DEVELOPMENT OF AN INOCULANT OF PHOSPHATE
ROCK-SOLUBILIZING BACTERIA TO IMPROVE MAIZE
GROWTH AND NUTRITION
Thèse
Paola Magallón Servín
Doctorat en microbiologie agroalimentaire -
Philosophiae doctor (Ph.D.)
Québec, Canada
iii Résumé
L'utilisation directe de roche phosphatée (RP) est une alternative viable pour remplacer les coûteux fertilisants chimiques dans les pays en voie de développement. L'utilisation de bactéries solubilisatrices de RP (BSRP) est un bon moyen pour augmenter la réactivité de la RP. L'objectif principal de ce travail a été d'obtenir des isolats provenant de la mycorhizosphère du maïs possédant une grande capacité de solubilisation de RP, compatibles avec la mycorhize arbusculaire (MA) et présentant des traits associés aux rhizobactéries favorisant la croissance de plantes (RFCP) pour le développement d'un inoculant bactérien pour le maïs.À partir de 118 isolats obtenus de maïs biologique cultivé au Québec, huit BSRP ont été identifiés comme Asaia
lannaensis Vb1, Pseudomonas sp. Vr14, Rahnella aquatilis (Vr7, Vr13 et Sr24) et Pantoea agglomerans (Vr9,
Ve16 et Vr25). En milieu liquide, les isolats ont dissous le P des RP selon leur réactivité (Gafsa>Tilemsi>Maroc). La solubilisation des RP s'est effectuée par la production d'acides organiques (OA) et l'abaissement du pH. Les meilleures BSRP de chaque groupe: (A. lannaensis Vb1, Pseudomonas sp. Vr14, R.
aquatilis Sr24 et P. agglomerans Vr25) ont été sélectionnées selon leur capacité élevée de solubilisation de la
RP et de leur production d'acide indolacétique (AIA) et de sidérophores. L‟importance des biofilms formés, ainsi que le degré de motilité variaient selon les isolats et tous étaient compatibles avec le Glomus irregulare (Gi). L‟étude de la colonisation des racines montre que R. aquatilis Sr24 et P. agglomerans Vr25 ont été les meilleurs colonisateurs. Lors des expériences en serre, certains mélanges contenant R. aquatilis Sr24, P.
agglomerans Vr25 et Gi, ont amélioré la biomasse, l'absorption des nutriments et la colonisation de la plante
en association avec un champignon mycorhizien indigène du maïs cultivé dans un sol non stérile et fertilisé avec la RP Marocaine. Nous attribuons ces résultats à leur capacité d'être de bonnes BSRP colonisatrices des racines. Elles sont aussi compatibles avec Gi et sont capables de produire de l'AIA et des sidérophores. Cette thèse démontre donc le potentiel d'utilisation de BSRP comme inoculant afin d'améliorer l'efficacité de l'utilisation directe de RP comme fertilisant phosphaté pour l'agriculture durable du maïs.
v Abstract
Phosphorous is the second most important nutrient for plant growth, but its availability is often reduced. Therefore high quantities of expensive soluble P-fertilizers are added to soil. Direct use of phosphate rock (PR) is an alternative to chemical P-fertilizers in developing countries and for sustainable agriculture. In order to increase PR reactivity the use of phosphate rock-solubilizing bacteria (PRSB) is a good alternative. Therefore, the main objective of this work was to obtain mycorrhizosphere-competent PRSB presenting other PGPR-associated traits to be used for the development of an inoculant to improve maize growth and P nutrition. Out of 118 isolates obtained from organically grown maize in Quebec, eight PRSB were identified as Asaia
lannaensis Vb1, Pseudomonas sp. Vr14, Rahnella aquatilis (Vr7, Vr13 and Sr24) and Pantoea agglomerans
(Vr9, Ve16 and Vr25). All isolates were able to mobilize P from different sparingly soluble P sources in solid media. In liquid medium the isolates were able to solubilize P from PRs according to their reactivity (Gafsa>Tilemsi>Morocco). PRs were solubilized by the production of organic acids (OAs) and by lowering the pH. The best PRSB from each group (A. lannaensis Vb1, Pseudomonas sp. Vr14, R. aquatilis Sr24 and P.3
agglomerans Vr25) were selected based on their high PR solubilization, and capacity for indolacetic acid (IAA)
and siderophore production. These four isolates presented different biofilm formation and motility capacities and were compatible with Glomus irregulare (Gi). A root colonization study showed that R. aquatilis Sr24 and
P. agglomerans Vr25 were the best root colonizers. Vr25 was very competitive when used with other PRSB. In
greenhouse trials, plant inoculation with R. aquatilis Sr24 and P. agglomerans Vr25 in addition to Gi, increased the biomass, nutrient uptake in non-sterile soil amended with Moroccan PR (MPR). We attribute these results not only to their PR-solubilizing capacity but also for their ability to be good PRSB, competitive root colonizers, compatible with Gi and to produce IAA and siderophores. This thesis shows that PRSB with AM fungi can be used as inoculants to improve the efficiency of the direct use of PR as P fertilizer for sustainable maize production.
vii Table of contents
Résumé ... iii
Abstract ... v
Table of contents ... vii
List of tables ... ix
List of figures ... xi
List of abbreviations ... xiii
Acknowledgments ... xvii
Foreword ... xix
CHAPTER I. INTRODUCTION ... 1
1.1 Inorganic phosphorus fixation and its management in agricultural soils ... 1
1.2 Adsorption and fixation of organic phosphorus in soils ... 2
1.3 The use of P fertilizers and the importance of phosphate rock (PR) ... 2
1.4 Phosphate solubilization by soil microorganisms ... 3
1.5 Maize as a model to study PSM ... 6
1.6 The importance of combining different PSMs to be used as biofertilizers ... 6
1.7 The importance of biofilm formation in PSM ... 9
1.8 Hypotheses ... 11
1.9 Objectives ... 12
1.9.1 General aim ... 12
1.9.2 Specific objectives ... 12
1.10 References ... 12
CHAPTER II. PHOSPHATE ROCK-SOLUBILIZING RHIZOBACTERIA ISOLATED FROM MAIZE (Zea mays) MYCORRHIZOSPHERE ... 21
2.1 Introduction ... 23
2.2 Materials and methods ... 25
2.2.1 Sampling and isolation of PRSB ... 25
2.2.2 PRSB inocula ... 26
2.2.3 Soil and PR chemical analyses ... 26
2.2.4 PRSB identification ... 26
2.2.5 Solubilization of PRs and organic acids production by PRSB ... 27
2.2.6 Effect of EDTA on bacterial solubilization of Morocco PR ... 28
2.2.7 Phosphatase and phytase production ... 28
2.2.8 Plant growth-promoting characteristics of the PRSB and their effect on maize seedlings ... 29
2.2.9 Statistical analysis ... 31
2.3 Results ... 32
2.3.1 Isolation and identification of PRSB ... 32
2.3.2 Solubilization of phosphate rocks by bacteria... 33
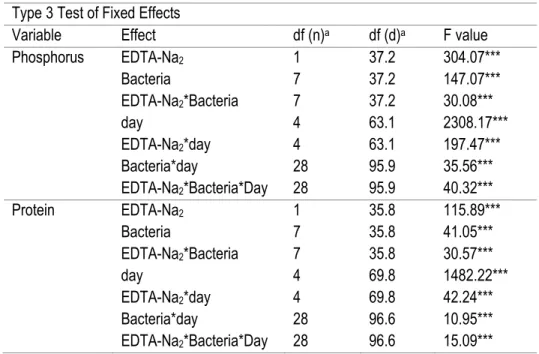
2.3.3 Effect of EDTA-Na2 on Morocco PR solubilization ... 40
2.3.4 PRSB phosphatase and phytase activities ... 42
2.3.5 Some in vitro characteristics of PRSB and their effects on maize seedling growth ... 44
2.4 Discussion ... 46
2.4.1 Isolation, and identification of PRSB, and evaluation of the selected strains for their capacity to dissolve different inorganic P sources ... 46
2.4.2 Determination of the solubilization of PR with different reactivity by PRSB ... 47
2.4.3 Effect of EDTA on Morocco PR solubilization ... 52
2.4.4 PRSB ability to mineralize organic P, some in vitro PGPR characteristics of the selected isolates and their effect on maize seedlings growth. ... 52
2.5 Conclusion ... 55
viii
CHAPTER III. COMPATIBILITY OF PR-SOLUBILIZING BACTERIA WITH THE ARBUSCULAR
MYCORRHIZAL FUNGUS (Glomus irregulare) ... 65
3.1 Introduction ... 67
3.2 Materials and methods ... 68
3.2.1 Compatibility of the selected PRSB with AMF in two-compartment Petri dish ... 68
3.2.2 Compatibility of the selected PRSB with AMF under greenhouse conditions ... 69
3.2.3 Statistical analysis ... 69
3.3 Results ... 70
3.3.1 Compatibility of the selected PRSB with Glomus irregulare in two-compartment Petri dish ... 70
3.3.2 Effect of the four selected PRSB on root mycorrhization of leek by Glomus irregulare under greenhouse conditions ... 71
3.4 Discussion ... 72
3.5 Conclusions ... 76
3.6 References ... 76
CHAPTER IV. DEVELOPMENT OF A COMBINED INOCULUM CONTAINING DIFFERENT PR-SOLUBILIZING BACTERIA BENEFICIAL TO MAIZE ... 81
