Mechanistic insights of SIDER2
retroposon-mediated mRNA decay in Leishmania
Thèse
Hiva Azizi
Doctorat en biologie cellulaire et moléculaire
Philosophiae Doctor (Ph. D.)
Québec, Canada
© Hiva Azizi, 2017
Mechanistic insights of SIDER2
retroposon-mediated mRNA decay in Leishmania
Thèse
Hiva Azizi
iii
Résumé
Leishmania est un pathogène important pour la santé humaine avec plus de
350 millions de personnes à risque dans le monde. Leishmania présente des caractéristiques uniques en termes de régulation génique constituant ainsi un excellent modèle pour l’étude des mécanismes de régulation génique. Chez
Leishmania ainsi que les autres Trypanosomatidae, il n’y a pas de controle au niveau
de l’initiation de la transcriptin et la regulation se fait pas presque exclusivement au niveau post-transcriptionnel. Les éléments régulateurs en cis situés dans les régions 3' non-traduites (3'UTRs) des ARN messagers chez Leishmania (ARNms) sont essentiels pour la régulation de la stabilité ou la traduction des transcrits. Malgré les efforts considérables déployés pour l’identification de ces éléments régulateurs, uniquement quelques centaines ont été caractérisées chez les eucaryotes. Nous avons identifiés une nouvelle classe de rétroéléments agissant en cis, appelés SIDERs (Short
Interspersed DEgenerate Retroposons) qui sont largement distribués dans le génome
du parasite (>2000 copies de SIDER1 et SIDER2), situés pour la plupart dans la région 3ʹUTR. Les transcrits contenant SIDER2 le région 3ʹUTR sont dégradés par un mécanisme indépendant de la déadénylation initié par un clivage endonucléolytique au sein de la séquence signature II (SII) qui est conservée parmi SIDER2. Mon travail a consisté à déterminer les séquences nécessaires pour le clivage endonucléolytique et à identifier les trans-régulateurs jouant un rôle dans la dégradation des ARNm dépendante de SIDER2. Nous avons adopté une approche de purification d'affinité d'ARN étiquetés MS2 permettant de capturer les facteurs trans-régulateurs. Parmi ces éléments liant spécifiquement SIDER2, la Pumilio protéine PUF6 est responsable de la dégradation du transcrit rapporteur possédant la séquence SIDER2 en 3ʹUTR. De plus, l’inactivation du gène PUF6 se manifeste par une augmentation de stabilité des transcrits, suggérant un rôle de PUF6 dans la dégradation des ARNm médiée par SIDER2. Des études de mutations au sein de la séquence conservée, signature II, responsable de la régulation de la dégradation, ont permis de souligner l’importance de sites de clivages putatifs, précédemment identifiés au niveau de SIDER2. De plus, deux régions additionnelles proches de l’extrémité terminale de la séquence SIDER2 se sont révélées de jouer aussi un rôle au niveau de la déstabilisation de l’ARNm.
iv
Enfin, nous avons investigué le rôle de la traduction au niveau de la dégradation des ARNm médiée par SIDER2 et nous avons montré que la dégradation des transcrits SIDER2 est liée à une traduction active, soulignant ainsi l’importance de la machinerie de la traduction au niveau de la régulation globale des transcrits contenants des éléments SIDER2 dans le 3’UTR..
v
Abstract
Leishmania spp. are important human pathogens which put lives of over 350
million people at risk, worldwide. Apart from being an important human pathogen,
Leishmania has unique features in terms of gene regulation, rendering it an excellent
model organism to study gene regulation mechanisms. Notably, Leishmania and other trypanosomatids lack control at the level of transcription initiation and therefore most of the regulation of gene expression takes place post-transcriptionally. Cis-acting elements in 3ʹ-untranslated regions (3ʹUTRs) of Leishmania messenger RNAs (mRNAs) are central to the regulation of mRNA decay or translation efficiency. We have identified a novel class of cis-acting retroposons, termed SIDERs (Short
Interspersed DEgenerate Retroposons) that are widely distributed in the parasite
genome (>2000 copies of SIDER1 and SIDER2), mostly within 3ʹUTRs. Transcripts bearing SIDER2 in their 3ʹUTR are degraded via a deadenylation-independent pathway involving endonucleolytic cleavage within the conserved signature II (SII) sequence of SIDER2 elements. My research project aimed at determining the sequence requirements for endonucleolytic cleavage and identifying the trans-acting factor(s) contributing to SIDER2-mediated mRNA decay. We employed a tethering approach using the MS2 system to capture the trans-acting proteins in vivo. Amongst the proteins specifically tethered to SIDER2, the Pumilio protein PUF6 was shown to downregulate the SIDER2-harboring reporter transcript. Furthermore, inactivation of the PUF6 gene resulted in upregulation and increased transcript stability, indicating that PUF6 contributes to SIDER2-mediated decay. Mutational analysis within the conserved SII region, known to regulate decay, highlighted the importance of the previously mapped putative cleavage sites in mediating degradation of SIDER2-containing transcripts. Furthermore, two additional regions closer to the end of the SIDER2 sequence were found to contribute to mRNA destabilization. Finally, we addressed the requirement of translation for SIDER2 mediated decay and showed that degradation of SIDER2 transcripts is linked to ongoing translation, underscoring significance of the translation apparatus in global regulation of SIDER2-containing transcripts.
vi
Table of contents
Résumé ... iii Abstract ... v Table of contents ... vi List of Tables ... ix List of Figures ... x Abbreviation list ... xi Avant-Propos ... xiv Acknowledgments ... xiv Contributions ... xviChapter 1: Leishmania and Leishmaniasis ... 1
1.1 Biology and life cycle of Leishmania spp... 1
1.1.1 New World Leishmania spp. ... 2
1.1.2 Old World Leishmania spp. ... 3
1.2 Leishmaniasis ... 4
1.2.1 Cutaneous Leishmaniasis (CL) ... 5
1.2.2 Visceral Leishmaniasis (VL) ... 6
1.2.3 Mucocutaneous Leishmaniasis (MCL) ... 7
1.2.4 Diffuse Cutaneous Leishmaniasis (DCL) ... 7
1.2.5 Post-Kala-azar Dermal Leishmaniasis (PKDL) ... 7
1.2.6 Leishmania/HIV co-infection ... 8
1.3 Diagnosis ... 8
1.4 Vaccine development and treatment ... 10
Chapter 2: Digenetic Life Cycle of Leishmania and Interaction with Vertebrate Host ... 14
2.1 Insect Life Stages ... 14
2.2 Subverting the host immune system and entry into macrophages ... 16
2.2.1 Inactivation of the host complement system ... 17
2.2.2 Uptake by neutrophils and transmission to macrophages ... 18
2.2.3 Entry to macrophages, differentiation to amastigotes and survival within macrophages ... 20
2.2.4 Manipulating macrophage signaling pathways ... 22
2.3 Environmental stress and amastigote differentiation ... 23
2.4 Leishmania virulence factors ... 24
Chapter 3: Genome Organization and Regulation of Gene Expression in Trypanosomatids ... 29
3.1 Genome Organization in Trypanosomatids ... 29
vii
3.1.2 Trans-splicing and mRNA Processing ... 34
3.1.2.1 Regulation of mRNA processing ... 37
3.1.3 mRNA export to the cytoplasm and regulation of mRNA export ... 38
3.1.4 mRNA decay ... 39
3.1.4.1 Mechanisms of mRNA Decay in Eukaryotes ... 39
3.1.4.2 Mechanisms of mRNA Decay in Leishmania and trypanosomes . 41 3.1.5 Co-translational mRNA surveillance mechanisms ... 59
3.1.5.1 Nonsense-mediated decay (NMD) ... 59
3.1.5.2 No-go decay (NGD)... 60
3.1.5.3 No-stop decay (NSD) ... 60
3.1.6 P-bodies and stress granules in trypanosomatids ... 61
3.2 Translation ... 63
3.2.1 Regulation of translation initiation ... 64
3.2.2 Regulation of translation elongation ... 69
3.2.3 Post-translation modification of proteins ... 69
3.3 Mitochondrial RNA editing in trypanosomatids ... 72
3.3.1 Mechanisms of mitochondrial RNA editing in trypanosomatids ... 74
Chapter 4: Transposable Elements ... 78
4.1 Transposable elements in higher eukaryotes ... 78
4.2 Impact of retrotransposons on gene regulation ... 79
4.3 Retrotransposons in trypanosomes ... 81
4.4 Leishmania retrotranspososns ... 83
4.4.1 SIDER2 elements and regulation of gene expression in Leishmania ... 86
Chapter 5: Hypothesis and Objectives ... 90
