Integration and function of adult-born olfactory bulb
neurons.
Thèse
Vincent Breton-Provencher
Doctorat en neurobiologie
Philosophiæ doctor (Ph. D.)
Québec, Canada
© Vincent Breton-Provencher, 2014
Résumé:
La récente découverte de la formation de nouveaux neurones dans certaines régions du cerveau adulte a remis en question notre conception des processus de plasticité neuronale. Dans le bulbe olfactif d’un adulte, il y a chaque jour des milliers de cellules qui envahissent le réseau bulbaire. La façon par laquelle ces neurones générés à l’âge adulte intègrent un réseau neuronal pré-existant demeure jusqu’à ce jour inexpliquée. De plus, nous ne savons toujours pas pourquoi il y a de nouveaux neurones qui sont formés dans le cerveau adulte. Nous avons examiné, dans un premier temps, le processus de formation des épines sur les dendrites des interneurones néoformés dans le bulbe olfactif. Nous avons démontré que les premières étapes de formation des épines dépendent grandement sur l’activité neuronale et que de cette façon le réseau bulbaire peut guider de façon efficace l’intégration des neurones générés à l’âge adulte. Dans un deuxième temps, nous nous sommes intéressés à la présence de plasticité structurelle une fois que ces nouveaux neurones ont déjà intégrés le réseau neuronal du bulbe olfactif. Nous avons découvert une nouvelle forme de plasticité structurelle qui consiste à un déplacement dans l’espace des épines vers des régions contenant des cellules principales plus actives. Ces résultats suggèrent une forme de plasticité capable de supporter les changements rapides qui ont lieu à l’intérieur du réseau neuronal du bulbe olfactif lorsque celui-ci s’adapte à un nouvel environnement sensoriel. Dans un dernier temps, nous nous sommes intéressés au rôle fonctionnel de l’arrivée incessante de nouveaux neurones dans le bulbe olfactif. Nous avons étudié les conséquences de l’arrêt de la neurogénèse adulte sur la transmission synaptique, sur l’activité neuronale et le comportement olfactif. Nos résultats révèlent que la neurogénèse adulte est importante pour maintenir l’inhibition sur les cellules principales qui en retour permet de synchroniser l’influx nerveux dans ces cellules. Cette influence sur la transmission synaptique à l’intérieur du réseau bulbaire est d’autant plus importante pour le maintien de la mémoire à court-terme des odeurs. Finalement, les résultats inclus dans cette thèse illustre la complexité que constitue l’intégration de nouveaux neurones dans un réseau neuronal pré-établi et l’importance de la neurogénèse adulte sur l’olfaction.
Abstract
The recent discovery of the formation of new neurons in specific regions of the adult brain has challenged the way we perceived neuronal plasticity processes. In the olfactory bulb of adults, there are thousands of cells populating the neuronal network every day. There are as yet few clues on how adult-born cells mature and incorporate inside a pre-established network of neurons. Moreover, the function of new neurons formed in the adult brain remains elusive. We first investigated how connections in the adult brain are made by recording spine formation and motility on dendrites of adult-born interneurons in the olfactory bulb. We discovered that early steps of spine formation are dependent on network activity inside the olfactory bulb, as a way for the bulbar network to efficiently guide the integration of adult-born cells. Secondly, to understand how new neurons maintain their connections, we monitored the structural plasticity processes occurring once adult-born cells have integrated the network. We discovered a new form of structural plasticity by which spines translocate toward regions of increased neuronal activity. We thus provide an explanation for the rapid synaptic plasticity that can support the adaptation of the olfactory bulb network to the constantly changing odor environment of an animal. Finally, we investigated the functional role of adult-born cells in the olfactory bulb. We recorded the consequences of blocking adult neurogenesis on synaptic transmission, on the network activity and on olfactory behaviors. We reported that adult neurogenesis sustains inhibitory activity essential for synchronizing the principal cells of the olfactory bulb, and for short-term memory of odors. This thesis demonstrates the complexity of integrating and maintaining newborn cells in an adult neuronal network, and the importance of neurogenesis for olfactory functions.
Table of contents
Résumé: ... iii
Abstract ... v
Table of contents ... vii
List of figures ... xiii
List of abbreviations ... xv
Avant-propos ... xxi
1 - Introduction: ... 1
1.1 - Studying neurogenesis in the adult brain ... 1
1.2 - Physiology of the olfactory bulb ... 4
1.3 - The generation and migration of new neurons in an adult brain ... 10
1.3.1 - Proliferation of adult born cells ... 11
1.3.2 - The migration of adult-born neurons ... 13
1.4 - Integration of adult-born cells in the OB pre-existing network. ... 15
1.4.1 - Maturation of adult-born cells in the OB ... 15
1.4.1.1 – Morphogenesis of adult-born cells in the OB ... 15
1.4.1.2 – Electrophysiological characterization of adult-born cells ... 18
1.4.2 - Synaptogenesis on the adult-born granule cells ... 19
1.4.3 - Factors influencing the synaptogenesis on adult-born granule cells ... 23
1.4.4 – Synaptic competition during adult neurogenesis in the OB ... 27
1.5 - Survival of adult-born cells in the OB ... 30
1.5.1 – The role of sensory activity on the survival of adult-born cells ... 32
1.5.2 - Mechanisms regulating the adult-born neuron survival ... 34
1.6 - Function of adult-born cells in the olfactory bulb network ... 36
1.6.1 - Adult-born cells provide characteristic inhibition of mitral cells ... 37
1.6.2 - Long-term potentiation can be transiently induced in adult-born GCs ... 38
1.6.3 - Adult-born GCs are highly sensitive to olfactory activity ... 39
1.7 - Role of adult neurogenesis in olfactory behavior ... 41
1.7.1 - Role of adult neurogenesis in spontaneous odor behavior ... 43
1.7.2 - Role of adult neurogenesis in olfactory associative learning ... 46
1.7.3 - Role of adult neurogenesis in ethologically relevant odor behavior ... 49
1.8 - Adult neurogenesis: a form of plasticity adapted to the needs of the OB ... 51
2 - Results - Activity of the Principal Cells of the Olfactory Bulb Promotes Structural Dynamics on the Distal Dendrites of Immature Adult-Born Granule Cells via Activation of
NMDA Receptors ... 55
2.1a - Abstract ... 56
2.1b – Résumé ... 57
2.2 - Introduction ... 58
2.3 - Material and Methods ... 59
Animals. ... 59
Stereotaxic injections. ... 59
Time-lapse two-photon imaging. ... 60
Filopodial dynamics analysis. ... 61
Lateral olfactory tract stimulation. ... 61
Iontophoresis. ... 62
Patch-clamp recordings. ... 63
Statistical analysis. ... 63
2.4 - Results ... 64
2.4.1 - Spine density increases during the maturation of adult-born GCs in the OB ... 64
2.4.2 - Adult-born GCs integrating the OB display a high level of structural dynamics on their distal dendrites ... 65
2.4.3 - Filopodia dynamics of adult-born GCs at 7 DPI, but not 14 DPI depends on the activation of NMDARs ... 67
2.4.4 - The activity of the principal cells influences filopodial dynamics of adult-born GCs ... 68
2.4.5 - The increased filopodial dynamics of adult-born GCs at 7 DPI following the LOT stimulation depends on the activation of NMDARs ... 70
2.4.6 - The application of NMDA is sufficient to induce the formation of new filopodia on the distal dendrites of immature adult-born GCs ... 71
2.4.7 - Decreasing the extracellular Mg2+ concentration led to filopodia formation on the distal dendrites of mature adult-born GCs following NMDA iontophoresis ... 72
2.5 - Discussion ... 73
2.5.1 - Structural dynamics and maturation of adult-born GCs ... 74
