Systematic Review of Effects of Withdrawal of Immunomodulators or
Biologic Agents From Patients With Inflammatory Bowel Disease
Joana Torres,1,* Ray K. Boyapati,2,* Nicholas A. Kennedy,2 Edouard Louis,3 Jean-Frederic Colombel,1 and Jack Satsangi2
1 Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, New York;
2Gastrointestinal Unit, Centre for Molecular Medicine, Institute of Genetics and Molecular Medicine, Western General Hospital, Edinburgh, Scotland;
3Department of Gastroenterology, University Hospital CHU of Liège, Liège, Belgium
Abstract
Little is known about the optimal duration of therapy with an anti-tumor necrosis factor (TNF) agent and/or an immunomodulator for patients with inflammatory bowel disease (IBD). We performed a systematic search of the literature to identify studies reporting after de-escalation (drug cessation or dose reduction) of anti-TNF agents and/or immunomodulators in patients in remission from IBD. Studies were reviewed according to the type of IBD and drug. Rates of relapse, factors associated with relapse, and response to re-treatment were determined. Our search yielded 6315 unique citations; we analyzed findings from 69 studies (18 on de-escalation [drug cessation or dose reduction] of immunomodulator monotherapy, 8 on immunomodulator de-escalation from combination therapy, and 43 on de-escalation of anti-TNF agents, including 3 during pregnancy) comprising 4672 patients. Stopping immunomodulator monotherapy after a period of remission was associated with high rates of relapse in patients with Crohn's disease or ulcerative colitis (approximately 75% of patients experienced a relapse within 5 years after therapy was stopped). Most studies of patients with Crohn's disease who
discontinued an immunomodulator after combination therapy found that rates of relapse did not differ from those of patients who continued taking the drug (55%-60% had disease relapse 24 months after they stopped taking the immunomodulator). The only study in patients with ulcerative colitis supported continued immunomodulator use. Approximately 50% of patients who discontinued anti-TNF agents after combination therapy maintained remission 24 months later, but the proportion in remission decreased with time. Markers of disease activity, poor prognostic factors, and complicated or relapsing disease course were associated with future relapse. In
conclusion, based on a systematic review, 50% or more of patients with IBD who cease therapy have a disease relapse. Further studies are required to accurately identify subgroups of patients who are good candidates for discontinuation of treatment. The decision to withdraw a drug should be made for each individual based on patient preference, disease markers, consequences of relapse, safety, and cost.
Keywords : Crohn's Disease ; Ulcerative Colitis ; Patient Management ; Cessation.
Abbreviations used in this paper : anti-TNF, anti-tumor necrosis factor therapy ; CD, Crohn's disease ; CRP,
C-reactive protein ; FC, fecal calprotectin ; IBD, inflammatory bowel disease ; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses ; RCT, randomized controlled trial ; TNF, tumor necrosis factor ; UC, ulcerative colitis.
Therapeutic strategies in inflammatory bowel disease (IBD) have evolved considerably in the past few decades. The acknowledgment that subclinical and undertreated inflammation can lead to poor outcomes has underpinned a shift in treatment goals from symptomatic control to sustained clinical and endoscopic remission.1 Treatment strategies have changed accordingly, including the early introduction of immunomodulators and/or anti-tumor necrosis factor α therapy (anti-TNF), frequent assessment of disease activity, and rapid therapeutic escalation to achieve tight control of inflammation.2 Combination therapy is the most effective strategy in achieving remission in patients with moderate to severe Crohn's disease (CD)3 and corticosteroid-refractory ulcerative colitis (UC).4 Therefore, the current trend is to intervene early with an immunomodulator and/or anti-TNF, targeting a window of opportunity before the development of potentially irreversible intestinal damage.5 However, in this era of early and aggressive treatment, clinicians are increasingly being presented with questions about the feasibility and timing of stopping or reducing the dose of therapy once remission is achieved. Although it may seem
counterintuitive to stop or reduce the dose of therapy once the therapeutic goals have been achieved, this is often considered in clinical practice because of safety concerns, adverse effects, cost, national regulations, and special situations such as pregnancy. Herein, our goal was to review the literature about drug withdrawal and dose reduction strategies in a comprehensive, systematic, and meaningful way that would provide information on different clinical scenarios.
Materials and Methods
Eligibility Criteria and Literature Search
A broad systematic literature search was performed using a predetermined protocol (Supplementary Material) and in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist. In consultation with a qualified medical librarian, the comprehensive search query we composed included both subject headings and an exhaustive list of keywords related to IBD and stopping or dose reducing anti-TNF and/or an immunomodulator. We searched for relevant studies in the PubMed/MEDLINE, EMBASE (Excerpta Medica database), and Cochrane CENTRAL databases from their inception to March 16, 2015. The search combined disease- and medication-relevant terms as well as terms on drug cessation such as stopping or withdrawal. No restrictions were placed on language. Additionally, bibliographies of included articles were searched and experts in IBD were consulted to identify additional studies. An outline of the detailed search strategy performed in each database is available in the Supplementary Material.
Selection Criteria
Therapy de-escalation was defined as stopping the drug (anti-TNF or immunomodulator) or reducing the dose (reducing the dose of an immunomodulator or decreasing the dose and/or increasing the interval for anti-TNF). Immunomodulators included thiopurines (azathioprine or 6-mercaptopurine) and/ or methotrexate. Studies describing the outcomes of patients with CD or UC in whom therapy had been de-escalated during remission were eligible for inclusion. Studies reporting on long-term outcomes using anti-TNF and/or an
immunomodulator were included as long as information on outcomes after drug withdrawal was available. The outcomes of interest were rate of relapse, time to relapse, factors predictive of relapse, and response to re-treatment. Studies describing de-escalation strategies for other therapeutic classes other than an
immunomodulator and/or anti-TNF were not considered. Studies were excluded if the status of remission before discontinuation was unclear and/or not clearly defined or if outcomes after drug discontinuation were
unavailable. Studies reporting on dose reduction to the standard dose after a period of drug optimization with or without the use of therapeutic drug monitoring were believed to be outside the scope of our review and therefore excluded. Non-peer-reviewed articles, case reports, review papers, and expert opinion papers were also excluded, but review papers were further searched for relevant bibliography. Two reviewers (J.T. and R.K.B.)
independently conducted an initial screen of abstracts for eligibility and evaluated the full-text articles of identified abstracts for final eligibility. Retained articles were screened in DistillerSR (https://distillercer.com) using predesigned screening forms. Disagreements at any stage were resolved by consensus and, if necessary, involvement of a third party (N.K., J.-F.C, or J.S.).
Data Extraction and Quality Assessment
Both reviewers (J.T. and R.K.B.) extracted data from all studies that fulfilled the inclusion criteria using custom data extraction forms created in DistillerSR. Extracted data included study characteristics, participant
characteristics, and outcomes.
Of note, data on patient characteristics were retrieved only for the population of interest (patients de-escalating therapy) when and if available. Authors were contacted to supplement missing time point data whenever it was believed to be relevant. Randomized controlled trials (RCTs) were assessed for quality using the Cochrane method.6 All nonrandomized studies were evaluated for quality using the Newcastle Ottawa Scale.7 Study risk of bias and quality assessment was made independently by 2 of the investigators (J.T. and R.K.B.), and any discrepancies were resolved by joint reassessment of the original article. In evaluating RCTs, any study with one or more components perceived to be high or unclear for bias, was considered overall as presenting a high or unclear risk of bias. If all components were perceived as having a low risk of bias, the study was considered overall as having a low risk of bias.
Categorization of Studies
Studies were grouped into 3 different clinical scenarios: (1) patients de-escalating an immunomodulator while on immunomodulator monotherapy, (2) patients de-escalating an immunomodulator from combination therapy, and (3) patients de-escalating anti-TNF irrespective of concomitant immunomodulator use. Categorization of number 3 was not further subdivided into combination therapy and anti-TNF monotherapy because concomitant
immunomodulator use was variable or often not reported in these studies.