Chapter 6: SIDER2 retroposon-mediated mRNA decay in Leishmania is coupled to translation ... 94 Résumé ... 94 Succinctus ... 95 Abstract ... 96 Acknowledgements ... 104 References ... 105 Figure legends ... 108 Figures ... 111 ... 111
Chapter 7: RNA secondary structure and nucleotide composition within the conserved hallmark sequence of Leishmania SIDER2 retroposons are essential for endonucleolytic cleavage and mRNA degradation ... 114
Résumé: ... 114
Article ... 115
Abstract ... 116
Introduction ... 117
viii Results ... 121 Discussion ... 127 Acknowledgements ... 130 References ... 131 Figure legends ... 136 Figures ... 140 Supplementary data ... 146
Supplementary Figure Legends ... 148
Chapter 8: The Pumilio PUF6 protein contributes to SIDER2-mediated mRNA decay in Leishmania ... 151
Résumé ... 151 Article ... 153 Abstract ... 154 Introduction ... 155 Experimental Procedures ... 156 Results ... 159 Discussion ... 166 Acknowledgements ... 169 References ... 170 Tables ... 175 Figure legends ... 176 Figures ... 180 Supplementary Table ... 186
Supplementary Figure legends ... 188
Chapter 9: General Discussion ... 199
9.1 Cis-acting requirements of SIDER2-mediated mRNA decay ... 199
9.2 Trans-acting factor(s) contributing to SIDER2-mediated mRNA decay ... 205
9.3 Link of SIDER2-mediated mRNA decay to ongoing translation ... 210
Chapter 10: Conclusions ... 214 References ... 217 Appendix 1. ... 250 Appendix 2. ... 251 Appendix 3. ... 252 Appendix 4. ... 253 Appendix 5. ... 254
ix
List of Tables
Table 1: Classification of the Leishmania genus. ... 3 Table 2: Status of current vaccine candidates. ... 13 Table 3: Components of the main mRNA decay pathways originally discovered in T.
brucei and their orthologs in L. infantum. ... 43
Table 4: The cis-acting elements and trans-acting proteins involved in mRNA decay in trypanosomatids. ... 56 Table 5: Transposable elements identified in the trypanosomatids. ... 83
x
List of Figures
Figure 1-1: The life cycle of Leishmania. ... 2
Figure 1-2: Global endemicity of cutaneous leishmaniasis, 2013. ... 6
Figure 1-3: Global endemicity of visceral leishmaniasis, 2013. ... 6
Figure 2-1: Insect Leishmania life stages. ... 16
Figure 2-2: Inactivation of the complement system by Leishmania spp. ... 18
Figure 2-3: Implication of neutrophils in the Leishmania survival within the human host. ... 20
Figure 2-4: Virulence factors of Leishmania parasites and the mechanisms by which they subvert host macrophage defense. ... 26
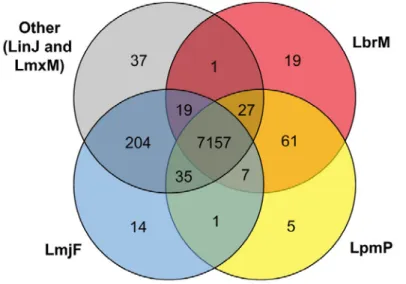
Figure 3-1: Distribution of orthologous groups among species of the L. (Leishmania) and L. (Viannia) subgenera using a simplified Venn diagram. ... 31
Figure 3-2: Mechanism of trans-splicing in Leishmania and other trypanosomatids. ... 35
Figure 3-3: Gene organization, expression and processing to mature mRNAs in Leishmania. ... 36
Figure 3-4: General pathways of mRNA decay in eukaryotic cells. ... 40
Figure 3-5: Major protein subunits of the CAF1-NOT complex in T. brucei. ... 45
Figure 3-6: Proposed model of mRNA decapping and recapping in T. brucei. ... 49
Figure 3-7: Model of the canonical pathway of eukaryotic translation initiation. ... 66
Figure 3-8: Classification of known RNA-editing across eukaryotes. ... 73
Figure 3-9: Mechanism of mitochondrial U insertion/deletion editing in trypanosomatids. ... 75
Figure 3-10: RNA editing core complex (RECC or editosome) components. ... 76
Figure 3-11: Proposed model for integration of RNA editing machinery with polyadenylation complex and the Ribosome. ... 77
Figure 4-1: Effects of retrotransposons on gene expression. ... 81
Figure 4-2: : A schematic representation of a SIDER2 retrotransposon in Leishmania. ... 85
xi
Abbreviation list
AGO : Argonaute proteinαIR : intergenic region of α-tubulin
APE1 : Apurinic/apyrimidinic endonuclease 1
APOBEC1 : apolipoprotein B-editing enzyme, catalytic polypeptide-1 ARE : AU-rich element
BCG : Bacillus Calmette Guerin BF : bloodstream form
BirA : bifunctional ligase/repressor BirA
BioID : proximity dependent biotin identification cDNA : complementary DNA
CHX : cycloheximide CL : cutaneous leishmaniasis CMI : cell-mediated immunity CNS : central nervous system cox : cytochrome oxidase CPs : cysteine proteases DAT : direct agglutination test DC : dendritic cells
DCL : diffuse cutaneous leishmaniasis DGC : directional gene cluster
DIRE : degenerated ingi related element EFs : Elongation factors
eIFs : eukaryotic translation initiation factors ELISA : enzyme linked immunoabsorbent assay eRFs : eukaryotic release factors
FDA : Food and Drug Administration G3BP : RasGAP SH3 binding protein
GCN2 : general control non-depressible-2 kinase GP63 : major surface glycoprotein of 63 kDa GRBC : gRNA binding complex
gRNA : guide RNA
HAART : highly active antiretroviral therapy HAT : histone acetyltransferases
HDAC : histone deacetylases
HIV : human immuno-deficiency virus HSGs : heat stress granules
HSP : heat shock protein
HYG : hygromycin B resistance gene IFN-γ : interferon gamma
IL : interleukin IR : intergenic region
IRE1 : Inositol-requiring enzyme 1 IV : intravenus
xii
KDAC : lysine deacetylases kDNA : kinetoplast DNA KO : knock out
LINE : long interspersed nulear element LPG : lipophosphoglycan
LPS : lipopolysaccharide LTR : long-terminal repeat
LUC : firefly luciferase reporter gene LYST : lysosomal trafficking regulator Mb : mega base pairs
MCL : mucocutaneous leishmaniasis
MRB : mitochondrial RNA binding complex mRNA : messenger RNA
mRNP : messenger ribonucleoprotein mt : mitochondrial
NE : neutrophil elastase
NEO : neomycin phosphotransferase gene NET : neutrophil extracellular traps NGD : no-go decay
NH : nucleoside hydrolase NHP : non-human primates NK : natural killer cells
NLS : nuclear localization signal NO : nitric oxide
NMD : nonsense-mediated decay NPC : nuclear pore complex NSD : non-stop decay
Oligo(dT) : oligonucleotide with 18 thymidines ORF : open reading frame
PABP : poly(A)-binding protein PAP : poly(A) polymerase
PARN : polyadenylate ribonuclease PBM : post-blood meal
PCR : polymerase chain reaction PE : primer extension
PERK : pancreatic endoplasmic reticulum eIF2 kinase PFE : paraflagellar rod regulatory element
PFR : paraflagellar rod PG : phosphoglycan PH : peckstrin homology PIC : pre-initiation complex
PKDL : post-kala-azar dermal leishmaniasis PM : peritrophic matrix
PMR1 : Polysomal ribonuclease 1 pol : polymerase
PPG : Proteophosphoglycan
xiii
PUR : puromycin
PUF : puffyeye, isoform F; pumilio protein PV : parasitophorous vacuole
REMC : RNA editing mediator complex RECC : RNA editing core complex
RESC : RNA editing substrate binding complex RIME : ribosomal mobile element
RNAi : RNA interference ROS : reactive oxygen species RRM : RNA recognition motif
RT-PCR : polymerase chain reaction with reverse transcribed RNA SGs : stress granules
SIDER : short interspersed degenerated retroposon SINE : short interspersed nuclear element
SL : spliced leader
SRP : signal recognition particle SSR : strand switch region
TbCe1 : T. brucei cytoplasmic capping enzyme TbCmt1 : T. brucei cytoplasmic methyltransferase 1 TbDcp2 : T. brucei decapping protein 2
tdMS2 : tandem MS2
tdMCP : tandem MS2 coat protein TC : ternary complex
TE : transposable element TF : transcription factor Th1/Th2 : T-helper cells TNF : tumor necrosis factor tRNA : transfer RNA
TREX : transcription/export complex TSD : target site duplication
TUTase : terminal uridylyltransferase URE : U-rich element
UTR : untranslated region UV : ultraviolet
VL : visceral leishmaniasis
VSG : variant surface glycoprotein WT : wild-type
XBP1 : X box-binding protein-1 ZEO : zeocin
xiv
Avant-Propos
Acknowledgments
Now that my thesis and PhD has finally come to an end and I am about to graduate, I would like to express my deepest gratitude to all those who helped me throughout these years to finish my PhD studies, successfully. My father used to tell me that there is nothing greater than continuing education to its highest level. He certainly has been the main source of inspiration for me, ever since I remember.
