Par
Adam Langlois
Thèse présentée au département de chimie en vue de l’obtention Du grade de docteur ès sciences (Ph.D.)
FACULTÉ DES SCIENCES
ii
By
Adam Langlois
Thesis presented to the department of chemistry in order to obtain the rank of Doctor of Science (Ph.D.)
FACULTY OF SCIENCE
iii Membres du jury
Professeur Pierre D. Harvey Directeur de recherche Département de chimie Dr. Dale Wood Évaluateur externe Département de chimie Université de Bishop
Professeur Jérôme Claverie Évaluateur interne Département de chimie
Professeur Patrick Ayotte Président-rapporteur Département de chimie
iv
To my family, friends and mentors.
v domaine des dispositifs photovoltaïques naturelles ou artificielles. En effet, le transfert d’énergie est un processus que l’on observe dans la nature au sein de tous les organismes phototrophes depuis les végétaux multicellulaires complexes jusqu’aux bactéries unicellulaires photosynthétiques. Par exemple, dans le cas des bactéries photosynthétiques pourpres, ces dernières utilisent un photosystème de deux protéines assemblées, la première étant appelé protéine collectrice de lumière 1 (LH1 pour light-harvesting protein 1) et la seconde appelé protéine collectrice de lumière 2 (LH2 pour light-harvesting protein 2) afin de capter suffisamment d’énergie lumineuse pour assurer leur survie. La protéine LH2 n’a pour vocation que d’absorber et de transmettre l’énergie lumineuse à la protéine LH1, qui contient une paire spéciale dans un centre réactionnel. Les transferts d’énergie sont des phénomènes essentiels à la survie des organismes. Un photon absorbé par une molécule de type bactériochlorophylle dans la protéine LH2 subira un transfert d’énergie efficace à d’autres bactériochlorophylles au sein de la même structure protéique. Les transferts d’énergie se dérouleront aussi bien entre différentes protéines LH2 qu’entre des protéines LH1 et LH2. Ces processus de transfert d’énergie servent à canaliser l’énergie lumineuse jusqu’au centre réactionnel qui devient à son tour excité par transfert d’énergie. Ces processus sont hautement efficaces et essentiels à la survie de l’organisme en question.
En science des matériaux, la conception d'un assemblage donneur-accepteur, covalent ou non, qui présente un transfert d'énergie efficace est un sujet d'intérêt pour des applications en photovoltaïque et diodes émettrices de lumière. En utilisant les bactéries pourpres photosynthétiques comme modèle, des structures similaires étudiant différents colorants permettant d'absorber et de transmettre de l'énergie lumineuse à un squelette central (un processus appelé effet antenne) font l'objet de recherches actives. Le principe étant que l'utilisation de ces antennes permet d'absorber plus de lumière dans les régions du spectre électromagnétique qu’il serait impossible d’obtenir avec un seul colorant. La conséquence est que, pour que le processus fonctionne efficacement, le transfert d'énergie entre l'antenne et le squelette doit être rapide, et parfois contrôlé.
Dans ce travail, nous étudierons les processus de transfert d'énergie entre des colorants oligopyrroliques reliés par un noyau truxène, qui montre une similarité structurale avec le
vi
le donneur et l'accepteur ouvre la porte à l’hypothèse de la présence d'un double processus de transfert d'énergie régi par les mécanismes Förster et Dexter. Nous démontrerons que l'utilisation de ce système conjugué conduit à un processus de transfert d'énergie très rapide malgré la distance importante séparant le donneur et l’accepteur. Nous démontrerons en outre que le processus, bien qu'il s'agisse d'un double processus, est dominé par le processus Dexter grâce au système conjugué reliant le donneur et l'accepteur qui fait office de pont communiquant. Les processus de transfert d'énergie rapides et efficaces suggèrent que, pour tirer pleinement parti de l'effet antenne dans des applications photovoltaïques, les designs devraient être basés sur l'utilisation de ponts conjugués reliant donneurs et accepteurs.
Le travail présenté dans cette thèse est divisé en huit sections. Dans l'introduction, une brève description des chromophores utilisés tout au long du présent travail sera fournie avec des concepts généraux non-exhaustifs pour la théorie de la fonctionnelle de la densité (DFT) qui a été utilisé comme outil tout au long des travaux actuels pour démontrer un certain degré de couplage orbitalaire. Le chapitre 1, intitulé Les principes fondamentaux de la photophysique, proposera une introduction à la théorie nécessaire à la bonne compréhension des travaux présentés dans cette thèse. Le chapitre 2 est simplement intitulé Instrumentation et fournira une description des instruments utilisés tout au long des travaux. Au chapitre 3: « Maple™-Assisted Calculations of
the J-integral: A Key Parameter for the Understanding of Excited State Energy Transfer in Porphyrins and other Chromophores », une description détaillée de l'intégrale J ainsi qu’un outil
pour le calcul à partir de données spectrales seront exposés.
L'étude des processus de transfert d'énergie entre les chromophores pontés par truxène commencera au chapitre 4: « Origin of the Temperature Dependence of the Rate of Singlet Energy
Transfer in a Three-Component Truxene-bridged Dyads ». Dans ce chapitre, nous étudierons le
transfert d'énergie entre un donneur de type zinc(II)-porphyrine et un ensemble d'accepteurs de porphyrine base libre. Des preuves circonstancielles indiquant que le processus de transfert d'énergie observé se produit à travers un double mécanisme qui peut être dominé par le mécanisme Dexter seront présentées.
vii transfert d'énergie entre un donneur BODIPY et deux accepteurs de type Zn-porphyrine. Dans ce chapitre, la comparaison du processus de transfert d'énergie à une dyade similaire qui contient un pont non-conjugué entre le donneur et l'accepteur sera effectuée et il sera démontré que la dyade ponté par truxène présente non seulement un taux plus rapide, mais que ce taux ne peut être expliqué que par un processus Dexter dominant.
Au chapitre 6 : « Very Fast Singlet and Triplet Energy Transfers in a Tri-chromophoric Porphyrin
Dyad Aided by the Truxene Platform », l'étude du transfert d'énergie entre une porphyrine de
palladium(II) donneuse et une paire d'accepteurs de type zinc(II)-porphyrine pontés par un noyau de truxène sera montré. Ici, un processus de transfert d'énergie triplet très rapide est observé, ce qui prouve que le système conjugué favorise le processus Dexter et conduit à un transfert efficace d'énergie du donneur vers l'accepteur.
Enfin, le chapitre 7 présentera le dernier travail inclus dans cette thèse. Le chapitre 7 est intitulé
« Excited State N-H Tautomer Selectivity in the Singlet Energy Transfer in a Zinc(II)Porphyrin-Truxene-Corrole Assembly » et exposera une dernière fois un processus de transfert d'énergie très
rapide et efficace. Dans ce travail, le transfert d'énergie se produit entre un donneur de type Zn-porphyrine et une corrole base libre acceptrice. Le processus de transfert d'énergie rapide présente une constante de vitesse qui se situe dans le même ordre de grandeur que ceux présentés dans les chapitres précédents, ce qui suggère que le processus se produit par le biais du même double mécanisme dominé par Dexter. Il est intéressant de noter que, dans ce dernier cas, le processus de transfert d'énergie s'est révélé sélectif sur l'une des deux espèces tautomériques du corrole. Ceci a mené à une étude sur les taux de tautomérisation de l'état excité de la corrole base libre conduisant à la premier mesure expérimentale du taux de tautomérisation de la corrole base libre.
Cette thèse s’achèvera par une discussion générale sur les travaux présentés dans ces pages ainsi que sur l'impact que les résultats ont eus dans communauté scientifique dans ce domaine.
viii
natural and laboratory photonic devices. Indeed, energy transfer is a process seen in nature in all photosynthetic organisms from complex multicellular plants to simple, single cell photosynthetic bacteria. For example, the purple photosynthetic bacteria uses two protein assemblies, referred to as the light-harvesting protein 1 (LH1) and the light-harvesting protein 2 (LH2), to collect light energy in order to survive. The LH2 protein serves only to absorb and transmit light energy to the LH1, which contains a special pair in a central reaction center. Energy transfer is essential to the survival of the organism. A photon of light absorbed by a bacteriochlorophyll molecule in the LH2 protein will undergo efficient energy transfer to other bacteriochlorophylls within the same protein structure. Energy transfer will also occur between different LH2 proteins and between the LH2 and LH1 protein. These energy transfer processes all serve to funnel the light to the reaction center which itself is excited by energy transfer. This process is highly efficient and essential to the organism’s survival.
In the area of material sciences, the design of a covalent or non-covalent donor-acceptor assembly that exhibits efficient energy transfer, is a topic of interest for application in solar energy and light emitting diodes. Using the purple photosynthetic bacteria as a model, designs that append different dyes that serve to absorb and transmit light energy to a central backbone (a process referred to as the antenna effect) are being investigated. The principle being that the use of these antenna allows for the absorption of more light in regions of the electromagnetic spectrum that we cannot necessarily obtain with a single dye. The fall-back is that, in order for the process to work efficiently, the energy transfer between the antenna and backbone must be rapid.
This work presents an investigation of the energy transfer processes between oligopyrrole dyes that are bridged by a truxene core, which exhibits a structural similarity to graphene. The aim of this work is to further understand the energy transfer processes between chromophores. We demonstrate in our work that the presence of a conjugated bridge between the donor and acceptor provides the possibility of a dual energy transfer process governed by both the Förster and Dexter mechanisms. We demonstrate that the use of this conjugated bridge leads to a very fast energy transfer process despite the large distance that separates the donor and acceptor. We further demonstrate that the process, although being a dual process, is dominated by the Dexter
ix antenna effect in man-made photonic devices, designs should be built upon the use of conjugated bridges between the donor and acceptor.
