Mechanism of action and recruitment of
antigen-presenting cells in a mouse model of multiple sclerosis
Thèse
Noopur Singh
Doctorat en médecine moléculaire
Philosophiæ doctor (Ph. D.)
Résumé
La sclérose en plaques (SEP) est largement acceptée comme étant une maladie auto-immune du système nerveux central (SNC) dirigée par les cellules T auto-réactives. Cette maladie est très répandue au Canada. L'encéphalomyélite auto-immune expérimentale (EAE) est le modèle animal le plus utilisé pour l’étude de la SEP. Malgré l’importance des cellules T dans cette pathologie, il est important de souligner que ce sont les cellules présentatrices d'antigènes (CPA) myéloïdes, et surtout les cellules dendritiques (CD), qui amorcent l’activation des cellules T auto-réactives dans les organes lymphoïdes secondaires ainsi que leur éventuelle réactivation dans le compartiment du SNC plus tard au cours de la maladie. Cependant, il existe une disparité dans l'identification des différentes sous-populations de CPA myéloïdes en raison de l'absence de marqueurs fiables et spécifiques, ce qui constitue une entrave dans le domaine de l'immunothérapie cellulaire. Par ailleurs, les mécanismes moléculaires responsables de la régulation du recrutement des CPA myéloïdes au niveau de la vasculature cérébrale suite à l'activation de l'endothélium du SNC sont encore mal connus. L'interleukine-6 (IL-6) est une cytokine pro-inflammatoire essentielle à la différenciation des lymphocytes TH17 auto-réactifs. Ces derniers sont des régulateurs clés de l'EAE et jouent un rôle important dans la SEP. Ainsi, le premier objectif de ma thèse était de démontrer que l'IL-6 joue un second rôle dans l'EAE en stimulant l'endothélium du SNC à exprimer les molécules nécessaires au recrutement des CPA myéloïdes. Nous démontrons que : (1) les cellules endothéliales du SNC expriment le récepteur de l’IL-6 (IL-6R) ; (2) l’ablation génétique de l’IL-6R spécifiquement dans l'endothélium atténue le recrutement des neutrophiles, des macrophages et des CDs, et prévient le développement de l'EAE ; et (3) l'IL-6 stimule les cellules endothéliales à produire CXCL1 et PTGS2, des protéines impliquées dans le recrutement et l'activation des cellules myéloïdes. Le deuxième objectif général de mon étude était d'obtenir une vue d'ensemble phénotypique et fonctionnelle des sous-populations de CD présentes pendant la phase initiale d'induction de l'EAE. À cette fin, nous avons utilisé la technologie de séquençage de l'ARN unicellulaire (scRNAseq) pour comparer la signature transcriptionnelle des cellules CD11c+ issues des nœuds lymphatiques au cours de la phase préclinique de l'EAE. Nous montrons que : (1) quatre grandes sous-populations de CD sont présentes, soit les CD115+ monocytaires (mDC), les SiglecH+ plasmacytoïdes (pDC), les XCR1+ conventionnelles de type 1 (cDC1) et les CCR7+ conventionnelles de type 2 (cDC2) ; (2) les cDC2, une sous-population spécifique de CPA, présentent des niveaux transcriptonnels élevés de cytokines pro-inflammatoires (IL-6, IL-12) et de marqueurs de maturation et de co-stimulation pour la présentation des antigènes (CD80, CD83, CD86, OX40L) ; (3) miR155, un microARN connu pour son rôle dans l'EAE, est principalement
exprimé dans les cDC2 ; et (4) que l'enzyme D-aminoacide oxydase (Dao), qui produit du peroxyde d'hydrogène (H2O2) à partir d’acides aminés D, est régulée à la hausse dans les cDC2 des souris miR155-/-. En résumé, mon travail de thèse met l'accent sur le rôle critique des CPA myéloïdes lors des premiers événements précliniques de l'EAE et il suggère un nouveau rôle important de la signalisation classique de l'IL-6 dans le développement de l'EAE et le recrutement des leucocytes. Finalement, il identifie en outre des cibles et des biomarqueurs potentiels à des fins diagnostiques et thérapeutiques.
Abstract
Multiple sclerosis (MS) is widely accepted as an autoreactive T cells driven autoimmune disorder of the central nervous system (CNS), highly prevalent in Canada. Experimental autoimmune encephalomyelitis (EAE) is the established animal model for studying MS. Antigen-presenting cells (APCs) of myeloid origin, most importantly, dendritic cells initiate the priming of autoreactive T cells in the secondary lymphoid organs, and their eventual reactivation in the CNS compartment later in the disease course. However, a disparity exists in identifying myeloid APCs subsets with accuracy due to absence of reliable and specific markers, causing a hindrance in the field of cell-based immunotherapy. In addition, more needs to be deciphered on the molecular mechanisms that regulate activation of CNS endothelium for recruitment of myeloid APCs. Interleukin-6 (IL-6) is a pro-inflammatory cytokine, essential for differentiation of self-reactive TH17 lymphocytes, which are key regulators of EAE and play an important role in MS. The first objective of my thesis was to demonstrate that IL-6 plays another role in EAE by stimulating the CNS endothelium to express molecules required for the recruitment of myeloid antigen-presenting cells. We show that: (1) endothelial cells in the CNS express IL-6 receptor (IL-6R); (2) genetic deletion of IL-6R specifically in the endothelium blocks neutrophils, macrophages and dendritic cells recruitment as well as EAE development; (3) ICAM1-expressing extravascular myeloid APCs are reduced in number during the pre-onset stage of EAE; and (4) IL-6 stimulates endothelial cells to produce CXCL1 and PTGS2, which are involved in recruitment and activation of myeloid cells. The second general objective of my study was to get a phenotypic and functional overview of DC subsets present during the initial induction phase of EAE. For that purpose, we used the single-cell RNA sequencing (scRNAseq) technology to compare the transcriptional signature of CD11c+ cells from lymph nodes during the pre-clinical phase of EAE. We show that: (1) four major subsets of DCs are present: CD115+ monocytic DC (mDC), SiglecH+ plasmacytoid DC (pDC), XCR1+ conventional DCs type-1 (cDC1) and CCR7+ conventional DCs type-2 (cDC2); (2) cDC2 exhibit elevated expression of pro-inflammatory cytokines (IL-6, IL-12), maturation marker (CD83) and co-stimulatory molecules for antigen presentation (CD80, CD86, OX40L); (3) miR155, a microRNA known to have a role in EAE, is predominantly expressed in cDC2; (4) the enzyme D-amino acid oxidase (Dao), that produces hydrogen peroxide (H2O2) from D-amino acids, is upregulated in cDC2 of miR155-/- mice. In summary, my thesis work emphasizes on the critical role of myeloid APCs during initial pre-clinical events of EAE. Moreover, it suggests additional important role of classical IL-6 signaling in EAE development and leukocyte recruitment. It further identifies potential targets and biomarkers for diagnostics and therapeutic purposes.
