Open Archive TOULOUSE Archive Ouverte (OATAO)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
This is an author-deposited version published in :
http://oatao.univ-toulouse.fr/
Eprints ID : 11013
To link to this article : DOI : 10.1016/j.nucengdes.2013.11.085
URL :
http://dx.doi.org/10.1016/j.nucengdes.2013.11.085
To cite this version :
Bertron, Alexandra and Jacquemet, Nicolas
and Erable, Benjamin and Sablayrolles, Caroline and Escadeillas,
Gilles and Albrecht, Achim Reactivity of nitrate and organic acids
at the concrete–bitumen interface of a nuclear waste repository cell.
(2014) Nuclear Engineering and Design, vol. 268 . pp. 51-57. ISSN
0029-5493
Any correspondance concerning this service should be sent to the repository
administrator:
staff-oatao@listes-diff.inp-toulouse.fr
Reactivity
of
nitrate
and
organic
acids
at
the
concrete–bitumen
interface
of
a
nuclear
waste
repository
cell
A.
Bertron
a,∗,
N.
Jacquemet
a,
B.
Erable
b,
C.
Sablayrolles
c,d,
G.
Escadeillas
a,
A.
Albrecht
eaUniversitédeToulouse;UPS,INSA;LMDC(LaboratoireMatériauxetDurabilitédesConstructions);135,avenuedeRangueil;F-31077;ToulouseCedex04,
France
bUniversitédeToulouse;INPT,UPS;CNRS;LaboratoiredeGénieChimique;4,AlléeEmileMonso,F-31030Toulouse,France
cUniversitédeToulouse;INP;LCA(LaboratoiredeChimieAgro-Industrielle);ENSIACET,4alléeEmileMonso,BP44362,31432ToulouseCedex4,France dINRA;LCA(LaboratoiredeChimieAgro-Industrielle),F-31029Toulouse,France
eAndra,1-7,rueJean-Monnet,92298Châtenay-Malabry,France
h
i
g
h
l
i
g
h
t
s
•Interactionsofcementpasteandorganicacid–nitratesolutionswereinvestigated.
•CementleachingimposedalkalinepH(>10)veryrapidlyintheliquidmedia.
•Aceticacidactiononcementpastewassimilartothatofclassicalleaching.
•OxalicacidattackformedCa-oxalatesalts;organicmatterinsolutiondecreased.
•Nitratewasstableunderabioticconditionsandwithorganicmatter.
a
b
s
t
r
a
c
t
Thisstudyinvestigatesthefateofnitrateandorganicacidsatthebitumen–concreteinterfacewithin repositorycellforlong-lived,intermediate-level,radioactivewastes.Theinterfacewassimulatedbya multiphasesysteminwhichcementitiousmatrices(CEMVcementpastespecimens)wereexposedto bitumenmodelleachatesconsistingofnitratesandaceticacidwithandwithoutoxalicacid,chemical compoundslikelytobereleasedbybitumen.Leachingexperimentswereconductedwithdailyrenewalof thesolutionsinordertoacceleratereactions.Theconcentrationsofanions(acetate,oxalate,nitrate,and nitrite)andcations(calcium,potassium)andthepHweremonitoredovertime.Mineralogicalchanges ofthecementitiousmatriceswereanalysedbyXRD.Theresultsconfirmedthestabilityofnitratesinthe abioticconditionsoftheexperiments.Theactionofaceticacidonthecementitiousmatrixwassimilarto thatofordinaryleachingintheabsenceoforganicacids(i.e.carriedoutwithwaterorstrongacids);no specificinteractionwasdetectedbetweenacetateandcementitiouscations.Thereactionofoxalicacid withthecementitiousphasesledtotheprecipitationofcalciumoxalatesaltsintheouterlayerofthe matrix.Theconcentrationofoxalatewasreducedby65%insidetheleachingmedium.
1. Introduction
InFrance,asignificantfractionoflong-lived,intermediate-level, radioactivewastes (bituminous wastes) consist ofa mixture of inorganicsaltsimmobilisedinabitumenmatrix.Theyarepoured incylindricalsteelcontainerscalledprimarypackages. Depend-ing onthedisposal concept, thesecontainers are groupedinto cuboidreinforcedconcreteoverpacks.Thesesecondarypackages
∗ Correspondingauthorat:LMDCINSAUPSGénieCivil,135avdeRangueil,31077 ToulouseCedex4,France.Tel.:+33561559931;fax:+33561559949.
E-mailaddresses:bertron@insa-toulouse.fr,alexandra.bertron@insa-toulouse.fr
(A.Bertron).
arethenplacedinsidewastecellsbuiltatadepthof450–550m within a Callovo-Oxfordian clay rock formation, using signifi-cantamountsofadditionalconcrete(i.e.lining,shotcrete)(Andra, 2005).
Afterclosureofthecells,waterresaturationthatprobablyfills thewastecellsfromthebottomup(bathtubeffect),reaching near-saturationwithinafewthousandyears,shouldenhancetherelease ofchemicalspecies.Amongthecompoundsprobablyreleasedinto theinterstitialaqueousmediumaresolublesalts(notablynitrates) andorganicmattersuchasorganicacids,phenols,etc.contained withinthebituminousmatrix(Walczaketal.,2001),togetherwith gas(mostlyH2)producedviaradiolysisoforganicmatterand/or
anaerobicsteelcorrosion(blackandstainlesssteelpresentin pri-marycontainersandconcretereinforcement).
Table1
CEMV/Acementcomposition.l.o.i.:lossonignition.
CaO SiO2 Al2O3 Fe2O3 MgO TiO2 Na2O K2O MnO SO3 l.o.i.