Results
Summary of Literature Search
Details of the literature search, article screening, article evaluation, data extraction, and data analyses described in Materials and Methods are summarized in the PRISMA diagram (Figure 1). The search strategy yielded 6315 unique citations. After exclusion of duplicates and screening of abstracts, 125 full-text articles were assessed for eligibility. Agreement between reviewers for title and abstract screening and for full-text review was 100%. Sixty-nine studies were included in the final qualitative systematic review, 3 of which were substudies of original trials reporting on predictive factors for relapse. Eighteen studies reported on de-escalating
immunomodulator monotherapy, 8 on withdrawing an immunomodulator from combination therapy, and 40 on withdrawing anti-TNF (including 3 in pregnancy). A further 3 studies were substudies of their original
counterparts, reporting on predictive factors for relapse. Overall, there were 7 RCTs, 16 prospective cohort studies, and 43 retrospective cohort studies. Supplementary Tables 1, 4, 7, and 10 summarize the study and population characteristics as well as definitions of remission and relapse in every study. Supplementary Tables 2, 5, 8, and 11 summarize the outcomes after de-escalation in these studies, and Supplementary Tables 3, 6, and 9 include detailed information about the specific inclusion/exclusion criteria for each study.
De-escalation of Immunomodulator Monotherapy
Overall, we identified 18 studies that reported on immunomodulator withdrawal, 15 of which were published as full articles. These comprised 5 RCTs8-12 and 13 retrospective cohort studies13-25 (one of which20 was an extension study from a previous RCT8). Eleven studies reported on immunomodulator withdrawal in CD 8-11,14,15,17,20,23-25 and 8 in UC.12,13,17-19,21,22,24 One study by Fraser et al reported on long-term outcomes of patients treated with methotrexate and presented relapse rates for IBD with no distinction between CD and UC.17
De-escalation of an immunomodulator in CD. All 4
RCTs in CD reported higher relapse rates after stopping the immunomodulator compared with continuing the drug.8-11 All RCTs focused on azathioprine exclusively and the longest follow-up was for 2 years after
withdrawal.10 The study by Lemann et al was designed as a noninferiority study, and the investigators concluded that they could not reject the hypothesis that placebo was inferior to continuing azathioprine.8 The other 3 RCTs showed higher relapse rates in the drug withdrawal arm compared with the drug continuation arm (P < .05), but statistical significance was lost in one study at longer follow-up.10 Of the RCTs, Treton et al20 reported the highest relapse rates at 12 months but was the only one that was not placebo controlled. Relapse rates after drug withdrawal in RCTs ranged from 8% to 25% at 6 months, 16.5% to 53% at 12 months, 21.3% to 31% at 18 months, and 31% at 24 months. Relapse rates of patients continued on an immunomodulator were 0% at 6 months, 4% to 15% at 12 months, 7.9% to 12% at 18 months, and 15% at 24 months. Lemann et al8 reported the lowest relapse rates after drug de-escalation, but this was still significantly higher compared with drug
continuation. This was also the largest RCT, was adequately powered, and had the longest period of remission before drug withdrawal.8
Despite the significant heterogeneity in study design and patient population in the retrospective cohort studies in CD, the relapse rates at 12 months for patients who stopped thiopurines were fairly similar, ranging from 14% to 38% at 12 months and 39% to 71% at 24 months. Five studies14,15,17,20,25 reported longer follow-up periods. The relapse rates at 3 years ranged from 53% to 85% for those stopping the drug, as compared with 22% to 55% for those kept on the drug. At 5 years, relapse rates for those stopping the drug ranged from 63% to 85%, as compared with 32% to 61% for those continuing the drug. The highest relapse rates at 5 years (both for those stopping and continuing the drug) were reported by Kim et al (85% and 61%, respectively).15 Bouhnik et al14 reported a cumulative probability of relapse of 61% (95% confidence interval, 53%-70%) and 75% (95% confidence interval, 65%-85%) at 3 years and 5 years, respectively, very similar to the results from the study by Fraser et al of 66% and 75%.17 Treton et al,20 in an extension study of the RCT by Lemann et al,8 studied the extended relapse rates of patients who were in the placebo arm of the original trial, as well as those in the active drug arm in the trial but thereafter stopped the drug. The cumulative probability of relapse was 52.8% (±7.1%) and 62.7% at 3 and 5 years, respectively. The study by Sokol et al25 described similarly high relapse rates of 73.3% at 5 years in those stopping the immunomodulator (compared with 44.4% in those continuing the drug). In all the previously mentioned studies, patients stopped the immunomodulator while in clinical remission (with slightly different definitions). One study,23 published as an abstract, studied withdrawal of azathioprine after clinical and endoscopic remission was achieved. After a median follow-up period of 46 months (interquartile range, 27.5-67.6 months), 46% presented with endoscopic relapse, 38% had a clinical flare, 17% underwent surgery, and 23% were hospitalized.
Most predictive factors for relapse reported for immunomodulator withdrawal in CD were reflective of non-symptomatic disease activity (elevated C-reactive protein [CRP] level, increased leukocyte or neutrophil count, decreased hemoglobin level), presence of poor prognostic factors (ileal disease location, perianal disease, younger age), or a worse disease course or disease more difficult to control previous to de-escalation (shorter duration of remission, prior complications, and need for corticosteroids in the previous 50 months) (Table 1). Male sex14,25 was associated with relapse in some trials. Higher doses of azathioprine,10 thiopurine taper before de-escalation,24 and, unexpectedly, absence of smoking20,25 were found to be associated with relapse.
Two studies reported on response to re-treatment with an immunomodulator after drug withdrawal.20,24
Reintroduction of thiopurine was successful in 74%24 and 96%20 of cases. The study by Treton et al20 reported a median duration of remission of 28 months (interquartile range, 17-45 months) with the second course of an immunomodulator.
De-escalation of an immunomodulator in UC. One RCT12 and 7 cohort studies13,17-19,21,22,24 reported on the rates of relapse in UC after immunomodulator withdrawal. In the only RCT, Hawthorne et al reported a relapse rate of almost 60% by the end of the first year in those who discontinued the drug while in corticosteroid-free clinical remission compared with 36% on continued thiopurine therapy.12 Cohort studies, with very
heterogeneous designs and follow-up times, reported discrepant relapse rates ranging from 11% to 77% at 12 months to 21% to 100% at 24 months. Lobel et al18 reported the highest rates of relapse at 12 months (77% in the immunomodulator cessation group vs 43% in the immunomodulator continuation group) and 24 months (100% in the immunomodulator cessation group vs 69% in the immunomodulator continuation group). The relapse rates at longer follow-up periods were higher, as expected, ranging from 43% to 65% at 5 years and up to 75% to 87% with longer follow-up periods.13,17 In one prospective study, published as an abstract, patients with UC stopping azathioprine after a minimum of 3 years of therapy in clinical, biological, and endoscopic remission (Mayo score
of 0) had a relapse rate of 37% after a mean follow-up of 55 months.21 The mean time to relapse was 26.4 months (12-72 months).
Among the identified predictive factors for relapse, most are reflective of disease activity (absence of biological remission, increased white cell count22,24), higher disease burden19,22 (pancolitis or extensive colitis compared with left-sided colitis or proctosigmoiditis), and worse disease control before withdrawal (need for therapy while on thiopurines,19 number of clinical relapses during treatment with thiopurines22). Factors associated with reduced rates of relapse were increasing age,12 biological remission, maintaining the drug,12,18 and longer duration with thiopurine treatment.
One study reported on drug reintroduction, with a response rate of 92%.24
De-escalation of an Immunomodulator From Combination Therapy
We identified 8 studies reporting on immunomodulator withdrawal from combination therapy, 5 of which were published as a full paper (Supplementary Tables 4-6). These comprised 2 RCTs26,27 and 6 retrospective cohort studies.28-33 Six studies reported on immunomodulator withdrawal from combination therapy in CD26,27,29-32and one study in UC33; one study did not differentiate between CD and UC when reporting outcomes.
De-escalation of an immunomodulator from combination therapy in CD. The 2 RCTs were open label and
not blinded.26,27 In the IMID trial, Van Assche et al randomized 80 patients on combination therapy for more than 6 months and in clinical remission to either stop or continue the immunomodulator.26 No difference was found between the 2 groups at 24 months for the primary end point, which was a combination of clinical relapse requiring dose escalation and stopping infliximab for adverse effects or loss of response (55% vs 60%). Notably, 6 months was the minimum combination therapy in this trial before discontinuation; however, the median duration of therapy was 25 months for the immunomodulator (range, 6-98 months) and 24.5 months for infliximab (range, 6-90 months). The group of patients that discontinued the immunomodulator had higher CRP and lower infliximab trough levels by the end of the trial. Kierkus et al27 randomized 84 pediatric patients to continue or withdraw the immunomodulator after 26 weeks of combination therapy. At 7 months after withdrawal, the proportion of patients who experienced a relapse or had loss of response to infliximab was 35.9% in those stopping the immunomodulator compared with 33.3% in those continuing combination therapy (P > .05). Despite the significant heterogeneity in the retrospective cohort studies reporting on outcomes in CD, the 2 studies comparing relapse rates in patients who continued combination therapy with those stopping the immunomodulator28,30 found no difference between the groups (similar to the findings of the RCTs). There were similar relapse rates at 12 months in the withdrawal group (38.8%-42.8%) and continuing drug group (40%-40.6%), although the abstract by Sokol et al included both CD and UC. Oussalah et al and Drobne et al reported cumulative relapse rates of 27% and 38% after a median follow-up period of 14 and 29 months, respectively. In the latter study,29 infliximab levels remained stable after withdrawal of the immunomodulator, although there was an increase in the proportion of those with very low or undetectable infliximab trough levels. An abstract by Fischer et al31 reported a higher relapse rate (cumulative 72.1% rate of relapse; median time to relapse, 28.1 months) in an observational study.
