© Xue Cheng, 2019
Role of NuA4 histone acetyltransferase complex in DNA
damage response pathways
Thèse
Xue Cheng
Doctorat en biologie cellulaire et moléculaire
Philosophiæ doctor (Ph. D.)
ii
Role of NuA4 histone acetyltransferase complex in
DNA damage response pathways
Thèse
Xue Cheng
Sous la direction de :
iii
Résumé
L’organisation du génome eucaryote sous forme de chromatine est intimement liée à la régulation de nombreux processus cellulaires. Le contexte chromatinien joue un rôle central dans les voies de réponse cellulaires aux dommages à l’ADN. NuA4 est un complexe histone-acétyltransférase (HAT) très conservé formé de nombreuses sous-unités, responsable de l’acétylation des histones H4 et H2A au sein des nucléosomes. Il joue un rôle important dans l’expression des gènes mais aussi dans la réparation efficace des cassures double-brin (CDBs) de l’ADN. Bien qu’il ait été montré que NuA4 est rapidement recruté sur la chromatine autour des CDBs, le mécanisme précis de ce recrutement et le rôle joué par NuA4 à ce niveau restent mal compris. Mon projet de doctorat se concentre sur l’étude du mécanisme de recrutement de NuA4 et sur les conséquences de ce recrutement. Au moyen d’approches in vitro et in vivo, nous avons montré que NuA4 est recruté au niveau d’une CDB par le complexe MRX et se propage en suivant la résection de l’ADN. Après son recrutement et sa propagation, NuA4 participe à la réponse aux dommages en acétylant les nucléosomes pour faciliter leur retrait. De plus, NuA4 acétyle des facteurs-clés de la réponse aux dommages à l’ADN, tels que RPA et Sae2, pour réguler leur dynamique et leurs fonctions. En étudiant l’implication de NuA4 lors de phases spécifiques de la réponse aux dommages, nous avons découvert que le complexe est impliqué dans différentes étapes de la réparation par recombinaison homologue, dont la résection et la formation de D-loops, en coopération avec une autre HAT, Gcn5/SAGA. Dans l’ensemble, les résultats présentés dans cette thèse décrivent les fonctions complexes de NuA4 dans les
voies de réponse aux dommages à l’ADN et permettent de mieux comprendre
comment la chromatine et sa régulation orchestrent les processus cellulaires lors de la réparation de l’ADN.
iv
Abstract
The organization of the eukaryotic cell genome into chromatin structure is intricately linked to the regulation of many cellular processes. DNA damage response (DDR) takes place in the context of chromatin and the latter plays a central role in the regulation of cellular DDR pathways. The NuA4 histone acetyltransferase (HAT) is a highly conserved multi-subunit complex responsible for acetylation of nucleosomal histone H4 and H2A. It is important for gene expression but also the efficient repair of DNA double-strand breaks (DSBs). Although it was shown that NuA4 is rapidly recruited to chromatin surrounding a DSB, the specific mechanism of this recruitment and NuA4 function after its recruitment remains unknown. My PhD project focuses on investigating NuA4 recruitment mechanism and the functional consequences of this recruitment. Taking advantage of in vitro and in vivo approaches, we found that NuA4 is recruited by the MRX complex to a DSB site and spreads along with DNA resection. After recruitment and spreading, NuA4 participates in DDR through acetylation of nucleosomes to assist their removal. In addition, NuA4 also acetylates key DDR factors, such as RPA and Sae2, to regulate their dynamics and functions. By dissecting NuA4 involvement in specific DDR steps, we found that the complex is involved in different stages of homologous recombination (HR) repair, including resection and D-loop formation, during which it cooperates with another HAT, Gcn5/SAGA. Altogether, the data presented in this thesis delineate the intricate functions of NuA4 in DDR pathways and extend our understanding on how chromatin and its regulation orchestrate chromatin-based cellular processes during DNA repair.
v
Table of Contents
Résumé ... iii Abstract ... iv Table of Contents ... v List of Figures ... ixList of Abbreviations ... xii
Acknowledgements ... xviii
Forewords ... xix
Introduction ... 1
1.1 Chromatin Structure and Regulation ... 2
1.1.1 Chromatin structure ... 2
1.1.2 Chromatin regulation ... 4
1.1.2.1 Histone PTMs ... 4
1.1.2.2 Histone variants ... 7
1.1.2.3 ATP-dependent chromatin remodeling ... 9
1.2 Histone acetyltransferase complex NuA4 ... 12
1.2.1 Subunits/Modules ... 12
1.2.1.1 Catalytic module- Piccolo NuA4 ... 13
1.2.1.2 Recruitment module- Tra1 ... 14
1.2.1.3 Scaffold subunit- Eaf1 ... 15
1.2.1.4 Swc4-Yaf9-Arp4-Act1 module and TINTIN ... 16
1.2.1.5 Human NuA4/TIP60 complex ... 17
1.2.2 Substrates of NuA4 and their functions ... 19
1.2.2.1 NuA4 histone substrates and functions ... 19
1.2.2.2 NuA4 non-histone substrates and functions ... 21
1.3 DNA damage response and DSB repair ... 21
1.3.1 DNA DSB and DNA damage response ... 21
1.3.2 DSB repair pathways ... 22
1.3.2.1 HR ... 23
1.3.2.1.1 Initial end processing by MRX/N and Sae2/CtIP .... 23
vi
1.3.2.1.3 Strand-invasion, DNA synthesis and resolution... 27
1.3.2.2 NHEJ ... 27
1.3.2.3 Other DSB repair pathways ... 28
1.3.2.4 Repair pathway choice ... 29
1.3.3 DDR in the context of chromatin ... 30
1.3.3.1 Chromatin modifications in DDR ... 31
1.3.3.2 Chromatin remodeling in DDR ... 33
1.3.3.3 Histone variants in DDR ... 34
1.4 PhD Project Hypothesis and Aims ... 35
Chapter 1 Phospho-dependent recruitment of the yeast NuA4 acetyltransferase complex by MRX at DNA breaks regulates RPA dynamics during resection ... 37
2.1 Résumé ... 38
2.2 Abstract ... 39
2.3 Introduction ... 40
2.4 Results ... 41
2.4.1 The Mre11-Rad50-Xrs2 complex is required for recruitment of NuA4 at a DNA break ... 41
2.4.2 DNA damage induces a phospho-dependent interaction of NuA4 with Xrs2 ... 44
2.4.3 After initial recruitment, NuA4 spreads on each side of the break during end resection ... 46
2.4.4 NuA4 and RPA interact on ssDNA during resection ... 48
2.4.5 NuA4 acetylates RPA and modulates its binding to ssDNA ... 49
2.5 Discussion ... 52
2.6 Materials and Methods ... 53
2.6.1 Yeast strains, materials and mass spectrometry ... 53
2.6.2 ChIP and ChIP-MS ... 53
2.6.3 GST pull-down assay ... 54
2.6.4 Biotin-ssDNA pull-down and gel shift assays ... 54
2.6.5 NuA4-Rfa1 co-immunoprecipitation ... 55
vii
2.8 Supplemental Information Appendix ... 56
Chapter 2 Conserved roles of lysine acetyltransferase complexes for repair of DNA breaks by homologous recombination in eukaryotes ... 69
3.1 Résumé ... 70
3.2 Abstract ... 71
3.3 Introduction ... 72
3.4 Results ... 73
3.4.1 NuA4 acetylates chromatin around a DNA break and favors nucleosome loss ... 73
3.4.2 NuA4 facilitates resection and subsequent recombination process ... 76
3.4.3 NuA4 plays a role in strand invasion at the homologous donor sequence ... 78
3.4.4 NuA4 cooperates with SAGA to regulate HR ... 80
3.5 Discussion ... 83
3.6 Materials and Methods ... 85
3.6.1 Yeast strains ... 85
3.6.2 ChIP ... 85
3.6.3 Plasmid-based NHEJ assay ... 86
3.6.4 Acetyl-lysine IP ... 86
3.6.5 DR-GFP HR assay ... 86
3.7 Author Contributions ... 87
3.8 Acknowledgements ... 87
3.9 Supplementary information ... 87
Chapter 3 NuA4-dependent acetylation of Sae2 regulates its proteasome-dependent degradation ... 94
4.1 Résumé ... 95
4.2 Abstract ... 96
4.3 Introduction ... 97
4.4 Results ... 98
4.5 Discussion and Perspectives ... 103
viii
Discussion and Perspectives ... 108
5.1 Challenges of NuA4 study in DDR ... 110
