the exchanges between the skin and its environment. The model presented in this work can serve as a starting point for many investigations not only in cosmetology but also in pharmacology, where use of microporous therapeutic films could have certain benefits in the treatment of certain pathologies, such as wounds or burns.
5 Medicine and forensics applications
From fluid mechanics point of view, blood is a complex colloidal suspension that behaves like a non-Newtonian fluid. Human blood is composed of different cells that form about 45% of whole blood volume and 25% of the total blood mass. They are classified as red blood cells (RBCs), or erythrocytes; white blood cells (WBCs), or leukocytes; and platelets (thrombocytes). The other 55% is blood plasma, which is composed of water (over 90%), ions, electrolytes (salts), plasma proteins (7%, most of which are by weight), and other substances transported
by blood. The main physical properties of whole human blood, such as the density, viscosity, and surface tension, can be found in the literature [62].
Hereafter, we focus on the type of blood drying in droplet or pool geometry. Blood droplets and pools of blood are of huge interest in forensic science to retrieve relevant information from a crime scene. The scientific objective has succeeded in performing an accurate reverse engineering. The question to be asked is simple: from a fluid already spread on a substrate and already drying or dried, is it possible to obtain accurate and relevant infor-mation? But blood droplets spreading and drying are also of the tremendous potential of biomedical applications. Here also, a reverse engineering work could potentially reveal the blood composition based on the patterns ob-served in the dried droplet. Here the blood composition is the main complexity to perform a simple reverse engineer-ing. Later we will present a few parameters that affect the final pattern of dried droplets.
5.1 Drop of blood
Evaporation of sessile drops of biological fluid is studied as a potential area of interest for medical applications. Most studies have been conducted on biological fluids and blood serum, but a few have focused on whole human blood. Sev-eral parameters influence the evaporation, gelation, crack-ing, and delamination processes of a drop of whole hu-man blood such, as for example, the surface roughness or the ambient humidity rate. The first parameter influences the triple line stability when moving for example during the spreading. The second clearly changes the evaporation rate and then the stresses encountered in the colloidal sus-pension under phase change.
5.1.1 Medical application
One of the parameters that influence the drying rate, and thus the final pattern, is the ambient humidity rate, RH. The influence of the relative humidity on the pattern at the end of the drying phase has been investigated by con-sidering the same volume of drops of blood evaporating in different RH levels. Final drop images are presented in fig. 15, which shows morphological and structural evo-lutions of a drying drop of whole blood for different val-ues of RH. For each RH, the bottom images are taken at the final stage of the drying process. One clearly ob-serves a structural change and, consequently, the morpho-logical evolution of the final drying pattern. At the final stage of the evaporation, the pattern is characterized by three distinguished regions: a central part composed of a sticking deposit and a network of small cracks, a corona composed of mobile plaques organized radially, and a fine periphery strongly adhering to the substrate. It can be observed that the width of mobile plaques in the corona and the fine periphery region become larger as the RH increases. Also, fig. 15 shows that mobile plaques depend on the drying rate. At low drying rates (high RH), more
Fig. 15. Final deposition morphology for various RH values (RH varying between 13.5% and 78.0%). Area between dashes in yellow represents the fine periphery corona. Figure repro-duced with permission from [63].
completely adhering mobile plaques are observed than at high drying rates (lower RH). Indeed, low adhering areas are highlighted by the circulated red light region that is found in each of the mobile plaques in the corona area. The final drying pattern and crack nucleation vary with the evaporation rate as presented in fig. 15. Under our ex-perimental conditions, the transfer of water in the air is limited by diffusion and is controlled by the RH in the surrounding air. The drying process of a sessile drop of blood is characterized by an evolution of the solution into a gel saturated with solvent. When the gel is formed, the new porous matrix formed by the aggregation of particles continues to dry through evaporation of the solvent that causes the gel to consolidate. As the liquid progressively recedes into the porous medium, it forms at first menisci at the air/solvent interface due to capillary tension be-tween the particles and then causes liquid bridges bebe-tween the particles. During solvent evaporation, the curvature of the solvent air menisci is responsible for a capillary pres-sure in the liquid phase. This depression induces shrink-age of the porous matrix that is constrained by the ad-hesion of the deposit to the glass substrate and the evap-oration of the solvent. As tensile stresses build up, the internal stresses become too great, and fractures appear so as to release mechanical energy. Assuming that gela-tion is due to particles accumulagela-tion, we attribute these differences in pattern formation to the competition be-tween the drying process and the adhesion of the gel on the substrate.