The work presented in this thesis is divided into eight sections. In the introduction, a brief description of the chromophores that are seen throughout the rest of this work, is provided along with some general concepts with regard to density functional theory (DFT), which was employed as a tool throughout the presented works to demonstrate a certain degree of molecular orbital coupling. Chapter 1, entitled The Basic Principles of Photophysics, provides an introductory explanation of the theory that is required to fully understand the works that are presented in this thesis. Chapter 2 is simply entitled Instrumentation and serves to provide a description of the instruments used throughout the works. In Chapter 3: Maple™-Assisted Calculations of the
J-integral: A Key Parameter for the Understanding of Excited State Energy Transfer in Porphyrins and other Chromophores a detailed description of the J-integral is provided and a tool for is
calculation from spectral data is presented.
The investigation of the energy transfer processes between truxene bridged chromophores begins in Chapter 4: Origin of the Temperature Dependence of the Rate of Singlet Energy Transfer in a
Three-Component Truxene-bridged Dyads. In this chapter, the energy transfer between a
Zn-porphyrin donor and a set of free-base Zn-porphyrin acceptors is investigated. Circumstantial evidence suggests that the energy transfer process that is observed, is occurring through a dual mechanism that may be dominated by the Dexter mechanism is provided.
Chapter 5: Antenna Effect in Truxene-bridged BODIPY Triarylzinc(II)porphyrin Dyads: Evidence
for a Dual Dexter-Förster Mechanism presents the investigation of the energy transfer processes
between a BODIPY donor and two zinc(II)-porphyrin acceptors. In this chapter the comparison of the the energy transfer process to a similar dyad, that contains a non-conjugated bridge between the donor and acceptor, is made and it is shown that the truxene bridged dyad not only presents a faster rate, but that this faster rate can only be explained by a Dexter dominant process.
In Chapter 6: Very Fast Singlet and Triplet Energy Transfers in a Tri-chromophoric Porphyrin
Dyad Aided by the Truxene Platform the investigation of the energy transfer between a
x
the donor to the acceptor.
Finally, Chapter 7 presents the last work that is included in this thesis. Chapter 7 is entitled Excited
State N-H Tautomer Selectivity in the Singlet Energy Transfer in a Zinc(II)Porphyrin-Truxene-Corrole Assembly and once again presents a very fast and efficient energy transfer process. In this
work the energy transfer occurs between a Zn-porphyrin donor and a set to free-base corrole acceptors. The rapid energy transfer process exhibits a rate constant that falls in the same order of magnitude of those presented in the earlier chapters, suggesting that the process is occurring through the same dual mechanism that is Dexter dominated. Interestingly, in this last the energy transfer process was found to occur selectively to only one of the two corrole tautomeric species. This prompted an investigation into the excited state tautomerization rates of the free base corrole and lead to the first report of an experimentally measured tautomerization rate from free-base corrole.
This thesis closes with a general discussion of the works presented within its pages and a discussion of the impact that the results have on the scientific community.
xi Firstly, I would like to thank my research supervisor Pr. Pierre D. Harvey whose supervision, encouragement and guidance has helped me to survive my thesis. Working not for, but alongside you has been an honour.
I would also like to thank all of the student members of the Harvey group that I have worked with in my long years as part of the Harvey group, you made working here a delight. Thank you to: Mohammed Abdelhameed, Shawkat Aly, Antoine Bonnot, Bertrand Brisset, Léo Bucher, Di Gao, Frank Juvenal, Antony Lapprand, Hu Lei, Peng Luo, Gabriel Marineau Plante, Adrien Schlachter, Ahmed Soliman, Loïc Tanguy, Gang Wang and Xiaorong Wang.
My thanks extend to all of the members (professors and support staff) of the chemistry departments at the Université de Sherbrooke. I would like to express a special thanks to Paul-Ludovic Karsenti who spent countless hours with me making ultrafast photophysical measurements and for teaching me that just because something looks simple does not mean that it is.
I would also like to extend thanks to the French and Chinese collaborators that I have had the honour of working with. Thank you Prof. Claude Gros, Prof. Roger Guilard, Dr. Romain Ruppert, Dr. Sébastien Richeter, Prof.Silvain Jugé, and Prof.Wai-Yeung Wong.
Thank you to the interns that put up with my supervision (Jude Deschamps and Antoine Buisson). You may not know it, but you taught me just as much, if not more, than what I taught you.
Thank you to the friends, both new and old that I have met along my journey through this thesis, may our friendships extend well beyond the walls of the university.
I would like to sincerely thank my past mentors who have guided me to where I am today. Jodi Coleman, my high school science teacher who opened my eyes to the word of the sciences at an age of twelve, I never would have taken this path had you not set me on it. Patrick Draper and Catherine Filteau, my CEGEP chemistry teachers who showed me the wonders of chemistry. Your outstanding teaching abilities made a mark on me and will stick with me all of my life. Dale Wood, my inorganic chemistry teacher at Bishop’s University, you helped me to grow not only as a chemist but as a person. You showed me how to have confidence in myself, without which I would never have made it to where I am today.
xii
science with you but you, have always been willing to listen anyway. You have helped me overcome many obstacles and have helped me to become the man I am today.
I would like to thank the funding agencies: Le Fonds de Recherche du Québec: Nature et Technologies (FQRNT), the Nature Sciences and Engineering Research Council of Canada (NSERC) that supported my work during my thesis. I would also like to thank NSERC for the Alexander Graham Bell scholarship. Finally, I would like to thank the Université de Sherbrooke for accepting me into their program in order to carry out my studies.
xiii
ABSTRACT --- vii
ACKNOLEDGEMENTS --- x
TABLE OF CONTENTS --- xii
LIST OF PUBLICATIONS INCLUDED IN THIS THESIS --- xvii
LIST OF PUBLICATIONS NOT INCUDED IN THIS THESIS --- xviii
LIST OF ABBREVIATIONS --- xx
LIST OF EQUATIONS--- xxiii
LIST OF FIGURES --- xxvi
LIST OF TABLES --- xxx
LIST OF SCHEMES --- xxi
INTRODUCTION --- 1
I.1 Oligopyrrole dyes --- 2
I.1.1 Porphyrin --- 3
I.1.1.1 Porphyrin structure --- 3
I.1.1.2 Porphyrin synthesis --- 5
I.1.1.3 Porphyrin properties --- 11
I.1.2 Boron-dipyrromethene (BODIPY) --- 14
I.1.2.1 BODIPY structure--- 14
I.1.2.2 BODIPY synthesis --- 15
I.1.2.3 BODIPY properties --- 17
I.1.3 Corrole --- 17
I.1.3.1 Corrole structure --- 17
I.1.3.2 Corrole synthesis --- 18
I.1.3.3 Corrole properties --- 20
xiv
I.2 Density Functional Theory --- 22
I.3 Objective of this thesis --- 27
CHAPTER 1: The Basic Principles of Photophysics --- 29
1.1 Photochemistry vs. Photophysics --- 29
1.1.1 Photochemical processes --- 29
1.1.2 Photophysical processes --- 30
1.1.3 Singlet and triplet states in diamagnetic molecules --- 31
1.2 Electronic transitions between states --- 32
1.2.1 The electromagnetic spectrum --- 32
1.2.2 Absorption of light --- 33
1.2.3 Absorption spectroscopy --- 34
1.2.4 Types of electronic transitions--- 36
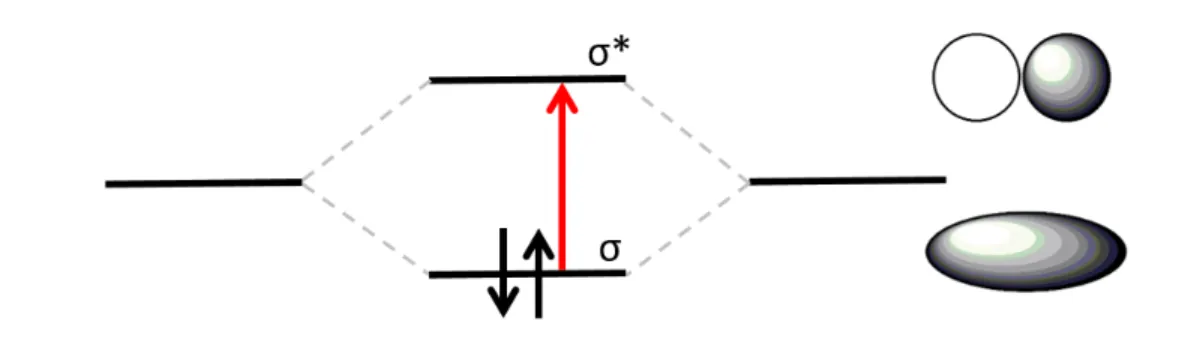
1.2.4.1 σ → σ* transitions --- 37
1.2.4.2 π → π* transitions --- 38
1.2.4.3 n → π* transitions --- 39
1.2.4.4 Charge Transfer (CT) transitions --- 39
1.2.4.5 Ligand field transition --- 41
1.3 Electronic transition probability --- 43
1.3.1 Absorption intensity and transitions probability --- 43
1.3.2 Calculation of the probability of transition --- 44