At the top of my list, I would like to first, sincerely thank my supervisor, Prof. Barbara Papadopoulou for accepting me and giving me the opportunity of being in her lab. She has been always available for me whenever I faced a challenge or an obstacle in my experiments and her advise was always fruitful. I very much appreciate the discussions we had, either in our personal meeting or group meeting which greatly contributed to my understanding on how to manage and troubleshoot research projects. I would also like to sincerely thank her to allow me to learn and improve my writing skills during preparation of my manuscripts and the thesis; and to teach me the importance of writing skills. I must also thank Prof. Marc Ouellette and Dr. Dave Richard for kindly accepting evaluation of my thesis and being on my thesis examiner's board.
I owe my gratitude to Dr. Reza Salavati (McGill University) who in every opportunity we met either in conferences held in Canada or US or through Skype or email communications, did not hesitate to share his knowledge and provide me with his useful comments. I also thank him for accepting to be the external referee for my thesis. My gratitude also goes to my former mentor, Dr. Sima Rafati who brought me to Leishmania field, let me develop my scientific skills and taught me how science creates humble and generous scientists.
I want to thank senior students, Dr. Aurélien Dupé and Dr. Serge Cloutier for their assistance in setting up experiments or sometimes devises and for their team work. I very much enjoyed their opposite, special characters which never deprived me from having fun with them. My special thanks goes to Dr. Prasad Padmanbhan
xv
and Dr. Mukesh Samant who I had the chance to meet them. Their modesty and "down to earth" attitude are unforgettable. I deeply appreciated all sorts of discussions I had with them, from the very scientific to very geographical-historical ones. I find myself very lucky to receive Dr. Padmanabhan back to BP team and profiting from his always available comments and suggestions. I am very thankful to Carole Dumas for being so helpful to me from the very beginning in helping to find accommodation to implementing the experiments and ordering lab stuff. More than a colleague, she has been a friend to me and for that I am very glad. I have also had great time with former post-doctoral fellows, Dr. Tatiany Romao. I should express my gratitude to Dr. Ouafa Zghidi Abouzid for many things, from helping me with carrying out the qPCR experiment on the Roche LightCycler 480 instrument to the translation of abstracts of my manuscripts and thesis into French and to her constant moral support during the last years of my PhD. I am also glad to have the junior PhD student, Bruno Guedes in our lab whose curiosity and enthusiasm is very encouraging.
I would like to express my deepest gratitude to my parents who never stopped their unconditional love and support. Two other persons whose contribution to the success of my PhD is undeniable and come probably before all; my spouse, Zeinab and my daughter Avin, I could have a tough time without their patience and understanding. My thesis is dedicated to both of you along my eternal love.
Finally, I would like to thank Canadian Institutes of Health Research (CIHR), Université Laval and Centre for Host-Parasite Interactions (CHPI) for their financial support throughout my PhD studies which without their support, this work could have never been accomplished.
xvi
Contributions
My thesis is presented in the form of articles. I have done most of the work myself under the direct supervision of my research director, Prof. Barbara Papadopoulou. Below are manuscripts listed with my contribution, which represent three different thesis chapters and one appendix (Appendix 5).
The manuscript presented in Chapter 6, entitled “SIDER2
retroposon-mediated mRNA decay in Leishmania is coupled to translation” has been
presented and written in form of a ''short communication'' and consists of 3 Figures. I designed, performed and analyzed most of the experiments (Figure 1 and 2). Michaela Müller-McNicoll carried out the experiments presented in Figure 3. I wrote the first draft of the manuscripts and Prof. Barbara Papadopoulou, revised and wrote the final draft and supervised the project. This manuscript has been accepted and recently published in the ''International Journal for Parasitology'' (IJP).
In the manuscript presented in Chapter 7 entitled “RNA secondary structure
and nucleotide composition within the conserved hallmark sequence of
Leishmania SIDER2 retroposons are essential for endonucleolytic cleavage and
mRNA degradation” I did most of the experiments and analysis of the results. Dr.
Tatiany Tatiany Patricia Romão and Dr. Osvaldo de Melo Neto designed and generated mutant constructs, M1, M2, M3, M4, M5 and M6. I generated additional mutant constructs, M6-1 and M6-2. Dr. Karen Santos Charet performed the in silico RNA structure predictions of the mutant SIDER2 signature 2, presented in Figure 2A. Michaela Müller-McNicoll implemented the primer extension experiments (Figure 4). Dr. Prasad Kotail Padmanabhan helped me with Northern blotting demonstrated in Figure 3. I wrote the first draft of the manuscript and Prof. Barbara Papadopoulou supervised the project as a whole and wrote the final draft. The manuscript has been submitted to PLoS ONE.
The work presented in Chapter 8 entitled“The Pumilio-domain protein
PUF6 contributes to SIDER2 retroposon-mediated mRNA decay in Leishmania“
xvii
almost all experiments. Carole Dumas helped with the Northern blots (α-tubulin) demonstrated in Figures 4 and 5. I wrote the first draft of the manuscript and Prof. Barbara Papadopoulou supervised the project and wrote the final draft.
The work presented in Appendix 5, entitled “A mitochondrial 3'-5'
exoribonuclease of the DEDD family plays a role in guide RNA (gRNA) biogenesis in Leishmania“ describes my side project which is awaiting pending experiments in order to publish the work. I have written the incomplete manuscript, peformed and analyzed all experiments. Prof. Barbara Papadopoulou has been supervising the project and revised my draft.