Final dried images presented in fig. 15 show that the fi-nal adhering area of mobile plaques is strongly dependent on the RH values. By changing the drying rate, the me-chanical properties of the drying gel are modified, such as
the adhesion energy of the gel to the glass substrate (ac-cording to the Griffith theory). Indeed, the surface area of mobile plaques of drops of blood dried at RH from 13.5% to 50.0% becomes progressively larger with a lower adhering region. This adhering region shrinks until the formation of a circular adhering region that leads to a delamination process. For images of RH above 50%, mo-bile plaques are smaller with a higher adhering region. This observation is due to the buckling process that is rapidly overcome by the adhesion of the gel to the sub-strate. In desiccated colloidal gel dried at RH = 70% and RH = 46%, Pauchard experimentally showed that com-plete adhesion or de-adhesion is a function of the cell sur-face area [64]. Moreover, the large fine periphery of the stick deposit onto the glass substrate is due to the reced-ing of the gel front, causreced-ing the absence of RBCs. This result is due to a change in the internal flow that trans-ports RBCs from the centre to the periphery of the drop of blood.
5.1.2 Forensic application
The first obvious parameter which influences the pattern of a dried drop of blood after impact is the impact velocity. The added kinetic energy for the same volume leads to a larger spreading of the droplet and to a decrease of its thickness. The evaporation is thus preferred at the contact line, which generates a surface tension gradient that drives a stronger re-circulation, namely Marangoni flow, and for the RBCs to homogenize. Moreover, the coffee ring effect is exposed to a timescale competition between the water evaporation and the movement of RBCs. A clear coffee ring effect cannot be successfully observed if the water evaporates faster than the RBCs movement towards the periphery of the droplet. Hence in the case of a droplet impacting at a higher velocity for the same volume, the thinning of the drip stain thickness induces a faster evap-oration and a change in the internal flows. The 3D profile visualisation of the dried bloodstain was used to charac-terize the features marking the rim dimensions in a radial cross section. Thus we were able to record the internal diameter of each droplet as shown in fig. 16, by averag-ing its measurement in different radial cross-sections, and
Fig. 16.View of dried blood drip stains with the bulging outer rim, from a healthy volunteer. Droplets of initial diameters of 2.4 mm, and of impact velocities from left to right of 0.74 m/s, 1.87 m/s and 4.75 m/s. On the left side with photography, on the right side with confocal microscopy that gives the 3D fea-tures by means of radial cross-sections. Figure reproduced with permission from [65].
![Fig. 1. (a) TEM image of a dried boehmite nanorod suspension [1]. (b) Casein micelles isolate](https://thumb-eu.123doks.com/thumbv2/123doknet/2991910.83287/3.892.86.807.136.408/image-dried-boehmite-nanorod-suspension-casein-micelles-isolate.webp)
![Fig. 3. Ball bearing parts reinforced by ceramic coatings [5,6].](https://thumb-eu.123doks.com/thumbv2/123doknet/2991910.83287/4.892.512.769.137.354/fig-ball-bearing-parts-reinforced-ceramic-coatings.webp)
![Fig. 5. Schematic phase diagram of a boehmite suspension with added cerium nitrate Ce(NO 3 ) 3 inspired from the exper-iments detailed in [1]](https://thumb-eu.123doks.com/thumbv2/123doknet/2991910.83287/5.892.94.404.139.294/schematic-diagram-boehmite-suspension-cerium-nitrate-inspired-detailed.webp)