THÈSE
Pour l'obtention du grade de
DOCTEUR DE L'UNIVERSITÉ DE POITIERS UFR des sciences fondamentales et appliquées
Institut de chimie des milieux et matériaux de Poitiers - IC2MP (Diplôme National - Arrêté du 25 mai 2016)
École doctorale : Sciences pour l'environnement - Gay Lussac (La Rochelle) Secteur de recherche : Chimie théorique, physique, analytique
Présentée par : Nihat Ege Sahin
Réduction électrochimique du dioxyde de carbone sur des électrocatalyseurs à base de cuivre
Directeur(s) de Thèse : K. Boniface Kokoh, Teko W. Napporn Soutenue le 08 décembre 2016 devant le jury Jury :
Président Meriem Anouti Professeur, PCMB, Université de Tours Rapporteur Marc Robert Professeur, Université Denis Diderot, Paris Rapporteur Shigenori Mitsushima Professeur, Yokohama National University, Japon Membre K. Boniface Kokoh Professeur, IC2MP, Université de Poitiers
Membre Teko W. Napporn Chargé de recherche CNRS, Université de Poitiers Membre Clement Comminges Maître de conférences, Université de Poitiers Membre Nicolas Glandut Maître de conférences, Université de Limoges
Pour citer cette thèse :
Nihat Ege Sahin. Réduction électrochimique du dioxyde de carbone sur des électrocatalyseurs à base de cuivre [En ligne]. Thèse Chimie théorique, physique, analytique. Poitiers : Université de Poitiers, 2016. Disponible sur Internet <http://theses.univ-poitiers.fr>
1
THESE
Pour l’obtention du Grade de DOCTEUR DE L’UNIVERSITE DE POITIERS (Faculté des Sciences Fondamentales et Appliquées)
(Diplôme National - Arrêté du 25 mai 2016)
École Doctorale : Sciences pour l’Environnement Gay Lussac. Secteur de Recherche : Chimie Théorique, Physique, Analytique
Présentée par : Nihat Ege ŞAHİN
***********************************************************************
Réduction électrochimique du dioxyde de carbone sur des
électrocatalyseurs à base de cuivre
Electrocatalytic reduction of carbon dioxide on copper-based catalysts
***********************************************************************Directeur de Thèse : K. Boniface KOKOH
Co-directeurs de thèse : Têko W. NAPPORN & Clément COMMINGES ************************
Soutenue le 8 décembre 2016 devant la Commission d’Examen ************************
JURY
Marc ROBERT, Professeur Rapporteur
(Université Paris Diderot, Paris)
Shigenori MITSUSHIMA, Professeur Rapporteur
(Yokohama National University, Japon)
Mériem ANOUTI, Professeur Présidente
(Université de Tours)
Nicolas GLANDUT, Maître de Conférences Examinateur
(Université de Limoges)
Têko W. NAPPORN, Chargé de Recherches-HDR au CNRS Examinateur (Université de Poitiers)
Clément COMMINGES, Maître de Conférences Examinateur
(Université de Poitiers)
K. Boniface KOKOH, Professeur Examinateur
3
Foremost
This Thesis research was financially supported by Region-Poitou Charentes Council (France) and studied at Institut de Chimie des Milieux et Matériaux de Poitiers (IC2MP) at the Department of Chemistry, University of Poitiers in the period of 2013-2016. Ecole Doctorale Gay-Lussac and Equipe du Site Actif au Matériau Catalytique (SAMCat) were administrativly facilitated the PhD period in terms of all experimental platforms. Likewise, some congressional expenses were supported by Centre National de la Recherche Scientific, France (CNRS). It is esteemed for me to thank to all the people working at the administrative offices for their helpful decisions during my PhD period in historical and natural Poitiers.
It was a mysterious, exacting and staggering period to initiate the CO2 electrolysis
and unearth the first scientific data for my thesis. I have gained considerable experience in Synthesis and Characterization of nanostructured&mesoporous architectures as
electrocatalysts and evaluation of their electrocatalytic performance towards conversion of CO2 using lab-scale electrolyzer. In the meantime, I have attended many international
conferences, summer schools, collaborations and useful internships where I have fall deeply into the scientific world. I have enthusiastically implemented the suggestions and principles contained therein. In this way, I have had opportunity to meet some of eminent scientists.
Thank you everyone making this meaningful.
4
5
Dedicated to
M. Faraday’s life story
7
Acknowledgement
First and foremost, I wish to thank my dear family for their understanding and patience. They (Asya- Arif- Yayla- Naim- Aysu- Nedim- Nilgun- Emircan- Ağbal Sahin-) infinitely encourage and trust me on all my current life decisions and scientific life. I desire you to feel that this is yours PhD degree, too.
I would like to sincerely express my deepest appreciation to my thesis supervisor, Prof. Dr. Boniface Kokoh, for encouraging me in the freedom at scientific research throughout my PhD thesis in his research group at University of Poitiers. I am extremely grateful for his academic advice and guidance during my thesis research.
I am gratefully indebted to my thesis co-advisers, Dr. Clément Comminges and Dr. Teko W. Napporn for technical assistance, scientific discussions and amicable activities during my thesis research. I am also grateful for the all hospitable invitations during my stay in Poitiers. I also wish to express my thanks to Dr. Julien Parmentier, for giving me the opportunity to synthesize novel meso-structured materials in his research group (IS2M) at University of Haute-Alsace. It was a valuable experience both in scientific research and never-to-be forgotten the social activities in Alsace region. Among the so many different white wines,
Alsacien-white wine is the favorite one with its geographical history of the soil it grows. I also wish to express my thanks to the Jury’s President, Prof. Dr. Mériem Anouti. I have gained a new perspective as to thesis content thanks to her precious premonitions.
I also wish to express my thanks Prof. Dr. Marc Robert, Prof. Dr. Shigenori Mitsushima and
Dr. Nicolas Glandut for serving on my examining committee. Their explanatory instructions were more valuable contributed to the thesis content.
I would like to thank to physical characterization platform of IC2MP, IS2M and IPCMS for the technical supports, namely to Mr. S. Pronier (Electron microscopy), Mrs. N. Guignard (Raman), Mrs. S. Arrii-Clacens (XRD), Mrs. C. Roudaut (DT/TG), Prof. O. Ersen (Electron tomography), Mr. A. Le Valant (XRD modeling) and Prof. F. Lima (DEMS) for their guidance in the evolution of the physical characterization results. I gratefully thank to S. Hebie, Y. Ogihara-san,, K. Kumar, P. Corradini, H. Touati, Y. Holade, C. Lemoine, S. Lankiang, W. Pech-Rodriguez, D. Gonzalez-Quijano, S. Dessources, N. Mayet, I. Abidat, G. Ferreira, L. Estidullo-Wong, K. Servat, A.Habrioux, C. Morais, J-C. Guillon, N. Alonso-Vante, Claude and all colleagues for their friendly supports during the thesis period. It has been pleasure to work with all of you.
It is also important for me to thank all friends around me for their continuous support and perception during my educational life.
Finally, I thank to all reputable scientists preparing the whole infrastructure to contribute for development and accelerate of scientific researches.