2.5.2 - NMDARs play a role in the structural dynamics of adult-born GCs ... 76
2.5.3 - A critical period for the integration of adult-born GCs ... 78
2.6 - References ... 79
2.7 - Figures ... 83
3 – Results - Principal cell activity induces spine relocation of adult-born bulbar interneurons ... 97
3.1a – Summary ... 98
3.1b – Résumé ... 99
3.2 – Introduction ... 100
3.3 – Material and methods ... 101
Animals ... 101
Stereotaxic injection ... 101
Time-lapse two-photon imaging ... 102
Image analysis ... 103
Stimulation of mitral cells ... 104
Iontophoresis and puff application ... 105
Unilateral nostril occlusion ... 105
Immunohistochemistry ... 106
In situ hybridization ... 106
Statistical analysis ... 107
3.4 – Results ... 107
3.4.1 - Dendritic spines of adult-born GC can relocate in the OB network ... 107
3.4.2 - MC stimulation induces the spine displacement of adult-born GC and is preceded by the directional growth of SHF. ... 108
3.4.3 - Glutamate released from MC controls the motility of SHF via activation of AMPA receptors. ... 110
3.4.4 - MC-derived BDNF plays a role in the directional growth of SHF and the spine displacement of adult-born GC. ... 111
3.4.5 - Sensory deprivation increases SHF dynamics. ... 113
3.5 - Discussion ... 114
3.5.1 - The dual dependence of SHF on MC-derived glutamate and BDNF. ... 114
3.5.2 - The displacement of the spines of adult-born GC may be a faster way to adapt the functioning of the OB network to changing environmental conditions ... 115
3.5.3 - The relevance for other sensory systems ... 116
3.6 – References ... 118
3.7 – Figure ... 125
4 – Results - Interneurons Produced in Adulthood Are Required for the Normal Functioning of the Olfactory Bulb Network and for the Execution of Selected Olfactory Behaviors ... 145
4.1a - Abstract ... 146
4.1b - Résumé ... 147
4.3 - Materials and Methods ... 150
Animals ... 150
Stereotaxic injection ... 150
Western blot analysis ... 150
Patch-clamp recordings ... 151
Local field potential recordings ... 152
Immunohistochemistry and BrdU labeling ... 153
Behavior ... 154
Locomotion. ... 154
Object recognition. ... 154
Novelty suppressed feeding behavior. ... 155
Tail suspension. ... 155
Odor detection threshold. ... 156
Odor discrimination. ... 156
Long-term odor-associative memory. ... 156
Olfactory short-term memory. ... 157
Statistical analysis ... 157
4.4 - Results ... 158
4.4.1 - Treatment with an antimitotic drug abolished the arrival of new neurons in the OB ... 158
4.4.2 - Ablation of OB neurogenesis does not affect the pre-existing population of interneurons ... 158
4.4.3 - Suppression of OB neurogenesis alters IPSCs received by the mitral cells ... 159
4.4.4 - Suppression of neurogenesis decreases the number of GABAergic synapses on the mitral cells ... 161
4.4.5 - Ablation of neurogenesis reduces the frequency of the induced gamma oscillations in the OB ... 162
4.4.6 - AraC treatment does not impair the general exploratory activity, anxiety, and motivation of animals ... 163
4.4.7 - Continuous neurogenesis in the OB is required for some but not all odor-associated behavior ... 164
4.5 - Discussion ... 167
4.5.1 - Roles of neurogenesis in the structural and functional maintenance of the OB circuitry ... 167
4.5.2 - Newborn neurons are involved in the execution of selected olfactory behaviors170 4.6 - References ... 173
4.7 - Figures ... 177
5 - Discussion ... 191
5.1 - Future perspectives ... 192
5.1.1 - Integration of adult born cells in the OB ... 192
5.1.2 - Function of adult-born cells in the OB ... 199
5.2 - General conclusion ... 201
References ... 203
ANNEX A - Newborn neurons in the adult olfactory bulb: Unique properties for specific odor behavior ... 227
A.1 - Abstract ... 228
A.2 - Introduction ... 229
A.3 - The unique nature of adult-born granule cells in the OB ... 230
A.3.1 - Survival, targeting, and synaptic maturation of adult-born GCs are different from their pre-existing counterparts ... 230
A.3.2 - GCs generated during adulthood display delayed acquisition of firing activity but greater excitability when fully matured ... 231
A.3.3 - Long-term potentiation can be transiently induced in adult-born GCs ... 232
A.3.4 - Adult-born GCs generated during 28 days provide 45% of inhibition received by mitral cells ... 233
A.3.5 - Adult-born GCs are highly sensitive to olfactory activity ... 233
A.4 - Role of adult neurogenesis in olfactory behavior ... 234
A.4.1 - Role of adult neurogenesis in spontaneous odor behavior ... 235
A.4.2 - Role of adult neurogenesis in olfactory associative learning ... 237
A.4.3 - Role of adult neurogenesis in ethologically relevant odor behavior ... 239
A.5 - Adult neurogenesis: a form of plasticity adapted to the needs of the OB ... 240
A.6 - Conclusion ... 241
A.7 - References ... 242
List of figures
Chapter 1
Figure 1.1: First discovery of a rostral migratory stream in the adult-brain. Figure 1.2: Overview of the olfactory pathway.
Figure 1.3: Neural elements of the mammalian olfactory bulb.
Figure 1.4: A schematic diagram showing the working mechanisms of the olfactory dendrodendritic reciprocal synapses between a mitral and a granule cell.
Figure 1.5: Adult-generated cells are constantly arriving into the olfactory bulb (OB). Figure 1.6: Illutrastion of the morphogenesis of adult-born granule cells in the olfactory bulb.
Figure 1.7: Electron micrographs of synapses in the EPL.
Figure 1.8: Sensory Deprivation Reduces the Dendritic Length and Spine Density of Newly Generated Granule Cells.
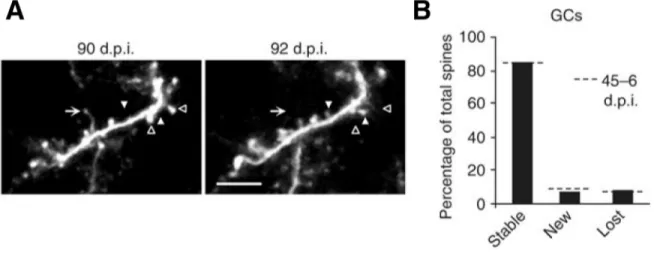
Figure 1.9: Stable granule cells remain structurally dynamic at 90 d.p.i.
Figure 1.10: Labeled cells in the GCL of the olfactory bulb at different survival points after [3H]-thymidine injection.
Figure 1.11: Illustration showing the distinct properties of the adult-born granule cells.
Chapter 2
Figure 1: Spine density increases on the distal dendrites of adult-born GCs during their maturation.
Figure 2: Time-lapse two-photon imaging reveals the rapid formation and retraction of filopodia on the dendrites of adult-born GCs.
Figure 3: NMDAR activity is required for the BL filopodial dynamics at early maturational stages.
Figure 4: Mitral cell firing activity controls the integration of the adult-born GCs.
Figure 5: The mitral cell activity-induced filopodial dynamics on the dendrites of immature adult-born GCs is dependent on the activation of NMDARs.
Figure 7: Decreasing the extracellular Mg2+ concentration makes filopodia on the dendrites of mature adult-born GCs sensitive to NMDA iontophoresis.
Chapter 3
Figure 1: Spines on adult-born GCs are translocating in the OB.
Figure 2: Ultrastructural imaging reveals the target of spine head filopodia.
Figure 3: The activity of a single MC stabilizes the nearby SHF and promotes spine displacement.
Figure 4: The glutamate release from MC stabilizes the SHF. Figure 5: BDNF application promotes spine displacement.
Figure 6: The activity of BDNF lacking MC does not induce spine displacement.
Figure 7: Sensory deprivation increases the number of SHF in a BDNF dependent manner.
Chapter 4
Figure 1: Infusion of antimitotic drug, AraC, abolishes neurogenesis in the adult olfactory bulb but not in the hippocampus.
Figure 2: The pre-existing population of interneurons is unaffected by the AraC treatment. Figure 3: Suppression of OB neurogenesis reduces inhibition on mitral cells.
Figure 4: Reduced number of GABAergic postsynaptic sites on the mitral cells lateral dendrite after the suppression of neurogenesis.
Figure 5: Mice with ablated neurogenesis show reduced network oscillations.
Figure 6: AraC treatment does not affect general locomotor and exploratory activities, anxiety, and motivation of the mouse.
Figure 7: Reduced odor memory in mice with ablated neurogenesis.