CEMV/A42.5N 46.4 30.0 11.2 3.6 2.8 0.6 0.2 1.2 0.1 2.8 2.1
Thepresenceofnitratesinthevicinityofwastepackagesmay resultinoxidisingconditionsfavourabletothemobilityofaseries ofradionuclides(Se,U,Tc,Pu,Np,etc.)Albrechtetal.(2013). How-ever,inthegeochemicalconditionsprevailinginthecell,different redoxreactionsarelikelytooccur,includingnitratereduction driv-ingthesystembacktoareducingenvironmentfavourabletosafe repository.Reductionofnitrate(NO3−)mayoccur(i)fromabiotic
processes,withironfromthesteeland/orH2 actingaselectron
donors,andsurface catalysisprovidedby thedifferenttypesof steelandcorrosionproductspresentinthewastecell,and/or(ii) frombiologicalcatalysisthroughbacterialactivity.Thereactions mayleadtotheformationofnitrite(NO2−),gaseousnitrogen(N2)
and/orammonium(NH4+),dependingonavarietyofparameters
notyetwellunderstood,particularlyinconcrete-dominated sys-tems(Devlinetal.,2000;Trucheetal.,2013a,b;Alquieretal.,2014; Libertetal.,2011; Bertronetal.,2013).Bothtypesofreactions (abioticandbiotic)involveelectrondonorsandnumerous inor-ganicandorganiccandidatesareavailableinthewastecellorin thehostrock(organicacids,H2,zero-valentmetals,etc.)Albrecht
etal.(2013).Allreactionsoccurinanenvironmentinfluencedby thealkalineconditionsimposedbytheconcretephases.Theoverall project,ofwhichthispaperisapart,aimstoinvestigatethe reduc-tionofnitrateswithinasystemcomparabletoa“real”waste-cell wherebacterialactivityislikelytooccur(denitrifyingalkaliphilic bacteria)andnotablytodeterminethephenomenologyand kinet-icsofreactionsandtheroleoftheelectrondonor(i.e.organicacids releasedbybitumen,suchasaceticoroxalicacids)inthereactions (Alquieretal.,2012,2013,2014).
However,previousstudieshaveshownthatstronginteractions occurbetweencementitiousmaterialsandorganicacids(Bertron etal.,2005a,b;Bertronetal.,2007;Larreur-Cayoletal.,2011a,b; OueslatiandDuchesne,2012;BertronandDuchesne,2013).These reactionscouldmodifynotonlythebioavailabilityoforganic mat-ter – and thus influence thereactivity of nitrates under biotic conditions–butalsothecompositionoftheconcreteanditsability tobufferthechemicalsystem(i.e.pH).
An important prerequisite was therefore to investigate the interactionsoforganicacidswiththecementitiousmatrixinthe presenceofnitratesunderaerobicconditionsandwithoutbacteria. Reactionsandtherelatedequilibriaofmultiphasesystems repre-sentativeofthebitumen–concreteinterfacewereinvestigatedin simplifiedbatchsystemsandinshorttermexperiments.CEMV pastespecimenswereimmersedinsolutionsmadeofaceticand/or oxalicacidsandnitratesthatsimulatedbitumenmodelleachates. Changesinthecementpastesandliquidphasecompositionswere investigated.
2. Materialsandmethods 2.1. Materials
2.1.1. Cementpastes
ThespecimenswereCEMV/A42.5(S-V)NCEPM-ES-CP1cement pastes(slagandflyashcementfromtheAirvaultCalciafactory, chemicalcompositiongiveninTable1)withawater/cementratio of0.40.Theywerecastincylindricalplasticmoulds50mmhighand 27mmindiameter(volume=28.6cm3andsurfacearea=53.9cm2)
withoutdemouldingoilandwerevibratedtoevacuateairvoids. Thespecimensweretakenoutoftheirmoulds24hafterpouring andstoredinwaterat20◦Cfor28days.Theywerethensubjectedto
theleachingtestsdescribedbelow.Inparallel,somecontrol speci-menswerekeptinwaterat20◦C.
2.1.2. Bitumenmodelleachatesolutions
Uncertainties remain on the release of organic and inor-ganicmatterbybitumen–saltmixtures.Experimentalstudieshave shownthatthedegradationofbitumengivesrisetotherelease oforganicmatter(naphthalene,alcohols,linearcarboxylicacids, aromaticsandglycols)andsalts(NaNO3,Na2SO4,etc.)(Walczak
etal.,2001;VanLoonandKopajtic,1990;LibertandWalczak,2000; Nakayamaetal.,2003;Marienetal.,2008;Walczak,2000;Kagawa etal.,2000).
Onthebasisofthisliteraturedata,leachingofbitumenwas sim-ulatedbyaqueoussolutions madeoforganicacidsandnitrates. Linearcarboxylic acids were considered(i) since theyare eas-ily assimilatedby (Ma etal., 2012;Daniel etal., 2007) and(ii) becauseoftheirprobablestronginteractionswithcementphases. Experimentsofbitumendegradationbywateruptakeand/or radi-olysishaveshownthepresenceofacetic,formicandoxalicacids in the leachates (Walczak et al., 2001; Van Loon and Kopajtic, 1990;Walczak,2000;Kagawaetal.,2000).Amongexperimental parametersinfluencingthequantityandnatureofacidsare:pH oftheleachingsolution,irradiation,temperatureanddurationof experimentand presenceor lackofoxygen. Inleaching experi-mentsperformedatambientormoderatetemperature(≤45◦C),
concentrations ofacids of theorder of tenmilligrams perlitre havebeenfoundintheleachates.Oxalicacidwasnotablydetected afterradiolysisofbitumeninthepresenceofoxygen;incoherence withexperimentsyieldingoxalateproductionduringirradiationof formicacidunderoxygen.VanLoonandKopajtic(1990)concluded thattheproductionofoxalateintheabsenceofoxygenwas ques-tionable.Oxygeninitiallypresentinthewastecellsafterclosure willberapidlyconsumedeitherreactingwithcomponentsofthe clayhostrockorwithmetalspresentinthereinforcedconcrete, butitmustbekeptinmindthatthewastecontainerswillbestored forseveraldecadesinanoxygen-bearingatmosphere.
Calciumsaltsofformic andacetic acidsbeinghighlysoluble inwater(LangeandDean,1985),itislikelythatbothacidshave similareffectsonthecementitiousmatrix(BertronandDuchesne, 2013).Incontrast,calciumoxalatesaltsareonlyslightlysolublein water(Seidell,1919;Streitetal.,1998)andarethuslikelytobe producedduringtheattackofcementitiousmatrixbyoxalicacid. Aceticandoxalicacidswerethereforeconsideredinthepresent study.