1.4 Selection rules --- 45
1.4.1 Spin section rule --- 45
1.4.2 The breakdown of the spin selection rule (spin-orbit coupling) --- 46
1.4.3 Laporte selection rule --- 49
xv
1.5 The Franck-Condon term and its relationship to the shape of spectra. --- 54
1.5.1 The Frank-Condon Principle (radiative transitions between states) --- 54
1.5.2 Franck-Condon Principle (non-radiative transitions between states) --- 57
1.5.2.1 Internal conversion within a given state --- 57
1.5.2.2 Internal conversion between states --- 58
1.6 The Jablonski diagram --- 60
1.7 Luminescence --- 63
1.8 Excited state kinetics --- 64
1.8.1 Excited state lifetimes --- 64
1.8.2 Quantum yields --- 64
1.9 Bimolecular photophysical processes --- 68
1.9.1 Bimolecular energy transfer --- 69
1.9.1.1 Trivial energy transfer --- 72
1.9.1.2 Energy transfer by electron exchange --- 73
1.9.1.3 Energy transfer by dipole-dipole interaction --- 77
1.9.1.4 Mixed energy transfer mechanisms --- 79
CHAPTER 2: Instrumentation --- 81
2.1 Introduction --- 81
2.2 Photo-detection --- 81
2.2.1 Photomultiplier tube (PMT) --- 81
2.2.2 Photodiodes --- 88
2.2.3 Charge coupled device (CCD) --- 88
2.3 Light sources --- 89
2.4 Monochromators --- 91
2.5 Emission quantum yield measurements --- 92
xvi
2.6.1.2 Methodology --- 100
2.6.2 Multi-channel scaling --- 101
2.7 Instrumentation --- 102
2.7.1 UV-Visible absorption spectroscopy --- 102
2.7.2 Fluorescence / Phosphorescence spectroscopy --- 104
2.8 Ultrafast time-resolved spectroscopy --- 106
2.8.1 Ultrafast photoluminescence--- 107
2.8.2 Transient absorption spectroscopy --- 109
CHAPTER 3: Maple™-Assisted Calculations of the J-integral: A Key Parameter for the Understanding of Excited State Energy Transfer in Porphyrins and other Chromophores --- 115
3.1 The project --- 115
3.2 Article published in Journal of Porphyrins and Phthalocyanines. 2014, 18, 666-674. --- 116
3.3 Supporting Information --- 135
CHAPTER 4: Origin of the Temperature Dependence of the Rate of Singlet Energy Transfer in a Three-Component Truxene-bridged Dyads. --- 149
4.1 The project --- 149
4.2 Article published in Journal of Porphyrins and Phthalocyanines. 2014, 18, 94-106. --- 151
xvii
4.1 The project --- 185
4.2 Article published in Dalton Transactions. 2014, 42, 8219-8229. --- 187
4.3 Supporting Information --- 209
CHAPTER 6: Very Fast Singlet and Triplet Energy Transfers in a Tri-chromophoric Porphyrin Dyad Aided by the Truxene Platform. --- 221
4.1 The project --- 221
4.2 Article published in Journal of Porphyrins and Phthalocyanines. 2015, 19, 427-441. --- 223
4.3 Supporting Information --- 250
CHAPTER 7: Dyads Excited State N-H Tautomer Selectivity in the Singlet Energy Transfer in a Zinc(II)Porphyrin-Truxene-Corrole Assembly. --- 295
4.1 The project --- 295
4.2 Article published in Chemistry A European Journal. 2017, 23, 5015-5022. --- 297
4.3 Supporting Information --- 334
CHAPTER 8: Discussion and Conclusions. --- 371
8.1 Synthesis --- 371
8.2 Energy transfer --- 376
8.3 Temperature dependence --- 377
8.4 Through bond energy transfer (TBET) --- 383
8.5 Excited state tautomerization --- 385
CONCLUSION --- 387
xviii
1. Langlois, A; Harvey, P. D. Maple Assisted Calculation of the J-Integral Overlap: A Key Parameter for the Understanding of Photo-induced Energy Transfer. J. Porphyrins
Phthalocyanins. 2014, 18, 666-674.
2. Langlois, A; Xu, H-J; Brizet, B; Denat, F; Barbe, J-M; Gros, C. P; Harvey, P.D. Origin of the Temperature Dependence of the Rate of Singlet Energy Transfer in a Three Component Truxene-bridged Dyads. J. Porphyrins Phthalocyanines. 2014, 18, 94-106.
3. Xu, H-J; Bonnot, A; Karsenti, P-L; Langlois, A; Abdelhameed, M; Barbe, J-M; Gros, C. P; Harvey, P.D. Antenna Effect in Truxene-bridged BODIPY Triarylzinc(II)porphyrin Dyads: Evidence for a Dual Dexter-Förster Mechanism. Dalton Trans. 2014, 42, 8219-8229.
4. Langlois, A; Xu, H-J; Karsenti, P-L, Gros, C. P; Harvey, P.D. Very Fast Singlet and Triplet Energy Transfers in a Tri-chromophoric Porphyrin Dyad Aided by the Truxene Platform.
J. Porphyrins Phthalocyanines, 2015, 19, 427-441.
5. Langlois, A; Xu, H-J; Karsenti, P-L; Gros, C. P; Harvey, P.D. Excited State N-H Tautomer Selectivity in the Singlet Energy Transfer in a Zinc(II)Porphyrin-Truxene-Corrole Assembly. Chem. Euro. J., 2017, 23, 5010-5022.
xix 1. Lei, H; Langlois, A; Fortin, D; Karsenti, P-L; Aly, S. M; Harvey, P.D. Rendering the
Cross-conjugated Azophenine Derivatives Emissive to Probe the Silent Photophysical Properties of Emeraldine. Phys. Chem. Chem. Phys. 2017, 19, 21532-21539.
2. Langlois, A; Camus, J-M; Karsenti, P-L; Guilard, R; Harvey, P. D. Metal-dependence on the bidirectionality and reversibility of the singlet energy transfer in artificial special pair-containing dyads. Inorg. Chem. 2017, 56(5), 2506-2517.
3. Juvenal, F; Langlois, A; Bonnot, A; Fortin, D, Harvey, P. D. Luminescent 1D- and 2D-Coordination Polymers using CuX Salts (X = Cl, Br,I) and a Metal-containing Dithioether Ligand. Inorg. Chem. 2016, 55(21), 11096-11109.
4. Dekkiche, H; Buisson, A; Langlois A; Karsenti, P-L; Ruhlmann, L; Harvey, P. D; Ruppert, R. Ultrafast singlet energy transfer in porphyrin dyads. Inorg. Chem. 2016, 50(20), 10329-10366. 5. Deschamps, J; Langlois, A; Martin, G; Bucher, L; Desbois, N; Gros, C. P; Harvey, P. D.
Cyclotriveratrylene-containing Porphyrins. Inorg. Chem. 2016, 55 (18), 9230–9239.
6. Tan, G; Shuming Chen, S; Siu, H; Langlois, A;, Yongfu Qiu, Y; Fan, H; Cheuk-Lam Ho, C-L; Harvey, P. D; Lo, Y.H; Liu, C-L; Wong, W-Y. Platinum(II) cyclometallates featuring broad emission bands and their applications in color-tunable OLEDs and high color-rendering WOLEDs. J. Mater. Chem. C. 2016, 25(4), 6016-6026.
7. Dekkiche, H; Buisson, A; Langlois, A; Karsenti, P-L; Rhulmann, L; Ruppert, R; Harvey, P. D. Metal Linkage Effect on Ultrafast Singlet Energy Transfer. Chem. Eur. J. 2016, 22(30), 10484– 10493.
8. Deschamps, J; Chang, Y; Langlois, A; Desbois, N; Gros, C. P; Harvey, P. D. The First Example of Cofacial Bis(dipyrrinphenol)s. New. J. Chem. 2016, 7(40), 5835-5845.
9. Longevial, J-F; Langlois, A; Buisson, A; Devillers, C. H; Clément, S; van der Lee, A; Harvey, P. D; Richeter, S. Synthesis, Characterization and Electronic Properties of Porphyrins Conjugated with N-Heterocyclic Carbene (NHC) Gold(I) Complexes. Organometallics. 2016, 35 (5), 663-672.
10. Bayardon, J; Maronnat, M; Langlois, A; Rousselin, Y; Harvey, P. D.; Jugé, S. Modular Chirogenic Phosphine-Sulfide-Ligands: Clear Evidence for Both Electronic Effect and
P-xx
11. Wu, F; Tong, H; Li, J; Li, Z; Adachi, C; Langlois, A; Harvey, P. D; Wong, W-Y and Zhu, X. Phosphorescent Cu(I) complexes based on bis(pyrazol-1-yl-methyl)-pyridine derivatives for organic light emitting diodes. J. Mater. Chem. C. 2015, 3, 138-146.
12. Abdelhameed, M; Langlois, A; Karsenti, P-L; Richeter, S; Ruppert, R; Harvey, P. D. Ultrafast Energy Transfer in a Pd(II)-Bridged Bisporphyrin Dyad. Chem. Commun. 2014, 50 (93), 14609-14612.
13. Abdelhameed, M; Langlois, A; Fortin, D; Karsenti, P-L; Harvey P. D. Drastic Substituent Effect on the Emission Properties of Quinone diimine Models and Valuable Insight on the Excited States of Emeraldine. Chem. Commun. 2014, 50, 11214-11217.
14. Abdelhameed, M; Karsenti, P-L; Langlois, A; Lefebvre, J-F; Richeter, S; Ruppert, R; Harvey, P. D. Unexpected Drastic Decrease in the Excited State Electronic Communication Between Porphyrin Chromophores Covalently Linked by a Palladium(II) Bridge. Chem. Eur. J. 2014, 40, 12988-13001.
15. Brizet, B; Desbois, N; Bonnot, A; Langlois, A; Dubois, A; Barbe, J-M; Gros, C; Goze, C; Denat, F; Harvey, P. D. Slow and fast singlet energy transfers in BODIPY-gallium(III)corrole dyads linked by flexible chains. Inorg. Chem. 2014, 53(7), 3392-3403.
16. Camus, J-M; Langlois, A; Aly, S; Guillard, R; Harvey, P. D. Is the special pair structure a good strategy for the kinetics during the last step of the energy transfer with the nearest antenna? A chemical model approach. Chem. Commun., 2013, 49, 2228-2230.