Predictive factors for relapse identified included those reflective of disease activity and younger age at diagnosis (Table 1). In addition, a shorter duration of combination therapy before withdrawal,31,32 combination therapy with methotrexate compared with azathioprine,31 and discontinuation of infliximab due to loss of response were associated with disease relapse. Higher infliximab levels at withdrawal were associated with lower rates of relapse.29
Table 1. Factors Predictive of Relapse in CD and UC After Drug De-escalation
Univariable analysis Multivariable analysis
Reflective of disease activity at de-escalation or during follow-up: CD
Stopping the IM Neutrophil count ≥4.0 x 109/L20 Elevated CRP level (<20 mg/L vs ≥20 mg/L: RR,
16.9 [2.7-104.3]8; ≥20 mg/L: HR, 58.6 [7.5-45a7]20; >3 mg/L: HR, 4.05 [1.98-8.27]23; HR, 1.04 [1.00-1.07]24; ≥14 mg/L: HR, 3.2 [1.48-7.05]24) Neutrophil count <4000/mL vs ≥4000/mL: RR, 7.9 (1.0-60.8)8 Platelet count ≥235 x 109/L20 CRP level ≥20 mg/L20
Hemoglobin level ≤12 g/dL20 Low hemoglobin level (<12 g/dL vs ≥12 g/dL: RR, 8.7
[1.6-48.8]8;≤12 g/dL: HR, 4.8 [1.7-13.7]20) Hemoglobin level <12 g/dL vs ≥12 g/dL: RR, 4.6 (0.9-22.7)8
CRP level at time of drug withdrawal (P = .005)24 Elevated neutrophil or WBC count (neutrophil count ≥4.0 x 109/L [HR, 3.2 [1.6-6.3])20; WBC count: HR, 1.18 [1.04-1.33]24; WBC count ≥6.6 x 109/L: HR, 3.75 [1.87-7.54]24)
CRP level <20 mg/L vs ≥20 mg/L: RR, 12.3 (2.3-64.3)8 Combination Stopping
anti-TNF
Elevated CRP level at start of anti-TNF: OR, 2.44 (1.11-5.36)43 Hemoglobin level <14.5 g/dL: HR, 6.0 (2.2-16.5)47
FC level >250 µg/g during follow-up62,a Increased leukocyte count (>6 x 109/L: HR, 2.4 [1.2-4.7]47; >5.25 x 109: HR, 2.01 [1.10-3.66]54)
Elevated FC level (PPV of 66.7% with FC level >50 µg/g)49
Complete mucosal healing48,13 CD Endoscopic Index of Severity >0: HR, 2.3 (1.1-4.9)47
Elevated WBC count >5.25x 109/L: 2.55 (1.42-4.59)54 Elevated CRP level37 (high-sensitivity CRP level
≥5 mg/L: HR, 3.2 [1.6-6.4])47; CRP level >10 mg/L: OR, 2.44 [1.31-4.54])43
Elevated FC level: HR, 1.82 (1.03-2.82)54
Wall thickening at magnetic resonance enterography: 9 vs 1 mm (P = .015)82
Elevated FC level (>50 µg/g: HR, 3.04 [1.26-7.37])54; ≥300 µg/g: HR, 2.5 [1.1-5.8])47
Rising FC34 level and rising CRP level after de-escalation65,66 (see text); Absence of mucosal healing37
Stopping the IM WBC count >4.88 x 109/L: HR, 4.2232 CRP level >5 mg/L at the time of withdrawal (increased risk for
[0.38-94.67); discontinuation of IFX because of loss of response: HR, 7.99 (2.19-29.25)29 Neutrophil count >3.167 x 109/L: HR, 4.6832 Platelet count >298 x 109/L: HR, 5.1432 CRP level >5 mg/L: HR: 9.2932 Factors reflective of disease/poor prognostic features: CD
Stopping the IM Ileal and colonic disease: P = .06210 Age ≤31 y: RR, 2.7 (1.1-6.7)14
Younger age: P < 0.02215; >40 y vs 3-40 y vs ≤30 y: RR, 2.5 (1.1-5.7)8
Perianal disease at diagnosis of CD: HR, 2.24 (1.06-4.72)23 Combination Stopping
anti-TNF
Perianal disease40,44 Smoking (HR, 1.91 [1.11-3.27]41; OR, 2.74 [0.99-7.59]43'0)
Smoking41: OR, 2.34 (1.09-5.01)43 Perianal disease: HR, 1.72 (1.02-2.89)41
Ileal disease62, a Disease location (ileocolonic disease: OR,
3.18 [1.15-11.14])49; colonic vs ileal or ileocolonic disease: OR, 0.16 [0.03-072]57)
High anti-Saccharomyces cerevisiae antibody levels62, a Age at diagnosis ≥25 y48, b
Disease duration >5 y: OR, 0.12758 Age at diagnosis ≥25 y: HR, 1.83 (1.03-3.25)48,b
Younger age at diagnosis39 Age at diagnosis <22 y: HR, 2.24 (1.33-3.78)54
Age at diagnosis <22 y: HR, 2.71 (1.66-4.43)54 Isolated L4 disease: HR, 5.43 (1.65-17.93)54 Perianal disease: HR, 0.54 (0.3-0.99)54 Stricturing disease: HR, 1.93 (1.09-3.40)54
Stopping the IM - Age at diagnosis (<16 y vs >40 y): HR, 4.55 (1.18-17.62)31
Previous disease course: CD
Stopping the IM Higher daily dose of 6-MP during remission: P < .00615 Time without corticosteroids ≥50 mo vs <50 mo: RR, 5.2 (1.5-18.1)8
Time without corticosteroids ≥50 mo vs <50 mo: RR, 4.0 (1.2-13.3)8
Prior bowel complication: HR, 1.74 (1.02-2.96)23 Duration of remission on AZA/6-MP <4 y vs >4 y: P = .02)14,20 Higher AZA dose: HR, 2.2 (1.06-4.42)10
Duration of remission <4 y: RR, 4 (0.9-17.4)14 Combination
stopping the anti-TNF
History of antimetabolite failure41 Previous antimetabolite failure: HR, 1.78 (1.07-2.97)41
Dose intensification49 previous to de-escalation: OR, 15.4 (1.83-129.9)43
Corticosteroid use 12 and 6 mo before baseline: HR, 3.5 (1.1-10.7)47
Corticosteroids at the start of anti-TNF: OR, 3.43 (1.4-8.4)43 Time to relapse with previous anti-TNF course: OR, 2.77 (1.53-5.0)43
Previous anti-TNF: OR, 3.05 (1.23-7.52)43
History of prior surgery62,a Previous anti-TNF (OR, 8.96 [1.48-72.47]49; OR, 4.23
[1.39-12.84]43) Longer disease duration at first IFX infusion:
HR, 1.1 (1.0-1.1)52 <1 y between diagnosis and start of anti-TNF48,b
Dose intensification previous to de-escalation: 12.96 (1.39-120.5)43 Surgery for inflammatory bowel disease: HR, 11.43 (1.38-94.67)29 No previous surgical resection: HR, 4.0 (1.4-11.4)47
Other: CD Stopping the IM Male sex: P= .00114 Male sex (RR, 4.9 [1.9-12.6]14; OR, 2.4225)
Nonsmoking status20 Absence of smoking: OR, 2.78; P = .00625
Thiopurine taper before de-escalation: P = .00424 Combination Stopping
anti-TNF
Female sex: HR, 2.84 [1.28-6.28)41 Male sex: HR, 3.7 (1.9-7.4)47
Lack of normalization of mucosal cytokines
(TNF-a: HR, 4.3: lack of normalization of IL-17A: HR, 3.3)63
IFX trough level >2 mg/L: HR, 2.5 (1.1-5.4)47
Lack of normalization of mucosal cytokines (TNF-a: HR, 4.1; IL-17A HR, 3.4)63
Female sex (P < .02)55
Lack of maintenance of an IM after discontinuation of anti-TNF (P < .01 )55
Epidermal growth factor level ≤39.5 U/mL
(HR, 2.7 [1.2-6.6]; sensitivity, 39%; specificity, 86%; PPV, 75%; NPV, 58%)83
Shorter duration of anti-TNF before cessation39
IFX trough concentrations <6 µg/mL48,b Treatment with an IM after discontinuation of anti-TNF: HR, 0.5 (0.3-0.7)55
Positive serum vascular cell adhesion protein 1 (>0.67 µg/mL) at time of IFX discontinuation48,b
Stopping the IM Step-up strategy: HR, 3.9632 Duration of IFX/AZA therapy before withdrawal <27 mo: HR,
6.77-7.4632 Duration of IFX/AZA therapy before withdrawal <27 mo: HR,
5.7432
Combination therapy <6 mo: HR, 5.68 (1.58-20.36)31
Combination therapy with methotrexate as opposed to AZA: HR, 3.37 (1.14-9.96)31
Elevated (>5 µg/mL) IFX trough levels at withdrawal of IM: HR, 0.36 (0.14-0.91)29
Discontinuation of IFX because of loss of response: HR, 7.99(2.19-29.25)29
Factors reflective of disease activity at de-escalation: UC
Stopping the IM WBC count at withdrawal (P = .007)24 Biological remission at withdrawal: HR, 0.004 (0.0001-0.14)22
WBC count (HR, 1.44 [1.11-1.87]24; ≥9.1 x 109/L: HR, 6.7 [1.86-24.2])24
Factors reflective of poor disease/prognostic features: UC
Stopping the IM Increasing age (HR for every year older: 0.95 [0.93-0.98])12
Extensive colitis vs left-sided colitis and distal colitis: HR, 1.79 (1.06-3.02) and 2.02 (1.10-3.72)19
Pancolitis: HR, 5.01 (1.95-26.43)22 Previous disease
course: UC
Stopping the IM Total duration of treatment with thiopurines22 Lack of sustained remission while on AZA (no need for acute therapy during AZA therapy): HR, 2.35 (1.43-3.85)19
Time of corticosteroid-free clinical remission before withdrawal22
No. of clinical relapses during treatment with thiopurines: HR, 1.3 (1.01-1.66)22