8
LIST OF TITLES
Foremost ... 3
Thesis scope... 15
CHAPTER I ... 18
Lite rature Review ... 18
1.1. Properties of carbon dioxide... 19
1.2. Electrochemical reduction of carbon dioxide ... 19
1.3. Electrocatalytic reduction pathways of CO2... 23
1.4. Hydrogen evolution reaction ... 29
1.5. The role of the supporting electrolyte ... 30
1.6. The role of the carbon material as electrocatalyst support ... 31
1.7. The influence of metallic electrodes on CO2 electrocatalysis ... 32
1.8. Pd and CuxPd100-x electrocatalysts ... 36
1.9. Synthesis of the electrocatalysts ... 39
1.9.1. Microwave-assisted polyol method ... 40
1.9.2. Synthesis of mesoporous carbon materials ... 44
CHAPTER II ... 51
Synthesis Processes, Characterization Techniques, Experimental Methods ... 51
2.1. Synthesis Methods ... 51
2.1.1. Synthesis of ordered mesoporus silica SBA-15... 51
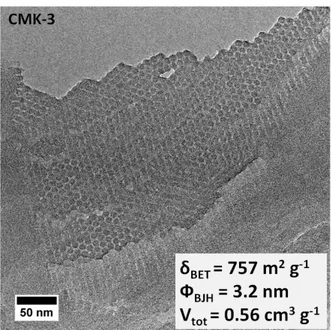
2.1.2. Hard-templating synthesis of oredered mesoporous CMK-3 carbon ... 52
2.1.3. Microwave-assisted polyol synthesis of Cu/C and Cu/CMK-3 nanomaterials ... 52
2.1.4. One-pot soft templating synthesis of nanostructured Cu/FDU-15 electrocatalysts.... 53
2.1.5. Direct synthesis of tannin-based mesoporous carbons by evaporation-induced self-assembly (EISA) method ... 54
2.1.6. In-situ nucleation and growth of copper nanoparticles in the porous network by organic-inorganic interface ... 54
2.1.7. One-pot microwave-assisted polyol synthesis of binary Cux-Pd100-x/C electrocatalysts ... 58
2.2. Physicochemical characterization methods ... 59
2.2.1. Differential thermal and thermo-gravimetric (DT/TG) analysis ... 59
2.2.2. X-ray diffraction (XRD) analysis ... 59
2.2.3. Determination of the metallic specific surface area of copper catalysts ... 61
9
2.2.5. Cross-section transmission electron microscopy (CS-TEM) analysis... 65
2.2.6. Energy dispersive X-ray spectroscopy ... 65
2.2.7. Electron tomography (3D-TEM) analysis ... 65
2.2.8. N2 sorption analysis ... 65
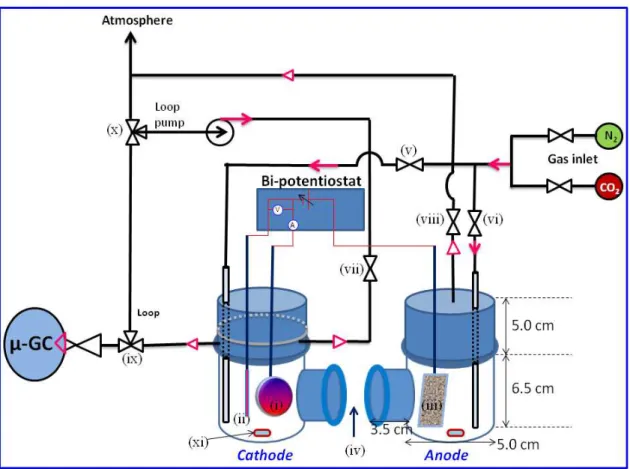
2.3. Electrochemical characterization methods ... 67
2.3.1. Electrochemical measurements ... 67
2.3.2. Faradaic efficiency of liquid and gaseous products... 68
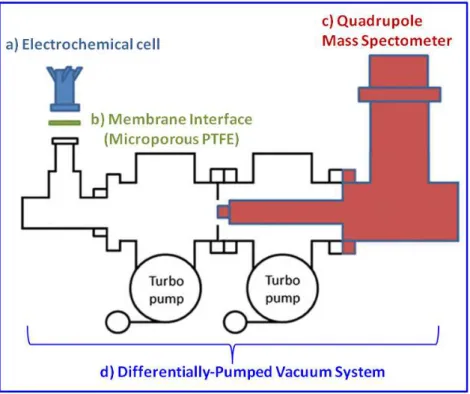
2.3.3. Differential electrochemical mass spectrometer (DEMS) ... 69
2.4. Analytical and spectroscopic characterization methods ... 70
2.4.1. High Performance Liquid Chromatography (HPLC) ... 70
2.4.2. Gas chromatography (GC)... 71
2.4.3. Raman spectroscopy ... 72
CHAPTER III ... 74
Dimensionally stable carbon supported nanostructured copper electrodes for selective CO2 reduction reaction ... 74
3.1. CO2 Electroreduction at Cu/Vulcan XC-72R Electrode ... 75
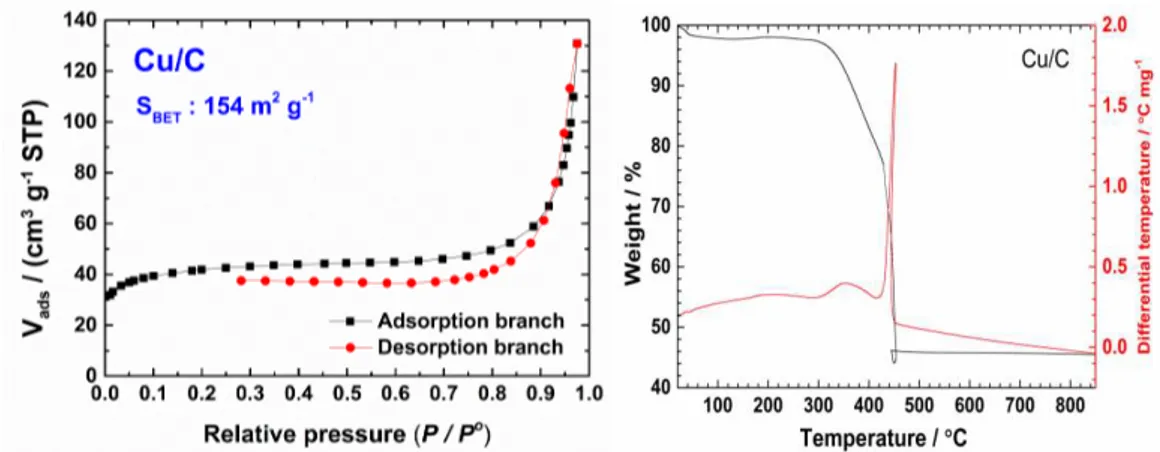
3.1.1. Physicochemical characterization of the Cu/C electrocatalyst ... 75
3.1.1.1. N2 sorption and diffrential thermal and thermo-gravimetric (DT/TG) analysis .. 75
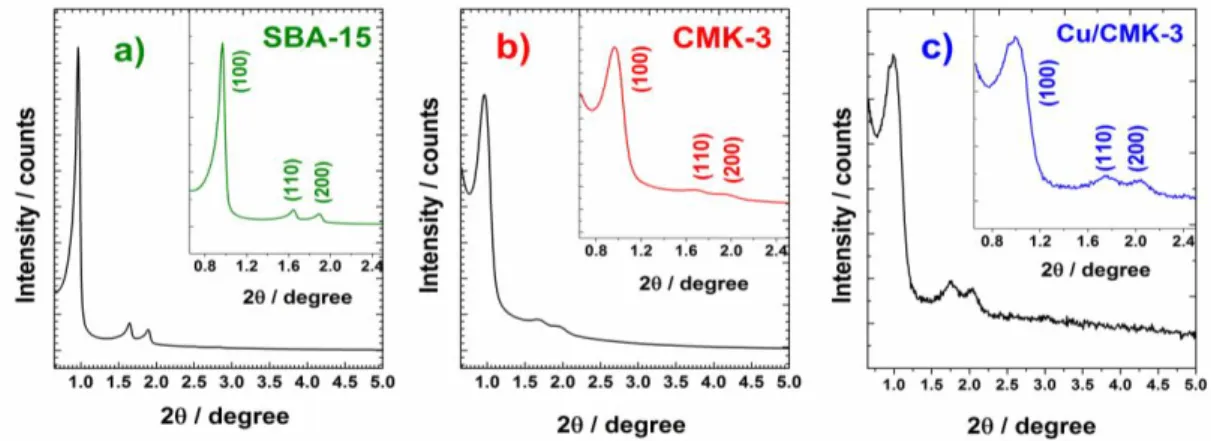
3.1.1.2. X-ray diffraction (XRD) analysis... 76
3.1.1.3. High resolution transmission electron microscopy (HRTEM) analysis ... 77
3.1.2. Electrochemical characterization of the Cu/C electrocatalyst ... 78
3.1.2.1. Cyclic Voltammetry ... 78
3.1.2.2. Differential Electrochemical Mass Spectrometer (DEMS)... 80
3.1.2.3. CO2 Electrolysis at Cu/C electrocatalyst... 82
3.1.3. Conclusion ... 85
3.2. CO2 Electroreduction at Cu/CMK-3 Electrode ... 87
3.2.1. Physicochemical characterizations of the Cu/CMK-3 electrocatalyst... 87
3.2.1.1. N2 adsorption / desorption analysis ... 87
3.2.1.2. X-ray diffraction (XRD) analysis... 89
3.2.1.3. Differential thermal and thermo-gravimetric (DT/TG) analysis... 90
3.2.1.4. Cross-section transmission electron microscopy (CS-TEM) analysis ... 91
3.2.2. Electrochemical characterization of the Cu/CMK-3 material ... 92
3.2.2.1. Cyclic Voltammetry ... 92
3.2.2.2. Differential Electrochemical Mass Spectrometry ... 93
10
3.2.3. Conclusion ... 97
3.3. CO2 electroreduction at Cu/FDU-15 electrodes ... 99
3.3.1. Physicochemical characterization of the Cu/FDU-15 material ... 99
3.3.1.1. N2 sorption analysis... 99
3.3.1.2. Differential thermal and thermo-gravimetric (DT/TG) analysis... 101
3.3.1.3. X-ray diffraction (XRD) analysis... 101
3.3.1.4. Cross-section transmission electron microscopy (CS-TEM) analysis ... 103
3.3.1.5. Three Dimensional Electron Tomography (3D-TEM) Observations ... 106
3.3.2. Electrochemical characterization of the Cu/FDU-15 electrocatalysts ... 109
3.3.2.1. Cyclic Voltammetry ... 109
3.3.2.2. CO2 Electrolysis at the Cu/FDU-15 cathode materials ... 110
3.3.3. Conclusion ... 114
3.4. CO2 electroreduction at Cu/Tannin electrode as “green electrocatalyst” ... 116
3.4.1. Physicochemical characterization of the Cu/IS2M-1-400 ... 116
3.4.1.1. N2 sorption analysis ... 116
3.4.1.2. Raman spectroscopy analysis... 117
3.4.1.3. Differential thermal and thermo-gravimetric (DT/TG) analysis... 119
3.4.1.4. X-ray diffraction (XRD) analysis... 121
3.4.1.5. High resolution transmission electron microscopy (HR-TEM) analysis ... 122
3.4.2. Electrochemical characterization of the Cu/IS2M-1-400 green electrocatalyst ... 124
3.4.2.1. Cyclic Voltammetry ... 124
3.4.2.2. CO2 Electrolysis at Cu/IS2M-1-400 green electrocatalyst... 126
3.4.3. Conclusion ... 128
CHAPTER IV ... 130
Correlation of nanostructured CuxPd100-x/C Catalysts in controlling of product selectivity toward formic acid... 130
4.1. CO2 electrocreduction at nanostructured binary CuxPd100-x/C electrodes ... 131
4.1.1. Physicochemical characterizations of the binary CuxPd100-x/C catalysts... 131
4.1.1.1. N2 sorption analysis... 131
4.1.1.2. X-ray diffraction (XRD) analysis... 131
4.1.1.3. Transmission electron microscopy coupled by energy dispersive X-ray spectroscopy (TEM-EDS) ... 134
4.2. Electrochemical characterization... 138
4.2.1. Cyclic Voltammetry... 138
4.2.2. CO stripping measurements... 141
11
4.3. Conclusion ... 144
CHAPTER V... 146
General Conclusion ... 146
13
