Different Modes of Vancomycin and D-Alanyl-D-Alanine Peptidase
Binding
to
Cell Wall Peptide and
a
Possible Role for the
Vancomycin Resistance
Protein
JAMES R.
KNOX'*
ANDR. F. PRATT2Departmentof Molecular and Cell Biology, The University of Connecticut, Storrs, Connecticut 06269-3125,1 and
Department of Chemistry, Wesleyan University,
Middletown, Connecticut 064572 Received 26 February 1990/Accepted 11 April 1990Acomparison wasmadeof the bindingmodesof the bacterial cell wailprecursorL-lysyl-D-alanyl-D-alanine
totheglycopeptide antibiotic vancomycin andtotheD-alanyl-D-alanine-cleaving peptidase of Streptomycessp.
strainR61,amodel for cellwail-synthesizingenzymeswhose X-ray three-dimensionalstructureisestablished. In eachof thetwopairings(vancomycin with peptide and DD-peptidase with peptide), polypeptide backbones
wereantiparallel, and the antibioticorenzymeenveloped thepeptide substrate fromopposite sides.
Hydrogen-bondinggroupsonthe substrate whichareinvolvedwith theDD-peptidasewereshowntobe different from the
ones reported from nuclear magnetic resonance studies to be involved with vancomycin. Because of steric hindrance, the bindingof either moleculetothesubstratepreventsthebinding of the other molecule. Binding tothe substrate byaD-alanyl-D-alanine-recognizing protein inamannersimilartothat usedby theDD-peptidase couldexplainrecentobservations ofvancomycinresistance, in whicha newmembrane-associated protein has
beendetected.
Glycopeptide antibiotics of the vancomycin family (in-cluding ristocetin, avoparcin, and teicoplanin) are increas-ingly used against gram-positive bacteria, especially those resistantto 1-lactams. Like the ,-lactams, the glycopeptides inhibit the synthesis of bacterial cell wall peptidoglycan. However, they inhibit synthesis not by inactivating the biosyntheticenzymesbut, rather, by noncovalentbindingto theD-alanyl-D-alanine (D-ala-D-ala)terminusofa pentapep-tide cell wallprecursor. Itis unclearwhetherthe antibiotics prevent transmission of the disaccharide pentapeptide monomeracrossthe inner membranetotheregion of the D-alanyl carboxypeptidases and transpeptidases (DD-pepti-dases) orwhether the binding of antibiotic to the peptide occurs on the outer side of the membrane where direct
competition with the D-ala-D-ala-recognizing enzymes is likely (14, 22).
Structuralaspectsof the complexation of vancomycin and ristocetin with the tripeptide terminus have beenprobed by nuclearmagneticresonance(NMR)spectroscopy(2, 19, 20), andkinetic studies of thecomplexation havebeenreported (16, 19). With the recentdetermination ofan X-ray crystal structureofaDD-peptidase and themodeling of tripeptideto the binding site of the enzyme (6), it is possible to ask whether aDD-peptidase and the much smaller vancomycin-typeantibiotics interactwith a common cell wall substrate in a similar fashion. The DD-peptidase-substrate interaction could also serve as amodel for vancomycin resistance(9, 11, 18, 21),inwhichanewD-ala-D-ala-recognizing proteinmay bindtothepeptide terminus of themonomer atsomepoint onitsway tobecomingacross-linkedpeptidoglycan.
MATERIALS AND METHODS
Three-dimensional structures ofvancomycin and DD-pepti-dase.Intheabsence ofanX-raycrystallographicstructureof
*Corresponding author.