Absence of sustained remission19
Time from diagnosis of UC to starting therapy with thiopurines: HR, 1.01 (1.01-1.02)22
Combination stopping anti-TNF
Previous IFX therapy: OR, 6.81 (1.15-40.4)70
Other: UC Stopping the IM Male sex19 Continued treatment with 6-MP: OR, 0.36 (0.15-0.87)18
AZA withdrawal due to drug-related toxicity Continued treatment with AZA: HR, 0.43 (0.20-0.93)12 Duration of AZA treatment 3-6 mo vs 48 mo: HR, 2.78 (1.27-6.11)19
Longer treatment with thiopurines: HR, 0.15 (0.03-0.66)22 IM, immunomodulator; RR, relative risk (95% confidence interval); CRP, C-reactive protein; HR, hazard ratio (95% confidence interval); WBC, white blood cell; OR, odds ratio (95% confidence interval); FC, fecal calprotectin; PPV, positive predictive value; IFX, infliximab; L4, jejunal disease as per Montreal classification; 6-MP, 6-mercaptopurine; AZA, azathioprine; NPV, negative predictive value.
aType of analysis not specified.
bPredictive factors of sustained clinical remission and not relapse after withdrawal. cOnly significant after exclusion of corticosteroid-dependent patients (n = 13).
De-escalation of an immunomodulator from combination therapy in UC. One study reported on
immunomodulator withdrawal from combination therapy in UC.33 In this retrospective analysis, patients in remission after primary response to infliximab induction and at least 6 months of remission with scheduled infliximab therapy in association with azathioprine and who subsequently discontinued the immunomodulator were included. There was a significantly higher rate of relapse, reported as trimesters with clinical flare, in the discontinuation cohort (12% vs 3%; P = .049). The mean time to relapse was also longer with combination therapy compared with infliximab alone (16.6 vs 7 months; P < .05). This study did not report on factors associated with relapse.
De-escalation of Anti-TNF
Thirty-seven studies that reported outcomes after anti-TNF withdrawal were identified, 21 of which were published as a full article (Supplementary Tables 7-9). Twenty-two were retrospective cohort studies, and 15 were prospective cohort studies. One study34 comparing two different maintenance anti-TNF regimens (8 weekly vs 10 weekly dosing) had a control arm, but all others did not. No RCTs were found. Three additional studies specifically included pregnant patients (Supplementary Tables 10 and 11).
De-escalation of anti-TNF in CD. Thirty-two studies presented data on outcomes after withdrawing anti-TNF
for CD. In only one study, in a group of patients with surgically induced remission, was anti-TNF de-escalated after use as monotherapy.35 In 6 studies, the proportion of patients on a concomitant immunomodulator was not specified34,36-39; in the remaining studies, it ranged from 20 to 100%. Three studies40-42 compared relapse rates after infliximab withdrawal after induction alone ("induction group") with relapse rates after withdrawal after at least 1 year of scheduled maintenance therapy ("maintenance group"). There were no significant differences in the rates of relapse between the induction and maintenance groups in each study, although the actual rates of relapse varied between studies. A study in a pediatric population found high relapse rates of 75% and 73% in the induction and maintenance groups, respectively, after 1 year.42 The other 2 studies40,41 reported relapse rates of 22% to 29% (induction group) and 31% to 44% (maintenance group) after 1 year; the difference between the groups was not statistically significant. Molnar et al found a similar relapse rate of 45% in a study of withdrawal after 3 induction doses of infliximab.43
Domenech et al found significantly increased relapse rates after stopping scheduled biologic therapy in patients with perianal disease compared with those with luminal disease only (82% vs 17%) after a mean follow-up period of 8.8 months.40 This finding is also supported by a study by Molnar et al.44
Of the remaining studies, 2 were in a pediatric population.45,46 Both examined the long-term effectiveness of anti-TNF and reported on outcomes after cessation in a small subset of patients. Crombe et al45 reported a cumulative probability of relapse of 14.8% in 27 patients after a median of 125 months of follow-up, whereas Nuti et al46 reported a relapse rate of 61.5% at 12 months in 13 patients.
In most studies in the adult population, anti-TNF was discontinued while patients were in clinical remission. In the STORI trial,47 Louis et al prospectively followed 115 patients with CD on combination therapy for at least 1 year who discontinued anti-TNF after being in corticosteroid-free clinical remission for at least 6 months. The relapse rates at 12 and 24 months were 43.9% ± 5.0% and 52.2% ± 5.2%, respectively, with a median time to relapse of 16.4 months. It is interesting to note that across all adult studies reporting on anti-TNF withdrawal from clinical remission, despite heterogeneous study designs, patient populations, and variable use of immunomodulators, the 1- and 2-year relapse rates were reasonably consistent. One exception is the study by Papamichael et al,48 which reported the lowest relapse rates after drug discontinuation; the cumulative probability of maintaining sustained clinical remission after the first, second, third, fourth, and fifth year was 96%, 93%, 88%, 79.9%, and 72.8%, respectively. In other studies in which relapse rates were available at 12 and 24 months,47,49-55 the rates ranged from 21.1% to 39% and from 37%% to 55.7%, respectively. Interestingly, except for one study published in abstract form that reported a lower relapse rate of 16% after a mean follow-up of 19 ± 13 months,56 most studies, including the one by Papamichael et al, reported high cumulative relapse rates of between 49% and 88% by the end of follow-up,36,37,47,48,52,54,57-59 with higher rates with longer follow-up periods. The median time to relapse was between 4.8 and 16.4 months across the studies reporting this
outcome.38,47,49,53,55,57-61 Five studies39,57,62-64 included patients withdrawn from therapy while in both clinical and endoscopic remission. Radiological healing as assessed by magnetic resonance imaging was also shown in one study.62 Rismo et al63 reported higher rates of relapse (52% at 6 months and 74% at 12 months), potentially relating to short treatment duration before withdrawal. The other studies reported relapse rates of 18% at 6 months,57 22% to 41% at 12 months,57,62 47% to 49% at 24 months,57,62 and 50% at 36 months,39 very similar to that of those withdrawn while in clinical remission. The median time to relapse was also similar to that of those
in who only clinical remission was documented. Two studies explored the possibility of reducing the dose of therapy. Sorrentino et al35 examined anti-TNF withdrawal monotherapy in the postoperative setting. Twelve patients commencing anti-TNF therapy 2 weeks postoperatively continued anti-TNF for 3 years, at which point it was withdrawn. The relapse rate at 6 months after withdrawal was 83.3%; in all cases, patients were re-treated successfully with a lower dose of infliximab (3 mg/kg). Mantzaris et al34 reported relapse rates in patients with CD in deep long-term remission who had their infliximab spaced from 8 to 10 weeks; the relapse rate was 6.25% at 12 months for the drug spacing group and 6.7% for the drug continuation group.