4.1 Introduction ... 83
4.2 Material and methods ... 84
4.2.1 Bacteria ... 84
4.2.2 Biocompatibility assay ... 84
4.2.3 Determination of the PR solubilization capacity of the four different PRSB individually and in combination ... 84
4.2.4 Determination of specific biofilm formation capacity on abiotic surfaces by the traditional crystal violet method ... 85
4.2.5 Determination of biofilm formation capacity on MPR surface ... 85
4.2.6 Effect of different PRSB isolates, individually and in mixture, on maize (Zea mays L. cv. SENECA Horizon) root colonization in growth pouches... 86
4.2.7 Greenhouse experiment to determine the effects of different mixtures of the PRSB on the growth of forage maize (Zea mays L. cv. Focus) in the presence of the AMF Glomus irregulare ... 87
4.2.8 Statistical analysis ... 89
4.3 Results ... 89
4.3.1 Biocompatibility ... 89
4.3.2 Phosphate rock solubilization in liquid media by PRSB used individually or in mixtures ... 89
4.3.3 Specific biofilm formation (SBF) index of the selected PRSB on abiotic surfaces, individually and in mixture, calculated by the traditional crystal violet (CV) method ... 94
4.3.4 Determination of biofilm formation capacity on MPR surface ... 94
4.3.5 Effect of the PRSB on growth and root colonization of two-week-old maize seedlings (Zea mays L. cv. SENECA Horizon) in growth pouches ... 98
4.3.6 Greenhouse experiment ... 100
4.4 Discussion ... 107
4.4.1 Solubilization of PR by the selected PRSB as single or multiple species inoculants in liquid media 107 4.4.2 Specific biofilm formation (SBF) index of the selected PRSB ... 109
4.4.3 Effect of PRSB on growth and root colonization of two week old maize seedlings (Zea mays L. cv. SENECA Horizon) in growth pouches ... 110
4.4.4 Greenhouse experiment ... 111
4.5 Conclusion ... 113
4.6 References ... 114
GENERAL CONCLUSION ... 119
ANNEX I. Complementary material and statistical analysis ... 121
ix List of tables
Table 1. Phosphate-solubilizing fungi. ... 4 Table 2. Phosphate-solubilizing bacteria. ... 5 Table 3. Synergism between rhizobacteria and AM fungi in various crops. ... 8 Table 4. Phenotypic characteristics of colonies of phosphate rock-solubilizing bacteria (PRSB) and their P
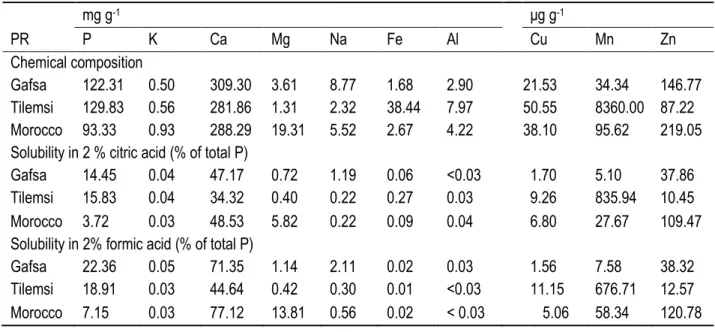
dissolution activity on solid NBRIP medium supplemented with different P sources. ... 32 Table 5. Chemical composition of phosphate rocks and extractability of P and other elements with 2% (wt/vol)
citric or 2% (wt/vol) formic acid. ... 34 Table 6. ANOVA (SAS Mixed Procedure) of the effects of Morocco, Gafsa, and Tilemsi phosphate rocks on P
solubilization by the selected PRSB in NBRIP liquid medium... 35 Table 7. Effect of inoculation with phosphate rock-solubilizing bacteria on the pH of the culture filtrate of
NBRIP supplement with different phosphate rocks, after 5 days of growth at 28°C ... 37 Table 8. ANOVA (SAS MIXED procedure) of the effect of the addition of 12 mM of EDTA-Na2 on the Morocco
PR solubilization by PRSB in NBRIP liquid medium. ... 40 Table 9. Phosphatases and phytase production by phosphate rock-solubilizing bacteria (PRSB) ... 43 Table 10. Indolacetic acid (IAA) and siderophore production, specific biofilm formation (SBF) and swimming
motility in phosphate rock-solubilizing bacteria. ... 45 Table 11. Effect of inoculation with PRSB on dry matter yields of two-week old maize seedlings (Zea mays cv.
SENECA, Horizon). ... 45 Table 12. Effect of inoculation with PRSB on fresh and dry matter yields of 70-day-old leek plants (Allium
ampeloprasum var. porrum cultivar „Lancelot‟) in presence or absence of G. irregulare, under
greenhouse conditions. ... 72 Table 13. Effect of inoculation of PRSB on the percentage of Glomus irregulare root colonization of 30-, 43-
and 70-day-old leek plants under greenhouse conditions. ... 72 Table 14. Effect of inoculation with phosphate rock-solubilizing bacteria on the pH of the culture filtrate of
NBRIP-MPR, after 7 days of growth at 28 ºC ... 92 Table 15. Effect of biofilm conformed by PRSB over MPR particles on the pH of the culture, after 5 days at
28ºC. Tested strains were Asaia lannaensis (Vb1), Pseudomonas sp. (Vr14), Rahnella aquatilis (Sr24) and Pantoea agglomerans (Vr25). ... 96 Table 16. Effect of inoculation with PRSB (used individually or in mixed culture) on dry matter yields of
2-week-old maize seedlings (Zea mays cv. SENECA, Horizon). ... 99 Table 17. Summary of analyses of variance of shoot fresh and dry matter, AM colonization (% AM), and total
N, P and K uptake by corn (Zea mays cv. FOCUS) fertilized with MPR and inoculated with different PRSB, individually and in mixture, in the presence or absence of Glomus irregulare (Gi). ... 101 Table 18. Effect of inoculating maize (Zea mays cv. FOCUS) plants with PRSB, either individually or in
different combinations, on shoot fresh and dry matter, nutrient uptake and PRSB root colonization in a soil amended with MPR in the presence and absence of Glomus irregulare (Gi) under greenhouse conditions ... 105 Table 19. Summary of analyses of variance for numbers of potential P solubilizing bacteria (PPSB) present in
the rhizosphere of maize (Zea mays cv. FOCUS) fertilized with MPR and inoculated with different PRSB, individually and in combination, in the presence or absence of Glomus irregulare (Gi). ... 107
xi List of figures
Figure 1. PR solubilization (a) by the different PRSB and bacterial growth (b) expressed as μg mL-1 protein,
after 5 days incubation in NBRIP-PR liquid media. Error bars are + standard deviation (n=3). Soluble P from uninoculated control was subtracted for each PR used. Asaia lannaensis (Vb1), Pseudomonas sp. (Vr14), Rahnella aquatilis (Vr7, Vr13, and Sr24) and Pantoea agglomerans (Vr9, Ve16, and Vr25). ... 36 Figure 2. Production of 2-ketogluconic (a) and D-gluconic acid (b) by phosphate rock solubilizing bacteria,
after 5 days incubation in NBRIP-PR liquid cultures. Error bars are + standard deviation (n=3). Asaia
lannaensis (Vb1), Pseudomonas sp. (Vr14), Rahnella aquatilis (Vr7, Vr13, and Sr24) and Pantoea agglomerans (Vr9, Ve16, and Vr25). ... 39
Figure 3. Effects of adding 12-mM EDTA-Na2 on the solubilization of Morocco PR by PRSB isolates, after 5
days incubation in the NBRI-Morocco medium. Error bars are ± standard error (n=3). Asterisks (*) indicate significant (P < 0.001) differences between the presence and absence of EDTA-Na2. Presented
values were substracted from the level of P obtained from the uninoculated controls. ... 41 Figure 4. Effect of adding 12-mM EDTA-Na2 on the growth (total protein) of phosphate rock-solubilizing
bacteria after 5 days incubation in liquid NBRIP-Morocco phosphate rock. Error bars are + standard deviation (n=3). (*) indicates significant differences (P < 0.01) between the presence and absence of EDTA-Na2. ... 42
Figure 5. Hyphae colonization of Glomus irregulare DAOM 197198 in two-compartment Petri dish system by (A) A. lannaensis Vb1; (B) Pseudomonas sp. Vr14; (C) P.agglomerans Vr9; (D) R. aquatilis Vr13; incubated 5 days at 25ºC in M medium without carbon source or vitamins. Yellow arrows indicate the inoculation point and light-blue arrows indicated un-colonized hyphae. Scale bars represent 1000 µm. This observations were determined by using a bright-field microscope without coloration (Zeiss Upright microscope Axio Imager). ... 71 Figure 6. PR solubilization by the different PRSB individually and in mixed culture (a) and bacterial growth (b)
expressed as μg protein mL-1, after 7 days incubation in NBRIP-MPR liquid media. Error bars are +
standard deviation (n=3). Soluble P from uninoculated control was subtracted from the respective treatments. Different letters indicate significant differences (P<0.05) according to Tukey‟s test. Tested strains were Asaia lannaensis (Vb1), Pseudomonas sp. (Vr14), Rahnella aquatilis (Sr24) and Pantoea
agglomerans (Vr25). ... 90
Figure 7. Production of organic acids by the selected PRSB used individually and in mixture: (a)