List of abbreviations
4AP 4-Aminopyridine 5T4 Oncofetal protein
AMPA 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid D-APV 2-amino-5-phosphonopentanoic acid
AraC Cytosine-beta-D-arabinofuranoside BDNF Brain derived neurotrophic factor BL Baseline
BMI Bicucullin methiodide BrdU 5-bromo-2-deoxyuridine CC Corpus callosum;
cfos Cellular oncogene response CTGF Connective tissue grotwh factor DAB Diaminobenzidine
DCS D-cycloserine, Dcx Doublecortin
DDI Dendrodendritic inhibition DG Dentate Gyrus
DNA Deoxyribonucleic acid DPI Days post-injection DTF Discrete fourier transform EGF Epidermal growth factor EPL External plexiform layer; EPSC Excitatory post synaptic current GABA Gamma aminobutyric acid GAD Glutamic acid decarboxylase
GC Granule cell GCL Granule cell layer
GFP Green fluorescent protein GL Glomerular layer;
IPSC Inhibitory post synaptic current Kyn Kynurenic acid
LFP Local field potential LOT Lateral olfactory tract LTP Long-term potentiation LV Lateral ventricle; MC Mitral cell
MCL Mitral cell layer;
mEPSC Miniature excitatory post synaptic current mGluR2 metabotropic glutamate receptors
mIPSC Miniature inhibitory post synaptic current NA Nucleus accumbens;
NBQX 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline -7-sulfonamide NCAM Neural cell adhesion molecule
NeuN Neuronal nuclei NMDA N-Methyl-D-Aspartate NSC Neural stem cells OB Olfactory bulb OB Olfactory bulb;
ONL Olfactory nerve layer; OSN Olfactory sensory neurons Pax6 Paired box gene 6
PSA-NCAM Polysialylated neuronal cell adhesion molecule PSD95 Postsynaptic density protein 95
RMS Rostral migratory stream SE Septum.
SEM Mean standard error SHF Spine head filopodia Sp8 Specificity protein 8 STIM Stimulation
SVZ Subventricular zone Tbr1/2 T-box, brain, 1/2 TBS Theta burst stimulation TEA Tetraethylammonium TH Tyrosine hydroxylase
TrkB Tropomyosin related kinase B TTX Tetradotoxin
Val66Met Variant brain-derived neurotrophic factor VEGF Vascular endothelial growth factor
À ma famille
Avant-propos
La première partie des résultats présentés dans cette thèse a fait l’objet d’une publication tout récemment dans le Journal of Neurosciences en janvier 2014. Dans cette publication, je suis le seul étudiant impliqué, les deux autres co-auteurs étant mes deux directeurs de recherches avec qui j’ai écrit le manuscrit.
La deuxième partie constitue un manuscrit qui est présentement aux étapes de finalisation afin d’être publié dans les prochaines semaines. Plusieurs personnes ont pris part à cette publication dont je suis le premier auteur. J’ai réalisé l’ensemble des manipulations d’imagerie et d’électrophysiologie. L’étudiante Delphine Hardy a été impliquée dans les expériences de dépravation sensorielle. Marina Snapyan a aidé pour les expériences de biologie moléculaire du papier.
Finalement, la troisième partie de ma thèse comporte ma première publication parut en décembre 2009 dans le Journal of Neurosciences. Plusieurs personnes encore une fois ont permis de réaliser cet article. J’ai réalisé la majeure partie des expériences de l’article. Morgane Lemasson a réalisé la partie comportementale et Modesto Peralta a aidé pour les marquages par immunofluorescence. Il est à noter que depuis sa parution l’article a été listé comme « must read » sur le site web « Faculty of 1000 prime » et a été l’objet de 3 commentaires. J’ai aussi en lien avec cet article publié une revue de la littérature dans Behavioural Brain Research en 2012. Une partie de cette revue a été modifiée et incluse dans l’introduction de la thèse. La version intégrale se retrouve également en annexe.
Je remercie tout d’abord mon directeur de recherche, Dr. Armen Saghatelyan, pour avoir cru en mon potentiel et m’avoir enseigné une pléiade de techniques qui me seront utiles tout au long de ma carrière. De plus, j’apprécie la rigueur scientifique qu’il m’a transmise durant la réalisation de mon doctorat. Je remercie aussi mon co-directeur, Dr. Daniel Côté, qui m’a beaucoup aidé avec les différentes techniques d’imagerie. Je remercie les membres du laboratoire d’Armen présents et passés : Marina, Morgane, Pierre-Olivier, Mireille, Lynda, Dino, Luciné, Sofia, Jivan, Filippo, Caroline, Hèlia, Archana, Delphine, Karen et
Arthur. Tous les membres du laboratoire de Daniel ainsi que tous mes collègues du CRULRG qui ont rendu mon séjour des plus agréables. Par ailleurs, j’aimerais dire merci à mes parents et mes amis qui m’ont supporté durant ces études. Finalement, un merci tout spécial à ma femme, Charlie, qui a toujours été là pour me conseiller et surtout me dire de ne pas lâcher.
Cette thèse constitue l’accomplissement de sept années de travail sur la neurogénèse adulte dans le bulbe olfactif. Arrivant du baccalauréat en Génie physique, je recherchais de nouveaux défis lorsque j’ai joint le laboratoire du Dr. Armen Saghatelyan. J’ai découvert en la neuroscience un monde fascinant, où il est possible de combiner les connaissances de plusieurs domaines afin de répondre à des questions toujours plus enlevantes les unes des autres.
1 - Introduction:
1.1 - Studying neurogenesis in the adult brain
Most of our knowledge on the generation of new neurons in the adult brain is only two decade old. At the end of the 19th century, the renowned neuro-anatomist Ramon y Cajal concluded from his work that neurons were generated exclusively in embryogenesis, and in the early postnatal brain (Parent, 2009). His conclusions progressively created a dogma that prevented any research on adult neurogenesis. The first report describing adult neurogenesis in mammals was obtained almost 50 years ago by the group of Joseph Altman. By using injection of [3H]-thymidine, that incorporates only in the DNA of dividing cells, they observed a large proportion of interneurons that were formed after birth in the rat (Altman and Das, 1965a; Altman and Das, 1965b). He confirmed these results in many subsequent studies and identified the neurogenic corridor located between the subventricular zone (SVZ) and
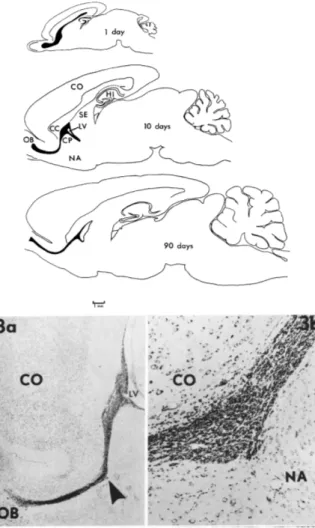
Figure 1.1: First discovery of a rostral migratory stream in the adult-brain.
Top – Tracings of sagittal sections of the brain from rats of different ages to show the position of the subependymal and “rostral migratory stream” (black). CC, corpus callosum; CP, caudate-putamen; HI, hippocampus; LV, lateral ventricle; NA, nucleus accumbens; OB, Olfactory bulb; SE, septum. Bottom – Left: Low-power photomicrograph of the subependymal layer of the lateral ventricle (LV) and the rostral migratory stream (arrow) in sagittal section in a 21 day old rat. Cresyl violet, X16. Rigth: Rostral migratory stream at higher magnification. X101. (Adapted from Altman, 1969).
the olfactory bulb (OB) (Altman, 1969) (Figure 1.1). Other groups confirmed the presence of adult neurogenesis also in rats (Kaplan and Hinds, 1977), as well as in other species, e.g. dogs (Blakemore and Jolly, 1972), and birds (Goldman and Nottebohm, 1983). Even though more and more studies were showing the evidence of neurons generated long time after birth, the scientific community mostly ignored these results. Moreover, the presence of neurogenesis was questioned in primates and humans. In this perspective, the work from Pasko Rakic’s laboratory halted further discoveries on adult neurogenesis by showing with [3H]-thymidine injections that neurogenesis was absent during the last two months of prenatal brain formation and in the adult brain of primates (Rakic, 1974; Rakic, 1985).
The interest of adult neurogenesis was brought back to life in the 90s by the discovery of the neurogenic potential of the SVZ in rodents (Lois and Alvarez-Buylla, 1993; Reynolds and Weiss, 1992). The existence of a pool of stem cells in adults and the availability of more advanced techniques were in a way the trigger for extensive research on adult neurogenesis. The discovery of adult neurogenesis has been since extended to the dentate gyrus of the hippocampus (Gould et al., 1999; Gould et al., 1998), the OB (Bedard et al., 2002; Kornack and Rakic, 2001; Pencea et al., 2001) the olfactory tubercule (Bedard et al., 2002) and the amygdala and adjoining cortex (Bernier et al., 2002) of primates, resolving the initial debate of the 80s.