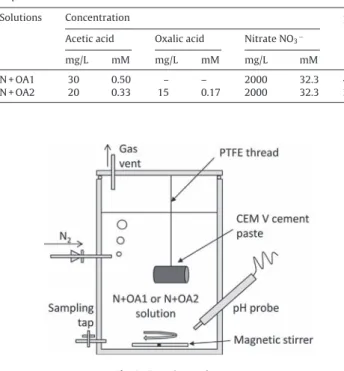
Twotypesofmodelleachatesolutionswereconsidered.Thefirst typewasmadeofaceticacid(0.50mMto30mg/L)andsodium nitrate(32.3mM).TheinitialpHofthissolution,notedasN+OA1, was4.Thesecondtypewasamixofaceticacid(0.33mM),oxalic acid(0.17mM)(sametotalconcentrationofacidsof0.50mM)and sodiumnitrate(32.3mM).ThepHofthissolution,notedasN+OA2, was3.6.Thenitrateconcentration(32.3M)wasonlyanestimateof thelikelyconcentrationinawastecell(resultsofcoupled chemi-calandtransfermodelling)(Sercombeetal.,2006;Gwinneretal., 2006).Table2summarisesthecompositionofthetestsolutions.
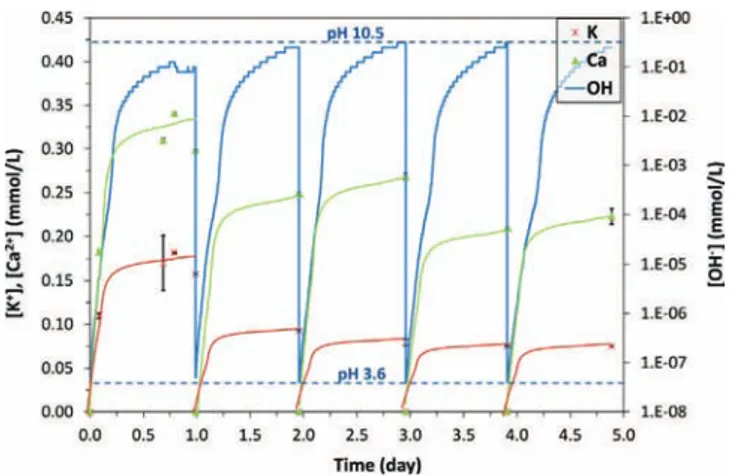
2.2. Experimentalset-upandleachingprocess
TheexperimentaldeviceisshowninFig.1.Batchsystemswere implemented.
Table2
Compositionsofbitumenmodelleachates.
Solutions Concentration pH
Aceticacid Oxalicacid NitrateNO3−
mg/L mM mg/L mM mg/L mM
N+OA1 30 0.50 – – 2000 32.3 4.0
N+OA2 20 0.33 15 0.17 2000 32.3 3.6
Fig.1.Experimentalset-up.
Thereactor,filledwith1Lofnitrateandorganicacidsolution (N+OA1orN+OA2)wasequippedwithanoutletforsolution sam-pling,agasinletwithcheckvalve(usedforN2bubblingtoimpose
anoxicconditionsinsidethereactorsimilartothosethatwill pre-vailinsidearepositorycell),agastighttapforapHprobe,anda hermeticallyclosedlidfittedwithagasvent.
ThepHprobewasconnectedtoadataacquisitionsystem (Con-sort,D230DataAcquisitionSystem,v1.1.13).Thesolutioninthe reactor was continuously stirred using a magnetic barrel. The cementpastespecimenwassuspendedinthesolutionbyaPTFE thread. The solid/liquid volume ratio wasabout 3% (similar to thatimplementedin thebiotic partof thestudy(Alquieretal., 2012)).Theexperimentaldevicewaskeptinanair-conditioned room(20◦C)duringthewholeexperiment.
N+OA1 (acetic acid 0.5mM; NO3− 32.23mM) and N+OA2
(acetic0.33mMandoxalic0.17mMacids;NO3−32.23mM)
solu-tionswerereneweddailyfor5days.Theseshorttermexperiments werechosenbecausethekeyphenomenaimpactingbacterial reac-tion(exploredinthebioticpartoftheoverallstudyofwhichthis paper is partof (Alquier et al., 2013,2014)) occurin the very shorttermoftheexperiment(becauseoftheverysevere condi-tionsrapidlyimposedbythecementitiousmaterialsintheliquid medium).Moreover,itshouldbenotedthattheseshortterm leach-ingexperimentswerefavouredwiththeviewtolimittheseverityof themediaandensuremicrobialgrowth(andthusmicrobial activ-ity)inthecorrespondingbiotictesting(Alquieretal.,2013,2014).
Table4
Detectionandquantificationlimits(DLandQL)ofions(mmol/L)(S/N=Signal/Noise). Acetate Oxalate Nitrite Nitrate Potassium Calcium DL(3<S/N) 0.68 0.79 1.74 2.42 0.402 0.764 QL(10<S/N) 2.04 2.61 6.09 8.06 1.339 2.546
Duringthefirstdayofexposure,thesolutionwassampled4times (20–25mLeachsample).Thesolid/liquidratiochangedverylittle. Ondays2–5,aliquidsamplewastakenfromthesolutionjustbefore renewal.ConcentrationsofCa,K,acetate,nitrateandnitritewere measuredoneachsample.
Controlexperimentswerecarriedouttoinvestigatethe possi-blesorptionofnitratesonthecementpasteinthesameconditions (NO3−32.3mM,solid/liquidratio3%,durationoftheexperiment:
15dayswithnorenewalofthesolution).Nodecreaseofnitrate concentrationwasobservedinthesolution.Moreover,the cemen-titiousspecimenswererinsedthreetimesandtherinsingsolutions wereanalysedbyHPICtodeterminenitrateandnitrite concentra-tion.Theconcentrationswerelowerthanthedetectionlimitsofthe equipment(Table3).
2.3. Analyticalmethods
2.3.1. Methodsfordeterminingionconcentrationsofsolutions Concentrationsofanions(acetate,oxalate,nitrateandnitrite) andofcations(calciumandpotassium)weremeasuredbyHigh PerformanceIonChromatography(HPIC)coupledtoa conducti-metricdetectorfittedwithchemicalsuppressor(DionexICS-2000 andICS-3000).TheanalyticalconditionsaresummarisedinTable3. Quantificationwasperformedbyexternalcalibrationoverthe concentration range 1–40mg/L witha regression coefficient of 0.999calculatedfrompeakareas.Repeatabilitywasassessedon 5injectionsandwasbetterthan98%.Biaserrorwasbetween5and 10%.Thedetectionlimit(definedasinjectedquantitygivinga sig-nal/noiseratioof3)andquantificationlimit(definedasinjected quantitygivingasignal/noiseratioof10)werecalculatedforeach compound(Table4).