5.2 The Anchor-away system as a valuable tool to study NuA4 in DDR ... 112
5.3 NuA4 involvement in post-resection steps of HR repair ... 114
5.4 Depicting global picture of NuA4-dependent non-histone acetylation in DDR 116 5.5 Where and how the DSBs are generated make a difference? ... 119
5.6 NuA4 in chromatin mobility and relocation of DSBs? ... 121
5.7 Outlook ... 122
Reference ... 123
Appendix I………152
Appendix II………..………155
ix
List of Figures
Figure 1-1. Chromatin structure and several levels of condensation. ... 3
Figure 1-2. Reader domains of histone PTMs. ... 7
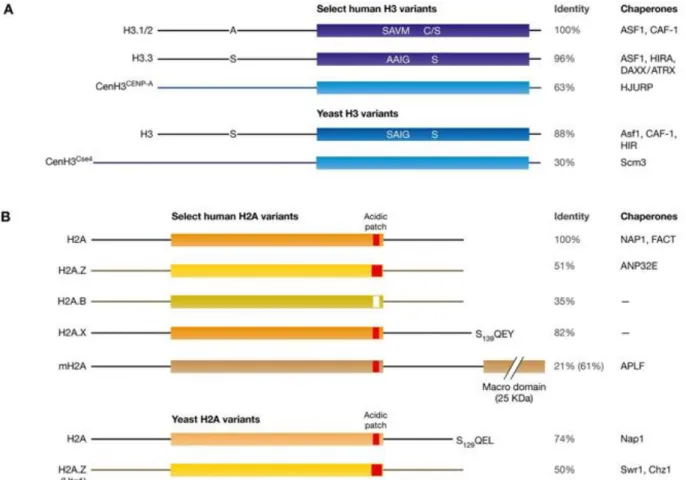
Figure 1-3. Schematic representation of key H3 and H2A variants in yeast and human. ... 8
Figure 1-4. Simplified functional classification of chromatin remodelers. ... 11
Figure 1-5. Schematic representation of yeast NuA4 complex and submodules... 13
Figure 1-6. Model for the evolution from yeast NuA4 HAT complex to human TIP60 HAT complex. ... 18
Figure 1-7. Schematic representation of DSB repair by NHEJ, MMEJ, SSA and HR. ... 23
Figure 1-8. Schematic representation of domain arrangement in Mre11, Rad50, Xrs2 and Sae2. ... 24
Figure 1-9. DSB repair pathways and main factors in human cells. ... 30
Figure 1-10. Hypothesis of PhD project. ... 36
Figure 2-1. NuA4 recruitment around DNA breaks depends on MRX. ... 43
Figure 2-2. DNA damage induced phosphorylation of NuA4 is required to interact with Xrs2... 45
Figure 2-3. NuA4 recruitment at DNA breaks occurs mainly in G2 and is followed by spreading during resection. ... 47
Figure 2-4. NuA4 interacts with RPA upon DNA damage. ... 49
Figure 2-5. NuA4 displaces RPA from ssDNA in an Ac-CoA-dependent manner. 51 Figure S2-1. Three potential modes of NuA4 recruitment to DSB site based on ATM-related factor Tra1. ... 62
Figure S2-2. NuA4 mutants for Mec1/Tel1 phosphorylation sites are not sensitive to DNA damage. ... 63
Figure S2-3. Effect of DNA end resection on chromatin surrounding the HO break. ... 64
Figure S2-4. NuA4 subunit Swc4 can bind resected DNA end structures and 9-1-1 clamp or clamp loader do not significantly affect NuA4 enrichment near DNA breaks. ... 65
x
Figure S2-5. Acetylation sites on RPA subunits. ... 67
Figure 3-1. NuA4 is required for H4 acetylation and nucleosome loss upon DSB induction. ... 74
Figure 3-2. NuA4 is involved in DNA end resection and recombination during HR. ... 77
Figure 3-3. NuA4 is present at the donor homologous sequence during HR. ... 79
Figure 3-4. NuA4 collaborates with the SAGA HAT complex during HR. ... 82
Figure S3-1. Using NuA4 mutants in Gal-HO system. ... 89
Figure S3-2. NuA4 shows genetic interaction with resection and recombination factors. ... 91
Figure S3-3. NHEJ assay, FRB-Tra1 data, BrdU assay validation and HO cleavage efficiency. ... 92
Figure S3-4. Knockdown of PCAF accumulates cells in S/G2... 93
Figure 4-1. Sae2 is acetylated by NuA4. ... 99
Figure 4-2. Acetylation of Sae2 by NuA4 regulates its abundance independent of DNA damage. ... 100
Figure 4-3. Acetylation of Sae2 on K239 and K266 steers it away from proteasome-dependent degradation. ... 101
Figure 4-4. Sae2 acetylation by NuA4 directly or indirectly stabilizes Mre11 at break site. ... 103
Figure S4-1. Sae2 is functionally linked to NuA4. ... 106
Figure S4-2. Sae2 K239, K266 mutations do not render cell sensitive to DNA damaging agents. ... 107
Figure 5-1. Model of NuA4 roles in DNA damage response pathways………….110
Figure 5-2. Schematic representation of the anchor-away (AA) technique in budding yeast. ... 112
Figure 5-3. Mating type switching in Saccharomyces cerevisiae and the primer positions to be used to monitor HR repair. ... 115
xi
List of Tables
Table 1-1. Members of HAT families in yeast and human* ... 5
Table 1-2. Members of HDAC families in yeast and human*... 6
Table 1-3. Chromatin remodeler subfamilies and key members in yeast and human ... 10
Table 1-4. Saccharomyces cerevisiae NuA4 subunits, modules and their human homologs ... 13
Table 1-5. Major histone PTMs involved in DDR ... 31
Table 1-6. Summary of key budding yeast chromatin remodelers involved in DDR ... 34
Table S2-1: Yeast strains used in this study... 58
Table S2-2: List of primers used in ChIP ... 60
xii
List of Abbreviations
53BP1 Tp53 binding protein 1 AA anchor-away AcK acetyl-lysine Act1 actin 1ADE adenine requiring
ARP actin related protein
ATG autophagy related
ATM ataxia telangiectasia mutated
ATP adenosine triphosphate
ATR ataxia telangiectasia and Rad3 related
ATRIP ATR interacting protein
Bar1 barrier to the alpha factor response 1
Bdf1 bromodomain factor 1
BLM bloom syndrome protein
BRCA breast cancer
BRCT BRCA1 C terminus
BRD8 bromodomain 8
Cas9 CRISPR-associated 9
CDK cyclin-dependent kinase
CenH3 centromeric histone 3
CHD chromodomain
ChIP chromatin immunoprecipitation
CK2 casein kinase 2
c-Myc cancer-myelocytomatosis
CPT camptothecin
CRISPR clustered regularly interspaced short palindromic repeats
CtIP CTBP-interacting protein
DDR DNA damage response
D-loop Displacement-loop
DNA deoxyribonucleic acid
Dna2 DNA replication ATP-dependent helicase/nuclease 2
DNA-PKcs protein kinase DNA dependant catalytic subunit
Dnl4 DNA ligase 4
DSB double-strand break
Eaf Esa1p-associated factor
EPC enhancer of polycomb
Esa1 essential SAS family acetyltransferase 1
xiii
FHA forkhead associated domain
FKBP12 FK506 binding protein 1A, 12kDa
FRB FKBP12-rapamycin binding
Frp1 FK506-sensitive proline rotamase
Fun30 function unknown now
GAL galactose metabolism
Gcn5 general control nonderepressible 5
GNAT Gcn5 N-acetyltransferase related
GST Glutathione S-transferase
HAT histone acetyltransferase
HBO1 histone acetyltransferase bound to ORC1
HDAC histone deacetylase
HELB DNA helicase B
HML hidden mat left
HMR hidden mat right
HO homothallic switching endonuclease
HR homologous recombination
HSA helicase SANT associated
HSS HAND–SAND–SLIDE
INO80 inositol requiring 80
Ioc4 localization of mRNA
IP immunoprecipitation
IR Ionizing radiation
ISWI imitation switch
KAT5 lysine acetyltransferase 5
KD/KO knock-down/knock-out
Lcd1 lethal, checkpoint-defective, DNA damage sensitive
Lif1 ligase interacting factor 1
LIG3/4 DNA ligase 3/4
MAT mating type
MDC1 mediator of DNA damage checkpoint 1
Mec1 mitosis entry checkpoint
MG132 Z-L-Leu-D-Leu-L-Leu-al
MMEJ microhomology-mediated end joining
MMS methyl methane sulfonate
MOF male absent on the first
Mre11 meiotic recombination 11
MRX/N Mre11-Rad50-Xrs2/NBS1
MS mass spectrometry
mTOR mammalian target of rapamycin
xiv
MYST MOZ, Ybf2/Sas3, Sas2, TIP60
NAD nicotinamide adenine dinucleotide
Nbs1 Nijmegen breakage syndrome 1
NCP nucleosome core particle
Nej1 Non-homologous end joining defective 1
NER nucleotide excision repair
NHEJ Non-homologous end joining
NuA3/4 nucleosome acetyltransferase of H3/H4
ORF open reading frame
p300/CBP E1A binding protein p300/CREB binding protein
p400 E1A binding protein p400
p53 tumor protein p53
PALB2 partner and localiser of BRCA2
Pck1 phosphoenolpyruvate carboxykinase 1
PHD plant homeodomain
Pho phosphate metabolism
PI3K phosphatidylinositol 3 kinase
PIKK PI3K related kinases
poly-Q poly-glutamine
PTM post-traductional modification
PWWP Pro-Trp-Trp-Pro
qPCR quantitative PCR
Rad2/XPG radiation 2/xeroderma pigmentosum, complementation group G
Rad radiation sensitive
rDNA ribosomal DNA
Rfa replication factor A
Rmi1 RecQ mediated genome instability
RNA PolII RNA polymerase 2
RNF ring finger protein
RPA replication protein A
Rpd3 reduced potassium dependency 3
Rpl13a ribosomal protein of the large subunit 13
