Peptide Inhibitors of
Streptomyces
DD-Carboxypeptidases
By MANUEL NIETO and HAROLD R. PERKINSNational Institute for MedicalResearch, Mill Hill, London NW7 1AA, U.K.
and MItLINA LEYH-BOUILLE, JEAN-MARIE FR-RE and JEAN-MARIE GHUYSEN Service deMicrobiologie, Faculte de Medecine, Institutde Botanique, Universiteide Liege,
SartTilman, 4000Liege, Belgium (Received 14 August 1972)
1.Peptides thatinhibittheDD-carboxypeptidases fromStreptomyces strains albus G and R61 weresynthesized.They arecloseanalogues ofthe substrates of these enzymes. The enzymes fromalbus G and R61 strainsarein general inhibited by thesamepeptides, but the enzyme fromstrain R39differsconsiderably. 2.Thetwo C-terminal residues ofthe peptidesubstratesandinhibitors appeartobemainly responsiblefor the initialbinding ofthe substrate tothe enzymesfrom albus Gand R61 strains. The side chain inthe thirdresiduefromthe C-terminusseemscriticalininducing catalytic activity.3. Experi-mentalevidence is presented suggesting thattheamide bondlinking thetwoC-terminal residueshas a cisconfiguration when bound totheenzymesfrom strains albus G and R61. 4. Thepeptide inhibitors are notantibiotics against thesamemicro-organisms.
Bacterial DD-carboxypeptidases catalyse the cleav-age of the C-terminal D-Ala-D-Ala dipeptide in the
un-cross-linkedpeptide chainsofthecell-wall muco-peptide (peptidoglycan). In most bacteria these enzymes appear tobe bound tothecell membrane, butmanyStreptoinyces strainsexcretethem into the culturemediuminasoluble form.Advantage of this
facthasbeen takentopurify and study the substrate specificity of the DD-carboxypeptidases from
Strepto-myces strains albus G (Ghuysenet al., 1970;
Leyh-Bouilleetal., 1970), R61 (Leyh-Bouilleetal., 1971) R39 andK 1I(Leyh-Bouilleetal., 1972). Although in general, all these enzymes show similar specificity
profiles, their kinetic parameters (Km and Vrnax.) differconsiderably. Theyalsodifferintheir sensitivity to the penicillin and cephalosporin group of
anti-biotics; whereas the enzyme fromstrain albus G is
not inhibited by the antibiotics, the DD-carboxy-peptidasesfromstrains R61,K1I and R39are very
sensitive although their kinetics of inhibition differ. We now report the synthesis of peptides that are
analoguesofthesubstratesof thecarboxypeptidases and thatinhibitbothpenicillin-resistant and penicil-lin-sensitive types ofenzyme. These substrate
ana-logues are shown to be better inhibitors of the penicillin-resistantenzyme.
Materials and Methods Enzymes
The enzyme preparations and units of enzymic
activity used in this work have been previously described(Ghuysenet of., 1970; Leyh-Bouille etal.,
1971, 1972). D-Alanine, liberated during enzymic
hydrolysisof the standard substrate ce-Ac2-L-Lys-D-Ala-D-Ala, was converted into the Dnp derivative
and measured as previously described. (Ghuysen
et al., 1970) or by using ocE-Ac2-L-Lys-D-Ala-D-[14C]Ala.In the lattercaseafterincubationwiththe
enzyme,theD-[14C]alaninecleavedwasseparated by paper electrophoresis (0.25M-formic acid, pH 1.9, 1OV/cm for 2-3h), eluted with water (0.3-0.4ml) and radioactivity was measured in a Packard Tri-Carb liquid-scintillation counter, with 10ml of a
solution in dioxan(final volume 1 litre) of180gof
naphthalene, 4.Og of2,5-diphenyloxazole and 1.Og
of 1,4-bis-(4-methyl-5-phenyloxazol-2-yl)benzene as
scintillant. Efficiencyofcountingwas70-73%. Amino acids
These were puriss quality, from Koch-Light Laboratories Ltd. (Colnbrook, Bucks., U.K.). Dicyclohexylcarbodi-imide was purchased from
BDH Chemicals Ltd. (Poole, Dorset, U.K.) and N-hydroxysuccinimide, benzyloxycarbonyl chloride andD-cycloserine (D-4-amino-3-isoxazolidone) were
from Sigma Chemical Co., St. Louis, Mo., U.S.A. D-[14C]Alanine (3OmCi/mmol) was purchasedfrom
TheRadiochemicalCentre, Amersham, Bucks.,U.K. Allother chemicals usedinthisworkwereof thebest quality commerciallyavailable.
Aminoacid derivatives
The synthesis of thet-butoxycarbonyl derivatives ofD-alanine and L-lysine and their N-hydroxysuc-cinimide esters, as wellas that ofthey-benzyl ester
of D-glutamic acid have been described (Nieto & Perkins, 1971). Dibenzyloxycarbonyl-L-lysine was prepared as described by Schroder et al. (1961). Benzyloxycarbonyl-D-alanine was prepared as
de-scribed by Greenstein & Winitz (1961, p. 895) for
L-threonine.Itrecrystallizeseasily from ethyl acetate-hexane, yield 70% of theoretical, m.p. 72-75° C, [oc]i,5 +16.8 (c 2 in acetic acid) (Found: C, 59.2; H, 5.8; N, 6.3;
Cj1H13N04
requires
C, 58.9; H, 5.9;N, 6.9%). The
N-hydroxysuccinimide
ester ofdi-benzyloxycarbonyl-L-lysine
and benzyloxycarbonyl-D-alanine were prepared by the general method of Anderson et al. (1964) in a 86-90% yield and re-crystallized frompropan-2-ol. Dibenzyloxycarbonyl-L-lysine N-hydroxysuccinimide ester had m.p. 111-113° C and[a]22s
-15.4 (c 1 in acetone) (Found:C, 60.9; H, 5.7; N, 8.1; C26H29N308
requires
C,61.1; H, 5.7; N, 8.2%). TheN-hydroxysuccinimide esterofbenzyloxycarbonyl-D-alaninehad m.p.
