IDENTIFICATION AND CHARACTERIZATION
OF NOVEL DRUGS FOR THE TREATMENT OF
PEDIATRIC GLIOMAS
Thèse
Norbert Fonya Ajeawung
Doctorat en biologie cellulaire et moléculaire
Philosophiae Doctor (PhD)
Québec, Canada
iii
RÉSUMÉ
Les astrocytomes (gliomes) sont les tumeurs cérébrales primaires les plus communes chez les adultes et le deuxième neoplasme le plus fréquent chez l’enfant. Malgré les traitements qui sont disponibles par chirurgie, chimiothérapie et radiothérapie, la résistance, la toxicité ainsi que les taux de guérison faibles conduisent à rechercher de nouvelles drogues plus efficaces. J'ai effectué un test de viabilité chimique avec une banque de molécules sur des lignées cellulaires dérivées de tumeurs cérébrales. J'ai découvert quatre nouveaux composés anti-cancéreux, à savoir: DK16, Bpv(pic), EM011 et le Targetin, qui ont démontré une efficacité élevée et constante dans toutes les lignées de cellules tumorales du cerveau testées. En utilisant une variété de techniques de biologie moléculaire et cellulaire j'ai découvert que ces composés ont une efficacité significative pour inhibent des voies de progression clés dans les lignées de cellules de gliome pédiatrique de bas grade, à savoir: la viabilité, la prolifération, la migration et invasion, et avec seulement peu d'effets indésirables sur les cellules normales. En outre, tous les composés peuvent traverser la barrière hémato-encéphalique et inhiber la croissance de cellules de gliome pédiatrique de bas grade cultivées dans l'agarose mou. Une diminution de la viabilité cellulaire a été simultanément accompagnée à la fois pas l'arrêt du cycle cellulaire et l'apoptose avec la modification concomitante de l'expression de nombreux gènes qui favorisent la progression du cancer. De plus, une induction de l'apoptose impliqué dans translocation phosphatidylsérine, augmentation du ratio BAX/BCL2, dépolarisation de la membrane mitochondriale et translocation nucléaire du AIF a été observé. Comme EM011, le Targetin est également doté de propriétés anti-angiogéniques. Les résultats pré-cliniques de cette thèse montrent que les composés: DK16, Bpv(pic), EM011 et Targretin, peuvent être utiles pour le traitement des gliomes pédiatriques. Les résultats de cette thèse servent de base pour futures études « in vivo » et des essais cliniques chez les patients pédiatriques atteints de gliomes.
v
ABSTRACT
Astrocytomas (Gliomas) are the most common primary brain tumors among adults and second most frequent neoplasm among children. Although gliomas are treated aggressively with surgery, chemotherapy and radiation, treatment resistance, drug toxicity and poor response rates among pediatric glioma patients, continue to drive the need to discover new and more effective chemotherapeutic agents. In line with this notion, I undertook a chemical viability screen involving a library of compounds to discover and characterize novel compounds with high efficacies in retarding the viability of a panel of brain tumor cell lines. I subsequently discovered four new anti-cancer compounds, namely DK16, Bpv(pic), EM011 and Targetin, which demonstrated high and consistent potency in the panel of all brain tumor cell lines and cancer stem cells tested. Using a variety of in-vitro molecular and cell biology techniques, I discovered that these compounds have significant efficacies in abrogating key cancer progression pathways in pediatric low grade glioma cell lines, namely: cell viability, proliferation, migration, and invasion, but with little/no adverse toxicity on normal cells. Furthermore, all compounds can cross the blood brain barrier and inhibit anchorage independent growth of pediatric low grade glioma cell lines in soft agarose. A decrease in cell viability was concurrently accompanied by both cell cycle arrest and apoptosis with the concomitant alteration in the expression of numerous genes that promote cancer progression. Moreover, an induction of apoptosis involved increases in phosphatidylserine translocation, the upregulation of BAX/BCL2 ratio and depolarization of the mitochondrial membrane. In addition, glioma cell lines treated with DK16 and EM011 showed further evidence of mitochondrial dissipation leading to the release and nuclear translocation of the apoptotic inducing factor (AIF), with subsequent nuclear fragmentation. Furthermore, two compounds namely, EM011 and Targetin were found to perturb angiogenesis. These pre-clinical findings suggest DK16, Bpv(pic), EM011 and Targetin, can be suitable for the treatment of pediatric gliomas and serve the basis for future in-vivo studies and clinical trials to further validate the mechanism of action and their efficacies among Pediatric patients with gliomas.
vii
TABLE OF CONTENTS
RÉSUMÉ ... iii
ABSTRACT ... v
TABLE OF CONTENTS ... vii
LIST OF FIGURES ... xv
LIST OF TABLES ... xvii
LIST OF ABBEVIATIONS ... xix
ACKNOWLEDGEMENT ... xxv
FORMAT OF THE THESIS ... xxvii
CHAPTER 1 ... 1
INTRODUCTION ... 1
1.1 Definition of brain tumors ... 2
1.2 Incidence of brain tumors ... 2
1.3 Glioma biology ... 3
1.3.1 General overview and origin of gliomas ... 3
1.3.2 Classification of astrocytomas ... 4
1.3.3 Pediatric gliomas/astrocytomas ... 9
1.3.4 Differences between Pediatric and Adult gliomas ... 15
1.4 Glioma therapy ... 16
1.4.1 Surgery ... 17
1.4.2 Radiotherapy ... 18
1.4.3 Chemotherapy ... 18
1.5 Rationale and statement of hypothesis of this dissertation ... 36
1.6 Overall Research Objectives ... 39
CHAPTER 2 ... 41
Viability screen on pediatric low grade glioma cell lines unveils a novel anti-cancer drug of the steroid biosynthesis inhibitor family ... 41
2.1 Preface to Chapter 2 ... 42
2.2 Résumé ... 43
2.3 Abstract ... 44
viii
2.5 Materials and method ... 47
2.5.1 Chemical and reagents ... 47
2.5.2 Tissue culture ... 47
2.5.3 Trypan blue viability assay ... 48
2.5.4 Methylene blue viability assay ... 48
2.5.5 BrdU ELISA proliferation assay ... 48
2.5.6 Wound healing assay ... 49
2.5.7 Boyden chamber invasion assay ... 49
2.5.8 Cell cycle analysis ... 50
2.5.9 Quantification of apoptotic cells ... 50
2.5.10 Measurement of mitochondrial membrane potential ... 50
2.5.11 Soft agarose assay anchorage independent growth ... 50
2.5.12 Real time reverse transcription (RT) PCR ... 51
2.5.13 Evaluation AIF release from the mitochondria ... 51
2.5.14 Statistical analysis ... 52
2.6 Results ... 52
2.6.1 Chemical genetic screen identifies DK16 as a potent inhibitor of cell viability ... 52
2.6.2. DK16 decreases proliferation and modifies the cell cycle ... 54
2.6.3 DK16 induces apoptosis ... 56
2.6.4 DK16 mediated apoptosis is associated with mitochondrial release and nuclear relocation of AIF ... 59
2.6.5 DK16 blocks migration and invasion of glioma cells ... 59
2.6.6. DK16 inhibits anchorage independent growth ... 60
2.6.7 DK16 perturbs the expression of key genes involved in cancer progression ... 61
2.7 Discussion ... 64
2.7 Acknowledgements ... 66
2.8 Supplemental Figures ... 67
2.9 References ... 70
CHAPTER 3 ... 77