1
Chapter 1:
Leishmania and Leishmaniasis
1.1 Biology and life cycle of Leishmania spp.
Leishmania is a protozoan parasite belonging to the Trypanosomatidae family
and the order Kinetoplastidae. Members of the Trypanosomatidae family share a common structure termed kinetoplast defined as a network of DNA engulfed in mitochondrion. Leishmania is a unicellular eukaryotic pathogen that infects primarily macrophages and causes a variety of clinical manifestations depending on the species regrouped under the term leishmaniasis. Leishmania alternates between an invertebrate host, which is usually a sandfly of the genus Phlebotomus or Lutzomya and a mammalian host such as human, dog or rodents. In the gut of the sandfly, the parasite exists as extracellular flagellated form called promastigote. Promastigotes migrate from the midgut to the pharynx; undergoing metacyclogenesis and differentiate into the non-dividing and highly infective metacyclic forms. Once the sandfly takes a blood meal, metacyclic promastigotes are injected into the skin where they are taken up by neutrophils, monocytes and macrophages, as well as dendritic cells and fibroblasts [1]. The definitive host cell is the macrophage. Upon entry into macrophages, Leishmania are engulfed in acidic phagolysosomes where they readily differentiate into replicative unflagellated intracellular amastigotes. These amastigotes proliferate by binary fission, rupture the host cell and infect new macrophages until they are taken up by a new sand fly where they transform back to promastigotes preparing for another round of infection [2] (Figure 1-1). Infected monocytes and macrophages circulating in the peripheral blood are assumed to be carriers of the parasite to distal sites of the body [3].
2
Figure 1-1: The life cycle of Leishmania.
Infective promastigotes are transmitted by female phlebotomine sandflies when they take a blood meal. The sandflies inject the parasites from their proboscis during blood meals. Promastigotes are then entering macrophages through phagocytosis and transform into intracellular amastigote forms which then rupture the macrophage and infect new cells. Infected cells could be taken up by the sandfly, released in the midgut where amastigotes transform to promastigotes to make their way to proboscis and getting ready for the next cycle of infection. From [2].
1.1.1 New World Leishmania spp.
Over 30 Leishmania species have been identified to date from which 20 are pathogenic to humans and are subdivided into the ‘New World’ (Americas) and ‘Old World’ (Asia, Africa and Europe) species (Table 1). The genus Leishmania consists of three subgenera L. (Leishmania), L. (Viannia) and L. (Sauroleishmania), the latter
3
designates lizard parasites. The species of the subgenus L. (Viannia) are exclusive to the New World and include the:
- L.braziliensis complex with the species L. braziliensis and L. peruviana
-L. guyanensis complex with species L. guyanensis, L. panamensis and L. shawi - L. mexicana complex with the species L. mexicana, L. amazonensis, L. arstidesi, L.
venezuelensis and L. forattinii [4, 5] (Table 1).
New World Leishmania species are transmitted by the vector sandfly of Lutzomyia spp. which are found throughout Americas. Lutzomyia genus, which is divided into 25 subgenera and species, belongs to the Phlebotominae subfamily [5].
Table 1: Classification of the Leishmania genus.
Updated classification based on the references [4, 5].
1.1.2 Old World Leishmania spp.
4
-L. donovani complex with the species L. donovani, L. archibaldi, and L. infantum
-L. major complex with L. major, L. gerbilli, L. arabica and L. turanica
- L. tropica complex with L. tropica, L. killicki and L. aethiopica
These parasites are all transmitted by the sandfly vector of the genus Phlebotomus. Sandfly vectors of Phlebotomus and Lutzomyia species both belong to the phlebotominae subfamily. Phlebotomus of the Old World is further divided into 12 subgenera. Sandflies of Phlebotomus or Lutzomyia spp. share some commonalities with other arthropod vectors in ecological and physiological aspects. For instance, taking up of blood meal is solely for the purpose of generating eggs and therefore only female vector takes blood. However, unlike mosquito vectors, sandfly vectors do not require an aquatic environment to complete their life cycle [5]. As such, the sandfly can find proper habitants in many domestic communities.
1.2
Leishmaniasis
Leishmaniasis is a collective term referring to a widespread spectrum of the disease caused by Leishmania species. Clinical manifestations of leishmaniasis vary from self-healing cutaneous scars to devastating visceral forms and are mainly determined by the Leishmania species causing the disease. Depending on the disease manifestation, leishmaniasis is divided into three categories:
-Cutaneous leishmaniasis (CL) occurring in both the Old and New World.
-Mucocutaneous leishmaniasis (MCL), which is endemic in central and southern America.
-Visceral leishmaniasis (VL), which also occurs both in the New and Old World and is fatal, if left untreated.
From nearly 20 pathogenic Leishmania species, five of them are of main importance in terms of human transmission and disease development. These include
L. tropica, L. major, L. braziliensis, L. donovani and L. infantum chagasi [6]. It has
5
with up to 400,000 new cases of visceral leishmaniasis and 1.2 million cases of the cutaneous form annually. In 2013, more than 62,500 people died from visceral leishmaniasis [8, 9]. Leishmaniasis is an endemic disease in more than 98 countries and is a major health problem in some developing countries. Several factors have recently contributed to the dissemination of leishmaniasis such as climate changes, immigration and increasing population size along harming ecosystems, which naturally protect humans from spreading vector expansion. It is estimated that 350 million people live in endemic areas and are currently at risk of Leishmania infections.
1.2.1 Cutaneous Leishmaniasis (CL)
CL is the most common form of leishmaniasis. It is usually caused by L.
major and L. tropica in the Old World and by L. mexicana and L. braziliensis
complexes in the New World. CL is distributed in three main epidemiological regions: the Americas, the Mediterranean basin and Western Asia from the Middle East to Central Asia. Ten countries with the highest estimated case counts, Afghanistan, Algeria, Colombia, Brazil, Costa Rica, Ethiopia, Peru, Iran, North Sudan and Syria, together account for 70 to 75% of global estimated CL incidence [10] (Figure 1-2).
6
Figure 1-2: Global endemicity of cutaneous leishmaniasis, 2013.
From World Health Organization (WHO); http://www.who.int/leishmaniasis/burden/en/.
CL first appears with a papule at the sandfly bite, which then develops to an ulcer, often severe, that can take sometimes up to a year to heal. This ulcer usually leaves a prominent scar, which could be cosmetically damaging if bite happens on the face. However, because the Leishmania remains at the site of infection, no serious morbidity is associated with that.
1.2.2 Visceral Leishmaniasis (VL)
VL is a systemic leishmaniasis, which is fatal if not treated. It is found in both Eastern and Western hemispheres. In the New World, it is caused by L. infantum
chagasi and in the Old World by L. donovani. 90% of global VL cases take place in
six countries: India, Bangladesh, Sudan, South Sudan, Ethiopia and Brazil [10] (Figure 1-3).
Figure 1-3: Global endemicity of visceral leishmaniasis, 2013.
7
The disease also named kala-azar (black fever in Hindi) is pathogenically and clinically quite distinct from the CL form because it involves many internal organs.
Leishmania in this form does not retain in the site of sandfly bite, instead parasites
disseminate through the host’s reticuloendothelial system and are capable of invading the spleen and liver, resulting in prolonged hepatosplenomegaly. Abdominal swelling is the most noticeable feature of this form of disease.
1.2.3 Mucocutaneous Leishmaniasis (MCL)
MCL is a severe disfiguring variant of CL caused by L. braziliensis and occasionally by L. panamensis. It is found in tropical and sub-tropical regions of South America, Central America and parts of Mexico and is transmitted by
Lutzomyia sandflies [11]. In this form, L. braziliensis parasites can migrate away
from the site of sandfly bite and eventually involve mucous membrane tissues. If the patient is left untreated, the nasal septum, lips and soft palate could be severely damaged or even destroyed, which could further cause asphyxiation due to airway collapse or secondary bacterial infection, ultimately resulting in death.
1.2.4 Diffuse Cutaneous Leishmaniasis (DCL)
DCL is a profound form of CL caused by L. mexicana and L. braziliensis in the New World and L. aethiopica in the Old World. This rare form of CL is the result of the absence of specific cell-mediated immunity (CMI) to Leishmania. The chronic manifestation of CL appears as non-ulcerating lesions that expand locally and hematogenously to areas such as face and limbs and may even cause destruction of deep tissues [12]. Lack of T cell response renders these patients with lesions full of parasites which is a consequence of low levels of TH1 cytokines [13]. Although DCL
shares some feature with VL such as inactivity of specific CMI response and high parasite burden, however, VL responds well to treatment while DCL patients are generally resistant to classical therapies [14].