Among predictive factors for relapse, most were reflective of nonsymptomatic disease activity at withdrawal (elevated CRP level, increased leukocyte or neutrophil count, elevated fecal calprotectin [FC] level, decreased hemoglobin level, absence of mucosal healing, wall thickening at magnetic resonance elastography), presence of poor prognostic factors (younger age; ileal, jejunal, or ileocolonic disease location; perianal disease; stricturing phenotype; active smoking; and elevated anti-Saccharomyces cerevisiae antibody levels), or factors reflecting a worse disease course or a more difficult to control disease previous to de-escalation (use of corticosteroids before withdrawal or at the start of anti-TNF, history of antimetabolite failure, need for dose escalation before withdrawal, past use of anti-TNF before the current course, history of surgery) (Table 1). Shorter duration of anti-TNF and longer duration of disease before starting anti-TNF seem to predict relapse, whereas a shorter interval between disease diagnosis and starting anti-TNF and maintaining treatment with an immunomodulator were associated with remission.48,55 Only 3 studies24,47,61 specifically reported the rate of simultaneous
introduction of the immunomodulator with anti-TNF. In the STORI trial, being naive to immunomodulator therapy was not found to be a predictor of relapse on univariate analysis.47 On the other hand, another study found previous antimetabolite failure to be predictive of relapse.41 Higher infliximab trough levels at withdrawal was associated with relapse in 2 studies.47,48 One study looked at molecular healing; elevated expression levels of TNF-α and interleukin-17A in endoscopic healed mucosa were associated with a 3- and 4-fold increased risk of relapse, respectively.63 Two studies reported on factors predictive of relapse during follow-up rather than at drug discontinuation.65,66 De Suray et al followed 113 patients in the STORI trial with bimonthly measurements of CRP and FC levels until relapse or 18 months; the investigators found that the evolution of CRP and FC levels was significantly different between those who experienced a relapse and those who did not. In those who experienced a relapse, a sudden and pronounced increase in CRP and FC levels was observed during the 4 months that preceded relapse. The median value for FC and CRP before relapse was 534 µg/g and 8 mg/L for those who experienced a relapse and 66.9 µg/g and 3.7 mg/L for those who did not experience a relapse, respectively65 The optimal cutoffs to predict relapse were 6.1 mg/L for CRP (sensitivity of 71% and specificity of 66%) and 305 µg/g for FC (sensitivity of 70% and specificity of 74%). These results were corroborated by Molander et al.66
A significant proportion of the patients who experienced a relapse were re-treated in 15 studies. With the exception of the study by Sorrentino et al,35 in which endoscopic remission was reported (100% remission at 80 weeks), most studies only provided rates of clinical remission/ response after re-treatment; in many cases, the information was restricted to the response rates, with no further details.42,43,50,51,54,58,59,62 The studies by Molnar et al43 and Monterubbianesi et al51 reported the lowest response rates (54.7% and 63.3%, respectively) and the highest infusion reaction rates (6% and 10%, respectively). Excluding these studies, remission was safely achieved in a high proportion of patients in all other studies (ranging from 78.3% to 100%). Studies describing longer follow-up remission rates (at least 1 year) reported clinical remission rates between 80% and 92% at 1 year49,55,64
De-escalation of anti-TNF in UC. Fourteen studies described outcomes after withdrawal of anti-TNF in
UC36,37,52-55,58,59,64,67-71 5 of which were retrospective cohort studies. There was significant heterogeneity in study design and patient population, including the definition of relapse and clinical remission before entry. The duration of clinical remission before entry was only stated in 2 studies (both with a minimum of 6 months).54,64 In 3 studies, the proportion of patients on combination therapy was not specified36,37,68 and in further studies ranged from 20% to 100%. One study reported on a pediatric population.67 The primary goal of this study was to compare the effectiveness of infliximab in avoiding colectomy in acute severe UC and with "chronically stable" corticosteroid-dependent UC. Eighty percent had a favorable response to infliximab and were eventually weaned off anti-TNF after a mean of 9.8 infusions. After a mean 10-month follow-up after the last infusion, no patient required additional treatment with infliximab or corticosteroids, and all were maintained with immunomodulator monotherapy.67 Studies in adult populations reported relapse rates between 14% and 41.8%52-54,68 at 12 months and between 25% and 47.1%54,68 at 24 months.54,68 In studies in which mucosal healing was used as part of the definition of remission before drug de-escalation, the relapse rates at 12 and 24 months were between 17% and 25% and between 25% and 35%, respectively.64,68 Overall, the cumulative probability of relapse varied from 35% to 45%36,37,52,54,70 with median times of follow-up between 16 and 29 months. Steenholdt et al52 reported
relapse rates at longer follow-up of 4.5 years, with 60% of patients experiencing a relapse at this time point.52 Studies with a lower proportion of patients on combination therapy or in which this information was not specified reported similar relapse rates to those studies with a higher proportion on combination therapy. Exposure to anti-TNF before the existing course of therapy was the only factor found to be significantly associated with time to relapse.70 Molander et al,66 in a follow-up of a previous study,64 showed that persistently normal FC concentrations during follow-up were highly predictive of clinical and endoscopic remission. An FC level greater than 200 µg/g measured 2 to 4 months before relapse was predictive of relapse (sensitivity of 83% and specificity of 50%); no differentiation was made between CD and UC.66
Re-treatment rates were reported in 4 trials,53,54,68,70 with response rates ranging from 67% to 100%. Detailed information was available in only 2 studies. In one study,68 clinical remission was achieved by 100% of patients by week 12; Dai et al53 reported a mean time of 3 months to clinical remission after drug re-introduction.
Special situations: pregnancy. The 3 studies specifically investigating de-escalation in pregnancy reported
outcomes after anti-TNF withdrawal.72-74 All studies had low rates of concomitant immunomodulator use (17.5%-36%). Seirafi et al retrospectively analyzed 85 patients with IBD who had anti-TNF withdrawn and primarily reported on pregnancy outcomes rather than relapse; 14% experienced a relapse during the last trimester and 32% within 3 weeks postpartum.74 Zelinkova et al retrospectively examined 23 patients who discontinued anti-TNF during the second trimester and found that 8.7% experienced a relapse by gestational week 36.72 In a recently published prospective study of predominantly patients with CD, in part consisting of patients in the study by Zelinkova et al, 9.8% of patients who had been in remission for more than 8 months before discontinuation had a relapse by the end of pregnancy and 15.7% had a relapse by 3 months postpartum. This was compared with a cohort of patients who were not in clinical remission and had anti-TNF continued throughout pregnancy; the relapse rates were not different between the groups.73
In the one study reporting response to postpartum retreatment, 100% remission rates were achieved72; however, one patient had an allergic reaction and 2 patients required a switch to adalimumab due to adverse events.72 Although de Lima et al did not specifically describe remission rates after re-treatment, 1.9% had an allergic reaction and 1.9% had loss of response.73
Discussion
This systematic review comprehensively summarizes the available literature on de-escalation of an immunomodulator or anti-TNF during remission in patients with IBD. Based on the studies with extended periods of follow-up, relapse rates after cessation appear high across all therapeutic classes, suggesting that complete discontinuation in the long-term may only be feasible in select patients. Withdrawing
immunomodulator monotherapy in CD and UC results in higher short-term relapse rates compared with continuing, and relapse rates increase steadily over longer periods of follow-up, with only 15% to 37% of patients maintaining clinical remission after 5 years. In patients receiving combination therapy in CD, there seems to be no difference between stopping or continuing the immunomodulator, with relapse rates of
approximately 40% by the end of the first year. Less evidence is available for UC. Finally, approximately 40% to 50% of patients who discontinue anti-TNF will experience a relapse within 2 years; studies with longer follow-up of 7 and 10 years after withdrawal show remission rates of 35% and 12%, respectively52,60 Studies in pregnancy show that a short drug holiday is not associated with high rates of relapse or loss of response upon restarting.