Pre-clinical evaluation of Dipotassium bisperoxo (picolinato) oxovanadate V for the treatment of Pediatric Low Grade Gliomas ... 77
ix
3.2 Résumé ... 79
3.3 Abstract ... 80
3.4 Introduction ... 81
3.5 Materials and Methods ... 83
3.5.1 Cell culture, chemicals & reagent ... 83
3.5.2 Viability assays ... 83
3.5.3 BrdU ELISA ... 83
3.5.4 Cell cycle investigation ... 84
3.5.5 Cell death assays ... 84
3.5.6 Migration assay ... 84
3.5.7 Invasion assay ... 84
3.5.8 Soft agarose assay ... 84
3.5.9 Real-time PCR ... 85
3.5.10 CDC25A activity inhibition assay ... 85
3.5.11 Immunofluorescent cytochemistry ... 85
3.5.12 Blood–brain barrier assay ... 85
3.6 Results ... 86
3.6.1 Bpv(pic) inhibits viability ... 86
3.6.2 Bpv(pic) inhibits proliferation ... 87
3.6.3 Bpv(pic) disrupts microtubule networks & blocks cellular transition within the S & G2M cell cycle phases ... 88
3.6.4 Bpv(pic) induces apoptosis... 89
3.6.5 Bpv(pic) inhibits migration & invasion ... 92
3.6.6 Bpv(pic) inhibits anchorage-independent growth ... 94
3.6.7 Bpv(pic) alters the expression of key genes involved in cancer progression ... 94
3.6.8 Bpv(pic) alters the expression & activity of PTPs implicated in cell cycle regulation ... 96
3.7 Discussion ... 96
3.8 Conclusion ... 100
3.9 Future perspectives ... 100
3.10 Executive summary ... 101
3.11 Acknowledgements ... 101
x
3.13 Ethical conduct of research ... 102
3.14 Supplementary Figures ... 103
3.15 References ... 114
CHAPTER 4 ... 121
The microtubule binding drug EM011 inhibits the growth of pediatric low grade gliomas ... 121
4.1 Preface to Chapter 4 ... 122
4.2 Résumé ... 123
4.3 Abstract ... 124
4.4 Introduction ... 125
4.5 Materials and method ... 126
4.5.1 Cell lines and drugs ... 126
4.5.2 MTS cytotoxic assay ... 127
4.5.3 Cell death/viability assessment... 127
4.5.4 Methylene blue assay ... 127
4.5.5 BrdU ELISA proliferation assay ... 127
4.5.6 Cell cycle analysis ... 128
4.5.7 Apoptotic assay ... 128
4.5.8 Mitochondrial membrane permeability tests ... 128
4.5.9 Migration ... 128
4.5.10 Invasion ... 129
4.5.11 Anchorage independent growth ... 129
4.5.12 Immunofluorescent cytochemistry ... 129
4.5.13 Western blotting ... 129
4.5.14 Gelatin zymography ... 130
4.5.15 Real time reverse transcription (RT) PCR ... 130
4.5.16 Statistical analysis ... 130
4.6 Results ... 131
4.6.1 EM011 inhibits cell viability and proliferation ... 131
4.6.2 EM011 induces S and G2M growth arrest ... 134
4.6.3 EM011 induces apoptosis ... 136
4.6.4 EM011 mediated apoptosis is associated with transient release of AIF from the mitochondria ... 136
xi
4.6.5 EM011 blocks migration and invasion ... 137
4.6.6 EM011 inhibits anchorage independent growth ... 138
4.6.7 EM011 dysregulated the expression of key cancer progression genes and disrupted microtubule formation ... 139
4.6.8. EM011 inhibits matrix metalloproteinase activity ... 140
4.7 Discussion ... 141 4.8 Grant support ... 145 4.9 Acknowledgement ... 145 4.10 Supplemental Figures ... 146 4.11 References ... 152 CHAPTER 5 ... 159
Investigation of Targetin, a Microtubule Binding Agent which regresses the growth of Pediatric High and Low Grade Gliomas ... 159
5.1 Preface to chapter 5 ... 160 5.2 Résumé ... 161 5.3 Abstract ... 162 5.4 Introduction ... 163 5.5 Methods ... 164 5.5.1 Tissue culture ... 164
5.5.2 Determination of the half maximal inhibitory concentration (IC50) ... 164
5.5.3 Assessment of Cell proliferation and clonogenicity ... 164
5.5.4 Measurement of apoptosis ... 164
5.5.5 Cell cycle analysis ... 164
5.5.6 Mitochondrial membrane permeability determination ... 165
5.5.7 Assessment of cell migration and invasion ... 165
5.5.8 Immuno-fluorescent cytochemistry ... 165
5.5.9 PCR array and gene expression analysis ... 165
5.5.10 Determination of CDC25A activity ... 165
5.5.11 Statistical Analysis ... 166
5.6 Results ... 166
5.6.1 Targetin retards cell proliferation, viability and anchorage independent growth ... 166
5.6.2 Targetin alters microtubule networks, and induces cell cycle arrest, phosphatidyl serine externalization and dissipation of mitochondrial membranes ... 167
xii
5.6.3 Targetin interferes with cell migration and invasion ... 172
5.6.4 Targetin perturbs the expression of genes involved in cancer progression ... 172
5.7 Discussion ... 174 5.8 Conclusion ... 175 5.9 Summary points ... 175 5.10 Acknowledgements ... 176 5.11 Sources of funding... 176 5.12 Supplemental Figures ... 177 5.12 References ... 179 CHAPTER 6 ... 183
In-Vitro and Ex-Vivo Investigations of the Microtubule Binding Drug Targetin on Angiogenesis ... 183
6.1 Preface to chapter 6 ... 184
6.2 Resume ... 185
6.3 Abstract ... 186
6.4 Introduction ... 187
6.5 Materials and Methods ... 188
6.5.1 Cell Lines ... 188
6.5.2 Cell Viability Assay ... 188
6.5.3 Cell Proliferation Assay ... 188
6.5.4 Immunofluorescent Cytochemistry ... 188
6.5.5 Endothelial Tube Formation Assay ... 189
6.5.6 Ex-Vivo Angiogenesis Assay ... 189
6.5.7 Statiscal Analysis ... 189
6.6 Results ... 190
6.6.1 Targetin Disrupts Endothelial Cell Viability, Proliferation and Microtubule Network ... 190
6.6.2 Targetin Disrupts Endothelial Cell Capillary-Like Structure Formation ... 191
6.6.3 Targetin Abrogates Vascular Outgrowth in a Rat Aortic Model. ... 193
6.7 Discussion ... 195
6.8 Summary Points ... 196
6.9 Acknowledgments ... 196
6.10 References ... 198
xiii
GENERAL CONCLUSIONS ... 203
7.1 The hypothesis and summary of conclusions ... 204
7.1.1 Effect of DK16, Bpv(pic), EM011 and Targetin on cell proliferation ... 206
7.1.2 Effect of DK16, Bpv(pic), EM011 and Targetin on cell cycle ... 207
7.1.3 Effect of DK16, Bpv(pic), EM011 and Targetin on apoptosis ... 209
7.1.4 Effect of DK16, Bpv(pic), EM011 and Targetin on cell migration and invasion ... 210
7.1.5 Effect of DK16, Bpv(pic), EM011 and Targetin on non cancerous cells or normal cells ... 211
7.2 Identification of the most therapeutically useful compound(s) ... 212
7.3 Significance ... 214
7.4 Future directions ... 216
Bibliography ... 219
xv
LIST OF FIGURES
FIGURE 1.1:AGE SPECIFIC INCIDENCE OF SELECTED NERVOUS SYSTEM TUMORS. ... 3
FIGURE 1.2:DISTRIBUTION OF GLIOMAS BY HISTOLOGICAL TYPES. ... 5
FIGURE 1.3:HISTOLOGIC CLASSIFICATION OF ASTROCYTIC TUMORS. ... 7
FIGURE 1.4:MOLECULAR CLASSIFICATION OF ASTROCYTOMAS. ... 8
FIGURE 1.5:CHEMICAL STRUCTURE OF DK16. ... 32
FIGURE 1.6:CHEMICAL STRUCTURE OF BPV(PIC) ... 34
FIGURE 1.7:CHEMICAL STRUCTURE OF NOSCAPINE,EM011 AND TARGETIN. ... 36
FIGURE 1.8:OPERATIONAL FRAME WORK. ... 38
FIGURE 2.1:EFFECT OF DK16 ON CELL VIABILITY. ... 53
FIGURE 2.2:EFFECT OF DK16 ON CELL PROLIFERATION AND CELL CYCLE. ... 55
FIGURE 2.3:EFFECT OF DK16 ON APOPTOSIS. ... 57
FIGURE 2.4:ASSESSMENT OF MITOCHONDRIAL MEMBRANE POTENTIAL AND BAX/BCL2 RATIO. .. 58
FIGURE 2.5:EFFECT OF DK16 ON CELL MIGRATION AND INVASION. ... 60
FIGURE 2.6:EFFECT OF DK16 ON GENE EXPRESSION. ... 62
FIGURE 2.6:EFFECT OF DK16 ON GENE EXPRESSION. ... 63
FIGURE 3. 1: EFFECT OF DIPOTASSIUM BISPEROXO (PICOLINATO) OXOVANADATE V ON CELL VIABILITY AND PROLIFERATION OF THE R259 CELL LINE OVER A 5DAY PERIOD. ... 87