2-ketogluconic and (b) D-gluconic acid produced by the PRSB isolates after 7 days of incubation in liquid cultures. Error bars are + standard error (n=3). Different letters show significant differences by Tukey‟s test (P<0.05). Tested strains were Asaia lannaensis (Vb1), Pseudomonas sp. (Vr14), Rahnella aquatilis (Sr24) and Pantoea agglomerans (Vr25). ... 93 Figure 8. Specific biofilm formation index determined by the traditional CV method of the selected PRSB used
individually and in combination. Error bars are + standard deviation (n=5). Uninoculated control values were subtracted from the respective treatments. Different letters show significant differences by Tukey‟s test (P<0.05). Tested strains were Asaia lannaensis (Vb1), Pseudomonas sp. (Vr14), Rahnella aquatilis (Sr24) and Pantoea agglomerans (Vr25). ... 94 Figure 9. Amount of P solubilized (a) and exopolysaccharides (μg per mL-1) produced by the different PRSB
isolates used individually or in mixture (b). Error bars are + standard deviation (n=3). Uninoculated control values were subtracted from the respective treatments. Significant differences according to the Tukey test, at P<0.01 for exopolysaccharides and P<0.05 for phosphorus, are indicated by different letters. Tested strains were Asaia lannaensis (Vb1), Pseudomonas sp. (Vr14), Rahnella aquatilis (Sr24) and Pantoea agglomerans (Vr25). ... 96 Figure 10. Root colonization of two week-old corn seedlings (Zea mays L. cv SENECA, Horizon) by the
PRSB. Values (CFU g-1 of dry roots) are means of n=3. Different letters indicate significant (P<0.05)
differences based on Tukey‟s test. 1E10 equals 1x1010 CFU gr-1 dry roots. ... 100
Figure 11. Effect of PRSB inoculation (used individually or in mixture) on the percentage root mycorrhization by the iAM treatment. Error bars are + standard deviation (n=4). Different letters indicate significant
xii
(P<0.05) differences based on LSMeans test. Tested strains were Asaia lannaensis (Vb1),
Pseudomonas sp. (Vr14), Rahnella aquatilis (Sr24) and Pantoea agglomerans (Vr25). ... 102
Figure 12. Effect of PRSB inoculation (used individually or in mixture) on the percentage root mycorrhization by the iAM +Gi treatment. Error bars are + standard deviation (n=4). Different letters indicate significant (P<0.05) differences based on LSMeans. Tested strains were Asaia lannaensis (Vb1), Pseudomonas
xiii List of abbreviations
ABS: absorbance
ANOVA: analysis of variance
%AM: percentage of root AM colonization AM: arbuscular mycorrhizae
iAM: indigenous AM BS: bacterial suspension BSA: bovine serum albumine CEC: cation exchange capacity CFU: colony forming units df: degrees of freedom dw: distilled water
EDTA: ethylenediaminetetracetic acid
Gi: Glomus irregulare
GLM: General Linear Model HCN: hydrogen cyanide Hxa: Hydroxyapatite IAA: indolacetic acid
LSD: least significance difference LSMeans: least squares means MHB: mycorrhizae helper bacteria MPR: Moroccan phosphate rock
NBRIP: national botanical institute‟s phosphate growth medium
NBRIP-Hxa: NBRIP medium supplemented with hydroxyapatite as a sole P source
NBRIP-PR: NBRIP medium supplemented with PR as sole P source
OD: optic density OA: organic acid OM: organic matter P: phosphorous pb: pair of bases
PCR: polymerase chain reaction
PGPR: plant growth promoting rhizobacteria PR: phosphate rock
PRS: phosphate rock solubilizer
PRSB: phosphate rock solubilizing bacteria PS: P solubilizer
PSB: P solubilizing bacteria PSM: P solubilizing microorganism RAE: relative agronomic effectiveness rpm: revolutions per minute
RT: room temperature
SBF: specific biofilm formation index TCP: Tricalcium phosphate
TLC: Thin-layer chromatography TSB: tryptic soy broth
TSA: tryptic soy agar TPS: triple superphosphate
To my beautiful family and friends from Quebec, Mexico and beyond.
xvii Acknowledgments
I want to acknowledge my thesis director, Dr Hani Antoun, for all his help, support and enthusiasm during my research. Thank you for giving me the opportunity to realize this project and to open the doors of your laboratory, to your knowledge and experience. I want to thank Dr Patrice Dion for all his help at the beginning of the project and for his helpful comments in the pre-lecture. I want to thank Marie-Claude Julien for all her support, knowledge and courage that help me to advance in my research and to let me work with her in order to learn more about soil bacteria; you always gave me advice and good comments and a wonderful friendship. Finally I want to thank Dr Henri Fankem for all his counseling, friendship, good advice and participation in my research.
Many thanks to Dr. Martin Trépanier for all his help, friendship and his practical advices in all my experiments. Thank you for sharing your passion and knowledge in mycorrhizae. Also, I want to recognize Marie-Pierre Lamy for her support in the statistical analysis and good counceling in the greenhouse experiments. I especially want to thank Taylor Morey which help in the English editing and his good advices.
I want to thank all my laboratory teammates: Antoine Dionne, Fardin Ghobakhlou, Robert Kawa, and Jean Martin for all his help in the soil analysis; Rejean and David for all your support; Hélène Crépeau for all her help in the statistical analyses; Nicolas Gruyer for his help, friendship, support and advice; Madeleine Roy for all her help, support and friendship; Salma Taktek for her friendship and all the advices that helped me to finish my research, especially for her huge help during my greenhouse experiments; thanks Salma for giving me energy and courage to finish my research, your friendship means a lot to me; thanks to Anne-Marie Busque-Dubois for working hard in my project and for all her questions that improved my work; Jean-Sébastien Gauthier for all his help in the last part of my thesis;Pierre-Alain Girard and Melissa Girard for their huge help in the French translations. Thanks Melissa and Marine for all your support, time, help, advice, courage and love, you are exceptionally a good friends.
I want to acknowledge in a special manner all the people in the Envirotron that helped me for finishing my work and to do my greenhouse experiments: Carole Martinez for all your help and collaboration, thanks to Claudette Roy, Rachel Daigle, and Anne-Catherine and to all the people that work in the greenhouses of the Envirotron.
I want to thank the Natural Sciences and Engineering Research Council of Canada for the financial support; they allowed the development of this project. Also, I want to thank the Ministère de l’Éducation, du Loisir et du
xviii
Sport (Quebec) for giving the scholarship that supported me during my project and to M. Yves Bernier for all
his help.
Thanks to my family and friends for your advice, friendship and love. My friends Antoine, Salma, Marine, Melissa, Joseph, Andrée, Jonathan, Geneviève and Sandra that not only support me during this project but for giving me their friendship when I need it the most. I want to thank my husband Pierre-Alain for working in the greenhouse experiments and working with me during the weekends. Thanks to Émilien for being with me during this process, you give me the strength and the courage to continue. Thanks for all the members of my family in Quebec and in Mexico, you are very important for me, thanks to you I got the emotional support to work on this project. To my comadritas (Gaby, Vane, Melissa and Llumi), you were always supporting me. Thanks to Rejean and Louise, for all the days and nights that you help me and support me and to Louise, Jacques and Marilie for everything, I just love you guys. Finally, I want to acknowledge my parents and my aunt Callita in a special manner for teaching me everything that I know, being an inspiration to me, and give all your love, thanks.
xix Foreword
The work performed in this thesis was presented in national and international conferences and two publications are in preparation.
