© Nathan Haag, 2020
Displacement of the invasive zebra mussel by the
similarly invasive quagga mussel: patterns and
biological factors
Thèse
Nathan Haag
Doctorat en biologie
Philosophiæ doctor (Ph. D.)
Québec, Canada
Displacement of the invasive zebra mussel by the similarly invasive
quagga mussel:
patterns and biological factors
Thèse
Nathan E. Haag
Sous la direction de:
iii
Résumé
Depuis leur arrivée à la fin des années 1980, les moules zébrées et quagga, Dreissena
polymorpha (Pallas) et Dreissena bugensis (Andrusov) respectivement, sont devenues deux des
espèces envahissantes les plus connues en Amérique du Nord. Leur nouveauté au Canada et aux États-Unis en tant que premiers mollusques épifaunaux filtrants leur a permis de concurrencer les espèces indigènes pour l’espace et la nourriture, ce qui a entraîné des changements importants au niveau du substrat, de la clarté et de la qualité de l’eau, des populations indigènes, des réseaux trophiques et des économies locales des écosystèmes dulcicoles. Ces mollusques très féconds ont atteint des rivières et des lacs dans plus de 30 États et 3 provinces par les eaux de ballast et par la dissémination en aval et par voie terrestre, mais malgré la vitesse de leur propagation, l’invasion est en mouvement. La moule zébrée autrefois dominante a été déplacée par son congénère dans une grande partie du benthos des Grands Lacs Laurentiens. Dans les lacs Érié et Ontario, la couverture de moules quagga a parfois atteint 100 % du fond. Les populations restantes de moules zébrées sont généralement limitées dans des zones peu profondes exposées aux forces hydrodynamiques comme les vagues et les forts courants. Bien que le remplacement d’un envahisseur par un autre puisse sembler insignifiante, il est important de comprendre quels facteurs font de la moule quagga un meilleur compétiteur et de déterminer si ce déplacement se produit différemment dans les rivières. Le fleuve Saint-Laurent sert d’environnement privilégié pour l’observation de la dynamique entre ces deux espèces envahissantes, et cette thèse examine les deux espèces sur l’ensemble de la portion dulcicole de la rivière en ce qui concerne les facteurs physiques, y compris le substrat, la profondeur et la distance par rapport à la population supposée source. Un inventaire combiné à une expérience de survie a révélé que le déplacement des moules zébrées progresse en tant qu’intégration, chaque espèce occupant des sections différentes de la rivière, même 20 ans après son arrivée dans la rivière. De plus, les résultats indiquent que certaines combinaisons de facteurs physiques peuvent limiter la dominance de la moule quagga dans les parties inférieures de la rivière, en particulier la diminution de la taille du substrat dans les zones à vitesse accrue. L’étude a également révélé que la population de moules quagga a augmenté de façon spectaculaire depuis la dernière étude effectuée plus de dix ans auparavant. Cette augmentation extrême de l’abondance a donné lieu à une étude expérimentale examinant l’effet de la vie dans les bancs de moules afin de déterminer le niveau de concurrence entre les espèces de dreissenidés et entre elles. En utilisant des dispositifs expérimentaux pour
iv
étudier à la fois l’exploitation et la compétition d’interférence, il a été révélé que le contact physique entre les moules est tout aussi important, sinon plus, que l’exploitation de la nourriture. De plus, les deux espèces poussent davantage lorsqu’elles vivent avec des congénères que des conspécifiques. Enfin, pour comprendre les coûts et les avantages de la capacité des dreissenidés à s’attacher à des matériaux nouveaux et plastiques, des cages ont été construites pour manipuler expérimentalement l’écoulement des moules pendant plusieurs mois sur le terrain. Le
dénombrement des fils byssaux et la force nécessaire pour enlever les moules ont été enregistrés et les analyses montrent que les moules zébrées poussent davantage à des vitesses d’eau
croissantes, ce qui confirme la plus grande propension des moules zébrées à la plasticité, tout en indiquant également que le coût de fixation est faible. Une étude simultanée sur le terrain a examiné les tailles et les forces d’attachement des moules prélevées in situ. Les résultats indiqués que les moules quagga deviennent plus grosses à des vitesses de courant plus élevées — un résultat contraire aux attentes et à l’expérience, ce qui suggère que d’autres études sur le terrain sont nécessaires pour mieux comprendre comment chaque espèce réagit entre eux et aux conditions changeantes le long du fleuve. Ensemble, en examinant la dynamique des
populations, les interactions concurrentielles et la plasticité phénotypique, cette thèse contribue à la compréhension de l’invasion continue de dreissenidés, ainsi que du déplacement des moules zébrées par les moules quagga dans le fleuve Saint-Laurent.
v
Abstract
Since arriving in the late 1980s the zebra and quagga mussels, Dreissena polymorpha (Pallas) and Dreissena bugensis (Andrusov) respectively, have become two of the most notorious invasive species in North America. Their novelty in Canada and the United States as the first epifaunal filtering mollusks has enabled them to outcompete native species for space and food, resulting in extensive changes to the substratum, water clarity and quality, native populations, food webs, and local economies of freshwater ecosystems. These highly fecund mollusks have reached rivers and lakes in over 30 states and 3 provinces through ballast water and downstream and overland dispersal, yet despite the speed of their spread, the invasion is in flux. The once dominant zebra mussel has been displaced by its congener across much of the benthos of the Laurentian Great Lakes. In Lake Erie and Lake Ontario the cover of quagga mussels has sometimes reached 100% of the bottom. Remaining zebra mussel populations are generally restricted to shallow areas exposed to large hydrodynamic forces from waves and strong currents. While the replacement of one invader by another may seem inconsequential, understanding the factors that make the quagga mussel a better competitor, and determining whether the mechanisms by which this displacement occurs are different in rivers are important for developing management strategies. The St. Lawrence River serves as a model environment for observing the dynamics between these two invasive species, and this dissertation examines the populations of both species over the entire freshwater portion of the river with respect to physical factors including substratum, depth, and distance from the source population. A survey combined with a survival experiment revealed that the displacement of zebra mussels is not as complete as expected, with each species occupying different sections of the river, even 20 years after both species were introduced. Further, the results indicate that certain combinations of physical factors may limit quagga mussel dominance in lower portions of the river, specifically decreasing substratum size in areas of increased velocity. The survey also revealed that the population of quagga mussels has increased roughly fivefold since the last survey over ten years before. This extreme increase in abundance inspired an experimental investigation of the effects of living in closely packed mussel beds to ascertain the level of intraspecific and interspecific competition in these species. Using field cages modified to study both exploitative and interference competition, it was shown that physical contact among mussels is just as, if not more, important than exploiting available food. Further, both species grew more when living
vi
with congeners than conspecifics. Finally, to understand the costs and benefits of dreissenid mussels’ novel and plastic attachment ability, I experimentally manipulated the flow experienced by mussels in the field. Counts of byssal threads and force required to remove mussels showed that zebra mussels grew more in increasing water velocities, reaffirming the zebra mussel’s greater propensity for plasticity, while also indicating that the cost for attachment is low. A concurrent field study examined the size and attachment strengths of mussels collected in situ. The results indicated that quagga mussels grow larger in higher current velocities – a result contrary to expectations and the experiment, suggesting that further field studies are required to develop a better understanding how each species responds to each other and to changing
conditions along the river. Together, by examining population dynamics, competitive
interactions, and phenotypic plasticity, this thesis contributes to our understanding of the ongoing dreissenid invasion, as well as the displacement of zebra mussels by quagga mussels in the St. Lawrence River.