114-1170C, [oc]25 +32.7(c1 inacetone) (Found: C, 56.1;
H, 5.3; N, 8.5. Calc. for C15H16N206: C, 56.2; H,
5.0; N, 8.7%).
Synthesisofnon-radioactive
peptides
ThesynthesisofL-Lys-D-Ala-D-Ala,
Gly-D-Ala-D-Ala and L-Gly-D-Ala-D-Ala-D-Gly-D-Ala-D-Ala-D-Gly-D-Ala-D-Ala has beendescribed
pre-viously (Nieto & Perkins, 1971). The remaining
peptides
weresynthesized bythe methodofAnderson etal. (1964) withoutprotecting
the carboxyl group of the amino-donor amino acid. A mixture of acetonitrile-water (1.3:1, v/v) was used as solvent. Theintermediates wereisolated and purified as de-scribed by Nieto&Perkins (1971)but ingeneraltheywerenotcharacterized.Removalof
protecting
groups and acetylation or succinylation (3-carboxypro-pionylation)werecarriedout aspreviouslydescribed (Nieto&Perkins, 1971).Theprocedure for glutaryla-tion(4-carboxy-n-butyrylation)
was similar to thatfor succinylation. Whatman no. 3 paper required for preparative purposes was first exhaustively washed by irrigation with 1 M-ammonium acetate followed by water. Additional information is as follows.
(a) In the synthesis of L-Lys-D-Glu-D-Ala,
di-benzyloxycarbonyl-L-Lys-D-Glu y-benzyl ester was synthesized as described above and then coupled to D-alanine benzyl ester by means of dicyclohexyl-carbodi-imide as described by Nieto & Perkins (1971). Carbodi-imidecoupling was also usedinthe synthesis of myristoyl-D-Ala-D-Asp from myristic acid,t-butoxycarbonyl-D-alanineandD-asparticacid dibenzyl ester.
(b) The protecting group in t-butoxycarbonyl-D-Ala-D-cycloserine was removed with 10% (v/v) tri-fluoroacetic acid in dichloromethaneat0° C for 15 min,
followedby 10min at room temperature. Even under these conditions about 15 % of the D-cycloserine in the peptidewas degraded. Free D-cycloserine, how-ever, seemed stable. Also, after succinylation of
D-Ala-D-cycloserinein the presence oftriethylamine,
paperelectrophoresis at pH7 and 10V/cm, showed two close anionic bands. Their relative mobilities with respect to glutamic acid were 0.3 and 0.7
respectively. Under identical conditions the relative mobility with respect to glutamicacidof
Ac-D-Ala-D-Gluwas 1.4andthatof Ac-D-Ala-D-Ala,0.78.The succinylated product was obtained as a mixture of thetwo bands. After acidhydrolysis ofthemixture
andcorrection fordestruction ofserine an
alanine/
serine ratio of 1:1 wasobtained. Nofurtherattempt was made to identify the chemical nature of the compound.
A similar treatment asfor the D-cycloserine
pep-tide was used to remove protecting groups of di-t-butoxycarbonyl-L-Lys-racemic- cyclodiaminoadipic
acid. No ring opening was observed. The rotations of most of the peptides synthesized are given in Table 1. The methods used for identification of
peptideswere asdescribed byNieto&Perkins (1971).
Table 1. Optical rotations ofsyntheticpeptides
Theconcentration of peptidewasestimated by automatic amino acid analysis inall cases. Abbreviations:Ac, acetyl; Z, benzyloxycarbonyl. Peptide D-Ala-D-Glu Ac-D-Ala-D-Glu Z-D-Ala-D-Glu D-Ala-D-Asp Ac-D-Ala-D-Asp D-Ala-D-cycloserine L-Lys-D-Ala-D-Glu L-Lys-D-GIu-D-Ala a-Ac-L-Lys-D-Glu-D-Ala Ac2-L-Lys-D-Glu-D-Ala
[khD
-12.68 at24° C (c 0.5inwater, pH3.9) +77.8at26.5° C(c1in water, pH 1.6) +10.7 at23.5° C (c 0.5 inaceticacid) -1 1.9 at 22° C(c1.6 in water, pH 2.75) +63.5at23.0° C(c0.8in water, pH 1.8) -5.5 at22.6° C(c0.2in water, pH 5.3) +92.4 at 24.1° C(c0.5in water, pH4.35) +103.2at23.0° C(c0.5in water, pH4.35) +8.25at240C (c0.8 inwater,pH4.2) +17.9at25° C (c1 in water, pH12.45)All peptides gave correct amino acid analyses, but some were obtained in too small an amount for measurement ofrotation. Acetyl- and succinyl-pep-tides were separated from the parent pepsuccinyl-pep-tides by paperelectrophoresis (Nieto & Perkins, 1971) and identified by their mobilities, and by their correct amino acidanalysis.
(c) Phenylacetylationwasperformedasfollows.A sample ofracemic-diaminoadipicacid lactam
(9,umol
in 1 ml water) was adjusted to pH9.5 by addition of
triethylamineandplacedin anice bath.Then
phenyl-acetylchloride
(5,ul)
wasadded and themixturewasshaken, beforebeing evaporatedtodryness in vacuo. Afteracidification with HCI, excess ofphenylacetic
acidwas removedbyetherextraction. The aqueous layer was thenapplied to paper forelectrophoresisin
acetic acid-collidine-water (2.65:9.1:1000,byvol.),
pH7, at 10V/cm for 2h. Apart from a little
un-changed lactam, the main product was an anionic band thatreactedstrongly withthe
chlorine/starch-iodide procedure of Rydon & Smith (1952). The
peptidewaseluted from the paper. Onacid
hydro-lysis (4M-HCI, 1050C, 1h) this material yielded
di-aminoadipicacidand wasthereforetakentobe the N-phenylacetyl-cyclodiaminoadipic acid (racemic mixtureofDD-andLL-isomers).