Liquidsampleswerefilteredat0.2mm(MinisartPES,Fisher Sci-entific)toremovesuspendedsolidmatter.
2.3.2. Mineralogicalanalysesofcementitiousspecimens
To explore the mineralogical changes, cement paste sam-pleswereanalysedbyX-Raydiffraction(SiemensD5000;copper cathode;anodevoltage40kV;currentstrength30mA)afterthe leachingtests.Themeasurementswereperformedaccordingtothe distanceinwardsfromtheplanecylindersurfaceincontactwiththe solutions.Thefirstanalysiswasmadeontheplaneexternalfaceof thespecimen,whichwasthenabradedwithapolishingdiscand subjectedtothenextanalysis.Thedepthofanalysiswasmeasured usingacallipersquare.Thelastanalysis,whichwasonthecoreof thespecimen,wascarriedoutatadepthof5mm(afterthe speci-menhadbeensawnperpendicularlytoitsaxis).Acontrolspecimen wasalsoanalysed5weeksafterpouring.
Table3
HPICtestconditions.
Eluant(1mL/min) Precolumn Chromatographic
column
Suppressor
Anions KOH(1×10−3mol/L)
elutiongradient:10%mobilephaseto 60%mobilephasein25min
NG1(4mm×50mm, Dionex)+IonPacAG11-HC (4mm×50mm,Dionex) IonPacAS11-HC (4mm×250mm, Dionex) ASRS300(4mm, Dionex)+CRD200 (4mm,Dionex) Cations Methylsulfonicacid(3010−3mol/L)
isocratic(30min) NG1,(4mm×50mm, Dionex)+IonPacCG16 (4mm×50mm,Dionex) IonPacCS16 (4mm×250mm, Dionex) SuppressorCSRS300 (4mm,Dionex)
Fig.2.Macroscopicobservationofthecementpastespecimens.(a)Control spec-imenkeptinwater,(b)specimenafter5daysofleachingbyN+OA1(aceticacid 0.50mM,nitrate32.3mM)solutionand(c)specimenafter5daysofleachingby N+OA2(acetic0.33mMandoxalic0.17mMacids,nitrate32.3mM)solution.
3. Resultsanddiscussion 3.1. Macroscopicobservations
Thecementitiousmatrix,initiallygrey,hadturnedtoayellowish colourattheendoftheleachingexperiments(Fig.2).Thisis charac-teristicoftheleachingofacementitiousmatrixbyacidicsolutions whereFe(III)ofhydratedandor/anhydrousphasesprecipitatesin theformoforangey-colouredironhydroxide.InCEMV-type anhy-drouscement,ironiscontainedinflyashesandintheC4AF-phase
(brownmillerite)ofclinker.Iron-bearingphasesofcementhydrate moreslowlythansilico–calcicphases.Inahydratedcementitious matrix,ironisfoundinanhydrousresidualphasesandaspartial substitutioninhydrates.TheouterlayerofleachedCEMIandCEM Vpastesisenrichediniron(relativeenrichment)(Bertronetal., 2007;Moudilou,2000;Pavlík,1994).Ironintheouterlayeris prob-ablycontainedinresidualanhydrousflyashes,inC4AF(thisphase
ismoreresistanttoleachingthanotherhydratedandanhydrous phasesofPortlandmatrix(Bertronetal.,2005a)andinthesilica gel,issuedfromleachedcalciumsilicatehydrates(mainhydrated phaseofcementitiousmatrices).
3.2. CompositionandpHofthetestsolutions
Concentrationsof K+ and Ca2+of N+OA1 and N+OA2
solu-tionsincementpasteimmersiontestsareshownversustimein
Figs.3and4.ConcentrationsofOH−ions,calculatedfrom
contin-uousmeasurementofpHarealsorepresented.Concentrationsof
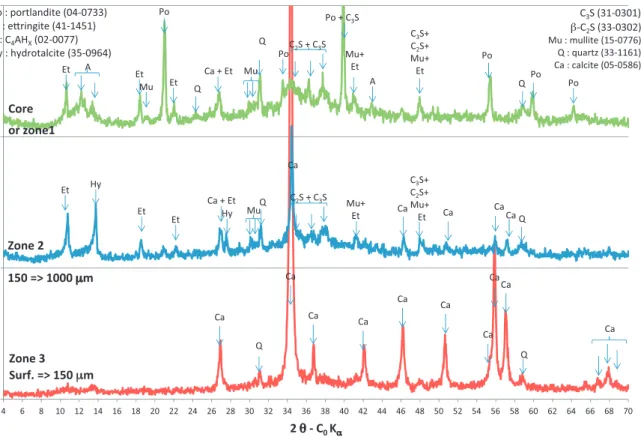
Fig.3. ConcentrationsofK+,Ca2+andOH−inN+OA1(aceticacid0.50mM;nitrate
32.3mM)leachingsolution.Thesolutionwasreneweddaily.Solidarea/liquid vol-ume≈50cm2/L.ConcentrationofOH−wascalculatedfromthepHvalues.Fordays
2–5,variationsofKandCaconcentrationsarebasedonthefinalconcentrationand anevolutionsimilartoday1.
Fig.4.Concentrations ofK+, Ca2+ andOH−
in N+OA2 (acetic 0.33mM/oxalic 0.17mMacids;nitrate32.3mM)leachingsolution.Thesolutionwasreneweddaily. Solidarea/liquidvolume≈50cm2/L.ConcentrationofOH−wascalculatedfromthe
pHvalues.Fordays2–5,variationsofKandCaconcentrationsarebasedonthefinal concentrationandanevolutionsimilartoday1.
nitrate,nitrite,acetateand oxalateofthesolutionsaregiven in
Table5.