vancomycin (Fig. 1), one mustbuild vancomycin fromthe crystal structure ofadegradation product, CDP-I, in which anasparagineatposition3 hasbeen convertedtoisoaspartic acid. The CDP-I structure has been published previously (17), and its coordinates are available at the Brookhaven Protein Structure Data Bank (Upton, N.Y.). Initial coordi-nates of a vancomycin model were kindly provided by Robert Sheridan(Lederle Laboratories, Pearl River, N.Y.). They were derived from the X-ray structure of the CDP-I analog, and energy was minimized in the presence of N-acetyl-D-ala-D-ala. For the presentstudy, this starting van-comycin modelwasalteredatthe Nterminus andfitted to L-lysyl-D-ala-D-alatoagreewithNMRresults(2,19, 20). The interactivefittingprogramFRODO(4)wasusedon a graph-icssystem(PS330; Evans &Sutherland). The X-ray crystal-lographicstructuresofthepenicillin-sensitive, 38-kilodalton DD-peptidase of Streptomyces sp. strainR61 and five of its
,3-lactam complexes
have beenexperimentally
mapped
at resolutions of 2.3 A (0.23 nm) and2.8 A (0.28 nm) (6, 7). Basedon thesemaps, thebindingoftheL-lysyl-D-ala-D-ala substrate to the catalytic site of the enzyme has been modeled previously (6). Atomic coordinates of the com-plexes described below have been deposited at the BrookhavenDataBank.RESULTS
Interaction of the DD-peptidase with the cell wail peptide. Analysis ofthe atomic-level interactions between the DD-peptidaseand thetripeptide substrate (6) showsthat impor-tantfeaturesare,first,anantiparallel hydrogen bondingwith ,-strandb3(residues298to303) ofthepeptidase (Fig. 2A). Inparticular, thebackbone amideNHandcarbonylgroups of Thr-301 bind to the amide carbonyl and NH of the
penultimateD-alanine. Thecarbonyl of this D-alanine forms asecondhydrogen bond withtheamideNHofSer-62onthe adjacenta2helix(Fig. 2B).Theresulting polarization ofthe 1342
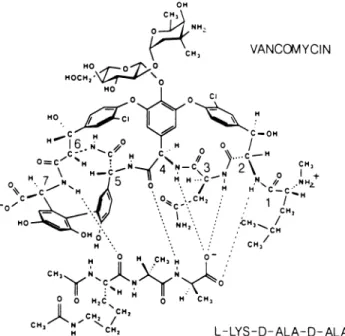
\CH, VANCOMYCIN Ho 0 HOCH, O HO 0 0 c C vW,m C-OH H:!O3 >I + ~ 16 , H H 0 / C=H1 Ccm,\~ H HHN...-4C3 2\N NH 0* N J5 / H C-C.tm O,Ht XH /-HYCH.. -AL-D, L
FIG. 1. Chemical structure ofvancomyc ho N
0 0CHC,Nc 0 ~~~
CH3N~~~~~~~~~C OH
signed interactions with diacetyl-L-lysyl-D-alanyl-D-alanine sub-strate(adapted from Kannan etal. [5]).
carbonyl bond of the substrate facilitates nucleophilic attack by the Ser-62 hydroxyl group. A second feature of the binding is the immobilization of the C-terminal carboxylate group of the peptide via interactions with the imidazole group of His-298, the hydroxyl group of Thr-299, and, possibly, the positively charged side chain of Lys-65 on the a2 helix. The enzymatic environment of the two D-methyl substituents is generallyhydrophobic, with the penultimate methyl group being somewhat more buried. The L-lysyl side chain lies in a hydrogen-bonding and negatively charged region containing Thr-227, Glu-228, and Gln-303. The re-mainder of thepeptidoglycan is expected to extend outward from Fig. 2, with the oligosaccharide running horizontally along the bottom of the enzyme (see also Fig. 3C).
Interaction of vancomycin with the cell wall peptide. Two schemes are considered for the interaction between the two molecules. One model (Fig. 3A) is derived from the fact that vancomycin and DD-peptidase have two structural features in common. (i) Both molecules contain an extended peptide chain which is aligned with substrate in ahydrogen-bonding array, and (ii) both molecules contain a protonated amine functionwhich, in the antiparallel alignment with substrate, falls near the carboxylate
ggrup
ofthe substrate (compare Fig. 2A and 3A). The model shown in Fig. 3A does not agree, however, with published NMR assignments for thevancomycin-substrate complex (2, 19, 20), especially with regard to the
hydrogen-bonded
atomsindicatedin Fig. 1. Analternative model for the interaction of the two molecules with the
NMR-assigned
hydrogen bonds found previously (2, 19, 20) is shown in Fig. 3B. Specifically, thecarbonyl of residue 4 ofvancomycinandtheamide NH ofresidue7bind to theC-terminalD-alanyl amide NH andthelysyl carbonyl of the substrate, respectively. Here, too, the two peptide strands align in an antiparallel fashion. Another feature in thebindingisashielding oftheionizedcarboxylategroup of the substrate by the N-terminal D-leucyl portion of vanco-mycin,theresult ofwhich isoptimizationof the electrostaticH I S 9THR XTHR a~~~~~~~~~~~~~~ 6~~~~~ u u~~~s 22GL ~~~~~~~~~22G
FIG. 2. (A) Stereoviewof theP-strandb3(residues298 to303)of theDD-peptidase ofStreptomyces strain R61aligned with L-lys-D-ala-D-alahavingtheconformation taken from Kellyetal. (6),with
(+,Ol) equal(-,960),(1450,-120°), and(80°,300)from theNtothe C terminus. Hydrogenatoms arenot shown. (B) Expanded
stere-oview of the binding site for the L-lysyl-D-ala-D-ala substrate (or P-lactaminhibitor) in the DD-peptidaseofStreptomyces strainR61
(6).