14 The global warming being arise from the massive increase of the greenhouse gases because of the natural forces and anthropogenic events adversely affects the environment. The anthropogenic emissions of carbon dioxide (CO2) dramatically increase, because of the
continued use of the fossil resources as the major energy supply for energy production, industrial products and transportation. With the growing population and developing the industrialization, global demand for better energy converting technologies is rapidly increasing, on account this the natural resources difficulty in keeping up with demands [1, 2]. According to the recently reported studies, global CO2 emissions have grown to 35-40
gigatons of CO2 (GtC) per year [3, 4]. The amount of CO2 emissions as concerns human
activities and deforestation are estimated at 5.5 and 1.5 Gt carbon per year, respectively. In an effort to mitigate the dependence on fossil fuels, there are different strategies and technologies to be developed for reducing the energy consumption through controlling the current energy supply/demand. These include changeover from traditional power plants to renewable energy sources to store in the form of chemical energy for sustainable carbon recycling system. In 1958, Charles David Keeling began tracking CO2 level in the atmosphere at two of the
world’s last wildernesses: the South Pole and the summit of the Mauna Loa volcano in Hawaii. This steady rise in CO2has come to be called the “Keeling Curve”. The data recorded
in Hawaii with the jagged upward plot display increasing the CO2 levels in the atmosphere
that continued without interruption from 1958 to nowadays. In order to diminish greenhouses gases emissions; The Kyoto Protocol was adopted in Kyoto, Japan, on December 11, 1997 and entered into force on February 16, 2005. It is critical to note that the scientific investigations are not the only solution to minimize the global warming effects; it is also necessary to administer fair, clear and unselfish politics on sociology, geopolitics, and economics.
The management of the demands and the supplies of energy are also crucial since the highly accessible renewable energy sources include solar, hydro, wind, geothermal, and wave for energy production. Therefore, the advanced-renewable technologies can solve the carbon and climate problem in the first half of this century by using fundamental scientific, technical, and industrial facilities [5]. The global carbon emissions of CO2 from fossil fuel combustion
and the key technologies for reducing CO2 emissions until 2050 are shown in Fig. 1.0 [6].
Basically, the main key technologies and their ability for reducing CO2 emissions include CO2
Capture and Storage (CCS) with 19 %, renewable technologies with 17 %, nuclear technology with 6 %, power generation efficiency and fuel switching with 5 %, end-use fuel switching 15 % and end-use fuel and electricity efficiency with 38 %.
15
Figure 1.0. Key technologies for reducing CO2 emissions [6].
Nowadays, to reduce the CO2 emissions, the researchers focus strategically on
development of CO2 utilization processes which maintains conversion of CO2 to a variety of
useful products. Among the various processes, electrochemical reduction of CO2 has been
attracted significantly attention as the reaction occurs under ambient temperature, low pressure and in neutral pH. However, a large overpotential, difficulty in controlling the selectivity and competition with side reaction being hydrogen evolution reaction is the main challenges, giving rise to poor efficiency in electrochemical processes. Moreover, there are particular limitations being composed of high costs of CO2 capture, separation, purification
and transportation for CO2 utilization process. The marketing size limitations and investment
incentives are still the major challenges. For this reason there is necessary to extend the wide range of practical knowledge on developing a favorable electrode material for CO2
electroreduction in order to generate targeted energetic molecules and to minimize the energy required for electro-chemical transformations. In this regard, significant efforts are dedicated to the development of lab-scale prototype systems that can be operated under different conditions.
Thesis scope
This Thesis focuses deeply on the development of novel mesostructured carbon materials supported copper nanocatalysts for sustainable and efficient reduction of carbon dioxide. The extensive studies were given to the synthesis of tunable mesostructured carbon materials impregnated copper nanoparticles. Furthermore, valued efforts were performed for reaching the optimum operating conditions on lab-scale electrolyzer under mild conditions.
16 Given the high surface area and uniform mesopore size of the ordered mesoporous carbon supported Cu nanoparticles were found superior performance in the electrochemical reduction of carbon dioxide.
Chapter 1 presents fundamental information about electrochemical reduction of carbon
dioxide and its utilization challenges both in thermodynamic and kinetic aspects. The first part of this chapter discusses general principles and technical drawbacks of CO2 electrocatalysis.
In the following parts describe why carbon supported metallic catalysts are extremely important for converting CO2 into useful chemicals relevant to the scope of this thesis. In the
last part of this chapter, the general synthesis methods are discussed.
In the chapter 2, the synthesis processes, including microwave-heated polyol synthesis, hard-tempalting, soft templating, and evaporation-induced self-assembly approaches used to prepare of novel mesostructured carbon materials are highlighted as well as advanced-methods used to characterize these electrode materials. Furthermore, preparation of electrode and using of the electrolyzer with operating conditions of CO2 electrocatalysis are elucidated.