Table of contents
Résumé ... ii
Abstract ... iv
Table of contents ... v
List of figures ... viii
List of Abbreviations ... x
Acknowledgements ... xvii
Avant-propos ... xix
Preface ... ii
Introduction ... 1
1.1. Central Nervous System ... 2
1.2. Myelin ... 3 1.3. Multiple Sclerosis ... 5 1.3.1. Diagnosis of MS ... 7 1.3.2. MS Risk Factors ... 9 1.3.3. MS Epidemiology ... 10 1.3.4. Types of MS ... 10 1.3.5. MS Treatment ... 12 1.4. Animal models of MS ... 14
1.4.1. Virus induced demyelination ... 14
1.4.2. Toxin induced demyelination ... 14
1.4.3. Experimental autoimmune encephalomyelitis (EAE) ... 15
1.5. Meninges ... 18
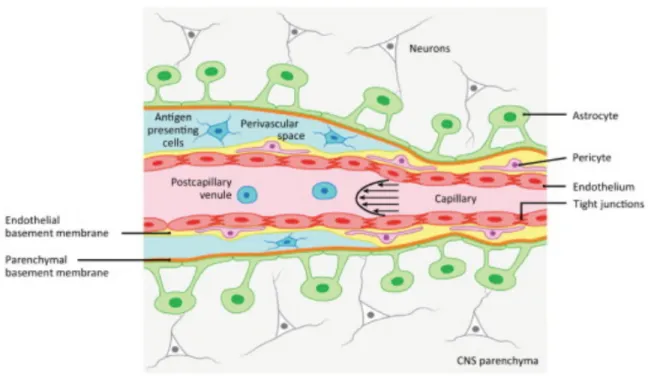
1.6. Blood Brain Barrier (BBB) ... 20
1.6.1. Endothelial-leukocytes interaction mediates extravasation to the CNS parenchyma ... 23
1.7. Antigen presenting cells (APCs) ... 28
1.7.1. Neutrophils ... 28
1.7.2. Macrophages ... 31
1.7.3. Dendritic cells (DCs) ... 33
1.8. Antigen-processing and presentation by APCs to the T cells ... 38
1.9. General pathology of EAE ... 41
1.10. Cytokines and Chemokines ... 44
1.10.1. IL-6 ... 44
1.11. miRNAs in MS and EAE ... 49
Chapter 1 ... 54
Endothelial IL-6 signaling is critical for leukocyte recruitment and activation in a
mouse model of autoimmune demyelination ... 54
Résumé ... 55
Abstract ... 56
2.1. Introduction ... 57
2.2. Results ... 58
2.2.1. IL-6Rα is expressed in the cerebral endothelium and it can be selectively deleted in chimeric IL-6Rαfl/flTie2cre mice ... 58
2.2.2. IL-6Rα expression by endothelial cells is critical for EAE initiation. ... 61
2.2.3. The source of IL-6 remains same between WT→WT and WT→IL-6Rαfl/flTie2cre mice.63 2.2.4. T cell priming and leukocyte mobilization is not affected in WT→IL-6Rαfl/flTie2cre mice. ... 64
2.2.5. Endothelial IL-6R signaling regulates the recruitment of myeloid antigen-presenting cells (APCs) into the CNS. ... 67
2.2.6. Classical IL-6R signaling stimulates endothelial cells for up-regulation of molecules involved in myeloid cells recruitment and activation. ... 68
2.3. Discussion ... 71
2.4. Materials and methods ... 75
2.4.1. Animal ... 75
2.4.2. Generation of bone marrow chimeras ... 75
2.4.3. EAE induction and clinical evaluation ... 75
2.4.4. Adoptive transfer ... 76
2.4.5. Flow cytometry ... 76
2.4.6. RT-qPCR ... 77
2.4.7. RNAScope Assay ... 78
2.4.8. Immunostaining and confocal microscopy ... 78
2.4.9. Statistical analysis ... 78
Chapter 2 ... 80
Single-cell profiling identifies miR155 targets in conventional dendritic cells type 2 in
a mouse model of multiple sclerosis ... 80
Résumé ... 81
Abstract ... 82
3.1. Introduction ... 83
3.2. Results ... 85
3.2.1. cDC2s are main antigen-presenting cells in EAE. ... 85
3.2.2. miR155 is chiefly expressed in cDC2. ... 88
3.2.3. miR155 does not regulate the activation and maturation of cDC2. ... 89
3.2.4. miR155 does not regulate the phagocytosis or pinocytosis pathway of antigen-processing in cDC2s. ... 92
3.3. Discussion ... 94
3.4. Materials and methods ... 98
3.4.1. Animals ... 98
3.4.2. EAE induction ... 99
3.4.3. Bone-marrow DC (BMDC) culture ... 99
3.4.4. Phagocytosis and pinocytosis assay ... 99
3.4.5. Antigen-presentation assay ... 100
3.4.6. Flow cytometry ... 100
3.4.7. Western Blot ... 101
3.4.8. Single cell RNA sequencing ... 101
3.4.9. RT-qPCR ... 101
3.4.10. Statistical analysis ... 102
Chapter 3 ... 104
Discussion ... 104
4.1.1. IL-6 receptor is expressed in the cerebral endothelium. ... 106
4.1.2. Classical IL-6 signaling by the CNS endothelium is critical for extravascular ICAM1 expressing myeloid APCs recruitment. ... 107
4.1.3. IL-6 acts on the endothelial cells for upregulation of genes for chemokine CXCL1 and prostaglandin-synthesizing enzyme, PTGS2 (COX-2) to regulate the recruitment of ICAM1 expressing extravascular myeloid APCs. ... 108
4.2.1. cDC2 are primary APCs for T cell priming in secondary lymphoid organs of EAE. .... 110
4.2.2. miR155 is primarily expressed in cDC2s. ... 111
4.2.3. Dao, gene for D-amino acid oxidase enzyme is upregulated in miR155-/- cDC2. ... 111
Conclusion ... 113
List of figures
IntroductionFigure 1.1: Myelin sheath proteins are targeted as antigens during MS………..4
Figure 1.2: MS lesions at CNS sites……….7
Figure 1.3: Immune cells infiltration of acute and chronic lesions of MS………...9
Figure 1.4: Types of MS on the basis of clinical course……….…...12
Figure 1.5: A schematic showing few drugs available for MS treatment along with their site of action………..13
Figure 1.6: Anatomy of cerebrovascular barriers………...19
Figure 1.7: Tight junctions of BBB………21
Figure 1.8: Highly specialized endothelial cells line the BBB………...22
Figure 1.9: In the bone marrow, myeloid (MP) and lymphoid progenitors (LP) are generated from hematopoietic stem cells (HSCs)………37
Figure 1.10: T cells reactivation in the CNS compartment………42
Figure 1.11: IL-6 signaling……….47
Figure 1.12: A schematic summarizing previous works done by Vallieres lab on the role of IL-6 in immune cells and CNS endothelial crosstalk……….48
Figure 1.13: Schematic structure of human pri-miRNA-155……….50
Chapter 1 Figure 2.2.1: IL-6Rα is expressed in the cerebral endothelium and it can be selectively deleted in chimeric IL-6R-/- Tie2cre mice ……….60
Figure 2.2.2: IL-6Rα expression by endothelial cells is critical for EAE induction………..62
Figure 2.2.3: The source of IL-6 remains same between WT→WT and WT→IL-6Rαfl/flTie2cre mice………64
Figure 2.2.4: T cell priming and leukocyte mobilization is not affected in WT→IL-6Rαfl/flTie2cre mice………66
Figure 2.2.5: Endothelial IL-6Rα is required for the recruitment of myeloid APCs into the CNS…68 Figure 2.2.6: Classical IL-6R signaling stimulates endothelial cells to upregulate molecules involved in myeloid cells recruitment and activation……….70
Chapter 2 Figure 3.2.1: scRNAseq of lymph node cells from mice 6 days after immunization with or without MOG35-55……….87
Figure 3.2.2: miR155 is chiefly expressed in cDC2…….………..89
Figure 3.2.3: miR155 does not regulate activation and maturation of cDC2…………..……..…….91
Figure 3.2.4: miR155 does not regulate the phagocytotic and macropinocytic uptake of cDC2…...93
Figure 3.2.5: Dao is upregulated in cDC2s in absence of miR155.………...94
Supplementary Figure: Quantification of bone marrow derived DC (BMDC) for the activation markers……….103
Chapter 3
Discussion
List of Abbreviations
ADAMTS A Disintegrin And Metalloproteinase With Thrombospondin Motifs ADEM Acute Disseminated Encephalomyelitis
ADP Adenosine Diphosphate
AIDS Acquired Immunodeficiency Syndrome ALCAM Activated Leukocyte Cell Adhesion Molecule ANOVA Analysis Of Variance
APC Antigen-Presenting Cells ARE AU-Rich elements
ASPRV1 Aspartic Peptidase Retroviral-like 1 ATP Adenosine Triphosphate
BAC Bacterial Artificial Chromosome BAM Border-Associated Macrophages
BATF3 Basic Leucine Zipper Transcriptional Factor ATF-Like 3 BBB Blood brain barrier
BCR B Cell Receptor
BCSFB Blood-Cerebrospinal Fluid Barrier BGS Bovine Growth Serum
BLMB Blood-Leptomeningeal Barrier
BM Bone Marrow
BMDC Bone-Marrow Derived Dendritic Cells BSF B-Cell Differentiation Factor
CADM1 Cell Adhesion Molecule 1 CAM Cell Adhesion Molecule CCL CC Motif Chemokine Ligand CCR CC Chemokine Receptor CD Cluster Of Differentiation cDC1 Conventional Dendritic Cell 1 cDC2 Conventional Dendritic Cell 2
CDC42 Cell Division Control Protein 42 Homolog CDP Common DC Precursor
CEACAM1 Carcinoembryonic Antigen-Related Cell Adhesion Molecule 1 CFA Complete Freund’s Adjuvant