RSC remodel the structure of chromatin
Rtt107 regulator of Ty1 transposition 107
Rvb1/2 RuVB-like 1/2
Sae2 sporulation in the absence of spo eleven 2
SAGA Spt, Ada, Gcn5 acetyltransferase
SANT Swi3, Ada2, N-Cor et TFIIIB
Sas2 something about silencing 2
SDSA Synthesis-dependant strand annealing
xv
sgRNA single guide RNA
Sgs1 slow growth suppressor 1
Sic1 substrate/subunit inhibitor of cyclin-dependent protein kinase 1
Sir3 silent information regulator 3
SMARCAD1 SWI/SNF-related, matrix-associated actin-dependent regulator of chromatin, subfamily A, containing DEAD/H box 1
SMC structural maintenance of chromosome
Spo11 sporulation 11
SSA Single-strand annealing
ssDNA Single-strand DNA
STR Sgs1-Top3-Rmi1
Swc Swr Complex
SWI/SNF switching/sucrose non-fermenting
SWR1 Swi2/Snf2-related ATPase 1
Tbf1 TTAGGG repeat-binding factor 1
Tel1 telomere maintenance 1
TINTIN trimer independent on NuA4 involved in transcription interactions with
nucleosomes
Tip60 Tat-interacting protein 60kDa
Top3 topoisomerase 3
TopBP1 DNA topoisomerase II binding protein 1
Tor1 target of rapamycin 1
Tra1 similar to human TRRAP 1
TRRAP transformation/transcription domain associated protein
TSS transcription start site
UBC13 ubiquitin-conjugating 13
UDR ubiquitination-dependent recruitment
ULK1 Unc-51 like autophagy activating kinase 1
UPS ubiquitin-proteasome system
UV ultraviolet
VPA valproic acid
WB western blot
WT wild type
XLF XRCC4-like factor
XRCC4 X-ray repair cross complementing 4
Xrs2 X-ray sensitive 2
Yaf9 yeast homolog of the human leukemogenic protein AF9
Yng2 yeast ING1 homolog 2
xvi
To
François, the harbour of warmth and love and
xvii
学而不思则罔,思而不学则怠。
- 孔子
To learn without thinking is blindness, to think without learning is in danger.
xviii
Acknowledgements
My graduate study is filled with supports from many great people, to all of whom I owe a debt of immense gratitude. First of all, sincere thanks from the bottom of my
heart to my supervisor Dr. Jacques Côté. Years of graduate study under his
guidance has been an amazing journey and I am utterly grateful for his excellent scientific mentoring, patience, encouragement and inspiration. He is kind, showing me the example of being a great scientist as well as a good person. He is able to see the best in everybody, making people want to be better. I cherish his encouragement when I made advancement in my work, and I am forever grateful for his understanding when the times were difficult.
I am also grateful to my current and previous colleagues in the lab: Marie-eve, Anne-lise, Dorine, Wajid, Karine, Pierre, Céline, Mathieu, Valérie, Catherine, Salar, Jonathan, Maeva, Suk Min, Deepthi and Anahita. I am fortunate to share the equipment, reagents and laughs with you. In the pursuit of science, we met; through our discussions, I grew.
I would like to thank Dr. Amine Nourani, Dre. Amélie Fradet-Turcotte and Dr. Jean-Yves Masson for their expertise, precious suggestions and heartwarming encouragement throughout the years.
I am grateful to my little family. Thanks to my husband François, who has been so supportive during the whole course of my study. I am grateful for your trust in me and the encouraging words that lift me up. I am grateful for the warm dinners ready on the table after a long lab day. I am grateful for you being a stay-at-home dad for 7-month to take care of Amelia so I could go back to the lab for my study. I am also grateful to my baby girl Amelia, for being such a beautiful being in my life and bringing me the joy that words simply cannot describe, the joy that fuels my days.
In the end, I would like to thank all my thesis jury members, Dr. Jacques Côté, Dr. Jean-Yves Masson, Dre. Amélie Fradet-Turcotte and Dr. Hugo Wurtele for accepting to evaluate my thesis in your busy schedules and for contributing your time and expertise to my study.
xix
Forewords
The article presented in Chapter 1 has been published in journal Proceedings of the National Academy of Sciences of the United States of America (PNAS) in September 2018 (Proc Natl Acad Sci USA. 2018 Oct 2;115(40):10028-10033). I am the co-first author of this publication along with Olivier Jobin-Robitaille. I was involved in the experiments presented in fig. 1 (total), 3A, 3C-D, 4B-E, 5D-F and supplementary fig. S1 (total), S2B-D, S3B-C, S4C-F, S5B-D, S5F-I. Olivier Jobin-Robitaille was involved in the experiments presented in fig. 1 (total), 2B, 2D-F, 3 (total), 4D-E and supplementary fig. S2A, S3 (total), S4G-H. Pierre Billon was involved in the experiments presented in fig. 2A, 4A and S4B. Valérie Côté provided critical technical assistance in preparation of the experiments. Contributions from collaborators include: Rémi Buisson from Dr. Jean-Yves Masson’s lab was involved in the experiments presented in fig. 5A-C. Hengyao Niu from Dr. Patrick Sung’s lab provided reagents used in fig. 5A-C and S5A-B. Nicolas Lacoste was involved in the experiments presented in fig. 2C. Nebiyu Abshiru from Dr. Pierre Thibault’s lab, Dr. Stephen J. Kron and Dr. Christopher J. Brandl provided critical support to the study and helped with data analysis. The article was written by me and Dr. Jacques Côté. The revision of the paper was performed by me, Valérie Côté and Dr. Jacques Côté. The manuscript presented in Chapter 2 has been submitted to journal Nucleic Acids
Research. I am the first-author of this manuscript. I was involved in experiments
presented in all the figures presented. The manuscript was written by me and Dr. Jacques Côté.
The manuscript presented in Chapter 3 describes preliminary results that require additional experiments before being considered for submission. I was involved in experiments in all the figures presented.
Unless otherwise specified, in this thesis “NuA4” refers to the NuA4 complex in
Saccharomyces cerevisiae; “TIP60” refers to the NuA4 complex in human cells and
“Tip60” refers to the catalytic subunit of the complex. The protein representation format XX/YY indicates budding yeast protein XX and its human protein homolog YY.
1
2
1.1 Chromatin Structure and Regulation
In 1879, German cytologist Walther Flemming first coined the term “chromatin” to
describe the heavily dyed “threads” he observed in the nucleus (Flemming, 1879). He also predicted that this term would only be temporary and “may serve until its chemical nature is known”. The definition of chromatin evolved with the elucidation of its chemical
property in the 19th century, but unlike Flemming’s prediction, the term “chromatin” was
inherited throughout time. In fact, we now appreciate that chromatin hosts many fundamental processes in eukaryotic cells and the study of chromatin biology is one of the most flourished fields of scientific research in recent years.
1.1.1 Chromatin structure
Eukaryotic cell genome is organized into chromatin structure in order to compensate for the limited space within the nucleus. The basic unit of chromatin is the nucleosome, which consists of 146bp of DNA wrapped around two copies of four core histones, H2A, H2B, H3 and H4 (Kornberg, 1974; Steunou et al., 2014). The primary structure of chromatin is the “beads-on-a-string” conformation with various lengths of linker DNA in between the nucleosomes (Woodcock and Ghosh, 2010). With the help of linker histone H1, this primary chromatin structure can be further compacted into several higher-order structures, and eventually condensed about 10,000-fold to form mitotic/meiotic chromosomes (Fyodorov et al., 2018; Jansen and Verstrepen, 2011) (Figure 1-1).