17. Camus, J-M; Langlois, A; Aly, S; Guillard, R; Harvey, P. D. Evidence for reverse pathways and equilibrium in singlet energy transfer between artificial special pair and antenna. J.
Porphyrins Phthalocyanines. 2013, 17, 722-732.
18. Harvey, P. D; Langlois, A; Filatov, M; Fortin, D; Ohkubo, K; Fukuzumi, S; Guilard, R. Decoupling the Artificial Special Pair to Slow Down the Rate of Singlet Energy Transfer. J.
Porphyrins Phthalocyanines, 2012, 16, 685–694.
19. Du, B; Langlois, A; Fortin, D; Stern, C; Harvey, P. D. Complete Quenching of the Pd3(dppm)3(CO)2+ Cluster Emission via Electrostatic Host-Guest Assemblies With Carboxylate-Containing Tetraphenylporphyrins of Ni(II) and Fe(III). J. Cluster Sci. 2012, 23, 737-751.
xxi
%T --- Percent Transmittance [c] --- Sample concentration A --- Absorbance
A --- Ground state energy acceptor A* --- Excited state energy acceptor Ae --- Ground state electron acceptor Ae* --- Excited state electron acceptor BO --- Bond order
BODIPY -- Boron dipyrromethene
c --- The speed of light in a vacuum
CCD --- Charge coupled device CT --- Charge Transfer
D --- Ground state energy donor D* --- Excited state energy donor De --- Ground state electron donor De* --- Excited state electron donor De+ +Ae- --- Radical ion pair
DFT --- Density Functional Theory E --- Energy
eV --- Electronvolt FC --- Franck-Condon
FHG --- Fourth harmonic generation FRET --- Förster Resonance Energy Transfer
fs --- Femtosecond (1 x10-15 s) FWHM ---- Full width at half maximum
g --- gerade (symmetric with respect
to a center of inversion) H --- Hamiltonian operator
h --- Plank’s constant
(6.626 x 10-34Js) HDex --- Dexter operator HFör --- Förster operator
HOMO--- Highest occupied molecular orbital
HSO --- Spin-orbit operator Hz --- Hertz
ℎ𝜈 --- A photon of light i --- center of inversion
I --- Ground state intermediate I* --- Excited state intermediate IC --- Internal conversion
ICCD --- Intensified charge coupled device
Ifinal --- Final light intensity
Iinit --- Initial light intensity
IP --- Internal conversion between states of different multiplicity IRF --- Instrument response function ISC --- Intersystem crossing
J --- Joules
J --- Spectral overlap integral
K --- Pre-exponential factor in Dexter
xxii
kFör --- Rate of Förster Energy transfer kHz --- Kilohertz (1 MHz =103 Hz) kIC --- Internal conversion rate constant (states of the same multiplicity) kIP --- Internal conversion rate constant (states of different multiplicity) kISC --- Intersystem crossing rate constant
kP --- Phosphorescence rate constant L --- Orbital angular momentum
l --- Sample pathlength
L --- Sum of Van der Waals radii
LMCT --- Ligand-to-Metal Charge Transfer
LUMO ---- Lowest un- occupied molecular orbital
MCP --- Micro-channel plate
MHz --- Megahertz (1 MHz =106 Hz) MLCT --- Metal-to-Ligand Charge Transfer
ms --- Electron quantum spin number ms --- Millisecond (1 x10-3 s)
n --- non-bonding orbital
n --- Refractive index Na --- Avogadro’s number (6.022 x1023 Mol-1)
Nantibonding- Number of antibonding electrons Nbonding--- Number of bonding electrons nm --- Nanometers
OPA --- Optical parametric amplifier P --- Ground state product molecule P --- Probability of transition P* --- Excited state product molecule PCS --- Photoconductive switch PMT --- Photomultiplier tube ps --- Picosecond (1 x10-12 s)
R --- Ground state reactant molecule R* --- Excited state reactant molecule rDA --- Donor-acceptor distance s --- second
S --- Spin angular momentum S0 --- Singlet ground state SFM --- Single frequency mixing SGH --- Second harmonic generation Sn --- The nth singlet excited state
T --- Transmittance
T0 --- Triplet ground state TD-DFT --- Time-Dependant Density
Functional Theory
THG --- Third harbonic generation Tn --- The nth triplet excited state TRES --- Time resolved emission spectroscopy
TSCPC--- Time correlated single photon counting
xxiii UV-Vis ---- Ultraviolet-visible absorption
spectroscopy
Z* --- Effective nuclear charge ZnTPP --- tetraphenylporphyrin zinc(II) ΔQ--- Excited state distortion
ε --- Molar extinction coefficient -or-
Absorption coefficient ζSO --- Spin-orbit coupling constant λ --- Wavelength of light in nanometers
μ --- Transition operator μA --- Acceptor transition dipole μD --- Donor transition dipole μL --- Orbital magnetic moment μs --- Microsecond (1 x10-6 s) μS --- Spin magnetic moment ν = n --- (n = 0, 1, 2, 3…) ground state vibronic energy level
ν’ = n --- (n = 0, 1, 2, 3…) excited state vibronic energy level
π --- Bonding π-orbital π* --- Anti-bonding π-orbital σ --- Bonding σ-orbital σ* --- Anti-bonding σ-orbital τS1 --- Singlet excited stare lifetime
Φe --- Quantum yield of emission ΦF --- Quantum yield of fluorescence ΦIC --- Quantum yield of internal conversion (singlet state) ΦIP --- Quantum yield of internal conversion (triplet state) ΦISC --- Quantum yield of intersystem crossing
ΦP --- Quantum yield of phosphorescence
Ψ --- A molecular wavefunction Ψe --- Electron wavefunction Ψn --- Molecular wavefunction for state n (n=1 or 2)
Ψne --- Electronic wavefunction for state n (n=1 or 2)
Ψns --- Spin wavefunction for state n (n=1 or 2)
Ψnv --- Vibrational wavefunction for state n (n=1 or 2)
ΨS --- Singlet spin wavefunction Ψs --- Spin wavefunction
ΨT --- Triplet spin wavefunction Ψv --- Vibrational wavefunction
xxiv [I.2] 𝐻̂ =1 2∑ ∇𝑖 2− ∑ ∑ 𝑍𝐴 𝑅𝑖𝐴+ ∑ ∑ 1 𝑟𝑖𝑗 = 𝑇̂ + 𝑉̂𝑁𝑒+ 𝑉̂𝑒𝑒 𝑁 𝑗>𝑖 𝑁 𝑖=1 𝑀 𝐴=1 𝑁 𝑖=1 𝑁 𝑖=1 --- 23 [I.3] 𝐸𝑡𝑜𝑡 = 𝐸𝑒𝑙𝑒𝑐+ 𝐸𝑛𝑢𝑐 where 𝐸𝑛𝑢𝑐 = ∑ ∑ 𝑍𝐴𝑍𝐵 𝑅𝐴𝐵 𝑀 𝐵>𝐴 𝑀 𝐴=1 --- 23 [I.4] 𝐸0< ⟨Ψ′|𝐻̂|Ψ′⟩ = ⟨Ψ′|𝐻̂′|Ψ′⟩ + ⟨Ψ′|𝐻̂ − 𝐻̂′|Ψ′⟩ = 𝐸′0+ ∫ 𝜌(𝑟⃗)[𝑉𝑒𝑥𝑡(𝑟⃗) − 𝑉𝑒𝑥𝑡′ (𝑟⃗)]𝑑𝑟⃗ --- 24 [I.5] 𝐸′0< ⟨Ψ|𝐻̂′|Ψ⟩ = ⟨Ψ|𝐻̂|Ψ⟩ + ⟨Ψ|𝐻̂′ − 𝐻̂|Ψ⟩ = 𝐸0+ ∫ 𝜌(𝑟⃗)[𝑉𝑒𝑥𝑡(𝑟⃗) − 𝑉𝑒𝑥𝑡′ (𝑟⃗)]𝑑𝑟⃗ --- 24 [I.6] 𝐸0+ 𝐸0′ < 𝐸0′+ 𝐸0 --- 24 [1.1] 𝐸 = ℎ𝜈 --- 34 [1.2] 𝐸 =ℎ𝑐 𝜆 --- 34 [1.3] 𝑇 =𝐼𝑓𝑖𝑛𝑎𝑙 𝐼𝑖𝑛𝑖𝑡 = 10 −𝜖𝑙[𝑐] --- 35 [1.4] %𝑇 = 100𝑇 --- 35 [1.5] 𝐴 = − log (𝐼𝑓𝑖𝑛𝑎𝑙 𝐼𝑖𝑛𝑖𝑡) --- 35 [1.6] 𝐴 = − log(𝑇) = −log (%𝑇 100) --- 35 [1.7] 𝐴 = 2 − log (%𝑇) --- 35 [1.8] 𝐴 = 𝜖𝑙[𝑐] --- 35 [1.9] 𝐵𝑂 =𝑁𝑏𝑜𝑛𝑑𝑖𝑛𝑔−𝑁𝑎𝑛𝑡𝑖𝑏𝑜𝑛𝑑𝑖𝑛𝑔 2 --- 37 [1.10] 𝐻Ψ = 𝐸Ψ --- 44 [1.11] Ψ = Ψ𝑒Ψ𝑣Ψ𝑠 --- 44 [1.12] 𝑃 = ∫ Ψ1|𝜇|Ψ2𝑑𝜏 = ∫ Ψ1𝑒Ψ1𝑣Ψ1𝑠|𝜇|Ψ2𝑒Ψ2𝑣Ψ2𝑠𝑑𝜏 --- 44 [1.13] 𝑃~ ∫ Ψ1𝑒|𝜇|Ψ2𝑒𝑑𝜏𝑒× ∫ Ψ1𝑣Ψ2𝑣𝑑𝜏𝑣× ∫ Ψ1𝑠Ψ2𝑠𝑑𝜏𝑠 --- 45 [1.14] ∫ Ψ𝑆Ψ𝑇𝑑𝜏 = 0 --- 46 [1.15] ∫ Ψ𝑆Ψ𝑇𝑑𝜏 = 0 --- 47 [1.16] 𝐻𝑆𝑂~ 𝜁𝑆𝑂𝜇𝑆𝜇𝐿 --- 47 [1.17] 𝑔 → 𝑔 = 𝑠𝑦𝑚𝑚𝑒𝑡𝑟𝑦 𝑓𝑜𝑟𝑏𝑖𝑑𝑑𝑒𝑛 --- 49 [1.18] 𝑢 → 𝑢 = 𝑠𝑦𝑚𝑚𝑒𝑡𝑦 𝑓𝑜𝑟𝑏𝑖𝑑𝑑𝑒𝑛 --- 49