FIGURE 3. 2: EFFECT OF DIPOTASSIUM BISPEROXO (PICOLINATO) OXOVANADATE V ON THE CELL CYCLE OF THE RES259 CELL LINE OVER A 5DAY PERIOD. ... 89
FIGURE 3.3:EFFECT OF DIPOTASSIUM BISPEROXO (PICOLINATO) OXOVANADATE V ON APOPTOSIS IN THE RES259 CELL LINE OVER A 5DAY PERIOD USING FLOW CYTOMETRY ANALYSES. ... 91
FIGURE 3. 4: EFFECT OF DIPOTASSIUM BISPEROXO (PICOLINATO) OXOVANADATE V ON MITOCHONDRIAL MEMBRANE PERMEABILITY AFTER 48 H OF TREATMENT USING FLOW CYTOMETRY ANALYSIS. ... 92
FIGURE 3. 5: EFFECT OF DIPOTASSIUM BISPEROXO (PICOLINATO) OXOVANADATE V ON CELL MIGRATION, INVASION, ANCHORAGE-INDEPENDENT GROWTH AND CDC25A ACTIVITY. ... 93
FIGURE 4.1:EFFECT OF EM011 ON CELL VIABILITY. ... 132
FIGURE 4.2:EFFECT OF EM011 ON CELL PROLIFERATION AND CELL CYCLE REGULATION... 133
FIGURE 4.3:EFFECT OF EM011 ON APOPTOSIS. ... 135
FIGURE 4. 4:EFFECT OF EM011 ON CELL MIGRATION, INVASION AND ANCHORAGE INDEPENDENT GROWTH. ... 138
FIGURE 4.5:EFFECT OF EM011 ON THE EXPRESSION OF KEY CANCER PROGRESSION GENES, USING A REAL-TIME PCR BASED CANCER PATHWAY FINDER ARRAY. ... 140
xvi
FIGURE 5.2:REPRESENTATIVE IMAGES OF IMMUNOFLUORESCENT CYTOCHEMISTRY STUDIES DONE ON THE SF188 CELL LINE TREATED WITH VARYING DOSES OF TARGETIN, AND LABELLED WITH THE DM1A ALPHA TUBULIN ANTIBODY (GREEN). ... 169
FIGURE 5.3:TARGETIN DELAYS THE TRANSITION OF CELLS IN THE S AND G2M PHASES OF THE CELL CYCLE. ... 170
FIGURE 5.4:TARGETIN INDUCES APOPTOSIS. ... 171
FIGURE 5.5:TARGETIN ATTENUATES CELL MIGRATION AND INVASION, AND FURTHER ALTERS THE EXPRESSION OF CANCER PROGRESSION GENES. ... 173
FIGURE 6. 1: TARGETIN PERTURBS METABOLIC ACTIVITY AND HINDERS PROLIFERATION OF ENDOTHELIAL CELLS. ... 191
FIGURE 6. 2: TARGETIN DISRUPTED ENDOTHELIAL CELL PRE-ESTABLISHED CAPILLARY-LIKE STRUCTURES. ... 192
FIGURE 6. 3: TARGETIN ABROGATED THE FORMATION OF NEW VESSELS FROM PRE-EXISTING VASCULATURE. ... 194
xvii
LIST OF TABLES
TABLE 1:LOCATION AND GENETIC DIFFERENCES IN MALIGNANT GLIOMAS. ... 16
xix
LIST OF ABBEVIATIONS
AIF ALT ATCC AV+ BCL2 BCNU bFGF Bpv(phen) Bpv(pic) BRAF BrdU CBTRUS CDKN2A CDKN2A CDKN2B CHD5 CNS COX-2 CT DioC6(3) DIPG DMEM DMSO E2F EGFR EM011 FDA FOXO3 G1apoptosis inducing factor
alternative lengthening of telomere american type culture collection annexin V positive
B-cell CLL/lymphoma 2
1,3-bis(2-chloroethyl)-l-nitrosourea basic fibroblast growth factor
potassium bisperoxo (1,10-phenanthroline) oxovanadate dipotassium bisperoxo (picolinato) oxovanadate V v-raf murine sarcoma viral oncogene homolog B1 bromodeoxyuridine
central brain tumor registry of the United States cyclin-dependent kinase inhibitor 2A
cyclin-dependent kinase inhibitor 2A cyclin-dependent kinase inhibitor 2B
chromodomain helicase DNA binding domain 5 central nervous System
mitochondrially encoded cytochrome c oxidase II computerized tomography
3-Hexyl-2-[3-(3-hexyl-2(3H)benzoxazolylidene)-1-propenyl diffuse intrinsic pontine gliomas
dulbecco's modified eagle's medium dimethyl sulfoxide
transcription factor 1
epidermal growth factor Receptor 9-bromo-noscapine
Food and Drug Administration forkhead box O3
xx G2M GBM GDP GDP GSK-3β GSTP1 HDCT HGG HIF1A HIPK2 HOXA10 HOXA9 HPLC hSKPs HSV-TK HUVEC KRAS LGG MAPK MCL1 MDM2 MGMT MLH1 MRI MSH2 mTOR NADP NADPH NF1 NHA NPC Gap2-Mitosis Glioblastoma guanosine-5'-diphosphate guanosine-5'-triphosphate (GTP) glycogen synthase kinase 3 beta
single photon emission computed tomography high dose chemotherapy
high grade glioma
hypoxia inducible factor 1, alpha subunit homeobox interacting protein kinase homeobox A10
homeobox A9
high-performance liquid chromatography multipotent Skin-derived precursors herpes simplex virus thymidine kinase human umbilical vein endothelial cells
v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog low grade glioma
mitogene activated protein kinase myeloid cell leukemia sequence 1 oncogene, E3 ubiquitin protein ligase
O-6-methylguanine-DNA methyltransferase
mutL homolog 1, colon cancer, nonpolyposis type 2 magnetic resonance imaging
mutS homolog 2, colon cancer, nonpolyposis type 1 mammalian target of rapamycin
nicotinamide adenine dinucleotide phosphate
reduced Nicotinamide adenine dinucleotide phosphate neurofibromatosis type 1
normal human astrocytes neural progenitor cell
xxi OS P53 PA PDGF PDGFR PET PFS PHLDB1 PI+ PI3K PMS2 PTEN PTK PTP PTPN11 RAF Rb RTK RTKs S SOS1 SPECT SRGAP3 Targetin TERT TMZ TP53 VEGF VEGF VEGFR VEGFR2 overall survival tumor protein p53 pilocytic astrocytoma
platelet-derived growth factor
platelet derived growth factor receptor positron emission tomography
progression free survival
pleckstrin homology-like domain, family B, member 1 propidium iodide positive
phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha ostmeiotic segregation increased 2
phosphatase and tensin homolog protein tyrosine kinase
protein tyrosine phosphatase
protein tyrosine phosphatase, non-receptor type 11 v-raf murine sarcoma viral oncogene homolog B1 retinoblastoma 1
receptor tyrosine Kinase receptor tyrosine kinases synthesis
son of sevenless homolog 1
single photon emission computed tomography SLIT-ROBO Rho GTPase activating protein 3 9-amino folate Noscapine
telomerase reverse transcriptase temozolomide
tumor protein p53
vascular endothelial growth factor receptor vascular endothelial growth factor
vascular endothelial growth factor receptor vascular endothelial growth factor receptor-2
xxii
VP16 WHO
etoposide
xxiii
To my sweet-heart lily and my lovely triplet, Delight, Kayren and Kaela-Zoey
xxv
ACKNOWLEDGEMENT
I wish to express my sincere gratitude to Dr. Deepak Kamnasaran for giving me the opportunity to undertake this dissertation in his laboratory. Over the years, you have moulded me from nothing to something. You have been a source of inspiration. Furthermore, your constant direction and suggestions have served as a catalyst to drive this research to success. I am very thankful to have had you as my research director and for all the sacrifices you made to enable me to suceed with my studies.
I am also very grateful for the immense support from our collaborators: Dr. Donald Poirier, Dr. Rene Maltais, Dr. Robert Faure and Dr. Joshi Harish, who have provided helpful comments, as well as the library of drugs used in my dissertation.
To all the part-time internship and summer students whom I co-supervised during my doctoral studies- Angela, Ewa, Helena, Saleh, Gueorgui, Christina, YuChen, Lotta, Andrea, Christian, Anna-laura, Sofia, Chiara, Elisa, Luis, Francesco and Ameer, I extend my thanks for your encouragement, questions and for creating an excellent laboratory atmosphere during your research internships. Your presence was a source of motivation and your contributions are highly appreciated.
I extend a warm thanks to Dr. Manjit Rana for all the support you have given me during the initial years of my studies. I still wonder whether I would have been so passionate about science if our paths would have never crossed.
To my beautiful and lovely wife, I joyfully extend my thanks. You kept me motivated and have been the “backbone” of my success. This work is a product of your endless prayers.