Chromatin can be further classified into two structurally and functionally distinguishable categories based on their condensation level and gene density: less condensed, gene-rich euchromatin and highly condensed, gene-poor heterochromatin (Wang et al., 2016). Euchromatin is typically more accessible to transcription, whereas heterochromatin is generally transcriptionally silent. The size and complexity of heterochromatin varies greatly among different organisms. In budding yeast Saccharomyces cerevisiae, the majority of the genome is in the euchromatin status and only the silenced mating type loci (HML and HMR), rDNA repeats, and the sub-telomeric regions are heterochromatin (Hickman et al., 2011). On the other hand, the structure and regulation of heterochromatin is more complex in human cells. Human heterochromatin can be further divided into facultative heterochromatin that are subject to decondensation based on cellular context,
3
and constitutive heterochromatin which maintains high levels of compaction (Wang et al., 2016).
Figure 1-1. Chromatin structure and several levels of condensation.
DNA is wrapped around core histone octamer to form nucleosomes. Nucleosomes are connected by linker DNA to form “beads-on-a-string” chromatin primary structure. For further condensation, this primary structure is folded into a 30nm-diameter fiber-like form. These 30nm fibers can be further compacted into higher-order structures that eventually form chromosomes during mitosis and meiosis. It is worth mentioning that the native existence of a 30nm fiber in vivo remains controversial (Ausio, 2015). Modified from (Jansen and Verstrepen, 2011).
? ?
4
1.1.2 Chromatin regulation
Rather than a steady state, the chromatin structure is highly dynamics and constantly being modulated to meet the requirement of all DNA-based cellular activities, including transcription, DNA replication and repair. Cells have evolved several mechanisms to regulate chromatin structure, including histone post-translational modifications (PTMs), chromatin remodeling, histone variant incorporation, histone chaperones and DNA methylation (Rothbart and Strahl, 2014). The structure of certain chromatin status is often from a combined action of several modulations. The following section presents three major chromatin regulation mechanisms that are closely related to my PhD project.
1.1.2.1 Histone PTMs
The N-terminal and C-terminal tails of the histones protrude from the nucleosomes, providing a platform for different types of post-translational modifications (PTMs), including acetylation, methylation, phosphorylation and ubiquitination (Zhao and Garcia, 2015) (Figure 1-2). Histone PTM states are highly dynamic and being regulated by a series of “writer” enzymes that deposit the PTMs (such as acetyltransferase, methyltransferase and kinase), as well as the respective “eraser” enzymes to remove the modification from the histones (deacetylase, demethylase and phosphatase). It has been well-accepted that different histone modifications, on one or more histone tails, form a “histone code” that could specify unique downstream events (Strahl and Allis, 2000). Histone PTMs regulate chromatin structure and function by two general mechanisms. Some histone modifications can directly modulate chromatin by altering the charge on histones, thereby reducing the interaction between histone tails and the negatively charged DNA (Rothbart and Strahl, 2014). Histone acetylation is one of these PTMs. Histone acetylation is regulated by the antagonistic actions of two classes of enzymes: histone acetyltransferases (HATs) and histone deacetylases (HDACs). HATs are divided into the GNAT, MYST and p300/CBP subfamilies (Table 1-1), whereas HDACs belong to
one of four groups: Zn2+-dependent class I, II and IV HDACs and NAD+-dependent class
III HDACs (also known as Sirtuins) (Steunou et al., 2014) (Table 1-2). Acetylation of lysine on its ε-amino group neutralizes the positive charge of the residue, attenuating the
5
interaction between DNA and histones (Pelletier et al., 2008). This generally leads to less stable nucleosomes susceptible to mobility/loss and increases the DNA accessibility for other proteins such as transcription activators to favor gene expression. Therefore, the presence of histone acetylation at gene promoter regions is often associated with active transcription (Steunou et al., 2014).
Table 1-1. Members of HAT families in yeast and human*
* Modified from (Steunou et al., 2014).
Name Saccharomyces
cerevisiae (Sc)
Homo sapiens (Hs)
KAT1 Hat1 HAT1
KAT2 Gcn5
KAT2A GCN5
KAT2B PCAF
KAT9 Elp3 ELP3
KAT10 Hpa2
KAT14 CSRP2BP
HAT4
KAT5 Esa1 TIP60/PLIP
KAT6 Sas3
KAT6A MOZ/MYST3
KAT6B MORF/MYST4
KAT7 HBO1/MYST2
KAT8 Sas2 MOF/MYST1
KAT3A CBP KAT3B p300 KAT11 Rtt109 GNAT family MYST family p300/CBP family HAT family
6
Table 1-2. Members of HDAC families in yeast and human*
* Modified from (Steunou et al., 2014).
The second mechanism is mediated by the recruitment of histone PTM “reader” proteins as effectors. Different histone PTMs provide a landing platform for the “docking” of reader proteins through their reader domains to carry out specific downstream events in the context of chromatin (Figure 1-2). For examples, budding yeast Rad9 is an adaptor protein in DNA damage checkpoint cascade and also functions as an inhibitor of resection during DNA double-strand break (DSB) repair (Lazzaro et al., 2008; Vialard et al., 1998). Rad9 can be recruited to the chromatin through binding to H3K79me2-containing nucleosomes via its tudor domain. Moreover, the BRCT (BRCA1 Carboxyl-Terminal) domain of Rad9 can also specifically interact with damage-induced H2AS129ph. Both domains are important for Rad9 chromatin association to realize its checkpoint adaptor and resection inhibitor functions (Hammet et al., 2007; Javaheri et al., 2006; Nnakwe et al., 2009; Wysocki et al., 2005). The recruitment of 53BP1, the human ortholog of Rad9,
HDAC1 HDAC2 Hos1 Hos2 HDAC3 HDAC8 Hos3 HDAC4 HDAC5 HDAC7 HDAC9 Hda1 HDAC6 HDAC10 SIRT1 SIRT2 SIRT3 Hst1 Hst2 Hst3 Hst4 II SIRT4 III SIRT5 SIRT6 SIRT7
HDAC Class IV HDAC11
Sir2 Rpd3
HDAC family
HDAC Class I
HDAC Class II
HDAC Class III (Sirtuins)
A
B
I
7
to the chromatin also involves multivalent interactions (Wilson and Durocher, 2017). Like Rad9, 53BP1 contains a tudor domain that can interact with H4K20me1/2-containing nucleosomes (Botuyan et al., 2006). Moreover, 53BP1 consists of an ubiquitination-dependent recruitment (UDR) region that not only directly recognizes the RNF168-catalysed ubiquitylation of H2AK15, but also interacts with the acidic patch of the nucleosome core particle (NCP) (Fradet-Turcotte et al., 2013; Wilson et al., 2016). These three interactions (Tudor-H4K20me2, UDR-H2AK15ub and UDR-NCP) are all essential for the recruitment of 53BP1 to chromatin (Wilson and Durocher, 2017). The BRCT domain of 53BP1, on the other hand, can interact with H2A.XS139ph, but this interaction is dispensable for 53BP1 recruitment or function (Ward et al., 2006; Zgheib et al., 2009). As for acetylation, it is worth mentioning that, besides directly changing chromatin physical property, acetylation can also function by recruiting downstream reader proteins through their acetyl-binding domains such as bromodomains (Steunou et al., 2014).
Figure 1-2. Reader domains of histone PTMs.
Recognition of the methylated (me) lysine (K), methylated (me) arginine (R), acetylated (ac) lysine (K) and phosphorylated (ph) serine (S) and threonine (T) residues by indicated reader domains. Histone H3 N-terminal tail is shown as an example since it has many residues subject to different types of PTMs. Adapted from (Musselman et al., 2012).
1.1.2.2 Histone variants
In addition to the four canonical histones (H2A, H2B, H3, and H4), there are also several histone variants that can be incorporated into chromatin and profoundly change chromatin
8
properties (Mattiroli et al., 2015; Talbert and Henikoff, 2017) (Figure 1-3). Histone variants differ from canonical histones in many aspects. To start with, histone variants have various degrees of primary protein sequence divergence compared to their respective canonical histones, with H2A and H2B variants showing most profound differences (Mattiroli et al., 2015). These differences are fundamental to their functional specialization. For instance, human histone variant H2A.X contains a short C-terminal extension that includes an important residue, Ser139. S139 is phosphorylated during DNA damage response, an event that is crucial for the DDR cascade (see section 1.3 for more details). Although budding yeast does not have a H2A.X variant, its canonical H2A contains the equivalent short C-terminal extension and serine 129 that can also be phosphorylated upon DNA damage (Figure 1-3).
Figure 1-3. Schematic representation of key H3 and H2A variants in yeast and human.
Blue shades in H3 variants and orange shades in H2A variants represent the conserved histone-fold domain. The N- and C-terminal tails are shown as lines. Human H2A.B is also referred as H2A.Bbd. Different shades of color indicate the level of sequence divergence.
9
H2A acidic patch is shown in red (where the acidic residues are present) and white (for H2A.B which lacks these residues). H2A.Z has an extended acidic region. Reported chaperones involved in their deposition are listed on the right. Adapted from (Mattiroli et al., 2015).