CIS Clinically Isolated Syndrome
CLEC4C C-Type Lectin Domain Family 4 Member C CLIP Class II-Associated Invariant-Chain Peptide CLR C-Type Lectins
CNS Central Nervous System
COX Cyclooxygenase
CSF Cerebrospinal Fluid
CVO Circumventricular Structures CX3CR1 CX3C Chemokine Receptor 1 CXCL C-X-C Motif Chemokine Ligand CXCR C-X-C Motif Chemokine Receptor DAG 1,2-Diacylglycerol
DAMP Danger-Associated Molecular Patterns DAPI 4′,6-diamidino-2-phenylindole
DC Dendritic Cells
DEC Lymphocyte Antigen 75
DGCR8 DiGeorge syndrome critical region gene 8 DMF Dimethyl Fumarate
DMT Disease-Modifying Therapies DNA Deoxyribonucleic Acid
DPBS Dulbecco's Phosphate Buffered Saline
DRB1 DR Beta 1
DTR Diptheria Toxin Receptor
EAE Experimental Autoimmune Encephalomyelitis EBP Enhancer-Binding Protein
EBV Epstein-Barr Virus EC Endothelial Cells ECM Extra Cellular Matrix
EDSS Expanded Disability Status Scale EDTA Ethylenediaminetetraacetic Acid ELISA Enzyme-Linked Immunosorbent Assay ELR Glutamate-Leucine-Arginine Motif EP4 E-Type Prostanoid Receptor 4 ER Endoplasmic Reticulum
ERK Extracellular Signal-Regulated Kinase ESAM EC Adhesion Molecule
FACS Fluorescence-Activated Cell Sorting FAD Flavin Adenine Dinucleotide
FAK Focal Adhesion Kinase
FDA Food And Drug Administration FLT3L FMS-Like Tyrosine Kinase 3 Ligand FRET Forster Resonance Energy Transfer G-CSF Granulocyte Colony-Stimulating Factor GAPDH Glyceraldehyde 3-Phosphate Dehydrogenase GEF1 Rho Guanine Nucleotide Exchange Factor 1 GFP Green Fluorescent Protein
GM-CSF Granulocyte Macrophage Colony-Stimulating Factor GPCR G-Protein Coupled Receptor
GTP Guanosine Triphosphate
HIV Human Immunodeficiency Virus HLA Human Leukocyte Antigen
HPRT1 Hypoxanthine-Guanine Phosphoribosyltransferase HSC Hematopoietic Stem Cells
ICAM Intercellular Adhesion Molecule 1 ID2 DNA Binding Protein Inhibitor 2
IFN Interferon
IL Interleukin
IL6R Interleukin-6 receptor
IRAK1 Interleukin 1 Receptor Associated Kinase 1 IRF Interferon Regulatory Factor
ITAM Integrin Subunit Alpha M
ITG Integrins
JAK Janus Kinase
JAM Junctional Adhesion Molecules JNK Janus Kinase
KLF4 Kruppel-Like Factor 4 L-NMMA L-N-Mono Methyl Arginine
LAMP Lysosome-Associated Membrane Protein LARC Liver And Activation-Regulated Chemokine LBRC Lateral Border Recycling Compartment LFA1 Lymphocyte Function-Associated Antigen 1 LIF Leukaemia Inhibitory Factor
LN Lymph Node
LP Lymphoid Progenitors
LPAM1 Lymphocyte Peyer’s Patch Adhesion Molecule 1 LPS Lipopolysaccharide
MACS Magnet-Activated Cell Sorting
MAdCAM Mucosal Addressin Cell Adhesion Molecule MAG Myelin-Associated Glycoprotein
MAPK Mitogen-Activated Protein Kinase MBP Myelin Basic Protein
MCAM Melanoma Cell Adhesion Molecule MCP Monocyte Chemoattractant Protein mDCs Monocyte-Derived DCs
MerTK MER Receptor Tyrosine Kinase MFI Median Fluorescence Intensity MHC Major Histocompatibility Complex MHV Mouse Hepatitis Virus
MIIC MHC Class II Containing Antigen-Processing Compartment MIP3 Macrophage Inflammatory Protein-3 Alpha
miRNA Micro RNA
MMR Mannose Receptor
MOG Myelin Oligodendrocyte Glycoprotein MP Myeloid Progenitors
MPO Myeloperoxidase
MRF Myelin Regulatory Factor MRI Magnetic Resonance Imaging mRNAs Messenger RNAs
MS Multiple Sclerosis
MSFC Multiple Sclerosis Functional Composite NADPH Nicotinamide Adenine Dinucleotide Phosphate NET Neutrophil Extracellular Traps
NFAT Nuclear Factor Of Activated T-cells
NH3 Ammonia
NK Natural Killer Cells NKT Natural Killer T cells
NMDAR N-methyl-D-aspartate receptor
NMOSD Neuromyelitis Optica Spectrum Disorder
NO Nitric Oxide
NOD Nucleotide-Binding Oligomerization Domain NOS Nitric Oxide Synthase
NVU Neurovascular Unit
OCBs Oligoclonal Immunoglobulin Bands
OH Hydroxide
OPC Oligodendrocyte Precursor Cells
OSM Oncostatin M
OX40L OX40 Antigen Ligand
PAMP Pathogen-Associated Molecular Patterns PBMC Peripheral Blood Mononuclear Cells PBS Phosphate Buffered Saline
PECAM Platelet Endothelial Cell Adhesion Molecule-1 PG Prostaglandin
PGE2 Prostaglandin E2 (Dinoprostone) PGES Prostaglandin E Synthase PGH2 Prostaglandin H2
PI3K Phosphoinositide 3-Kinase PKC Protein Kinase C
PL Phospholipids PLCγ Phospholipase Cγ PLP Proteolipid Protein PMA Phorbol Myristate Acetate PPMS Primary-Progressive MS
PRR Pathogen Recognition Receptors PSGL-1 P-Selectin Glycoprotein-1
PTGS2 Prostaglandin-endoperoxide Synthase 2 PTX Pertussis Toxin
PVS Perivascular Space
RANK Receptor Activator Of NFkB
RANKL Receptor Activator Of NFkB Ligand RAPL Rap-1 Binding Molecule
RIAM Rap-1 Interacting Adaptor Molecule RIG Retinoic Acid-Inducible Gene RIS Radiologically Isolated Syndrome RISC RNA-Induced Silencing Complex RNA Ribonucleic Acid
RNS Reactive Nitrogen Species ROR RAR-Related Orphan Receptor ROS Reactive Oxygen Species RRMS Relapsing-Remitting MS
RT-PCR Real Time Polymerase Chain Reaction
RT-qPCR Quantitative Reverse Transcription Polymerase Chain Reaction SASPase Skin Aspartic Protease
SC Spinal Cord
SEM Standard Error Of Mean
SIRPα Signal-Regulatory Protein Aipha SOCS3 Suppressor Of Cytokine Signaling 3 SOM Self-Organizing Maps
SPMS Secondary-Progressive MS
STAT Signal Transducer And Activator Of Transcription STED Stimulated Emission Depletion Microscopy TAP1 Transporter Associated With Antigen Processing TBI Total Body Irradiation
TCR T Cell Receptor
TF Tissue Factor
TGF Transforming Growth Factor TLR Toll-Like Receptor
TMEV Theiler’s Murine Encephalomyelitis Virus TNF Tumor Necrosis Factor
TNFSF4 TNF Superfamily Member 4 TRAF6 TNF Receptor Associated Factor 6
TRANCE TNF-Related Activation-Induced Cytokine tSNE T-Distributed Stochastic Neighbour Embedding UMI Unique Molecular Identifier
UTR Untranslated Region
VCAM Vascular Cell Adhesion Molecule VEGF Vascular Endothelial Growth Factor VLA Very Late Antigen
WT Wild type
XCR1 Chemokine XC Receptor 1
‟A man should look for what is, and not for
what he thinks should be.”
- Einstein
“One has to keep all one’s will-power and
reason to deal with them, and they will all be
overcome at the time when once one has
familiarized oneself with the minutest details
of the business…”
- Dostoevsky
Acknowledgements
This has been an incredible journey and it goes without saying that none of it would have been possible without the support of my supervisor Dr. Luc Vallières. I take this opportunity to express my sincere gratitude and thankfulness to Dr. Vallières for giving me this opportunity to work in his laboratory for my doctoral training. His scientific temperament and knack for the subject has been a constant inspiration to learn and acquire the research skills, I am able to present in my thesis form today. His patience with my physical struggles during times of sickness, words of kindness and courage kept me motivated towards my research. He has shaped my scientific outlook as an aspiring researcher and his continuous zeal for excellence, passion for science, constant curiosity for recent technology developments and therapeutics discovery is something I would like to carry forward. For everything that I have learned from him, in research and on interpersonal level, I feel highly obliged to him.
I take this opportunity to express my sincere thankfulness to my doctoral committee members, Dr. Manu Rangachari and Dr. Fawji Audojit. I am grateful to them for providing their valuable time and appraisal of my doctoral thesis work. Their constructive criticism has valuably contoured the direction of my research work and addressed the missing points in the study.