Synthesis ofpeptides of
D-[14C]alanine
D-Ala-D-[U-14C]Ala.
To50,uCi
ofD-[U-14C]alanine
in water(100,ul) wereadded NaHCO3 (3.2mg) and
t-butoxycarbonyl-D-Ala
N-hydroxysuccinimide
ester (3.2mg) inacetonitrile(120,ul),
andthemixturewas agitated for 2h at room temperature. The clearsolution was concentrated to dryness and treated
with 50% (v/v) trifluoroacetic acid in dichloro-methane(300ul) for 5minat0° C followed by10min at roomtemperature. Thesolutionwasconcentrated to dryness, redissolved in 80% (v/v) acetic acid
(300,il) and evaporation was repeated.
Finally
the reaction mixturewasdissolvedin0.25M-formic acidand applied to washed Whatman no. 3 paper for purification bypaperelectrophoresisin 0.25M-formic acid (10V/cm, 3h). Undertheseconditionstheratio of mobilities
(D-Ala-D-Ala)/(D-Ala)
is 1.22. Betterseparation
was obtained in aceticacid-pyridine-water(10:1:1000,by vol.),pH3.5 (53 V/cm, Ih), the
ratioofmobilitiesbeing3.0 inthiscase.Theposition
of thepeptide was determined by radioautography. After elutionof the
peptide
withwater aconversion ofradioactivity
fromD-[14C]Ala
intoD-Ala-D-[14C]-Alaof 93%wasobtained.
Similarly, by reaction between di-t-butoxycar-bonyl-L-Lys
N-hydroxysuccinimide
esterandD-Ala-D-[U-14C]Ala
thepeptideL-Lys-D-Ala-D-[U-_4C]Ala
was obtained and purified. Acetylation was carried out as described
by
Nieto & Perkins(1971).
TheVol.
overall recovery of radioactivity in acetylated
tri-peptidewas 78% and thespecific radioactivity of the peptideshould be identical with that ofthestarting
labelledamino acid.
2,5-Diaminoadipic acidlactam. The parent acid was synthesized via dimethylacc-diphthalimidoadipateas described by Greenstein & Winitz (1961, p. 2510). The mesoandracemicforms of theintermediatewere
separatedby fractionalcrystallization, the final m.p.
of recrystallized samples being 210° C (Kofler) for
the meso-isomer (lit. 211° C) and 178.5° C for the
racemic mixture ofDD- and LL-isomers. Thevalue of 165° C given by Greenstein & Winitz (1961, p. 2510) for thelattercompound is evidently toolow. Thefreediaminoadipicacidsobtained by
hydrazino-lysis and subsequent acid hydrolysis of the above intermediates were examined by paper
chromato-graphy in solvent A [methanol-water-pyridine-conc.HCI(32:7:4:1, byvol.) (Rhulandetal.,1955)] orsolvent B [methanol-water-pyridine-98% (w/v)
formic acid (80:19:10:1, by vol.) (Perkins, 1965)]. The meso-isomer was rather insoluble but gave a single ninhydrin-positive spot in each solvent with
Rmeso-diaminopimelic
acid
0.79(solvent
A)
or0.91(solvent
B), whereas the racemic mixture of DD- and LL-isomersgavetwospots withRmeso-diaminopimelic acid0.79 and 0.97 (solvent A) or 0.91 and 1.11 (solvent B).
Thusoptical isomersofdiaminoadipicacid separate in these solvents, as first described for diamino-pimelic acid (Rhuland et al., 1955). On
chromato-gramsdevelopedinsolventA and thentreatedwith
ninhydrin and heatedat105° C, diaminoadipic acids
gave
cherry-red
spots like thatgiven
by ornithine (Perkins & Cummins, 1964). The identity of thevarious compounds was confirmed by elution of samples
chromatographed
in solventBfollowed by conversion into thedi-Dnp
derivatives. The deriva-tivesfrom both slow- andfast-moving spots foundinthe racemic mixtureranin oneposition
(RF
0O.39) on a chromatogram (Whatman no. 1 paper) in solvent C[butan-1-ol-water-aq. NH3 (sp.gr.0.880)(20:19:1, by vol.)] whereas the
di-Dnp
derivative ofthe meso-isomer ran with RF 0.50 (cf. the di-Dnp derivatives ofdiaminopimelic acidinthesamesolvent meso-isomer,RF0.13;LL- orDD-isomer, RF0.19).
Todeterminewhichoftheracemic pair of isomers of
diaminoadipic
acidranfaster in solventsAandB, asample ofthedi-Dnp derivative of the fasterspot was dissolved in 0.125M-NaHCO3 and its opticalrotatory dispersion was compared with that of the
di-Dnp derivative of LL-diaminopimelic acid. The
shape of the curve was identical; hence the faster
spot was the LL-isomer ofdiaminoadipic acid. The molar rotations of the di-Dnp derivatives,
[M]350
were -35000° for LL-diaminopimelic acid and
-79
7000
forLL-diaminoadipicacid.Anattemptwasmadetocyclize
racemic-diamino-adipic
acidby
using
asoluble carbodi-imide. Racemicacid(23mg)in water(5ml)wastreated with
triethyl-amine
(36,ul)
and 1-cyclohexyl-3-(2-morpholino-ethyl)carbodi-imide methotoluene -p-sulphonate(33mg), and left in the dark at room temperature. After 3 days a samplechromatographedinsolventA and treated with ninhydrin showed the cherry-red
spots of the unchanged DD- and
LL-isomers,
butalso a faster-running purple spot
(RDD-isomer
2.25)and oneattributabletothe reagents(RDD-isomer 2.7).