attraction of the carboxylate to the three backbone amide NH groupsof residues 2, 3, and 4. Recent data suggest that themethylated N-terminal amine becomes solvated by water as ahydrophobic cavity is formed around the substrate (5, 20). Thus, in theNMR-compatiblemodel shown inFig. 3B, the D-leucyl amine is directed away from the carboxylate group of the substrate.
The vancosamine sugarhelps direct the specificity of the antibiotic foraD-configured methylgrouponthe C-terminal alanine (20). We propose that a role of the entire di- to hexasaccharide unit found in theseglycopeptide antibiotics mightbetohelporientthepeptidebackboneofthe antibiotic alongsidethesubstrate backbone(Fig.3C). Becausethe long axis of theoligosaccharide isgenerallyperpendicularto the peptide backbone (as seen in the X-ray structure ofCDP-I [17]), and because the N-acetylmuramic acid-N-acetylglu-cosamine oligosaccharide axis in peptidoglycan is likely to be perpendicular to the pentapeptide (as proposed from conformational modelingstudies [12]), if thetwo glycopep-tides were broughttogether, itwouldplacehydroxylgroups of the saccharides within hydrogen-bonding distance. This mutual alignment of the two saccharide units and the two
A
a 5 0 iD-ALA 0 6 03L-LYS3~~~~~~~~~~~~~~~~~~~~
IA IINFIG. 3. (A) A model for the vancomycin-substrate interaction
derived from theinteraction of the substrate with theDD-peptidase
(6), as diagrammedin Fig. 2A. Forclarity, onlytheheptapeptide
backbone and disaccharide ofvancomycin are drawn. Hydrogen
atoms are alsoomitted. (B)A model of thevancomycin-substrate
complexbasedonpreviousNMR-derivedproposals (19, 20),with solvation of the methylated N-terminal D-leucyl amine. (C) A
possiblemutualalignment of saccharide units andpeptideunits in
vancomycin-peptidoglycan binding.Dashedlines andstripsindicate
directions of hydrogen binding. Glucosylvancosamine (G-V) is joined viatheglucosyl (G)to thephenolicsidechain ofposition4'in
vancomycin (left). N-Acetylglucosamine (NAG) and
N-acetylmu-ramic acid (NAM) are joined via N-acetylmuramic acid to the
pentapeptidesubstrate(right).
peptideunits could initiatecomplexation, stabilize
complex-ation,orboth. The attractivenessof such analignment(Fig. 3C) further supports the peptide-to-peptide binding mode shown in Fig. 3B over that in Fig. 3A, in which the disaccharide of the antibiotic would be unabletohydrogen bond the N-acetylmuramic acid-N-acetylglucosamine oli-gosaccharide.
DISCUSSION
Comparison of the interactions. Figure4A shows that the antibiotic andenzymeeachcontain anoligopeptideof five to seven residues in length which aligns antiparallel to and along opposite sides ofa commonsubstrate. Each molecule uses atleasttwohydrogen bondstobindtothebackboneof the substrate, and the enzyme uses a thirdhydrogen bond from its a2 helix. Somewhat different modes are usedforthe interaction of each molecule with the C-terminal carboxylate group of the substrate. Theenzymesurrounds the anion with threechargedorhydrogen-bondinggroups(Lys-65,His-298,
Thr-299). In addition, a weak field of positive charge is generated in the carboxylate-binding area by the dipole momentof thelong helixbeginningatSer-62.BetweentheN terminusof this helix andthe
p-strand
b3 isanoxyanionhole which immobilizes the carbonyl group of the cleavablepeptidebond of the D-ala-D-ala substrate.