Publications in preparation
Magallon-Servin, P., Fankem, H., Trépanier, M., Antoun, H. Phosphate rock solubilizing rhizobacteria isolated from maize (Zea mays) mycorrhizosphereMagallon-Servin, P., Fankem, H., Trépanier, M., Antoun, H. Development of an inoculum by mixing different phosphate rock solubilizing bacteria able to enhance the growth and nutrient uptake of maize (Zea mays L.).
Conferences
Magallon-Servin, P., Fankem, H., Trépanier M., Antoun, H. Rock phosphate solubilizing rhizobacteria associated with corn. Rhizosphere 3, 25-30 September 2011, Perth, Australia.
Magallon-Servin, P., Fankem, H., Trépanier M., Antoun, H. Mobilization of phosphorus from rock phosphates by mixtures of rhizobacteria. The Canadian Society of Microbiologist (CSM) 2011, June 2011, University of Newfoundland St. John's, Canada.
Paola Magallon Servin, Salma Taktek, Martin Trépanier, Antoine Dionne Et Hani Antoun. 2011. Dissolution biologique des phosphates : état des connaissances et perspectives d‟avenir. 25e congrès annuel de l‟AQSSS,
25 au 27 mai 2011, Wendake, Qc.
Magallon-Servin, P., Trépanier, M., Hichem, Ch., Dion, P., Antoun, H. Microorganismes associés au maïs dissolvants les phosphates organiques et inorganiques. Congrès conjoint AQSSS-SPPQ, 1-3 juin 2010, Oka, Québec, Canada.
1 CHAPTER I. INTRODUCTION
1.1 Inorganic phosphorus fixation and its management in agricultural soils
In agricultural crop production, phosphorus (P) is second only to nitrogen in importance as a fertilizer (Goldstein 2007). Agricultural soils are often rich in insoluble mineral phosphates but deficient in soluble orthophosphate. In calcareous soils, H2PO4- and HPO4-2 react with Ca+2, producing the precipitate CaHPO4
with the release of H+ ions, lowering the pH and affecting the relative distribution of the phosphate ionization
states. The magnitude of phosphate precipitation is dependent on: the phosphate concentration, cation exchange capacity (CEC), pH, and the type of soil cation (Cho 1991). A study performed in two alkaline calcareous soils showed that the presence of Ca+2 ions increases P precipitation (Tunesi et al., 1999). When
choosing monocalcium phosphate, superphosphate, and diammonium phosphate for application to alkaline and calcareous soils, monocalcium phosphate is less recommended because its application results in the precipitation of Ca phosphates (Castro and Torrent 1994).
Aluminium oxides and iron hydroxides have been recognized as the most important components of P fixation in acid soils. In this case, the phosphate replaces the hydroxyl oxygen on the Fe-OH, this reaction being increased at low pH (Li and Stanforth 2000). Fertilizers like superphosphate contain sufficient amounts of calcium phosphate to precipitate their own P in acidic soil conditions, and additional precipitation of P in the presence of Al and Fe is possible (Chabot et al., 1996a). Beside P fixation by the inorganic phases of the soil, P can be absorbed by organic matter (OM) constituents such as humic substances (Gerke and Hermann, 1992). However, this is strongly influenced by the presence of organically complexed metals including Ca, Mg, Zn, Fe, and Al (Riggle and von Wandruszka 2005).
2
1.2 Adsorption and fixation of organic phosphorus in soils
Much of the organic phosphorus present in soils is in the form of inositol phosphates. Inositol phosphates, and specifically inositol-hexakisphosphate or inositol hexaphosphoric acid (phytate), can represent up to 50% of total P soil content (Turner et al., 2002). Phytates are a major part of soil P reserves and can be used as direct P fertilizer to improve plant nutrition in various crops, such as maize, Mexican sunflower, wheat, buckwheat, and rye, achieving the same plant-biomass yield as Ca(H2PO4)2 (Steffens et al., 2010). However, phytate can
be very strongly fixed by soils, clays, and peats as compared to inositol-monophosphate or other organic sources of P (i.e. glucose-1-phosphate and α-glycerol-phosphate), so it is less likely to be available for plants (McKercher and Anderson 1989). In acid soils, the sorption of inositol phosphates is related to amorphous Al and Fe oxides content (Anderson et al., 1974), while in neutral and basic soils, the amount of phytate fixed seems to be governed by clay, OM, and Ca content. Phytates can also react with humic and fulvic acids present in the soil. In some volcanic soils, free inositol phosphate can be associated with these fractions of OM (Borie et al., 1989). It seems that humic acids are able to form complexes with inositol phosphates through a metal link (i.e. Fe+3). Furthermore, the presence of humic acids in the presence of Fe (Pospisil and Hrubcová
1975) or Al (He et al., 2006) leads to inhibition of phytase activity leading to phytate accumulation in soil. Nevertheless, a recent study shows that organic anions produced by plants can release phytate sorbed to goethite, thus increasing its concentration and making it more available to hydrolysis by plant phytases (Giles et al., 2012).
1.3 The use of P fertilizers and the importance of phosphate rock (PR)
The majority (76-90%) of soluble P fertilizers used for agricultural production precipitates after application, by forming metal cation complexes (Khan et al., 2007). Because P chemical fertilizers are expensive, the cheaper direct use of PR with high reactivity is an interesting alternative (Babana and Antoun 2006a,b; Sagoe et al., 1998; Smalberger et al., 2010; Vassileva et al., 1997). PR composition ranges from fluorapatite Ca5(PO4)3F to
fully substituted carbonate apatite Ca9.4Na0.4Mg0.2 (PO4)4.5(CO3)1.5F2.6)0.5+ (Nye and Kirk 1987). It is the cheapest
P fertilizer but because of its low reactivity, acid pre-treatment is required to reach the effectiveness of other P fertilizers (Rajan et al., 1996). However, its reactivity also depends on the PR source, particle size, and soil conditions (Garth 1984).
PR is generally applied in acid soils and can take a few years of annual application before it is as effective as superphosphate (Garth 1984; Ghani et al., 1994). One of the main obstacles to direct application of phosphate rocks (PRs) to soil is the failure of PRs to release P in sufficient quantity to support plant growth. The relative agronomic effectiveness (RAE) of the PR depends on soil characteristics and PR reactivity (Bolland and Gilkes 1997; Smalberger et al., 2010). Several alternatives for increasing PR reactivity have been tried: (a)
3 incorporation of additives, (b) partial acidulation (Kpomblekou-A and Tabatabai 2003; Sagoe et al., 1998), (c) compaction of PR with water-soluble P fertilizers (Kpomblekou-A and Tabatabai 2003; Rajan et al., 1996), and (d) the use of phosphate- solubilizing microorganisms (PSM) (Ghani et al., 1994; Vassileva et al., 1997). PSMs are a very attractive approach for using PRs as fertilizer because they are able to solubilize P by excreting organic acids (Reyes et al., 2001). Among the most common low-molecular weight organic acids (OAs) identified in soil are the oxalic, succinic, tartaric, fumaric, malic, citric, synaptic, caffeic, syringic, salicylic, gallic,
p-coumaric, gentisic, protocatechuic, p-hydroxybenzoic, and ferulic (Kaurichev et al., 1963). These organic
acids exhibit different chelation and complexing properties, by forming soluble complexes with polyvalent cations from rocks and minerals. Hence, these organic acids play important roles in dissolution, transportation, and concentration of elements. The reactions involved in the P release process are not only pH dependent, but also are related to the structural characteristics of the OAs (Kpomblekou-A and Tabatabai 1994) and the chemical characteristics of PR (Kpomblekou-A and Tabatabai 2003).
It has been concluded that certain tri-carboxylic acids (citric and cis-aconitic) and di-carboxylic acids (oxalic, malonic, fumaric and tartaric) can solubilize different PRs, but the presence of Ca and Mg ions (as PR impurities) contribute to a decrease in P solubility, especially in PRs that contain substantial amounts of alkaline-earth carbonates (Kpomblekou-A and Tabatabai 2003). Among these, citric and tartaric acids are very efficient in solubilizing PR (Sagoe et al., 1998). Gerke (1992; 1993) proved that the addition of organic acids such as citric acid increases the solubilization of Al and Fe-P precipitates, so the potential secretion of organic acids by root exudates or microbes seems to be a good option to increase the solubility of P in soil, when PR is added as fertilizer.
1.4 Phosphate solubilization by soil microorganisms
Rhizobia and bradyrhizobia are well known for their beneficial effect on legumes resulting from their ability to fix N2, but studies indicate that these bacteria have many of the attributes of plant growth-promoting
rhizobacteria (PGPR) including the ability to solubilize phosphates and to stimulate the growth of non-legumes (Alikhani et al., 2006; Antoun et al., 1998; Chabot et al., 1996a). Some other plant growth-promoting microorganisms and fungi with important phosphate-solubilizing capacity are listed in Tables 1 and 2. Chelation (excretion of organic acids or production of siderophores) and/or acidification (through the ionization of organic acids or the release of protons accompanying respiration or NH4+ assimilation) are the main
4
Table 1. Phosphate-solubilizing fungi.