1.2.5 Post-Kala-azar Dermal Leishmaniasis (PKDL)
PKDL is a complication of visceral leishmaniasis, which is characterized by a macular, maculopapular and nodular rash, typically on the face, but it may subsequently spread to all parts of the body. Increasing evidence suggest that the
8
pathogenesis is greatly immunologically mediated. PKDL is mainly seen in Sudan and India. The latent period for which PKDL appears is 0-6 months in Sudan and 2-3 years in India. The main known risk factor associated with emergence of PKDL is previous treatment for VL with antimonials, though it may occur following treatment with other medicines. Due to the high parasite load of skin lesions, PKDL patients are highly infectious [15].
1.2.6 Leishmania/HIV co-infection
The first report of leishmaniasis associated with HIV infection dates back to 1985, while the number of cases since then has increased significantly throughout the world. So far 35 countries have reported cases of co-infection. Fortunately, after the introduction of highly active antiretroviral therapy (HAART) in Europe, the incidence rate of the co-infection where the disease is endemic sharply decreased. However, the problem has expanded to other main areas of leishmaniasis due to an increased overlap of the two diseases. VL has emerged as an important opportunistic disease connected to HIV. Many VL patients residing in endemic areas are asymptomatic. A simultaneous infection with HIV can increase the risk of developing active VL between 100 and 2320 times. In Southern Europe, 70% of adult VL patients are associated with HIV infection [16]. Both diseases are reciprocally reinforcing; HIV-infected persons are greatly vulnerable to VL, while VL stimulates and accelerates HIV replication and therefore progression to AIDS. Moreover, probability of treatment failure for VL is highly independent of the drug used. Therefore, all co-infected patients will eventually die unless they are treated with an antiretroviral therapy [17-19].
1.3 Diagnosis
There are several methods of laboratory diagnosis of leishmaniasis, including parasitological, immunological and molecular techniques, which are summarized below:
-Microscopic examination: Diagnosis of leishmaniasis relies on the microscopical demonstration of amastigotes in the corresponding tissue aspirates or biopsies such as
9
bone marrow, spleen or lymph nodes or in the peripheral blood buffy coat [20]. The samples could then be smeared onto a microscope slide and stained with Romnowsky’s, hemotoxyline eosine, immunoperoxidase or Giemsa’s stain. However, Giemsa’s stain is the most appropriate method as it can readily stain amastigotes [21]. The immunoperoxidase stain has been show to provide improved sensitivity in cases of cutaneous and mucocutaneous leishmaniasis [22].
- Culture examination: Promastigotes can be cultured from specimens on solid NNN medium containing 20-30% rabbit blood. Promastigotes grow in 90% of aspirates from spleen and liver in active kala-azar patients [23]. Schneider’s medium or M199 medium has also been successfully used for in vitro culture of L. donovani. However, the biggest issue is culture contamination at early stages, even in best laboratory setups.
- Direct fluorescent antibody test: This is one of the commonly used tests to detect the antigen (Leishmania) in tissue sections or smears containing amastigotes and is performed using fluorescent dye conjugated antibodies. This technique is more applicable in CL, MCL and PKDL patients.
- Direct agglutination test: The direct agglutination test (DAT) is highly specific and sensitive. Cheap price and ease of use makes it ideal for both field and laboratory use. In this method, dilutions of serum from the patient are mixed with whole stained promastigotes either in suspension or in freeze-dried forms. Presence of
anti-Leishmania antibody in the serum results in agglutination within 18h. DAT has been
shown to be 91-100 per cent sensitive and between 72- 100 per cent specific in various studies [24] .
- Enzyme linked immunoabsorbent assay (ELISA): It is one of the most sensitive tests for the serodiagnosis of visceral leishmaniasis. It could be used for both laboratory and field analysis when screening high number of samples in relatively short period of time is aimed. The sensitivity and specificity of ELISA greatly depend on the antigen used. A recombinant antigen, rK39, has been shown to be specific for antibodies arising in VL caused by the members of the L. donovani complex. The
10
term rK39 stands for a 39 amino acid repeat of kinesin-related protein, conserved among members of the L. donovani complex [25]. Other recombinant forms of L.
chagasi kinesin have been generated, including rK26 and rK9 [26] and more recently
rKE16 from the L. donovani strain KE16, the latter shown 98 per cent sensitivity in diagnosis of Indian kala-azar and PKDL [27].
- Polymerase chain reaction (PCR): PCR is a molecular technique aimed at amplifying a target DNA sequence. PCR is highly advantageous compared to other techniques due to its high sensitivity, fast performance and the ability to use various types of clinical specimens. Serological tests, despite their availability and diversity are not suitable for detection of CL and MCL because antibodies generally tend to be undetectable due to week humoral response [28, 29]. Diagnostics in chronic CL patients are challenging because they often have very low or no anti-Leishmania antibodies and therefore serological tests are not recommended for them. PCR, however, has been shown to be 100 per cent sensitive in one such study [30]. One great advantage of PCR-based techniques is the ability to identify and differentiate at the level of strain, thanks to the recent accomplishments in whole genome sequencing of various Leishmania strains. Having strain-specific sequences available enables us to design primers that exclusively amplify target genes of the desired strain. Quantitative real time PCR (qPCR) has contributed further to the sensitivity of the technique by using fluorescent DNA binding dye allowing amplicon identification in real time.
1.4
Vaccine development and treatment
As noted before, some forms of leishmaniasis, particularly VL, can culminate in death of the patient, if not treated. Due to the lack of an effective vaccine, chemotherapy has remained the only choice. Organo-antimonial compounds constitute the first line of treatment against all forms of leishmaniasis for over 60 years. The modern era of antimony use goes back to 1905 when Plimmer and Thompson showed effectiveness of sodium and potassium tartrate against African trypanosomes. Use of trivalent antimonials for the treatment of CL was first reported by Vienna in 1913 [31] and its efficacy was later shown against VL by Di Cristina
11
and Caronia in Sicily [32] and by Rogers in India in 1915 [33]. Nonetheless, this drug was later found to be highly toxic as well as highly unstable in tropical regions [34] which eventually led to the discovery of pentavalent antimonials. Synthesis of pentavalent antimony compounds by Brahmachari in 1920 and their successful effect against Indian kala-azar saved millions of lives in India [35, 36]. Professor Brahmchari was later nominated for the Nobel Prize in 1929 in appreciation for this discovery and contribution to cure kala-azar in India [37]. Despite its popularity and use as first choice drug in the treatment of leishmaniasis for decades, the cellular and molecular mechanisms of their action is poorly understood [38]. In recent years, an increasing number in clinical resistance to pentavalent antimonials (Sbv) have been reported [39, 40]. For instance, in India 65% of patients who have never been treated before, did not promptly respond or relapse after antimonial therapy [41]. This urged the development of new drugs, which termed as second-line drugs, comprising pentamidine and amphotericin B. Amphotericin B is an antimicrobial isolated from
Streptomyces that was originally used to treat systemic fungal infections. Liposomal
amphotericin B (AmBisome®) is a form of the drug that is administered intravenously (IV) and exhibits milder side effects and increased efficacy. It was approved by the Food and Drug Administration (FDA) in 1997 and is currently used for treatment of VL cases resistant or refractory to pentavalent antimonials. However, side effects and also the high cost has restricted its use [42, 43]. Miltefosine (hexadecylphosphocholine) originally developed for cancer therapy is the first oral drug against leishmaniasis approved by the FDA in 2014 for the treatment of cutaneous, mucosal, and visceral leishmaniasis. Miltefosine shows promising efficacy in both antimony-responding and non-responding patients [44]. Nevertheless, due to long half-life and potential teratogenic effects, which have been shown in animals, its use is strictly forbidden in pregnant women and those women becoming pregnant during the course of treatment. Considering that only a few affordable anti-leishmanial drugs are currently available, the increased incidence of resistant
Leishmania strains against any of the first-line drugs could have a big impact on the
12
order to understand molecular and biochemical mechanisms of clinical drug resistance hoping to tackle this immense issue [45].