Besides protein sequence, canonical and variant histones also differ in their timings of expression and deposition into chromatin. While canonical histones are expressed almost exclusively during S-phase of the cell cycle and mainly incorporated along with replication, histone variants are expressed throughout the cell cycle and incorporated independently of replication (Talbert and Henikoff, 2017). The incorporation of histone variants involves histone chaperones and ATP-dependent chromatin remodelers. Histone chaperone proteins can bind histones and facilitate their interactions to form the nucleosome, while ATP-dependent chromatin remodelers reorganize the chromatin and exchange canonical histones to histone variants (Mattiroli et al., 2015).
The incorporation of histone variants diversifies chromatin signals in a temporally and spatially regulated manner and contributes to chromatin complexity by creating specialized nucleosomes that dictate specific functions. Histone variant CenH3, for example, is a histone H3 variant that is localized exclusively to centromeres. The incorporation of CenH3 in both yeast and human is important for centromere identity and kinetochore assembly during chromosome segregation and division (Mattiroli et al., 2015; Sarma and Reinberg, 2005). Histone H2A variant H2A.Z, on the other hand, is localized at two specific nucleosomes, one upstream and one downstream of the transcription start site (−1 and +1 nucleosomes to TSS), to regulate gene transcription (Billon and Cote, 2013).
1.1.2.3 ATP-dependent chromatin remodeling
Chromatin structure and function can also be regulated by chromatin remodelers. While all chromatin remodeler enzymes contain ATPase activity, the presence of additional domain organisation in these enzymes further classifies them into four distinct subfamilies: SWI/SNF (SWItch/Sucrose Non-Fermentable), ISWI (Imitation SWItch), CHD (Chromodomain Helicase DNA-binding) and INO80 (INOsitol 80) (Rother and van Attikum, 2017) (Table 1-3). SWI/SNF members contain an HSA (Helicase-SANT-Associated)
10
domain for the binding of ARPs (actin-related proteins) and a bromodomain that interacts with acetylated histones. ISWI remodelers contain HSS (HAND-SANT-SLIDE) domains, which allow for histone and DNA binding within nucleosomes. CHD subfamily of remodelers possess an ATPase domain similar to that of the ISWI remodelers, but also contain a unique N-terminus chromodomain which recognizes methylated histones. Finally, INO80 remodelers feature an HSA domain and a large insertion in their unique ATPase region, which permits the binding of helicase-related Rvb1/2 (AAA+ ATPase) and ARPs (Clapier and Cairns, 2009; Nair and Kumar, 2012; Rother and van Attikum, 2017).
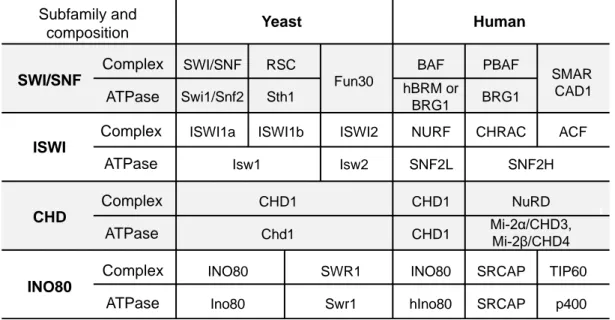
Table 1-3. Chromatin remodeler subfamilies and key members in yeast and human
*Modified from (Clapier and Cairns, 2009; Costelloe et al., 2012)
Chromatin remodelers modulate chromatin structure by regulating nucleosome assembly and organization (slide and space nucleosomes), chromatin access (eject partial or full nucleosomes) and nucleosome editing (incorporating or removing histone variants) (Clapier et al., 2017). One important aspect of chromatin remodelers is that they often reside in multi-subunit complexes. The diverse reader domains comprised in the enzymes and associated subunits can assist complex localization to specific genomic regions by interacting with certain histone PTMs (Clapier and Cairns, 2009; Clapier et al., 2017). For example, yeast Snf2 ATPase of the SWI/SNF subfamily uses its C-terminus
Complex ATPase Complex ATPase Complex ATPase Complex ATPase Yeast Human SWI/SNF Subfamily and composition ISWI CHD INO80 SWI/SNF Swi1/Snf2 RSC Sth1 Fun30 SMAR CAD1 BAF PBAF hBRM or BRG1 BRG1
ISWI1a ISWI1b ISWI2 NURF CHRAC ACF
Isw1 Isw2 SNF2L SNF2H CHD1 Chd1 CHD1 CHD1 NuRD Mi-2α/CHD3, Mi-2β/CHD4 INO80 INO80 Ino80 hIno80 SWR1 Swr1 SRCAP TIP60 SRCAP p400
11
bromodomain to promote the targeting of SWI/SNF activity to nucleosomes that are acetylated on histone H3 (Bennett and Peterson, 2015; Chatterjee et al., 2011; Hassan et al., 2002). H3 acetylation also enhances the recruitment of the RSC complex through its bromodomain containing Rsc4 subunit to increase RSC-mediated nucleosome mobilization and H2A/H2B displacement, favoring gene activation (Chatterjee et al., 2011; Kasten et al., 2004). Besides acetylation, other histone PTMs also interact with chromatin remodeling complexes. For instance, Isw1b chromatin remodeling complex of the ISWI subfamily can be specifically recruited to the gene open reading frames (ORFs) through the interaction between the PWWP domain of its Ioc4 subunit and transcription-coupled H3K36me3, maintaining chromatin integrity during transcription elongation by RNA Pol II (Smolle et al., 2012).
Figure 1-4. Simplified functional classification of chromatin remodelers.
ISWI and CHD subfamilies generally function in nucleosome assembly via random deposition of histones, the maturation of nucleosomes and their spacing (left). SWI/SNF subfamily primarily regulates chromatin access by repositioning nucleosomes, evicting histone dimers or octamers (middle). INO80 subfamily can carry out nucleosome editing by exchanging canonical and variant histones, such as the incorporation of H2A.Z variants (yellow). Some chromatin remodelers, such as INO80, ISWI and certain CHD remodelers can also regulate chromatin access. Adapted from (Clapier et al., 2017).
12
1.2 Histone acetyltransferase complex NuA4
NuA4 is a large histone acetyltransferase (HAT) complex that is conserved from yeast to human. It was first reported in 1997 by Workman’s lab as budding yeast “complex 2” which shows substrate specificity for histone H2A and H4 (Grant et al., 1997). Soon after, this 1.3MDa complex was named as “NuA4”, standing for “Nucleosomal Acetyltransferase of histone H4”, and found to function in transcription activation and cell cycle progression (Allard et al., 1999; Clarke et al., 1999; Utley et al., 1998). Its human homologous complex, the TIP60 complex, was purified and shows high structural and functional conservation with yeast NuA4 (Doyon et al., 2004). Subsequent studies over the past two decades have identified NuA4/TIP60 involvement in many other cellular activities besides transcription, including DNA repair, cellular metabolism, autophagy, stem cell maintenance, chromatin boundary maintenance and chromosome segregation (Cheng et al., 2018; Fazzio et al., 2008; Jacquet et al., 2016; Lin et al., 2012b; Squatrito et al., 2006; Steunou et al., 2014; Yi et al., 2012).
1.2.1 Subunits/Modules
The multifunctional nature of the NuA4 complex is rooted from its multi-subunit assembly. Yeast NuA4 can be divided into several submodules, including catalytic module Piccolo NuA4, recruitment subunit Tra1, scaffold subunit Eaf1, Swc4-Yaf9-Arp4-Act1 module and TINTIN (Eaf5-Eaf7-Eaf3) module (Auger et al., 2008; Brown et al., 2001; Cheng and Cote, 2014; Rossetto et al., 2014) (Figure 1-5; Table 1-4). This section will introduce in more details the modules mentioned above, as well as the human TIP60 complex.
13
Figure 1-5. Schematic representation of yeast NuA4 complex and submodules.
A) NuA4 subunits and submodules. Tra1 (shaded in yellow) is the recruitment module of NuA4. Eaf1 (shaded in red) is the scaffold subunit of NuA4. Esa1 (in red letters) is the catalytic subunit of NuA4. B) Schematic of the locations of NuA4 modules based on cryo-EM. Scale bar, 50 Å. Modified from (Setiaputra et al., 2018).