The corresponding spot
(RDD-i,omer
2.25) from a chromatogram run insolventB waseluted. Ont.l.c. on silicagelG in solvent D[propan-1-ol-water-aq.NH3 (sp.gr.0.880) (14:3:3, by vol.)] it gave a spot at
RF
0.43, yellowon heating withninhydrin,con-trastingwith thepurplespot of
diaminoadipic
acid,RF 0.12.Thatthis productwasthelactamsoughtwas shownbythefollowingprocedures. (a)Onhydrolysis
inacid it yielded theparent
diaminoadipic
acid (later the optimum conditions wereshown to be 4M-HCIat 105° C for0.5-1
h);
(b)
ontreatmentwithfluoro-dinitrobenzene in the presence of
triethylamine
it gave aDnp derivative extractable intotdiethyl
ether from acid solution. After t.l.c. on silica gel G inchloroform-methanol-acetic acid
(95:5:1,
by vol.) it gave a spot atRF
0.08. This was eluted andhydrolysed in 4M-HClfor 3 h.
During
ether extractiontheyellowcolour nowremainedintheaqueouslayer.
On t.l.c. as
before,
it now had RF 0.41, the same value as authenticmono-Dnp-diaminoadipic
acid, and onspraying
withninhydrin
the spot turnedbrown,
indicating
afree amino group.It was observed that old marker solutions of diaminoadipic acid in propan-2-ol-water
(1:4,
v/v) contained a small amount of thecyclic
compound identified by the above criteria. Furtherinvestigation
showed thatheating
an aqueous solution(or
sus-pension) of
racemic-diaminoadipic
acid(2mg/ml)
at90° C in a sealed tube for 22h led to
considerable
conversion into the lactam.Heating
the drycom-pound or
refluxing
under dry benzene was noteffective. To check that
heating
at 90° C had not resulted in a change ofconfiguration
at theasym-metric carbon atoms, a sample of the lactam was
hydrolysed to
diaminoadipic
acid and convertedinto its di-Dnp derivative.
Chromatography
in sol-vent C showedthat no more thana trace ofmeso-compound had been formed. Longer
periods
of heat-ingcausedincreasing racemization,
nodoubt via thelactamitself.
Racemic-diaminoadipicacid(200 jtmol)was heated in water as described above, and theresultinglactam was separated from unchanged
diaminoadipic
acid by chromatography on a cation-exchange resin. A column (21cmx1.5cm) of Zeo-Karb 225 (H+ form)wasequilibrated at 2° Cwith0.2M-pyridine-acetate buffer, pH3 (16.1ml ofpyridine addedto
SOOml
of water, adjustedtopH3 withacetic acid and diluted to 1litre),
and thesample
containing diaminoadipic
acid lactam was applied. The column was eluted with thesame buffer and 3.5 ml fractions werecollected. Fractions 36-41 contained lactamand no diamino-adipic acid,asshown by t.l.c. in solvent D. The buffer solution was then changed to0.2M-pyridine-acetate buffer, pH 5, which soon eluted the diaminoadipic
acid. The combined fractions containing lactam were concentrated to dryness by rotary evaporation and dried in vacuo over conc. H2SO4 and NaOH. The sample was dissolved in water and shown by t.l.c. in solvent D to contain nodiaminoadipicacid. The concentration was determined by hydrolysis to di-aminoadipic acid as described above, followed by measurementof
E5s0
in the acid-ninhydrin procedure of Work(1957),areaction notgivenby the lactam. The final yield of purified lactam was 90,umol(45%).
Crystalline lactam was also prepared as follows.
Racemic-diaminoadipic acid (100mg) was dissolved inwater(35 ml)andheatedin asealedtube at 90° C for 22h.Aftercooling,thesolution was evaporated successively to 20ml,5ml and 1.5ml and after each evaporation it was kept at 2° C for 2h, during which time unchangeddiaminoadipic acidwas precipitated. It was removed bycentrifuging.The final supernatant solutioncontained only lactam(t.l.c. insolvent D). It was evaporated to dryness, redissolved in water (5ml) withwarming,and asmall residuewasremoved bycentrifuging.The solution wasagain concentrated
to 1.5ml, and crystallization of the lactam was
initiated bydropwiseaddition of80%(v/v)ethanol and completed at 2° C. Thecrystalswerewashedwith ethanol and ether and finally dried in vacuo over P205. On t.l.c. in solvent D a sample showed no
diaminoadipic acid. The lactam had m.p. 171° C
(Found: C, 40.3; H, 6.7; N, 16.1%. This is the analysis required by diaminoadipic acid, or the lactammonohydrate).
A similar procedure was used for preparing the lactam of meso-diaminoadipicacid except that, since the parentcompoundwasso much lesssolublethan the racemic-isomer mixture, the suspension after
heatingat 105° C wasevaporatedundervacuumto a small volumeandadjustedto pH 3 withaceticacid,
whenvirtuallyall the lactam present was in solution.
The final separation was on cation-exchange resin asdescribedabove.
Measurementofinhibition constants
KI values were calculated from plots of1/vversus
inhibitor concentration in the presence of different concentrations of standardsubstrate
oce-Ac2-L-Lys-D-Ala-D-Ala (range 0.4-1.7mM). With enzyme from strain albus G, the concentration of the inhibitor Ac-D-Ala-D-Glu rangedfrom 0.3to0.9mM and the
experiments were carried out in 0.02M-Tris-HCI
strain R61,concentrationof theinhibitor Ac-D-Ala-D-Asp ranged from 2.8 to 6.3mm and the experiments were carried out in 0.01M-Tris-HCl buffer, pH 7.5. Incubations were performed at 37° C.
Results
To facilitate the description of the results, the
amino acid residues in the peptides are numbered as H-3-2-1-OH.