vii
Table of Contents
Résumé ... iii
Abstract ...v
Table of Contents ... vii
List of Tables ... ix
List of Figures ... xi
Acknowledgements ... xiv
Foreword ... xvi
Introduction ...1
Chapter 1: Where are they now? Examining the large-scale displacement of one invasive Dreissenid mussel by its congener in the St. Lawrence River 20 years later. ...10
1.1 Résumé ... 10
1.2 Abstract ... 11
1.3 Introduction ... 12
1.4 Methods ... 16
1.4.1 Adult mussel survey ... 16
1.4.2 Survival study ... 17
1.4.3 Analysis ... 17
1.5 Results ... 18
1.5.1 Survey ... 18
1.5.2 Survival... 20
1.5.3 Comparisons between studies... 21
1.6 Discussion ... 22
1.7 Tables ... 28
1.8 Figures ... 35
Chapter 2: Intraspecific and interspecific competition in congeneric invaders: field experiments on interactions of zebra and quagga mussels in a riverine environment ...42
2.1 Résumé ... 42
2.2 Abstract ... 43
2.3 Introduction ... 44
2.4 Methods: ... 46
2.4.1 Mussel Filtration Tubes ... 46
2.4.2 Artificial Mussel Beds ... 47
2.4.3 Sample Processing ... 48
2.4.4 Analysis ... 48
2.5 Results ... 49
2.5.1 Mussel Filtration tubes ... 49
2.5.2 Artificial Mussel Beds ... 50
2.6 Discussion ... 51
2.7 Tables ... 57
2.8 Figures ... 63
Chapter 3: Adhere or add there? Cage and in-situ experiments on attachment and growth in response to different current velocities in the invasive dreissenid mussels ...72
3.1 Résumé ... 72
viii
3.3 Introduction ... 73
3.4 Methods ... 76
3.4.1 Attachment-plasticity Tubes ... 76
3.4.2 Field attachment study ... 78
3.4.3 Analysis ... 79
3.5 Results ... 79
3.5.1 Attachment-plasticity Tubes ... 79
3.5.2 Field attachment study ... 80
3.6 Discussion ... 81 3.8 Tables ... 85 3.8 Figures ... 90 General Conclusions ...99 Future perspectives ...102 Literature Cited ...106
ix List of Tables
Chapter 1
Table 1.1. Substratum type, size, and calculated phi value (–log2d) (adapted from Jones and Ricciardi 2005)... 28 Table 1.2. Univariate results for the effect of river kilometer (RK), substratum (phi scale), and depth (m) on zebra and quagga mussel biomass (g/m2), adult density (ind./m2), juvenile density (ind./m2), and shell length (mm) for mussels in the 2013 river survey. Shell length vs. RK was better modeled as a quadratic equation. All values in parentheses are standard error. ... 29 Table 1.3. Generalized Linear Model results for the effect of species (zebra and quagga mussels) crossed with substratum (Sub: phi scale), depth (m), and river kilometer (RK) on mussel biomass (g/m2), adult density (ind./m2), juvenile density (ind./m2), and shell length (mm) for mussels in the 2013 river survey. ... 30 Table 1.4. Multivariate results for the effect of river kilometer (RK), substratum (phi scale), and depth (m) on zebra and quagga mussel biomass (g/m2), adult density (ind./m2), juvenile density (ind./m2), and shell length (mm) for mussels in the 2013 survey. ... 31 Table 1.5. Univariate results for the effect of river kilometer and 2013 substratum (phi scale) data on zebra and quagga mussel survival (%), shell growth (mm), and juveniles (ind./m2) in 2014, as well as the effects of these factors on each other for mussels in the 2014 survival cage experiment... 32 Table 1.6. Generalized Linear Model results for the effect of species (Z/Q) crossed with river kilometer, growth (mm), and juvenile density (ind./m2) on survival, growth (mm), and juvenile density (ind./m2) for mussels in the 2014 survival cage experiment. ... 33 Chapter 2
Table 2.1. Three-way Chi-square/nominal regression results for the effect of species (zebra and quagga mussels), location (edge, group, center isolation), and neighbor treatment (zebra mussels, quagga mussels, or empty shells) on survival of mussels in Mussel Filtration Tubes (MFTs). ... 57 Table 2.2. Three-way ANOVA results for the effect of species (zebra and quagga mussels), location (edge, group, center isolation), and neighbor treatment (zebra mussel, quagga mussel, or empty shells) on percent unidimensional growth (length, height, and width), proportionate growth (length:height, length:width, height:width), and total wet relative mass (g mm-1) and dry relative mass of soft tissue and shell (g mm-1) of surviving mussels in Mussel Filtration Tubes. Statistically significant values are in bold. ... 58 Table 2.3. Three-way Chi-square/nominal regression results for the effect of species (zebra and quagga mussels), initial level (bottom, 3 cm deep, or top), and sediment treatment (high,
medium, low) on survival of mussels in Artificial Mussel Beds. ... 60 Table 2.4. Two-way ANOVA results for the effect of species (Z/Q) and initial level (bottom, 3 cm deep, or top) on percent unidimensional growth (length, height, and width), proportionate growth (length:height, length:width, height:width), and total wet relative mass (g mm-1) and dry relative mass of soft tissue and shell (g mm-1) of surviving mussels in Artificial Mussel Beds (AMBs). Statistically significant values are in bold. ... 61
x
Table 2.5. Two-way Chi-square/nominal regression results for the effect of species (zebra and quagga mussels) and initial level (bottom, 3 cm deep, or top) on movement in the cup as measured as a difference between initial level and final level at the conclusion of the Artificial Mussel Bed experiment (AMBs). ... 62
Chapter 3
Table 3.1 One-way ANOVA results for the effect of tube type (A/B/C/D) on percent clod card loss (i.e. dissolution) and Three-way Chi-square/logistic regression results for the effect of species, tube type, and %Clod dissolution on survival of mussels. Statistically significant values are in bold. The Wald p value indicates the amount of variation ... 85 Table 3.2. Three-way ANCOVA results for the effect of species, tube type (A/B/C/D), and %Clod dissolution on percent unidimensional growth (length, height, and width), proportionate growth (length:height, length:width, height:width), total wet relative mass (g mm-1), byssal thread count, and force required for detachment (N) of surviving mussels. Statistically significant values are in bold. ... 86 Table 3.3. Multivariate correlations among the factors of percent clod dissolution, shell length, and detachment force (N) in mussels pulled from rocks in the field. Kendall’s τ test was used to determine significant correlations. Statistically significant values are in bold. ... 88 Table 3.4. Two-way ANCOVA results for the effect of species and site on log transformed detachment force and length values from mussels pulled from rocks in the field. Sites without both species were removed from this analysis. Statistically significant values are in bold. ... 89
xi
List of Figures
Chapter 1
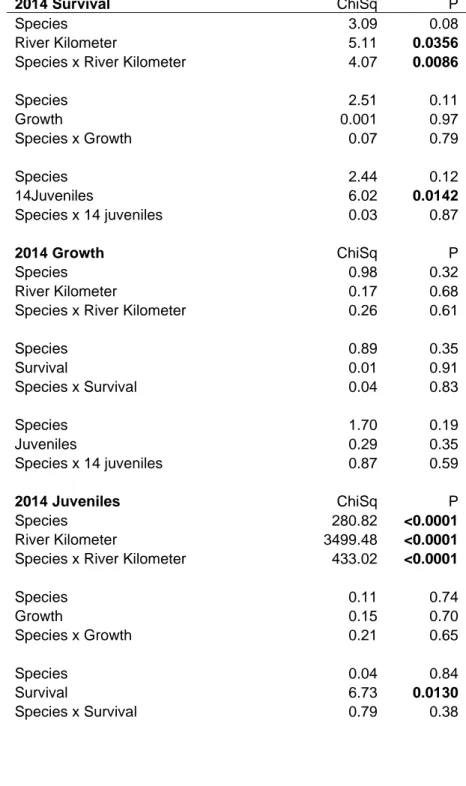
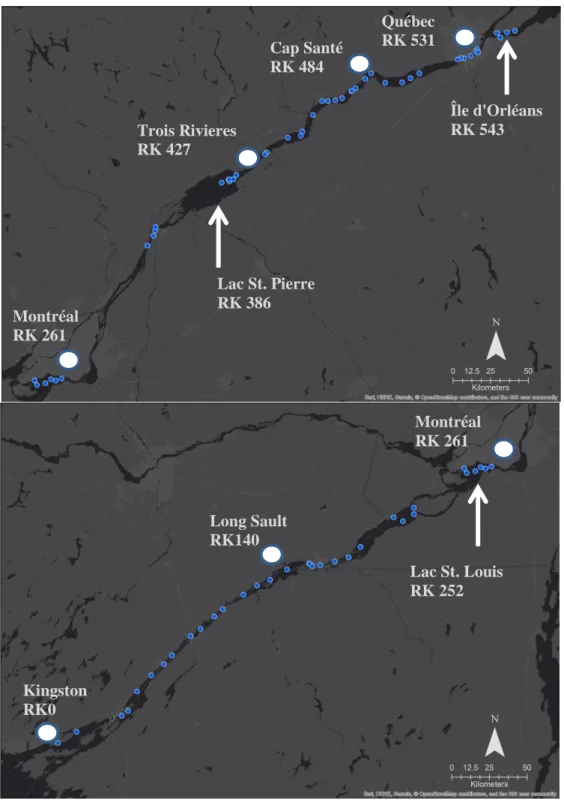
Figure 1.1. Map of 2013 dive sites along the St. Lawrence River from Montréal to Île d’Orléans (above) and Kingston to Montréal (below). ... 35 Figure 1.2. Histograms of zebra mussel (white) and quagga mussel (grey) shell length (mm) along the length of the St. Lawrence River. Each histogram represents roughly 1/9th of the individuals of either species, highlighting variation in population density along the river. Each bin increment is 2mm... 36 Figure 1.3. Distribution of dreissenid mussels in the St. Lawrence River (Canada/USA): (A) Log-transformed biomass (g/m2), (B) adult density (individuals/m2) and (C) juvenile density (individuals/m2) of zebra (white squares, dotted line) and quagga (gray circles, solid line)
mussels vs. river kilometer. Asterisks indicate significantly different slopes between species. .. 37 Figure 1.4 . A) Log-transformed biomass (g/m2), B) adult density (individuals/m2) and C) juvenile density (individuals/m2) of zebra (white squares, dotted line) and quagga (gray circles, solid line) vs. substratum for mussels collected during the 2013. Asterisks (*) indicate a