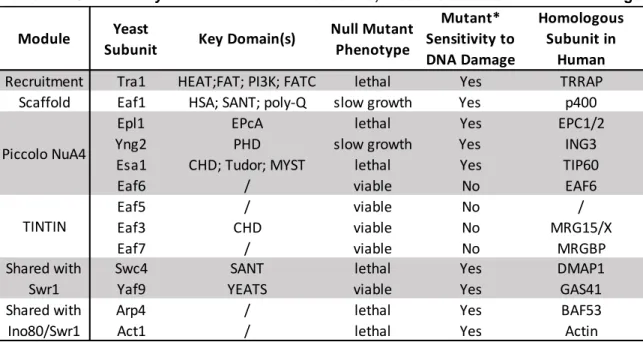
Table 1-4. Saccharomyces cerevisiae NuA4 subunits, modules and their human homologs
1.2.1.1 Catalytic module- Piccolo NuA4
Piccolo NuA4 represents a sub-complex of NuA4 that contains four subunits: Epl1, Yng2, Eaf6 and the catalytic subunit Esa1 (Auger et al., 2008; Boudreault et al., 2003). Esa1 is
Module Yeast
Subunit Key Domain(s)
Null Mutant Phenotype Mutant* Sensitivity to DNA Damage Homologous Subunit in Human
Recruitment Tra1 HEAT;FAT; PI3K; FATC lethal Yes TRRAP
Scaffold Eaf1 HSA; SANT; poly-Q slow growth Yes p400
Epl1 EPcA lethal Yes EPC1/2
Yng2 PHD slow growth Yes ING3
Esa1 CHD; Tudor; MYST lethal Yes TIP60
Eaf6 / viable No EAF6
Eaf5 / viable No /
Eaf3 CHD viable No MRG15/X
Eaf7 / viable No MRGBP
Swc4 SANT lethal Yes DMAP1
Yaf9 YEATS viable Yes GAS41
Arp4 / lethal Yes BAF53
Act1 / lethal Yes Actin
* Mutant: Null mutant or hypomorphic mutant Piccolo NuA4 TINTIN Shared with Ino80/Swr1 Shared with Swr1 A B TINTIN
14
the only essential HAT in yeast and several thermosensitive (ts) mutants, such as
esa1-L254P and esa1-414, are often used to study its function (Clarke et al., 1999). Although
Esa1 alone is highly active on free histones, it is unable to acetylate nucleosome substrates. The association with Yng2 and Epl1 subunits allows Piccolo NuA4 to bind and modify chromatin (Boudreault et al., 2003; Huang and Tan, 2013; Selleck et al., 2005). More specifically, the conserved EPcA domain of Epl1, the N-terminal of Yng2 and the Tudor domain of Esa1 play critical roles in the binding and activity of Piccolo NuA4 on nucleosome substrates (Boudreault et al., 2003; Huang and Tan, 2013; Selleck et al., 2005; Xu et al., 2016). Consistent with this, depletion of Yng2 or Epl1 strongly cripples NuA4 activity on chromatin (Boudreault et al., 2003; Choy et al., 2001; Nourani et al., 2001; Selleck et al., 2005). Eaf6 is a small enigmatic 13kDa protein with little reported functions. Eaf6 is also present in NuA3, a HAT complex that targets histone H3 (Taverna et al., 2006). It is a subunit of Piccolo NuA4 but is dispensable for Piccolo NuA4 activity
in vitro (Boudreault et al., 2003; Selleck et al., 2005). In addition, null mutation of EAF6
shows no apparent phenotype in yeast (Rossetto et al., 2014). While its conservation during evolution hints for its functional importance (Doyon et al., 2004), additional work is required to unveil Eaf6 function within and beyond the context of NuA4.
The specificity and substrate targeting of chromatin modifying enzymes are often realized by their associated subunits within the complexes (Lalonde et al., 2014; Xu et al., 2016). Since Piccolo NuA4 is concise in structure but functionally active on chromatin, it has been proposed that Piccolo NuA4 is responsible for global histone acetylation while NuA4 carries out targeted acetylation events, either through chromatin-binding domains presented within associated subunits or transcription factor-directed recruitment by Tra1 (Boudreault et al., 2003; Cheng et al., 2018; Downs et al., 2004; Friis et al., 2009; Selleck et al., 2005; Steunou et al., 2016).
1.2.1.2 Recruitment module- Tra1
Tra1 and its human homolog TRRAP are members of the phosphatidylinositol 3-kinase-related protein kinase (PIKK) family but are catalytically inactive since Tra1/TRRAP lack critical residues essential for protein phosphorylation (Knutson and Hahn, 2011; Saleh et
15
al., 1998). Tra1 is one of the largest proteins in yeast cells and is essential for cell viability (Saleh et al., 1998). Besides NuA4, Tra1 is also a subunit of another HAT complex, SAGA, which is mainly responsible for the acetylation of nucleosomal H3 through its catalytic subunit Gcn5 (Grant et al., 1998). Nuclear depletion of Tra1 affects the acetylation level of both H3 and H4, but the magnitude is smaller than the sum of single Esa1 and Gcn5 depletion, potentially due to the existence of their catalytic subcomplexes, piccolo NuA4 and the ADA complex (the catalytic submodule of SAGA) (Bruzzone et al., 2018).
One important role of Tra1 is to recruit HAT complexes to gene promoter regions by interacting with transcription activators, enabling their transcription co-activator function (Allard et al., 1999; Brown et al., 2001). Supporting this recruitment role during transcription, depletion of Tra1 leads to a clear decrease in Esa1 enrichment at ribosomal protein gene promoters (Bruzzone et al., 2018). Tra1 human homolog TRRAP also interacts with transcription factors and functions as an essential cofactor for c-Myc- and E2F-mediated oncogenic transformation (McMahon et al., 1998; Murr et al., 2007). The recruitment function of Tra1 could also be observed beyond transcription. Several PIKK kinases (Mec1/ATR, Tel1/ATM and DNA-PKcs) can be recruited by a conserved module presented in specific DNA damage sensor proteins (more details in section 1.3) (Falck et al., 2005). Tra1, also a member of the PIKK protein family, is argued to recruit NuA4 to DSB sites through an interaction with DSB sensor protein complex MRX (Cheng et al., 2018), underlining the importance of Tra1 in directing NuA4 to specific regions of the genome to realize its function.
1.2.1.3 Scaffold subunit- Eaf1
Eaf1 is the scaffold protein for NuA4 assembly and is essential for complex integrity (Auger et al., 2008; Mitchell et al., 2008; Wang et al., 2018a). It is also the only subunit unique to NuA4 (Auger et al., 2008), providing a valuable tool to investigate NuA4 function without affecting subunits shared with other chromatin regulating complexes (e.g. INO80, SWR1, SAGA, NuA3) or sub-complexes (e.g. Piccolo NuA4, TINTIN) (Table 1-4). For this reason, the mutants of EAF1 are often adopted to investigate NuA4-specific functions and the antibody raised against the Eaf1 protein is routinely used to detect the presence of NuA4 (Auger et al., 2008).
16
Eaf1 contains an HSA domain, a SANT domain and a C-terminal glutamine-rich (poly-Q) domain (Auger et al., 2008). To fulfill its scaffold function and maintain NuA4 complex architecture, Eaf1 binds to Tra1 through its SANT domain and associates with the Arp4/Act1 module via its HSA domain. The interaction with the TINTIN (Eaf5/7/3) module depends on N-terminal of Eaf1, thus a N-terminal truncation of Eaf1 fully disassociates TINTIN from the rest of NuA4 (Setiaputra et al., 2018; Wang et al., 2018a). Piccolo NuA4, on the other hand, relies on the HSA domain of Eaf1 and Tra1 to stay connected within the complex (Wang et al., 2018a).
1.2.1.4 Swc4-Yaf9-Arp4-Act1 module and TINTIN
NuA4 shares the Swc4-Yaf9-Arp4-Act1 module with the SWR1 chromatin remodeling complex and the Arp4-Act1 subunits with the INO80 chromatin remodeling complex (Auger et al., 2008). Swc4 and Yaf9 interact through their C-terminal domains and the mutation of neither subunits affects bulk H4 acetylation levels, suggesting their roles in the targeted functions of NuA4 (Auger et al., 2008; Bittner et al., 2004; Zhang et al., 2004). Swc4 contains a SANT domain related to the one present in the telomere-binding protein Tbf1 and is essential for cell viability (Aasland et al., 1996; Auger et al., 2008). Yaf9 is a non-essential protein which contains a YEATS domain associated with the binding of H3K27ac and histone succinylation (Klein et al., 2018; Wang et al., 2018b). Arp4 and Act1 are linked to NuA4 through the HSA domain of Eaf1 and bound to SWR1 via the HSA domain of its Swr1 subunit (Szerlong et al., 2008). Arp4 has been shown to interact with damage-induced γH2A, contributing to the accumulation of NuA4, as well as the INO80 and SWR1 chromatin remodelers at DSB sites (Downs et al., 2004).