xxv [1.21] 𝑘𝑆1 = 𝑘𝐹+ 𝑘𝐼𝐶+ 𝑘𝐼𝑆𝐶 --- 64 [1.22] 𝜏𝑆1 = 1 𝑘𝐹+𝑘𝐼𝐶+𝑘𝐼𝑆𝐶= 𝑘𝑆1 −1 --- 64 [1.23] 𝑘𝑇1 = 𝑘𝑃+ 𝑘𝐼𝑃 --- 64 [1.24] 𝜏𝑇1 = 1 𝑘𝑃+𝑘𝐼𝑃 = 𝑘𝑇1 −1 --- 64 [1.25] Φ𝐹 = 𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑝ℎ𝑜𝑡𝑜𝑛𝑠 𝑒𝑚𝑖𝑡𝑡𝑒𝑑 𝑏𝑦 𝑓𝑙𝑢𝑜𝑟𝑒𝑠𝑐𝑒𝑛𝑐𝑒 𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑝ℎ𝑜𝑡𝑜𝑛𝑠 𝑎𝑏𝑠𝑜𝑟𝑏𝑒𝑑 --- 65 [1.26] Φ𝐼𝐶 = 𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒𝑠 𝑡ℎ𝑎𝑡 𝑟𝑒𝑙𝑎𝑥 𝑏𝑦 𝐼𝐶 𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑝ℎ𝑜𝑡𝑜𝑛𝑠 𝑎𝑏𝑠𝑜𝑟𝑏𝑒𝑑 --- 65 [1.27] Φ𝐼𝑆𝐶 = 𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒𝑠 𝑡ℎ𝑎𝑡 𝑝𝑜𝑝𝑢𝑙𝑎𝑡𝑒 𝑡ℎ𝑒 𝑡𝑟𝑖𝑝𝑙𝑒𝑡 𝑠𝑡𝑎𝑡𝑒 𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑝ℎ𝑜𝑡𝑜𝑛𝑠 𝑎𝑏𝑠𝑜𝑟𝑏𝑒𝑑 --- 65 [1.28] Φ𝐼𝑆𝐶 = 𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒𝑠 𝑡ℎ𝑎𝑡 𝑝𝑜𝑝𝑢𝑙𝑎𝑡𝑒 𝑡ℎ𝑒 𝑡𝑟𝑖𝑝𝑙𝑒𝑡 𝑠𝑡𝑎𝑡𝑒 𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑝ℎ𝑜𝑡𝑜𝑛𝑠 𝑎𝑏𝑠𝑜𝑟𝑏𝑒𝑑 --- 65 [1.29] Φ𝐼𝑃 = 𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒𝑠 𝑡ℎ𝑎𝑡 𝑟𝑒𝑙𝑎𝑥 𝑏𝑦 𝐼𝑃 𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒𝑠 𝑡ℎ𝑎𝑡 𝑝𝑜𝑝𝑢𝑙𝑎𝑡𝑒 𝑡ℎ𝑒 𝑡𝑟𝑖𝑝𝑙𝑒𝑡 𝑠𝑡𝑎𝑡𝑒 --- 65 [1.30] Φ𝐹+ Φ𝐼𝑆𝐶+ Φ𝐼𝐶 = 1 --- 65 [1.31] Φ𝑃 + Φ𝐼𝑃 = Φ𝐼𝑆𝐶 --- 65 [1.32] Φ𝐹 = 𝑘𝐹 𝑘𝐹+𝑘𝐼𝐶+𝑘𝐼𝑆𝐶 --- 66 [1.33] Φ𝐼𝐶 = 𝑘𝐼𝐶 𝑘𝐹+𝑘𝐼𝐶+𝑘𝐼𝑆𝐶 --- 66 [1.34] Φ𝐼𝑆𝐶 = 𝑘𝐼𝑆𝐶 𝑘𝐹+𝑘𝐼𝐶+𝑘𝐼𝑆𝐶 --- 66 [1.35] Φ𝐹 = 𝑘𝐹𝜏𝑆1 --- 66 [1.36] Φ𝐼𝐶 = 𝑘𝐼𝐶𝜏𝑆1 --- 66 [1.37] Φ𝐼𝑆𝐶 = 𝑘𝐼𝑆𝐶𝜏𝑆1 --- 66 [1.38] Φ𝑖 = Φ∗× 𝑘𝑖 Σ𝑘𝑑 --- 66 [1.39] Φ𝑃 = Φ𝐼𝑆𝐶× 𝑘𝑃 𝑘𝑝+𝑘𝐼𝑃 --- 67 [1.40] Φ𝐼𝑃 = Φ𝐼𝑆𝐶× 𝑘𝐼𝑃 𝑘𝑝+𝑘𝐼𝑃 --- 67 [1.41] Φ𝑃 = Φ𝐼𝑆𝐶𝑘𝑃𝜏𝑇1 --- 67
xxvi [1.44] 𝐷∗+ 𝐴 {𝑒𝑙𝑒𝑐𝑡𝑟𝑜𝑛 𝑒𝑥𝑐ℎ𝑎𝑛𝑔𝑒} → 𝐷 + 𝐴∗+ ℎ𝑒𝑎𝑡 --- 68 [1.45] 𝐷𝑒∗ + 𝐴 𝑒{𝑒𝑙𝑒𝑐𝑡𝑟𝑜𝑛 𝑡𝑟𝑎𝑛𝑠𝑓𝑒𝑟} → 𝐷𝑒++ 𝐴𝑒− --- 68 [1.46] 𝐷𝑒 + 𝐴𝑒∗{ℎ𝑜𝑙𝑒 𝑡𝑟𝑎𝑛𝑠𝑓𝑒𝑟} → 𝐷 𝑒++ 𝐴𝑒− --- 68 [1.47] 𝜏𝐷(𝑆1) = 1 𝑘𝐹+𝑘𝐼𝐶+𝑘𝐼𝑆𝐶+𝑘𝐸𝑇(𝑆1) --- 71 [1.48] 𝜏𝐷(𝑇1) = 1 𝑘𝑃+𝑘𝐼𝑃+𝑘𝐸𝑇(𝑇1) --- 71 [1.49] 𝑘𝐸𝑇(𝑆1) = 1 𝜏𝐷(𝑆1)− 1 𝜏𝑆1 --- 72 [1.50] 𝑘𝐸𝑇(𝑇1) = 1 𝜏𝐷(𝑇1)− 1 𝜏𝑇1 --- 72 [1.51] ∫ Ψ𝐷∗Ψ𝐴|𝐻𝐷𝑒𝑥|Ψ𝐷Ψ𝐴∗𝑑𝜏 = ⟨Ψ𝐷∗Ψ𝐴|𝐻𝐷𝑒𝑥|Ψ𝐷Ψ𝐴∗⟩ --- 75 [1.52] 𝑘𝐷𝑒𝑥 = ⟨Ψ𝐷∗Ψ𝐴|𝐻𝐷𝑒𝑥|Ψ𝐷Ψ𝐴∗⟩2 --- 75 [1.53] 𝑘𝐷𝑒𝑥 ∝ 𝑒−2𝑟𝐷𝐴 --- 75 [1.54] 𝑘𝐷𝑒𝑥 = 𝐾 ∙ 𝐽 ∙ 𝑒−2𝑟𝐷𝐴𝐿 --- 75 [1.55] 𝑘𝐹ö𝑟 = ⟨Ψ𝐷∗Ψ 𝐴|𝐻𝐹ö𝑟|Ψ𝐷Ψ𝐴∗⟩2 --- 78 [1.56] 𝐻𝐹ö𝑟 ∝ 𝜇𝐷𝜇𝐴 𝑟𝐷𝐴3 --- 78 [1.57] 𝑘𝐹ö𝑟 ∝ 1 𝑟𝐷𝐴6 --- 78 [1.58] 𝑘𝐹ö𝑟 = Φ𝐹°(𝐷)𝜅2 𝜏𝐹°(𝐷)𝑟𝐷𝐴6 ∙ ( 9000 ln(10) 128𝜋5𝑁 𝑎𝑛4) ∙ 𝐽 --- 78 [1.59] 𝑘𝐹ö𝑟 ≅ 8.785 × 10−25∙ ( 𝑘𝐹°(𝐷)𝜅2 𝑟𝐷𝐴6 𝑛4 ) ∙ 𝐽 --- 78 [1.60] 𝑘𝐸𝑇 = 𝛼⟨Ψ𝐷∗Ψ𝐴|𝐻𝐹ö𝑟|Ψ𝐷Ψ𝐴∗⟩2+ 𝛽⟨Ψ 𝐷∗Ψ𝐴|𝐻𝐷𝑒𝑥|Ψ𝐷Ψ𝐴∗⟩2 --- 79 [2.1] Φ𝐹𝑆= 𝐹𝑆𝑓𝑅𝑛𝑆2 𝐹𝑅𝑓𝑆𝑛𝑅2Φ𝐹 𝑅 --- 92 [2.2] 𝐼𝑡 ∝ [𝑆1]𝑡=𝑡 --- 95 [2.3] 𝐼0 ∝ [𝑆1]𝑡=0 --- 95 [2.4] ln (𝐼𝑡) = −𝜏𝑆1 −1𝑡 + ln(𝐼 0) --- 95 [2.5] 𝐼𝑡 = 𝐼0𝑒−𝜏𝑆1−1𝑡 --- 95
xxvii Figure 2. Structure of the porphyrin macrocycle. --- 3 Figure 3. A periodic table showing the elements that were known to form porphyrin
complexes as of the year 19993, 5. --- 4 Figure 4. Absorption and emission spectra of a free-base porphyrin (left), deprotonated
porphyrin (center) and zinc(II)-porphyrin (metalloporphyrin) (right). --- 12 Figure 5. A) The Gouterman orbitals for porphyrin. B) The four orbital model showing
the electronics transitions leading to the Soret and Q-bands. C) A representation of the transition dipole moments in porphyrin and the
symmetry of a free-base (D2h) and metallo-porphyrin (D4h) --- 13
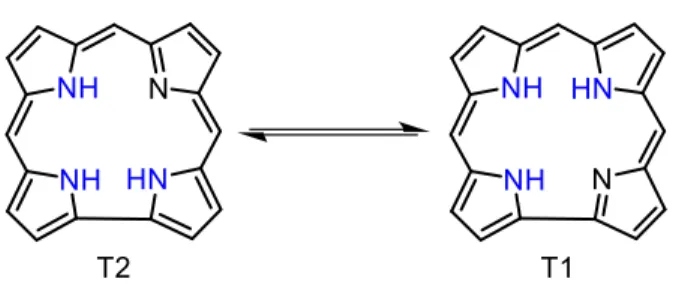
Figure 6. Structures of dipyrromethene, BODIPY, dipyrromethene and s-indacene. --- 15 Figure 7. Structures of the T1 and T2 tautomers of free-base corrole. --- 18 Figure 8. Structures of fluorene, truxene and C60 fullerene. The fluorene and truxene
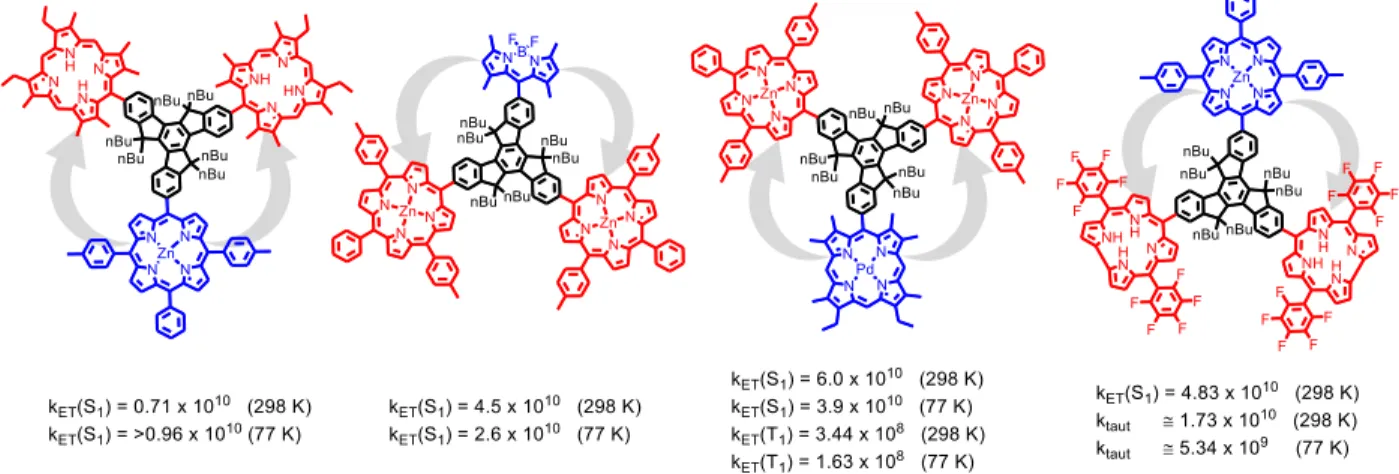
substructures are highlighted in truxene and fullerene respectively. --- 21 Figure 9. Structures and energy transfer rates of the investigated donor-acceptor
assemblies built upon a truxene spacer. --- 28 Figure 10. Schematic representation of a photochemical process. --- 29 Figure 11. Schematic representation of a simple photophysical process. --- 30 Figure 12. Schematic representation of a competing photophysical and photochemical
process. --- 31 Figure 13. Electronic configurations of an excited singlet state (left) and excited triplet
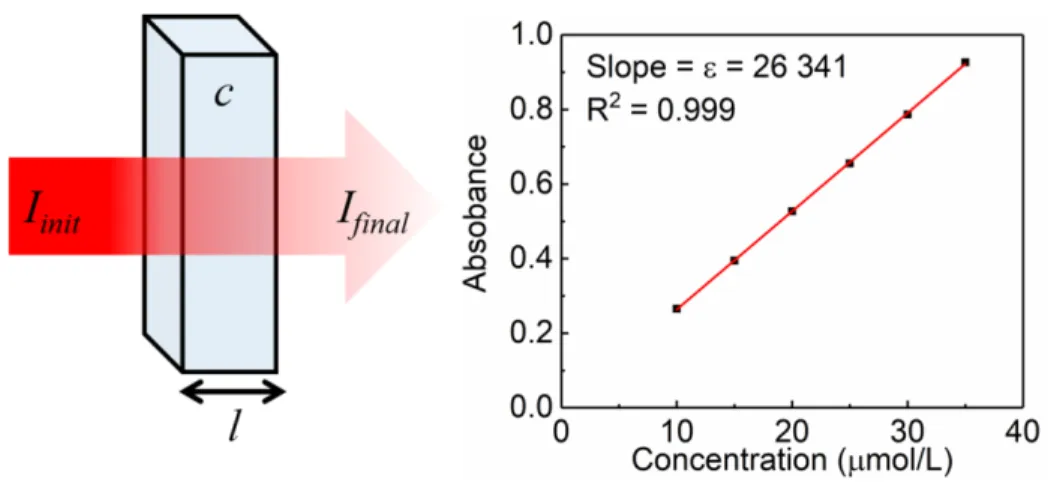
state (right). The electron spins are equal to +½(↑) and -½(↓). --- 32 Figure 14. The electromagnetic spectrum --- 33 Figure 15. Schematic representation of the absorption of light as it passes through a
sample (left) and the linear relationship between absorption intensity and
sample concentration for a fictitious sample (right). --- 36 Figure 16. Molecular orbital energy diagram for a simple σ → σ* transition. --- 37
xxviii
Figure 18. Structure of trans-butadiene in its excited state. --- 39 Figure 19. Molecular orbital energy diagram for the simple n → π* transition seen in
formaldehyde. --- 39 Figure 20. Metal-to-Ligand Charge Transfer in a d5 octahedral complex. --- 40 Figure 21. Ligand-to-Metal Charge Transfer in a d6 octahedral complex. --- 41 Figure 22. Ligand field transition (d → d) in a d6 octahedral complex. --- 41 Figure 23. A molecular orbital energy diagram showing three possible electronic
transitions for a d6 octahedral complex. --- 42 Figure 24. A representation of forbidden, partially forbidden, allowed and totally allowed
electronic transitions. Figure modified from reference 55 --- 43 Figure 25. Schematic diagram showing an electron in a Bohr orbit around a central
nucleus. The orbiting electron produces a magnetic moment μL. The
rotation of the election on its own axis yields a magnetic moment μS. --- 47 Figure 26. Visualization of the S1 and T1 potential wells in the absence (left) and presence
(right) of spin-orbit coupling.--- 48 Figure 27. Centrosymmetry in a cylinder and an octahedral structure. --- 49 Figure 28. Diagram showing the two symmetry allowed (solid arrows) and two symmetry