ThevariationsofpHweresimilarforthefive24-hcycles:from theinitialvaluesof4.0forN+OA1and3.6forN+OA2,pHincreased rapidlyduringthefirst6hofleaching,toreachabout9.5, then increasedslowly toreach 10.6 in N+OA1 and 10.5 in N+ OA2 after24h(Figs.3and4).LongerreactiontimeswouldallowpHto increaseevenfurther.Alkalineconditionswerethusveryrapidly imposedintheleachingmedium.TheincreaseinpHwasmostly duetothereleaseofhydroxideionsbythedissolvingcementpaste matrix.
Concentrationsof K+ and Ca2+ variedthe sameway.During
thefirstleachingcycle,variationsfollowedthoseofpHand con-centrationsreached[Ca2+]≈0.25mmol/L and[K+]≈0.15mmol/L
in N+OA1 and [Ca2+]≈0.33mmol/L and [K+]≈0.17mmol/L in
N+OA2. For leaching days 2–5, concentrations at the end of thecycle decreased progressivelytoreach [Ca2+]≈0.25mmol/L
and [K+]≈0.09mmol/L in N+OA1and [Ca2+]≈0.22mmol/L and
[K+]≈0.08mmol/LinN+OA2attheendofthe5thleachingcycle.
Concentrations of nitrates in N+OA1 and N+OA2 (Table 5) remainedequivalenttotheinitialvalue(32.3mM).Nitriteswere notdetectedineithersolution.Moreover,cationchromatograms didnot reveal anypeak due toammonium NH4+ produced by
abioticreductionofnitratesunderanoxicconditions.Ammonium hasnotablybeenobservedby(Alquieretal.,2012;Trucheetal., 2013a,b)inthepresenceofvarioustypesofsteelactingas reac-tioncatalysts.Resultsobtainedinthisstudyindicatethatnoabiotic reductionofnitrateoccurredintheconditionsoftheexperiment.
Table5
Evolutionofconcentrations(mmol/L)ofanionsinN+OA1andN+AO2solutions withtimeofcementpasteimmersion.Solutionreneweddaily.Solidarea/liquid volume≈50cm2/L.<DL:lowerthanthedetectionlimit.
Time(day) N+OA1solution N+OA2solution
[Ac] [NO3] [NO2] [Ac] [Ox] [NO3] [NO2]
0.00 0.57 31.6 <DL 0.37 0.17 31.8 <DL 0.08 0.53 31.9 <DL 0.30 0.05 32.1 <DL 0.67 0.62 31.4 <DL 0.26 0.07 31.7 <DL 0.79 0.60 31.7 <DL 0.34 0.07 31.7 <DL 1.00 0.61 31.6 <DL 0.34 0.06 31.8 <DL 2.04 0.55 31.8 <DL 0.32 0.05 31.7 <DL 3.04 0.52 31.7 <DL 0.28 0.06 31.9 <DL 3.99 0.47 31.7 <DL 0.31 0.05 31.7 <DL 4.97 0.56 31.6 <DL 0.30 0.05 31.8 <DL
70 68 66 64 62 60 58 56 54 52 50 48 46 44 42 40 38 36 34 32 30 28 26 24 22 20 18 16 14 12 10 8 6 4
2
θ
-
C
0K
α CaCore
or zone1
Zone 2
150 => 1000
μm
Ca Ca Ca Ca Ca Ca Ca Ca Ca Et A Po Et Et Po : portlandite (04-0733) Et : e=ringite (41-1451) A : C4AHX(02-0077) Hy : hydrotalcite (35-0964) Po + C3S Po Po Po Ca + Et Po C2S + C3S Mu+ Et C3S+ C2S+ Mu+ Et Q C3S (31-0301) β-C2S (33-0302) Mu : mullite (15-0776) Q : quartz (33-1161) Ca : calcite (05-0586) Mu Mu Q A Q Q Q Et Et Et Ca Ca + Et Mu+ Et C3S+ C2S+ Mu+ Et Ca Ca Q C2S + C3S Ca Ca Hy Mu Q HyZone 3
Surf. => 150
μm
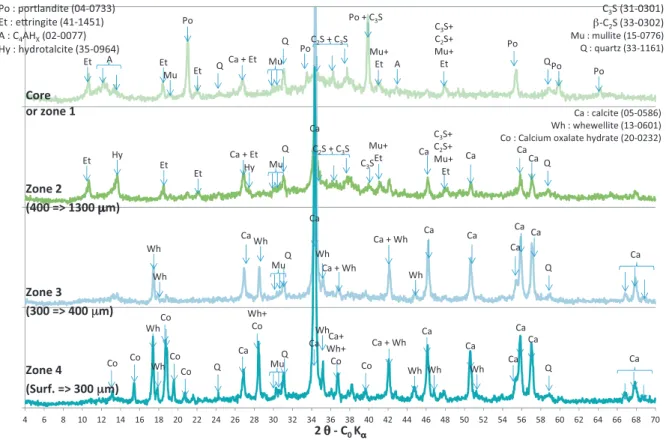
Fig.5. XRDmineralogicalanalysesofCEMVpastespecimenafter5-dayleachinginN+OA1(aceticacid0.5mM,nitrate)solutionatvariousdistancesinfromthesurfaceof thespecimen.
ConcentrationsofacetateinN+OA1andN+OA2werealmost constant throughouttheexperiment and weresimilartoinitial concentrations(Table2).Incontrast,theconcentrationofoxalate, whichwasinitially0.17mmol/L,haddecreasedatthefirst sam-plingtime(0.08day∼2h)andremainedrelativelystable(equalto 0.06±0.01mmol/L)inthefollowingsamples.
3.3. Mineralogicalchangesinthecementpastes
Figs. 5 and 6 present the mineralogical characterisation by XRD ofthe CEM Vpaste specimens atthe end of theleaching experimentsbyN+OA1andN+OA2solutionsrespectively. Diffrac-togramsarepresentedaccordingtothedistancefromthesurfacein contactwiththetestsolution.Theanalysesshowedthatalterations ledtoamineralogicalzonationofthespecimens.
Diffractogramsofcontrolzones(“coreorzone1”onFigs.5and6) performedat5mmdepthpresenttypicalpeaksofanhydrous(C3S
or3CaO·SiO2,C2Sor2CaO·SiO2,mullite3Al2O3·2SiO2)andhydrated
(ettringite 3CaO·Al2O3·3CaSO4·32H2O, portlandite or Ca(OH)2,
hydratedcalciumaluminatesC4AHxor4CaO·Al2O3·×H2O)
cemen-titiousphasesandofquartz.