I take this opportunity to thank my lab members who helped me with immersion in initial days of research work. Their patience during my training phase and presence as valuable aids in final execution of work has been remarkable. Without them this thesis work would not be possible, Aline, Alex, Ryder, Catherine, Françoise, Yu and Patrick. I would like to thank Dr. Alexandre Brunet and Vincent Desrosiers, without whom none of the flow cytometry work would have been possible. I would also like to express my thankfulness to Joanie and Nicola for saving my experiments during critical times when I almost ran out of reagents or our orders were delayed, Mark for his patience with my cryostat sessions.
I thank my friends in Quebec, who have been constant emotional and intellectual support throughout this PhD terrain, and made me smile during this challenging phase: Linda, Lucie, Karima, Marishka, Banshi, Mena, Fernando, Minja, Olus, Sai, Maria, Irene, Katerina, Flora, Asmita, Prenitha, Pooja, Jason, Jonathan, Johanna, Nico, Dafni, Jehane, Anais, Melanie, Laura, Andre, Kaushik, Kanchan, Marie-kim, Micael, Mina, Sophie, Gab, Irshad, Sarah and Giacomo.
As a final note, I would like to thank my dad for always believing in me, supporting my decisions, and being my rock, however disagreeable the situations may have appeared to him. My mum, for
her ever so composed countenant, her patience and her strength, I wish to inculcate that. My siblings, Kirti and Dipshi, I cannot imagine my life without them. My friends back at home or elsewhere, specially, Charu, Tanya, Neelu, Ankur, Stavan, Anne, Debo, Divu, Priyanka, Shanah, Sarah, Abhishek, Alok and rest of the family, Nanu, who would have been pleased to see this if he was with us, Khushi and Aaryan, for showing an exceptional amount of courage while we suffered loss of a life as a family during this pandemic – you inspire me everyday, to my teachers, from when I was a toddler till this moment. The space requires conciseness, and I may have already violated the rules for this section. So, I would like to end with saying, I feel you, I remember you, and I am ever so thankful to you for being my hope during bleak times.
Avant-propos
La thèse est divisée en cinq parties et contient les résultats scientifiques obtenus par moi au cours des quatre dernières années en tant que doctorante dans le laboratoire du Dr Luc Vallières.
Introduction
La thèse commence par un aperçu général du système immunitaire dans le SNC et la périphérie, et présente les conséquences du trouble auto-immun dans l'approche centrée sur les cellules présentatrices d'antigènes myéloïdes.
Chapitre 1
Le chapitre 1 est constitué du manuscrit intitulé "La signalisation endothéliale IL-6 est essentielle pour le recrutement et l'activation des leucocytes dans un modèle de démyélinisation auto-immune". Cette étude met en évidence le rôle critique de la signalisation classique de l'IL-6 dans le recrutement des cellules présentant l'antigène myéloïde au cours de la phase pré-départ de la démyélinisation auto-immune et donc l'initiation de la maladie par l'utilisation d'un modèle de souris transgénique avec une déficience du récepteur endothélial de l'IL-6 dans l'endothélium. La plupart des expériences, l'analyse des données et la génération de figures ont été réalisées par moi. Le Dr Aline Dumas a été d'une grande aide dans la réalisation des expériences en tant que professionnelle de la recherche sur le projet. Le Dr Jean-Françoise Richard a réalisé quelques expériences initiales sur le projet avant que je ne rejoigne le laboratoire et la figure 2.1 contient des données issues de certains de ses précieux travaux. Le Dr Luc Vallières a constamment assuré sa précieuse supervision dans la rédaction du manuscrit et a énormément contribué à la conception scientifique du travail.
Chapitre 2
Le chapitre 2 correspond au manuscrit intitulé "Single-cell profiling identifies role of miR155 in antigen processing pathway of conventional DCs type 2 as primary antigen-presenting cells in demyelinating animal model of multiple sclerosis". Dans ce travail, nous identifions des biomarqueurs spécifiques aux sous-ensembles de CD présents lors de la phase initiale d'amorçage de l'EAE. En outre, nous sommes en mesure d'expliquer pour la première fois le rôle du miR155 de manière spécifique aux DC de type 2 conventionnelles, car l'absence de miR155 entraîne une
altération du profil de traitement de l'antigène de ces sous-ensembles de DC. J'ai réalisé la plupart des expériences avec l'aide précieuse des professionnels de la recherche du projet, le Dr Catherine Bélanger et le Dr Aline Dumas. Le séquençage d'ARN unicellulaire et l'analyse des données bioinformatiques ont été effectués par Génome Québec et les membres du laboratoire de Jacques Corbeil, Elsa Rousseau et Juan Manuel Dominguez. Mon directeur de recherche, le Dr Luc Vallières, a conçu l'approche scientifique de ce projet, a contribué à l'analyse bioinformatique et a été un guide constant dans la planification, l'exécution et la présentation des travaux.
Chapitre 3
Le présent chapitre examine les conclusions des chapitres 1 et 2.
Conclusion
Preface
The thesis is divided into five parts and contains the scientific results obtained by me during the last 4 years as a PhD student in the laboratory of Dr. Luc Vallières.
Introduction
The thesis begins with a general background of the immune system in the CNS and periphery, and introduces the consequences of autoimmune disorder in the myeloid antigen-presenting cells centered approach.
Chapter 1
The chapter 1 consists of the manuscript titled “Endothelial IL-6 signaling is critical for leukocyte recruitment and activation in a model of autoimmune demyelination”. This study unravels a critical role of classical IL-6 signaling in the myeloid antigen-presenting cells recruitment during pre-onset stage of autoimmune demyelination and thus the disease initiation by the use of a transgenic mice model with endothelial IL-6 receptor deficiency in the endothelium. Most of the experiments, data analysis and figure generation were carried out by me. Dr. Aline Dumas has been a great help in carrying out the experiments as research professional on the project. Dr. Jean-Françoise Richard has performed few initial experiments on the project before I joined the lab and Figure 2.1 contains data from some of his valuable work. Dr. Luc Vallières has constantly given his valuable supervision in the manuscript writing and contributed immensely in scientific design of the work.
Chapter 2
The chapter 2 corresponds to the manuscript titled “Single-cell profiling identifies miR155 targets in conventional dendritic cells type-2 in a mouse model of multiple sclerosis.” In this work, we identify biomarkers specific to DC subsets present during initial induction phase of EAE. Furthermore, we are able identify potential targets of miR155 for the first time in conventional DC type 2 specific manner, as the absence of miR155 results in upregulation of Dao, a gene for D-amino acide oxidase. I performed most of the experiments with valuable assistance from research professionals on the project, Dr. Catherine Bélanger and Dr. Aline Dumas. Single-cell RNA sequencing and the bioinformatics data analysis was done by Genome Quebec and Dr. Jacques Corbeil’s lab members Dr. Elsa Rousseau and Juan Manuel Dominguez. My research director, Dr. Luc Vallières designed the scientific approach of this project, contributed in bioinformatics analysis and has been a constant guide in the planning, execution and presentation of the work.
Chapter 3
This chapter discusses the findings from chapter 1 and chapter 2.
Conclusion
This chapter consists of the conclusions obtained from the work performed within the dimensions of this thesis.
Introduction
From early 20th century, the central nervous system (CNS) was considered to be an immunoprivileged site protected from exogenous and endogenous antigens by physiological barriers such as the blood-brain barrier (BBB).1 However, Medawar’s graft transplant rejection experiment in 1948 and the discovery of lymphatics in the CNS during year 2015 established that the CNS is not completely isolated from the peripheral immune response, but rather has a more robust and complex physiological regulation of immune-neurovascular unit (NVU).2,3,4 Multiple neurological disorders are autoimmune in nature with still unknown or partially known triggers for initiation of pathogenesis. T cells are major components of immunosurveillance and they are considered as instigators of most neurological disorders of autoimmune nature. Multiple sclerosis (MS) is an exemplary model for T cell mediated neurodegenerative autoimmune disorders.5 Active lesions found in different compartments of the CNS during MS are full of autoreactive myelin specific T cells. However, it is myeloid antigen-presenting cells (APCs) that educate the T cells for a specific immune response by providing necessary activation signals. Myeloid APCs, primarily dendritic cells (DCs), process antigenic peptides and present them in major-histocompatibility complex (MHC) restricted manner to the T cells. Other co-stimulatory molecules and pro-inflammatory cytokines provide accessory activation signals for T cells expansion into specific phenotypes (e.g. TH1, TH17 etc.).5,6 The immune cell activation is initiated by cellular crosstalk, which is generally mediated by secretion of cytokines (e.g. IL-6) and chemokines. Once activated, these cells are equipped with adhesion molecules, chemokines and cytokines receptors to migrate towards the blood-brain barrier (BBB) for interaction with endothelial cells to get entry into the perivascular space, where T cells are reactivated by cognate antigen-presentation from peripheral myeloid APCs, and finally the pathogenesis is initiated.7,8,9 Most of past MS research has been focused on T cells, but recent decade has seen some promising work into characterization of the role of myeloid APCs. This thesis work is an attempt to look into the role of myeloid APCs at two levels: (1) at preclinical T cell reactivation stage, but more specifically, in the context of myeloid APCs interaction with CNS endothelium under influence of cytokine IL-6 signaling, showing a critical role of IL-6 in initiation of experimental autoimmune encephalomyelitis (EAE), an animal demyelinating disease model for MS, (2) myeloid APCs phenotypic and functional characterization during initial induction phase of EAE in the secondary lymphoid organ. This study adds to the fundamental understanding of underlying mechanisms in establishments of MS and EAE, and provides potential targets for biomarkers and immunotherapeutics development.