forbidden (dotted arrows) π → π* transitions in trans-butadiene.--- 50 Figure 29. Absorption spectrum of [Cr(NH3)6]3+ in aqueous solution. Figure modified
from reference 56. --- 52 Figure 30. The interactions of the electric field of an electromagnetic wave with the
electronic cloud of a hydrogen atom producing an induced dipole moment. --- 53 Figure 31. Schematic representation of the Franck-Condon principle. --- 55 Figure 32. Absorption and emission spectra of anthracene in dichloromethane at 298 K
(left) and a schematic diagram showing the vibronic progression of
anthacene (right). --- 57 Figure 33. Internal conversion between an upper and lower excited state. --- 59 Figure 34. Kasha’s rule showing rapid upper excited state internal conversion. --- 60 Figure 35. A Jablonski diagram including vibronic levels, showing many possible
xxix Figure 37. Simplified Jablonski diagram showing the electronic configurations of the
indicated states. --- 63 Figure 38. A frontier molecular orbital representation of photoinduced electron and hole
transfers. --- 69 Figure 39. Schematic representations of singlet-singlet (top) and triplet-triplet (bottom)
energy transfer. --- 70 Figure 40. Jablonski diagram illustrating both singlet-singlet and triplet-triplet energy
transfer. --- 71 Figure 41. Electron exchange mechanism. --- 74 Figure 42. Förster resonance energy transfer mechanism. --- 77 Figure 43. Schematic representation of the basic operation of a photomultiplier tube. --- 82 Figure 44. Electron ejection by transmission (for head-on type PMTs) and reflection (for
side-on type PMTs). --- 83 Figure 45. A 9 stage circular-cage PMT and a schematic diagram of the dynode
architecture. --- 85 Figure 46. Schematic representation of a microchannel plate (left) and of the electron
multiplication process inside the microchannel. --- 87 Figure 47. Schematic diagram of a monochromator. --- 91 Figure 48. The basic principle of operation of TCSPC. --- 97 Figure 49. A histogram generated by TCSPC showing the exponential decay of emission
intensity. --- 97 Figure 50. Repetition rate that is too fast (left) leading to overlapping decay traces and a
correct reputation rate (right) allowing complete relaxation between
excitation events. --- 99 Figure 51. Pulse pile-up due to a high stop rate resulting in a bias favoring the counting
of fast photons. Lost photons are not counted. --- 100 Figure 52. The basic operating principle of the diode array spectrophotometer. --- 102 Figure 53. The basic operating principle of the Cary 300 Bio spectrophotometer. --- 103 Figure 54. Schematic diagram of the FLS980 Phosphorimeter by Edinburgh Instruments --- 104
xxx
Figure 56. A schematic diagram showing the operating principle of a Streak camera. --- 108 Figure 57. Schematic layout of the transient absorption setup at the Université de
Sherbrooke prepared with the help of Paul-Ludovic Karsenti
(instrumentation laboratory coordinator). --- 109 Figure 58. State diagram showing the transient absorption process for a single
chromophore (left) and for a donor-acceptor assembly (right). --- 111 Figure 59. Structures and names of all of the investigated dyads and model compounds. --- 372 Figure 60. The overlap of the π-system between an energy donor and truxene. Left, partial
overlap due to a dihedral angle. Right, no overlap due to a 90° angle. --- 378 Figure 61. A proposed modified Dexter mechanism. --- 379
xxxi Laporte and spin selection rules55-56. --- 52 Table 2. Spectral properties of common PMT photocathode and window materials62. --- 84 Table 3. Comparison of some of the key parameters of the R928 and R3809U-50 PMTs --- 88 Table 4. Energy transfer rates of the investigated dyads. --- 376 Table 5. Description of the proposed structures for temperature dependence
measurements. --- 382 Table 6. Description of the possible results from temperature dependence measurements
xxxii
tetraphenylporphyrin) --- 6 Scheme 2. Proposed mechanism by Lindsay et al for the oxidation of a porphyrin by 2,
3-dichloro-5, 6-dicyano-1, 4-benzoquinone (DDQ)15,20. --- 8 Scheme 3. Preparation of a dipyrromethane. --- 9 Scheme 4. Different combinations of dypyrromethane and functionalized benzaldehydes
for the preparation of various porphyrins. --- 10 Scheme 5. Common synthesis method for the preparation of a BODIPY, illustrated here
for 4, 4-difluoro-1, 3, 5, 7-tetramethyl-8-phenyl-4-bora-3a,
4a-diaza-s-indacene (1, 3, 5, 7-tetramethyl-8-phenyl-BODIPY). --- 16 Scheme 6. Preparation of mono- and di-styryl BODIPY derivatives. --- 16 Scheme 7. The synthesis of corrole. --- 19 Scheme 8. Preparation of corrole bearing different aryl-groups. --- 19 Scheme 9. The synthesis of truxene from 1-indanone. --- 21 Scheme 10. Alkylation of truxene. --- 22 Scheme 11. The preparation of the truxene precursor compounds for the investigated
dyads (R = n-buthyl). --- 373 Scheme 12: Preparation of [Zn-Fb2], (R = n-buthyl). --- 374 Scheme 13. Preparation of [BOD-Zn2], (R = n-buthyl). --- 374 Scheme 14. Preparation of [Pd-Zn2], (R = n-buthyl). --- 375 Scheme 15. Preparation of [Zn-COR2], (R = n-buthyl). --- 375 Scheme 16. Proposed preparation of [Pdm-Zn2f]. --- 380 Scheme 17. Proposed preparation of [Pdf-Zn2f]. --- 381 Scheme 18. Proposed preparation of [Pdm-Zn2m]. --- 381
xxxiii This page is intentionally left blank
xxxiv
1 Energy transfer is a process of great importance in both natural and synthetic photovoltaic devices. In Nature, plants and photosynthetic bacteria use absorbed solar energy in order to produce the nutrients needed for their survival. To do this, several light absorbing dyes known as chlorophyll and bacteriochlorophyll absorb the light and transfer its energy to a reaction center where a photo-induced electron transfer occurs, thus starting the process that allows for the production of the carbohydrates that are essential to the organisms survival. To carry out this process efficiently the energy that is absorbed by the pigments must be rapidly transported from one dye to the other to ultimately arrive at the reaction center. This transportation is carried out by a process known as excited state energy transfer. Man-made photovoltaic devices also profit from energy transfer processes. Similar to natural photovoltaic devices man has begun using multiple dyes that are able to communicate absorbed energy to an electron acceptor, where an electron transfer occurs, thus creating a usable current. Much like in the natural system, the efficiency of theses synthetic devices is dependent on the ability of the individual dyes to transfer its absorbed energy to the reaction center.