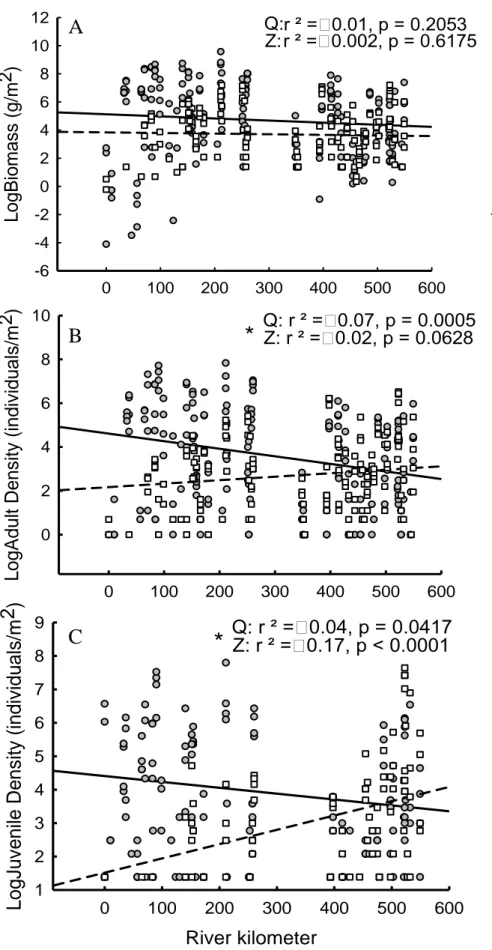
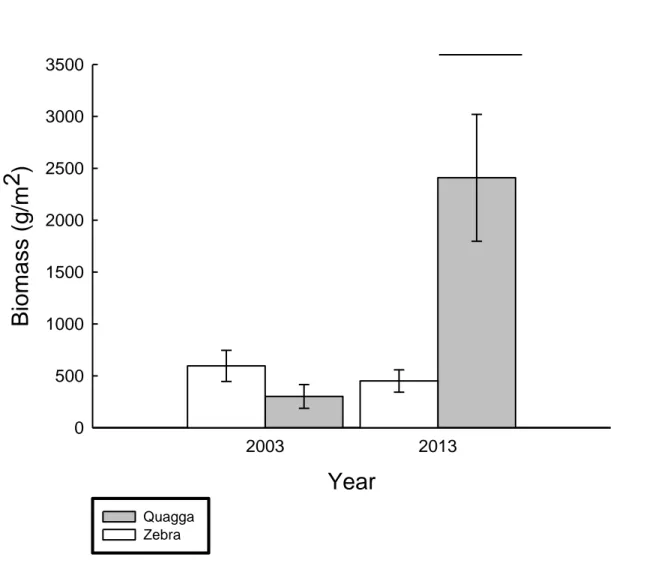
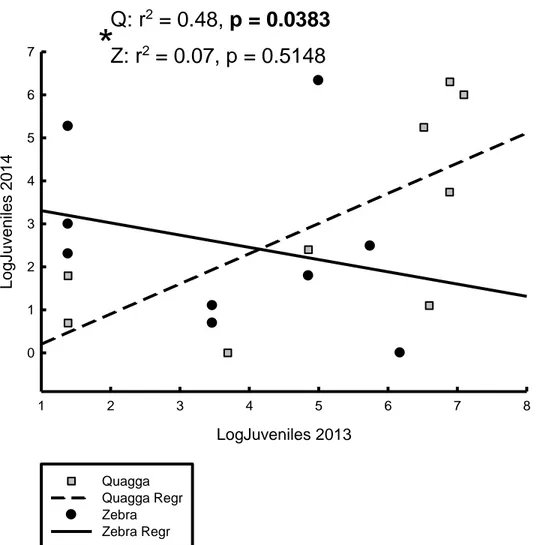
significant difference in slopes between species. ... 38 Figure 1.5. Growth (mm) of zebra (white squares, dotted line) and quagga (gray circles, solid line) mussels vs. river kilometer in the St. Lawrence River for mussels collected during the 2014 survival experiment. ... 39 Figure 1.6. Biomass (g/m2) of zebra (white) and quagga (grey) mussels collected in 2003 (Jones and Ricciardi 2005) and 2013 (this study). Bar designates significant difference from other groups (Species: F1,161=2.5314, p=0.1136, Year: F1,161=3.1858, p=0.0762 , Species x Year: F1,161=4.4787, p=0.0359) ... 40 Figure 1.7. Log transformed juvenile zebra (white) and quagga (grey) mussel densities found on 2014 survival cages vs. 2013 substratum data. Asterisk indicates significant difference in slopes between species. ... 41
Chapter 2
Figure 2.1 Diagram of Mussel Filtration Tubes (MFTs). Each ABS plastic tube apparatus consisted of three isolation chambers and two group chambers. Isolation chambers contained only one target mussel of each species. Group chambers were filled with one of three neighbor types (ZN = zebra, QN = quagga, ESN = empty mussel shells) with three target mussels of each species evenly spaced in the chamber. Each chamber was separated by 3-mm-opening mesh. Three MFTs (one of each neighbor type) were attached to a cinder block for deployment. Letters indicate positioning of target mussel pairs (I=isolation, G=Group). Each mussel was uniquely marked with oil-paint markers. ... 63 Figure 2.2. Diagram of Artificial Mussel Beds (AMBs). Each cup apparatus consists of an ABS “cup” secured by friction inside an ABS coupler. Cups were constructed from ABS piping by gluing one of three treatment types to one end: solid plastic bottom (high sedimentation), and
3-xii
mm mesh bottom (medium sedimentation) and 3-mm mesh bottom with holes drilled into the ABS coupler (low sedimentation). Three target mussels of each species were placed at three levels in the cup (on the bottom, 3 cm deep, and on the top). Cups were covered with 3-mm mesh. The X’s indicate positioning of target mussel pairs. ... 64 Figure 2.3. Survival of adult zebra (white) and quagga (grey) mussels recovered from the MFT experiment. Letters indicate statistical similarity. Dotted lines are visual aides to separate neighbor. Neighbor treatments: Empty shell, live quagga mussel, live zebra mussel. Chamber location codes: E = edge, G = group, C = central. ... 65 Figure 2.4. Percent growth in length, height, and width (± SD) of surviving adult zebra (white) and quagga (grey) mussels recovered from the MFT experiment. Letters indicate statistical similarity. Bars lacking letters are similar to all groups. Dotted lines are visual aides to separate neighbor treatments. Neighbor treatments: Empty shell, live quagga mussel, live zebra mussel. Chamber location codes: E = edge, G = group, C = central. ... 66 Figure 2.5. Change in length:height, length:width, and height:width ratios (±SD) of surviving adult zebra (white) and quagga (grey) mussels recovered from the MFT experiment. Letters indicate statistical similarity. Bars lacking letters are similar to all groups. Dotted lines are visual aides to separate neighbor treatments. Neighbor treatments: Empty shell neighbors, live quagga mussel neighbors, live zebra mussel neighbors. Chamber location codes: E = edge, G = group, C = central. ... 67 Figure 2.6. Biomass (wet weight in g/shell length in mm) (±SD) of surviving adult zebra (white) and quagga (grey) mussels recovered from the MFT experiment. Letters indicate statistical similarity. Bars lacking letters are similar to all groups. Dotted lines are visual aides to separate neighbor treatments. Neighbor treatments: Empty shell neighbors, live quagga mussel neighbors, live zebra mussel neighbors. Chamber location codes: E = edge, G = group, C = central. ... 68 Figure 2.7. Percent survival of adult zebra (white) and quagga (grey) mussels recovered from the AMB experiment. Letters indicate statistical similarity. Dotted lines are visual aides to separate neighbor treatments. Treatment codes: B = bottom layer, M = middle layer, T = top layer; 1 – high sediment (solid “cup” bottom), 2 = medium sediment (mesh “cup” bottom), 3 = low
sediment (mesh “cup” bottom and holes drilled in housing). ... 69 Figure 2.8. Percent growth in length, height, and width (±SD) of surviving adult zebra (white) and quagga (grey) mussels recovered from the AMB experiment. Letters indicate statistical similarity. Bars lacking letters are similar to all groups. Treatment codes: B = bottom layer, M = middle layer, T = top layer ... 70 Chapter 3
Figure 3.1 Diagram of attachment-plasticity tube device showing the bundled arrangement of individual mussel tubes (8 plus one empty space surrounding a central cylindrical clod card) inside an ABS pipe (above) and design for manipulation of flow regime (below). Four mussels of each species were placed in the acrylic tubes with their siphons pointed upstream and allowed to attach before field deployment. Bundles were separated from the rest of the pipe by 3-mm-opening mesh. Each ABS pipe was fitted with a different sized ABS coupler (A=7.6 cm downstream, closed upstream; B=5.1 cm; C=3.8 cm; D=10.2 cm). Four APTs (one of each coupler type) were attached to a cinder block. ... 90
xiii
Figure 3.2 Boxplot of %clod card lost in each of the four tube types in the tube attachment experiment (pipe opening A=7.6 cm downstream, closed upstream; B=5.1 cm; C=3.8 cm; D=10.2 cm). Dotted lines within boxes indicate mean values, solid lines within indicate median, box edges indicate quartiles, and whiskers represent 95% confidence intervals. ... 91 Figure 3.3. Survival of adult zebra (white) and quagga (grey) mussels recovered from the tube attachment experiment vs. flow treatment type (pipe opening A=7.6 cm downstream, closed upstream; B=5.1 cm; C=3.8 cm; D=10.2 cm). Letters indicate statistical similarity. Species x flow treatments without letters were not significantly different from any other treatment
combination... 92 Figure 3.4. Percent growth in length of surviving adult zebra (white squares, dotted line) and quagga (grey circles, solid line) mussels recovered from the APT experiment vs. percent clod dissolution. Asterisk indicates significantly different slopes. ... 93 Figure 3.5. Byssal thread counts (# of plaques, top) and detachment force (N, bottom) of
surviving adult zebra (white squares, dotted line) and quagga (grey circles, solid line) mussels recovered from the tube attachment experiment vs. percent clod dissolution. ... 94 Figure 3.6. Detachment force (N) vs. thread count of surviving adult zebra (white squares, dotted line) and quagga (grey circles, solid line) mussels recovered from the tube attachment experiment... 95 Figure 3.7. Byssal thread count (top) and detachment force (N, bottom) vs. percent growth (length) of surviving zebra (white squares, dotted line) and quagga (grey circles, solid line) mussels recovered from the attachment-plasticity experiment. ... 96 Figure 3.8. Dreissenid detachment force (N) vs. site (arranged by increasing clod dissolution) of zebra (white) and quagga (grey) mussels pulled from rocks in the field. Letters indicate