I also wish to express my sincere thanks to Fonds de la recherche en santé du Québec (FRSQ), Quebec Black Medical association (QMBA), Fondation de etoiles, Club de Recherches Cliniques du Québec (CRCQ), and l’Université Laval, for the scholarships and scholastic awards that were generously given to me in support of my Doctorate studies at Laval University.
xxvii
FORMAT OF THE THESIS
This thesis consist of seven chapters and adopts a manuscript-based format according to the guidelines of the “Faculté des études supérieures et postdoctorales de l’Université Laval”. Chapter 1 reviews relevant background information on the biology and current therapies for gliomas. In addition, this chapter outlines the justification, hypothesis and objectives of this dissertation and discusses the preliminary research which has led to the identification of four new anti-cancer compounds for the treatment of Pediatric low and high grade astrocytomas; compounds that were further examined for their mechanism of action in Chapters 2, 3, 4, 5 and 6 using a variety of in-vitro cancer assays.
Chapters 2, 3, 4, 5 and 6 represent findings on characterizing the mechanism of action of the four anti-cancer drugs: DK16, Bpv(pic), EM011 and Targetin, using a variety of in-vitro assays on a panel of Pediatric low and high grade glioma cell lines. All data from each of these Chapters are published in peer-reviewed journals. Briefly, Chapter 2 characterizes the drug DK16 and its content was published in Cancer Letters (2013 Mar 1; 330(1):96-105). Chapter 3 characterizes the drug Bpv(pic) and its content was published in Future Oncology (2013 Aug;9(8):1215-29). Chapter 4 characterizes the drug EM011 and its content was published in Cancer Letters (2013 Jul 10; 335(1):109-18). Chapter 5 characterizes the effect of the drug Targetin on pediatric glioma cells and its contents was published in the Journal of Pediatric Oncology (2013) 1(1): 41-47. DOI: http://dx.doi.org/ 10.14205/ 2309-3021.2013.01.01.6. While Chapter 6 further characterizes the effect of Targetin on angiogenesis and its contents has also been published in the Journal of Pediatric Oncology (2013) - 1(1):32-40. DOI: http://dx.doi.org/10.14205/ 2309-3021.2013.01.01.5.
Chapter 7 outlines the general conclusion and future perspectives of this dissertation. Chapter 8 (bibliography) has the list of references cited in the introduction and conclusion.
The Appendix (Chapter 9), contains a bar chart of 50 compounds used in the chemical viability screening experiment, a list of glioma cell lines and the calculated IC50 values of each drug as well as supplemental data in support of the research undertaken in Chapters 2, 3, 4, 5 and 6.
CHAPTER 1
2
1.1 Definition of brain tumors
The term brain tumor refers to the abnormal growth of cells into a benign or malignant mass within the brain. Virtually, every region of the brain is susceptible to the development of a tumor and as a result, patients with brain tumor may experience an assortment of symptoms including headache, fatique, seizures and focal deficits etc. (Lovely et al., 2004). The type and severity of the symptoms depend on factors, including the location, size and pathological properties of the tumor. As early as 1957, the World Health Organization (WHO) initiated a resolution aimed at establishing a gradation scheme for human neoplasms. This subsequently resulted in 1979 to the onset of a histology based classification scheme for the diagnosis of Central Nervous System (CNS) tumors (Zulch, 1979). However, as more knowledge advances this field of research, this classification scheme is currently being continuously revised, with the objective of maximing the accuracy of clinical diagnosis and the proposition of treatment modalities (Kleihues et al., 1993; Kleihues et al., 2000; Louis et al., 2007). Since the focus of this dissertation is on identifying and characterizing new chemotherapeutic agents for the treatment of Pediatric astroctyomas, this Chapter will focus on relevant background information pertaining to the biology and treatment of Pediatric gliomas. However, given that some similarities are evident between Pediatric and Adult gliomas, inferences from Adult glioma data will be occasionally used to provide perspectives on Pediatric gliomas.
1.2 Incidence of brain tumors
Brain tumors are among the top 10 lethal cancers in Canada (Canadian cancer statistics, 2013) and the third most frequent cancer among children/adolescents less than 19 years of age (Canadian Cancer statistics 2011). Aproximately, 16% of newly diagnosed childhood cancer cases in Canada are from the CNS and CNS tumors account for about 27% of all cancer related deaths among Canadian children (Canadian Cancer Statistics, 2011). There is a current epidemiological augmentation in the incidence of primary brain tumors which could potentially be as a result of improved cranial imaging detection technologies leading to more effective diagnosis. According to the central brain tumor registry of the United States (CBTRUS), the incidence of primary brain tumors is about 20.6 cases per 100 000 and by the end of 2013, an estimated 69,720 new cases with
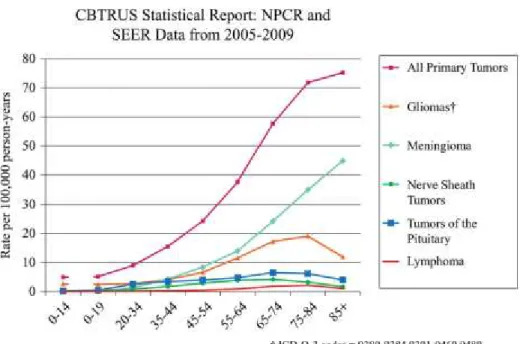
primary CNS neoplasms are expected to be diagnosed in the United States (http://www.cbtrus.org/factsheet/factsheet.html) (Dolecek et al., 2012). A graphical representation of the age-specific incidence of primary brain and nervous system tumors is shown in Figure 1.
Figure 1. 1: Age specific incidence of selected Nervous system Tumors. Adapted from page v12, Neuro Oncol 2012; 14(5): v1-v49, with permission.
1.3 Glioma biology
1.3.1 General overview and origin of gliomas
Gliomas represent a spectrum of histologic and molecularly heterogenous tumors originating from glial cells; the most common CNS cell type. Glial cells compose of astrocytes, oligodendrocytes and ependymal cells. Tumors originating from these glial cell types are respectively refered to as astrocytomas, oligodendrocytomas and ependymomas. However, the term "astrocytomas" is commonly referred to as "gliomas" by investigators worldwide, and as well as in this dissertation. Glial cells have pivotal roles by providing essential support namely immune protection, mechanical support, nutrients and oxygen supply and so on, to neuronal cells (van Meir et al., 2010). Glial cells also assist neurons to mediate complex processes such as signal transduction and neurotransmission (van Meir et al., 2010). Astrocytes further provide structural
4
scaffolds for the migration of neurons into their respective networks during development (van Meir et al., 2010).
The cellular ontogeny of gliomas is unclear. Most reports have used theoretical postulations to describe the putative cell or cell types, from which gliomas could originate from. One of such theories referred to as the "dedifferention hypothesis" posist that mature glial cells acquire genetic aberrations that permits their dedifferention into immature-like cells that have “stem cell like” properties. It has also been suggested that neural progenitors and even mature neural stem cells could acquire mutations to promote spurious proliferation and differentiation leading to cellular transformation and enhanced tumorigenicity. Further evidence suggest that these neural “stem-like” cells haboring aberrancies in growth factor signalling, cell death and proliferation are highly invasive and can self-renew or differentiate into different glial progenies; hence referred to as glioma stem cells (GSC) or glioma initiating cells (GIS) (van Meir et al., 2010; Stiles and Rowitch 2008; Dirks 2001 and 2008).
1.3.2 Classification of astrocytomas
1.3.2.1 Histologic classification of astrocytomas
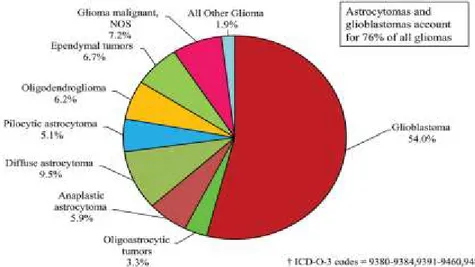
According to the World Health Organization (WHO), tumors of a glial origin can be histologically stratified into four gradations, namely Grades I to IV; with astrocytomas being the most common (Figure 1.2). The least aggressive forms are classified as WHO grade I and II tumors and referred to as low grade gliomas (LGG) or astrocytomas (LGA). On the hand, the most aggressive and lethal astrocytomas are pathologically categorized as grade III and IV, and designated high grade gliomas (HGG) or astrocytomas (HGA). These tumors are comprised of anaplastic astrocytomas and glioblastomas; and represent about 76% of all gliomas (Dolecek et al., 2012) (Figure 1.2).