In this thesis the energy transfer process between pigments that are bridged by a conjugated truxene core is examined in order to develop our understanding of the energy transfer process. The pigments that are examined in this work belong to a group of dyes known as oligopyrrole dyes, and share structural similarities with natural pigments used by plants and photosynthetic bacteria. The objective is to further develop our understanding of the energy transfer processes in order to determine what structure-property relationships can be used to favour efferent energy transfer between oligopyrrole dyes.
Before examining the photophysical theories behind energy transfer we will first examine the individual pigments that are employed throughout this work. We will also briefly examine the truxene spacer and define the importance of density functional theory. Finally, we will describe the objectives of this thesis.
2
I.1 Oligopyrrole dyes
An organic dye is often a conjugated organic chemical compound that is known for its strong colour. These strong colours arise from the strong absorptions (Chapter 1, Section 1.2) that the conjugated system evokes. A luminescent dye is a strongly coloured species that is capable not only of absorbing light to give it its characteristic colour, but is also capable of emitting light after absorption. This characteristic of a luminescent dye makes it a compound that is of importance in the study of photophysical phenomena. The photophysical characteristics of luminescent dyes allow for the applications of these dyes in many material fields such as: organic light emitting diodes (OLEDs)1, solar cells2, and other optoelectric devices, such as photodiodes, phototransistors and lasers.
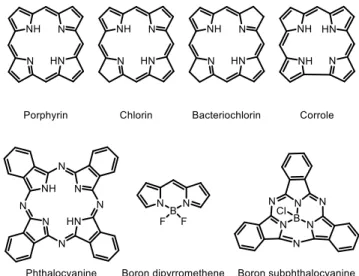
This work concerns itself with the photophysical behaviours of a certain class of organic dyes known as oligopyrrole dyes. The name oligopyrrole is used to describe this class of dyes as their chemical structures can be broken down into the repeating units of a pyrrole-containing oligomer. Some examples of oligopyrrole dyes are given in Figure 1, although this work is only concerned with porphyrin, boron dipyrromethene (BODIPY) and corrole, each of which is described in more detail in the following sections.
3 I.1.1 Porphyrin
I.1.1.1 Porphyrin structure
Porphyrin is a planar macrocyclic compound, composed of four pyrrole rings each of which is bridged by a methine (=CH-) carbon, and has the empirical formula C20H14N4 as shown in Figure 2. The carbons in the porphyrin macrocycle are subdivided into three groups: 1) The four meso-carbons located between the pyrrole rings (the methine bridges), 2) the eight α-meso-carbons, located in the α-positions of the pyrrole rings and 3) the eight β-carbons, located in the β-positions on the pyrrole rings. The porphyrin macrocycle contains eleven carbon-carbon double bonds, nine of which form the aromatic cycle (Hückel's rule: 4𝑛 + 2 , 𝑤ℎ𝑒𝑟𝑒 𝑛 = 4 → 18𝜋 𝑒𝑙𝑒𝑐𝑡𝑟𝑜𝑛𝑠) that is highlighted in Figure 2. The porphyrin macrocycle forms a central cavity with the four pyrrole nitrogen atoms pointing inwards. In its free base form, the central cavity is occupied by two pyrrolic hydrogen atoms. However, upon the deprotonation of these nitrogens the porphyrin is able to host a metal ion within its central cavity where the metal ion forms four coordination bonds (one with each of the nitrogens) to the porphyrin.
Figure 2. Structure of the porphyrin macrocycle.
Coordination complexes containing over 80 different metal ions in the central porphyrin cavity are known. Porphyrin is generally known to host metal ion in both +2 and +3 oxidation state. However, complexes with metals in higher oxidation states are also known. For example porphyrin
4
complexes with chromium ions in the +2 to +5 oxidation states and molybdenum porphyrin complexes are known in the +2, +4, +5 and +6 oxidations states3-4. Metal ions in oxidation states higher than +2 generally either contain axial ligands to form a penta- or hexa- valent complex or are accompanied by a counter ion. The periodic table in Figure 3 shows the elements that had been reported as having formed porphyrin complexes as of the year 1999. The figure illustrates the elements that have formed porphyrin complexes as well as those that are known to form complexes in two or more oxidation states. Interestingly, although porphyrin is known to host many metal ions, there are only 14 metal ions (Figure 3) that have been shown to form dimeric porphyrin species exhibiting metal-metal bonds, sometimes exhibiting up to a quadruple bond between metals.5 One should note that in the case of carbon porphyrin complexes, no complex that contains a single carbon bonded to the four central nitrogen atoms of a porphyrin is known. However, N-alkylated and N-N-carbon bridged porphyrin compounds are known to exist6-7.
Figure 3. A periodic table showing the elements that were known to form porphyrin complexes as of the year 19993, 5.
Since 1999 the chemistry of porphyrin complexes has continued to grow and even more elements have been shown to form complexes with porphyrin. The growth is most noted in the f-block elements for which many complexes have now been formed. As a matter of a fact, porphyrin
5 complexes are known with all of the non-synthetic f-block elements with the exception of protactinium8-11.