In humans, the first discovery of adult neurogenesis was obtained in the hippocampus by the injection of 5-bromo-2'-deoxyuridine (BrdU), a thymidine analog (Eriksson et al., 1998). This discovery was confirmed recently by showing that one third of hippocampal neurons are replaced during the life of humans (Spalding et al., 2013). For OB neurogenesis, the presence of a migratory pathway as well as newborn cells is still controversial with some studies arguing for (Curtis et al., 2007; Kam et al., 2009) and others against (Bergmann et al., 2012; Sanai et al., 2004). However, adult-born cells and proliferative cells were observed in OB and olfactory peduncle of adult humans, even though they are generated at a much lower rate than in rodents or than in the hippocampus of humans (Bedard and Parent, 2004; Curtis et al., 2007; Sanai et al., 2011). This lower rate of newborn cells added to the human OB could be due to evolution, since the size of the OB is much smaller in humans and olfaction
is not as essential as it is for rodents. Interestingly, while we are not sure that there is substantial neurogenesis in the OB, the SVZ of humans is preserved during adulthood and displays neurogenic potential (Bernier et al., 2000; Sanai et al., 2004).
The discovery of a pool of progenitor cells in the brain of adult humans shows a lot of promise in finding a cure for neurodegenerative diseases or brain injuries. Researchers are hoping one day to target these newborn neurons in specific regions of the brain and replaced the damaged populations of neurons. In this prospect, the existence of neurogenesis in the adult brain of humans is of high interest. However, in order to unveil the mechanisms underlying this process, and to eventually use it in cell replacement therapy, we use animal models. Indeed, because of the limited techniques currently available to explore the human brain, we use species such as rodents, to precisely monitor the mechanisms involved in adult neurogenesis.
In rodents, the adult neurogenesis is mainly observed in two regions of the brain: the subgranular zone of the dentate gyrus of the hippocampus and the subventricular zone of the lateral ventricles (Lledo et al., 2006; Ming and Song, 2005; Zhao et al., 2008). Both neurogenic regions have interesting and distinct features. However, neurogenesis in adult rodents is much more prominent in the OB. Indeed, approximations from study made with DNA-based analogs such as BrdU and [3H]-thymidine indicate that 20,000 to 30,000 cells arrive every day to the OB (Petreanu and Alvarez-Buylla, 2002; Winner et al., 2002). Moreover, up to half of the population of interneurons in the OB is renewed during one year (Imayoshi et al., 2008) implying a strong role in network plasticity for this adult-born population of neurons. This intense turnover of cells makes adult neurogenesis in the OB a perfect model to study the physiological mechanisms of cell replacement in an intact network of neurons.
Even though adult neurogenesis has been described extensively, we still lack proper understanding of the mechanisms regulating the different steps of neuron formation in a pre-existing network. The goal of my doctoral studies was to elucidate the ultimate step of adult neurogenesis, when newborn cells integrate the OB network, and to understand the
mechanisms underlying the formation, stabilization or elimination of new spines/synapses. I also aimed to reveal the function of adult-born neurons in the OB. All these three studies are presented in the following chapters (2, 3 and 4). In the next sub-chapters of the introduction I will describe the OB physiology, the different steps of bulbar neurogenesis, the synaptogenesis in the OB and elsewhere in the brain, and the function of adult-born neurons in olfaction.
1.2 - Physiology of the olfactory bulb
The olfactory system is one of the oldest sensory modalities, and is essential for the survival of many animal species. Olfaction provides vital information about food location, and influences social and sexual behaviors. In mammals, olfactory sensory neurons (OSNs), located in the olfactory epithelium, detect odor molecules, and send the sensory information to the OB (Shepherd et al., 2004). The OB is a functional equivalent to the early thalamic
Figure 1.2: Overview of the olfactory pathway.
The olfactory bulb receives input from the olfactory sensory neurons in the olfactory epithelium and projects to the olfactory cortex. The diagram indicates some essential aspects of the projection patterns between the regions, as well as the main neural elements within the OB. Note that the olfactory epithelium is arranged in overlapping populations of olfactory sensory neurons which project to individual glomeruli. Some of the central olfactory connections to limbic brain structures are also indicated. c.f., centrifugal fiber; G, granule cell; M, mitral cell; OSN, olfactory sensory neuron; P, pyramidal cell; PG, periglomerular cell; r.c., recurrent axon collateral; T, tufted cell. (Adapted from Shepherd et al. 2004).
relay present in other sensory modalities (Kay and Sherman, 2007). In order to process the olfactory signal, the OB is arranged in different layers, each characterized by their cellular composition (Shepherd et al., 2004) (Figures 1.2 and 1.3). From the surface to the center of the OB, these layers are: the olfactory nerve layer (ONL), the glomeruli layer (GL), the external plexiform layer (EPL), the mitral cell layer (MCL), the internal plexiform layer, the granule cell layer (GCL) and the rostral migratory stream of the OB (RMSOB).
Originating in the nasal epithelium, the axons from the OSNs converge in the ONL, the most superficial layer of the OB. These axons project to the GL, where they form synapses with the principal cells – the mitral (MC) and the tufted cells. OSN sensitive to a specific odorant molecule are scattered in the different part of the nasal epithelium, but groups together in distinct glomerule of the OB (Mombaerts et al., 1996; Mori et al., 2006; Treloar et al., 2002). Thus, the GL represents a map of the different olfactory receptor present in the epithelium. This means that one odorant activate specific regions of the OB. Intrinsic neurons reside in the GL, the short-axon cells and the periglomerular cells (PGCs). Short-axon cells lateral dendrites innervate glomeruli and their axons reach to glomeruli further away. Their anatomy allows them to synchronize the activity of two glomeruli expressing an identical olfactory receptor within one bulb (Shepherd et al., 2004). PGCs generally do not have axons and their dendrites innervate only one or two glomeruli. They contact the apical dendrites of principal cells and the axons of OSNs. This strategic positioning of PGCs allows them to control the activity of different glomeruli and take part in odor detection and discrimination processes (Aungst et al., 2003; Murphy et al., 2005; Vucinic et al., 2006; Wachowiak and Shipley, 2006). Different subpopulations of PGCs have developed to perform these complex and variable functions at the initial stages of odor processing in the OB. Subsets of PGCs can express GABA, glutamate, calretinin, calbindin and parvalbumin (Bagley et al., 2007; Brill et al., 2009; Kosaka et al., 1995; Kosaka et al., 1998; Whitman and Greer, 2007a). In addition, some GABAergic PGCs co-express tyrosine hydroxylase (TH), a rate limiting enzyme for dopamine synthesis (Kosaka et al., 1995). The specific function of most of these subpopulations of interneurons in the OB circuitry is still poorly understood. However, recent studies have specified a role for parvalbumin expressing PGCs in modulating the gain
of OB inputs, using optogenetics to selectively activate this subpopulation of interneurons (Kato et al., 2013; Miyamichi et al., 2013).
Located under the GL, the EPL mostly consists of lateral and primary dendrites of principal cells as well as the dendrites of interneurons. Scattered somas of interneurons and tufted cells are also present. Right below, the MCL is the niche for MC somas. MCs and tufted cells – hereafter, we refer to MCs or principal cells for simplicity – contact olfactory inputs via glutamatergic synapses formed at the tip of their primary dendrites (Figure 1.3B). MCs convey the olfactory signal to other regions of the brain such as the piriform cortex, amygdala, hippocampus, and
enthorinal cortex via the lateral olfactory tract (Davis, 2004; Neville and Haberly, 2004). Each MC also gives rise to long (up to 1 mm in mice) lateral dendrites that innervate the EPL and contact the dendrites of interneurons.
The GCL is the location of granule cell (GC) somas. In general, a GC has short basal dendrites where it receives inputs from other brain regions. A primary dendrite extends toward the EPL, where it divides into secondary, and tertiary branches called the distal dendrites (Figure 1.3C). In the EPL, the GC makes dendrodendritic contact with the lateral dendrites of MCs, providing recurrent and lateral inhibition on these principal neurons (Arevian et al., 2008; Isaacson and Strowbridge, 1998; Jahr and Nicoll, 1982; Schoppa and Urban, 2003; Tan et al., 2010; Urban, 2002). The inhibition provided by the GCs synchronizes MC activity, allowing for fine spatio-temporal tuning of the responses of these principal cells to odors (Arevian et al., 2008; Urban, 2002; Yokoi et al., 1995). Compared to PGCs, fewer GC subpopulations exist. Based on their molecular content, GCs are more homogeneous with all cells expressing GAD protein, an enzyme responsible for the production of GABA. One third of these GAD+ cells selectively express mGluR2 and few cells express also calretinin or the glycoprotein 5T4 (Batista-Brito et al., 2008; Imamura et al., 2006; Murata et al., 2011; Yoshihara et al., 2012). Also sparingly represented in the GCL, the deep short-axon cells are also a population of GABAergic interneurons. Their morphology is similar to the short-axon cells of the GL with dendrites and axons innervating the GCL within one bulb. The function of the deep short-axon cells in the OB is not well understood. However, recordings from the Blanes cells, a subpopulation of deep short-axon cells, have demonstrated that they receive strong glutamatergic inputs from centrifugal fibers
<< Figure 1.3: Neural elements of the mammalian olfactory bulb.