1.1. Central Nervous System
The central nervous system (CNS) consists of brain and spinal cord, which are surrounded by a protective layer of bone, meninges and cerebrospinal fluids.10,11 The brain is divided into three major parts: the cerebrum, the cerebellum and the brain stem. Spinal cord (SC) forms the caudal part of the CNS and extends from the basal part of the skull up to the lumbar vertebra. The gray matter of the SC is constituted of nerve cell bodies, the dorsal horn of which is responsible for receiving sensory inputs from the periphery, while the ventral horn innervates specific muscles to execute motor functions. The white matter surrounds the gray matter and contains longitudinal tracts of axons covered with myelin sheath to form the ascending pathways for transport of sensory information between the brain and the periphery. The SC is considered to be the “final common pathway”, as all higher motor controlling activity must be administered by brain through the spinal motor neurons.12 Meninges covering the CNS have distinct immunomodulatory properties from the CNS.13
The cellular components of the CNS consists of approximately 1011 neurons, with 10 times higher abundance of glial cells, which include microglia, astrocytes, oligodendrocytes, ependymal cells, schwann cells, satellite cells and other CNS myeloid cells such as, leptomeningeal, perivascular and choroid plexus macrophages.13,14,15 Microglia are major innate immune cells of the CNS. They are derived from yolk sac or FL-derived monocytes and maintain physiological properties of the brain by acting as professional phagocytes.16,17
Maintenance of cellular homeostasis in the CNS is essential for proper functioning of the neurons. Therefore, the CNS is protected from peripheral body by a set of barriers to hinder unwanted substances from entry into the CNS parenchyma. Three types of physiological barriers exist between the CNS and the peripheral circulation; microvessels of the CNS contain endothelial blood-brain barrier (BBB), meningeal microvessels contain the blood-leptomeningeal barrier (BLMB), and the choroid plexus is lined with epithelial blood-cerebrospinal fluid barrier (BCSFB). These barriers restrict the migration of immune cells into the CNS parenchyma during homeostatic conditions.18 These barriers are used for removal of toxic materials produced by physiological reactions and act as an interactive interface between the immune system and the CNS.19,20 Based on the anatomical barrier location, the immune response to the CNS varies.
(TCR) on their surface, undergo apoptosis by positive and negative selection in the thymus. Similarly, undesirable neurons are directed towards apoptosis by synaptic pruning. Lymphocytes reside mostly in the fluid and membranous lining interfaces of the CNS which are located in the meninges and choroid plexus, where very few peripheral immune cells (T cells, B cells, NK cells, macrophages, dendritic cells) enter the CNS parenchyma with minimal antigen-presentation capacity.3,21 However, once the primary signal of inflammation is initiated in the peripheral lymphoid tissue, the commencement of immune response takes place. This initiates the mobilization of leukocytes towards the CNS, which eventually increase their interaction with the CNS barriers such as BBB, BLMB, BCSFB for entry into the CNS parenchyma, and depending on the initiated immune response, CNS pathology evolves.22
1.2. Myelin
In the CNS, oligodendrocytes synthesize enormous amount of plasma membranes. These highly stable stacks of membranes act as a wrap of multiple layers around an axon of a neuron and are made from hydrophobic lipids and proteolipid proteins. This highly ordered structure is called myelin sheath, which insulates the axons and clusters the sodium channels into the nodes of Ranvier, enabling fast saltatory transmission of action potential by their jump from one node to another. The hydrophobic nature of lipids and proteins in the myelin sheath helps in the structural maintenance by intermolecular attraction of lipids, repulsion of aqueous cytosol and extracellular fluid.23
Myelin is divided into compact (extracellular space of 2 nm) and non-compact (extracellular space of 12-14 nm) form and consists of few specific proteins, such as, myelin-associated glycoprotein (MAG), myelin basic protein (MBP), proteolipid protein (PLP), and myelin oligodendrocyte glycoprotein (MOG). MOG is a transmembrane glycoprotein located at the external surface of the myelin sheath and triggers T and B cell mediated immune response as one of the main autoantigens of MS (Figure 1.1).24,25,26
Figure 1.1. Myelin sheath proteins that are targeted as antigens during MS. Reproduced with
permission of Hemmer et. al. New concepts in the immunopathogenesis of multiple sclerosis. Nat
Rev Neurosci. 2002.
The biochemical studies done on myelin in the past have revealed that myelin has molecular composition of lipid content up to 73-81%. Its plasma membrane is unique in that it has a protein to lipid dry weight ratio of ~0.25, which is much lower than general plasma membrane composition range between 1 to 4. Myelin is comprised of three main lipid components namely; cholesterol, glycosphingolipids (GalC), glucosylceramides (GlcC), and phospholipids (PLs). Myelin is enriched in glycosphingolipids (~27%) and plasmalogens (~16%), a specific structural form of phosphatidylethanolamine unique to myelin. The glycosphingolipids GalC and sulfatide are distinct to myelin by having a headgroup based on galactose instead of glucose and high levels of fatty acid hydroxylation.27,28,29
According to the prevailing view, oligodendrocytes provide the necessary molecular cues required for myelination.30 Oligodendrocyte precursor cells (OPCs) enter into terminal differentiation stage and become premyelinating oligodendrocytes to synthesize myelin components. However, the
extrinsic and intrinsic factors that regulate the neuron-oligodendrocyte crosstalk for myelin formation are believed to be highly complex and remain to be investigated. Transcription factors such as, Sox5/6, Hes5, Id2/4 are involved in terminal differentiation of OPCs, myelin regulatory factor (MRF) regulates myelin biogenesis by postmitotic oligodendrocytes and Zfp191 regulates late stage of oligodendrocyte differentiation. These controlling factors are mostly regulated by miRNAs, histone deacetylases and signaling pathways. In peripheral nervous system, myelin sheath formation is performed by Schwann cells on signals from axonal neuregulin-1.27,30 Interestingly, OPCs derived from SC form longer myelin sheaths than the OPCs derived from cortex area.30 Studies in the past have shown that monocytes and macrophages also contribute in regeneration of myelin and promotion of oligodendrocytes precursor activity.9,31 There are vast array of diseases of inter and intra-individual heterogeneous origin that arise from dysfunction of the CNS, mostly by insult of immunological nature on the myelin.32 These demyelinating disorders include; acute disseminated encephalomyelitis (ADEM), clinically isolated syndrome (CIS), neuromyelitis optica spectrum disorder (NMOSD) and multiple sclerosis (MS).33
1.3. Multiple Sclerosis
Multiple sclerosis (MS) is most prevalent autoimmune chronic inflammatory demyelinating disorder of the CNS. During this disease, myelin sheath of the neurons, the basic components of nerve impulse transmission by nerve fibers, come under assault.34 Almost 150 years ago, MS was established as a dissemination of plaque like sclerosis across time and space. ‘Dissemination in space’ is referred to as the disease related changes found in multiple anatomical locations of the CNS (white matter, gray matter, brain stem, spinal cord, and optic nerve) and ‘dissemination in time’ is considered to be CNS associated appearance of lesions over time (Figure 1.2).35 Typical symptoms of MS include, fatigue, visual loss, motor deficits such as limb weakness, numbness, impaired ambulation or ataxia due to cerebellar lesion, spasticity, tremor, pain, loss of bladder control, depression, sexual and brain-stem dysfunction etc. The evolution of the disease over time results in cognitive impairment. The chronic condition also leads to neuronal loss and alterations in the integrity of axons etc. with reduced brain volume.9,33,36,37 The cerebral cortex contains subpial demyelinated lesions, which are considered very specific to MS.38
MS is a composite of interplay between two most intricate biological systems, namely; the immune system and the nervous system.39 An active MS lesion contains focal demyelination areas with