In the chapter 3, copper embedded inside the mesoporous structure electrode materials were prepared via different synthesis methods to examine the influence of carbon support morphology on the electrocatalytic performance of the CO2 reduction reaction. Here, using
novel characterization techniques (CS-TEM and 3D-Electron tomography) provide opportunities in order to improve the identification of the link between porosity and nanoparticle location. For this purpose, commercial Vulcan-XC-72R carbon black and highly organized mesoporous carbon materials, namely CMK-3, FDU-15 and IS2M-1 are used. The superior electro-catalytic activity was ascribed to the mesopore system that facilitates the diffusion of CO2 molecules towards active sites of copper nanostructure in the reaction.
The influence of atomic composition of the copper/palladium nanoparticles (CuxPd 100-x/C) on Faradaic selectivity was investigated in chapter 4. The copper/palladium bimetallic
nanoparticles with different atomic compositions are served as well-defined platform to examine their Faradaic selectivity of CO2 reduction reaction. Among the CuxPd100-x/C
bimetallic nanoparticles, Cu50Pd50/C nanoparticles show the highest formic acid Faradaic
efficiency in CO2-saturated 0.5 mol L-1 NaHCO3 electrolyte.
This Thesis contributes to understanding the effect of copper nanoparticles decorated by mesostructured carbon substrates on the CO2 electrolysis under mild conditions. Chapter 5
17 emphasizes the comparison of the as prepared electrode materials on the performance of the CO2 reduction reaction.
18
CHAPTER I
19
1.1. Properties of carbon dioxide
Compare to fossil fuels, using the molecular carbon dioxide (CO2) as an alternative
carbon source has received great attention due to its bi-functional chemical property. The CO2
belongs to the Dhpoint group and is a linear molecule (O=C=O) in which the two oxygen atoms are covalently double bounded to a carbon atom. The Molecular Orbital (MO) diagram of CO2 is based upon a C atom and an O-O ligand component. Carbon 1s and oxygen 2s are
assumed to be non-bonding whereas carbon 2s and 2pz orbitals are hybridized to form sp
orbitals. The σ-bonding interacts between oxygen 2pz and carbon sp orbital, while π-bonding
is formed between oxygen 2px or 2py orbitals with empty orbitals of the carbon. Fig. 1.3a
presents the role of the polar C-O bonds on charge separations, which marked with blue vectors. The CO2 posseses two different reaction sites: the positively charged carbon atom is
an eletrophile, whereas the negatively charged oxygen atoms are nucleophiles. Hereby, one may assume that the molecular CO2 is expected to act as an electron acceptor rather than an
electron donor due to its high ionization potential [7]. To address these fundamental issues, CO2 activation could be investigated by taking into account physical, chemical, and especially
electronic properties. According to the MO phenomena [8], the population of the lowest unoccupied molecular orbital (LUMO) as may occur in electronically excited CO2, the radical
anion CO or the adduct of CO2 2 with an electron rich species, leads to the distortion of the
molecule from linearity [9]. This bent geometry with the angles close to 133 ° minimizes the electron repulsion and the molecular energy. In this regard, carbon atom intends being the activation site in electrochemical attacks.
1.2. Electrochemical reduction of carbon dioxide
The electrochemical reduction process [10-15] is outperformed among a variety of CO2
conversion processes includes chemical [16-19], photochemical [20-22], biochemical [23-25], bioelectrochemical [26, 27], and photoelectrochemical [28-30]. When aiming to establish an electrocatalytic conversion of CO2 in useful chemicals in high yields for targeted applications,
the fundamental challenges in terms of thermodynamic stabilityand kinetic inertnessare to be handled [11]. Primarily, it is necessary to cope with the activation of the molecular carbon dioxide with an ideal mechanistic pathways, which explores how to break the C=O bond and then how to form C-H and/or C-C bonds towards higher-order products [31]. Secondarily, it is essential to discuss the interaction between a metallic catalyst and CO2 at electrode/electrolyte
20 interface for the capability of CO2 reduction in terms of reaction rate and catalyst selectivity.
So, developing of an effective electrocatalyst that catalyzes CO2 with sufficiently low
overpotentials at high current densities to obtain energetic efficiency is one of the key points. After all, the economic feasibility for CO2 conversion process, including expense of designing
electrolyzers and of developing electrocatalysts should be laid deeply out. In general, temperature and pressure are the most important parameters in development of lab-scale prototype electrolyzers. These types of electrolyzers allow controlling the operative conditions, such as low/high temperature and low/high pressure.
Figure 1.1. Publications per year on electrochemical reduction of carbon dioxide. The data was extracted from the ISI Web of Science in December 2016 (search: electrochemical reduction of CO2).
One of the significant advantages of the electrochemical reduction of CO2 is the
reaction can proceed by two-electron, four-electron, six-electron and eight-electron reduction pathways, leading to various products. The controllable technical issues such as electrode composition [32-34], catalyst morphology [35-39], nature of the electrolyte [40-46], and the other operating parameters that include applied potential, targeted current density, electrolysis time, pressure and temperature [13, 47, 48] allow yielding of high Faradaic selectivity. Additionally, the electrochemical converting systems can be easily adapted for scale-up application [49]. Coupling the electrochemical process to renewable energy systems could allow storing the excess of electricity in the form of liquid fuels or chemical products. Fundamental researches of the electrochemical reduction of carbon dioxide started as early as the 19th century and numerous reports were published in the last three decades. As seen in Fig.
1.1 the number of papers published related to the electroreduction of carbon dioxide slightly increased in 1980s and 1990s, and then it is continuously increasing in 2010s and thereafter.
21 The scientific literature related to electrochemical reduction of CO2 is underlined that
investigations are increasing towards environmentally sustainable energy networks.
Carbon dioxide is an amphoteric molecule possessing both acidic and basic properties. The thermodynamic equilibrium of CO2 and its electro-reactive species in aqueous solution
are illustrated in Eqs. 1.1 and 1.6.
H O HCO H
CO2 2 3 (1.1)
The pH value related to gaseous CO2 with HCO3- is expressed by Eq. 1.2.
3
2
1 logHCO logCO
pK
pH a (1.2)
where K is the equilibrium constant of the reaction (a1 pKa16.35) at 25 °C expressed in Eq. 1.1. Because
CO2 is related to the partial pressure of CO2 with Henry constant at 25 °C (1 1 2 10 38 . 3 molL atm h ).
log ( ) log log 3 2 1 h HCO PCO pK pH a (1.3) CO H HCO 2 3 3 (1.4)The pH value for bicarbonate and carbonate species is evaluated by using Eq. 1.5 with the equilibrium constant of Ka2 and ( pKa2 10.33) at 25 °C.
2 3 3 2 log CO /HCO pK pH a (1.5)Here, P(CO2) can be expressed in Eq. 1.6 by combining the Eq.1.3 and Eq.1.5.
2 3 3 3 2 12) log log log /
(
logPCO pKa pKa h HCO CO HCO (1.6)
Admittedly, the noticeable advantageous of the electrochemical processes is to select the electrode potential toward a desired value to obtain the optimal reaction rate and product selectivity. According to Nernst equation, the theoretical equilibrium pote ntials decrease when the pH value is increasing. Fig. 1.2 illustrates the thermodynamic pH-potential diagram (so-called Pourbaix diagram) for CO2/H2O systems that presents the equilibrium potentials
over the range of pH values at which the electrochemical reactions occur. The Pourbaix diagram evidently highlighted the CO2 reaction competes with hydrogen evolution reaction
22 one of the critical point is to evaluate the effect of the pH value on the CO2 reduction reaction.