Grouped according to subdivision into (A) afferent fibers, (B) principal cells and (C) local interneurons. ONL, olfactory nerve layer; GL, glomerular layer; EPL, external plexiform layer; MCL, mitral cell layer; IPL, internal plexiform layer; GRL, granule cell layer. A, ON olfactory nerve fibers. Centrifugal afferents are from the contralateral anterior olfactory nucleus (cAON), ipsilateral anterior olfactory nucleus (iAON), tenia tecta (TT), olfactory cortex (OC), horizontal limb of the diagonal band (HDB), locus coeruleus (LC), and raphe nucleus (Ra). pE, pars externa of the AON; pM, pars medialis of the AON. B, dendrites and axon collaterals of a mitral cell (M), and internal tufted cell (iT, or a displaced mitral cell, dM), a middle tufted cell (mT), and an external tufted cell (eT) are illustrated, a, axon; d, dendrite. C, GI, GII, and GIII, three types of granule cells; PG, periglomerular cell; SA(S), superficial short-axon cell; SA(B), Blanes cell. (Adapted from Shepherd et al., 2004)
and inhibit the GCs (Boyd et al., 2012; Pressler and Strowbridge, 2006).
The OB circuitry is characterized by the presence of reciprocal synapses. Indeed, the inhibition provided by the dendrodendritic synapse of both PGC and GC spines is mediated by this type of synapse (Isaacson and Strowbridge, 1998; Jahr and Nicoll, 1982; Price and Powell, 1970) (Figure 1.4). It is defined by a synaptic loop of transmission between spines of interneurons, and dendrites of principal cells. Following a depolarization in the lateral dendrites of MCs, glutamate vesicles are released from the MC dendrite onto the spine of a GC (or a PGC). The ensuing activation of NMDA and AMPA receptors located on the GC Figure 1.4: A schematic diagram showing the working mechanisms of the olfactory dendrodendritic reciprocal synapses between a mitral and a granule cell.
spine creates a local depolarization, which in turn activates the voltage-gated calcium channels. This activation elicits the release of GABAergic vesicles, located inside the spine of the GC, back onto the GABAA receptors of mitral cell lateral dendrites. Metabotropic
glutamate and GABAB receptors are also present on the post-synaptic part of the MC and GC
synapse, respectively, to modulate this reciprocal inhibition (Dong et al., 2009; Isaacson and Vitten, 2003) (Valley et al., 2013). The purpose of the reciprocal inhibition in the OB is to maintain the synchronous activity of the principal cells (Lagier et al., 2004; Lagier et al., 2007; Lepousez and Lledo, 2013). All in all, the reciprocal inhibition is a key component in synaptic transmission in the OB as well as in the computation of the sensory activity.
There are several inputs originating from the brain and projecting to the OB (Shepherd et al., 2004) (Figure 1.3A). Most of these centrifugal inputs are innervating the GCL and the GL and connect the local interneurons. These connections originate, in part, from brain regions responsible for olfactory computation: the anterior olfactory nucleus and the olfactory cortex. Also, cholinergic, noradrenergic and serotoninergic innervations project to the OB from the horizontal limb of the diagonal band, the locus coeruleus and the raphe nucleus respectively. These cortical feedback projections have an active role in modulating the response of the interneurons during sensory discrimination (Boyd et al., 2012; Markopoulos et al., 2013; Nunez-Parra et al., 2013; Winner et al., 2002).
A remarkable feature of the OB circuitry is the constant addition of PGCs and GCs during adulthood, which provides increased plasticity to the network throughout life. In the following sections, I will detail how these adult-born interneurons are generated and integrated, but also their unique function in the bulbar circuitry as well as in olfactory behavior.
1.3 - The generation and migration of new neurons in an adult brain
The generation of adult-born cells occurs outside the OB. The progenitor cells originates
from stem cells located close to the lateral ventricles (LV). They have to migrate over relatively long distances in the anterior direction to arrive at their final destination and to develop into fully mature neurons in the OB (Figure 1.5). This section describes the mechanisms regulating the initial steps of adult neurogenesis: the proliferation and the migration of cells in an adult-brain.
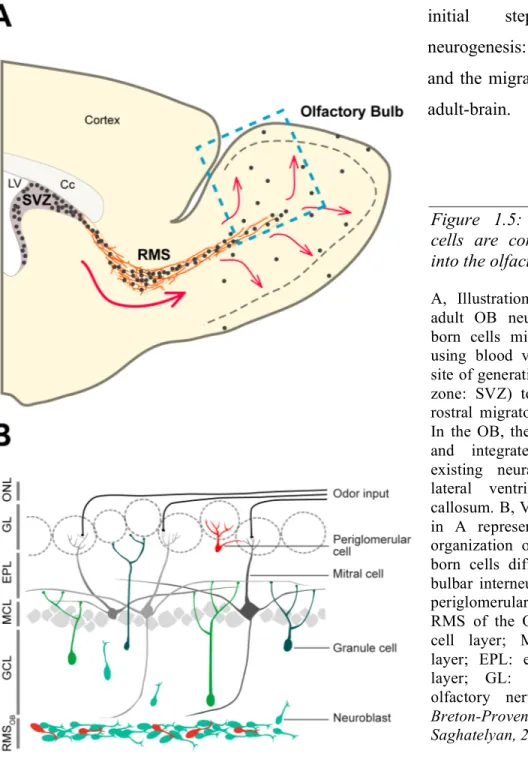
Figure 1.5: Adult-generated cells are constantly arriving into the olfactory bulb (OB).
A, Illustration representing the adult OB neurogenesis. Adult-born cells migrate tangentially, using blood vessels, from their site of generation (subventricular zone: SVZ) to the OB via the rostral migratory stream (RMS). In the OB, they migrate radially and integrate into the pre-existing neural network. LV: lateral ventricle; Cc: corpus callosum. B, View of boxed area in A representing the laminar organization of the OB. Adult-born cells differentiate into the bulbar interneurons, granule and periglomerular cells. RMSOB:
RMS of the OB; GCL: granule cell layer; MCL: mitral cell layer; EPL: external plexiform layer; GL: glomeruli; ONL: olfactory nerve layer. (from:
Breton-Provencher and Saghatelyan, 2012)
1.3.1 - Proliferation of adult born cells
Bordering the LV, the SVZ is the principal neurogenic region responsible for the production of adult-born neurons of the OB. Different cellular types compose the SVZ, each having an important role in adult neurogenesis: the neural stem cells (NSCs), the transient amplifying cells, the neuroblasts, the astrocytes, the blood vessels, and the ependymal cells (Ming and Song, 2011). The proliferation in the SVZ is first initiated from astrocyte-like NSCs (Type B cells) that are slow dividing cells or quiescent cells (Doetsch et al 1999). The Type B cells originate from the radial glia responsible for cortical neuron production in embryogenesis that mutates into the SVZ NSC after birth (Merkle et al., 2004; Young et al., 2007). The NSCs divide into transit-amplifying cells (Type C cells) (Doetsch, 2003; Fuentealba et al., 2012). The transit amplifying cells in turn divides rapidly into other transit amplifying cells, and neuronal precursors (Type A cells). These neuroblasts are forming chains ensheathed by astrocytic processes, within the SVZ, and especially in the rostral migratory stream (RMS), as a mean to migrate anteriorly toward the OB. Other cells, the ependymal and endothelial cells, have a role in the neurogenic activity by secreting molecular factors that preserve neurogenesis in the SVZ. Even though the SVZ is the main neurogenic region in adult OB neurogenesis, there are also stem cells found all along the RMS, producing specific interneuron subtypes (Alonso et al., 2008; Hack et al., 2005).