The peripheral part of cement pastes immersed in N+OA1 (aceticacid)solutionshowedtwozoneswithdifferent mineralog-icalcompositions (zones 2and 3 inFig. 5).Thetotal thickness of thetwo zoneswas about1mm. In zone 2(from 150mmto 1000mm from the surface) portlandite had disappeared and a hydrotalcite-type (Mg4Al2(OH)14·3H2O) compound had
precipi-tated.Precipitation of hydrotalcite(carbonated ornot)hasalso beenobservedbyFauconetal.(1996)indegradedzonesofcement pastespecimensleachedatpH7.Calcitealsoprecipitatedinzone 2,probablybecauseofa re-combinationofcalciumreleasedby portlanditedissolutionwithcarbonatereleasedbythedissolution of carbonatedAFm.Zone 3(from thesurface to150mm deep), wasmorestronglycarbonated.Allcrystallisedcementitiousphases
haddisappeared.Thisthincarbonatedlayerwasalsoobservedon controlspecimenskeptinwater(carbonationofspecimen’sskin becauseofambientCO2 dissolvedin repositorywater).Neither
Ca-acetatenorNa-acetatecharacteristicpeakswerefoundonthe diffractogram.
The peripheral zone of the specimen immersed in N+OA2 (acetic/oxalicacids)solutionshowed3zoneswithdifferent min-eralogicalcharacteristics(zones2–4onFig.6).Thesethreezones togethermadeup atotal a thicknessof approximately1.3mm. As for specimens immersed in N+OA1, portlandite had disap-peared in zone 2 (from 400mm to 1300mm deep) where a hydrotalcite-likecompoundhadprecipitatedtogetherwithcalcite. Zone3(300–400mmdeep)wascharacterisedbyettringite disso-lution.Calciteand calciumoxalatemono-hydrateorwhewellite (CaC2O4·H2O)hadprecipitated.Theouterlayerofthespecimenor
zone4(fromthesurfaceto300mmdeep)showedtypicalpeaks ofcalcite,whewelliteand calciumoxalatetri-hydrateorcaoxite (CaC2O4·3H2O).NeitherCa-orNa-acetatenorNa-oxalatesaltpeaks
werefoundinthediffractograms.
Precipitation of whewellite in cement paste specimens immersed in oxalic acid solutions (0.28M) has been observed experimentallyby Larreur-Cayolet al.(2011a,b).Ca-oxalate tri-hydrate, mentioned by Streit et al. (1998) is not very stable (Deganelloet al., 1981)and is veryeasily transformedinto di-or mono-hydrate Ca-oxalate (Echigo et al., 2005; Tomazic and Nancollas,1979).Nevertheless,theprecipitationofCa-oxalate tri-hydrate jointly with mono-hydrate has already been observed (Heijnenetal.,1985;Opalkoetal.,1997).
Finally,theresultsofcementpastemineralogicalanalyses cou-pled with those of chemical analyses of the solutions showed thattheexposureofCEMVpastespecimenstoN+OA1solution wassimilartoaleachingofcementpaste(BertronandDuchesne, 2013;Fauconetal.,1996;Moranvilleetal.,2004;Duchesneand Bertron,2013).Acetateiondidnotcombinewithcationsreleased
70 68 66 64 62 60 58 56 54 52 50 48 46 44 42 40 38 36 34 32 30 28 26 24 22 20 18 16 14 12 10 8 6 4
2
θ
-
C
0K
αCore
zone 1
or
Et A Po Et Et Po : portlandite (04-0733) Et : e=ringite (41-1451) A : C4AHX(02-0077) Hy : hydrotalcite (35-0964) Po + C3S Po Po Po Ca + Et Po C2S + C3S Mu+ Et C3S+ C2S+ Mu+ Et Q Ca Ca Ca+ Wh+ Co Ca Ca Ca Ca + Wh Ca Ca Q Et Et Et Ca Ca + Et Ca Ca Ca Ca Ca + Wh Ca Ca Ca Ca + Wh Ca Ca Q Ca Wh Wh Wh+ Co Wh Wh Wh Wh Wh Wh Wh Co Co Co Co Co Q Co C3S (31-0301) β-C2S (33-0302) Mu : mullite (15-0776) Q : quartz (33-1161) CaZone 2
=> 1300
(400
μm)
Zone 3
(300 =>
40
0
μm)
Zone 4
(Surf.
=>
30
0
μm)
Wh Wh Q Ca : calcite (05-0586) Wh : whewellite (13-0601) Co : Calcium oxalate hydrate (20-0232)C3S C2S + C3S Ca Ca Hy Mu Mu Mu Mu Mu A Q Q Q Q Q Hy Mu+ Et C3S+ C2S+ Mu+ Et
Fig.6. XRDmineralogicalanalysesofCEMVpastespecimenafter5-dayleachinginN+OA2(acetic0.33mM/oxalic0.17mMacids,nitrate)solutionforvariousdistancesin fromthesurfaceofthespecimen.
bythecementitiousmatrix.Thisresultisinaccordancewiththose obtainedbyotherauthorswhohaveworkedontheattackofacetic acidbutwithdifferentconcentrationsofacids(Bertronetal.,2007; OueslatiandDuchesne,2012;Pavlík,1994).Moreover,itshouldbe notedthatanumericalanalysisbyLarreur-Cayoletal.(2011a,b)
suggestedthatnoCa-acetateaqueouscomplexformsinsolutions withacetateconcentrationsrangingfrom10−5Mto0.3M.Thus,
regardingtheframeofthestudy,thereactionsbetweenaceticacid releasedbybitumenshouldnotmodifyacetatebioavailabilityfor bacteriathatmaybepresentinsidethewastecells.
Incontrast,whenoxalicacidwaspresentinsolution,reactions ofoxalateanionwithcementitiouscations(calciumatleast)did occurandledtotheformationofvariousformsofcalciumoxalate salts.Precipitationofcalciumoxalatesaltinoxalicacidsolution containingcalciumhasbeenconfirmednumericallyby Larreur-Cayol(2012)foralargerangeofoxalateactivity(10−5M–0.3M).