Microorganism Characteristics* Reference
Rhizopus sp. PS (Chabot et al., 1996a)
Penicillium rugulosum PRS (Reyes et al., 2001)
P. bilaiae PS, PRS (Wakelin et al., 2004) P.simplicissimum PS, PRS P. griseofulvum PS, PRS Talaromyces flavus PS, PRS Penicillium radicum PS, PRS P. minioluteum PS, PRS Penicillium sp. PS, PRS
Aspergillus niger PS, PRS (Ogbo 2010; Vassilev et al., 1996; Vassilev et al., 1997; Vassilev et al.,
1998; Vassileva et al., 1997 and 1998)
Aspergillus awamori PS,PRS, IAA (Babana and Antoun 2006 a and b; Mittal et al., 2008)
Penicillium chrysogenum PS, PRS (Babana and Antoun 2006 a and b)
P. citrinum PS, PRS, IAA (Mittal et al., 2008)
Yarowia lipolytica
(yeast)
PRS (Vassileva et al., 2000)
5 Table 2. Phosphate-solubilizing bacteria.
Microorganism Characteristic* Type Reference
Rhizobium leguminosarum bv. phaseoli
PS, S, HCN, IAA, PhyS PGPR (Alikhani et al., 2006; Antoun et al., 1998; Chabot et al., 1996a; Chabot et al., 1998)
R. leguminosarum bv. trifolii PS, S, HCN, IAA, PhyS PGPR (Alikhani et al., 2006; Antoun et al., 1998)
(Alikhani et al., 2006; Antoun et al., 1998)
R. leguminosarum bv. viciae PS, S, HCN, IAA, PhyS PGPR
B. japonicum PS, S, IAA, PhyS PGPR (Antoun et al., 1998)
Sinorhizobium meliloti PS, S, IAA, PhyS PGPR (Alikhani et al., 2006; Antoun et al., 1998)
Bradyrhizobium sp. PS, S, IAA, PhyS PGPR (Alikhani et al., 2006; Antoun et al., 1998)
Mesorhizobium cireri & M. mediterraneum
PS, PhyS PGPR (Alikhani et al., 2006)
Artic rhizobia IAA, S PGPR (Antoun et al., 1998)
Enterobacter sp. PS, S, IAA PGPR (Chabot et al., 1996a)
Serratia sp. PS, S, IAA, PRS PGPR (Chabot et al., 1996a;
Hameeda et al., 2006)
Pseudomonas sp. PS,S, IAA, PRS, MHB PGPR (Babana and Antoun 2006a
and b; Chabot et al., 1996a; Hameeda et al., 2006; Postma
et al., 2010)
Bacillus sp. PS SB (Postma et al., 2010)
Serratia marcescens PS R (Mamta et al., 2010)
Enterobacter cloacae PRS E (Hameeda et al., 2006)
Burkholderia sp. PS SB (Mamta et al., 2010; Postma et
al., 2010)
*Production of : S (siderophore), IAA (indole acetic acid), HCN (hydrogen cyanide); MHB (mycorrhizae helper
bacteria); PRS (phosphate rock solubilizer); PS (Phosphate solubilizer); PhyS (Phytate mineralization); PGPR (plant growth promoting rhizobacteria), SB (soil bacteria), R (rhizobacteria), E (enterobacteria)
Some organic acids, such as oxalic, citric, tartaric and lactic, can solubilize P efficiently; these compounds can either dissolve the mineral phosphate directly as a result of the exchange of the phosphate anion by an acid anion or by the chelation of iron and aluminum ions associated with phosphate, lowering the pH (Khan et al., 2007). Among bacteria that solubilize phosphates through production of organic acids are: Burkholderia
6
2009), Serratia marcescens (Pérez et al., 2007) and others (Table 2). Recently, Streptomyces griseus,
Streptomyces cavourensis, and Micromonospora aurantiaca were recognized for their ability to solubilize PR;
the solubilizing mechanism involved is the production of siderophores (Hamdali et al., 2008).
Bacteria are not the only microorganisms that can solubilize phosphates and increase plant growth and yield. It has been proven that certain fungi have a mineral phosphate-solubilizing phenotype (MPS+). For example, Penicillium rugulosum readily dissolves hydroxyapatite (HA, Ca5P3O13H) and PR through excretion of gluconic
and citric acids and protons (Reyes et al., 1999 a,b; 2001; 2002). Penicillium simplicissimum and Penicillium
bilaiae RS7B-SD1 were isolated from wheat roots (Tricum aestivum cv. Krichauff) for their ability to dissolve
PR (Wakelin et al., 2004). Immobilized mycelium of Aspergillus niger NB2 can dissolve PR in supplemented olive mill wastewater-based medium. In a soil plant-experiment, this fungus improved the growth and P uptake of Trifolium repens fertilized with PR (Vassilev et al., 1998). An inoculant containing a mixture of two
Aspergillus awamori strains and a mixture of four strains of Penicillium citrina that solubilize PR in the
presence of Rhizobium ciceri stimulates growth in chickpea (C. arietinum L.cv GPF2) and increases P- and N-uptake. When the Aspergillus and Penicillium strains are mixed together, the seed P content and P available in soil increased after harvest (Mittal et al., 2008).
1.5 Maize as a model to study PSM
Several studies of the rhizospheric and endophytic bacterial communities of maize (Zea mays), have identified the presence of PGPR belonging to the following genera: Acinetobacter, Agrobacterium, Bacillus,
Corynebacterium, Enterobacter, Klebsiella, Pseudomonas, and Serratia (Lalande et al., 1989). Chabot et al.
(1996 a and b) also reported maize root colonization and dry matter yield increase by strain P31 of R.
leguminosarum bv. phaseoli. Supporting this, a bioluminescent mutant (R1 Lux+1) and its wild type (R1) Rhizobium leguminosarum bv. phaseoli were able to colonize maize roots and to increase plant dry matter at
different P-fertilization rates (Chabot et al., 1998). In a soil-plant microcosm, inoculation of maize with
Penicillium rugulosum IR-94MF resulted in increases of dry matter yields ranging from 3.6 to 28.6% when PRs
were used as a P source (Reyes et al., 2002). Under greenhouse and field conditions, Hameeda et al. (2008) showed that two phosphate-solubilizing bacteria, Serratia marcescens EB 67 and Pseudomonas sp. CDB 35, increased the biomass of maize fertilized with PR.
1.6 The importance of combining different PSMs to be used as biofertilizers
In natural ecosystems, bacteria do not exist as solitary cells, but live in organized biofilms, flocs, or granules where they contribute to biogeochemical processes (Paerl and Pinckney 1996). Pandey and Maheshwari (2007) observed that under in vitro conditions the growth of Sinorhizobium meliloti PP3 was increased in the
7 presence of Burkholderia sp. MSSP in a mixed culture. Although growth of strain MSSP was not affected by S.
meliloti PP3, more indoleacetic acid (IAA) was produced in mixed culture, and inoculation of Cajanus cajan
with this consortium significantly increased seedling growth and fresh shoot weight after 40 days of growth. The use of synergistic microorganisms has been applied in various areas. In wastewater treatment, the use of more than one microorganism can be advantageous, because it allows the exploitation of metabolic versatility (Fong and Tan 2000). In bioremediation, the biodegradation of complex hydrocarbons (alkanes) is enhanced by the presence of a bacterial consortium (three Bacillus sp. strains plus two Pseudomonas aeruginosa and one Micrococcus sp.) in diesel and oil contaminated soils (Ghazali et al., 2004). In agriculture, the presence of synergistic microorganisms in soils seems to be responsible for suppressing certain plant diseases (Mendes et al., 2011).
Associations of different microorganisms in agriculture can improve plant growth and nutrient uptake. Increased growth and P and N uptake of plants has been observed when the AM (arbuscular mycorrhizae) fungi consortium of Acaulospora scrobiculata, Gigaspora albida and Glomus irregulare was used to inoculate
Eucalyptus hybrid plants (Sastry et al., 2000). Villegas and Fortin (2001; 2002), mentioned that when G. irregulare interacts with P. aeruginosa and P. putida the P solubilization is increased, although these species
are rather inefficient P solubilizers individually. Plant growth, seed yield, grain protein and N and P uptake of wheat (Triticum aestivum L) under field conditions were significantly increased by inoculation with a mixture containing a N2 fixing bacterium (Azotobacter chroococcum), a phosphate-solubilizing bacterium (Bacillus sp.),
and the AM fungus (Glomus fasciculatum). The combination of these microorganisms resulted in higher growth promotion as compared to individual inoculation effects, suggesting a possible synergism (Khan and Zaidi 2007). Mamta et al. (2010) reported that growth stimulation and P content of Stevia rebaudiana was more pronounced when inoculated with a mixture of tricalcium-phosphate-solubilizing bacteria, such as Burkholderia
gladioli, Serratia marcescens, and Enterobacter aerogenes, than individual treatments. The effect of the
8
Table 3. Synergism between rhizobacteria and AM fungi in various crops.