significantly different groups. Groups without letters are not significantly different from any other group. ... 97 Figure 3.9. Detachment force (N) vs. length (mm) of adult zebra (white squares, dotted line) and quagga (grey circles, solid line) mussels pulled from rocks at both (A) less and (B) more exposed sites based on clod card dissolution. ... 98
xiv
Acknowledgements
This dissertation was made possible thanks to funding from the Canadian Aquatic Invasive Species Network (CAISN), Québec-Océan, and the Natural Science and Engineering Research Council of Canada (NSERC).
First and foremost, I would like to thank my advisor, Ladd Johnson, and my thesis committee members Warwick Vincent and Nadia Aubin-Horth. Ladd, you are famous all over the city of Québec, and the world of academia, but you never let it give you a big head. I was intimidated coming to work in your lab, but your confidence in me immediately inspired me to get to work. While you emphasized important skills like experimental accuracy and precision, you also taught me to step back and look at the bigger picture – in science and life. You and your family made it possible for my wife and I to have Dash, and we are eternally grateful for your help along the way. Warwick, I owe you a great deal of thanks for taking me on as your TA for the limnology course. Through talking with you and interacting with your students, I found a great sense of optimism, self-confidence, and joy in studying biology. Nadia, from our first meeting, to the time in your ecological development course, I felt that you believed in my abilities, and I will forever be grateful, especially since Ladd is probably still questioning whether chapter 3 is actually phenotypic plasticity.
I would also like to thank Mike Russell, my MS advisor at Villanova University. Mike, you taught me everything from experimental design to presenting my findings, but most importantly, you taught me how to think critically.
I need to give an enormous thank you to everyone involved in the field work required for this dissertation. Perhaps most deserving is my primary dive buddy for over 100 dives along the St. Lawrence River, Nacho Garrido. Nacho, you know the river was a dark, rough, and dangerous place, but you never faltered. I had never done dry-suit or river diving, but your calm diving made it feel natural for me. I was also lucky to have help from DFO divers Geneviève P and Matthieu H as well as lab members Anne-Sara S, Thew S, Katie M, Filippo F, Benoit D, Carla N. I would be remiss if I did not also thank the people who had some sense of sanity and stayed out of the water, but spent hours driving boats up and down the river or sitting in the hot sun on the docks at Bassin-Louise, picking, measuring, and marking mussels: Leo M, Nico L, Sam C, Heather H, Kevin M, Manon P, Laurie D, Charlotte C, Marie-Helene S, Emy A, Audrey D (1), Audrey D (2), and Jessica G. This project would not have been possible without such a great
xv
group of lab mates and assistants always willing to help, but also always asking questions and pushing me to think more deeply about my research. These people are all incredible scientists and friends who always want to help you think around corners and solve problems with data, figures, or presentations. I will always remember and be grateful for your camaraderie.
I also want to thank the friends I had outside of Laval. Skip, Josh, Fox, and Avni, I will forever cherish the ridiculous conversations we have on google chat that always help me process academic life.
A very special thank you goes to my family, specifically my mom who has had faith in me since day one. I grew up hearing about how you earned advanced degrees and worked in foreign countries while learning new languages, and I have always been inspired by these stories. Without your example, and your assurances, I might never have reached outside of the US for this degree. Thank you for teaching me German from such a young age, because it made learning French that much easier, but more than that, it gave me perspective. Thank you also to my
grandmother, Oma, for always challenging me while believing in me. I would also like to thank my father-in-law, Brian, and all three of my brothers-in-law as their examples have pushed me to build a balance between family and work.
Finally, I would like to give the biggest thank you to my wife, Heather, and my son, Dash. Heather, you have been the one constant I can always rely on, the rock on which I stand. You have pushed me to stick with this, to carry out my mission and achieve my goals. I can never thank you enough for your love and support, but I will try. Dashiel, you may never decide to crack open your dad’s old PhD dissertation and read this, but in case you do, remember that your questions made me want to be a better teacher. Through you I found that I loved helping people understand the world around them. Thank you, Dash, high five!
xvi
Foreword
This dissertation has a general introduction and conclusion and three chapters written in English as scientific articles. Each of the three thesis chapters (1-3) will be submitted for publication following the dissertation defense. In all the presentations listed below I am the first author. Experimental design, data collection and analysis, and chapter preparation were conducted by Nathan Haag under the supervision of Ladd Johnson.
Results of the Ph.D. have been presented in international and national conferences:
Oral presentations
Haag, N., LE. Johnson (2016). Comparing performance, dispersal, and population dynamics of two competing invasive Dreissenid mussels. Canadian Aquatic Invasive Species Network (CAISN) annual general meeting, Windsor, Ontario, Canada
Haag, N., LE. Johnson (2016). Comparing performance, dispersal, and population dynamics of two competing invasive Dreissenid mussels. 45th Benthic Ecology Meeting, Portland, Maine, United States
Haag, N., LE. Johnson (2015). Comparing performance, dispersal, and population dynamics of two competing invasive Dreissenid mussels in the St. Lawrence River. Canadian Aquatic Invasive Species Network (CAISN) annual general meeting, Halifax, Nova Scotia, Canada
Haag, N., LE. Johnson (2014). Comparing performance, dispersal, and population dynamics of two competing invasive Dreissenid mussels. Canadian Aquatic Invasive Species Network (CAISN) annual general meeting, Gatineau, Québec, Canada
Haag, N., LE. Johnson (2014). Comparing performance, dispersal, and population dynamics of two competing invasive Dreissenid mussels. 5th Colloque de Biologie, Laval University, Québec, Québec, Canada
Poster Presentations
xvii
competing invasive Dreissenid mussels. Québec-Océan annual general meeting, Québec, Québec, Canada
Haag, N., LE. Johnson (2015). Comparing performance, dispersal, and population dynamics of two competing invasive Dreissenid mussels. 44th Benthic Ecology Meeting, Québec, Québec, Canada
1
Introduction
Invasion biology is frequently defined as the study of species that, once introduced to an area outside of their native range, successfully survive, reproduce, and expand beyond their introduction point. While non-native species introductions can be innocuous, it is the expansion of certain of these species that prompts scientific discussion. Indeed, Charles Elton, the oft-labeled father of invasion biology, characterized these invasion events as “ecological
explosions,” as these species tend to dramatically increase their population sizes (Elton 1958). From introduction, to local adaptation, to the efficiency of reproduction and dispersal, invasion biology studies the drivers and effects of these explosions, which almost invariably produce negative impacts for the ecology and economy of the new host habitat.