ongoing inflammation and glial reaction. These reactions are mostly mediated by parenchymal and perivascular infiltrates of T cells, B cells, myeloid cells such as, macrophages, dendritic cells and neutrophils from the periphery and activated microglia from the CNS.40 Furthermore, these active lesions also contain activated astrocytes, which contribute to the tissue injury and inflammation.38 As a result, inflammatory cytokines such as, IL-6, IFN-γ, IL-23 and IL-17 are produced. Axonal injury and inflammation mediated demyelination also results in the production of reactive oxygen/nitrogen species (ROS/RNS), prostaglandins and vasoactive factors with few acute lesions showing severe oligodendrocyte loss.39 In progressed form of the disease, oxidative stress induced mitochondrial malfunction occurs due to oligodendrocyte apoptosis, demyelination, axonal damage and lack of remyelination.41 In MS, autoimmune reaction occurs on myelin sheath of the neurons by autoreactive CD4+ TH1 and TH17 cells.42 Myelin is not just exclusive to white matter of the CNS and involves demyelination of gray matter as well. Most of the peripheral immune cell invasion takes place due to initial myelin damage (Figure 1.3).41
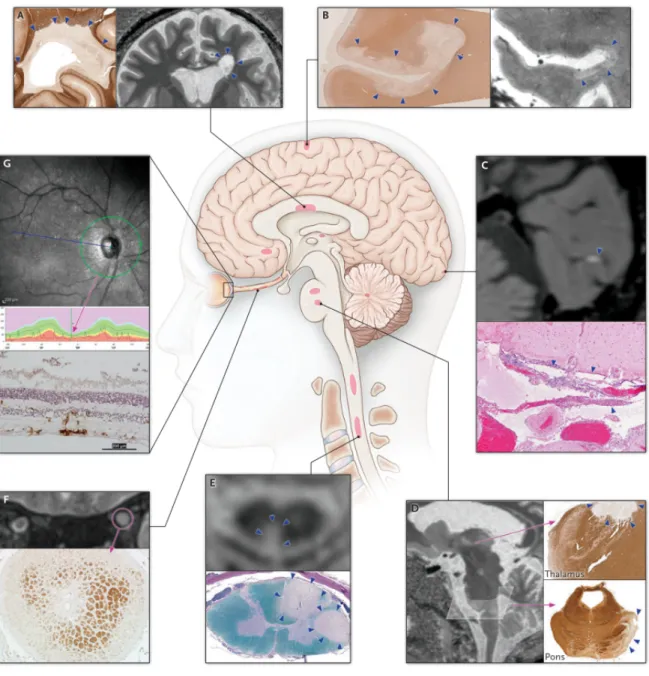
Figure 1.2. MS lesions at CNS sites. A) Periventricular white matter, B) subpial cortex, C)
leptomeninges, D) thalamus and pons, E) spinal cord, F) optic nerve, and G) retina. Reproduced with permission of Longo et. al. Multiple Sclerosis. N Engl J Med. 2018.
1.3.1. Diagnosis of MS
MS is heterogeneous in nature with variation in the clinical and imaging manifestations between subjects, and within the same individual over time. McDonald’s criteria are implemented for the diagnosis of MS.35 However, the integration of multiple tools such as; clinical, laboratory (e.g.
immunohistochemistry) and neuroimaging (MRI) etc. has made it possible to diagnose MS with better accuracy. Despite this, a clinic-radiological paradox is observed, as MRI confirms approximately 60-70% of white matter lesions observed with the help of histopathology. However, it is difficult to identify the gray matter lesions, subpial lesions are often underestimated by MRI. Apart from lesions observed on MRI, oligoclonal bands (OCBs) present in cerebrospinal fluid (CSF) of MS patients are used as primary diagnostic tools for MS. 9,33,36,43,44,45,46
Currently, research is also being carried out on blood serum or CSF based potential biomarkers for prognosis and diagnosis of MS (e.g. neurofilaments).47,48 A biomarker is generally considered as an indicator of a pathology, biological process or improvement to therapeutics administered. The characteristics of ideal biomarkers include, easy measurement by non-invasive methods with accuracy and reproducibility. Molecular biomarkers can include mRNAs, DNAs, miRNAs and proteins that can be isolated from blood serum and CSF of MS patients. However, the variability based on patient population, sample processing and sensitivity of assay used etc. makes them difficult clinical targets for MS prognosis.49,50 The lack of early biomarkers and suitable immunotherapy presents a major hindrance in the treatment of MS.
Expanded disability status scale (EDSS) is used for the assessment of endpoints for clinical trials
and therapeutic intervention decisions in MS patients. Ordinal rating system with 0-10 numerical range is used, where 0 represents normal neurological status, and 10 represents death due to MS. Patients are scored with 0.5 increment scale on the basis of neurological disability, with score up to 5 based on neurological imaging disability and above 6, dependent on motor deficit.51
Figure 1.3. Acute and chronic lesions of MS with sites of peripheral immune cell and complement
system activation mediated inflammation causing myelin sheath degeneration. Reproduced with permission of Longo et. al. Multiple Sclerosis. N Engl J Med. 2018.
1.3.2. MS Risk Factors
There is no single known cause of MS, as several genetic and environmental risk factors associated with MS have been identified. Sex is one such risk factor, as females are three times more susceptible to MS than males.52 However, males are observed to have severe cognitive deficit and prevalence of progressive form of MS in comparison to females.53,54 MS etiology is complex with almost 200 gene variants, most of them are associated with immune pathway such as, antigen-processing and presentation. Human leukocyte antigen (HLA)-DRB1*1501 is the allele for major histocompatibility complex class II (MHCII), and also considered to be the most preeminent genetic component for MS. Apart from that, other MHC class II associated genes such as HLA-DQ6 etc. also create a stronger genetic susceptibility to MS. The patients with presence of MHCII associated risk factors have more lesion formation with greater reduction in brain volume.9,32,33,37,55,56 In
addition, genome-wide association studies (GWAS) indicate numerous cytokine pathways as risk alleles, which are implicated in TH1/TH17 differentiation: 6/STAT3, 12/STAT4 and IL-23/STAT3 etc.57,58 Moreover, micro RNA (miRNA)-microarray analysis identified nine miRNAs as risk factors for MS, which are further supported by bioinformatics analysis to have strong correlation for disease progression, these miRNAs are: let-7e, miR23b, miR31, miR99b, miR125a, miR146b, miR155, miR193b and miR221.59 Additionally, MS patients have increased level of miR155 and miR326 in their brain.60
Furthermore, serological evidence of remote Epstein-Barr virus (EBV) infection (mononucleosis), low serum level of 25(OH) vitamin D caused by geographic latitude, exposure to smoking, changes in gut microbiota etc. add to susceptibility for MS. Presence of antibodies against the CNS such as, CSF specific OCBs are found in 82-89% of patients (identified by isoelectric focusing on agarose gel and immunodetection) and serum neurofilaments serve as another hallmark of MS.9,32,33,37,55,61
1.3.3. MS Epidemiology
According to 2016 data, an estimated 2.2 million of world population is living with MS, with a prevalence rate of 30.1 cases per 100,000 people.55 Canada has the highest rate of MS in the world with a total of 77,000 adult MS patients. Every 2.6 individuals per 1000 adult population are living with the disease with an estimate of 1 in every 385 Canadians. 60% of the diagnosed adults are between the age range of 20-49 years with almost 11 individuals diagnosed everyday.34,52 The mean age of diagnosis is 30 years.55 There is about 2-4% risk of people developing MS if they have relatives with MS in comparison to 0.1% risk in the general population, while monozygotic twins have a risk factor of 30-50%. The incidence of MS in children ranges from 0.07 to 2.9 per 100,000 children and is called paediatric MS. MS is usually detected in the youth. Yet 3-5% of patients receive their diagnosis during childhood with 0.3% being diagnosed before the age of 10. Over past few decades, the geographical gradient of MS has been increasing with high prevalence rate across North America and Europe, and high incidence is generally reported in the females.55
1.3.4. Types of MS
For describing the clinical course of MS, diagnostic and prognostic purposes and administration of clinical trials, the clinical phenotypes of MS were outlined first in 1996 by US National Multiple
Sclerosis Society (NMSS) Advisory Committee. At that time, MS was divided into 4 different subtypes, named as relapsing-remitting (RR), secondary-progressive (SP), primary-progressive (PP) and progressive-relapsing (PR)MS. PRMS was considered to be a rare form of MS similar to PPMS with the exception of remission phase and acutization.62 In October, 2012, a revision to phenotypic classification of MS was made by the MS phenotype group and with time, the revisions in McDonald’s criteria of 2010 and 2017 have added to their definitions to improve upon the early diagnosis and patient-care. MS progression starts even before the symptoms become clinically evident. However, the disease-associated manifestations can be exposed accidentally by means of radiological examinations at this window period mostly referred to as radiologically isolated syndrome (RIS). MS is classified into 4 major types (Figure 1.4).35,63,64,65
1.3.4.1. Clinically isolated syndrome (CIS); It refers to a single episode of neurological symptoms,
generally considered to be the earliest form of MS. The inflammatory demyelination can be mono or multifocal in nature, can develop acutely or sub acutely for at least 24 hours of duration and can occur in the absence of fever or infection with no known earlier symptoms of MS in the patient. The lesions detected by MRI during this phase are mostly leukocortical in nature, with hardly any subpial injury.35
1.3.4.2. Relapsing-remitting MS (RRMS); It is characterized by unpredictable but clear episodes of
exacerbations or flare-ups also considered as relapses. It generally leads to appearance of new lesions or symptoms or the worsening of pre-existing symptoms. During the period of relapse, recovery is either complete or partial in nature towards the pre-relapse condition, also known as remission. About 85% of MS patients have this form of the disease.66 During this disease occurrence, a hyperintense lesion in observed by MRI in the cervical, thoracic and lumbar region of the spinal cord.35
1.3.4.3. Secondary progressive MS (SPMS); Most patients with RRMS eventually progress towards
a progressive form of neurological deterioration with fewer relapses and minor remissions. The disease progresses steadily, sometimes with plateaus. Approximately 50% of the patients with RRMS develop SPMS in 10-20 years of diagnosis.