The smaller vancomycin has fewer options available for interacting with the carboxylate of the substrate. The
sec-ondary amine of the N-terminal D-leucine of vancomycin maybe the counterpart of the conserved amine (usually a
lysineor,inthiscase, His-298)of thecell wall-synthesizing enzymes(6), andonewouldexpect anelectrostatic interac-tion between theleucyl-ammoniumionand the carboxylate.
In theNMRmodel, however, the ammoniumion is turned away fromthecarboxylateandisexposedtosolvent in order tofold theN-methylandleucylgroups overthe carboxylate groupandtoinvolve the backbone amides of residues 2and 3 inhydrogenbond interactions with thecarboxylate (5, 19,
20). Incontrast, the DD-peptidaseuses nobackbone amide NH bonds for the carboxylate interaction. Because the
bindingsite of theenzymeisnotparticularly deeporsolvent inaccessible (Fig. 2B), His-298 may be solvated in the substrate-free enzyme, but any solvent is displaced by the
carboxylate of the substrate. To maximize the binding en-ergy with the substrate, the enzyme is presumablyable to make use of numerous other interactions which are not availabletothesmallervancomycin molecule.Nevertheless, the Km(and Ks)of theenzyme-substrate complexis several ordersofmagnitude largerthan thedissociationconstantof the vancomycin-substrate complex (3, 14). This difference
presumablyreflects therequirementsforrapid catalysis and turnoverin theformercase versusthe needfortightbinding in the lattercase.
Theglycopeptide antibiotics,unlikeDD-peptidases, donot
stronglybind D-ala-D-alaanalogssuchaspenicillins (13, 15),
probably because of the inabilityof the
P-lactam
nitrogen atomtobeahydrogenbonddonorandbecause of unfavor-able steric interactions between the thiazolidine ring of apenicillinand thephenolic ringsof glycopeptideatpositions
2, 4, and 6. It is interesting that the structural differences between peptide and penicillin, which in part preclude
bindingof the latterto theglycopeptide antibiotics,are not moreeffectivelyusedby DD-peptidasestoresist ,-lactams.
Presumably, the structural changes required by catalysis,
viz., the change in geometry at the amide nitrogen on formation of the tetrahedral intermediate and the need to
A
VANCOMYCIN
CH3NH+
2*..
N, ; \ X H(CH)q,
L-LYS-D-ALA-D-ALASUBSTRATE
DD-PEPTIDASE
HIS 298 LYSNN LEU HN -NH NH 3+>$
- THR 3./~~~ ~ ~ ~ ~~~~~~~299 -N--
HO\..20Q-
SER-2
CHOA
62 4OIH'N..
GLY 4/ SH3C-m~CH
. H C=O- ---- --H-N . C H-N 5. HCH-CH THR 1 3 301 N-H---- O=C -,O=Ci
I 6 ,,~~~~~~~HC
GLU\ VA 228 N,H7.!
Ioc
302,1f
GLN ,303 NH2FIG. 4. (A)Juxtaposition ofthemutuallyexclusivebindingmodes usedbyvancomycin (left)orDD-peptidase (right)forinteractionwith their commonsubstrate.Hydrogenbondsareindicatedbydashes;distancesarenot toscale.Arrowsshowdirection ofadjacent polypeptides. (B)Stereoviewshowingtherelativesizesofvancomycin (1.5kilodaltons),theDD-peptidase(38kilodaltons)ofStreptomycesstrainR61,and theirtripeptide substrate.Becauseof sterichindrance,thebindingofonetothesubstrate would exclude thebindingof the other. Molecules
bindtheacylgroup acceptor, do not allow such discrimina-tion.
DD-Peptidaseand thevancomycinresistanceprotein. Induc-ibleresistancetoglycopeptide antibiotics hasrecentlybeen discovered in various Enterococcus species (9, 11, 18, 21). Theresistance appearstobeassociated with atransferable
plasmidwhich carries the gene fora new39-to40-kilodalton membrane-associatedprotein. Thisprotein doesnotappear to
destroy
the antibiotics (21). Rather, it is reported to behomologous (38%)to a D-ala-D-alaligase (S.Dutka-Malen, A. Brisson-Noel, C. Molinas, and P. Courvalin, Program
Abstr. 29thIntersci. Conf. Antimicrob. AgentsChemother., abstr.no.272, 1984), anobservation whichsuggests several
possibilities for theresistancemechanism.