The cellular organization of the SVZ is essential to maintain the proliferative activity during adulthood. In the SVZ microenvironment, ependymal cells surround the NSCs, forming a pinwheel-like structure (Mirzadeh et al., 2008; Shen et al., 2008). The NSCs extend two main processes: one that contacts the cerebrospinal fluid present in the LV, and one that reaches a nearby blood vessel (Mirzadeh et al., 2008; Shen et al., 2008). Blood vessels of the SVZ release soluble factor that maintains the self-renewal capabilities of NSCs, and induces neuronal production (Kokovay et al., 2010; Shen et al., 2004). For this reason, all three types of progenitor cells agglomerate in the periphery of the SVZ blood vessels (Shen et al., 2008). The microenvironment organization of the SVZ facilitates communication between different cells by conferring different signaling domain for the stem cells (Fuentealba et al., 2012). Numerous molecular factors act at these cellular microdomains, and are involved in the
proliferation or differentiation of the cells. These factors include morphogens, neurogenic factors, gliogenic factors, neurotransmitters, growth factors, hormones, etc (Lledo et al., 2006) (Fuentealba et al., 2012; Ming and Song, 2011). From these identified factors, the ependymal growth factor (EGF) plays a prominent role in activating the proliferation of transit amplifying cells (Type C cells) (Doetsch et al., 2002; Gampe et al., 2011; Paliouras et al., 2012). On the other hand, ciliary neurotrophic factor inhibits neurogenesis in type B cells specifically (Lee et al., 2013). Morphogens like BMP acts as a regulator of gliogenesis in the SVZ, and noggin inhibits its action (Colak et al., 2008; Lim et al., 2000). The notch signaling pathway maintains the neurogenic activity, and the removal of notch receptors leads to depletion of all neural stem cells (Imayoshi et al., 2010). Neurotransmitters are also essential for proliferation in the SVZ. Release of GABA by SVZ neuroblasts reduces the proliferation of the Type B cells of the SVZ (Liu et al., 2005). Interestingly, projections originating from other regions of the brain also releases neurotransmitter in the SVZ. For example. innervations from dopamine neurons of the midbrain promote proliferation in the SVZ (Lennington et al., 2011). Thus the microenvironment of the SVZ is tightly regulated by a multitude of factors and altogether they ensure sustained neurogenesis throughout life.
On a larger scale, the SVZ can be divided in several regions each characterized by cells having distinct fate once they integrate the OB (Merkle et al., 2007). Indeed, the dorsal, ventral, or medial portion of the SVZ the progenitor cells generates different cellular subtypes in the OB. Depending on the region of the SVZ the cells express specific intrinsic factors creating this regionalization of cellular fate (Merkle et al., 2007). Several transcription factors are involved in the cellular fate, and they each have distinct pattern of expression within selected regions of the SVZ. For instance, some of the known factors are Pax6, Tbr1/2, Dlx or Sp8, each known to be expressed at different locations along the LV wall, and to give rise to different class of interneurons in the OB (Brill et al., 2009; Brill et al., 2008; de Chevigny et al., 2012; Hack et al., 2005; Kohwi et al., 2005; Ninkovic et al., 2013; Waclaw et al., 2006). The overall destiny of adult-born interneurons is consequently determined before they reach their final destination by their proliferation location. This suggest that the fate of adult-born cells is predetermined before entering the OB, but the final step of synapse formation is determined during the integration process in the OB.
1.3.2 - The migration of adult-born neurons
The SVZ is located at a distance from the OB. In mice, the neuronal precursors - the neuroblasts - have to migrate over approximately 5 mm to reach the different layers of the OB (Lois and Alvarez-Buylla, 1994). To do so, neuroblasts travel along the RMS where they migrate tangentially, and, once they reach the OB, they start migrating radially to populate the granule cell or glomerular layers. Here, I briefly discuss the mechanisms governing the migration process of progenitor cells in an adult brain.
After the rapid division of transit amplifying cells in the SVZ, the generated neuroblasts migrate in the SVZ toward the RMS (Doetsch and Alvarez-Buylla, 1996). Different mechanisms take place in the brain to efficiently guide the cells towards the OB. After their generation, the neuroblasts form chains in the SVZ to facilitate migration (Doetsch and Alvarez-Buylla, 1996; Shen et al., 2008). To exit the neurogenic niche, the neuroblast chains follow the flow of cerebrospinal fluid in the LV (Sawamoto et al., 2006). Repulsive factors sends neuroblasts outside the SVZ, such as slit that is released from the septum (Wu et al., 1999). The existence of attractive cues released from the OB that would give, the same way as slit, a general direction to the neuroblast migration is still unclear. In fact, the removal of the OB does not affect the number of cells in the RMS arguing against an attractive factors guiding migration (Kirschenbaum et al., 1999). The direction of the migration would then be explained by factors that are expressed locally along the RMS pathway.
Once they exit the SVZ, neuroblasts migrate tangentially in the RMS by again forming chains that are highly dependent on the PSA-NCAM adhesion molecules (Cremer et al., 1994; Hu et al., 1996; Lois et al., 1996). These chains of neuroblasts migrate inside tube-like structures formed by specialized atrocytes (Kaneko et al., 2010; Lois et al., 1996). Some mechanisms exist to maintain this neuroblast-astrocyte architecture. Present on the neuroblast, the axon guidance molecule slit prevents the astrocyte from invading the neuroblast chains, by binding onto the receptor robo2 present on the astrocytic processes
(Kaneko et al., 2010). Interestingly, the tube of astrocytes surrounding neuroblasts releases or uptakes molecular factors promoting migration, guiding the neuroblast in the RMS, and controlling the survival of astrocytes. For example, the astrocytes, via the GABA transporters present on their membrane, uptake the GABA that is released from neuroblasts, a process essential for migration (Bolteus and Bordey, 2004; Snapyan et al., 2009). In addition, the astrocytes release glutamate, which plays an important role in the survival of neuroblasts during their migration in the RMS (Platel et al., 2010). In addition, the blood vessels guide neuroblasts in the RMS (Saghatelyan, 2009). Chains of neuroblasts follows at a close distance blood vessels in the RMS (Snapyan et al., 2009). The endothelial cells of blood vessels promote migration in the RMS by releasing brain-derived neurotrophic factor (BDNF) that is detected by p75NTR receptors located on neuroblasts. At the same time, astrocytes trap the extracellular BDNF to create local gradients and facilitate the migration along the RMS (Saghatelyan, 2009; Snapyan et al., 2009). These BDNF gradients have an essential role in efficiently guiding newborn cells to the OB.
Radial migration in the OB is the final migration step, before the progenitor cell develops into a fully mature interneuron. Upon arrival in the OB, neuroblasts detach from the tangentially migrating chains, and initiate their radial migration to reach their final position in OB (Lledo and Saghatelyan, 2005). Similarly to tangential migration in the RMS, neuroblasts migrate along blood vessels of the OB during radial migration (Bovetti et al., 2007; Snapyan et al., 2009). Some factors are specific to radial migration. For instance, the extracellular matrix glycoprotein tenascin-R, expressed around the RMS of the OB, is involved in both the detachment of neuroblasts from chains and in radial migration (David et al., 2013; Saghatelyan et al., 2004). The glycoprotein reelin that is highly expressed by the principal cells of the OB has also a role in the detachment of the RMS neuroblasts chains, and in the positioning of the neuroblast in the OB (Hack et al., 2002; Kim et al., 2002).
All along the SVZ-RMS-OB pathway, the movement of neuroblasts is characterized by periods of migration intermingled with stationary periods (Snapyan et al., 2009). During migration, the neuroblast first extends a leading process, and the cell nucleus translocates to the position of the leading process of the neuroblast (Wichterle et al., 1997). Many
intracellular mechanisms regulate the formation and stabilization of the leading process, or the movement of the nucleus during migration (Lalli, 2014). For example, the microtubule associated protein doublecortin stabilizes the leading process, and promotes nuclear translocation (Koizumi et al., 2006). The deletion of doublecortin in neuroblasts results in an accumulation in the RMS, and premature differentiation, reflecting its crucial role in migration (Belvindrah et al., 2011). Moreover, the Lis1/Ndel1 complex allows nuclear migration through the cytoplasmic microtubules (Hippenmeyer et al., 2010).
Altogether, these data demonstrate the complexity of migration in the RMS and its tight regulation by many intrinsic and extrinsic factors.
1.4 - Integration of adult-born cells in the OB pre-existing network.