1.3.4.4. Primary-progressive MS (PPMS); It is characterized by slow accumulation of disability,
without defined episodes of remission. About 15% of diagnosed MS patients suffer from this form of disease. Almost 5% of patients with PPMS have occasional relapses with steady worsening of
MS from the beginning. PPMS is generally diagnosed around 40 years of age and affects both sexes equally. CNS atrophy can be seen at much early stage of the disease in PPMS. There is currently no test available to confirm this form of MS.67
Inflammation, demyelination and neurodegeneration are the common features amongst relapsing and progressive form of MS.38
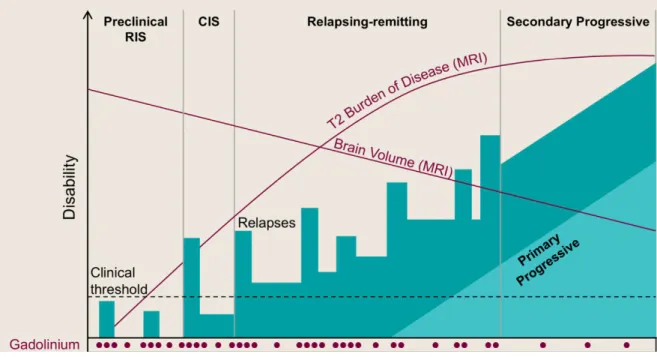
Figure 1.4. Types of MS on the basis of clinical course. X-axis represents time and Y-axis
represents the clinical course of disability. Gadolinium enhancing-lesions observed during MS disease course as seen in MRI are represented on X-axis along with T2 burden of disease and decrease in brain volume due to atrophy. Reproduced with permission of Baecher et. al. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 97, (2018).
1.3.5. MS Treatment
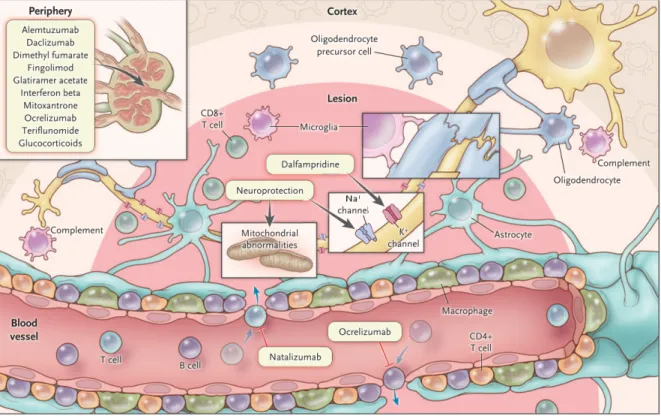
Drugs that limit the entry of activated leukocytes into the CNS parenchyma or cause inhibition of leukocytes activation, adhesion or egress from the periphery by disrupting their mechanism of action also slow down the disease progression. There are 16 disease-modifying therapies (DMT)
available to treat MS.50,68,69 Steroids are available as relapse management therapies to relieve from the inflammation of the CNS. Apart from that, therapies for symptom management, rehabilitation with lifestyle changes can slow the rate of disease progression in MS (Figure 1.5).70 And yet, we have not progressed enough to develop therapies that can fully prevent the disease or induce recovery from the neurological deterioration. Most of the available DMTs target the T and B cells (e.g. Natalizumab, Ocrelizumab).71 There is no specific DMT to specifically target myeloid APCs, dendritic cells that are most efficient for antigen-presentation and T cell activation. These shortcomings make the early diagnosis and proper management of MS progression of paramount importance. Younger population being the disease target also brings a huge cost burden on the economy.9,33,72
Figure 1.5. Schematic showing drugs available for MS treatment along with their site of action.
1.4. Animal models of MS
The complexity of MS is such that no single disease model ideally recapitulates myriad of genetic, environmental and immune heterogeneity seen in the patients living with it.9 Different animal models are available to study MS, and are selected on the basis of various aspects of MS pathogenesis to be studied, such as, inflammation, demyelination, remyelination or neurodegeneration in the CNS. 38
1.4.1. Virus induced demyelination
Even though no clear evidence of MS specific virus infection exists so far, there is strong epidemiological evidence for link between Epstein Barr virus infection and MS pathogenesis. Major animal models for study of a viral etiology are picornavirus Theiler’s murine encephalomyelitis virus (TMEV) and certain coronavirus mouse strains such as mouse hepatitis virus (MHV).73 Intracerebral TMEV injection of SJL/J mice leads to chronic-progressive demyelinating form of the disease. Demyelination of white matter neurons in the spinal cord occurs almost 50 days post-infection, which causes autoreactive CD4+ T cell response following epitope spreading from viral to myelin-specific response.38,73 Intracranial or nasal mode of immunization of animals with neurovirulent hepatitis virus strain causes disease development in 2 phases; (1) antiviral panencephalitis, and almost 4 weeks later, (2) T cell mediated immune response against myelin basic protein (MBP) or proteolipid protein (PLP) by molecular mimicry. Furthermore, it results in extensive microglial activation and oxidative injury. It has been observed that MHV strain shares 6 amino-acids (AA) with encephalitogenic peptide region of MBP.38,73
Neurotropic viral infection can cause demyelination as an aftermath of encephalitis. These models represent key pathological features of inflammatory demyelination similar to MS.38 However, the shortcomings of viral models are their complex viral and immune mediated pathogenesis.74
1.4.2. Toxin induced demyelination
Cuprizone or lyso-phosphatidylcholine (lysolecithin) animal models come under such category. Cuprizone is Copper chelating agent that causes oxidative stress mediated demyelination by
activation of microglia and astrocytes. It induces apoptosis of oligodendrocytes causing rapid remyelination. Lysolecithin is injected in the white matter of the CNS, which causes focal demyelinating plaque formation by damaging lipid rich myelin sheath. Another focal model of demyelination is the injection of ethidium bromide in the white matter of the CNS. These toxin-induced models are used for studying de- or remyelination, but they do not induce MS specific pathogenesis.38,42
1.4.3. Experimental autoimmune encephalomyelitis (EAE)
EAE is most extensively studied animal model of MS and it is considered to be very reliable. Development of EAE animal models led to a closer study of interaction of the CNS with peripheral immune system.2,75 We used this animal model for our study as EAE recapitulates key pathological features of neuro-immune interaction in MS with features of inflammation, demyelination, gliosis and axonal loss etc. EAE animal models have an immune cell-mediated organ specific approach towards establishment of the disease and generates a monophasic, relapsing-remitting or chronic form of the disease.42 This model has been successfully used in the development of various DMTs for MS.75 The EAE mice are sensitized with CNS immunogen, such as, myelin oligodendrocyte glycoprotein (MOG), myelin basic protein (MBP) or proteolipid protein (PLP).38,42,75 Depending on the research question of interest, different EAE induction methods can be used.
1.4.3.1. Passive EAE; T cells are isolated from immunized mice and are re-stimulated with myelin
antigens. These encephalitogenic T cells are then transferred intravenously or intraperitoneally into the naïve recipient animals for EAE induction. This model is used to study mechanism of immune surveillance, induction of inflammation and peripheral immune cells infiltration in the CNS. We used this mode of immunization for few experimental questions. However, the disadvantage of this model is that the disease pathogenesis is essentially limited to T lymphocytes.