First, the resistance protein may be another ligase. The resistance could then arise froman increased supply of D-ala-D-ala fragments. Since the binding of acyl-D-ala-D-ala species to glycopeptide antibiotics is not controlled by diffusion (16) and may be slower than binding to
DD-peptidases,
an increased rate of synthesis of these decoyfragments
may allow their steady-stateconcentration tobesufficiently
high (although low with respect to that under normalantibiotic-free conditions)topreventcelllysis.Shlaes and co-workers (18) have broadly theorized that the resistance protein prevents accessof the antibiotics to their
peptidoglycan
targets. Based on the aforementioned NMR andcrystallographic
data, which indicate thatvanco-mycin
andDD-peptidase
envelopthe peptidefromoppositesides,
ouranalysis
shows that steric factors wouldprevent mutualbindingof the three molecules(Fig. 4B).Hereinmay lie an alternative molecular mechanism of resistance tovancomycin.
Theligase-like
resistanceprotein
maynonco-valently
bind the D-ala-D-ala termini ina mannersimilarto that usedby
theDD-peptidase
describedhere. The normalligase
does bind D-ala-D-ala, the product of its reaction,although
notstrongly (K,,-1mM) (10).Theligaseshowsno obvioushomology
with the strain R61DD-peptidase,
butanX-raydetermination of the
ligase tertiary
structure,which is in progress (8), may reveal a common architecture at the substrate- orproduct-binding
site. The resistance protein,unlike thenormal
ligase,
isboundtothecytoplasmic
mem-brane(9,
11,18,
21). It may therefore serve to collectpeptidoglycan
monomers inside the cell and facilitatetheir transmissionthroughthemembrane.On theoutersideof themembrane,
the resistanceprotein might
then release the monomerunits(very tight bindingwould becounterproduc-tive)
close enough to the DD-peptidase to allow sufficienttransglycosylase-transpeptidase
activitytomaintain thecell wall. It ispossiblethatmuch of theundecaprenyl phosphate,the normal
cater,
may be tied up in monomer-antibioticcomplexes. Unlike the undecaprenyl phosphate-monomer
complex,
the resistance protein-monomer complex would not be sequestered by antibiotic because the resistanceprotein
and the antibiotic, like the DD-peptidase and theantibiotic,
may not be able to bind to a peptidoglycanmonomerunit simultaneously.
Thattheresistanceproteinand theDD-peptidasemaybind the cell wall peptide in similar fashion is supported by a recentreport(1)thatappearedafterthisarticlewas submit-ted. Fromtheir datashowingthetime-dependent
disappear-anceof
glycopeptide
bindingtothepentapeptideafter treat-mentwith membranes from resistantcells, Al-Obeidetal.(1) haveproposedthat the resistanceprotein functionsas aDD-peptidase.
We are indebted to Robert Sheridan for generously providing coordinates of a vancomycin model; and we thank N. E. Allen, T. I. Nicas, D. J.Tipper, and R. Williamson forhelpfuldiscussions.
LITERATURECITED
1. Al-Obeid, S., E. Coilatz, and L. Gutmann. 1990. Mechanism of resistance of vancomycin in Enterococcus faecium D366 and Enterococcusfaecalis A256. Antimicrob. Agents Chemother. 34:252-256.
2. Fesik, S. W., T.J. O'Donnell, R. T. Gampe, and E. T. Olejuic-zak. 1988. Determining thestructureofa glycopeptide-ac2-Lys-D-ala-D-ala complex using NMR parameters and molecular modeling. J. Am. Chem. Soc. 108:3165-3170.
3. Frere,J. M., and B. Joris. 1985. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. Crit. Rev. Microbiol. 11:299-396.
4. Jones, T. A. 1985. Interactive computer graphics: FRODO. Methods Enzymol. 115:157-171.
5. Kannan, R., C. M.Harris, T. M.Harris,J. P. Waltho, N. J. Skelton, and D. H. Williams. 1988. Function of the amino sugar and N-terminal amino acid of the antibiotic vancomycin in its complexation with cell wall peptides. J. Am. Chem. Soc. 110:2946-2953.