Inthepresentstudyconductedwithoxalicacidconcentrationof 0.17mM,65%ofoxalatewascombinedwithcementitiouscations. Consequently,withrespecttobioticstudiesconductedinthe pres-enceofcementitiousmatrices,oxalatecouldbelessbioavailableto bacteria.
4. Conclusion
The overall study, of which this paper is a part, aimed to investigatethereactivityofnitrateunderbioticconditions(biotic catalysis of nitrate reduction) in the context of repository of long-lived,intermediate-level,radioactivewastes.Theaimofthe presentpaperwastoinvestigatetheinteractionsbetweenthe var-iouschemicalspecies(nitrates,bitumenmodelleachates)andthe cementitiousmatrix underabiotic conditions,withtheviewto determiningthechemicalconditionstowhichbacterialcandidate speciescouldbeexposedinthebioticpartofthestudy.Leaching experimentswereconductedonbatchsystemscomprisingCEM
Vpastespecimensincontactwithsolutionsmadeofnitrateand aceticacidoraceticandoxalicacids(initialpHof4and3.6).Results showedthatanalkalinepH(>10)isveryrapidly(lessthan24h) establishedintheaqueousmedium.Theimpactofbitumenmodel leachatesmadeofaceticacid(0.50mM)wassimilartothosefound inclassicalleachingtestsofcementitiousmatrixasnoparticular interactionbetweencementitiouscationsandacetateanionswas observed.Thus,inexperimentsinvolvingbacteriaanda cementi-tiousmatrix,organicmatterwouldremaininsolutionandwould thereforemoreprobablybeavailableforoxidationandasacarbon sourceformicroorganisms.Incontrast,thestudyconductedwith themodelleachatemadeofacetic(0.33mM)andoxalic(0.17mM) acidsshowedastronginteractionbetweenoxalateanionsand cal-ciumcationsreleasedbythecement paste.65% ofoxalatewas combinedwithcalciumtoprecipitateascalciumoxalate mono-andtri-hydratesaltsintheperipheryofthematrix.Oxalatewould thusbelessavailableforbacteria.Finally,thestudyconfirmedthe stabilityofnitratesunderabioticconditionsinthepresenceofa cementitiousmatrixandorganicmatter.
Acknowledgements
TheauthorsthankAndra(FrenchNationalRadioactiveWaste ManagementAgency)foritsscientificandfinancialsupport.
References
Albrecht,A.,Bertron,A.,Libert,M.,2013.Microbialcatalysisofredoxreactionsin concretecellsofnuclearwasterepositories:areviewandintroduction.In:Bart, F.,Cau-di-Coumes,C.,Frizon,F.,Lorente,S.(Eds.),Cement-BasedMaterialsfor NuclearWasteStorage.Springer,NewYork,pp.147–159.
Alquier,M.,etal.,2012. Etudesexpérimentalesde laréactivitédesnitrates à l’interfacebitume–eaucimentaire–cimentenconditionsbiotiques,Rapport AndraLGC–LMDC–LCAnoCRPFSTR120031.
Alquier,M.,etal.,2014.Halomonasdesiderataasabacterialmodeltopredictthe possiblebiologicalnitratereductioninconcretecellsofnuclearwastedisposals. J.Environ.Manag.,http://dx.doi.org/10.1016/j.jenvman.2013.10.013. Alquier,M.,etal.,2013.Nitratereducingbacterialactivityinconcretecellsofnuclear
wastedisposal.In:L’Hostis,V.,Gens,R.(Eds.),EPJWebofConferences,vol.56, p.01003.
Andra, 2005. Dossier2005 Argile,Tome Architectureet Gestion dustockage géologique.RapportAndraC.RP.ADP.04.0001B.
Bertron,A.,Duchesne,J.,Escadeillas,G.,2005a.Acceleratedtestsofhardenedcement pastesalterationbyorganicacids:analysisofthepHeffect.Cem.Concr.Res.35 (1),155–166.
Bertron,A.,Duchesne,J.,Escadeillas,G.,2005b.Attackofcementpastesexposedto organicacidsinmanure.Cem.Concr.Compos.27(9–10),898–909.
Bertron,A.,Duchesne,J.,Escadeillas,G.,2007.Degradationofcementpastesby organicacids.Mater.Struct.40(3),341–354.
Bertron,A.,Duchesne,J.,2013.Attackofcementitiousmaterialsbyorganicacidsin agriculturalandagrofoodeffluents.In:Mark,A.,Alexandra,B.,Belie,N.D.(Eds.), PerformanceofCement-BasedMaterialsinAggressiveAqueousEnvironments. RILEMState-of-the-ArtReports.Springer,Netherlands,pp.131–173.
Bertron,A.,Erable,B.,etal.,2013.Catalysebiotiqueetabiotiquedelaréduction desnitratesenmilieualcalindanslecontextedustockageprofonddesdéchets radioactifs.Matériaux&Techniques101(1),104.
Daniel,S.L.,Pilsl,C.,Drake,H.L.,2007.Anaerobicoxalateconsumptionby microor-ganismsinforestsoils.Res.Microbiol.158(3),303–309.
Deganello,S.,Kampf,A.R.,Moore,P.B.,1981.Thecrystalstructureofcalciumoxalate trihydrate:Ca(H2O)3(C2O4).Am.Mineral.66(7–8),859–865.
Devlin,J.F.,Eedy,R.,Butler,B.J.,2000.Theeffectsofelectrondonorandgranulariron onnitratetransformationratesinsedimentsfromamunicipalwatersupply aquifer.J.Contam.Hydrol.46(1–2),81–97.
Duchesne,J.,Bertron,A.,2013.Leachingofcementitiousmaterialsbypurewaterand strongacids(HClandHNO3).In:Alexander,M.,Bertron,A.,DeBelie,N.(Eds.),
PerformanceofCement-BasedMaterialsinAggressiveAqueousEnvironments. RILEMState-of-the-ArtReports.Springer,Netherlands,p.22.
Echigo,T.,etal.,2005.Re-investigationofthecrystalstructureofwhewellite [Ca(C2O4)·H2O]andthedehydrationmechanismofcaoxite[Ca(C2O4)·3H2O].
Mineral.Mag.69(1),77–88.
Faucon,P.,etal.,1996.Leachingofcement:studyofthesurfacelayer.Cem.Concr. Res.26(11),1707–1715.