AM1 Rhizobacteria1 Plant Effect on plant P Fertilizer2 Reference
Glomus irregulare
Bacillus subtilis
(PSB)
Onion Increase in biomass accumulation P and N acquisition PR (Venezuela) Toro et al.,1997 G. mosseae or G. irregulare Azotobacter chroococcum (NFB) + B. megaterium (PSB) + B. mucilaginous (KSB)
Maize Increase in plant biomass and NPK uptake depending on the type of AM and fertilization rate PR + Farmyard manure Wu et al., 2005 AM natural consortium Pseudomonas jessenii (PGPR). Pseudomonas synxantha (PGPR)
Wheat Grain yield, N, P and K uptake increased Farmyard manure + diammonium phosphate Mäder et al., 2011 Glomus mosseae Rhizobium meliloti (NFB) Enterobacter sp. (PSB)
Alfalfa Increase in shoot biomass and N and P uptake PR (Venezuela) Barea et al., 2002 Glomus fasciculatum Azotobacter chroococcum (NFB) Bacillus sp. (PSB)
Wheat Increase in root and shoot dry matter and P and N content.
Diammonium phosphate
Khan and Zaidi 2007
1AM, Arbuscular mycorrhizae; PSB, phosphate solubilizing bacteria; NFB, nitrogen-fixing bacteria; KSB, potassium-solubilizing bacteria; PGPR, plant growth promoting rhizobacteria.
9 As mentioned before, one of the most important mechanisms for PSMs is the production of organic acids; however, not all the organic acids have the same solubilization effect on the different insoluble forms of P. The effectiveness of P solubilization by organic acids depends on their chelating and complexing abilities, which are affected by type and position of the functional ligand (Kpomblekou-A and Tabatabai, 1994). The fact that some organic acids can dissolve as much P as some inorganic acids, suggests that solubilization capacity cannot be explained by protonation only (Sagoe et al., 1998). This is especially true in the case of PRs (Kpomblekou-A and Tabatabai 1994; 2003), which have different reactivities (ease of solubilization) depending on the content of Ca, P, F, and accessory minerals like clays, carbonates, and quartz (Chien and Menon 1995; Zapata and Roy 2007). PR reactivity with different organic acids has different effects on PR solubilization (Kpomblekou-A and Tabatabai 2003; Sagoe et al., 1998). P. regulosum IR-94MF1 is capable of solubilizing different sources of inorganic P by releasing gluconic and citric acid (Reyes et al., 1999a). However, it has been observed that depending on the type of PR used, P. regulosum changes the production of organic acids; this behavior is mainly attributed to the intrinsic characteristics and reactivity of PR based on its mineralogy and chemical composition (Reyes et al., 2001). Because there are different types of P (not only PR) in soil, it is possible to hypothesize that a mixture of different PSMs has a broader spectrum of different organic acids than with individual bacteria, so the mixture might solubilize P more efficiently. Besides, it has been suggested that an alternative for PSMs as biofertilizers in sustainable agriculture is a mixture of cultures or co-inoculation with other microorganisms (e.i Azotobacter chroococcum, Bacillus sp. and Glomus fasciculatum), which results in a synergistic interaction between the microorganisms and higher solubilization of low solubility P sources (Khan et al., 2007).
1.7 The importance of biofilm formation in PSM
In a natural environment, bacteria cooperate (i.e biodegradative bacteria, spoilage biofilms, lichens, etc.) within a biofilm matrix rather than in a planktonic state (Caldwell, 1995; Davey and O'Toole 2000). Biofilms can be defined simply and broadly as communities of microorganisms that are attached to a surface, comprising single or multiple microbial species (Fletcher and Decho 2001; O'Toole et al., 2000; Ramey et al., 2004). Some recognized rhizobacteria are able to produce biofilms (Davey and O'Toole 2000), as in the case of
Azospirillum brasilense (Lerner et al., 2009), Bacillus subtilis (Branda et al., 2001), Paenibacillus polymyxa
(Haggag and Timmusk 2008; Timmusk et al., 2005) and nitrogen-fixing bacteria such as Sinorhizobium meliloti and Rhizobium leguminosarum bv. viciae (Fujishige et al., 2006). The ability of S. meliloti to form biofilms is related to its capacity to form nodules (Fujishige et al., 2006; Fujishige et al., 2008). Several physiological changes can occur during biofilm formation (Fletcher and Decho 2001) including cell motility (generally reduced), production of intercellular signals, and an increase in the resistance to antibiotics and production of lipopolysaccharides (Fletcher and Decho 2001; O'Toole et al., 2000).
10
Sometimes, species that form biofilms act in complementary physiological processes. This is the case for
Nitrosomonas and Nitrobacter (Fletcher and Decho 2001; Montràs et al., 2008) and Pseudomonas putida and Acinetobacter sp. (strain C6) (Hansen et al., 2007). This positive interaction is also possible when soil bacteria
and filamentous fungi interact (Seneviratne and Jayasinghearachchi 2003). Biofilm forming capacity has been observed in six species of common soil fungi (Aspergillus niger, A. nidulans, A. terreus and Aspergillus sp.,
Penicillium sp. and Mucor sp.) and in three bradyrhizobial strains (Bradyrhizobium japonicum TAL102, TAL
602 and B. elkanii SEMIA 5019) (Seneviratne and Jayasinghearachchi 2003). In a study of endophytic microorganisms isolated from rice it was observed that co-cultures of biofilm forming bacteria and fungi produced higher quantities of IAAs than those unable to form biofilms (Bandara et al., 2006).
Generally, nutritional conditions regulate the development of biofilms, as has been proven for S. meliloti (Fujishige et al., 2006; Rinaudi et al., 2006). Control of aggregation and surface attachment in response to the availability of key nutrients, such as P, is important in plant-associated bacteria (Danhorn and Fuqua 2007). In
Sinorhizobium meliloti, weak biofilm formation is observed when the P concentration is low (Rinaudi et al.,
2006). In contrast, the biofilm formation capacity of Agrobacterium tumefaciens C58 increases under low P conditions (Danhorn et al., 2004). Biofilms can also affect the availability of nutrients; in soil incubation studies, a biofilm of Bradyrhizobium elkanii SEMIA 5019 and Penicillium spp. had greater NH4+, NO3-, and PO4-3
availability and nitrogenase activity than the individual microorganisms of the biofilm (Seneviratne and Jayasinghearachchi 2005). This supports the idea that a mixture of microorganisms mineralizes N and P in soil better than individual microbial inoculants. Under laboratory conditions, Jayasinghearachchi and Sereviratne (2006) proved that by using a Penicillium sp. and Bradyrhizobium elkanii SEMIA 5019 biofilm, it was possible to increase the biosolubilization of PR more efficiently than by using individual microorganisms. By spraying tea plants (Camellia sinensis cv. DT1) field plots with biofilm based biofertilizers (Acetobacter spp.,
Azotobacter spp., Rhizobium spp., Bradyrhizobium spp. and non-pathogenic Colletotrichum ssp.) in
combination with half the recommended rate of chemical fertilizers added every two months a 20% increase in soil organic C was observed in comparison to the use of the full recommended rate chemical fertilizer alone (Seneviratne et al. 2011). All these studies support the idea that multiple-species inoculants could be more effective to mobilize P from sparingly soluble P sources as compared to single species inoculants.
11 1.8 Hypotheses
The hypotheses presented below were considered for this research:
a) A bacterial inoculum can be developed to improve the efficiency of the direct use of PR as P fertilizer for organic or sustainable maize production.
b) The selected bacteria should present the following characteristics:
To be able to colonize maize roots without causing any harm to the beneficial AM fungi symbiosis.
To present a good capacity to mobilize P from different sparingly-soluble P sources and from the soil organic P-pool.
To harbor other PGPR traits beneficial for maize growth. To form a beneficial biofilm
12
1.9 Objectives
Considering that PRSB have an excellent potential to be used for the improvement of the agricultural use of PR, the general aim and specific objectives of this work are as follows:
1.9.1 General aim
To obtain mycorrhizosphere-competent PRSB harboring other PGPR-associated traits, to be used individually or in a mixture for the development of an inoculant to improve maize growth and P acquisition from soil organic and inorganic P-pools.