Figure 1. 2: Distribution of gliomas by histological types. Adapted from page v8, Neuro Oncol 2012; 14(5): v1-v49, with permission.
Grade I astrocytomas
According to the World Health Organization, grade I astrocytomas, also known as low grade astrocytomas, comprise of a family of tumors namely: pilocytic astrocytomas, subependimal giant cell astrocytomas, polomixoid astrocytomas, difuse astrocytomas, pleomorphic xanthoastrocytomas. These tumors are mostly indolent, lack evidence of necrosis and extensive microvascular proliferation. Pilocytic astrocytomas (WHO grade I) are the most common childhood low grade gliomas and consist of lesions with well defined boundaries which grow slowly and occasionally disseminate into the spinal cord (von Hornstein et al., 2011). Tumors with pilocytic histology display features of low cellularity and portray a dual pattern consisting of varying degrees of densely packed elongated cells having defined rosenthal fibres intermingling with microcystic cells (figure 1.3).
Grade II astrocytomas
Diffuse astrocytomas (WHO grade II) consist of three histological sub-types namely, fibrillary astrocytoma, protoplasmic astrocytoma and gemistocytic astrocytoma. Diffuse astrocytomas are often well differentiated and moderately polymorphic. These tumors very often infiltrate neighbouring brain tissues and show evidence of progression into grade III tumors (Sanai et al., 2005) (Figure 1.3).
6
Grades III and IV astrocytomas
High grade astrocytomas refer to grade III and IV astrocytomas. Unlike low grade astrocytomas, these tumors have poorly differentiated densely packed cells which are highly proliferative with a high mitotic index and with evidence of nuclear atypia. However, grade IV astrocytomas, also known as glioblastomas differ from grade III or anaplastic astrocytomas by showing further histological evidence of necrosis and extensive microvascular structures (Louis et al., 2007; Kamnasaran et al., 2009; Pfister and Witt, 2009; Sievert and Fisher, 2009; Kros et al., 2011) (Figure 1.3). Recent findings established by examining radiological evidence of glioblastomas (Lim et al., 2007), further suggest four distinct patterns of how glioblastomas are located in the brain. Patterns 1 and 2 represent less than 40% of the observed cases of glioblastomas juxtaposing the subventricular zone with evidence of being either solitary or invasive. These tumors are hypothesized to arise from a transformation of neural stem cells, neuroprogenitors and other immature cell types which reside in the subventricular zone. Patterns 3 and 4 represent over 60% of the observed cases of glioblastomas present in the cortex but never juxtaposing the subventricular zone. In this manner, these tumors show evdience of either being solitary or invasive in the cortex. These tumors are hypothesized to arise from the transformation of glial or neuroprogenitors dispersed in the cortex, or even a dedifferentiation of an astrocyte in the cortex.
Figure 1. 3: Histologic classification of Astrocytic tumors. Adapted from page E167, Clin Invest Med. 2009 Apr 1;32(2):E166-79, with permission.
1.3.2.2 Molecular classification of astrocytomas
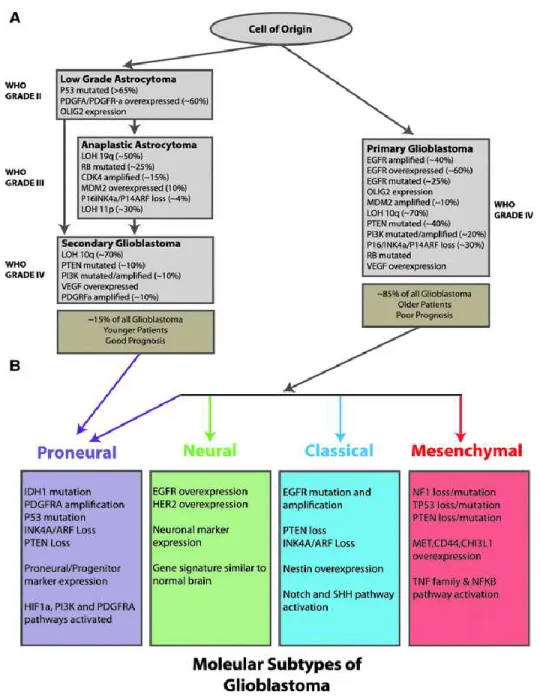
Based on specific molecular signatures, histological and clinical profiles, glioblastomas can further be stratified into primary and secondary glioblastomas. Primary glioblastomas are de-novo tumors, most commonly prevalent after 60 years of age and represent about 95% of glioblastomas. The remaining 5% represent secondary glioblastomas which are tumors originating from low grade lesions that eventually progress into high grade following the acquisition of secondary mutations as shown in Figure 1.4 (Ohgaki et al., 2004, Funari et al., 2007; Wen and Kesari 2008). Secondary glioblastomas are more prevalent among a younger age group. The advent of high throughtput genomics and proteomics platforms has recently contributed to revolutionizing how brain tumors are classified. In this manner, glioblastomas can further be sub-divided into four groups based on each group having distinct molecular signatures. This includes the proneural subtype depicted by IDH1 mutations, p53 mutations, PDGFRA gene amplifications, and the loss of INK4A/ARF and PTEN gene functions. The neural subtype has a similar genetic signature to that of the normal brain with the exception of EGFR gene amplifications and over expression of HER2. The classical subtype consists of tumors reminiscent of activated notch and sonic hedgehog pathways, over expression of EGFR as well as loss of expression of the PTEN and INK4a/ARF genes. Finally, the
8
mesenchymal subtype comprises of tumors with an activated TNF/NFkB pathway, as well as having loss function mutations in the NF1, p53 and PTEN genes, and with overexpression of the CD44, MET and CHI3L1 genes (Figure 1.4) (Verhaak et al., 2010; Agnihotri et al., 2013).
Figure 1. 4: Molecular classification of astrocytomas.
(A) Molecular properties of primary and secondary glioblastomas, as well as other low and grade astrocytomas. (B) Molecular properties of proneural, neural, classical and mesenchymal subtypes of glioblastomas. Adapted from page 28, Arch. Immunol. Ther. Exp. 2013; 61:25–41, with permission.
1.3.3 Pediatric gliomas/astrocytomas
Pediatric glomas as the name implies, are childhood tumors of a glial origin. Like Adult gliomas, they represent a heterogenous group of tumors, with distinct molecular characteristics, varying clinical attributes and varing levels of malignancy. Their histological profile mirrors those of Adult gliomas but the frequency of occurrence of distinct molecular signatures sets them apart from Adult gliomas (Pfister and Witt, 2009; Sievert, 2009, Bax et al., 2011, Jones et al., 2012a, Jones et al., 2012b; Paugh et al., 2010). Such inherent differences in the biology of Pediatric gliomas could partly explain why Pediatric astrocytomas tend to be unresponsive or have very low efficacies when administered with treatments tailored for Adult gliomas.
1.3.3.1 Epidemiology of pediatric gliomas
Pediatric gliomas are the most commonly encountered brain tumors among newborns, children and adolescents (ages 0 to 19), and accounts for about 40-60% of all CNS neoplasms in this population age range (ages 0 to 19) (Pfister and Witt, 2009; Hargrave et al., 2009). According to the Central brain tumor registry of the United States (CBTRUS), about 28 000 children are currently living with a brain tumor and an expected 4,300 new cases of childhood CNS neoplasms will be diagnosed in 2013. The current incidence rate of Pediatric brain tumors is about 5.1 per 100 000, with a slight increase in incidence observed among males when compared to females (Dolecek et al., 2012; http://www.cbtrus.org/ factsheet/factsheet.html). In fact, pilocytic astrocytomas are the most common childhood CNS neoplasms and affect about 18% of children with brain tumors within the age group of 0-14 years, and about 11% in the age group of 15-19 years (Dolecek et al., 2012). According to CBTRUS, the age adjusted incidence of childhood low grade astrocytomas, namely pilocytic astrocytomas and diffuse astrocytomas is about 0.8 and 0.27 per 100 000 respectively, and is higher than that of both anaplastic astrocytomas and glioblastoma (Dolecek et al., 2012).
Like most brain tumors, the etiology of childhood gliomas is not well understood and is based predominantly on risk factors calculated from mixed pools of patients with gliomas. Furthermore, data from epidemiological studies have unveiled a number of risk factors with the most prominent being exposure to X-ray radiation (Ohgaki and Kleihues 2005; Braganza et al., 2011) and certain hereditary predisposing syndromes (Pfister and Witt 2009), accounting for
10
potential causes of Pediatric brain tumors. For instance, about 15% of children with the genetic syndromes Neurofibromatosis type I (NF1) are susceptible to glioma development, mostly in the optic nerve (Pfister and Witt, 2009). Other genetic syndromes originating from mutations in the TP53 gene as in Li Fraumeni syndrome or MLH1, MSH2, PMS2 as in Turcot syndrome, also contribute to the onset of gliomas among Pediatric patients (Pfister and Witt 2009). With these genetic syndromes, an increased incidence is observed among first and second degree relatives of patients who developed gliomas (Scheurer et al., 2010; Sadetzki et al., 2013).