The thermodynamic electrochemical half-reactions of carbon dioxide reduction reaction and their associated standard equilibrium potentials (Eo, the standard equilibrium potential is
related to free energy change associated with the reaction expressed in E.q. 1.7 where n is the number of electrons involved in the reaction and F is the Faraday’s constant (96.485 C mol-1
equivalent)) vs. SHE at the metallic electrode surface are summarized by Eqs.1.8-1.19 at 1.0 atm, 25 º C and pH 7 [50, 51]. nFE G (1.7) 2 2 e CO CO E0= -1.90 V (1.8) HCOOH e H CO 2 2 2 E 0= -0.61V (1.9) O H CO e H CO2 2 2 2 E0= -0.52 V (1.10) 2 2 2 2 ( ) 2CO H e COOH E0=-0.87V (1.11) H e HCOO CO2 2 E0= -0.43V (1.12) O H HCHO e H CO24 4 2 E0= -0.48V (1.13) O H OH CH e H CO26 6 3 2 E0= -0.39V (1.14) O H CH e H CO28 8 42 2 E0= -0.24 V (1.15) O H OH H C e H CO2 12 12 2 5 3 2 2 E0= -0.33V (1.16) O H H C e H CO2 14 14 2 6 4 2 2 E0= -0.27V (1.17) O H H C e H CO2 12 12 2 4 4 2 2 E0= -0.34V (1.18) O H OH H C e H CO2 18 18 3 7 5 2 3 E0= -0.32V (1.19)
Electrochemical reduction of carbon dioxide in aqueous solution contains H2O, H+, and
OH-, and hence the equilibrium potential varies with respect to pH of the electrolyte. Thermodynamically stable regions of CO2 and HCOOH related species in accordance with
pH and potential are shown in Fig.1.2. It is clearly shown that the equilibrium potential of carbon dioxide reduction is in the same potential scale as HER in aqueous media. Because
23 alkaline media suppress the CO2 kinetics compare to HER, reaction properly follows the H2
production higher efficiency. However, in acidic conditions tend to promote yielding CO2
-derived products. HCOO- is predominantly produced from CO2 in neutral pH region instead
of HCOOH (pKa = 3.75).
Figure 1.2. Equilibrium potential versus pH relation (Pourbaix diagram) for the carbon dioxide reduction reaction at 25 °C adapted from [50, 52].
1.3. Electrocatalytic reduction pathways of CO2
A reaction rate can be improved by estimating the role of adsorption of reactants or intermediates in a given electrolyte and surface-modified electrodes, taking place through a heterogeneous reaction process. The key parameter in catalysis and electrocatalysis can be explained through adsorption phenomenon that occurs at the electrode/electrolyte interface, allowing definition of chemisorption and physisorption in terms of heat of adsorption. In the case of the chemisorption, the molecules are strongly adsorbed, resulting in a reaction product due to chemical, ionic chemical and covalent bond formation via sharing the electron density. The chemical potentials of the adsorbed species can be determined and controlled by using electrochemical techniques. From an electrochemical point of view, the electroactive species are first transferred and then accumulated by a weak interaction between adsorbed electroactive species and the electrode surface due to Van der Waals forces (polar and neutral molecules) and London forces (molecules with a permanent dipole moment). No chemical
24 bond is formed between electroactive species and the electrode surface by weakly adsorbed interaction, which suggests the product selectivity is strongly affected.
There are two main pathways for further reduction of adsorbed radical anion
2CO to
HCOO- and CO. In principle, CO2 may act as an electron acceptor via the lowest unoccupied
molecular orbital (LUMO) centered at the C atom, and an electron donor via the highest occupied molecular orbital (HOMO) centered at the O atoms. As the metallic catalysts being electron-rich nucleophiles in a low oxidation state strongly intend to interact with CO2 by
binding C atom. However, the electron-poor centers such as metal centers in high oxidation state and electron deficient molecules preferably attack one of the O atoms. CO2 molecule can
be chemisorbed as a bent CO2δ- molecule with different possible geometric structures, as
illustrated in Fig. 1.3. The targeted products are strongly affected by the so-called chemisorbed geometry with an electron-rich metallic surface. The latter surface can interact with carbon or oxygen atom or both in CO2 to form carbon coordination or oxygen
coordination, and mixed carbon-oxygen coordination. The oxygen atom in a bent CO2
molecule can be preferably attacked by protons, whereas the carbon atom in a bent CO2
molecule can be easily interacted with the adsorbed H atoms which require higher activation energy.
Figure 1.3. a) Polarity of the carbon dioxide. Possible structures for adsorbed CO2δ- on metals
b) carbon coordination, c) oxygen coordination, d) mixed coordination [53, 54].
The mechanistic insights of CO2 reduction to various products provide crucial
guidelines for preparing the effective and selective electrocatalysts. In a typical electroreduction of CO2 mechanism, CO2 may obtain electrons either directly from a
bare-cathode surface or a substrate supported nanocatalyst (heterogeneous catalysis), or indirectly from a soluble catalyst in the medium (homogeneous catalysis) to produce an intermediate
25 (CO2). To date, numerous studies have been focused on identifying the key reaction
intermediates and elucidating the possible reaction pathways of electrochemical reduction of carbon dioxide in both the theoretical and the experimental points of view [15, 55-57]. For this purpose, the understanding of the reaction mechanisms of CO2 reduction following the
C-O bond cleavage and C-C and C-H bonds formation through advanced in-situ, operando and
on-site characterization tools under electrochemical operations is of great importance. The possible reduction pathways of CO2 electroreduction were proposed in many reports [34, 36,
58, 59]. Khezri et al. [60] summarized the CO2 reduction pathways on metallic electrode surface in three categories presented in Fig. 1.4.
Figure 1.4. Proposed possible reaction pathways for CO2 electroreduction reaction to possible
26 The actual electrolysis potential of CO2 reduction occurs at much more cathodic
potential than the equilibrium one because of its stable structure. Pacansky and co-workers [61] discussed the stability of the radical anion
CO intermediate as a function of the bond 2
angle and bond length by using SCF ab initio molecular orbital and atomic population analysis of the radical anion
2
CO at the minimum energy geometry. They found that the radical anion
CO2
possesses the unpaired electron density at the highest occupied molecular orbital which is localized at C atom at 84 %. They suggested that the radical anion
2CO is ready to react as a nucleophilic reactant toward carbon atom. The reason is that the
intermediate species
CO2
which is formed by one-electron transfer to a CO2 molecule inEq. 1.20, proceeds as the first step at highly cathodic potential as confirmed by previously published studies [52, 62-64]. There is a characteristic change in geometry from linear CO2 to
a bent
CO configuration, causing a significant overpotential in the reduction of CO2
2 [65,66]. The standard potential of radical anion
CO2
formation was reported by several studies [67-69]. The intermediate
CO2
subsequently accepts an H+ and another electron, yielding HCOO- with the following Eqs. 1.21 and 1.22. 2( ) 2 e CO ads CO (1.20) H OHCOO OH CO2(ads) 2 (ads) (1.21) e HCOO HCOO(ads) (1.22)
Furthermore, formate can be formed directly by reaction with adsorbed hydrogen, which is supporting as an intermediate from hydrogen evolution reaction (in Eq. 1.23). Such pathway can appear in the case of radical anion
CO2
being adsorbed with oxygen coordination (Fig.1.3c) or being just close to the electrode.
H HCOO
CO2(ads) ads (1.23)
Here, the intermediate radical anion
CO2
intends to accept a H+ from H2O to the C atombecause of that the
CO2/COOH
acid-base couple has lower pKa (1.40) value [70]. Theadsorbed
) ( 2 adsCO also gains more negative charge on O atoms due to d electron contribution to metallic bond, and thus can be nucleophilic at O atoms, which facilitates protonation
27 yielding formate. Hori and Suzuki [52] noted that the rate determining step is the first electron transfer to form radical anion
2
CO with an assumption of the transfer coefficient of 0.25 and 0.67 for higher overpotential and lower overpotential regions, respectively.
Another reduction pathway occur when the hydrogen is reacted by O atom in radical anion
2CO , involving a first protonation (Eq. 1.24) and then reduction (Eq. 1.25). Such pathway appears more favorably on carbon coordination adsorption as depicted in Fig. 1.3b with the metal atom in complex homogeneous catalysts, yielding CO. Carbon coordination can facilitate protonation of oxygen via an additional charge transfer due to back donation from the metallic (Ni, Au, Zn, and Ag) d bond to CO2.
H O COOH OH CO2(ads) 2 (ads) (1.24) COOH e CO OH ads ads) ( ) ( (1.25)
Carbon monoxide can be formed directly by reaction with adsorbed hydrogen (in Eq. 1.26), which is supporting as an intermediate from hydrogen evolution reaction. Such pathway can appear in the case of radical anion
CO being adsorbed with oxygen coordination or being 2
just close to the electrode. Hori et al. [71] reported that the local pH near the Cu cathode could be substantially higher than that in the bulk electrolyte (0.1 M KHCO3), owing to the
production of OH− from CO2 reduction and hydrogen evolution.