Inhibition Of DD-carboxypeptidase from S. albus G
G DD-carboxypeptidase from S. albus G, an enzyme that is not inhibited by penicillins and cephalosporins (Ghuysen et al., 1970), can be
in-hibited by peptide analogues ofthe standardsubstrate
oce-Ac2-L-Lys-D-Ala-D-Ala. Table 2 summarizes the results obtained. The inhibition by Ac-D-Ala-D-Glu seems kinetically competitive, with KL 2.1x1O-4M. When one compares the behaviour of Ac-D-Ala-D-Glu orAc-Gly-D-Ala-D-Glu with that of
oeE-Ac2-L-Lys-D-Ala-D-Glu itisstrikingthattheacetylatedsidechain of L-lysine can change a non-substrate,
in-hibitory peptide into a very good substrate. The sameis observed for Ac-D-Ala-D-Ala,comparedwith
the standard substrate ae-Ac2-L-Lys-D-Ala-D-Ala. Also, succinylation instead of acetylation of the
abovelysine tripeptidesorlack of substitution of the
e-amino group decreased or suppressed their
sub-strateactivityand made thepeptidesinhibitors. The moststraightforward conclusionone candrawfrom
these results is that the C-terminal dipeptideis the
main portion of the molecule concerned with the
initial bindingto the enzymesurface, whereasaside
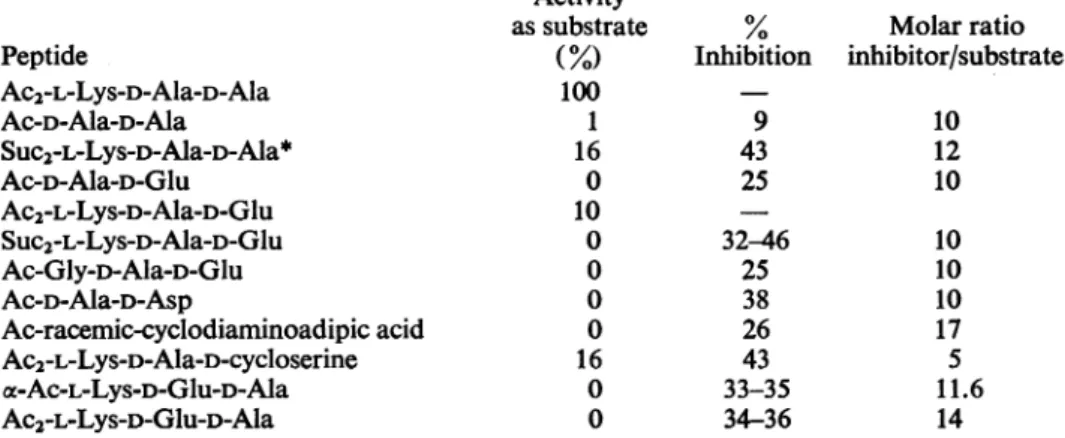
Table2.ActivityoftheDD-carboxypeptidase fromStreptomycesalbusG inthepresenceofpeptide inhibitors Ac2-L-Lys-D-Ala-D-Ala (15-20nmol)wasincubatedat37° Cfor30mininthe presenceof inhibitorandenough
enzymetoliberate 75%of itsterminal D-alanine in theabsence of inhibitor. The final volumewas
30,ul
and thebufferwas0.02M-Tris-HCl-2mM-Mg2+, pH7.5. % Inhibition is
100(AO-A/AO),
Abeing the alanine liberatedinthe presenceof the peptide inhibitor and
Ao
that inits absence. Activities as substrate weremeasured by incubating the inhibitors (150-200nmol) under the same conditions as described above and comparing the amount of terminal amino acid liberated with that cleaved under identical conditions from the standard substrate (Ac2-L-Lys-D-Ala-D-Ala). With standard substrate this represented a saturating concentration. Abbreviation: Suc,succinyl (3-carboxypropionyl).
Peptide Ac2-L-Lys-D-Ala-D-Ala Ac-D-Ala--Ala cee-Suc2-L-Lys-D-Ala-D-Ala Ac-D-Ala-D-Glu Suc-D-Ala-D-Glu c-Ac-L-Lys-D-Ala-D-Glu Ac2-L-Lys-D-Ala-D-Glu Oce-SUC2-L-Lys-D-Ala-D-Glu Ac-Gly-D-Ala-D-Glu Ac-D-Ala-D-Asp
Myristoyl-D-Ala-D-Asp
Ac-racemic-cyclodiaminoadipic
acidAc-meso-cyclodiaminoadipic
acidGlutaryl-racemic-cyclodiaminoadipic
acidAc2-L-Lys-racemic-cyclodiaminoadipic
acid D-CycloserineSuc-D-cycloserine
Suc-D-Ala-D-cycloserine*
Ac-D-Ala-D-cycloserineAc2-L-Lys-D-Ala-D-cycloserine
cx-Ac-L-Lys-D-Glu-D-Ala Ac2-L-Lys-D-Glu-D-AlaActivity
assubstrate(%)
100 0 2-8 0 0 0 100 0 0 0 0 0 0 0 0 0 0 0 0 3.5 0 0 Inhibition 0 10-20 50 88 52 45 88-96 84 59 70 52 0 17 43 0 0 88-100 8 59 67 87 Molarratio inhibitor/substrate 10 10 10 11.5 10 10 12 11 11 17 8.6 11 11 10 10 10 6 5 11.6 14*For discussion of the
purity
of thispeptide see the text.Table 3. Kinetics of hydrolysis
of
peptides withdifferent side chains at residue 3 by DD-carboxypeptidase fromS.albus G
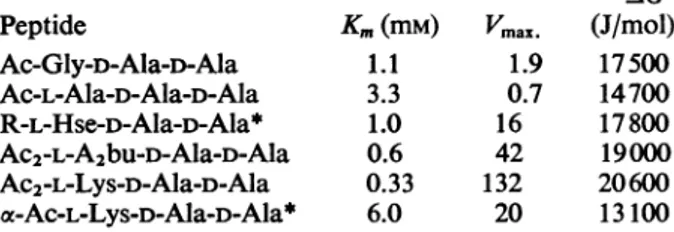
Vmax. is expressedinunitsof,umolof D-Ala-D-Alalinkage cleaved/h per mgofenzyme. Experimentalconditions are as described in Leyh-Bouille et al. (1970) and in the text. AG (Gibbs free energy)=-RTIn(lIKm).