Once the adult-born progenitor cells stop migrating, they start differentiating into neurons to eventually integrate the OB circuitry. The next section describes the steps of this maturation, and the mechanisms involved with synapse formation in a pre-existing network. The mechanisms of dendritic and synaptic development of adult-born cells are discussed in parallel with those governing maturation of neurons generated during embryogenesis.
1.4.1 - Maturation of adult-born cells in the OB
1.4.1.1 – Morphogenesis of adult-born cells in the OB
The neuronal precursors arriving in the OB resembles bipolar cells of only a few microns length. The neuroblast goes through different morphological stages, before it fully develops into an interneuron. The precursor cells differentiate mainly into two types of interneurons the GCs and the PGCs (Lledo et al., 2006; Lledo and Saghatelyan, 2005). As mentioned previously, some group of PGCs and GCs are characterized by their molecular content (Batista-Brito et al., 2008; Brill et al., 2009; De Marchis et al., 2007; Murata et al., 2011;
Yoshihara et al., 2012). These neurochemical subtypes are determined by transcription factors that are expressed in the cells before they enter the OB (Brill et al., 2009; Brill et al., 2008; de Chevigny et al., 2012; Hack et al., 2005; Kohwi et al., 2005; Merkle et al., 2007; Ninkovic et al., 2013; Waclaw et al., 2006). In addition to GCs and PGCs, there is new data suggesting that previously undescribed interneuron types are generated by adult neurogenesis (Merkle et al., 2014).
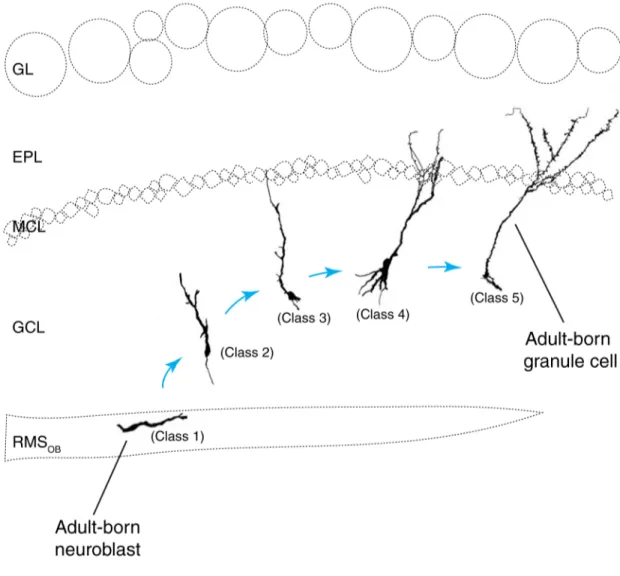
Figure 1.6: Illutrastion of the morphogenesis of adult-born granule cells in the olfactory bulb.
Each of the 5 classes are illustrated with their approximative position in the granule cell and external plexiform cell layer. GL, glomeruli layer; EPL, External plexiform layer; MCL, Mitral cell layer; GCL, granule cell layer; RMSOB, rostral migratory stream of the olfactory bulb.
For GCs, the morphological and physiological stages of integration are defined into 5 classes, from the progenitor cell to the fully mature interneuron (Carleton et al., 2003; Petreanu and Alvarez-Buylla, 2002) (Figure 1.6). Class 1 cells are defined as the neuroblast migrating tangentially in the RMSOB. Class 1 cells arrive in the core of the OB 5 days after being
generated in the SVZ. From 5 to 13 days after their generation, the progenitor cells detach from their tangentially migrating chains, and migrate radially to their final destination in the GCL, this constitute the class 2. Between 9 and 15 days after their generation, the neuronal precursors extend a primary dendrite toward the EPL, without crossing the MC, this defines class 3 cells. The later phases of integration happen between 11 to 30 days after the cells are generated in the SVZ. At these stages, immature GCs form secondary dendrites in the EPL that are almost devoid of spines (Class 4), and eventually generate spines to connect with OB circuitry (Class 5). At the end of the morphogenesis of adult-born GCs, the morphology of class 5 neurons resemble the morphology of mature GCs born during embryogenesis and shortly after birth. However, the location of adult-born GCs in the OB differs from their perinatal counterpart. GCs produced during the neonatal period are preferentially located in the superficial GCL (Lemasson et al., 2005), while the percentage of adult-born GCs is higher in the deep GCL (Imayoshi et al., 2008). Indeed, the genetic ablation of adult neurogenesis leads to the elimination of GCs in the deep, but not the superficial GCL (Imayoshi et al., 2008), implying that neonatal GCs positioned in the superficial GCL persist throughout life, whereas those positioned in the deep GCL are constantly replaced (Imayoshi et al., 2008). The differential targeting and replacement of neonatal and adult-born GCs may lead to different functional outcomes. Since the lengths of the primary dendrites of superficial and deep GCs remains relatively constant (Orona et al., 1983), these two subpopulations can preferentially target the dendrites of mitral or tufted cell, respectively (Greer, 1987; Shepherd et al., 2004).
The PGCs also undergo transitional morphological stages from the neuroblast to a cell with a complete dendritic arborization, but the scientific litterature lacks a proper classification of the different stages of PGC maturation (Belluzzi et al., 2003; Mizrahi, 2007). Interestingly, the PGC progenitors arrive in their final destination faster than the GC, even though they migrate radially over longer distances (Hack et al., 2005). Although they arrive quickly in the
GL, the PGCs might take up to 4 weeks after injection to form the complete arborization and mature dendritic spines (Mizrahi, 2007; Whitman and Greer, 2007a).
1.4.1.2 – Electrophysiological characterization of adult-born cells
Morphological distinctions between neonatal and adult-born cells have different implications for the functional circuitry of the OB. Supporting this fact, electrophysiological differences between these two subpopulations have been observed in the OB. Electrophysiological characterizations have revealed dissimilarities in terms of spiking activity and the development of sodium conductance on pre-existing versus adult-born interneurons in the adult OB (Belluzzi et al., 2003; Carleton et al., 2003).
The neuroblasts, when they arrive in the OB, are devoid of any post-synaptic currents either when they migrate tangentially or radially (Carleton et al., 2003). Ionotropic receptors are present, and are tonically activated at this stage. Both tangentially migrating, and radially migrating neuroblasts express AMPA and GABA receptors (Carleton et al., 2003). The presence of NMDA receptors is controversial in the tangentially migrating neuroblasts, (Platel et al., 2010) versus (Carleton et al., 2003), but they are present in the radially migrating cells. The presence of post-synaptic currents begins when GCs extend their primary dendrite. Interestingly at this stage, the inhibitory synaptic activity is more prominent than the excitatory one, denoting a possible role of GABA at this early stage of formation (Carleton et al., 2003; Panzanelli et al., 2009). The excitatory post-synaptic activity increases when the adult-born GC forms its dendrites and spines in the EPL (Carleton et al., 2003). The extension of the primary dendrite also corresponds to the appearance of sodium conductance in the cells, which will increase later on during dendrite formation in the EPL (Carleton et al., 2003). Once adult-born GCs distal dendrites are fully formed in the EPL, and are displaying a significant density of spines, their electrophysiological properties are almost indistinguishable from the mature GCs (Belluzzi et al., 2003; Carleton et al., 2003).
It has been also shown that neonatal GCs can fire action potentials around the time they finish migrating (Kelsch et al., 2008). In contrast, interneurons generated during adulthood acquire sodium conductance, and fire action potentials at later maturational stages, just before the emergence of output synapses and only after receiving synaptic inputs at their proximal dendrites (Belluzzi et al., 2003; Carleton et al., 2003; Kelsch et al., 2008), but see also (Wang et al., 2003). When both adult-born GCs and PGCs acquire their synaptic inputs, they develop significantly larger sodium currents than their neonatal counterparts (Belluzzi et al., 2003; Carleton et al., 2003; Saghatelyan et al., 2005), with steeper conductance-voltage relationships and more negative activation thresholds (Belluzzi et al., 2003). This results in a greater excitability of newcomers than of neonatal GCs, which would enhance their overall inhibitory drive on the principal cells of the bulbar network. Moreover, the excitability of adult-born GCs might be further increased by the modulation of the level of sensory activity (Saghatelyan et al., 2005). While odor deprivation induces a shift in the voltage dependency of the sodium currents toward more hyperpolarized values leading to the increased excitability of adult-born GCs, neonatal GCs remain unaffected (Saghatelyan et al., 2005). As such, adult-born cells display a distinct level of adaptation, which is not present in other interneurons in the OB.