1.9.2 Specific objectives
To isolate PRSB from the mycorrhizosphere of maize and to determine their ability to solubilize PRs with different reactivity.
To determine the effect (neutral, positive or negative) of the selected PRSB on the establishment of arbuscular mycorrhizal (Glomus irregulare) symbiosis in vitro and under greenhouse conditions.
To determine the PGPR traits of the selected PRSB and to evaluate growth promotion of maize by the selected isolates used individually or in different combinations in growth pouches with Glomus irregulare under greenhouse conditions.
1.10 References
Alikhani, H.A., Saleh-Rastin, N., Antoun, H. 2006. Phosphate solubilization activity of rhizobia native to Iranian soils. Plant and Soil 287: 35–41.
Anderson, G., Williams, E.G., Moir, J.O. 1974. A comparison of the sorption of inorganic orthophosphate and inositol hexaphosphate by six acid soils. Journal of Soil Sciences 25 (1): 51-62.
Antoun, H., Beauchamp, C.J., Goussard, N., Chabot, R., Lalande, R. 1998. Potential of Rhizobium and
Bradyrhizobium species as plant growth promoting rhizobacteria on non-legumes: Effect on radishes
(Raphanus sativus L.). Plant and Soil 204(1): 57-67.
Babana, A.H., Antoun, H. 2006a. Biological system for improving the availability of Tilemsi phosphate rock for wheat (Triticum aestivum L.) cultivated in Mali. Nutrient Cycling in Agrosystems 76: 285-295.
Babana, A.H., Antoun, H. 2006b. Effect of Tilemsi phosphate rock-solubilizing microorganisms on phosphorus uptake and yield of field-grown wheat (Triticum aestivum L.) in Mali. Plant and Soil 287: 51-58. Bandara, W.M., Seneviratne, G., Kulasooriya, S.A. 2006. Interactions among endophytic bacteria and fungi:
13 Barea, J.M., Toro, M., Orozco, M.O., Campos, E., Azcón, R. 2002. The application of isotopic (32P and 15N)
dilution techniques to evaluate the interactive effect of phosphate-solubilizing rhizobacteria, mycorrhizal fungi and Rhizobium to improve the agronomic efficiency of rock phosphate for legume crops. Nutrient Cycling in Agroecosystems 63:35-42.
Bolland, M.D.A., Gilkes, R.J. 1997. The agronomic effectiveness of reactive phosphate rocks 2: Effect of phosphate rock reactivity. Australian Journal of Experimental Agriculture 37(8):937-946.
Borie, F., Zunino, H., Martinez L. 1989. Macromolecule-P associations and inositol phosphates in some chilean volcanic soils of temperate regions. Communications in Soil Science and Plant Analysis 20:1881-1894.
Branda, S.S., Gonzalez-Pastor, J.E., Ben-Yehuda, S., Losick, R., Kolter, R. 2001. Fruiting body formation by
Bacillus subtilis. Proceedings of the National Academy of Sciences 98(20):11621-11626.
Caldwell, D.E. 1995. Cultivation and study of biofilm communities. In: Microbial biofilms. Lappin S. and J.W.Costerton (Eds.). Chapter 3. University Press, Cambridge, U.K. 64-79.
Castro, B., Torrent, J. 1994. Phosphate availability in calcareous vertisols and inceptisols in relation to fertilizer type and soil properties. Fertilizers Research 40(2):109-119.
Chabot, R., Antoun, H., Cescas, M.P. 1996a.Growth promotion of maize and lettuce by phosphate-solubilizing
Rhizobium leguminosarum biovar, phaseoli. Plant and Soil 184:311-321.
Chabot, R., Antoun, H., Kloepper, J.W., Beauchamp C.J. 1996b. Root colonization of maize and lettuce by bioluminescent Rhizobium leguminosarum biovar phaseoli. Applied Microbiology and Biotechnology 62(8):2767–2772.
Chabot, R., Beauchamp, Ch.J., Kloepper, J.W., Antoun, H. 1998. Effect of phosphorus on root colonization and growth promotion of maize by bioluminescent mutants of phosphate-solubilizing Rhizobium
leguminosarum biovar phaseoli. Soil Biology & Biochemistry 30(12): 1615-1618.
Chien, S.H., Menon, R.G.1995. Factors affecting the agronomic effectiveness of phosphate rock for direct application. Fertilizer Research 41: 227-234.
Cho, C.M. 1991. Phosphate Transport in Calcium-Saturated Systems: I. Theory. Soil Science Society of America Journal 55(5):1275-1281.
Danhorn, T., Fuqua, C. 2007. Biofilm formation by plant-associated bacteria. Annual Review of Microbiology 61:401-422.
Danhorn, T., Hentzer, M., Givskov, M., Parsek, M.R., Fuqua, C. 2004. Phosphorus limitation enhances biofilm formation of the plant pathogen Agrobacterium tumefaciens through the PhoR-PhoB regulatory system. Journal of Bacteriology 186(4):4492-4501.
Davey, M.E., O'Toole, G. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiology and Molecular Biology Reviews 64(4):847–867.
14
Fletcher, M., Decho, A.W. 2001. Biofilms. Encyclopedia of Life Sciences. John Wiley & Sons. 1-7.
Fong, K.P.Y., Tan, H.M. 2000. Isolation of a microbial consortium from activated sludge for the biological treatment of food waste. World Journal of Microbiology and Biotechnology 16: 441-443.
Fujishige, N.A., Kapadia, N.N., De Hoff, P.L., Hirsch, A.M. 2006. Investigations of Rhizobium biofilm formation. FEMS Microbiology Ecology 56(2):195-206.
Fujishige, N.A., Lum, M.R., De Hoff, P.L., Whitelegge, J.P., Faull, K.F., Hirsch, A.M. 2008. Rhizobium common nod genes are required for biofilm formation. Molecular Microbiology 67(3):504-515.
Garth, R.D. 1984. Comparison of water-insoluble phosphate fertilisers with superphosphate - a review. Journal of the Science of Food and Agriculture 35(3):265-271.
Gerke, J., Hermann, R. 1992. Adsorption of orthophosphate to humic-complexes and to amorphous Fe-oxide. Zeitschrift für Pflanzenernährung und Bodenkunde 155:233-236.
Gerke, J. 1992. Phosphate, aluminium and iron in the soil solution of three different soils in relation to varying concentrations of citric acid. Zeitschrift für Pflanzenernährung und Bodenkunde 155(4): 339-343. Gerke, J. 1993. Solubilization of Fe (III) from humic-Fe complexes, humic/Fe-oxide mixtures and from poorly
ordered Fe-oxide by organic acids - consequences for P adsorption. Zeitschrift für Pflanzenernährung und Bodenkunde 156(3):253-257.
Ghani, A., Rajan, S.S.S., Lee, A. 1994. Enhancement of phosphate rock solubility through biological processes. Soil Biology and Biochemistry 26(1):127-136.
Ghazali, F.M., Rahman, R.N., Salleh, A.B., Basri, M. 2004. Biodegradation of hydrocarbons in soil by microbial consortium. International Biodeterioration & Biodegradation 54(1):61-67.
Giles, C.D., Richardson A.E., Druschel, G.K. , Hill, J.A. 2012. Organic anion-driven solubilization of precipitated and sorbed phytate improves hydrolysis by phytases and bioavailability to Nicotiana
tabacum. Soil Science 177(10):591-598.
Goldstein, A.H. 2007. Future trends in research on microbial phosphate solubilization: one hundred years of insolubility. In: Velázquez, E., Rodríguez-Barrueco, C. (Eds.), First International Meeting on Microbial Phosphate Solubilization. Springer. Developments in Plant and Soil Sciences 102: 91-96.
Haggag, W.M., Timmusk, S. 2008. Colonization of peanut roots by biofilm-forming Paenibacillus polymyxa initiates biocontrol against crown rot disease. Journal of Applied Microbiology 104(4):961-969. Hamdali, H., Bouizgarne, B., Hafidi, M., Lebrihi, A., Virolle, M.J., Ouhdouch, Y. 2008. Screening for rock
phosphate solubilizing actinomycetes from moroccan phosphate mines. Applied Soil Ecology 38(1):12-19.
Hameeda, B., Harini, G., Rupela, O.P., Wani, S.P., Reddy, G. 2008. Growth promotion of maize by phosphate-solubilizing bacteria isolated from composts and macrofauna. Microbiological Research 163(2):234-242.
15 Hameeda, B., Reddy, Y.H., Rupela, O.P., Kumar, G.N., Reddy, G. 2006. Effect of carbon substrates on rock phosphate solubilization by bacteria from composts and macrofauna. Current Microbiology 53(4): 298-302.