Abbreviations: A2bu, 2,4-diaminobutyric acid; R, UDP-MurNAc-Gly-D-isoGlu. Peptide Ac-Gly-D-Ala-D-Ala Ac-L-Ala-D-Ala-D-Ala R-L-Hse-D-Ala-D-Ala* Ac2-L-A2bu-D-Ala-D-Ala Ac2-L-Lys-D-Ala-D-Ala a-Ac-L-Lys-D-Ala-D-Ala* -AG Km(mM) Vmax. (J/mol) 1.1 1.9 17500 3.3 0.7 14700 1.0 16 17800 0.6 42 19000 0.33 132 20600 6.0 20 13100
*Taken from Leyh-Bouille et
al.
(1970). The value ofVmax.
reported there for Ac2-L-Lys-D-Ala-D-Ala was slightly smaller (100).
chain of very definite molecular characteristics is
requiredinresidue 3toinduceenzymic action of the
moleculesboundtothe enzyme. Totesttheseviews the Km and Vmax. values fora series of tripeptides
differing in the side chain at residue 3 were
deter-mined (Table 3).Ifas afirstapproximationweaccept
the Michaelis constant Km as the dissociation con-stantof theenzyme-peptide complex, it isclear that
although residue 3 makessomecontribution tothe binding,mostof the freeenthalpy (Gibbs free energy)
ofbinding issupplied bythe interaction ofresidues
1 and2. Onthe other hand, anuncharged aliphatic sidechaininresidue 3longer thanonecarbonatom
inducesstrikingincreases intheVmax.value. Positive
charges in this position decrease both -AG and
Vmax. (ae-Ac-L-Lys-D-Ala-D-Ala). Negative chargesin thesamepositiondonotpreventpeptidesfrom bind-ingbut greatly decrease enzymic activityand hence
presumably thevalueof Vmax.. Thus
'cE-Suc2-L-Lys-D-Ala-D-Alais apoorer substrate than the diacetyl peptide, and whereasoe-Ac2-L-Lys-D-Ala-D-Glu isa good substrate the
corresponding
disuccinyl-peptide is nolongerasubstrate butaneffective inhibitor. Itis, ofcourse, difficulttodecide whethertheeffect of succinylgroupsis duetotheir negative charge or to theincreaseinthelengthofthe sidechainatresidue
3.The valuesforKmand Vmax. for Ac-L-Ala-D-Ala-D-Ala are slightly anomalous, without any obvious explanation.
The lactam ofdiaminoadipic acid (cyclodiamino-adipic acidinTable2)wassynthesizedas ananalogue
ofthe Ala-Alapeptideinwhich thetwomethylside chains have been linked covalently. Racemic-cyclo-diaminoadipicacid wouldcorrespondto amixture of L-Ala-L-Ala and D-Ala-D-Ala and
meso-cyclodi-aminoadipic
acid to amixture of D-Ala-L-Ala andL-Ala-D-Ala.Peptides with thetwolatterC-terminal
sequences are neither substrates nor inhibitors for the streptomyces enzymes(Leyh-Bouilleetal., 1970,
1971). Ring formation makes it obligatory for the
peptide bondtobe cis in allcases.
As could be predicted theacetyl-meso-compound
doesnot bindto the enzyme, whereas the
racemic-compound does, although presumably only the DD-isomer(Table 2). The mixturedesignated as SUC-D-Ala-D-cycloserine is the most inhibitory of the
peptides
listed in Table 2,and thatisthereasonwhy it is reported although the inhibitory compound(s)wasnotcharacterized.
Inhibition ofthe
DD-carboxypeptidasefrom
strain R61 Thecarboxypeptidase from strainR61 is inhibitedby penicillins or cephalosporins, andthisinhibition is kinetically competitive (Leyh-Bouille et al., 1971). As shown in Table 4 itis alsoinhibited by peptide analogues of its standard substrate AC2-L-LyS-D-Ala-D-Ala. The kinetics of the inhibition of the enzymeby Ac-D-Ala-D-Asp havebeen studied. The
inhibitionis competitive, witha Kgvalueof 3.2mM. Ingeneralthebehaviour of all the peptideinhibitors ontheactivityofcarboxypeptidases fromS. strains
albus G and R61 is similar, although inhibition of the latter isusually smaller. This can be correlated with the high Km values reported by Leyh-Bouille etal.(1971).Aswith the enzymefromstrain albus G,
residues 1 and2 appear to bethe ones mainly con-cerned with thebindingtothe enzyme, whereas size,
shapeandchargeonresidue3 appear tobe decisive forenzymic activity. Also,aswith the enzyme from strain albus G, Ac-racemic-cyclodiaminoadipic acid is aninhibitor ofcarboxypeptidasefrom strain R61.
Table 4. Activity of the DD-carboxypeptidase from Streptomyces strain R61 in the presence ofpeptide inhibitors Conditions were as described inTable 2 except that the buffer was0.01M-tris-HCI, pH7.5. The following compounds wereneither substrates nor inhibitors at a concentration of greater than 10 times that of the standard substrate: Suc-D-Ala-D-Glu; o-Ac-L-Lys-D-Ala-D-Glu; Ac-meso-cyclodiaminoadipic acid; Ac2-L-Lys-racemic-cyclodiaminoadipic acid; D-cycloserine; Suc-D-cycloserine; Ac-D-Ala-D-cycloserine; Suc-D-Ala-D-cycloserine. When D-glutamic acidwas liberated experiments were repeated in sodium phosphate buffer (0.01 M, pH8) to avoid confusion betweenDnp-glutamic acid andDnp-Tris.