Studies of chromosome aberrations in Pediatric brain tumor specimens have also defined non-random cytogenetic loci which may contribute to the biology of gliomas, chromosome intergrity and to the overall risk of developing gliomas (Melin, 2011). Examples include low penetrant cytogenetic loci at 5p15.33 (with the rs2736100 and TERT genes), 8q24.21 (with the rs4295627 and CCDC26 genes), 9p21.3 (with the rs4977756 and CDKN2A-CDKN2B genes), 20q13.33 (with the rs6010620 and RTEL1 genes), and 11q23.3 (with the rs498872 and PHLDB1, rs1695 and GSTP1 genes) and two loci at 7p11.2 (with the rs11979158, rs2252586 and EGFR genes), (Melin et al., 2011; Liu et al., 2010; Li et al., 2012a).
1.3.3.2 Cinical presentation and diagnosis of pediatric gliomas
Irrespective of the histologic grades, children with brain tumors present with a variety of generalized and specific symptoms. Generalized symptoms predominantly originate from ventricular obstruction leading to increased intracranial pressure. In this manner, the patient has headaches, nausea, vomiting, seizures or impaired conciousness. Depending on the tumor location, patients may manifest more specific symptoms ranging from weakness, somatosensory loss, visual loss and so on, depending on the location, size and pathological properties of the brain tumor. Moreover, symptoms may develop slowly as in the case of pilocytic astrocytomas or maybe sudden, as a secondary consequence of acute hemorrhage or brain herniation which results in a decline in conciousness and even comatosis. Clinical management is determined by factors such as the patients ‘ Karnofsky score which indicates the overall functional status of the patient, and with low scores indicative of a negative prognosis (Pfister and Witt, 2009; Sievert and Fisher, 2009; Schneider et al., 2010).
1.3.3.3 Diagnosis
The diagnosis of Pediatric gliomas is based on using techniques like Neuroimaging and is usually followed by a histologic assessment of surgically resected or tumor biopsies. Two tomographic approaches namely, Magnetic Resonance Imaging (MRI) and Computerized Tomography (CT), are often used to image tumors. However, MRI has emerged as the preferred method due its greater sensitivity, stronger constrast, enhanced depiction of the tumor bounderies and three dimensional (axial, sagittal and coronal) representation of the tumor (Scheineder et al., 2010).
Pediatric low grade gliomas display differential enhancement characteristics on Post-gadolinium sequences and appear to be hypointense on T1-weighted and hyperintense T2-weighted images. Unlike pilocytic astrocytomas which often show well defined doundaries, diffuse fibrillary astrocytomas are poorly-circumscribed tumors having poor enhancement (Sievert and Fisher, 2009). Moreover, some heterogeineity is seen in the appearance of high grade glial tumors and in general, their T1- and T2- weighted images are often hypo-/iso-intense and even hyperintense, when followed with contrast enhancement (Minturn and Fisher, 2013). Imaging of Diffuse intrinsic pontine gliomas (DIPG) often reveals distinct lessions within the pons which are hypo-/iso-intense on T1-weighted and hyperintense on T2-weighted images (Hipp et al., 2011).
Recent technologies such as MR proton spectroscopy are now being used to ameliorate the distinction between normal and neoplastic cells through the measurement of brain metabolites such as N-acetyl-aspartate, choline, creatine, lactate and lipids. For example, low grade gloimas can be distinguished from high grade gloimas by using MR spectroscopic data which often shows hovering choline peaks and augmented levels of the choline:N-acetylaspartate ratio. Moreover, areas with marked anaplasia as well as differences between the refractory tumor and radiation induced damages, can be accurately identified with the use of methods like amino acid PET and SPECT (Minturn and Fisher 2013; Morita et al., 2010).
1.3.3.4 Molecular genetics and signalling in pediatric gliomas
Enormous progress has been made towards understanding the underlying molecular biology of Adult gliomas. However, similar research on Pediatric gliomas is currently under represented. Despite this, current research efforts worldwide have elucidated potential genes that regulate cellular processes such as proliferation, migration, invasion, survival and angiogenesis which may
12
collectively contribute to the cause and prognosis of Pediatric gliomas (Jones et al., 2012a, Jones et al., 2012b). The use of high-throughput techniques with platforms such as Whole genome Exon Sequencing, cDNA Microarrays, Single Nucleotide Polymorphic (SNP) allele arrays, Fluorescence In Situ Hybridization (FISH), Spectral Karyotyping (SKY) and Comparative Genomic Hybridization (CGH) arrays, have contributed significantly to improving our understanding of distinct chromosome aberrations and genetic mutations between Pediatric high and low grade gliomas (Jones et al., 2012a, Jones et al., 2012b; Pfister and Witt, 2009; Hargrave, 2009). In fact, these studies have delineated two major pathways which are dysregulated in glioma namely, the mitogenic signalling pathway and the cell cycle regulatory pathway among Pediatric gliomas.
1.3.3.4.1 Mitogenic signalling pathways
Cellular proliferation, migration, invasion and even angiogenesis are dependent on mitogenic activation or the binding of ligands to cell surface receptors. Activation of these cell surface receptors results in transduction of cellular signals through the MAPK and PI3K/AKT signalling cascades to downstream effectors. Genomic analysis of tumor samples from Pediatric glioma patients have uncovered aberrations in several members of the mitogenic signalling cascade including over expression of activated AKT (Pollack et al., 2010a) and amplifications of genes such as EGFR (Bax et al., 2009; Liang et al., 2008; Gilbertson et al., 2003; Zarghooni et al., 2010), PDGFRA (Zarghooni et al., 2010; Paugh et al, 2010), HIPK2, BRAF (Deshmukh et al., 2008; Pfister et al., 2008), IGF1R, PDGFRB, PIK3CA and so on (Bax et al., 2010). In low grade gliomas, especially pilocytic astrocytomas, most of the mutations have been predominantly identified in genes of the MAPK pathway.
Initial reports linking the MAPK pathway with pilocytic astrocytomas emerged from the clinical assessment of patients with Neurofibromatosis type 1, an autosomal-dominant disorder affecting 1 in 3000 individuals and caused by germline mutations in the NF1 gene (Lewis et al., 1984; Huson et al., 1998; Takano et al., 1992). About 1 in 7 patient with Neurofibromatosis type 1 develops pilocytic astrocytomas mostly in the optic nerve (Lewis et al., 1984). Similar to Neurofibromatosis type 1, some studies have identified solid tumors including pilocytic astrocytomas in patients with Noonan syndrome, a genetic disorder with mutations of genes in the MAPK pathway such as PTPN11, SOS1 and KRAS (Fryssira et al., 2008; Sandford et al., 1999;
Schuettpelz et al., 2009). Moreover, the consitutive activation of BRAF kinase activity stemming from loss of the BRAF N-terminal autoregulatory domain, as a consequence of a genetic fusion of an uncharacterized gene KIAA1549 with BRAF, have been detected in 66% of pilocytic astrocytoma, and is even capable of transforming NIH-3T3 cells (Jones et al., 2008, Sievert et al., 2009b). The KIAA1549:BRAF gene fusion appears to be a reminiscent characteristic of low grade gliomas specifically pilocytic astrocytomas (Dougherty et al., 2010; Schiffman et al., 2010; Jacob et al., 2009, Lawson et al., 2010). Other gene fusions in pilocytic astrocytoma includes the fusion of FAM131B:BRAF (Cin et al., 2011) or RAF1 and SRGAP3 (Jones et al., 2009; Cin et al., 2011; Forsew et al., 2009) resulting in constitutively active oncogenic fusion proteins. Single amino acid missense mutations in BRAF, specifically a change from valine to glutamate at position 600 (V600E) in BRAF (Davies et al., 2002) or from glutamine to glutamic acid at position 61 (Q61E) of KRAS (Cin et al., 2011; Forsew et al., 2009; Maltzman et al., 1997; Janzarik et al., 2007; Sharma et al., 2005), have also been reported in Pediatric low grade gliomas, but with occurences at a low frequency.