H CO OH
CO2(ads) (ads) (ads) (1.26)
Studt et al. [56] investigated the electrochemical reduction of carbon dioxide to formic acid on twenty-seven different metal surfaces via density functional theory (DFT) calculations with thermodynamic arguments. Theoretically limiting potentials for electroreduction of CO2 to
HCOOH via either the HCOO or COOH intermediate are displayed in Fig. 1.5. The metal surfaces in the blue region orientate the formation of HCOOH via the HCOO intermediate, whereas those in the red region favor the production of HCOOH via the COOH
intermediate. The dashed lines represent the equilibrium potential for HCOOH formation from CO2. One can see that metal surfaces being close to the dashed lines require relatively
low overpotentials, thus are predicted to be ideal catalyst materials for electroreduction of CO2 to HCOOH.
28 Studt and co-workers [56] also highlighted that electrochemical reduction of CO2 to HCOOH
seems plausible when the reaction pathway occurs through the HCOO intermediate. Fig. 1.6.
illustrates the findings as regards with the free energies of HCOO and H . The blue and red
dashed lines show the equilibrium potentials for HCOOH and H2 formations, respectively.
One can see that the scaling (linear) relation is extremely weak, suggesting that the binding characteristics of HCOOand H are different, thus relatively independently tunable,
compared to those of COOH and H .
Figure 1.5. Theoretically limiting potentials for electro-reduction of CO2 to HCOOH via
either the HCOO* or COOH* intermediate. U represents the applied potential in RHE scale [56].
29
Figure 1.6. The scaling relation between the free energies of HCOO* and H* on various metal surfaces. ∆G represents the free energy in eV [56].
1.4. Hydrogen evolution reaction
Hydrogen evolution reaction (HER) is a two-electron process with at least two elemental steps, taking place in aqueous environment by cathodic polarization, and competing with CO2 reduction. The standard electrode potential of HER is -0.414 V vs. SHE at pH 7.0
and 25 °C. The electrochemical reduction of carbon dioxide to formate in aqueous solution is -0.43 V vs SHE, which is so close to the standard electrode potential of HER. The early mechanistic study of the HER on copper was reported by Bockris et al. [72] in both acidic and alkaline aqueous media. It is generally agreed that the overall HER proceeds through the electrochemical reduction of water molecule with the adsorption of hydrogen atom intermediate being the rate-determining step. The electrochemical desorption of hydrogen and chemical desorption proceeds via Volmer reaction (in Eq. 1.27 in acidic solution and in Eq. 1.28 in alkaline solution), Heyrovsky reaction (in Eq. 1.29 in acidic solution and Eq. 1.30 in alkaline solution) and Tafel reaction (in Eq. 1.31) mechanisms, respectively [73-76]. In the first step of the hydrogen evolution reaction, adsorbed active hydrogen is formed (in Eq. 1.27
30 in acidic solution and Eq. 1.28 in alkaline solution), thus physically adsorbed H+ can react with
2
CO radical chemisorbed on the electrode surface according to Eley-Rideal mechanism [77, 78]. The key factor is to control the formation of physically adsorbed H+ for advancing the electrochemical reduction reaction by adjustment of the nature of the electrolyte and its concentration [43, 79, 80] and operating conditions [81] resulting in accumulating the H+ ions at the electrode surface.
O H MH e O H M aq ads 2 * ) ( ) ( 3 (1.27) H O e MH OH M *ads ) ( 2 (1.28) O H H M e O H MH*ads 3 (aq) 2(g) 2 ) ( (1.29) H O e M H OH MH*ads 2 2(g) ) ( (1.30) ) ( 2 * ) ( * ) (ads MHads 2M H g MH (1. 31)
Here, M and H* represent a metallic electrode surface and a surface-bound hydrogen, respectively. Alternatively, adsorbed *
ads
H on the electrode surface can react with adsorbed CO2- radical at the adjacent electrode surface site via Langmuir-Hinshelwood mechanism at
which all the adsorption and desorption pressures are in equilibrium [82]. Then the reacted molecular structure may be desorbed from the catalytic surface due to the exothermic reaction. The stronger CO2 adsorption would intend the reaction step toward CO formation,
leading to formation of hydrocarbon products by means of hydrogenation of adsorbed CO on copper based catalysts [83-85].
1.5. The role of the supporting electrolyte
Substantial efforts have been devoted investigating the influence of supporting electrolyte composition and its concentration on the electrochemical reduction of CO2. Murata
and Hori [41] firstly examined the role of the cation radius in accordance with the degree of electrostatic adsorption that strongly affects the outer Helmholtz plane (OHP) potential at copper electrode in 0.1 mol L-1 aqueous solutions of LiHCO3, NaHCO3, KHCO3, CsHCO3.
They highlighted that HER is favorable with the sequence of Li+ > Na+ > Cs+ > K+ whereas
31 (CH4) and ethylene production are observed in NaHCO3 and CsHCO3 electrolytes,
respectively. More recently, Verma et al. [86] examined the role of the charge transfer resistance magnitude on stabilization of the rate limiting CO radical anion in the 2
electrolytes of KOH, KCl and KHCO3 through electrochemical impedance spectroscopy. The
observations showed that both charge resistance transfer and cell resistance decrease with increasing of KOH electrolyte concentration differing from 0.5 to 3.0 mol L-1. As improving
of the ionic conductivity leads to decreasing charge transfer resistance, intending an improved stabilization of the rate limiting CO radical by a higher concentration of cationic (K2 +) ions in the outher Helmholtz plane. Thorson et al. [42] reported the influence of a variety of alkali cations on the CO2 electroreduction to CO in an electrochemical flow reactor. They found that
the presence of large cations such as Cs+ and Rb+ suppress the H2 evolution while promote the
CO2 reduction by improving the partial current density. Additionally, Schizodimou and
Kyriacou [87] investigated influence of supporting electrolyte of 1.5 mol L-1 that contains
various cations on reaction rate. They noted that the reaction rate is dependent on the charge number of the supporting electrolyte in the order of Na+ < Mg2+ < Ca2+ < Ba2+ < Al3+ < Zr4+ <
Nd3+ < La3+.
1.6. The role of the carbon material as electrocatalyst support
The influence of the carbon materials in catalytic process [88], energy storage [89] and adsorption applications [90] has been investigated for many years. In general, Vulcan XC-72 carbon black, Kentjenblack EC-600JD, carbon nanotubes, graphene and ordered mesoporous carbon substrates are used as the supporting materials for a better attachment of metal nanoparticles in electrochemical conversion systems due to their excellent physical and chemical properties. Particularly, mesoporous carbon materials are ideal candidate for catalytic process since they possess unique characteristics, including tunable porosity, large surface area, better chemical and thermal stability, perfect electronic conductivity and better adsorption capacity [91]. The role of the carbon materials as catalyst supports is not only for providing better attachment of metal nanoparticles also the carbon structure can promote the catalytic activity in targeted process.
Carbon supporting materials can be inert or active towards catalytic reactions. Direct electrochemical reduction of carbon dioxide was investigated on different carbon materials
32 (such as glassy carbon [92, 93], graphite [94], and carbon nanotubes [95] electrodes without any catalyst. Hara et al. [93] observed carbon monoxide with a Faradaic efficiency of 44 % and formic acid (30 %) production under higher gas pressure (30 atm of CO2) in 0.1 mol L-1
KHCO3 aqueous electrolyte. Eggins et al. [96] observed the production of oxalic acid and
glycolic acid from carbon dioxide reduction at the graphite electrode surface at different potentials in aqueous solutions containing tetramethylammonium ions. Zhang et al. [95] noted that nitrogen-doped carbon nanotubes can catalyze the carbon dioxide conversion, resulting in the formate formation in the presence of polyethylenimine (PEI) in 0.1 M KHCO3 solution.
The overpotential value decreased and the current density was increased by using the PEI as a co-catalyst in CO2-saturated aqueous solution.