Often, the most reported aspect of species invasions is their ecological and economic impact (Johnson et al. 2012), which is logical as introduced species can lead to dramatic changes in their new host environment. Shifts in resource availability are among the most common, with examples across many taxa. A classic example is the Kudzu vine, which can grow up to one foot per day, and spreads so quickly that it smothers other plants and prevents access to the sun and soil nutrients (Webster et al. 2006). Invasive species can also reduce or extirpate native species through competition or predation, such as the loss of native unionid mussels and freshwater fish (e.g. benthic Hadley stickleback, deepwater cisco, Banff longnose dace) due to introduced fish (e.g. alewife, brown bullhead catfish, pumpkinseed sunfish), plants (e.g. Eurasian watermilfoil), and mollusks (e.g. zebra and quagga mussels) (Ricciardi et al. 1998, Dextrase and Mandrak 2006). Other examples include the losses of numerous bird species in Guam due to the invasive brown tree snake (Wiles et al. 2003), and several fish species in the Caribbean Sea after the introduction of the Lionfish (Ingeman 2016). Species invasions can also facilitate spread of parasites and pathogens such the citrus greening disease by the invasive Asian citrus psyllid in North America (Qureshi et al. 2008) or bovine tuberculosis by brushtail possum in New Zealand (Clout 1999). Perhaps more evident to the human populace are the enormous economic impacts of invasive species on both private and public industries, often costing several million dollars in repair or species removal (Pimentel et al. 2000). Blights and other tree diseases carried by nonindigenous insect species can result in millions of dollars of losses for timber companies (Holmes et al. 2009). Ecosystem alterations by aquatic invaders can result in decreases in water quality, negatively impacting ecosystem services such as water clarification (Connelly et al.
2
2007, Walsh et al. 2016). Altogether, impacts like these have likely contributed to the increased interest in the ecology of invasive species present in the literature (Ricciardi and MacIsaac 2008).
The increased interest in invasion biology has also focused on the spread of non-native species (Johnson et al. 2012). Most often linked to human travel and economic trade (Work et al. 2005), transport of non-native species is often accidental (Lehan et al. 2013). Other species invasions, however, are the result of remediation efforts for other invasive species, such as the cane toad, introduced in Australia to control cane beetles (reviewed in Kraus 2008). Regardless of the method or intention, invasive species are, by definition, the product of anthropogenic activity. In one of his seminal works, The Ecology of Invasions by Animals and Plants, Elton predicted that species invasions would be linked to human population growth (Elton 1958). Since then, the human population has more than doubled, and travel and trade have changed
dramatically (Perrings 2011). As predicted, the number of species has increased, inevitably resulting in multiple species invasions per host environment (reviewed in Johnson et al. 2009). These concurrent or sequential invasions can be by unrelated taxa, like bullfrogs and fish (Preston et al. 2012), distantly related species such as the Asian clam (Corbicula fluminea) and the golden mussel (Limnoperna fortunei) (Linares et al. 2017), or closely related species, such as the tunicates Ciona robusta and C. intestinalis (Bouchemousse et al. 2016). Ultimately, because the impacts of these co-invasions can be so negative, the focus has largely remained on how each invader impacts native populations, and where they might reach next.
Predicting where a single or multi-species invasion might occur is another topic
commonly examined in invasion biology (Parker et al. 1999, Kolar and Lodge 2001). However, the factors influencing an invasion, even after a nonnative species has become established, are numerous, making it difficult to both predict further spread and judge the efficacy of remediation efforts without periodic reassessment (reviewed in Johnson et al. 2012). It is often difficult and expensive, however, to monitor populations, especially when access is limited as is often the case in aquatic species. Therefore, predictive modelling has become a valuable tool, used in conjunction with field data, to map out potential invasion outcomes (Ricciardi 2003). These tools have helped elucidate the factors that limit and facilitate invasions (Jones and Ricciardi 2005, Vaclavik and Meentemeyer 2012, Murphy et al. 2016). Ultimately, however, each ecosystem is a unique and dynamic combination of environmental variables that can change over time.
3
Therefore, populations must be reassessed to validate and increase the efficiency of predictive tools, especially for introduced species with dramatic competitive advantages over the native species of the host habitat.
The advantages possessed by invasive species are often derived from a lack of key competitors or predators (Wolfe 2002, Callaway and Ridenour 2004, Callaway et al. 2011) and therefore may not persist or spread in patterns associated with their native range (Ricciardi 2003, Johnson et al. 2009). As populations of each invasive species change, so too can their impact on the host environment (Strayer et al. 2006, Johnson et al. 2012). Two invasive species with similar negative impacts on their introduced ecosystem may have additive or synergistic effects (Johnson et al. 2009, Preston et al. 2012, Beggel et al. 2016, Spencer et al. 2017, Wang et al. 2018). Some invaders can facilitate the arrival and success of other non-native species, resulting in what many refer to as an “invasion meltdown” (Simberloff and Van Holle 1999, Ricciardi 2001, Linares et al. 2017). Conversely, functionally similar invaders will likely compete for space or food in their new environment (Orlova et al. 2004, Arismendi et al. 2012, Heiler et al. 2012) sometimes resulting in a displacement of one or more competing invasive species (Huxel 1999, Shinen and Morgan 2009, Ram et al. 2012). While a displacement of one invader by another may seem like a zero-sum game, even closely related invasive species may have differences in adaptive ability, eradication tolerance, and ultimately, impacts on the host environment (Burlakova et al. 2014, Matthews et al. 2015). Therefore, examination of the interactions between and among invaders can provide valuable context for developing management strategies.
Perhaps the best example of competition between two aquatic invasive species is that of two freshwater mussels in the genus Dreissena: the zebra mussel (D. polymorpha) and the quagga mussel (D. bugensis). Originating from the Ponto-Caspian region of Europe, these bivalves were the first epifaunal filtering mollusks in North American fresh waters, and thus were able to exploit somewhat of an “empty niche”. Both species have had severely negative effects on native species and ecosystems (Bunt et al. 1993, Schloesser et al. 1996, Ricciardi et al. 1997, Stoeckel et al. 1997, Ricciardi et al. 1998, Glon et al. 2017, Dzialowski et al. 2018, Strayer and Malcom 2018). Able to attach to almost any solid surface, their massive and often mixed-species assemblages can cover the bottoms of lakes and rivers , dramatically altering the bottom composition, and effectively creating more substratum for dreissenid mussels (Ricciardi et al.
4
1997, Bially and MacIsaac 2000, Karatayev et al. 2002, Jones and Ricciardi 2005). Both species are also able to attach to and physically smother native mussels, potentially extirpating some unionid species (Schloesser et al. 1996, Ricciardi et al. 1998, Schloesser and Marsteller 1999, Rowe and Zanatta 2015, Burlakova et al. 2018, Dzierżyńska‐Białończyk et al. 2018). Further, both species are extremely efficient filter feeders, clearing 39-96% of the water column per day in some cases (Bunt et al. 1993). This ability enables them to dramatically alter both the food and chemical contents of the water column (Bunt et al. 1993, Baker and Levinton 2003, Higgins and Vander Zanden 2010). Such extreme filtration effectively relocates organic matter from the water column to the benthos (i.e. biodeposition) where it can serve as a valuable food resource for deposit feeders (Roditi et al. 1997), some of which themselves are nonindigenous (Higgins and Vander Zanden 2010, DeVanna et al. 2011). In some cases this biodeposition helps invasive species, but not the native analog (e.g. such as with the invasive rusty and native virile crayfish; Glon et al. 2017). Further, the dreissenid-driven clarification of the water column has increased light penetration, allowing for growth and spread of macrophytes (Zhu et al. 2006). The number of invasive species facilitated by the dreissenid invasion has led to discussion over whether some North American water bodies are now facing the aforementioned “invasion meltdown”
(Ricciardi 2001, DeVanna et al. 2011).
Another key aspect of the success of dreissenid invasions is their incredible fecundity, releasing up to a million eggs into the water column with each spawning event (Sprung 1991), with some able to spawn twice within one year (Borcherding 1991). Dreissenid larvae, or veligers, can spend several weeks (~1-9) in the water column (Carlton 1993, Martel et al. 1995), enabling them to travel up to 300km (Stoeckel et al. 1997). Further complicating control and remediation measures, both species are able to disperse over land via boat hulls, tangled macrophytes, live wells, and bilge water as both juveniles and adults (Ricciardi et al. 1995, Johnson et al. 2001, Collas et al. 2016), with adults surviving up to 3-5 days of aerial exposure in humid conditions (Ricciardi et al. 1995). Finally, the longevity of dreissenid mussels is estimated at about 2-4 years (Karatayev et al. 2006) meaning each mussel is likely to spawn more than once in its life.