1.3.3.4.2 The cell cycle regulatory pathways
The principal role of the mammalian cell cycle is to replicate DNA and to segregate the DNA equally during mitosis into daugther cells. The DNA synthesis (S) and mitotic phase (M) are respectively preceded by the preparatory G1 and G2 phase, during which enzymes and proteins necessary for cell replication and division are synthesized. While the G0 phase is reserved for non-dividing quiescent cells which may re-enter the cell cycle at a later point (Sherr, 2000). Two major regulatory pathways, namely the Rb and p53, modulate cell cycle entry and transition from the G1 to S phase (Sherr, 2000). However, genes of both pathways are frequently mutated or aberrantly expressed in gliomas, thereby causing the uncontrolled cycling of tumor cells.
The Rb pathway
In the quiescent state, the hypophosphorylated retinoblastoma (Rb) protein binds and sequesters the E2F protein to prevent E2F mediated transcriptional activation of genes necessary for cell cycle progression (Sheer et al., 2000). However, the mitogenic (Sheer et al., 2000) or aberrant activation of AKT and MAPK in Pediatric gliomas (Jones et al., 2012b) may result in the activation of cyclin D1 which forms a complex with CDK4 and CDK6, to phosphorylate Rb.
14
Phosphorylated Rb then relinquishes its inhibitory constraint on E2F, thereby enabling the transcription of genes required for transition from the G1 to S phase (Sheer et al., 2000).
Several genes of the Rb pathway are mutated in glioma. Loss of chromosome 13q14, which contains the Rb1 gene, occurs in about 30-40% of adult high grade astrocytomas (Henson et al., 1994; Yin et al., 2009). Moreover, Retinoblastoma protein activity can be inactivated by mutations in genes that regulate its function including the gene amplifications of CDK4, CDK6 as well as loss of the CDKN2A (p16Ink4a and p14ARF) gene, which occurs at varying frequencies in gliomas (Yin et al., 2009; Preusser et al., 2006). Furthermore, in Pediatric high grade gliomas and diffuse intrinsic pontine gliomas (DIPG), copy number mutations in critical mediators of the Rb pathways have been detected including amplifications of the CCND1, CCND2, CCND3, CDK4 and CDK6 genes, and deletions of the CDKN1C gene (Barrow et al., 2011; Bax et al., 2010; Zarghooni et al., 2010; Paugh et al., 2011; Warren et al., 2012a); suggesting that the Rb pathway may be crucial for the pathogenesis of Pediatric gliomas. Moreover, a defect in the Rb pathway in conjunction with other activating mutations could account for differences in the malignancies of Pediatric gliomas. For example, pilocytic astrocytomas are slow growing and posses an intact CDKN2A/CDKN2B locus, but they have predominant mutations in genes of the MAPK pathway, leading to the cellular transformation into tumors which are very indolent (Jacob et al., 2011; Raabe et al., 2011). On the contrary, deletions of the CDKN2A/CDKN2B genes and a V600E BRAF activating mutation are common in Pediatric high grade gliomas and diffuse intrinsic pontine gliomas (Bax et al., 2010; Paugh et al., 2010; Puget et al., 2010 and 2012; Barrow et al., 2011, Schiffman et al., 2010; Qu et al., 2010; Wong et al., 2006), which are highly aggressive tumors.
The p53 pathway
The human TP53 gene encodes a transcription factor (393 amino acids) which modulates the expression of >2500 genes (Hoh et al., 2002; Levine et al., 2006) with examples ranging from the Pro-apoptotic BCL-2 proteins (e.g. BAX, BAK, PUMA, NOXA), caspases, death receptors (e.g FAS), DNA repair proteins, cell cycle inhibitors such as p21, and as well as enzymes involved in proteosomal degradation such as ubiquitin E3 ligase and MDM2 that negatively regulates p53 function (Hoh et al., 2002; Levine et al., 2006; Essmann and Schulze-Osthoff , 2012). Given its ability to integrate signals from different extrinsic and intrinsic stressors, as well as to restrict the
proliferation of cells with unstable genomes by inducing apoptosis or G1 arrest, p53 has gained the recognition as being a major cellular stress sensor. As a result, disruption of p53 function could lead to increases in cell proliferation and genomic instability which could render cells more susceptible to neoplastic growth (Bögler et al., 1995). In gliomas, aberrations of the P53 pathway could originate from either germline or acquired mutations of the p53 gene, loss of chromosome 17p which has the p53 gene, or from genes that regulate p53 function (Louis 1994; Louis and Cavenee 1997; Royds and Iacopetta, 2006). For instance, patients with the autosomal dominant disorder, Li-Fraumeni syndrome, have germline mutations in the p53 gene, and have an increased propensity for developing cancers including astrocytomas (Royds and Iacopetta, 2006; Malkin et al., 1990; Srivastava et al., 1990). In fact, mutations in the p53 gene are very common in Pediatric anaplastic astrocytomas and glioblastomas, with a prevalance of 20-34% among glioma patients less than 17 years of age (Schiffman et al., 2010; Pollack et al., 2001) and 40% among children older than 3 years (Pollack et al., 2001). However, only the over expression of p53 and not loss of p53 gene function seems to diminish progression free survival among the Pediatric glioma cases (Pollack et al., 2002). Other studies have noted that the CDKN2A locus which encodes p16Ink4a and p14ARF, are often pristine in pilocytic astrocytoms but instead frequently deleted in Pediatric high grade gliomas and diffuse intrinsic pontine gliomas. Interestingly, the human p14ARF protein attenuates the function of MDM2, a ubiquitin ligase that negatively regulates p53; hence providing an alternative method to inactivate p53 in Pediatric high grade gliomas (Honda and Yasuda, 1999; Kamijo et al., 1998; Stott et al., 1998; Pomerantz et al., 1998). Similarly, gliomas with losses of chromosome 1p fail to express the tumor suppressor known as chromodomain helicase DNA binding domain 5 (CHD5) which functions to faciliate the expression of p14ARF; hence providing another strategy to dysregulate the p53pathway (Bagchi et al., 2007).
1.3.4 Differences between Pediatric and Adult gliomas
As mentioned above, Pediatric gliomas are histological similar to Adult gliomas, but posses distinct molecular signatures that occur at unique frequencies when compared to Adult gliomas. For instance, Pediatric pilocytic astrocytomas usually have a normal karyotype, but with mutations in genes such as Rb, PTEN and TP53 (Jones et al., 2006; Pfister et al., 2008; Sanoudou et al., 2000; Shlomit et al., 2000; Wong et al., 2005). However, gains of chromosomes 5, 6, 7, 11, 15, and 20
16
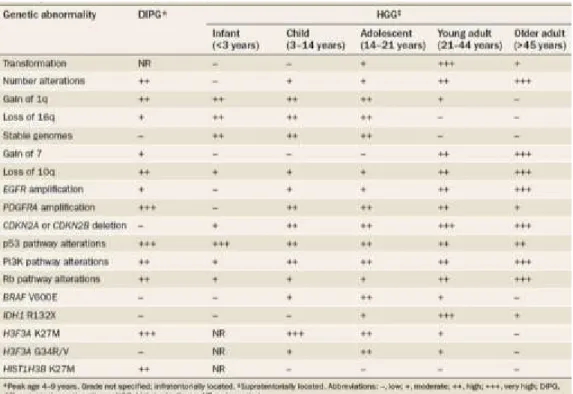
are frequent in Adult pilocytic astrocytomas (Jones et al., 2006; Pfister et al., 2008; Sanoudou et al., 2000; Shlomit et al., 2000; Wong et al., 2005). Apart from low grade gliomas, Table 1.1 provides examples of inherent molecular differences that can be further noted between Pediatric and Adult gliomas.
Table 1: Location and genetic differences in malignant gliomas.
Adapted from page 403, Nat Rev Clin Oncol. 2012;9(7):400-13, with permission.
1.4 Glioma therapy
The standard care of Pediatric patients with gliomas involves the surgical resection of tumors and coupled with adjuvant therapies like radiation and chemotherapy, when relevant. Most often the choice of therapy is dictated by the patient’s age, tumor location and tumor type. Patients with tumors having an indolent course such as optic pathway gliomas of Neurofibromatosis type 1, may be restricted to observation only since about 50% of these tumors fail to progress further (Hargrave, 2009). However, other tumors require an aggressive treatment modality in order to improve patient survival. It must be noted that overall, Pediatric glioma patients usually respond
better than Adult glioma patients to treatment (Ohgahi et al., 2004); possibly as a consequence of emerging evidence showing dissimiliar inherent molecular signatures and biology of tumors from these two age groups.