1.7. The influence of metallic electrodes on CO2 electrocatalysis
To work out aforementioned facilities and challenges, homogenous catalysis (metal-free surfaces, metal-organic frameworks or transition metal complexes) [97-102] and heterogeneous catalysis (metal or metal oxide surfaces) [4, 55, 79, 103-106] have been considered to be promising an efficient CO2 reduction reaction to generate useful chemicals
and fuels.
The electrode materials include various metals, metal oxides and alloys, which are the important key factor in determination of product selectivity. Hori et al. [107] classified the metal catalyst according to the products yielded in 0.1 mol L-1 KHCO
3 aqueous electrolyte.
According to their classification, the metallic electrodes were investigated by four groups in aqueous supporting electrolyte. The first metallic group includes Au, Ag, Zn, Pd and Ga being selective for production of carbon monoxide (CO) with a maximum Faradaic efficiency of 87.1, 81.5, 79.4, 28.3, and 23.2 %, respectively at the current density of 5.0 mA cm-2. Then formate was selectively produced at the Pb, Hg, In, Sn, Cd and Tl metallic surfaces. The Faradaic efficiency toward formate production is reached to 99.5 % at the potential of -1.51V vs. NHE with a current density of 5.0 mA cm-2. Other electrode materials such as Ni, Fe, Pt
and Ti are reported to be selective for hydrogen production with a Faradaic efficiency of 88.9, 94.8, 95.7, and 99.7 %, respectively. Differently, they reported that hydrocarbons and alcohols were generated on the copper electrode. Particularly, methane (CH4) and ethylene
(C2H4) were produced with a Faradaic efficiency of 33.3 and 22.5 %, respectively whereas
33 3.0 %, respectively at the potential of -1.44 V vs. NHE. One of the breakthroughs in using copper based materials is its fast deactivation after beginning of the electrolysis, causing the lower performance. Jermann and Augustynski [108] examined the electrochemical reduction of carbon dioxide at copper electrodes as a function of the electrolysis duration in hydrogen carbonate as electrolyte. They developed in-situ activation treatment through periodic anodic stripping of poisoning species from the electrode surface, allowing a long term electrolysis process with high faradaic efficiencies. It was noted that no deactivation was observed during a 48 h long term electrolysis measurements in CO2 saturated 0.1 mol L-1 NaHCO3 solution at
a potential of -1.72 V vs. NHE.
Furthermore, Chaplin and Wragg [34] and Ito et al. [109] classified the electrochemical CO2 reduction reaction according to both the nature of the cathode (sp or d group metals) and
the solvent used as supporting electrolyte (aqueous or non-aqueous solutions), which orientate the final reaction products. The sp metal group contains the principle group metals of the periodic table and d metal group includes the transition metals which have completely filled d orbitals. Ito et al. [109] reported the product distribution as a function of electrode materials (In, Pb, Zn, Sn) and the cathode potentials (-2.0 V and -2.4 V vs. SCE) for formic acid as a main product.From the literature, it has been emerged that the reduction products strongly depend on the electrode materials. The observed Faradaic efficiency data were demonstrated that the electrode surface with Cu [50, 71, 110, 111], Sn [43, 79, 81, 112-114], Pb [115-117], Hg [116], and In [116] have excellent ability towards the CO2 conversion into formate
(HCOO-) or formic acid (HCOOH) in aqueous solution.
The CO2 transformation into formic acid is more demanded because of the broad market
and wide application range. Formic acid is an excellent candidate fuel for low temperature fuel cells [118, 119], as well as one of the most promising compounds for hydrogen storage [120, 121]. Agarwal et al. [13] expressed the engineering and economic feasibility of the electrochemical reduction of carbon dioxide to formate and formic acid. They noted that the increase of the catalyst lifetime and the development of the reactor designs are the important issues to be handled for improving the current efficiency. The first studies on electrochemical CO2 reduction on different types of metal electrodes, resulting in formic acid as main product
was reported in 1900’s. Teeter et al. [122] investigated the electrochemical carbon dioxide reduction at the mercury electrodes at various constant current strengths, and reported in 1954 that formic acid was the only detectable reduction product. Additionally, Paik et al. [123] studied carbon dioxide reduction to formic acid on the Hg electrode in neutral and aqueous
34 electrolytes in 1969. DeWulf et al. [124] reported the hydrocarbon formation on copper electrode in alkaline electrolyte in 1989. However, the seminal research of Hori et al. [71] pointed out that metallic copper is a unique catalyst for electrochemical reduction of carbon dioxide since it is able to produce methane (CH4), ethylene (C2H4) and ethanol (C2H5OH)
with high rates and efficiencies in aqueous electrolytes at ambient temperatures. The electrochemical CO2 reduction process is also capable of providing several products (for
instance: carbon monoxide (CO) [44, 104, 125], methane (CH4) [126, 127], methanol
(CH3OH) [128, 129], ethylene (C2H4) [130, 131], ethanol (C2H5OH) [132], n-propanol
(C3H8O) [133], formaldehyde (CH2O) [134], oxalic acid (H2C2O4) or oxalate (C2O42-) [109],
ethylene glycol (C2H6O2) [135] and acetaldehyde (C2H4O) [132]) by controlling the operating
conditions.
Li and Kanan [136] studied influence of the thickness of the Cu2O layer toward CO2
reduction to carbon monoxide and formic acid. They observed that µm-thick Cu2O film layers
catalyze this reduction reaction with high Faradaic efficiencies at exceptionally low overpotentials. They reported a Faradaic efficiency of 45 % for CO production at the annealed Cu catalyst which was obtained at a potential ranging -0.3 - -0.5 V vs. RHE; this corresponds to an overpotential of 0.19−0.39 V. Similarly, the Faradaic efficiency for HCOOH production reached ca. 33 % at a potential domain of -0.45 - -0.65 V vs. RHE, corresponding to an overpotential of 0.25 - 0.45 V. Ethylene and ethane were also determined as reaction products with a Faradaic efficiency lower than 10 % at cathodic potentials lower than -0.6 V vs. RHE. Raciti et al. [137] worked on highly dense Cu nanowires for electrochemical CO2 reduction to
CO, achieving 60 % Faradaic efficiency with an overpotential of 0.3 V. On the other hand, high operation cost, low catalyst activity, insufficient catalyst durability, inability of product selectivity, and lack of mechanistic understanding make the CO2 conversion more complicate.
For this reason, it is needed to develop an ideal-electrocatalyst having sufficient stability with high catalytic activity at low overpotentials. Ren et al. [36] showed ethylene and ethanol production on Cu2O film electrodes at the potential values in the range of -0.59 and -1.19 V
vs. RHE in a 0.1 M KHCO3 solution. They demonstrated that optimum Faradaic efficiencies
of 34-39 % for ethylene and 9-16 % for ethanol respectively, were obtained on Cu2O film
layers at -0.99 V vs. RHE. Reske et al. [35] presented the dependence of the electrocatalytic activity and selectivity performance on the size-controlled copper nanoparticles. Selectivities of 20-25 for CO % and 60-70 % for H2 were observed on the nanoparticle sizes of 5-15 nm,
35 [138] investigated the electrochemical CO2 reduction on Cu(111), Cu(200) and (220) single
crystal surfaces prepared by chronoamperometric deposition. The working electrode potential was controlled at -1.0 V vs. RHE in 0.5 M KCl electrolyte. They determined methane and ethane as major products with Faradaic efficiencies of 26 % for methane at the Cu single surfaces and 43 % for ethane on a Cu/Cu electrode. Chung et al. [139] also prepared perpendicular Cu nanopillar structure from cathodic electrodeposition with a multi-current step technique for 2.5 and 5 h on a Cu foil substrate. The electrochemical CO2 conversion was
conducted at various constant potentials (between -0.2 and -0.9 V vs. RHE) in a 0.1 mol L-1 KHCO3 aqueous solution for 6 h. From the tested electrolysis results, the highest Faradaic
efficiency value for the formation of formic acid was reported as 28.7 % on the Cu electrode surface with a production rate of 13.17 µmol h-1 at -0.5 V vs. RHE. Besides the formic acid production under the same electrolysis conditions, H2 and CO were also detected with
Faradaic efficiencies of 65.3 and 2.8 %, respectively.