Even though the overall long-term results of discontinuing therapy may seem disappointing, there may be subgroups of patients who are better candidates for drug discontinuation. This review has found that most predictors of relapse reflect poor prognostic features, previous disease course, or markers of subclinical disease activity. Patients with poor prognostic features and those with a difficult-to-treat, relapsing course before de-escalation generally have a higher chance of relapse after drug withdrawal. However, it must be recognized that there is a notable lack of consistency across the studies in terms of individual prognostic markers, and caution is advised in the absence of a well-powered study to specifically examine this question. It has been argued that stopping therapy may be an option in patients with deep remission, as defined by clinical, biochemical, and endoscopic remission.47 Although it requires further investigation, it is important to acknowledge that recent studies of anti-TNF cessation in patients with deep remission (including some in radiological and even histological remission) have roughly similar relapse rates, with approximately 50% of patients experiencing a relapse after variable periods of follow-up.39,57,62-64After stopping therapy, it is possible that an individual's particular disease course will eventually manifest and follow its natural trajectory.75 Therapeutic drug monitoring
offers a promising avenue to identify candidates for de-escalation. Notably, while higher anti-TNF trough levels at the time of immunomodulator withdrawal were associated with lower rates of relapse,29 the opposite was true for anti-TNF withdrawal47,48 In this way, lower trough levels may identify patients in who remission has been achieved irrespective of drug therapy and who would therefore be promising candidates for withdrawal.76 Studies on re-treatment generally reported good response rates, mostly of clinical remission. However, it must be acknowledged that in most cases there is only short-term follow-up, and assessment of the rate of success based on objective data such as biomarkers, imaging, or endoscopy is lacking. Therefore, the true efficacy and impact of this strategy over the medium-term to long-term merits further investigation. More evidence is thus needed to show the safety and efficacy of re-treatment. These further data could lead to exploration of the concept of treatment cycles in patients with IBD. Over a lifetime of chronic IBD, these cycles could represent important sparing periods with regard to safety and costs. For example, a drug holiday from anti-TNF therapy, the main driver for direct costs in IBD,77 could be a way to optimize the cost-effectiveness of management of IBD with these agents. Likewise, an immunomodulator holiday could be an effective method of returning the risk of lymphoproliferative disorders back to baseline.78,79
This is by far the most extensive and comprehensive review of the literature on de-escalation strategies in patients with IBD to date, incorporating short-term and long-term results across different therapeutic strategies. However, this review has some limitations. Because we wanted to capture real-world data and long-term outcomes after drug de-escalation, we incorporated all studies reporting on drug de-escalation as long as it was clearly stated that this occurred during remission, irrespective of treatment duration or study design. The resulting heterogeneity in study methods, interventions, definitions of remission and relapse, study populations, and inclusion/exclusion criteria across studies precluded further meta-analysis and resulted in the inclusion of some studies with very few participants and low quality. For anti-TNF withdrawal in particular, it is difficult to draw firm conclusions about the effect of drug withdrawal in the absence of control data. Furthermore, questions around discontinuing anti-TNF monotherapy or dose-reducing anti-TNF remain unanswered due to the limited number of studies exploring these strategies. Additionally, many studies reporting predictive factors were limited by retrospective design and small sample size, precluding firm recommendations in this regard. Nevertheless, it is notable that for every class of therapy and independent of study design, the relapse rates (with some
exceptions) are fairly consistent and increase with time, with only a small proportion of patients being free of relapse in the long-term, independent of the therapy that is stopped.
Based on our review of the current literature, we believe that discontinuation of therapy in patients with IBD needs to be a personalized decision (Figures 2 and 3). Age, sex, and extent of disease modify the risk of various cancers linked to immunomodulator therapy, as illustrated by a recently developed Markov model.80 These risks must be weighed against an individualized assessment of the likelihood of successful withdrawal based on the presence of predictive factors of relapse and remission. An additional important consideration is patient preference. The willingness of an individual patient to accept risks associated with therapy must be weighed against their concerns about the risk of relapse with associated loss of quality of life and potential surgery. Uninterrupted, lifelong therapy with potent immunosuppressive agents is probably unsustainable in the long-term. Therefore, further studies and alternative strategies are needed for those patients reaching deep and sustained remission. After treatment de-escalation, early diagnosis of relapse before symptom development is supported by preliminary studies and warrants further confirmation, because this could potentially allow for early re-treatment and better outcomes. There is also a need to explore alternative de-escalation strategies such as dose reduction and drug withdrawal in early IBD. The latter strategy offers the enticing possibility of "resetting" the immunologic system to truly change the natural history of disease and has been pursued in other diseases such as rheumatoid arthritis. In the recently published double-blind, placebo-controlled OPTIMA trial of patients with early rheumatoid arthritis (<1 year) treated with combination therapy and achieving low disease activity withdrawal of anti-TNF did not result in a significant increase in disease activity or radiological or functional scores by the end of the study (week 78).81 Finally more prospective controlled studies are needed. The upcoming prospective, multicenter European Union-funded Biocycle project is expected to provide novel findings in this regard. It will incorporate the SPARE study a 3-armed trial in CD of
infliximab-immunomodulator continuation versus infliximab discontinuation versus infliximab-immunomodulator discontinuation during remission. Additionally Biocycle will explore the concept of using treatment cycles in patients with IBD. Considering the cyclic nature of IBD, this is an appealing strategy that may allow for safer and more cost-effective therapy based on a patient's individual disease course.
Figure 2. Factors involved in the decision to de-escalate drug therapy in patients with IBD, includes those
involving patient demographics, disease features, treatment history, current disease status, and patient preference. HSTCL, hepatosplenic T-cell lymphoma.
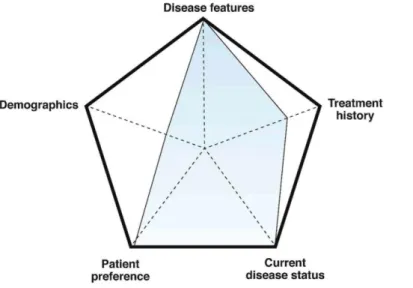
Figure 3. Schema illustrating the individualized decision on de-escalation in 2 patients with IBD based on
multiple areas of consideration. Along each of the 5 axes, the stronger the argument for continuation, the closer the point is to the edge of the pentagon. Overall, larger areas of the overlying shape favor continuation of therapy whereas smaller areas favor discontinuation.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of
Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/ j.gastro.2015.08.055. References
1. Ordas I, Feagan BG, Sandborn WJ. Early use of immunosuppressives or TNF antagonists for the treatment of Crohn's disease: time for a change. Gut 2011; 60:1754-1763.
2. Levesque BG, Sandborn WJ, Ruel J.etal.Converginggoals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015; 148:37-51.e1.
3. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med 2010;362:1383-1395.
agent in ulcerative colitis. Gastroenterology 2014;146:392-400.e3.
5. Pariente B, Cosnes J, Danese S, et al. Development of the Crohn's disease digestive damage score, the Lemann score. Inflamm Bowel Dis 2011;17:1415-1422.
6. Higgins JPT GSeCIHJ, Green S, eds. Chapter 8: assessing risk of bias in included studies. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 (updated March 2011) 2011. The Cochrane Collaboration, 2011. http://www.cochranehandbook.org.
7. Wells GA, Shea B, O'Connell D et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses, http://www.ohri. ca/programs/clinical_epidemiology/oxford.asp. Accessed April 2015.
8. Lemann M, Mary JY, Colombel JF, et al. A randomized, double-blind, controlled withdrawal trial in Crohn's disease patients in long-term remission on azathioprine. Gastroenterology 2005;128:1812-1818.
9. O'Donoghue DP, Dawson AM, Powell-Tuck J. Double-blind withdrawal trial of azathioprine as maintenance treatment for Crohn's disease. Lancet 1978;2:955-957.
10. Wenzl HH, Primas C, Novacek G, et al. Withdrawal of long-term maintenance treatment with azathioprine tends to increase relapse risk in patients with Crohn's disease. Dig Dis Sci 2015;60:1414-1423.
11. Vilien M, Dahlerup JF, Munck LK, et al. Randomized controlled azathioprine withdrawal after more than two years treatment in Crohn's disease: increased relapse rate the following year. Aliment Pharmacol Ther 2004; 19:1147-1152.