1.4.1 Surgery
Surgery remains the principal treatment for well-circumscribed resectable Pediatric high and low grade astrocytomas (Khatua et al., 2012; Mueller and Chang, 2009; Pollack et al., 2011a). It is often recommended for tumors with distinct boundaries which are amenable to gross total or near total resection such as those that are peripherally located in the cerebellar and cerebral cortex (Khatua et al., 2012; Mueller and Chang, 2009). In general, surgical resection can sometimes immensely decrease tumor volume, which is important to increase the efficacy of post-adjuvant treatments by radiation and/or chemotherapy on the residual unresected tumor. Surgical resection not only provides the tumorigenic tissues for histo-pathological diagnosis which is important for deciding follow-up therapies, but also decreases the size of the tumor mass, can prolong survival and sometimes can improve the overall quality of life for the patient (Hardesty et al., 2012; Stummer and Marcel, 2009; Carapella et al., 2011 ).
With the exception of pilocytic astrocytomas which have boundaries that are often well circumscribed, gliomas manifest predominantly a diffuse infiltrative behaviour with no definitive boundaries. Therefore, complete resection is often not feasible even with the aid of sophisticated imaging and intraoperative techniques. Moreover, the surgical resection of brain tumors among children is often associated with the significant risk of morbidity and mortality (Albright et al., 1986). Examples of post surgical complications include infections, bleeding, brain edema and stroke. Furthermore, neurological dysfunctions and even death can occur depending on what neighbouring functional neural networks at the resected tumor location are affected (Doxey et al., 1999; Mirnturn and Fisher, 2013). Considering that some astrocytomas are located at eloquent regions of the brain where surgical resection may be more harmful than beneficial, and the fact that gross or even partial resection can never completely remove the tumor mass, other therapeutic options such as chemotherapy and radiotherapy must be utilized to improve the overall survival of the patient.
18
1.4.2 Radiotherapy
Radiotherapy is recommended as a first line therapy for some unresectable Pediatric gliomas such as diffuse intrinsic pontine gliomas (DIPG) or as a post-operative treatment for residual disease (Urbanski et al., 2012; Mueller and Chang, 2009; Khatua et al., 2011). Generally, the procedure involves the use of computed tomography (CT) and magnetic resonance imaging (MRI) to first clearly define the tumor boundaries. This is then followed by the localized administration of 36-52 Gy photons (x-ray beams) in 1.8 G fractionations to the glioma mass of the Pediatric patient. Over the years, radiotherapy has undergone dramatic improvements as a therapeutic option for patients with brain tumors. For instance, the introduction of 3D treatment planning protocols which allows for a 30-40% reduction in volume of normal brain parenchyma exposed to irradiation compared to the 2D planning approach (Kortmann et al., 2000), could theoretically reduce long term morbidity and radiation associated sequelae. The use of rigid head immobilization and stereotactic irradiation approaches including stereotactic convergence therapy, fractionated stereotactic convergence therapy, fractionated conformal radiotherapy and intensity modulated radiotherapy can also permit the delivery of homogenously-focused irradiation fractionations to brain tumors of varying sizes, gradations and shapes (Kortmann et al., 2003). However, even though these advances have revolutionized radiotherapy, the physical property of X-rays including its high lateral side scatter and its toxic effect on normal tissues which are proximal and distal to the targeted tumor mass, limits its use among Pediatric patients who are younger than 3 years. Moreover, Pediatric patients treated by radiotherapy are often at risk of developing secondary malignancies and often succumb to neurodevelopmental abnormalities including serious long-term neurocognitive and neuroendocrine sequelae (Kortmann et al., 2003).
1.4.3 Chemotherapy
Chemotherapy plays a vital role in the treatment of cancers and is often administered adjuvantly or concomitantly with radiotherapy. It is usually recommended for children having disease progression after surgery and/or radiotherapy, as well as for those who are too young and therefore have been deferred from radiotherapy because of its associated toxicities (Burzynski, 2006). However, it must be noted that, no current Food and Drug administration (FDA) approved
chemotherapeutic agents show promising efficacies for the treatment of Pediatric astrocytomas by significantly improving overall survival; even when used in conjunction with other chemotherapeutic agents. Furthermore, dismal results still remain from current clinical trials; hence necessitating the identification and characterization of new chemotherapeutic agents for the treatment of Pediatric gliomas. Below is a review of the efficacy and safety of the most common therapeutic agents used for the treatment of Pediatric gliomas.
1.4.3.1 Alkylating agents
Akylating agents include analogues of nitrogen mustards, nitorsureas, platinum derivatives, thiopeta and so on (Mattes et al., 1986; Pizzo et al., 2006; Porquier 2011). They generally function by chemically modifying DNA with an alkyl group or by inducing monoadducts, intrastrand and interstrand crosslinks, which attenuates DNA replication leading to cell cycle arrest and apoptosis (Cruet-Hennequart et al., 2008; Pourquier 2011; Pizzo et al., 2006; Mattes et al., 1996). Summarized below, are the findings from clinical trials involving the most commonly used alkylating agents investigated to treat Pediatric gliomas.
1.4.3.1.1 Temozolomide
Following its discovery in the 1970s and subsequent pre-clinical studies demonstrating its anti-neoplastic properties, the alkylating imidazotetrazine derivative, temozolomide (TMZ), eventually became an FDA approved chemotherapeutic drug in 1999 for the experimental treatment of Adult patients with refractory anaplastic astrocytomas (Mutter et al., 2006). However, ongoing clinical trials, including the landmark Phase III trial conducted by the European Organization for Research and Treatment of Cancer (EORTC), unfortunately noted that the adjuvant post-operative use of temozolomide and radiotherapy only had a modest improvement in less than 20% of the patients by increasing the median overall survival to 14.6 months for newly diagnosed glioblastoma patients, when compared to 12.1 months among similar patients treated by post-operative radiotherapy only (Stupp et al., 2005). Since 2005, temozolomide is the current recommended drug for the treatment of Adult patients with newly diagnosed glioblastoma multiforme concomitantly with radiotherapy, and for use as maintenance therapy (Cohen et al., 2005). Temozolomide is administered orally, with a bioavailability of 96-100% and half-life of 1.8 hours (Brada et al., 1999; Stupp et al., 2001). At physiologic pH, temozolomide is converted to a reactive intermediate known
20
as 5-(3-methyl-1-triazeno) imidazole-4-carboxamide (MTIC) (Denny et al., 19 94; Stevens et al., 1987), which subsequently methylates the O6 or N7 -position of guanine as well as the N3 -position of Adenine. If left unrepaired by the cellular machinery, cells become susceptible to undergoing apoptosis (Roos et al., 2007; Hegi et al., 2008).
1.4.3.1.2 Clinical trials with Temozolomide in Pediatric glioma
Several clinical trials involving panels of Pediatric glioma patients were conducted to assess the efficacy and toxicity of temozolomide used alone or concomitantly with other chemotherapeutic agents and radiotherapy (Kuo et al., 2003; Lashford et al., 2002; Bartels et al., 2011; Nicholson et al., 2007; Jakacki et al., 2008; Kim et al., 2010; Jalili et al., 2010; Chiang et al., 2010). Unfortunately, only a minimal number of Pediatric patients showing signs of complete/partial response or disease stabilization when treated with temozolomide. Furthermore, adverse side effects are noted with the use of temozolomide, including thrombocytopenia, nausea, emesis, fatigue, myelosuppression, sepsis, pneumonia and even death (Kuo et al., 2003; Lashford et al., 2002).
According to the Canadian Paediatric Brain Tumor Consortium, about 96 children with glioma from a population of 137 pediatric brain tumor cases were experimentally treated with temozolomide between 2000 and 2006. The short-term responses appeared to be promising with disease stabilization noted in about 83% of patients (Bartels et al., 2011). For instance the overall survival at 1-year and 2-years post temozolomide treatment of high grade gliomas was 62% and 25.7% respectively, and 75% and 71.1% respectively for low grade gliomas (Bartels et al., 2011). However, complete response was rare and occurred in only one patient with low grade gliomas and three patients with high grade gliomas who eventually died of disease progression at a median of 26 months (Bartels et al., 2011). These dissappionting results were replicated in other studies including an early report on Pediatric patients diagnosed with progressive gliomas, treated with two different treatment regimens comprising of doses of temozolomide used daily at 150 mg/m2 for 5 consecutive days and 28 day cycle, or at 75 mg/m2 for 42 days (Kuo et al., 2003). Such study resulted in only partial responses and disease stabilization among Pediatric low grade glioma patients (Kuo et al., 2003; Nicholson et al., 2007). Furthemore, a multicenter Phase II clinical trial jointly conducted by the United Kingdom Children's Cancer Study Group and the French Society