Hansen, S.K., Rainey, P.B., Haagensen, J.A., Molin, S. 2007. Evolution of species interactions in a biofilm community. Nature 445:533-536.
He, Z., Ohno, T., Cade-Menun, B.J, Erich, M.S., Honeycutt, C.W. 2006. Spectral and chemical characterization of phosphates associated with humic substances. Soil Science Society of America Journal 70:1741-1751.
Jayasinghearachchi, H.S., Sereviratne, G.2006. Fungal solubilization of rock phosphate is enhanced by forming fungal-rhizobia biofilms. Soil Biology & Biochemistry 38(2):405-408.
Kaurichev I.S., Ivanova T.N., and Nozdrunova Y.M. 1963. Low molecular organic acid content of water-soluble organic matter in soils. Soviet Soil Science. 223-229.
Khan, M.S., Zaidi, A. 2007. Synergistic effects of the inoculation with plant growth-promoting rhizobacteria and an arbuscular mycorrhizal fungus on the performance of wheat. Turkish Journal of Agriculture and Forestry 31:355-362.
Khan, M.S., Zaidi, A., Wani, P.A. 2007. Role of phosphate-solubilizing microorganism in sustainable agriculture- A review. Agronomy for Sustainable Development 27:29-43.
Kpomblekou-A, K., Tabatabai, M.A. 1994. Effect of organic acids on release of phosphorus from phosphate rocks. Soil Sciences 158(6):442-453.
Kpomblekou-A, K., Tabatabai, M.A. 2003. Effect of low-molecular weight organic acids on phosphorus release and phytoavailabilty of phosphorus in phosphate rocks added to soils. Agriculture, Ecosystems & Environment 100:275-284.
Lalande, R., Bissonnette, N., Coutlée, D., Antoun, H. 1989. Identification of rhizobacteria from maize and determination of their plant-growth promoting potential. Plant and soil 115(1):7-11.
Lerner, A., Castro-Sowinski, S., Lerner, H., Okon, Y., Burdman, S. 2009. Glycogen phosphorylase is involved in stress endurance and biofilm formation in Azospirillum brasilense Sp7. FEMS Microbiology Letters 300(1):75-82.
Li, L., Stanforth, R. 2000. Distinguishing adsorption on surface precipitation of phosphate on goethite (α-FeOOH). Journal of Colloid and Interface Science 230(1):12-21.
Mäder, P., Kaiser, F., Adholeya, A., Singh, R., Uppal, H.S., Sharma, A.K., Srivastava, R., Sahai, V., Aragno, M., Wiemken, A., Johri, B.N., Fried, P.M. 2011. Inoculation of root microorganisms for sustainable wheat-rice and wheat-black gram rotations in India. Soil Biology and Biochemistry 43(3):609-619.
16
Mamta, R.P., Pathania, V., Gulati, A., Singh, B., Bhanwra, R.K., Tewari, R. 2010. Stimulatory effect of phosphate-solubilizing bacteria on plant growth, stevioside and rebaudioside-A contents of Stevia
rebaudiana Bertoni. Applied Soil Ecology 46(2): 222-229.
McKercher, R.B., Anderson, G. 1989. Organic phosphate sorption y neutral and basic soils. Communications in Soil Science and Plant Analysis 20:723-732.
Mendes, R., Kruijt, M., de Bruijn, I., Dekkers, E., van der Voort, M., Schneider, J.H.M., Piceno, Y.M., DeSantis, T.Z., Andersen, G.L., Bakker, P., Raaijmakers, J.M., 2011. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332:1097-1100.
Mittal, V., Singh, O., Nayyar, H., Kaur, J., Tewari, R. 2008. Stimulatory effect of phosphate-solubilizing fungal strains (Aspergillus awamori and Penicillium citrinum) on the yield of chickpea (Cicer arietinum L. cv. GPF2). Soil Biology and Biochemistry 40(3):718-727.
Montràs, A., Pycke, B., Boon, N., Gòdia, F., Mergeay, M., Hendrickx, L., Pérez, J.2008. Distribution of
Nitrosomonas europaea and Nitrobacter winogradskyi in an autotrophic nitrifying biofilm reactor as
depicted by molecular analyses and mathematical modeling. Water Research 42: 1700-1714. Nautiyal, C.S. 1999. An efficient microbiological growth medium for screening phosphate solubilizing
microorganisms. FEMS Microbiology Letters 170(1):265-270.
Nye, P.H., Kirk, G.J.D. 1987. The mechanism of rock phosphate solubilization in the rhizosphere. Plant and Soil 100:127-134.
O'Toole, G., Kaplan, H.B, Kolter, R. 2000. Biofilm formation as microbial development. Annual Review of Microbiology 54:49-79.
Ogbo, F.C. 2010. Conversion of cassava wastes for biofertilizer production using phosphate solubilizing fungi. Bioresource Technology 101(11):4120-4124.
Paerl, H.W., Pinckney, J.L. 1996. A mini-review of microbial consortia: Their roles in aquatic production and biogeochemical cycling. Microbial Ecology 31(3):225-247.
Pandey, P., Maheshwari, D.K. 2007. Two-species microbial consortium for growth promotion of Cajanus cajan. Current Science 92:1137-1142.
Pérez, E., Sulbarán, M., Ball, M.M., Yarzábal, L.A. 2007. Isolation and characterization of mineral phosphate-solubilizing bacteria naturally colonizing a limonitic crust in the south-eastern Venezuelan region. Soil Biology and Biochemistry 39(11):2905-2914.
Pospisil, F., Hrubcová, M. 1975. The influence of humic acids on phytase activity isolated from wheat seeds. Biologia Plantarum 17(6):468-474.
Postma, J., Nijhuis, E.H., Someus, E. 2010. Selection of phosphorus solubilizing bacteria with biocontrol potential for growth in phosphorus rich animal bone charcoal. Applied Soil Ecology 46(3):464-469.
17 Rajan, S., Watkinson, J.H., Sinclair, A.G., Donald, L.S. 1996. Phosphate rocks for direct application to soils. In
Advances in Agronomy. Academic Press. 57: 77-159.
Ramey, B.E., Koutsoudis, M., von Bodman, S.B., Fuqua, C. 2004. Biofilm formation in plant-microbe associations. Current Opinion in Microbiology 7(6):602-609.
Reyes, I., Bernier, L., Simard, R.R., Antoun, H. 1999a. Effect of nitrogen source on the solubilization of different inorganic phosphates by an isolate of Penicillium rugulosum and two UV-induced mutants. FEMS Microbiology Ecology 28(3):281-290.
Reyes, I., Bernier, L., Simard, R.R., Tanguay, P., Antoun, H. 1999b. Characteristics of phosphate solubilization by an isolate of a tropical Penicillium rugulosum and two UV-induced mutants. FEMS Microbiology Ecology 28:291-295.
Reyes, I., Baziramakenga, R., Bernier, L., Antoun, H. 2001. Solubilization of phosphate rocks and minerals by a wild-type strain and two UV-induced mutants of Penicillium rugulosum. Soil Biology and Biochemistry 33:1741-1747.
Reyes, I., Bernier, L., Antoun, H. 2002. Rock phosphate solubilization and colonization of maize rhizosphere by wild and genetically modified strains of Penicillium rugulosum. Microbial Ecology 44(1):39-48. Riggle, J., von Wandruszka, R. 2005. Binding of inorganic phosphate to dissolved metal humates. Talanta
66(2):372-375.
Rinaudi, L., Fujishige, N.A., Hirsch, A.M., Banchio, E., Zorreguieta, A., Giordano, W. 2006. Effects of nutritional and environmental conditions on Sinorhizobium meliloti biofilm formation. Research in Microbiology 157(9):867-875.
Sagoe, C.I., Ando, T., Kouno, K., Nagaoka, T. 1998. Relative importance of protons and solution calcium concentration in phosphate rock dissolution by organic acids. Soil Science and Plant Nutrition 44(4):617-625.
Sastry, M.S.R., Sharma, A.K., Johri, B.N. 2000 Effect of an AM fungal consortium and Pseudomonas on the growth and nutrient uptake of Eucalyptus hybrid. Mycorrhiza 10(2):55-61.
Seneviratne, G., Jayasinghearachchi, H.S. 2003. Mycelial colonization by bradyrhizobia and azorhizobia. Journal Biosciences 28(2):243-247.
Seneviratne, G., Jayasinghearachchi, H.S. 2005. A rhizobial biofilm with nitrogenase activity alters nutrient availability in a soil. Soil Biology and Biochemistry 37(10):1975-1978.
Seneviratne, G., Jayasekara, A.P.D.A., De Silva, M.S.D.L., Abeysekera, U.P. 2011. Developed microbial biofilms can restore deteriorated conventional agricultural soils. Soil Biology and Biochemistry 43(5):1059-1062.