The Eaf5/Eaf7/Eaf3 subunits of NuA4 can form an independent trimeric subcomplex referred as “TINTIN” (Trimer Independent of NuA4 involved in Transcription Interactions with Nucleosomes) (Cheng and Cote, 2014; Rossetto et al., 2014). While the Eaf5 and Eaf7 subunits are only found in either NuA4 or TINTIN, Eaf3 is also a component of the Rpd3S HDAC complex (Carrozza et al., 2005). The only defined domain in TINTIN is the chromodomain (CHD) of Eaf3, which can bind H3K36me2/3 (Carrozza et al., 2005; Joshi and Struhl, 2005; Keogh et al., 2005). Unlike NuA4, the enrichment of which is mainly around the promoter regions during transcription, TINTIN is enriched over the coding
17
region of active genes (Rossetto et al., 2014). Consistent with this localization, TINTIN is found to interact with the elongating RNA polymerase II (Pol II), H3K36me3 and proposed to assist the disruption of nucleosomes, RNA Pol II progression and nucleosome refolding in its wake (Rossetto et al., 2014). The TINTIN module also functions in the context of NuA4. The CHD domain of Eaf3 and the H3K4me3-binding PHD domain of Yng2 are required for the orientation of NuA4 HAT activity to specific H3 methylation-containing regions, allowing for the proper transition from transcription initiation to elongation (Steunou et al., 2016). Similar combined interactions of Eaf3-CHD and Yng2-PHD have also been proposed to recruit and regulate NuA4 at the site of a DSB (Su et al., 2016).
1.2.1.5 Human NuA4/TIP60 complex
The TIP60 complex, the human counterpart of yeast NuA4 complex, consists of at least 16 subunits, including 12 subunits that are homologous to yeast NuA4 (Table 1-4) (Doyon et al., 2004; Jacquet et al., 2016). Besides the HAT activity carried out by its Tip60 subunit (also called KAT5), the TIP60 complex also possesses ATP-dependent chromatin remodeling activity realized by the p400 ATPase subunit to incorporate H2A.Z (Billon and Cote, 2013). Interestingly, yeast H2A.Z incorporation is carried out by the SWR1 complex (with Swr1 as the ATPase subunit) and the human TIP60 complex possesses 9 subunits homologous to yeast SWR1 complex (Swc4-Yaf9-Arp4-Act1 module, Rvb1/2, Swc2/YL-1, Bdf1/BRD8 and Swr1/p400) (Auger et al., 2008; Jha and Dutta, 2009). Furthermore, human p400 shows homology with Swr1 and displays structural similarity with Eaf1, containing also the HSA, SANT and poly-Q domains (Auger et al., 2008; Jha et al., 2013). These lines of observation led to the proposition that the human TIP60 complex is actually a physical and functional merge of yeast NuA4 and SWR1 complexes through the fusion of scaffold proteins Eaf1 and Swr1 (Auger et al., 2008) (Figure 1-6). Indeed, the expression of artificial p400-like construct in yeast recaptures key functions of both complexes, in agreement with this model (Auger et al., 2008).
18
Figure 1-6. Model for the evolution from yeast NuA4 HAT complex to human TIP60 HAT complex.
The yeast NuA4 and SWR1 complexes (with or without Bdf1) are depicted. In human cells, the human TIP60 complex equals the physical merge between yeast NuA4 and the Bdf1-containing SWR1 by the fusion of the Eaf1 and Swr1 scaffold subunits into a single subunit, human p400. The human SRCAP complex corresponds to the yeast SWR1 lacking Bdf1, which does not possess chromatin acetylation activity. Note that the newly reported MBTD1 subunit of human NuA4/TIP60 complex, which binds to EPC1 (Jacquet et al., 2016), is not illustrated in this figure. MBTD1 does not have homologous subunit in either yeast NuA4 or SWR1. Adapted from (Auger et al., 2008).
19
1.2.2 Substrates of NuA4 and their functions
1.2.2.1 NuA4 histone substrates and functions
Recombinant protein of the catalytic subunit Esa1 is able to acetylate free histone H2A, H3 and H4 in vitro. However, when it resides in the context of a native complex, the main targets of NuA4 are the N-terminus tails of nucleosomal histone H2A (K4, K7 and K13) and histone H4 (K5, K8, K12 and K16) (Steunou et al., 2014). NuA4 is the only histone acetyltransferase enzyme that can acetylate H4K5,8,12 on chromatin in vivo while Sas2 HAT can also acetylate H4K16 (Boudreault et al., 2003; Sutton et al., 2003). Another histone acetyltransferase Hat1 is responsible for H4 K5/12 acetylation on newly synthesized free histones involved in nucleosome assembly (Benson et al., 2007). On the other hand, Tip60 targets on human histone H2A and H4 tails can be substrates of several HATs, including HBO1 for H4K5/8/12, MOF for H4K16 and p300/CBP for H2AK5, H4K5/8/12 (Steunou et al., 2014).
The “traditional” function of NuA4-mediated histone acetylation is to promote transcription activation. NuA4 can be recruited by transcription factors to gene promoters of many housekeeping genes as well as inducible genes (Bruzzone et al., 2018; Nourani et al., 2004; Steunou et al., 2014). Following this recruitment, NuA4-mediated histone acetylation leads to less stable nucleosomes to favor gene activation but can also interact with bromodomain-containing factors to regulate transcription. For example, Bdf1, a subunit of SWR1 complex, has been shown to interact with NuA4-mediated H4 acetylation through its bromodomain and promote transcription via recruitment of pre-initiation complex (Altaf et al., 2010; Durant and Pugh, 2007). RSC, another bromodomain-containing chromatin remodeler, can recognize nucleosomes acetylated by NuA4 and SAGA and facilitate passage of RNA Pol II through them (Carey et al., 2006). In addition, NuA4 acetylates nucleosomes at several inducible gene promoters (e.g.
PHO5 and ADE) and promotes the incorporation of H2A.Z before their activation,
pre-setting the chromatin structure for rapid transcriptional response (Altaf et al., 2010; Auger et al., 2008; Cheng et al., 2015; Nourani et al., 2004).
Besides transcription, histone acetylation by NuA4 has also been intensively linked to DNA repair and genome stability maintenance. NuA4 was shown to be physically
20
recruited to a DSB site and Esa1-dependent H4 acetylation is suggested to be important for DSB repair by homologous recombination (HR) as well as non-homologous end-joining (NHEJ) (Bird et al., 2002; Downs et al., 2004; House et al., 2014; Lin et al., 2008; Tamburini and Tyler, 2005; Torres-Machorro et al., 2015). However, the underlying mechanisms remain to be further examined. In addition, NuA4-dependent H4 acetylation is required to maintain CAG repeat stability and promote gap-induced sister chromatid recombination (House et al., 2014). In human cells, the TIP60 complex and Tip60-dependent H4 hyperacetylation are enriched around DSB sites and play a critical role in the recruitment of repair factors and HR (Chailleux et al., 2010; Hsiao and Mizzen, 2013; Jacquet et al., 2016; Murr et al., 2006; Tang et al., 2013; Xu et al., 2010). Furthermore, TIP60 can also acetylate H2AK15 to favor HR by blocking H2AK15 ubiquitination, a histone mark essential for 53BP1 binding (Fradet-Turcotte et al., 2013; Jacquet et al., 2016). Controversial results exist regarding to H4K16ac in DSB repair. A study by Greenberg’s lab showed that H4K16ac catalysed by TIP60 limits 53BP1 binding to the chromatin and promotes HR (Tang et al., 2013). On the other hand, another study reported that MOF, but not TIP60, is actually the enzyme responsible for H4K16ac in DDR and MOF is important for both HR and NHEJ (Sharma et al., 2010). Further investigation is needed to tackle this discrepancy.
In addition to core histones, NuA4/TIP60 also acetylates several histone variants. Yeast H2A.Z is acetylated (mostly at K14) by both NuA4 and SAGA and the acetylated H2A.Z is enriched at active gene promoters (Keogh et al., 2006; Millar et al., 2006). In Drosophila
melanogaster, dTip60 acetylates phosphorylated H2A.v (γH2A.v; H2A.v is the fly
homolog of human H2A.X and H2A.Z) at DSBs and exchanges it with an unmodified H2Av (Kusch et al., 2004). In human, DNA damage induces not only the phosphorylation of H2A.X, but also TIP60-dependent acetylation of H2A.X at lysine 5. This acetylation event is required for subsequent UBC13-dependent H2A.X ubiquitination, the release of H2A.X from damaged chromatin as well as NBS1 dynamics around DSBs (Ikura et al., 2015; Ikura et al., 2007).
21
1.2.2.2 NuA4 non-histone substrates and functions
Besides histone substrates, recent years of studies also identified a number of NuA4 non-histone substrates involved in various types of cellular activities (Downey et al., 2015; Lin et al., 2008; Mitchell et al., 2013). To start with, several subunits of NuA4 themselves are targets of NuA4 autoacetylation (Mitchell et al., 2013). Autoacetylation on Esa1 catalytic site on K262 is a prerequisite for all NuA4 HAT activity (Mitchell et al., 2013; Yuan et al., 2012). In addition, Esa1-dependent acetylation of Yng2 on K170 controls Yng2 protein stability and function, potentially regulating NuA4 dynamics around DSBs after its recruitment (Lin et al., 2008).