I.1.1.2 Porphyrin synthesis
The laboratory synthesis of porphyrin was first performed by Ruthemund in 1935 by the reaction of an aldehyde with pyrrole in a sealed glass tube at high temperatures. This method allowed for the production of approximately 1 mg of pure porphyrin for every 1 g of pyrrole used12-15, 19. In 1966 Adler and Longo developed a method known today as the Adler-Longo method for the preparation of tetraarylporphyrins. This method involves the mixing equimolar quantities of pyrrole and an appropriate aldehyde in refluxing propionic acid15-19 and became one of the most widely used methods for porphyrin synthesis as it allows for the simple preparation of porphyrin on the gram scale. However, the Adler-Longo method is not without its disadvantages. Firstly, the reaction conditions, although less extreme than Ruthemund’s method, are still quite harsh resulting in a complete failure of the reaction when aryl aldehydes bearing sensitive functional groups are used. Secondly, the Adler-Longo method produces a large amount of tar, which produces purification problems, especially when the desired porphyrin does not precipitate from the reaction mixture. Finally, the reaction yields are often highly variable and poorly reproducible and generally do not exceed approximately 20% conversion.
In 1986-7 Lindsey published his two step method for the preparation of tetraarylporphyrins15, 19-20. His objective was to adapt the Adler-Longo method in order to render the reaction conditions less extreme. In his 1986-7 publications, where he first published his method, Lindsey based the development of his method on three simple arguments.
1. The tetraarylporphyrinogen (un-oxidised porphyrin precursor) should be the thermodynamically favoured product when an aryl aldehyde and pyrrole are condensed under the appropriate reaction conditions. Therefore conditions that allow the reaction to attain its thermodynamic equilibrium state should favour the formation of tertaarylporphyrin.
2. Aryl aldehydes and pyrrole are reactive species and do not require high temperature in order to react with each other.
6
3. Reaction conditions that are sufficiently mild to reach equilibrium should also be mild enough to allow for the use aryl aldehydes bearing sensitive functional groups, making the preparation of a wide variety of porphyrins possible.
Based on these arguments, Lindsey then proposed a method that involved dissolving equimolar quantities of an aryl aldehyde and pyrrole (10-2 mol/L concentration) in dry dichloromethane, to which an acid catalyst (BF3 etherate or trifluoroacetic acid) is added. The reaction mechanism with acid catalyst is shown in Scheme 115, 19-20.
Scheme 1. Proposed mechanism for one-pot synthesis of a porphyrin (5, 10, 15, 20-tetraphenylporphyrin)
Lindsey then applies his first argument and allows the reaction to reach thermodynamic equilibrium by waiting a given period of time to allow for the formation of the desired octamer. The octamer can then either continue to polymerize or can form the cyclic porphyrinogen the latter of which is thermodynamically favoured under mild conditions. Lindsey showed that the degree
7 of polymerization was related to the concentration of the starting materials in solution and demonstrated that a 10-2 mol/L concentration was appropriate to allow for the formation of the octamer at room temperature but did not favour the formation of long polymers. More dilute solutions form less of the octamer leading to a decreased concentration porphyrinogen, and more concentrated solutions favoured further polymerisation (to a greater extent than the octamer) also leading to a decrease in the concentration of the porphyrinogen at equilibrium. This reaction was carried out at room temperature thus proving his second argument to be true. Finally, Lindsey was able to perform this reaction with many different aldehydes, justifying his third argument. Lindsey states that it is important that the reaction be carried out in the absence of oxygen, which can lead to the oxidative formation of a dypyrrylmethene moiety. This is problematic as the oxidation is non-reversible and can produce chain conformations that prevent the cyclisation of the octamer. He also proved that the condensation reaction was reversible under these conditions by preparing two solutions of porphyrinogen with different aryl groups. After confirming that most of the aldehyde had been consumed in the formation of the porphyrinogen, the two solutions were mixed and allowed to re-establish an equilibrium. Where six different porphyrin units were obtained due to porphyrinogen exchange. The ratios of the six porphyrins corresponded with both the ratio that was obtained when equal molar quantities of two aldehydes were mixed with pyrrole and the statistical expectations15, 20.
Once the porphyrinogen mixture has reached equilibrium, Lindsey applies the second step of his two step reaction method by adding the appropriate amount of an oxidizing agent (2,3,5,6-tetrachloro-1,4-benzoquinone [p-chloranil], or 2,3-dichloro-,5,6-dicyano-1,4-benzoquinone [DDQ]) to the mixture. This oxidising agent (see proposed mechanism in Scheme 2) serves to irreversibly oxidise the porphyrinogen to the porphyrin thus forming a maximum amount of the desired porphyrin. The porphyrin can then be purified by classical flash chromatography producing the desired tertaarylporphyrin in 45-50% yields15, 20.
8
Scheme 2. Proposed mechanism by Lindsay et al for the oxidation of a porphyrin by 2, 3-dichloro-5, 6-dicyano-1, 4-benzoquinone (DDQ)15,20.
Then in 1994 Lindsey described the facile one-pot synthesis of dipyrromethane21 (Scheme 3), which can then be used as a starting material for the preparation of mixed aryl group porphyrins. The dipyrromethane can simply be condensed with a different aldehyde to favour the production of the desired porphyrin. The preparation of the dipyrromethane is performed by placing the aldehyde in pyrrole (1:40 to 1:70 ratio) thus creating a large excess of the pyrrole and limiting the reaction to the formation of short oligomers (namely the dipyrromethane). This method allows for the formation of dipyrromethanes in a 50-80% yield21. Furthermore, in the same work Lindsey showed that the acidolysis of the dipyrromethane under porphyrin formation conditions was possible, but the degree to which it occurred during the time scale for porphyrinogen formation was negligible. This then opened the door to the facile preparation of porphyrins bearing different functional groups.
9 Scheme 3. Preparation of a dipyrromethane.
The preparation of dipyrromethane by Lindsey greatly simplifies the preparation of mixed aryl group porphyrins as illustrated in Scheme 4, which shows the advantages of using the dipyrromethane for the synthesis of trans-substituted (A2B2 and A2BC) porphyrins and A3B type porphyrins. Here, we see that the statistical reaction of two aldehydes to produce porphyrin will result in the production of six different porphyrins. Given that two aldehydes are used, the A4 and B4 porphyrins that are produced, are of no use, since they only contain one of the two desired functions leading to an automatic loss of 12.5% in yield. This leaves the A3B, cis-A2B2, and B3A porphyrins, which each make up 25% of the total porphyrin produced and the trans-A2B2 porphyrin which makes up 12.5% of the porphyrin. The situation becomes worse when we examine the statistical reaction of three aldehydes, which yields 21 different porphyrin structures. Clearly the statistical synthesis leads to problems with purification and a low yield regardless of which porphyrin it is that is saught. However, if you are you are seeking to synthesise a A2B2, trans-A2BC or a A3B type porphyrin, the use of a dipyrromethane will greatly simplify the task21.
10
Scheme 4. Different combinations of dypyrromethane and functionalized benzaldehydes for the preparation of various porphyrins.
If we use a dipyrromethane as illustrated in Scheme 4, we can favour the production of the A3B,
trans-A2B2 and trans-A2BC type porphyrins. In the case of the A3B and trans-A2BC type porphyrins, we will still produce three different porphyrins that need to be separated. However the reaction conditions here favour the formation of the desired porphyrin making it the major product. Furthermore, the separation of three porphyrins will be much easier to accomplish than the separation of the six or twenty-one different porphyrins that would have been obtained by the statistical method. Finally, in the case of a trans-A2B2 porphyrin, the use of a dipyrromethane under the correct reaction conditions leads to the exclusive formation of the desired product21.
11 Forty years have passed since Lindsey first published his porphyrin synthesis method and today his method is still the most employed for the production of meso-arylsubstituted porphyrin. The Adler-Longo method is still employed for the preparation of tetra-arylporphyrins even though it generally produces a lower yield than the Lindsey method. This is largely due to the facility of the Adler-Longo method and the fact that it is easily scaled up to produce large quantities of porphyrin. I.1.1.3 Porphyrin properties
As an organic dye porphyrin is most known for is strong purple coloration, as a matter of a fact, the name porphyrin comes from the Greek word “porphyra” which means purple22. Porphyrin possesses a strong absorptions in the visible light region of the electromagnetic spectrum (Chapter 1, Section 1.2) between 400 nm and 700 nm. The absorption spectrum of a porphyrin can be broken into two separate types of absorptions. The first, and most intense, is generally located around 400 nm and is known as the Soret absorption band (also referred to as the B-band). This absorption is generally labelled a S0 → S2 (ground state to second excited state) electronic transition, although formally in a free-base porphyrin the transition is more accurately described as an S0 → S3,4 absorption 23. The second set of absorption bands are known as the Q-bands, and are generally described as S0 → S1 (ground state to first excited state) transitions. Again, in the case of a free base porphyrin the Q-band absorptions are more accurately described as a pair of S0 → S1 and S0 → S2 absorptions. A free-base porphyrin (one with no metal ion in the central cavity) exhibits a
D2h symmetry and possesses four Q-band absorptions (S0 → S1 and S0 → S2) while a metalloporphyrin exhibits a D4h symmetry and possesses only two Q-band absorptions due to the
gain in symmetry, further explanation of this is provided below. The free-base porphyrin can be doubly protonated resulting in a change in colour from red-pink to emerald green. This change is colour is due to an out-of-plan deformation caused by the presence of the two extra protons in the central cavity changing the symmetry of the di-cation. The deprotonated porphyrin has an absorption spectrum that is composed of a red-shifted Soret band and a single red-shifted Q-band (see Figure 4). Each porphyrin also exhibits an emission spectrum upon excitation, emission is discussed further in Chapter 1, Section 1.7.