The result of this arsenal of advantages has been a dramatic increase in dreissenid populations. First arriving in the late 1980s via ballast water (Carlton 2008), the zebra mussel quickly spread to 20 states and 2 Canadian provinces (Connelly et al. 2007, Ram et al. 2012).
5
Arriving roughly two years later and spreading relatively slower (May and Marsden 1992, Mills et al. 1993), the quagga mussel has slowly become the dominant dreissenid, displacing much of the zebra mussel population where both species occur (Orlova et al. 2004, Ricciardi and
Whoriskey 2004, Orlova et al. 2005, Zhulidov et al. 2006, Ram et al. 2012). In some places, such as Lake Erie, Lake Michigan, and Lake Ontario, quagga mussels make up as much as 99% of the dreissenid population (Wilson et al. 2006, Karatayev et al. 2011a), pushing zebra mussels to shallower waters and onto macrophytes (Horvath and Lamberti 1997, Diggins et al. 2004).
While a majority of the literature on this dreissenid displacement in North America is based on studies performed in the Great Lakes (Naddafi et al. 2011, Karatayev et al. 2013,
Burlakova et al. 2014, Karatayev et al. 2014), studying their populations in rivers is slightly more complicated with more variable combinations of turbidity, nutrient input, and current velocity (Mellina and Rasmussen 1994). For example, because dreissenid mussels reproduce via broadcast spawning, their dispersal depends on currents, which are generally unidirectional in rivers. The downstream flow of genetic material results in a source/sink dynamic wherein dreissenid juveniles rely on upstream populations for continued existence (Horvath et al. 1996, Krkošek and Lewis 2010). There is also evidence that dreissenid settlement can be affected by turbidity (Kozarek et al. 2018) current velocity (Chen et al. 2011) and further, feeding in adults can also be negatively affected by current velocity (Ackerman 1999). Altogether, although lakes and rivers are often connected, they can be quite different environments.
At over 1000 kilometers, the St. Lawrence River is one of the longest and most important rivers for both Canadian and American commerce. This economic importance made the St. Lawrence River the original conduit of the dreissenid mussel Great Lakes invasion via ballast water release, which makes it as an excellent location to study the ecology and physiology of dreissenid mussels. Water in the St. Lawrence River comes primarily from the clear, calcium rich waters of Lake Ontario. However, between the lake and the Gulf of St. Lawrence, the river receives water and nutrients from over 58 tributaries, including a large supply of turbid, calcium-poor water from the Ottawa River. From the fluvial Lac St. Pierre to Île d’Orléans, the river is tidally influenced, resulting in a backflow of freshwater. Between Île d’Orléans and the Gulf, the river becomes an estuary and increases in salinity. Further, the river has been dredged to create and maintain a deep shipping channel (Morin and Côté 2003), which has added complexity to its benthos, creating a heterogeneous mix of boulders, gravel, sand, and mud. These combinations
6
of various water chemistries, velocities, and substrata make the St. Lawrence River a mosaic of abiotic conditions unlike the Great Lakes.
The two major surveys of dreissenid populations in the St. Lawrence River examined distribution patterns of zebra mussels over the entire portion of the river in the early 1990s (Mellina and Rasmussen 1994) and both species in the first half of the river in the early 2000s (Jones and Ricciardi 2005). Both of these studies focused on the establishment and spread of dreissenid mussels in relation to physico-chemical factors such as substratum size and calcium content. However, because invasive species, like any other species, can experience boom-and-bust changes in populations (Simberloff and Gibbons 2004, Pace et al. 2010, Carlsson et al. 2011), and because quagga mussels are displacing zebra mussels in many rivers and lakes of North America (Zhulidov et al. 2006, Ram et al. 2012, Burlakova et al. 2014, Burlakova et al. 2018), it becomes necessary to re-examine dreissenid populations periodically. Revisiting dreissenid populations in the river can both sharpen our understanding for shifting dispersal patterns and limitations of each species and inform future management decisions (Johnson et al. 2012, Burlakova et al. 2014).
In addition to monitoring the spread of invasive species, examining the underlying mechanisms that facilitate the dreissenid displacement will help predict potential outcomes of dreissenid co-invasions. Much of the work on the displacement focuses on differences in performance between the two species. The primary metric of performance has thus far been the efficacy of each species' exploitation of available food resources (Ackerman 1999, Diggins 2001, Baldwin et al. 2002). While Diggins (2001) found no difference in filtration rate in smaller individuals of both species (<15mm), among larger mussels, quagga mussels filtered significantly faster, and indeed, quagga mussels often reach a larger size faster than zebra mussels (Diggins 2001). Later research, however, found quagga mussels to be slower filterers (Stoeckmann 2003) or not significantly different from zebra mussels (Kemp and Aldridge 2018). Other suggestions for quagga mussel competitive advantages include broader spawning
temperatures (Roe and MacIsaac 1997, Orlova et al. 2004), higher food assimilation
(Stoeckmann and Garton 1997), and the ability to alter feeding organs in response to turbidity (Ouellette-Plante et al. 2017). At the same time, however, zebra mussels have exhibited higher reproductive output per soft tissue mass (Stoeckmann 2003). Zebra mussels have also exhibited thicker, stronger shells (Roe and MacIsaac 1997, Casper and Johnson 2010), and stronger
7
attachment (Peyer et al. 2009, Grutters et al. 2012). Further, in the presence of predators, zebra mussels can increase shell thickness and attachment strength faster than quagga mussels (Naddafi et al. 2010, Naddafi and Rudstam 2013, 2014), suggesting that one of the major advantages for quagga mussels in North America is the lack of abundant predators (but see Thorp et al. 1998, Andraso et al. 2011, Ruetz et al. 2012). Altogether, while the differences in dreissenid performance have been the focus, it has been difficult to develop a comprehensive picture of how much each of these characteristics contribute to the displacement of zebra mussels by quagga mussels.
Despite their widespread co-occurrence, few studies have examined how these two species perform in mixed assemblages, despite the obvious potential for direct competitive interactions. Conversely, competition studies are common in marine mussels, revealing both exploitative competition through feeding (Harger 1968, Ramon 1996, Fréchette and Despland 1999) and interference competition through impeding neighbor shell gape or outright smothering (Shinen and Morgan 2009, Comeau and Babarro 2014, Fuentes and Brante 2014). Studies of dreissenid competition, however, are limited to their impact on native unionid mussels (Mackie 1991, Schloesser et al. 1996, Schloesser and Marsteller 1999) or intraspecific competition (Burks et al. 2002, Tuchman et al. 2004, Kobak et al. 2009). Studies examining competition between zebra and quagga mussels are limited, and primarily examine exploitative competition (Baldwin et al. 2002, Karatayev et al. 2011b, Metz et al. 2018).
Examining the potential for interference competition between dreissenids can augment our understanding of dreissenid ecology and physiology because both species are most often found aggregating in large mussel beds that cover the benthos, and rarely exist in isolation (though see Tošenovský and Kobak. 2015). Mussels living in different parts of a bed can experience different predatory (Kobak and Kakareko 2011, Naddafi and Rudstam 2013) and hydrodynamic pressures (Carrington et al. 2008), and like the aforementioned marine mussels, likely experience competitive pressures associated with living in tightly packed assemblages. When living in high densities, marine mussels become elongated (Alunno-Bruscia et al. 2001, Cubillo et al. 2012), likely a plastic response to competition. Determining whether dreissenids exhibit similar adaptations will lend more insight about the dreissenid displacement in North America.
8
The aforementioned shell plasticity is not unique to marine mussels, as both zebra (Pathy and Mackie 1993, Lajtner et al. 2004) and quagga mussels (Claxton et al. 1998, Peyer et al. 2010) have some ability to alter shell morphology. Both mussels are able to alter their shape to facilitate attachment to hard substratum, but quagga mussels also have the ability to develop a profunda morphotype that enables them to live infaunally in soft sediments (Peyer et al. 2010). These results indicate that attachment is of utmost importance for successful invasion and spread in dreissenids, and lab studies have shown that zebra mussels tend to anchor faster and stronger than quagga mussels, and that zebra mussels are more responsive to higher velocities (Peyer et al. 2009, Collas et al. 2016). It is unknown, however, whether this stronger attachment in zebra mussels negatively impacts growth.
The displacement of zebra mussels by quagga mussels is no doubt affected by multiple factors ranging from functional differences to direct interspecific interactions. Examining the factors that differentiate these two species will facilitate proper management not only of dreissenids in the St. Lawrence River, but future dreissenid invasions in other rivers, as well as invasions of closely related species in general. This dissertation addresses the factors that may contribute to the dreissenid displacement from three different perspectives.