Gwinner,B.,etal.,2006.Modellingofbituminizedradioactivewasteleaching.Part II:Experimentalvalidation.J.Nucl.Mater.349(1–2),107–118.
Heijnen,W.,Jellinghaus,W.,Klee,W.E.,1985.Calciumoxalatetrihydrateinurinary calculi.Urol.Res.13(6),281–283.
Kagawa,A.,Fukumoto,M.,Kawamura,K.,2000.Influenceofchemicalandradiolytic degradationofbitumenonitsperformancefordisposal.J.Nucl.Sci.Technol.37 (10),934–937.
Lange,N.A.,Dean,J.A.,1985.Lange’sHandbookofChemistry.McGraw-Hill,New York,NY.
Larreur-Cayol, S., (Thèse de Doctorat. Doctoral thesis) 2012. Attaques des matériauxcimentairesparlesacidesorganiquesdeseffluentsagricoleset agro-alimentaires.UniversitéPaulSabatier,Toulouse.
Larreur-Cayol,S.,Bertron,A.,Escadeillas,G.,2011a.Degradationofcement-based materialsbyvariousorganicacidsinagro-industrialwaste-waters.Cem.Concr. Res.41(8),882–892.
Larreur-Cayol,S.,DeWindt,L.,Bertron,A.,Escadeillas,G.,2011b.Deteriorationof cementitiousmaterialsbyorganicacidsinagriculturaleffluents:experiments andmodelling.In:7thInternationalSymposiumonCementBasedMaterialsfor aSustainableAgriculture.CIGRInternationalSymposium,pp.38–46.
Libert,M.,etal.,2011.Molecularhydrogen:anabundantenergysourceforbacterial activityinnuclearwasterepositories.Phys.Chem.EarthPartsA/B/C36(17–18), 1616–1623.
Libert,M.,Walczak,I.,2000.Effectofradio-oxidativeageingandpHontherelease ofsolubleorganicmatterfrombitumen.In:ATALANTE2000Scientificresearch ontheback-endofthefuelcycleforthe21thcentury.Avignon,p.4.
Ma,J.,etal.,2012.AssimilableOrganicCarbon(AOC)insoilwaterextractsusing vibrioharveyiBB721anditsimplicationformicrobialbiomass.PLoSONE7(5), e28519.
Marien,A.,Smets,S.,Li,X.,Valcke,E.,2008.Processesrelatedtothewateruptake byEUROBITUMbituminisedradioactivewaste:Theoreticalconsiderationsand firstexperimentalresults.In:Lee,W.E.,Roberts,J.W.,Hyatt,N.C.,Grimes,R.W. (Eds.),ScientificBasisforNuclearWasteManagementXxxi.MaterialsResearch Society,Warrendale,pp.151–159.
Moranville,M.,Kamali,S.,Guillon,E.,2004.Physicochemicalequilibriaof cement-basedmaterialsinaggressiveenvironments—experimentandmodeling.Cem. Concr.Res.34(9),1569–1578.
Moudilou,E.,(ThèsedeDoctorat.Doctoralthesis)2000.Cinétiquesetmécanismes derelargagedesmétauxlourdsprésentsentracesdanslesmatricescimentaires. Universitéd’Orléans.
Nakayama,S.,Iida,Y.,Nagano,T.,Akimoto,T.,2003.Leachingbehaviorofasimulated bituminizedradioactivewasteformunderdeepgeologicalconditions.J.Nucl. Sci.Technol.40(4),227–237.
Opalko,F.J.,Adair,J.H.,Khan,S.R.,1997.Heterogeneousnucleationofcalciumoxalate trihydrateinartificialurinebyconstantcomposition.J.Cryst.Growth181(4), 410–417.
Oueslati,O.,Duchesne,J.,2012.TheeffectofSCMsandcuringtimeonresistanceof mortarssubjectedtoorganicacids.Cem.Concr.Res.42(1),205–214.
Pavlík,V.,1994.Corrosionofhardenedcementpastebyaceticandnitricacidspart II:formationandchemicalcompositionofthecorrosionproductslayer.Cem. Concr.Res.24(8),1495–1508.
Seidell,A.,1919.SolubilitiesofInorganicandOrganicCompounds:ACompilation ofQuantitativeSolubilityDatafromthePeriodicalLiterature.D.VanNostrand Company.
Sercombe,J.,etal.,2006.Modellingofbituminizedradioactivewasteleaching.Part I:constitutiveequations.J.Nucl.Mater.349(1–2),96–106.
Streit,J.,Tran-Ho,L.-C.,Königsberger,E.,1998.Solubilityofthethreecalciumoxalate hydratesinsodiumchloridesolutionsandurine-likeliquors.Monatsheftefür Chemie/ChemicalMonthly129(12),1225–1236.
Tomazic,B.B.,Nancollas,G.H.,1979.Astudyofthephasetransformationofcalcium oxalatetrihydrate-monohydrate.Invest.Urol.16(5),329–335.
Truche,L.,Berger,G.,Domergue,L.,Albrecht,A.,2013a.Abioticnitratereduction inducedbycarbonsteelandhydrogen:implicationsforenvironmental pro-cessesinwasterepositories.Appl.Geochem.28,155–163.
Truche,L.,Berger,G.,Albrecht,A.,Domergue,L.,2013b.Engineeredmaterialsas potentialgeocatalystsindeepgeologicalnuclearwasterepositories:Acase studyofthestainlesssteelcatalyticeffectonnitratereductionbyhydrogen. Appl.Geochem.35,279–288.
Van Loon,L.R., Kopajtic, Z., http://www.nagra.ch/en/shopproductdetail1.htm/s level/10390/sproduct/290181990.ComplexationofCu2+,Ni2+andUO
22+by
RadiolyticDegradationProductsofBitumen.Nagra.
Walczak,I.,(ThèsedeDoctorat.Doctoralthesis)2000.Determinationdesproduits organiquesd’altérationschimiquesetradiochimiquesdubitume:applications auxenrobesbitumes.INSA,Lyon.
Walczak,I.,Libert,M.,Camaro,S.,Blanchard,J.M.,2001.Quantitativeandqualitative analysisofhydrosolubleorganicmatterinbitumenleachates.Agronomie21(3), 247–257.