Very recently, aproteasome inhibitor, GNF6702, developed by Novartis, shown to have activity against the four main illnesses; VL, CL, Chagas disease and sleeping sickness caused by L. donovani, L. major, T. cruzi and T. brucei, respectively. GNF6702 reduces parasite burden by 90% in mice developing VL when administered at 10 mg/kg, orally. In mice infected with L. major, administration of the drug at similar concentrations reduced footpad swelling by 5-fold. GNF6702 was also tested in mice infected with T. cruzi. Almost all mice treated with the drug at 5 weeks post-infection had no detectable parasites in affected organs. More interestingly, mice infected with T. brucei at stage II where the parasites infect central nervous system (CNS) were also treated with GNF6702 at 100 mg/kg and the results were promising as no parasites were detectable in the blood or CNS upon completion of the treatment [46].
Currently, there is no licensed vaccine against human leishmaniasis. Despite advances with some vaccine candidates into clinical trials (Table 2), most are still at the research level. The idea of using whole killed Leishmania along with Bacillus
Calmette Guerin (BCG) as adjuvants, implemented on human individuals for both CL
and VL in Sudan [47] and for CL in Iran[48]; however, it was not shown to develop protection. This led to the development of second-generation vaccines that are based on Leishmania recombinant proteins adjuvanted in final vaccine formulation. Second-generation vaccines despite they had some success in terms of protection in animal models, did not advance further mostly due to indispensable requirement of adjuvant to complete the vaccine formulation [49]. Third-generation vaccines (naked DNA vaccines) are DNA-based and therefore safer as they do not contain any pathogenic material and have shown promising protection in rodents when administered by gene gun. Nevertheless, they have been unable to develop protection in non-murine models [50, 51]. LEISH-F2 is a candidate that made it to clinical trials. It is a modified version of LEISH-F1 constructed to improve results obtained by LEISH-F1 which itself is a fusion protein composed of three tandem polypeptides and formulated with
13
monophosphoryl lipid A-stable emulsion (MPL-SE). These proteins could be found in many Leishmania species including L. donovani, L. chagasi and L. braziliensis. The N-terminal histidine tag in LEISH-F2 has been removed and a potential proteolytic hot spot in Lys274 has been substituted to glutamine [52]. The LEISH-F3 candidate, comprise fusion of open reading frames (ORFs) of nucleoside hydrolase (NH) from L. donovani and sterol 24-c-methyltransferase (SMT) from L. infantum in tandem and formulated with glucopyranosyl lipid A (GLA-SE). In parallel, other groups are developing vaccine candidates based on sandfly salivary proteins, which have shown protection from VL and CL in experimental models [53-55]. Interestingly, a recent study showed a significant protection in non-human primates (NHP) against leishmaniasis, when monkeys were immunized with a 15kDa salivary protein, PdSP15, or just exposed to uninfected Phlebotomus duboscqi sand flies bites [56].
Table 2: Status of current vaccine candidates.
(adapted from ref. [9]).
Candidate name/identifier Preclinical Phase I Phase II Ref.
LEISH-F2 X [57]
LEISH-F3 X [58, 59]
Various Lutzomyia sandfly antigens X [60]
Various second generation protein based vaccines X [49]
14
Chapter 2:
Digenetic Life Cycle of Leishmania
and Interaction with Vertebrate Host
2.1 Insect Life Stages
After the sand fly takes a blood meal from infected mammal host, Leishmania amastigotes released from infected macrophages pass to the posterior abdominal midgut where they undergo several developmental stages migrating from the posterior midgut to the stomodeal valve (Figure 2-1) These stages are quite distinct morphologically and also functionally and are in fact parasite’s evolutionary response to three main natural barriers; i) secreted proteolytic enzymes, ii) the peritrophic matrix (PM) surrounding the ingested blood meal and iii) sand fly immune reactions.
Unfed sandfly midgut has a very low protease activity, whereas, the activity augments significantly 6h post-blood meal (PBM) and generally reaches a peak between 18-48h PBM depending on the sandfly species [61]. It has been reported that up to 50% of the released amastigotes die within the first day after sandfly’s blood feeding even in compatible vector-parasite combination [62]. This significant parasite loss is due to the proteolytic activity of the midgut since addition of soybean trypsin inhibitor to the blood meal elevated the survival rate of L. donovani in P. papatasi [63]. Leishmania parasites, on other had have evolved resistance mechanisms to diminish this proteolytic attack. Proteophosphoglycan (PPG) of phosphoglycans (PG) family, which is attached to the parasite cell surface through glycosylphosphatidylinositol lipid anchors, has been proposed as the major player in conferring resistance to the proteolytic activity in fully developed L.major procyclic promastigotes [64]. In spite of previous reports supporting a role of the other PG member, lipophosphoglycan (LPG) molecule which also constitutes cell surface, in parasite resistance against sandfly midgut proteases, it is now clear that LPG is only involved in the attachment of nectomonads to the midgut epithelium in specific vectors P. papatasi and P. duboscqi [65].
Survived amastigotes differentiate to procyclic promastigotes with short flagella and undertake a first round of multiplication in the fly. 48–72 hours later,
15
parasites begin to slow down their replication, then differentiate into strongly motile large slender nectomonads [62]. Nectomonads anchor themselves to epithelial cells lining the midgut, thereby escaping PM and migrate forward towards the anterior midgut and later develop into leptomonads. Leptomonads enter a second proliferation cycle [66]. These parasites further migrate and attach to the stomodeal valve which causes damage to the chitin lining and epithelial cells of the valve possibly through secretion of chitinases [67]. This interferes with the function of the valve facilitating reflux of parasites from the midgut. Thereafter, leptomonads transform into the highly infective non-dividing metacyclic promastigotes. Both sandfly and Leishmania secrete chitinases [68, 69]. However, the role of Leishmania chitinases in escaping PM has been recently argued by showing that PM opens similarly in infected and uninfected P. duboscqi sandflies [70]. Conversely, RNAi-mediated knock-down of P.
papatasi chitinase, PpChit1, led to a significant reduction of L. major in the midgut
120h PBM [71], which together favor the importance of sand fly chitinase activity and the opportunity of the parasite to take advantage of the host physiology.
The differentiation process resulting in the generation of metacyclic promastigotes refers to as ‘metacyclogenesis’, which provides with highly infective parasites ready to initiate infection in mammalian host. Unfortunately, very little is known about factors contributing to this process. Recently, however, it was claimed that absence of purines stimulates metacyclogenesis. In fact, it was shown that addition of adenosine in the sugar meal of infected sandflies inhibited metacyclogenesis. This effect was also consistently demonstrated on cultured promastigotes where by supplementing the medium with a strong antagonist of mammalian adenosine receptors, metacyclogenesis was strongly induced. Interestingly, addition of adenosine to a culture of metacyclics switched these non-dividing parasites to highly proliferative forms [72].
16
Figure 2-1: Insect Leishmania life stages.
From ref. [73]
2.2 Subverting the host immune system and entry into
macrophages
Leishmania metacyclics are eventually injected into the mammalian host skin
when an infected female sandfly takes a blood meal. From now on, the parasites enter a hostile environment and face a broad range of host defense mechanisms, including components of the innate and humoral immune responses designed to eliminate them from infected sites. Nonetheless, as a successful intracellular pathogen, Leishmania has developed various strategies to evade the host immune system so that it can survive within the host cell and propagate. These mechanisms help the parasite to establish a chronic form of the disease, which guarantees its survival and transmission to a new cycle. The role of sandfly saliva in facilitating infection and protection of the parasite is of particular interest, although it has not been much investigated. Nevertheless, in many studies, it was shown that saliva of both New and Old world sandfly vectors has hemostatic and immunomodulatory effects, generally resulting in increased migration of inflammatory cells to the site of deposition, thus enhancing the probability of phagocytosis [74]. It also decreases ‘tumor necrosis factor’ (TNF-α), interleukin (IL-10) and increases IL-6, IL-8 and IL-12 production in lipopolysaccharide (LPS) stimulated human macrophages [75] and down-regulates
17
nitric oxide (NO) production in LPS- or interferon (IFN-γ) activated macrophages [76].