Animal models with injection of respective autoantibodies along with passive transfer of encephalitogenic T cells are also reported. In such models, T cell mediated inflammation leads to damage of blood-brain barrier (BBB), which eventually causes the diffusion of antibodies and complement system components into the CNS. This passive co-transfer model gives rise to an EAE pathogenesis similar to MS.38
1.4.3.1. Active EAE; Animal models are sensitized with the CNS antigens and strong adjuvants such
as, complete Freund’s adjuvant (CFA) and pertussis toxin (PTX) to potentiate the humoral immune response.42 CFA provides a slow liberation of specific antigen from the inoculum, and contains inactivated mycobacterium tuberculosis, which creates a phagocytosis mediated uptake and presentation of antigens by APCs, eventually leading to CD4+ T cell activation and expansion mediated immune response.38
PTX is a hexameric protein obtained from Bordetella pertussis, a whooping cough causing bacteria.
The A promoter of PTX contains enzymatic activity, and is formed of S1 subunit. The B oligomer is made up of four subunits and binds to the cell surface.76 The A promoter is transported to the endoplamic reticulum (ER) after endocytosis where it blocks G protein-coupled receptor (GPCR) interaction by catalyzing ADP-ribosylation of G protein α subunit. It also acts on toll like receptor 4 (TLR4) for up regulation of P-selectin on the cerebral endothelium, and causes post-transcriptional up regulation of intercellular adhesion molecule 1 (ICAM-1) by secretion of IL-6 from stromal cells in the periphery.76,77 PTX mediates immune response sensitization, such as, inhibition of T cells anergy and regulatory function, innate functions and regulation of BBB, and helps in disease onset and severity.38,42
CNS antigen-specific EAE can be induced in different genetic backgrounds. PLP139-151 immunized SJL/J mice induces relapsing-remitting disease.42 However, the MOG35-55 peptide induced mice model is considered to be highly reproducible and popularly used for analysis of molecular mechanisms involved in MS pathogenesis. We used MOG35-55 antigen mediated active immunization for most of our study. This model generates acute and chronic form of inflammation with primary axonal damage mediated by MHC class II restricted CD4+ T cells, and elucidates the role of monocytes.38,42 This disease model can display 5 phases; (1) preclinical induction phase, T cells are primed in the primary lymphoid organs by myeloid APCs and migrate to the CNS, (2) onset phase, T cells interact with BBB, transmigrate to the CNS parenchyma and get reactivated after cognate antigens presentation by peripheral transmigrating APCs, (3) acute attack phase, activated myeloid cells initiate effector function, causing inflammation and demyelination of axons, (4) recovery phase, regulatory immune cells attempt to resolve the situation in a partial or complete manner, and (5) chronic phase, a progressive damage mediated by inflammation and demyelination without any or partial recovery with pathology perpetuation.78
Despite these advantages, this model has certain drawbacks that include; (1) Primary demyelination in MS patients is either sparse or absent, (2) this model has spinal cord restricted pathology, as brain stem, cerebellum and fore brain are scarcely damaged, (3) provides limited information for role of CD8+ T cells and B cells in EAE or MS, (4) few studies for evaluation of therapeutic treatments on neuronal growth etc. have been hard to interpret, and mostly off target.38,42 Interestingly, use of recombinant MOG1-125 as a sensitization peptide from the CNS has shown a more similar disease course to MS. The onset mechanism in this new model is mediated by encephalitogenic CD4+ T cells in combination with auto-antibody response against MOG.38
Certain modified EAE models also exist depending on the research question of interests, such as the role of gut microbiota in the CNS inflammation, B cells or T cells centered approach etc.
1.4.3.3. Spontaneous EAE; These models are generated by transgenic expression of TCR of
encephalitogenic origin, which eventually leads to spontaneous demyelinating disease in few months (e.g. 2D2 mice, they express Vα and Vβ chains reactive to MOG35-55).42 These animals can then be crossed with transgenic B cell receptors (BCR) to generate a combined autoantibody response against CNS antigens. These models are particularly useful for gut microbiota based inflammation studies, and sometimes used for passive EAE.38
1.4.3.4. Humanized EAE; Myelin proteins are largely conserved across species, thus intricacies of
antigen-presentation across species becomes very important. The activation of encephalitogenic T cells requires presentation of CNS antigens by MHC molecules on APCs. MHCs are highly polymorphic across species, and their validation for human patients becomes essential. Humanized mouse models offer transgenic mice expressing human MHC class I/II. However, these models do not contain humanization at antigen-processing stage and have animal model specific adhesion molecules and cytokines to regulate their inflammation. Due to this, such models have very limited application in research.38
1.4.3.5. Progressive EAE; Biozzi ABH mice show chronic disease form with moderate to high level
of neurological disability when injected with neurofilament-L. This is found similar to MOG35-55 and believed to generate CD4+ T cells mediated inflammatory response by molecular mimicry. A more reliable chronic EAE disease model is the non-obese diabetic (NOD) mouse that presents partially progressive disease form over the period of 70 days with 90% disease incidence.79
EAE score, EAE mice are generally characterized by ascending paralysis, which begins at tail and
progresses towards hind and fore limb paralysis assessed by use of a 5-point scale. Scoring follows from 0 to 5 depending upon the state of paralysis. Monitoring of clinical signs is performed on daily basis where the scorer remains blind to the treatment condition. Additionally, mice weight loss is taken into account as well, as a higher weight loss without recovery within observation period is considered as the endpoint.80,81 The atypical or non-classical EAE follows the similar 5-point scale, where ataxia and motor behaviours related to gaiting and rolling behaviour is taken into account.82
1.5. Meninges
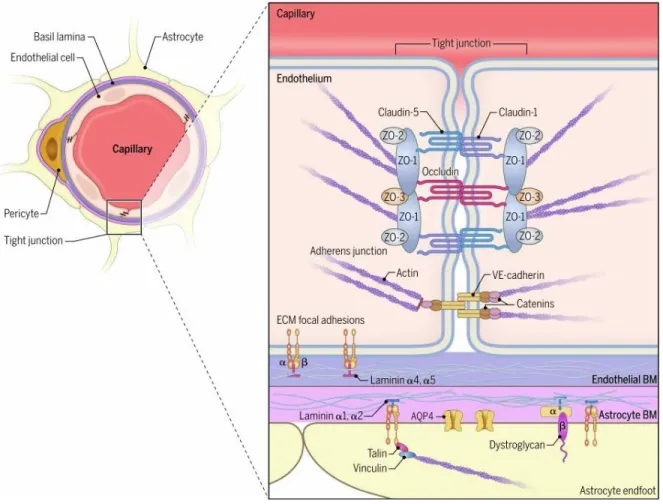
The meninges consists of dura, arachnoid and pia mater which are the membranous structures forming the meningeal vasculature. The meninges provide the major entry points into the CNS parenchyma. The dura mater is the external layer of the meninges attached to the skull from the inside. It contains fenestrated blood vessels and lymphatics, but lacks tight junctions. Underneath this is the arachnoid mater, which is made up of squamous epithelial layers and expresses laminin α5+ basement membrane. The epithelial layer of arachnoid mater contains tight junctions and efflux pumps. The subarachnoid space is encased by inner layer of meninges called pia mater, astrocyte endfeet as glia limitans on the inside and arachnoid mater as the outer layer of the meninges. The epithelial cells of pia mater produce laminin α1+ basement membrane and fibroblast like cells. It separates the cerebrospinal fluid (CSF) containing subarachnoid space from the perivascular space and the brain parenchyma underneath. The pia mater along with arachnoid mater forms leptomeninges. Leptomeningeal blood vessels lack astrocytic ensheathment, but their endothelial tight junctions are intact. The ependymal cells lining the parenchyma next to the ventricles overlay the choroid plexus and contain tight junctions to form BCSFB (Figure 1.6).20,22
Most of the lesions formed in the EAE models are located in the subpial region of the spinal cord. MS patient biopsy samples from early stage of pathogenesis confirm the presence of subpial lesions topographically related to meningeal aggregates causing inflammation. Meningeal lymphatic vessels represent an exit route for immune cells from the CNS parenchyma. Plausibility for maintaining basal immune regulation during homeostatic condition in the CNS is that T cells present in the meninges are highly specific to self-antigens. Partial leakage of the CNS interstitial fluid into the CSF results into its sampling by myeloid APCs in the meninges. These myeloid APCs present antigens to the T cells, which are then activated and help in regulation of basal cytokine
profile required for immunosurveillance during homeostasis and to maintain basal level of myeloid cells in the meninges.2 When this basal immune regulation mechanism fails due to an injury or disease, T cells acquire an effector profile geared to invade the parenchyma.
Figure 1.6. Anatomy of cerebrovascular barriers, with (A) depicting meningeal and cortical