Recently, Kuhl et al. [47] highlighted the electrochemical CO2 reduction on metallic
copper surface at various electrode potentials, ranging from -0.67 V to -1.18 V vs. RHE in 0.1 mol L-1 KHCO3. Different reaction products were reported to be dependent on the applied
potential. Hydrogen, carbon monoxide and formate were monitored potentials more positive than 0.75 V vs. RHE, while hydrocarbons were produced at potentials more cathodic than -0.75 V vs. RHE. When increasing the applied potential, the selectivity trends of the hydrocarbon products increase; however, the selectivity of carbon monoxide and formate decreases. The best selectivity was observed for methane and hydrogen at -1.18 V vs. RHE, with a total Faradaic efficiency over 90 %. Chen et al. [140] investigated the electrochemical reduction of carbon dioxide on Cu mesocrystals, electrodeposited Cu nanoparticles and electropolished Cu electrodes. Amongst these electrodes, Cu mesocrystals exhibited the best electrocatalytic performances at -0.99 V vs. RHE, producing ethylene with the average Faradaic efficieny of 26.90 % in 0.1 mol L-1 KHCO3 electrolyte. Cathodic electrolysis may
lead to degradation of the electrode surface because of the adsorption of reaction products. Shiratsuchi et al. [141] demonstrated that the pulsed electrolysis method can avoid deactivation of copper based electrodes during the electrolysis with suitable conditions. Jermann et al. [108] studied the cathodic reduction of carbon dioxide in hydrogenocarbonate aqueous solutions as function of the electrolysis duration. In order to avoid the degradation of the catalyst activity, in-situ anodic activation procedure was applied, allowing high product yields over a long-term electrolysis.
36 Conversion of carbon dioxide to liquid fuels and valuable chemicals are required innovator approaches for activating the CO2 molecule. Product distribution and reaction rates
can be improved by modifying the catalysts structure in terms of crystallographic characteristics and electronic structure. Moreover, bimetallic electrocatalysts have been investigated at various alloy compositions including Cu- Ni electrodes [142], Cu-Fe electrodes [142], Cu-Cd electrodes [143], Cu-Sn electrodes [144, 145], Cu-Zn electrodes [145], Cu-Au electrodes [146-148], and Cu-Pd electrodes [149-151] for the electrocatalytic reduction of CO2 reaction.
Watanabe and co-workers [152] investigated several Cu alloys for the CO2 reduction
reaction in 0.05 mol L-1 KHCO3 aqueous media. They prepared Cu/Ni, Cu/Sn, Cu/Zn, Cu/Cd,
Cu/Pb, Cu/Ag by electroplating process. CH3OH and HCOOH were selectively produced on
the Cu/Ni alloy, while HCOOH and CO were formed on Cu/Sn and Cu/Pb with enhanced reaction rates. HCOOH and CO can also be formed at the surface of copper with different onset potentials by adding Zn, Cd, Pb and Ag as co-metal. It was reported that the Cu electrode exhibited the highest Faradaic efficiency (ca. 30 %) for HCOOH at the potential of -1.5 V vs. SHE. It is worth of noting that the addition of 10 % Ni shifts the onset potential of HCOOH production towards a positive potential direction, about 0.2 V more positive than that on pure Cu; however, the Faradaic efficiency lowered to 20 % at -1.5 V vs. RHE. Moreover, these authors found a significant synergistic effect between Cu and Sn on the Faradaic efficiency and the overpotential of HCOOH formation. They observed a maximum Faradaic efficiency of 56 % for HCOOH at -1.3 V vs. SHE at the Cu/Sn (57/43) alloy electrode surface against a value of 16 % for CO formation. Kyriacou and Anagnostopoulos [146] have also investigated the effect of the current efficiency toward electrochemical CO2
reduction on Cu electrodes at 25 °C under atmospheric CO2 pressure in 0.5 mol L-1 KHCO3.
They found that the formation of hydrocarbons (CH4 and C2H4) and alcohol (CH3OH) were
suppressed on Cu/Au alloy catalysts, giving rise to the increase of CO formation.
1.8. Pd and CuxPd100-x electrocatalysts
As the concentration of hydrogen atoms can be controlled at the palladium (Pd) surface [153, 154], Pd based catalysts are hopeful materials for carbon dioxide valorization [155-160]. Recently, Liu et al. [151] investigated the electrochemical reduction of CO2 on graphene
supported Pd-Cu nanoparticles (8-10 nm) in 0.5 mol L-1 KHCO
37 catalytic performance of the Cu/graphene (7.5 ± 0.25 nm) was achieved on 1.0 wt. % Pd-2.0 wt. % Cu/graphene at the cathodic peak potential of -1.3 V vs. Ag/AgCl with -2.3 mA. Yin et al. [150] reported electrocatalytic reduction of CO2 on Pd/C, Pd85Cu15/C and
Pd56Cu44/C catalysts in CO2-saturated 0.1 mol L-1 KHCO3. The bimetallic Pd85Cu15/C and
Pd56Cu44/C cathode materials exhibited selective CO2 reduction to CO compared with Pd/C at
potentials ranging from -0.6 to -1.2 V vs. RHE. The higher Faradaic efficiency obtained for CO production was 16 % at -0.99 V vs. RHE. This value attained 73 and 86 % when the reaction was performed on Pd56Cu44/C and Pd85Cu15/C, respectively, at -0.89 V vs. RHE.
Toyoshima and Somorjai [161] investigated the heats of chemisorption of O2, H2, CO, and N2
on polycrystalline and single crystal transition metal surfaces. They noted that the heat of adsorption of CO on Pd is moderate and intermediate between Au and Pt which exhibit more suitable electroactive surface area for the electrochemical reactions.
Azuma et al. [162] observed not only carbon monoxide and formic acid, but also hydrocarbons when electrolysis was carried out on a Pd electrode in CO2-saturated 0.05 mol
L-1 KHCO3 and at more cathodic potentials (-2.0 V vs. SCE). The optimum Faradaic
efficiencies of HCOOH, CO, CH4 and C2H4 production were 8.6, 3.2, 0.31, and 0.061 %
respectively, at 20 °C. Ohkawa and co-workers [163] investigated the effect of hydrogen absorption on the catalytic activity of carbon dioxide reduction at palladium surfaces in aqueous KHCO3 solution. They noted that hydrogen evolution reaction can be suppressed by
hydrogen absorption in the palladium lattice structure, and the absorbed hydrogen can improve the yields of CO2 reduction products by reacting with the adsorbed intermediate
species. As shown in Fig. 1.7, the interactions of Pd and H pathways follow hydrogen adsorption in path I and hydrogen absorption in path II. One can assume that absorbed hydrogen directly reacts with reaction intermediates, resulting in increasing the electrochemical activity and CO2 reduction efficiency.
![Figure 1.3. a) Polarity of the carbon dioxide. Possible structures for adsorbed CO 2 δ- on metals b) carbon coordination, c) oxygen coordination, d) mixed coordination [53, 54]](https://thumb-eu.123doks.com/thumbv2/123doknet/7888452.264161/25.893.229.666.675.908/figure-polarity-possible-structures-adsorbed-coordination-coordination-coordination.webp)
![Figure 1.4. Proposed possible reaction pathways for CO 2 electroreduction reaction to possible products on transition metals [60]](https://thumb-eu.123doks.com/thumbv2/123doknet/7888452.264161/26.893.159.741.401.1066/proposed-possible-reaction-pathways-electroreduction-reaction-possible-transition.webp)
![Figure 1.7. Possible reaction pathways for electrochemical hydrogenation on the Pd/H alloy electrode [163]](https://thumb-eu.123doks.com/thumbv2/123doknet/7888452.264161/39.893.235.663.109.420/figure-possible-reaction-pathways-electrochemical-hydrogenation-alloy-electrode.webp)
![Figure 1.12. Schematic representation of a) the soft-templating pathway and b) the hard- hard-templating pathway leading to mesoporous materials [194]](https://thumb-eu.123doks.com/thumbv2/123doknet/7888452.264161/46.893.197.705.443.827/figure-schematic-representation-templating-pathway-templating-mesoporous-materials.webp)