2.2.1 Inactivation of the host complement system
Complement system is of the main components of the innate immune system against microbial infections and is the first defense line following contact with the parasite (Figure 2-2). Once Leishmania is injected to the host’s skin, it is crucial to escape or to deactivate the complement system before being phagocytized. It has to be noted that in contrary to procyclic promastigotes which are very susceptible to the lysis by the complement, metacyclics have been shown to be generally more resistant [77], underlining the impact of stage-specific requirements that are best suited to develop infection in host cells. Metacyclics employ three main mechanisms to counter the complement system; i) they express longer LPG molecules on the surface of the parasite [78] which prevents the insertion of the C5b-C9 membrane attack complex, rendering them very resistant to complement-mediated lysis [79]; and ii) surface-membrane metalloprotease, glycoprotein 63 (known as GP63, leishmanolysin, or major surface protease) which similarly to LPG is highly expressed in metacyclics, cleaves C3b molecule of complement system attached to its surface resulting in formation of inactive form, C3bi[80]. C3bi serves as an opsonin, which binds to CR3 receptor and transiently to CR1 on the surface of macrophages. By attaching to CR3 receptor rather than CR1, parasite inhibits induction of IL-12 [81], thus facilitating a silent entry into macrophages [82]. Involvement of CR3 receptor binding as a host evasion mechanism was shown in a recent study where CR3 deficient BALB/c mice infected with L. major exhibits increased resistant [83].
iii) Metacyclic parasites are known to express highest level of protein kinases. These
kinases were shown to phosphorylate many complement proteins including C3, C5 and C9, therefore deactivating classical and alternative complement pathways [84].
18
Figure 2-2: Inactivation of the complement system by Leishmania spp.
From Ref. [85].
2.2.2 Uptake by neutrophils and transmission to macrophages
Metacyclic parasites could be taken up by several types of phagocytes including neutrophils which are in fact the first cells recruited to the site of infection within a few minutes [86, 87] as the consequence of released alarmins (signal for tissue damage), chemokines and cytokines (depicted in Figure 2-3) and (reviewed in [88]). Neutrophils account for 80% of total infected cells in the dermis of C57BL/6 mice following infection with L. major [89] or L. mexicana [90]. These cells make up the first line of defense against intracellular pathogens and are capable of eliminating pathogens by either releasing their toxic granule content to the infection site or in the phagosome via reactive oxygen species (ROS) [91], neutrophil elastase (NE) as well as neutrophil extracellular traps (NET) [92]. Leishmania promastigotes on the other hand use their LPG and tartrate-resistant acid phosphatase present on the cell surface
19
to inhibit lysosome fusion, the respiratory burst and superoxide anion production in neutrophils [93]. Moreover, the parasites express a serine peptidase which has been shown to deactivate NE [94]. A role for L. infantum nuclease 3'-nucleotidase/nuclease has been recently proposed against NET defense mechanism [95] and was further deepened by the observation that a NET-destroying’ endonuclease (Lundep) from
Lutzomyia longipalpis saliva was capable of releasing parasites from NETs in vitro
[96]. Neutrophils are also being actively manipulated by Leishmania promastigotes residing within their phagosome. However, one has to remember that depending on the Leishmania species as well as the type of mammalian host and the origin of neutrophils, mechanisms and outcomes are sometimes completely different or even contrary. For instance mouse dermal neutrophils infected with L. major showed enhanced expression of phosphatidylserine (PS), an apoptotic marker which targets infected neutrophils to dermal dendritic cells for phagocytosis [89]. Similarly, L.
braziliensis was shown to induce apoptosis in neutrophils [97]. Based on these
observations the ‘Trojan Horse’ model was proposed suggesting a silent entry of
Leishmania-induced apoptotic neutrophils to macrophages as the mechanism of
transmission [98]. Recent in vivo data, however, have shown that neutrophils do not directly deliver their parasites to the macrophages, instead apoptotic neutrophils lyse, releasing their carrying promastigotes, which are then engulfed by the macrophages [86, 89]. On the contrary L. major has been reported to delay apoptosis in mouse peritoneal neutrophils [99] as well as human blood-derived neutrophils [100]. Besides, it was shown that unlike L.major, L. mexicana does not induce rapid apoptosis of dermal neutrophils in vivo [90] adding a species-specific dimension to the mechanisms of transmission to macrophages. Given the fact that in spite of delaying in apoptosis induced by the parasite, neutrophils will eventually undergo apoptosis, it is reasonable to conclude that parasites could enter macrophages through phagocytosis of both apoptotic infected neutrophils and promastigotes released from ruptured neutrophils (Figure 2-3, step 3)[90]. Despite the importance of neutrophils in early hours of Leishmania infection, one has to remember that metacyclic parasites are taken up simultaneously by mononuclear phagocytes including macrophages, dendritic cells (DCs) and monocytes. Notably, it has been shown that 24 hours
post-20
infection; macrophages represent the dominant infected population, underscoring the essential host cell for the parasite ensuring its survival and pathogenesis in long term [86].
Figure 2-3: Implication of neutrophils in the Leishmania survival within the human host.
Neutrophils are primary phagocytes uptaking promastigotes and main carriers for delivery to macrophages. From ref [101].
2.2.3 Entry to macrophages, differentiation to amastigotes and survival within macrophages
Leishmania promastigotes entering the main host cell phagosomes encounter a
highly acidic environment with elevated temperature along with high levels of ROS and NOS. Therefore, while significant number of changes at the level of parasite gene expression occur, the parasite concomitantly initiates evasion mechanisms on host cell in order to adapt and allow differentiation into amastigote forms, which are capable to resist acidic pH as well as 37°C temperature. Following initial attachment,
21
promastigotes are internalized by macrophages in caveolae-dependent manner [102]. In a first place, internalized parasites inhibit fusion of phagosome with lysosome transiently by inducing periphagosomal F-actin accumulation around the phagosome due to LPG-mediated deactivation of protein kinase C (PKC)α [103] which has been witnessed by the delayed recruitment of LAMP-1 and Rab7 [104]. Other reports have suggested a role for ‘lipid microdomains’ of the phagosome membrane that contain the ganglioside GM1. Integration of LPG molecules into lipid microdomains results in the exclusion of synaptotagmin V, an essential player in the recruitment of the vesicular proton ATPase (V-ATPase) that drives phagosome acidification [105]. Although it is generally perceived that acidification of phagolysosomes along with temperature shift triggers amastigote differentiation, one recent report suggests that temperature increase prevails over acidification on gene expression modulation leading to amastigote differentiation [106].
In addition to the mechanisms described here, it has been reported that
Leishmania of the New World may resist leishmanicidal effect of phagosomes by
modulating expression of the lysosomal trafficking regulator (LYST) protein. This notion stems from the observation that phagosome-containing parasites were markedly enlarged helping to dilute concentration nitric oxide, therefore, facilitating parasite survival. Interestingly, overexpression of LYST in mouse macrophages resulted in the reduction of parasite numbers in the phagosomes and the parasites contained within them were more susceptible to elimination by nitric oxide [107].
Although many promastigotes are killed by macrophages, in the end some escape the microbicidal power of the phagolysosome, consequently transforming this organelle into a parasitophorous vacuole (PV) that favors parasite growth [108, 109]. Within PVs, promastigotes convert into amastigotes and multiply via binary fission. When the host cell becomes inundated by parasites, it may either rupture and release amastigotes, or become apoptotic and pass the amastigote content to surrounding macrophages [110, 111].