12. Hawthorne AB, Logan RFA, Hawkey CJ, et al. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ 1992;305:20-22.
13. George J, Present DH, Pou R, et al. The long-term outcome of ulcerative colitis treated with 6-mercaptopurine. Am J Gastroenterol 1996;91:1711-1714.
14. Bouhnik Y, Lemann M, Mary JY, et al. Long-term follow-up of patients with Crohn's disease treated with azathioprine or 6-mercaptopurine. Lancet 1996; 347:215-219.
15. Kim PS, Zlatanic J, Korelitz Bl, et al. Optimum duration of treatment with 6-mercaptopurine for Crohn's disease. Am J Gastroenterol 1999;94:3254-3257.
16. Fraser AG, Morton D, McGovern D, et al. The efficacy of methotrexate for maintaining remission in inflammatory bowel disease. Aliment Pharmacol Ther 2002;16: 693-697.
17. Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut 2002;50:485-489.
18. Lobel EZ, Korelitz Bl, Xuereb MA, et al. A search for the optimal duration of treatment with 6-mercaptopurine for ulcerative colitis. Am J Gastroenterol 2004;99:462-465.
19. Cassinotti A, Actis GC, Duca P, et al. Maintenance treatment with azathioprine in ulcerative colitis: outcome and predictive factors after drug withdrawal. Am J Gastroenterol 2009;104:2760-2767.
20. Treton X, Bouhnik Y, Mary J, et al. Azathioprine withdrawal in patients with Crohn's disease maintained on prolonged remission: a high risk of relapse. Clin Gastroenterol Hepatol 2009;7:80-85.
21. Pratico C, Capozzi N, Rizzello F, et al. Prospective withdrawal trial of azathioprine (AZA) in ulcerative colitis (UC) (abstr). J Crohns Colitis 2014;8(suppl):S219-S220.
22. Moreno-Rincón E, Serrano-Ruiz FJ, Benitez JM, et al. Prognosis of patients with ulcerative colitis in remission after thiopurines withdrawal (abstr). J Crohns Colitis 2015;9(suppl 1):S355.
23. Qiu Y, Mao R, Chen Bl, et al. Predictors of disease relapse of patients with Crohn's disease in deep remission: who and when can withdraw thiopurine maintenance therapy? (abstr). J Crohns Colitis 2015;9(suppl 1):S61.
24. Kennedy NA, Kalla R, Warner B, et al. Thiopurine withdrawal during sustained clinical remission in inflammatory bowel disease: relapse and recapture rates, with predictive factors in 237 patients. Aliment Pharmacol Ther 2014;40:1313-1323.
25. Sokol H, Seksik P, Nion-Larmurier I, et al. Current smoking, not duration of remission, delays Crohn's disease relapse following azathioprine withdrawal. Inflamm Bowel Dis 2010;16:362-363.
26. Van Assche G, Magdelaine-Beuzelin C, D'Haens G, et al. Withdrawal of immunosuppression in Crohn's disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology 2008;134:1861-1868.
remission in children with moderate to severe Crohn disease. J Pediatr Gastroenterol Nutr 2015; 60:580-585.
28. Sokol H, Seksik P, Nion-Larmurier I, et al. Withdrawal of immunosuppression in inflammatory bowel disease treated with infliximab maintenance therapy (abstr). Gastroenterology 2009;136:A187-A188.
29. Drobne D, Bossuyt P, Breynaert C, et al. Withdrawal of immunomodulators after co-treatment does not reduce trough level of infliximab in patients with Crohn's disease. Clin Gastroenterol Hepatol 2015;13:514-521.e4.
30. Choi GJ, Park Dl, Park JH, et al. Withdrawal of azathioprine in luminal Crohn's disease treated with infliximab maintenance therapy: a retrospective case-control study (abstr). J Gastroenterol Hepatol 2010;25:A86.
31. Fischer M, Campbell SC, Johnson CS, et al. Risk factors for rescue therapy in Crohn's patients on combination therapy after discontinuation of the immunomodulator (abstr). Gastroenterology 2014;146:S-450.
32. Oussalah A, Chevaux JB, Fay R, et al. Predictors of infliximab failure after azathioprine withdrawal in Crohn's disease treated with combination therapy. Am J Gastroenterol 2010;105:1142-1149.
33. Filippi J, Laharie D, Michiels C, et al. Efficacy of sustained combination therapy for at least 6 months with thiopurines and infliximab in patients with ulcerative colitis in clinical remission: a retrospective multicenter French experience. J Crohns Colitis 2015;9:252-258.
34. Mantzaris GJ, Karatzas P, Kyriakos N, et al. Can we increase the dose interval of infliximab to 10 weeks without risking loss of response in patients with Crohn's disease? Prospective, single-center pilot study based on successive measurements of fecal calprotectin (abstr). Gastroenterology 2014;146:S-248.
35. Sorrentino D, Paviotti A, Terrosu G, et al. Low-dose maintenance therapy with infliximab prevents postsurgical recurrence of Crohn's disease. Clin Gastroenterol Hepatol 2010;8:591-599.e1.
36. Ciria V, Silva P, Leo E, et al. Factors influencing recurrence following suspension of biological treatment. Importance of mucosal healing (abstr). J Crohns Colitis 2014;8(suppl):S270.
37. Armuzzi A, Marzo M, Felice C, et al. Long-term scheduled therapy with infliximab in inflammatory bowel disease: a single-centre observational study (abstr). Gastroenterology 2010;138:S-691-S-692.
38. Lane C, Ramakrishnan S, Dougherty J. Outcomes of the use of infliximab and adalimumab in patients with Crohn's disease at a district general hospital (abstr). Gut 2014;63:A70.
39. Iimuro M, Nakamura S, Sato T, et al. Long term outcome of top-down therapy in Crohn's disease: a single-center experience (abstr). Inflamm Bowel Dis 2011; 17:S49-S50.
40. Domenech E, Hinojosa J, Nos P, et al. Clinical evolution of luminal and perianal Crohn's disease after inducing remission with infliximab: how long should patients be treated? Aliment Pharmacol Ther 2005;22:1107-1113.
41. Chauvin A, Le Thuaut A, Belhassan M, et al. Infliximab as a bridge to remission maintained by antimetabolite therapy in Crohn's disease: a retrospective study. Dig Liver Dis 2014;46:695-700.
42. Wynands J, Belbouab R, Candon S, etal. 12-month follow-up after successful infliximab therapy in pediatric crohn disease. J Pediatr Gastroenterol Nutr 2008;46:293-298.
43. Molnar T, Lakatos PL, Farkas K, et al. Predictors of relapse in patients with Crohn's disease in remission after 1 year of biological therapy. Aliment Pharmacol Ther 2013;37:225-233.
44. Molnar T, Farkas K, Miheller P, et al. Is the efficacy of successful infliximab induction therapy maintained for one year lasting without retreatment in different behavior types of Crohn's disease? J Crohns Colitis 2008;2:322-326.
45. Crombe V, Salleron J, Savoye G, et al. Long-term outcome of treatment with infliximab in pediatric-onset Crohn's disease: a population-based study. Inflamm Bowel Dis 2011 ;17:2144-2152.
46. Nuti F, Conte F, Cavallari N, et al. Long term efficacy of infliximab in inflammatory bowel disease at a single tertiary center. Dig Liver Dis 2010;42:S326-S327.
47. Louis E, Mary JY, Verniermassouille G, et al. Maintenance of remission among patients with Crohn's disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology 2012;142:63-70.e5.
48. Papamichael K, Vande Casteele N, Gils A, et al. Long-term outcome of patients with Crohn's disease who discontinued infliximab therapy upon clinical remission. Clin Gastroenterol Hepatol 2015;13:1103-1110.
49. Brooks AJ, Sebastian S, Cross SS, et al. Outcome of elective withdrawal of anti-tumour necrosis factor-α therapy in patients with Crohn's disease in established remission. J Crohns Colitis 2015 May 18 [Epub ahead of print].
50. Dart RJ, Griffin N, Taylor K, et al. Reassessment of Crohn' s disease treated with at least 12 months of anti-TNF therapy: how likely is treatment withdrawal? Frontline Gastroenterol 2014;5:176-182.
51. Monterubbianesi R, Papi C, Kohn A. Maintenance of clinical remission in Crohn's disease patients after discontinuation of antiTNF agents: results from a single centre cohort (abstr). J Crohns Colitis 2015;9(suppl 1):S345.
52. Steenholdt C, Molazahi A, Ainsworth MA, et al. Outcome after discontinuation of infliximab in patients with inflammatory bowel disease in clinical remission: an observational Danish single center study. Scand J Gastroenterol 2012;47:518-527.