The first chapter examines distributions of both dreissenid species along the entire
freshwater portion of the St. Lawrence River with respect to substratum type, depth, and distance from the assumed source population in Lake Ontario. The second goal of the field survey was to update previous accounts of dreissenid biomass in the St. Lawrence River. In addition, the field survey was complemented with an outplanting survival study designed to further elucidate the factors that affect dreissenids in the river by using a single source population from the lower portion of the river.
Using field experiments to manipulate mussel aggregations, the second chapter focuses on whether competition in dreissenids is primarily through interference or exploitative
competition, or both. This chapter also examines inter-/intraspecific competition, and whether one species exhibits any competitive advantages. In addition, this chapter includes an
investigation of the tolerance of each species for aggregation within a mussel bed and the ability to escape burial by other mussels.
Finally, the third chapter describes experimental cages used to manipulate water flow in the field to elucidate the potential tradeoffs associated with phenotypic plasticity in dreissenid
9
byssal attachment. In addition, this chapter also includes a field study wherein mussel
detachment force was recorded from individual zebra and quagga mussels removed from rocks collected in-situ. Together, these two components served to examine relative cost of attachment in dreissenid mussels and determine whether superior attachment in zebra mussels is detrimental to survival and/or growth.
Together, these chapters build a cohesive examination of the differences between two invasive congeners by focusing on the current state of the dreissenid invasion of the St.
Lawrence River, how it has changed, and two understudied factors that could contribute to these changes. The expectation based on the literature was that quagga mussels had already displaced zebra mussels in over 90% of the river, and that quagga mussels were simply more capable of surviving the various conditions in the river. The goal of the competition chapter was to examine whether such an advantage was limited to individual performance or included an interactive component. Previous work on marine mussels coupled with the dominance of quagga mussels in the Great Lakes lead to the hypothesis that quagga mussels physically interact with zebra
mussels. Finally, the attachment study sought to replicate lab-based studies in the field and determine costs of byssal threads. The rationale was that while zebra mussels have stronger reported attachment strength, perhaps the cost was too high in the long term, and reduced growth, thus reducing reproductive output. Ultimately, this thesis examines the big-picture dynamics of a dual invasion through the lens of the physiological abilities of the two players while reexamining long-held beliefs about each species strengths.
10
Chapter 1: Where are they now? Examining the large-scale displacement of
one invasive dreissenid mussel by its congener in the St. Lawrence River 20
years later.
1.1 Résumé
Bien qu’une grande partie de la recherche sur les espèces envahissantes porte sur les effets négatifs sur les écosystèmes locales, l’accent est moins mis sur la propagation et les dynamiques de population des envahisseurs avec le temps. De plus, comme la biologie de l’invasion se concentre souvent sur le rôle d’une seule espèce non indigène, les interactions qui influent sur la propagation de multiples espèces envahissantes peuvent être négligées ou supposées. L’un des cas les plus frappants d’interactions entre espèces envahissantes a été le déplacement de la moule zébrée (Dreissena polymorpha) par son congénère, la moule quagga (Dreissena bugensis). Alors que la moule zébrée s’est répandue rapidement dans les cours d’eau nord-américains, la moule quagga s’est répandue plus lentement, mais est devenue dominante dans de nombreuses zones benthiques des lacs américains et canadiens. La dynamique qui affecte le déplacement des Dreissenas dans les rivières est moins bien comprise. À l’aide de relevés des populations de moules en 2013 et d’expériences en cage de survie en 2014, cette étude examine l’étendue et la nature de la dominance de la moule quagga dans la rivière St Saint-Laurent. En comparant les populations avec les facteurs physiques de la rivière (type de substrat, distance par rapport aux populations sources, profondeur), nous avons constaté que le type de substrat demeure le facteur le plus important limitant les moules envahissantes, mais que les moules zébrées, peut-être par leur capacité d’attache, sont mieux en mesure de coloniser des substrats plus fins dans la rivière. Il est probable que le substrat interagit avec de multiples facteurs, en particulier la profondeur et la vitesse du courant, pour affecter l’invisibilité de diverses zones de la rivière. Comparativement aux relevés antérieurs effectués au début des années 1990 et 2000, nous constatons que les populations de la moule quagga ont considérablement augmenté, mais que la moule zébrée persiste et demeure abondante dans les parties inférieures du fleuve et que l’invasion du fleuve Saint-Laurent par la moule quagga prendra donc plus longtemps que dans les Grands Lacs et peut jamais le déplacement complet du congénère.
11
1.2 Abstract
While much of the research on invasive species focuses on their negative effects on local ecosystems, there is less emphasis on the spread and population dynamics of invaders over time. Moreover, because invasion biology often focuses on the role of a single nonnative species, interactions that impact the spread of multiple invaders can be overlooked or assumed. One of the most striking cases of interactions between invasive species has been the displacement of the zebra mussel (Dreissena polymorpha) by its congener the quagga mussel (Dreissena bugensis). While the zebra mussel spread quickly across North American waterways, the quagga mussel has spread more slowly but has displaced the zebra mussel in many benthic zones in American and Canadian lakes (Strayer et al. 2019). Less understood is the dynamics affecting the dreissenid displacement in rivers. Using SCUBA surveys of mussel populations in 2013 and survival cage experiments in 2014, this study examined the extent and nature of quagga mussel dominance in the St. Lawrence River. By comparing populations living in habitats with different physical factors of the river (substratum type, distance from Lake Ontario, depth), we found that substratum type continues to be the most important factor limiting invasive mussels, but that zebra mussels, perhaps through attachment abilities, are better able to colonize finer substrata in the river. It is likely that substratum interacts with multiple factors, particularly depth and current velocity to affect the invasibility of various areas in the river. In comparison with earlier surveys in the early 1990s and 2000s, we find that quagga mussel populations have increased
dramatically, but that zebra mussels still persist and remain at high abundance in the lower portions of the river. The quagga mussel invasion of the St. Lawrence River has taken much longer than in the Great Lakes and may not result in the complete displacement of its congener.
12
1.3 Introduction
Much of the effort in invasion biology research is focused on detection or management of a single species, and its impacts on its new host environment. As the number of species invasions increases, however, it becomes inevitable that invasive species will interact (Ricciardi 2006). Multiple species arrive together or sequentially and can have additive or synergistic effects on native populations (Beggel et al. 2016, Spencer et al. 2017, Wang et al. 2018). Some of these co-invaders have no impact on one another (Cope and Winterbourn 2004), but others can have antagonistic interactions (Braks et al. 2004, Ross et al. 2004). Invaders can also facilitate the arrival and success of other invaders, resulting in an “invasion meltdown” (Simberloff and Von Holle 1999, Ricciardi 2001, Linares et al. 2017). These synergies, antagonisms, and facilitations among nonindigenous species are difficult to predict, however, as invasive species are often benign in their native range (Ricciardi 2003, Bruno et al. 2005, Johnson et al. 2009) and become problematic once escaping predation (Wolfe 2002), competition (Callaway et al. 2011), or a combination of these and other conditions that typically control their numbers in their native ranges (Sih et al. 2010). Monitoring such interactions can promote a greater understanding of the combined impacts multiple invaders can have on native species, ecosystems, and economies, and ultimately inform prediction and management decisions.
One of the most ecologically devastating joint invasions is the spread of the Ponto-Caspian dreissenid mussels, the zebra mussel (Dreissena polymorpha) and quagga mussel
(D. bugensis). Arriving in North America in the late 1980s (Carlton 2008), zebra mussels quickly spread to waterways in over 23 American states and 2 Canadian provinces (Karatayev et al. 2011a, Ram et al. 2012). Since its arrival in North America in 1989, the quagga mussel, which generally exhibits slower colonization rates (Karatayev et al. 2011a), has reached 10 states (Mills et al. 1993). Quagga mussels have exhibited the ability to colonize deeper waters in the
Laurentian Great Lakes than zebra mussels (Dermott and Munawar 1993, Patterson et al. 2005). Despite their slow start and spread, and their affinities with the conditions of deeper water, quagga mussels have displaced zebra mussels over much of their distribution in North America (Mills et al. 1999, Stoeckmann 2003, Karatayev et al. 2011a, Ram et al. 2012, Burlakova et al. 2014, Karatayev et al. 2014, Karatayev et al. 2015), making up 70-100% of the benthos of the Great Lakes (Diggins et al. 2004, Burlakova et al. 2018). This dreissenid displacement, or the transition of dreissenid mussel populations from zebra mussel dominated to quagga mussel
13
dominated, has gained some interest in the species’ native range, but has become especially compelling in understanding the process of multi-species invasions.