After transforming to amastigotes within the phagolysosome of macrophages,
22
required for its growth and survival. Once such essential nutrient is iron [112]. Nramp1is an efflux pump of murine macrophages, which actively translocates Fe2+ from the phagolysosome to the cytosol, rendering the parasite with no iron supply. However, via activation of its own iron transporters, LIT1 and LIT2, Leishmania amastigotes are able to counterbalance the host’s iron sequestering mechanism [88]. Arginine, which is essential for parasite survival as is required for the synthesis of polyamines represents another interesting example of how amastigotes exploit and evade the host macrophage defense. Arginine is also used by the macrophage for iNOS production [113, 114]. Interestingly, Leishmania amastigotes express an arginase enzyme which was shown not only to facilitate arginine supply for the parasite survival, but also by competing with the host cell on available intracellular arginine, it attenuate iNOS mediated killing mechanism of the infected macrophages [115, 116].
2.2.4 Manipulating macrophage signaling pathways
Once inside macrophages armed with lots of microbicidal factors, Leishmania sets out diverse strategies to manipulate the host cell signaling pathways in its favor.
L. donovani GP63 has been shown to enter the macrophage cytosol using host cell
‘lipid rafts’ and selectively cleave host protein phosphatases such as SRC homology 2 domain phosphotyrosine phosphatase 1 (SHP1), resulting in its activation. SHP1 has a pivotal role in the regulation of inducible nitric oxide synthase (iNOS) and hence its increased activity contributes to parasite survival. SHP1 has been shown to bind extracellular signal-related kinase 1 (ERK1), ERK2 and IL-1 receptor-associated kinase-1 (IRAK1), regulating these important components of the innate immune system [117, 118]. Besides, SHP1 has been shown to dephosphorylate and thus deactivate Janus Kinase 2 (JAK2) in infected macrophages [119]. JAK2 binds to Signal Transducer and Activator of Transcription-1 (STAT1) protein, a component of JAK/STAT pathway, activation of which leads to the production of IFN-γ, which down-regulate nitric oxide production, hence facilitating parasite survival inside macrophages [120]. Reactive oxygen species (ROS) are another defense mechanism against pathogens. Induction of ROS requires phosphorylation of p47 and p67
23
subunits of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex by protein kinase C (PKC) [121]. However, LPG of Leishmania promastigotes has been shown to inhibit phosphorylation of the two subunits by interfering with binding of PKC with its natural substrates, Ca2+ and diacyl glycerol, ultimately assisting the silent entry to the macrophages [122]. A role for GP63 in inhibition of PKC activity has also been proposed by showing that GP63 cleaves PKC substrates, myristoylated alanine rich C kinase (MARCKS) and MARCKS-related protein during macrophage infection [123]. Another mechanism of hijacking host signaling pathways by Leishmania comes from the studies of the nuclear factor kB (NF-kB) transcription factor which plays a crucial role in regulating cytokine and chemokine expression and its absence has been shown to be associated with high parasite load and low NO production in macrophages [124]. Thanks to its GP63 surface protease, Leishmania cleaves the p65 subunit of NF-kB to generate the smaller p35 form which then induces expression of chemokines beneficial for the parasite survival [125]. Activator protein 1 (AP-1) is an example of another transcription factor targeted by Leishmania during macrophage infection. Once infected with promastigotes, AP-1 which is comprised of dimmers of Fos and Jun family members, translocates to the nucleus and induces expression of pro-inflammatory cytokines IL-1β, TNF-α, and IL-12 aimed to eradicate the infection [126, 127]. However, Leishmania-derived GP63 has been reported to enter the nucleus of macrophage and cleave c-Jun and c-Fos subunits of AP-1 making them inactive [127]. Now, one may appreciate how by using multiple mechanisms,
Leishmania can interfere with host’s signal transduction and transcription pathways,
ultimately subverting the host immune system.
2.3 Environmental stress and amastigote differentiation
As mentioned earlier, Leishmania surviving host cell-mediated immunity, eventually transform into intracellular amastigote forms inside phagolysosomes of macrophages. Although most of the molecular mechanisms imposing this transformation in vivo have remained unknown, using a host-free system for L.
24
with respect to this process have been addressed. Differentiation is associated with profound morphological and physiological changes including loss of flagellum, decreasing cell size and becoming round shape along with differential expression of many surface coat matrix components. This time course process has been divided morphologically into 4 distinct stages: I, signal perception (0-5 h after exposure); II, movement cessation and aggregation (5-10 h); III, amastigote morphogenesis in which promastigotes change morphologically into amastigote-shaped cells and return synchronously to the cell cycle and to normal growth (10-24 h); and IV, maturation (24-120 h). Recently, a study on the phosphoproteome of Leishmania during differentiation has revealed interesting evidences suggesting that a coordinated process takes place during this process. These data showed extensive differences in phosphorylation between promastigotes and amastigotes. Based on these findings, the authors have suggested that a phosphorylation pathway is activated upon receipt of signal, which initiates differentiation process [128]. However, further work is needed to understand what specific mechanisms are involved in this process.
2.4 Leishmania virulence factors
Virulence factors assist the parasite to escape host immune defense by subverting host’s immune mechanisms eventually leading to the establishment of pathogenesis. Thus far, several virulence factors have been identified with some of them such as GP63 mediating diverse range of actions to counteract host’s defense. An overview of these factors is depicted in Figure 2-4.
Leishmania glycoprotein63 (GP63): also called leishmanolysin
or Leishmania Major Surface Protease, is a zinc-dependent metalloprotease that is expressed at the surface of the parasite via a glycophosphatidylinositol (GPI) anchor. It could also be secreted to extracellular milieu. GP63 is the most abundant surface corresponding to 1% of the total parasite proteome [109, 129]. Genes encoding GP63 are organized in tandem array with differences in the C-terminal region and the 3′-untranslated regions (3ʹUTR) [130, 131], the latter accounting for differential expression stemming from developmental life stages of the parasite [132]. GP63 is
25
considered promastigote-specific as its expression markedly decreases following conversion to amastigotes, though detectable [118]. The highest GP63 expression occurs during metacyclogenesis [129], consistent with various functions attributed to these proteins during early steps of infection and host cell attachment. Direct evidence supporting GP63 role in pathogenesis comes from both in vitro and in vivo studies using L. major GP63−/− mutants which revealed significant decrease in virulence compared to the control [117]. Cleavage of complement protein, C3b into inactive iC3b which shelter the parasite from complement mediated-lysis [80] is also another important function of this protein during injection of metacyclics to the host skin. Upon entry to the macrophages, GP63 targets several host factors including protein tyrosine phosphatases (PTPs), transcription factors (TFs), as well as the mammalian target of rapamycin (mTOR), involved in translation, collectively resulting in modulation of macrophage signaling in the benefit of the parasite [127, 133, 134]. More recently, it was shown that GP63 cleaves several nucleoporins comprising nuclear pore complex (NPC) [135] providing a basis on how the parasite influences transcription factors [127]. Lastly, inhibition of antigen cross-presentation was attributed to GP63 by observing direct cleavage of Snare Vamp8, a critical phagosomal protein by GP63, leading to evasion of host immunity [136].
26
Figure 2-4: Virulence factors of Leishmania parasites and the mechanisms by which they subvert host macrophage defense.
From ref.[137].
Lipophosphoglycan (LPG): accounts for the most abundant surface glycoconjugate
of Leishmania promastigotes, likewise GP63 is among the main virulence factors identified to date. Also, similar to GP63, LPG expression down-regulates significantly in intracellular amastigotes [138], an indication that the main functions of these molecules are in sandfly midgut as wells as in early steps following mammalian host infection [132]. Direct evidence in supporting a role of LPG molecules in pathogenesis stems from experiments carried out on lpg1−/− and lpg2−/− mutants of L. donovani which showed involvement of these molecules in Mitogen Activated Protein Kinases (MAPKs) inhibition [139]. These evidences along with other observations like the inhibition of NADPH oxidase complex formation [140] and exclusion of proton-ATPase from the phagosome which hinders its acidification