53. Dai C, Liu WX, Jiang M, et al. Mucosal healing did not predict sustained clinical remission in patients with IBD after discontinuation of one-year infliximab therapy. PLoS One 2014;9:e110797.
54. Kennedy NA, Warner B, Johnston E, et al. Anti-TNF withdrawal in IBD: Relapse and recapture rates and predictive factors from 160 patients in a pan-UK study (abstr). J Crohns Colitis 2015;9(suppl 1):S41-S42.
55. Casanova MJ, Chaparro M, Garcia-Sanchez V, et al. Evolution after anti-TNF drug discontinuation in patients with inflammatory bowel disease (IBD): a multicenter long-term follow-up study (abstr). J Crohns Colitis 2015; 9(suppl 1):S329.
56. Ramos L, Hernandez Camba A, Carrillo Palau M, et al. Outcome of treatment with biological agents in Crohn's disease: 117 patients in 5 years from a tertiary referral center (abstr). J Crohns Colitis 2014;8(suppl):S231.
57. Duricova D, Bortlik M, Machkova N, et al. Deep remission in Crohn's disease does not prevent disease relapse after withdrawal of anti-TNFa therapy (abstr). Gastroenterology 2015;148(suppl 1):S-61.
58. Luppino I, Spagnuolo R, Marasco R, et al. Withdrawal of infliximab (IFX) after achieving remission: outcome in a cohort of inflammatory bowel disease (IBD) patients. Dig Liver Dis 2013;45:S100-S101.
59. Zucchi E, Fabbro M, Pinese E, et al. Outcome of infliximab discontinuation in IBD patients and therapy rechallenging in relapsers: single centre preliminary data. Dig Liver Dis 2014;46:S31.
60. Waugh AWG, Garg S, Matic K, et al. Maintenance of clinical benefit in Crohn's disease patients after discontinuation of infliximab: long-term follow-up of a single centre cohort. Aliment Pharmacol Ther 2010; 32:1129-1134.
61. Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn's disease: results from a single-centre cohort. Gut 2009;58:492-500.
62. Echarri A, Ollero V, Rodriguez JA, et al. Predictors of relapse after discontinuing anti-TNF therapy in Crohn's disease patients on deep remission. J Crohns Colitis 2013;7(suppl):S171.
63. Rismo R, Olsen T, Cui G, et al. Normalization of mucosal cytokine gene expression levels predicts long-term remission after discontinuation of anti-TNF therapy in Crohn's disease. Scand J Gastroenterol 2013;48:311-319.
64. Molander P, Farkkila M, Salminen K, et al. Outcome after discontinuation of TNFalpha-blocking therapy in patients with inflammatory bowel disease in deep remission. Inflamm Bowel Dis 2014;20:1021-1028.
65. De Suray N, Salleron J, Vernier-Massouille G, et al. Close monitoring of CRP and fecal calprotectin is able to predict clinical relapse in patients with Crohn's disease in remission after infiximab withdrawal. A sub-analysis of the stori study. Gastroenterology 2012;142(suppl 1):S-149.
66. Molander P, Farkkila M, Ristimaki A, et al. Does fecal calprotectin predict short-term relapse after stopping TNFalpha-blocking agents in inflammatory bowel disease patients in deep remission? J Crohns Colitis 2015; 9:33-40.
67. Fanjiang G, Russell GH, Katz AJ. Short- and long-term response to and weaning from infliximab therapy in pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr 2007;44:312-317.
68. Munoz Villafranca C, Bravo Rodriguez MT, Ortiz De Zarate J, et al. Mucosal healing in patients with ulcerative colitis treated with infliximab. What happens after treatment is discontinued? J Crohns Colitis 2014;8(suppl): S234-S235.
69. Caviglia R, Ribolsi M, Rizzi M, et al. Maintenance of remission with infliximab in inflammatory bowel disease: efficacy and safety long-term follow-up. World J Gastroenterol 2007;13:5238-5244.
70. Farkas K, Lakatos PL, Nagy F, et al. Predictors of relapse in patients with ulcerative colitis in remission after one-year of infliximab therapy. Scand J Gastroenterol 2013; 48:1394-1398.
71. Farkas K, Lakatos PL, Szucs M, et al. Frequency and prognostic role of mucosal healing in patients with Crohn's disease and ulcerative colitis after one-year of biological therapy. World J Gastroenterol 2014; 20:2995-3001.
72. Zelinkova Z, van der Ent C, Bruin KF, et al. Effects of discontinuing anti-tumor necrosis factor therapy during pregnancy on the course of inflammatory bowel disease and neonatal exposure. Clin Gastroenterol Hepatol 2013; 11:318-321.
73. de Lima A, Zelinkova Z, van der Ent C, et al. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut 2015 May 12 [Epub ahead of print].
74. Seirafi M, Treton X, De Vroey B, et al. Anti-TNF therapy and pregnancy in inflammatory bowel disease: a prospective cohort study from the GETAID. Gastroenterology 2011 ;140(suppl 1):S-175.
75. Sorrentino D, Nash P, Viladomiu M, et al. Stopping anti-TNF agents in patients with Crohn's disease in remission: is it a feasible long-term strategy? Inflamm Bowel Dis 2014;20:757-766.
76. Papamichael K, Vermeire S. Withdrawal of anti-tumour necrosis factor alpha therapy in inflammatory bowel disease. World J Gastroenterol 2015;21:4773-4778.
77. van der Valk ME, Mangen MJ, Leenders M, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFalpha therapy: results from the COIN study. Gut 2014;63:72-79.
78. Khan N, Abbas AM, Lichtenstein GR, et al. Risk of lymphoma in patients with ulcerative colitis treated with thiopurines: a nationwide retrospective cohort study. Gastroenterology 2013;145:1007-1015.e3.
79. Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009;374:1617-1625.
80. Kirchgesner J, Carrat F, Cosnes J, et al. Withdrawing or continuing maintenance treatment with thiopurines in patients with Crohn's disease in sustained clinical remission: a lifetime risk-benefit analysis (abstr). Gastroenterology 2015;148(suppl 1):S-231.
81. Smolen JS, Emery P, Fleischmann R, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet 2014;383:321-332.
82. Annunziata ML, Papparella LG, Sansoni I, et al. Normalized wall thickness at MRE predicts clinical remission in Crohn's disease after infliximab discontinuation: a 5 years' follow-up study. Gastroenterology 2014; 146(suppl 1):S-246.
83. Papamichael K, Claes K, de Bruyn M, et al. Serology panel for prediction relapse after discontinuation of infliximab in patients with Crohn's disease achieving clinical remission. J Crohns Colitis 2015;9(suppl 1 ):S295-S296.
Author names in bold designate shared co-first authorship. Acknowledgments
The authors thank Rachel Pinotti, MLIS, in the Gustave L. and Janet W. Levy Library at the Icahn School of Medicine at Mount Sinai for her assistance in helping design the comprehensive search strategy for this project. The Biocycle project receives funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 633168 - BIOCYCLE (PHC-13-2014).
Dr Nicholas A. Kennedy is funded by a Wellcome Trust Research Training Fellowship [Grant 097943].
Conflicts of interest
The authors disclose the following: Joana Torres has received consultant fees from Abbvie. Nicholas A.
Kennedy has received speaker fees from MSD and travel support from Abbvie. Edouard Louis has received fees for: Educational Grant: MSD, Abbvie. Speaker Fees: Abbvie, Ferring, MSD, Chiesi, Mitsubishi Pharma, Hospira, Janssen. Advisory Board: Abbvie, Ferring, MSD, Takeda, Mitsubishi Pharma, Celltrion. Jean-Frederic Colombel has served as consultant, advisory board member or speaker for Abbvie, ABScience, Amgen, Bristol Meyers Squibb, Celgene, Celltrion, Danone, Ferring, Genentech, Giuliani SPA, Given Imaging, Immune Pharmaceuticals, Janssen, Kyowa Kirin Pharma, Medimmune, Merck & Co., Millennium Pharmaceuticals Inc., Navigant Consulting, Neovacs, Nestle, Nutrition Science Partners Ltd., Pfizer Inc., Prometheus Laboratories, Protagonist, Receptos, Sanofi, Schering Plough Corporation, Second Genome, Shire, Takeda, Teva
Pharmaceuticals, Tigenix, UCB Pharma, Vertex and Dr. August Wolff GmbH & Co. Jack Satsangi has received speaker fees from MSD, Takeda, and research support from Abbie Vie. Ray K. Boyapati has no conflicts to disclose.