6. Kelly, J. A., J. R. Knox, H. Zhao, J. M. Frere, and J. M.
Ghuysen. 1989. Crystallographic mapping of ,B-lactams boundto aDD-peptidase target enzyme. J. Mol. Biol. 209:281-295. 7. Knox, J. R., J. A. Kelly, P. C. Moews, H. Zhao, J. Moring,
J.K. M.Rao,J.C.Boyington,0. Dideberg, P. C. Charlier, and M.Lipert. 1987. Crystallography of penicillin-binding enzymes, p. 64-82. In Y. litaka and A. Itai (ed.), Three dimensional
structureanddrug action. University of Tokyo Press, Tokyo. 8. Knox,J. R.,H.Liu, C. T.Walsh, andL.E. Zawadzke. 1989.
D-Alanine: D-alanine ligase (ADP) from Salmonella
typhimu-rium.Over-production, purification, crystallization and prelim-inaryx-rayanalysis. J. Mol. Biol. 205:461-463.
9. Leclercq, R., E. Derlot, J. Duval, and P. Courvalin. 1988. Plasmid-mediated resistancetovancomycin and teicoplanin in Enterococcusfaecium. N. Engl. J. Med. 319:157-161. 10. Neuhaus,F. C., C. V.Carpenter,J.M.Miller, N. M. Lee, M.
Gragg, and R. A. Stickgold. 1969. Enzymatic synthesis of
D-alanyl-D-alanine. Control of D-alanine:D-alanine ligase (ADP). Biochemistry8:5119-5123.
11. Nicas,T.I., C.Y. E.Wu,J.N.Hobbs,D. A.Preston,andN.E. Allen. 1989. Characterization ofvancomycin resistance in
En-terococcusfaecium and Enterococcus faecalis. Antimicrob. Agents Chemother.33:1121-1124.
12. Oldmixon, E. H., S. Glauser, and M. L. Higgins. 1974. Two proposed general configurations for bacterial cell wall pepti-doglycans shown by space-filling molecular models.
Biopoly-mers13:2037-2060.
13. Perkins, H. R. 1969. Specificity of combination between mu-copeptideprecursorsandvancomycinorristocetin. Biochem. J. 111:195-205.
14. Perkins, H. R. 1985. Vancomycin and related antibiotics, p. 115-132.InD. J.Tipper (ed.),Antibiotic inhibitors of bacterial cell wallbiosynthesis.International encyclopedia of pharmacol-ogyandtherapeutics.Pergamon Press, Oxford.
15. Popieniek,P.H.,and R. F. Pratt.1987.Afluorescentligand for bindingstudies withglycopeptide antibioticsofthe vancomycin class. Anal. Biochem. 165:108-113.
16. Popieniek,P.H.,and R. F. Pratt.1988.Ratesofspecific peptide bindingtothe glycopeptideantibiotics vancomycin, ristocetin andavoparcin. J. Am. Chem. Soc. 110:1285-1286.
17. Sheldrick,G.M.,P. G.Jones,0. Kennard,D. H.Williams,and
G. A. Smith. 1978. Structure ofvancomycinand its complex withacetyl-D-alanyl-D-alanine.Nature(London) 271:223-225. 18. Shlaes,D.M.,A.Bouvet,J.H.Shiaes, C. Devine, S. Al-Obeid,
and R.Williamson. 1989. Inducible, transferable resistance to
vancomycin in Enterococcusfaecalis A256. Antimicrob. Agents Chemother.33:198-203.
19. Waltho,J. P., J. Cavanagh, and D. H. Williams. 1988. Aspects of molecular recognition: use ofa truncated driven pseudo-NOESY experiment to elucidate theenvironment of
intermo-lecular electrostatic interactions in vancomycin.J. Chem. Soc.
Chem. Commun. 1988:707-709.
20. Williams, D. H.,and J. P.Waltho. 1988. Molecularbasis of the
activity of antibiotics of the vancomycin group. Biochem.
Pharmacol.37:133-141.
21. Williamson, R., S. Al-Obeid, J. H. Shiaes, F. W. Goldstein, and D. M. Shlaes. 1989. Inducible resistance to vancomycin in
Enterococcusfaecium D366. J. Infect. Dis. 159:1095-1104. 22. Zeiger, A. R. 1988. Vancomycinprevents ,-lactamantibiotics
fromcausing soluble peptidoglycan secretion by
Staphylococ-cusaureuscells,p. 563-567. In P.Actor,L.Daneo-Moore, M.
Higgins,M. R. J.Salton, and G.D.Shockman (ed.), Antibiotic inhibition of bacterial cell surface assembly and function. Amer-ican Society for Microbiology, Washington, D.C.