Several non-histone substrates of NuA4 are involved in cellular stress responses (Lin et al., 2009). In yeast cells, starvation induces Esa1-dependent transient acetylation of K19 and K48 on autophagy signaling component Atg3 and regulates autophagy by controlling Atg3 and Atg8 interaction and lipidation of Atg8 (Yi et al., 2012). The requirement of NuA4 activity in autophagy seems to be conserved in human, since TIP60 acetylates and stimulates protein kinase ULK1, which is essential for autophagy (Lin et al., 2012b). Phosphoenolpyruvate carboxykinase Pck1 was initially identified as an in vitro NuA4 substrate in protein acetylation microarrays and further verified as an in vivo NuA4 target. Acetylation of Pck1 controls its activity and is essential for the regulation of cellular metabolic network during nutrient deprivation (Lin et al., 2009). This is also a conserved acetylation event and the acetylation of human PCK1 by TIP60 plays a critical regulatory role in glucose production (Lin et al., 2009). Finally, when cells face genotoxic stress, TIP60 acetylates p53 on K120 to selectively favor proapoptotic gene expression (Sykes et al., 2006) and acetylates ATM on K3016 to activates its kinase activity (Sapountzi and Cote, 2011; Sun et al., 2005; Sun et al., 2007).
1.3 DNA damage response and DSB repair
1.3.1 DNA DSB and DNA damage response
DNA double-strand breaks (DSBs) are among one of the most cytotoxic types of damage towards our genetic information carrier DNA. Undesired DSBs can arise from both
22
exogenous (e.g. ionizing radiation (IR), certain chemotherapy) and endogenous sources (e.g. collapsed replication forks) (Mehta and Haber, 2014). Moreover, programmed DSBs are also generated under physiological conditions such as yeast mating-type switching, meiosis recombination and the diversification of immunoglobulins (Chapman et al., 2012; Haber, 2000). Failure or defects in DSB repair process can lead to cell death, immunological, developmental, neurological disorders, and cancer (Chapman et al., 2012).
Cells have evolved a number of mechanisms, collectively termed as DNA damage response (DDR), to combat DNA damage and preserve genomic integrity. DDR involves the recognition of DNA break by sensor proteins, signaling the damage presence through transducer proteins and eventually activating a series of cellular responses through effector proteins. The flow of DDR cascade coordinates DNA repair with checkpoint-dependent cell cycle arrest, transcription modulation, and cell fate determination (Sirbu and Cortez, 2013).
1.3.2 DSB repair pathways
There are two major pathways to repair DSBs in eukaryotic cells: homologous recombination (HR) and non-homologous end-joining (NHEJ). Alternative pathways can also be used by the cells at a lower frequency. Figure 1-7 provides a brief illustration of these DSB repair pathways.
23
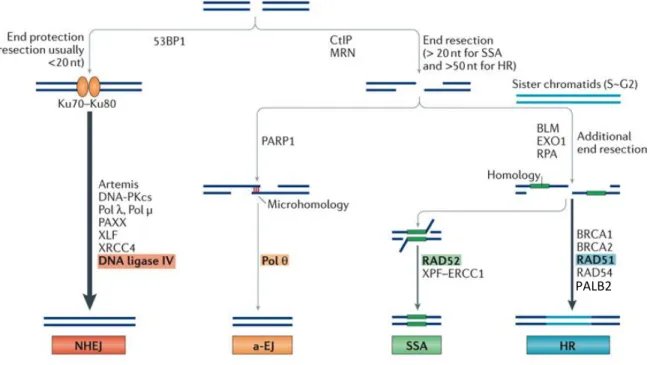
Figure 1-7. Schematic representation of DSB repair by NHEJ, MMEJ, SSA and HR.
In G1 phase of the cell cycle when DNA end resection is less active, DSBs are mostly repaired by the direct ligation of DNA ends via NHEJ. In S-G2 phase of the cell cycle, DNA end resection is activated. Resected ssDNA is firstly bound by RPA, and then replaced by Rad51 to carry out homologous pairing and strand invasion (HR). Alternatively, annealing of ssDNA that shares microhomology results in repair by MMEJ (microhomology-mediated end-joining), or by SSA (single-strand annealing) if longer direct repeats are present around the DSB. The orange lines represent direct-repeated sequences flanking the DSB. STR: Sgs1-Top3-Rmi1. Adapted from (Symington, 2016).
1.3.2.1 HR
HR utilizes an undamaged homologous DNA duplex sequence as template to repair the break. HR is initiated by the degradation of 5’-DNA strands on both sides of the DSB, generating long stretches of single-strand DNA (ssDNA), a process termed as DNA end resection. Following resection, ssDNA searches for homology in the genome, invades donor DNA duplex and copies the donor homologous sequence to repair the break.
1.3.2.1.1 Initial end processing by MRX/N and Sae2/CtIP
It is generally accepted that DSB resection takes place by a two-step mechanism: initial end processing and the subsequent extensive resection (Mimitou and Symington, 2008,
24
2009). DSB resection is initiated by the MRX/N complex and Sae2/CtIP (Figure 1-8). The MRX/N complex consists of three members: the Mre11 and Rad50 subunits are well conserved from bacteria to human, while the third component, Xrs2 in yeast and Nbs1 in human, is less conserved and only present in eukaryotic cells. In agreement with their involvement in the same pathway, deletions of any MRX complex subunits yield similar phenotypes and their effects are epistatic (Boulton and Jackson, 1998).
Figure 1-8. Schematic representation of domain arrangement in Mre11, Rad50, Xrs2 and Sae2.
The Mre11-D16A, Mre11-D56N, Mre11-H125N, Mre11-H213Y and Mre11-6 mutants are nuclease-defective (nd) in vitro. CDK-dependent S267 phosphorylation is essential for Sae2 function. Modified from (Fu et al., 2014; Symington, 2002).
Mre11 contains phosphodiesterase motifs at the N-terminus of the protein and displays
Mn2+-dependent 3’-5’ dsDNA endonuclease activity and ssDNA exonuclease activity in
vitro (Furuse et al., 1998; Moreau et al., 1999). Several nuclease-defective (nd) mutants
1-25
8 legend). Supporting the essential role of Mre11 nucleases activity in DSB end processing, these mre11-nd mutants are unable to remove Spo11 from DSB ends to allow for the processing of meiosis DSB (Furuse et al., 1998; Moreau et al., 1999; Tsubouchi and Ogawa, 1998; Usui et al., 1998). Moreover, mre11-nd mutants show less sensitivity
to DNA damage than mre11Δ null mutant (Llorente and Symington, 2004; Moreau et al.,
1999), indicating the presence of additional Mre11/MRX functions in DDR besides its nuclease activity, such as functions in checkpoint regulation and recruiting other resection factors (Moreau et al., 1999; Oh et al., 2016).
Rad50 is a member of the SMC (Structural Maintenance of Chromosomes) ATPase protein family, which features in Walker A and B nucleotide-binding motifs at the N- and C-terminal ends of the protein. The long stretches of amino acids in between the Walker A/B motifs form an extended coiled-coil structure that folds back on itself, leading to the intramolecular association of the ATPase domains at one side and a hook or hinge domain at the other side (Figure 1-8) (Alani et al., 1989; Hopfner et al., 2002). ATP binding and hydrolysis in Rad50 induce large conformational changes that influence Mre11 nuclease activity (Lammens et al., 2011; Lim et al., 2011; Williams et al., 2011).
Unlike Mre11 and Rad50, Xrs2/NBS1 itself does not have enzymatic activity. However, Xrs2/NBS1 is required for nuclear localization of Mre11 and has several protein-protein interaction motifs, suggesting that it functions mainly as a scaffold for protein interactions (Carney et al., 1998; Oh et al., 2016; Tsukamoto et al., 2005). Yeast Xrs2 contains a phospho-binding FHA (fork head-associated) domain at the N-terminus of the protein. Besides the FHA domain, human NBS1 has an additional BRCT domain at its N-terminus (Figure 1-8) (Symington, 2016). These domains are involved in several phospho-dependent interactions, including the interactions with phosphorylated Sae2/CtIP, Lif1 and MDC1 (Chapman and Jackson, 2008; Lloyd et al., 2009; Palmbos et al., 2008; Wang et al., 2013; Williams et al., 2009). Moreover, the C-terminus of Xrs2/NBS1 contains a conserved motif that recruits the PIKK-family checkpoint kinase Tel1/ATM to regulate DNA damage signaling (Falck et al., 2005).
Sae2 is less conserved during evolution and CtIP is considered to be its functional ortholog in human (Sartori et al., 2007). Sae2 possesses a N-terminal multimerization