Peptide Ac2-L-Lys-D-Ala-D-Ala Ac-D-Ala-D-Ala SUC2-L-LyS-D-Ala-D-Ala* Ac-D-Ala-D-Glu Ac2-L-Lys-D-Ala-D-Glu Suc2-L-Lys-D-Ala-D-Glu Ac-Gly-D-Ala-D-Glu Ac-D-Ala-D-Asp Ac-racemic-cyclodiaminoadipic acid Ac2-L-Lys-D-Ala-D-cycloserine oc-Ac-L-Lys-D-Glu-D-Ala Ac2-L-Lys-D-Glu-D-Ala Activity assubstrate
(%)
100 1 16 0 10 0 0 0 0 16 0 0%
Molarratio Inhibition inhibitor/substrate 9 43 25 32-46 25 38 26 43 33-35 34-36 10 12 10 10 10 10 17 5 11.6 14*Inthis experimentthesubstrate was
Ac2-L-Lys-D-Ala-D-[tC]Ala
and hence alanine liberated fromitwas measured as radioactivityand couldbe distinguished from alanineliberated from theinhibitor. Table 5. Activity
of
the DD-carboxypeptidasefrom
Streptomyces strain R39onseveralpeptides
Peptide (200nmol) was incubated with the enzyme (150 units; Leyh-Bouille et al., 1972) inTris-HCl
buffer (0.03M) containing 3mM-MgCl2 at pH7.5; final volume
30,ul.
Activity is expressed as a per-centage of thatonthe standardsubstrate, AC2-L-Lys-D-Ala-D-Ala. Thefollowing compoundswereneithersubstratesnorinhibitors for theDD-carboxypeptidase fromstrain R39; Ac-D-Ala-D-Asp;Suc-D-Ala-D-Glu; phenylacetyl-D-Ala-D-Glu; L-Lys-D-Glu-D-Ala;
a-Ac-L-Lys-D-Glu-D-Ala; Ac2-L-Lys-D-Glu-D-Ala; D-Ala-D-cycloserine; D-cycloserine;
Ac-D-Ala-D-cyclo-serine; Suc-D-Ala-D-cycloAc-D-Ala-D-cyclo-serine; racemic-cyclodi-aminoadipic acid;
Ac2-L-Lys-racemic-cyclodiamino-adipic
acid. Peptide Ac-D-Ala-D-Ala Suc2-L-Lys-D-Ala-D-Ala Ac-D-Ala-D-Glu x-Ac-L-Lys-D-Ala-D-Glu Ac2-L-Lys-D-Ala-D-GluSUC2-L-Lys-D-Ala-D-Glu
Activity(/)
5 36 4 110 67 5Assumingthatonly the DD-isomer is inhibitory, the cyclic peptide would be about three timesasgoodan
inhibitorasAc-D-Ala-D-Ala.
DD-Carboxypeptidase from
strain R39. The DD-carboxypeptidase from strain R39 is inhibited by penicillin in a kinetically non-competitive manner. Noneof thepeptide inhibitors ofthe enzymes fromstrains R61 and albus G had any effecton the DD-carboxypeptidase from strain R39. Infactsome of
themweregood substratesfor this enzyme
(Table
5). DD-Carboxypeptidase inhibitors as antibiotics. Thepeptide
inhibitors listedinTable2aswellas benzyl-oxycarbonyl-D-Ala-D-Glu, benzyloxycarbonylD-Ala-D-Asp, phenylacetyl-D-Ala-D-Glu, and benzyl-oxycarbonyl-D-Ala-D-cycloserineweretestedas anti-bioticsagainst S. strain albus Gbythe hole-in-the-plate method
(Perkins,
1969), eachbeingpresentasS0,ul
ofapprox. 13mM solution. Inaddition,
AC-D-Ala-D-Asp was testedagainst
S. strain R61. None of themwasactive.Discussion
Mechanism of the action
Of
DD-carboxypeptidasesfrom strains S. albus G and R61
ThebindingofAc-DD-cyclodiaminoadipic acidto theDD-carboxypeptidases from strainsalbus G and R61 is stronger than that of Ac-D-Ala-D-Ala, as judged by the inhibition caused byboth of them.It seemsreasonabletohypothesize from thisfact that thesecarboxypeptidases combine only with
peptides
having a cisconfiguration in the C-terminal amidebond. Although the free peptides in solution have most likely a predominantly trans configuration in
all their amide linkages, the combination with the enzyme can provide enough freeenthalpytoalter the
normal distribution of the isomers. The peptide
XE-Ac2-L-Lys-D-Ala-D-Ala is a very good substrate for enzymes from strains R61 and albus G, but
ocE-Ac2-L-Lys-racemic-cyclodiaminoadipic acid is not a sub-strate atall, i.e.the amide linkage in the ring is not
cleavedby theenzymes. This can beaccounted for if we assume thatafterthe initialbindingofthe
pep-tide to the enzyme occurs, the amide bond to be broken is forced by the enzyme to adopt a
con-figurationintermediate between cis and trans. For a
normalpeptide this involves overcoming an energy barrier of about 30-4OkJ(Scheliman & Schellman, 1964), but for the cyclic peptide the energy involved
would beso muchhigher, owingto therestrictions imposed bythe ring, thathydrolysiswould not take place.
A simplifiedsequence of events leading to hydro-lysis ofpeptide substrates by the DD-carboxypeptid-ases from strains albus G and R61 can be imagined
as follows.
(a) The enzymes bind the molecules of peptide thathave a cisconfigurationintheC-terminal amide
linkage. The interaction with the enzyme of the residues 1 and 2 would supply most of the free enthalpyofbinding.