In North America, several studies of the Great Lakes have examined various factors shaping dreissenid displacement ranging from differences in physiologies to reproductive capacities. One of the leading ideas is that quagga mussels are simply more efficient filterers, with lower filtration rates when food availability is low (Summers et al. 1996), better respiration rates (Stoeckmann 2003), and higher food sorting capabilities (Ouellette-Plante et al. 2017). Another suggestion is that lower energetic investment in shell building makes the quagga mussel the superior performer as predators in North America are limited to fellow invasive Ponto-Caspian gobies (Roe and MacIsaac 1997, Casper and Johnson 2010, Naddafi and Rudstam 2013). Zebra mussels, by contrast, have stronger attachment, enabling them to dominate in areas of higher hydrodynamic stress (Diggins et al. 2004, Peyer et al. 2009, Karatayev et al. 2013). Zebra mussels also produce more eggs and allocate a higher proportion of soft tissue to
reproduction than quagga mussels (Stoeckmann 2003) . Further, studies examining physical and chemical stressors have concluded that zebra mussels have a higher salinity tolerance than
quagga mussels (Mills et al. 1993, Orlova et al. 1998, Ellis and MacIsaac 2009) and temperatures (Spidle et al. 1995). Despite a seemingly balanced set of competitive advantages between
species, the displacement of zebra mussels by quagga mussels continues in lakes across North America under a variety of environmental conditions (e.g. flow, salinity, and temperature regimes).
While this displacement has been well described for the lentic environments of the Great Lakes and smaller inland lakes (Burlakova et al. 2014, Burlakova et al. 2018), few studies have examined this phenomena in small or large rivers, making generalizations on dreissenid
displacement difficult. The stronger current velocities of lotic environments (driven by gravity rather than wind) and generally shallower depths of rivers result in systems with markedly different environments relative to lakes, especially in terms of hydrodynamics. Indeed,
organisms from various taxa that inhabit both lakes and rivers exhibit adaptation for increased velocity in riverine populations (Brinsmead and Fox 2002, Pavey et al. 2010, Collin and
Fumagalli 2011, Guan et al. 2018). The faster, unidirectional current in rivers also leads to much shorter water residence times, and thus, decreased nutrient retention (Saunders and Kalff 2001).
14
Conversely, while both rivers and lakes can have varying levels of turbidity, rivers have increased perimeter to volume ratios, which can lead to increased nutrient input and mixing (Ryder and Pesendorfer 1989, Saunders and Kalff 2001, Hayes et al. 2017). Rivers can also stretch for long distances, taking on water from tributaries from several different water sheds, each discharging water of different physical and chemical characteristics (Ryder and Pesendorfer 1989). While lakes certainly have currents, mixing, and turbidity, the combination of these characteristics in rivers often leads to more horizontal, rather than vertical mixing in rivers, resulting in a mosaic of conditions along in their course. Therefore, while lentic dreissenid displacement studies can lend valuable insight to lotic environments, the two are different enough to merit special attention.
The St. Lawrence River, one of the longest and most economically important rivers in Canada, is a particularly heterogeneous river (Lachance et al. 1979). Its primary source is the calcium-rich waters of Lake Ontario, but it also receives the discharge of over 58 tributaries, including the Ottawa River, which deposits silty, low-calcium water into the fluvial lake Lac St. Louis where the two rivers meet near Montréal, Québec (Thorp et al. 2005). Further, the portion of the river between Lac St. Pierre and Île d’Orléans is tidally influenced, with tides of up to 6 meters in some places. Beginning at Île d’Orléans, the river transitions to an estuary, where fresh water from the river mixes with saltwater from the Gulf of St. Lawrence. In addition, the
riverbed is a patchwork of stone, gravel, sand, and mud. Further, the dredging of the shipping channel in the 1950s displaced tons of substratum, making some areas more heterogeneous, like the transition of muddy to rocky bottoms in Lac St. Louis, whereas others are more homogenous, such as the long sandy stretch between Montréal and Lac St. Pierre (pers. obs.; see also Mellina and Rasmussen 1994). Together, this patchwork of abiotic conditions creates a system unlike that of the Laurentian Great Lakes, likely resulting in slower progress of the dreissenid displacement along the length of the river.
The unidirectional flow of rivers also affects the ability of zebra and quagga mussels to maintain populations within a river. As is the case for many marine invertebrates, both mussel species reproduce through the release of gametes directly into the water column (i.e. broadcast-spawning), which results in a planktotrophic larval stage known as a veliger that must live and feed in the water column for weeks before developing a stage capable of settling on the bottom.
15
Although capable of weak swimming, this life stage cannot swim upstream, meaning dispersal is unidirectional in rivers (Stoeckel et al. 1997). Rivers can thus generally act as a conduit for downstream dispersal (i.e. between reservoirs), but the maintenance of lotic populations usually depends on larvae produced in upstream lakes or reservoirs, essentially a source/sink dynamic (Horvath et al. 1996, Krkošek and Lewis 2010). However, the situation in large rivers is less clear, especially if fluvial or “in-line riverine” lakes allow sufficient residency time for larval development (Stoeckel et al. 1997, Stoeckel et al. 2004). Stoeckel et al. (2004) suggest that dreissenid populations in the Mississippi River are dependent upon source populations in the “in-line riverine lake” Lake Pepin, and Martel and Madill (2018) suggest that retention times in locks along the Rideau River aid in structuring a dreissenid metapopulation, or a network of connected populations. In the case of the St. Lawrence River, which serves as the conduit connecting the Great Lakes to the Gulf of St. Lawrence (Mills et al. 1993), the expectation is that if Lake Ontario is dominated by quagga mussels then the St. Lawrence River will eventually be
dominated by quagga mussels (Thorp et al. 2002, Jones and Ricciardi 2005). However, because the St. Lawrence River has three large fluvial lakes, it is possible that the populations of both species along the river may be more complex than a simple source/sink dynamic. Periodically reexamining the patterns of dominance along the length of the river, including within these fluvial lakes, will explicate the intricacies of the dreissenid displacement in the river.
The first comprehensive survey after the arrival of zebra mussels in the St. Lawrence River recorded their presence along the entire freshwater potion of the river, related them to various physical and chemical factors, and found that substratum was a promising predictor of zebra mussel abundance (Mellina and Rasmussen 1994). This study, however, was conducted before quagga mussels were known to be in the river (Ricciardi and Whoriskey 2004). A decade later, a similar, but more limited, survey between Lake Ontario and Montréal was conducted that included quagga mussels (Jones and Ricciardi 2005). The latter study supported the findings of the former but predicted that substrata would likely become less important as mussel beds
continued to coat the river-bottom, and suggested the trend of quagga mussels dominating deeper waters would continue.
The purpose of this study was to examine the extent and nature of the dreissenid displacement in the St. Lawrence River by assessing populations over the entire length of the
16
river, comparing our findings with previous studies and reevaluating suggested predictors of mussel performance and dispersal in the river. Specifically, we sampled adult and juvenile mussel of both species at 77 sites and compared their wet biomass, density, and length and to local substratum size, depth, and river kilometer (i.e. distance from Lake Ontario, the presumed source population). The following year, we measured adult mussel survival and recruitment at a select number of sites. We also compared our results to earlier surveys to assess the evolution of the dreissenid displacement. Based on the evidence in these earlier surveys and our own
preliminary observations, the prediction was that the dreissenid displacement in the St. Lawrence River had progressed so that quagga mussels would be the dominant dreissenid mussel from Lake Ontario all the way to Québec City, with zebra mussels almost entirely relegated to the most shallow locations. Finally, we expected that dreissenid mussels had colonized enough of the benthos that the substratum type would not impact mussel populations and performance in either survey.
1.4 Methods
1.4.1 Adult mussel survey
Between early June and early October 2013, 77 sites along the St. Lawrence River were sampled by SCUBA diving from small boats. These sites were distributed along the entire length of the freshwater portion of the St. Lawrence River from its origin near Kingston, Ontario, to Île d’Orléans, Québec, where the St. Lawrence Estuary begins (~500 km, Figure 1.1). Because no official distance markers exist for the St. Lawrence River, river kilometers were measured for each site as the distance along the river, including all bends and turns, from the first site near Kingston, ON (44°15'39.48"N, 76°16'34.90"W, RK0). Dive sites ranged between 1.5 to 11.2 m in depth with an average of 4.9 m ± 2.0 (SD). At each dive site, a 0.5 m2 quadrat was
haphazardly placed on the bottom, and all mussels within collected in mesh bags. This process was repeated four times at each site with at least 2 m between quadrats, again placed haphazardly due to strong currents and low visibility. When time was limiting, rocks covered by mussels were brought to the surface for collection. Mussels were kept on ice and frozen within 24 hours of collection. Substratum composition was visually determined in situ for each quadrat, and