(b) If thesize, shapeandcharge of the side chain atresidue3wereappropriate, it would inducea
con-formational change in the enzyme, which,inturn,
would result in the previously cis-amide linkage
adopting
a configuration intermediate between cisandtrans, thuslosingalldouble-bond character. The
conformationalchangeinthe enzyme does not need tobe aspectacularone, buttogether withthe inter-actionsbetween enzyme groupsandpeptidesubstrate
it hastosupplyanenergyofsome30-40kJ.
(c)
The active intermediate of the previous stepwould break down into an acyl-peptide and the
terminal aminoacid.Thisfinalreaction isprobably madeupofmorethanone step, assuggestedbythe fact that transpeptidation can occur with enzyme
from strain R61 if supplied with the appropriate carboxylacceptor(Pollocketal.,1972).The
proposed
mechanism does not necessarily apply to all DD-carboxypeptidases, since the enzyme from strainR39 was not inhibited by any of the analogues examined.
Lee (1971), in attempting to addsupportto the
'structure analogue' hypothesis for the mode of action ofpenicillin (Tipper&Strominger,1965), has proposed thattranspeptidasesmight bindmaximally
to acyl-D-Ala-D-Ala peptides when the latter have
theirC-terminalamide bond suitably distorted so as tolose most of itsdouble-bondcharacter. In contrast, weproposethat theenzymewould simply select the
small population of cis isomer and thus displace the configuration equilibrium. Distortion of the amide bond would then only occur once the substrate was bound to the enzyme. The corresponding change in the enzyme would only occur in the presence of bound substrate. Thus, accordingtoour proposal, the free enzymeneed notnecessarilyhave any affinity for the
transitionstate of the substrate.
Role of DD-carboxypeptidases as transpeptidases The work of Pollock et al. (1972) has shown that the DD-carboxypeptidases of Streptomyces strains
R61 and R39 can function as transpeptidases in vitro,and further,thistranspeptidasereactionwasas sensitive to penicillin as the carboxypeptidase re-action. It is evident that, if suchatranspeptidase is indeed the enzyme involved in the cross-linking of nascentmucopeptide, thenaninhibitorof the enzyme
should also be an antibiotic. Some of the peptides describedinthe present paperweregood inhibitors
ofthe DD-carboxypeptidase activity of the enzyme from S. strain albus G and poorer inhibitors of that from strain R61, but the same peptides did not inhibit growth of the parent organisms on agar plates. So far asstrain albus Gis concerned, it has not been possible to demonstrate transpeptidation
with theisolated enzymeandany acceptor(Pollock etal., 1972; H. R. Perkins, M. Nieto, J. M. Frere, M. Leyh-Bouille & J. M. Ghuysen, unpublished work), so thatin thiscase thetranspeptidase func-tioning in vivo may well have a different inhibitor
spectrum. TheDD-carboxypeptidase from strainR61 wasinhibited less than50%byahigh concentration
ofAc-D-Ala-D-Asp,andsoperhaps itisnotsurprising
thatgrowth of cellsshowednoinhibitionbythesame substance,evenifitwereabletoreach the
appropriate
region of the cell.Wethank Mr. I. D. Bird and Mr. C. S. Gilbert for excellent technical assistance. The work in Liege was
supported by grants from the Fonds de la Recherche Fondamentale Collective, Brussels (no. 1000) and the Institut pourl'Encouragementdela RechercheScientifique dans l'Industrieetl'Agriculture,Brussels(nos. 1699and 2013)toJ.-M. G.
References
Anderson, G. W., Zimmerman, J. E. & Callahan, F. M. (1964)J. Amer. Chem. Soc. 86, 1839-1842
Ghuysen, J.-M., Leyh-Bouille, M., Bonaly, R., Nieto, M., Perkins, H. R., Schleifer,K. H. &Kandler, 0. (1970) Biochemistry 9, 2955-2961
Greenstein, J. P. &Winitz, M. (1961) Chemistry of the
Amino Acids, John Wiley and Sons Inc., New York and London
Leyh-Bouille, M., Ghuysen, J.-M., Bonaly, R., Nieto, M., Perkins, H. R., Schleifer, K. H. & Kandler, 0. (1970) Biochemistry 9, 2961-2970
Leyh-Bouille, M., Coyette, J., Ghuysen, J.-M., Idczak, J., Perkins, H. R. & Nieto, M. (1971) Biochemistry 10, 2163-2170
Leyh-Bouille, M., Nakel, M., Frere, J.-M., Johnson, K., Ghuysen, J.-M., Nieto, M. & Perkins, H. R. (1972) Biochemistry 11, 1290-1298
Nieto, M. & Perkins, H. R. (1971) Biochem. J. 123, 789-803
Perkins, H. R.(1965) Nature(London) 208, 872-873 Perkins, H. R. (1969) Biochem. J. 111, 195-205
Perkins,H.R. &Cummins, C. S.(1964)Nature(London) 201,1105-1107
Pollock, J. J., Ghuysen, J.-M., Linder, R., Salton, M. R. J.,Perkins, H. R., Nieto, M., Leyh-Bouille, M., Fr6re, J.-M. & Johnson, K. (1972)Proc. Nat. Acad. Sci. U.S. 69, 662-666
Rhuland, L. E., Work, E., Denman, R. F. & Hoare, D. S. (1955) J. Amer. Chem. Soc. 77, 4844 4846
Rydon, H. N. & Smith, P. W. G. (1952) Nature(London) 169, 922-923
Schellman, J. A. & Schellman, C. (1964)Proteins, 2nd edn., 1, 1-137
Schroder, E., Klieger, E. & Gibian, H. (1961) Justus Liebigs Ann. Chem. 646, 101-118
Tipper, D. J. & Strominger, J. L.(1965)Proc.Nat.Acad. Sci. U.S.54, 1133-1141
Work,E.(1957)Biochem. J.67, 416-423