1.4.2 - Synaptogenesis on the adult-born granule cells
The adult-born cells functionally integrate the OB (Carlen et al., 2002; Magavi et al., 2005). The expression of immediate early genes, a marker for neuronal activity, in the adult-born neurons of the OB during the execution of olfactory behavior denotes that these cells play a role in processing the sensory information, which is only possible by the formation of active synapses. During my thesis, I focused on the integration of GCs since they constitute 97% of the total population of adult-born cells in the OB (Winner et al., 2002). I will thus discuss, in the following section, how specifically adult-born GCs are functionally integrating the synaptic circuitry of OB. I will also compare the processes of synaptogenesis of adult-born and early-born GCs.
The synapse formation on the different parts of adult-born GCs occurs at different times during maturation. The first synaptic inputs appear on the proximal part of the primary dendrite (Kelsch et al., 2008; Whitman and Greer, 2007b). These synapses are either GABAergic or glutamatergic (Katagiri et al., 2011; Kelsch et al., 2008; Pallotto et al., 2012; Panzanelli et al., 2009; Whitman and Greer, 2007b). They originate from projections of
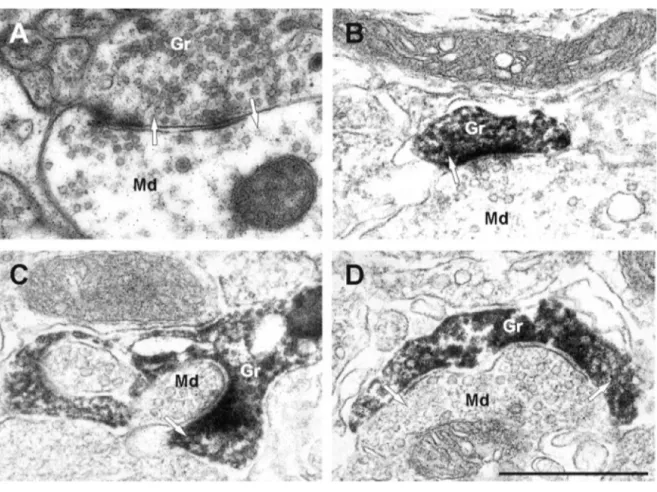
Figure 1.7: Electron micrographs of synapses in the EPL.
A, A dendrodendritic synapse in the EPL between a mitral cell dendrite and a granule cell spine, unlabeled, for reference. The mitral to granule synapse is defined by a collection of small spherical vesicles closely apposed to the presynaptic membrane of the mitral cell secondary dendrite, and an asymmetrically thick membrane specialization in the granule cell spine head. The reciprocal granule to mitral synapse is defined by the elliptical cluster of vesicles in the spine head and symmetrical thickenings in the presynaptic and postsynaptic membranes. B, C, Examples of mitral to granule excitatory synapses on labeled spines of adult-born GCs. Virally labeled adult-born cells were marked by GFP immunohistochemistry and DAB to form an electron dense product, so spines of new granule cells are darkly stained. D, An example of a bidirectional dendrodendritic synapse on the same spine head. On the right, the mitral to granule synapse can be seen, and on the left, the granule to mitral inhibitory synapse. Arrows indicate the direction of the synapse. Scale bar, 1 µm. Md, Mitral cell dendrite; Gr, granule cell spine. (Adapted from Whitman and Greer, 2007)
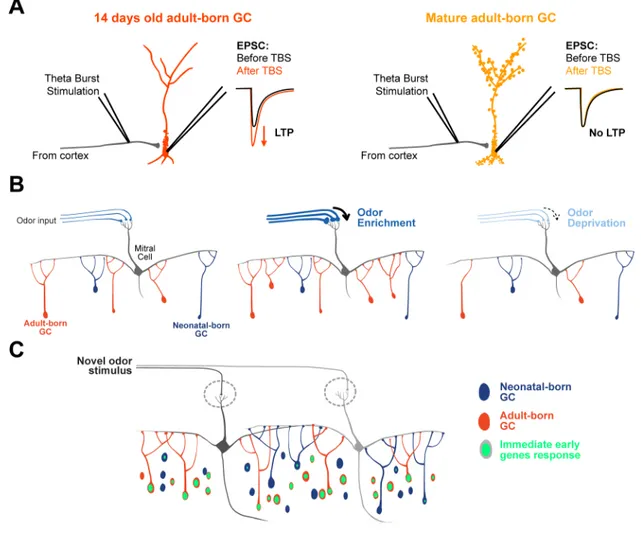
anterior olfactory nucleus neurons, and from pyramidal cells or smooth multipolar cells of the piriform cortex and from local interneurons such as Blanes cells and other short-axon cells (Deshpande et al., 2013; Whitman and Greer, 2007b). The GABAergic synapses on the proximal part of the apical dendrite are more abundant than glutamatergic synapses at early maturational stages, during the formation of the primary dendrite (Panzanelli et al., 2009). This presence of GABAergic synapses early in the development of the adult-born GCs is essential for the proper dendritic and spine formation in the EPL (Pallotto et al., 2012). On the other hand, the proximal glutamatergic inputs, forming slightly after the GABAergic ones, might have a role during the critical period for survival of the adult born GCs. Indeed, LTP can be induced between the centrifugal fibers and the adult-born GCs during a restricted period (2 weeks after birth), which corresponds to a period of massive elimination of adult-born cells (Nissant et al., 2009). The receptor composition of the glutamatergic synapse is also changing during the maturation of GCs (Katagiri et al., 2011).
Shortly after connections surrounding the soma are established, the synapses on the distal dendrites of the born GCs become visible (Kelsch et al., 2008). On the spines of adult-born GCs distal dendrites, the existence of dendrodendritic synapses with MC lateral dendrites have been demonstrated with ultra-structural microscopy (Panzanelli et al., 2009; Whitman and Greer, 2007b) (Figure 1.7). By using optogenetics for targeted-stimulation of adult-born cells, the GABAergic output provided by the adult-born GCs onto the MCs has been monitored. (Alonso et al., 2012; Bardy et al., 2010; Valley et al., 2013). So far no study has shown a direct recording of glutamatergic inputs specifically from distal synapses of the adult-born GCs, but the presence of PSD95 puncta, a marker for glutamatergic synapses, on spine heads as well as the electron microscopy data argue in that sense (Panzanelli et al., 2009; Whitman and Greer, 2007b). The sequence of formation of reciprocal synapses on the distal dendrites of adult-born GCs, and its underlying mechanisms are still poorly understood. It was shown that there is an initial increase in spine density right after the adult-born GCs integrate the OB followed by a decrease once they reach maturity (Pallotto et al., 2012; Whitman and Greer, 2007b). At the same time, the proportion of spines devoid of glutamate receptors or presynaptic GABA vesicles is greater at the beginning of integration with the OB network than later on, arguing for the formation of extra spines looking for a
synaptic partner at this stage (Panzanelli et al., 2009; Whitman and Greer, 2007b). In this regard, the strength of output synapses of adult-born GCs peaks long after they acquire spine on their dendrites (Bardy et al., 2010). At an early maturational stage, the spine turnover, that gives an indication on the stability of synapses, is greater than at later maturational stages (Mizrahi, 2007). 2 weeks after the generation of adult-born GCs only 55% of their spines observed in vivo on adult-born GCs are stable over a period of 24h (Mizrahi, 2007). Altogether, these data suggest that spines forms on dendrites of GCs before the formation of a synapse with the lateral dendrites of MCs. Once the spines contact a MC, they stabilize and they recruit receptors as well as vesicles to establish synapses. This strengthening of synapses is accompanied by an increase in spine head volume. After some time, the spines that fails to establish a connection are trimmed, which would explain the high spine turnover shown at early maturational stages, and the decrease in the number of spines at later maturational stages.
To elucidate what happens with spine and synapse formation at early versus later maturational stages, we conducted a longitudinal study of spine dynamics on adult-born GCs at different time after generation (Breton-Provencher et al., 2014). We found that the number of spines is increasing drastically between the first two weeks of integration of adult-born GCs in OB. Also, at early maturational stages spines have mostly a thin/filopodial shape, characteristic of immature spines. They acquire a mature morphology of mushroom/stubby spines as the cell matures in the OB. These data are in line with study on morphogenesis of spines during development. In the developing brain, different models exist to explain spine formation and synapse assembly (Yuste, 2010). One model states that the precursors of spines would be filopodia. These thin protrusions are highly present during development and they are extremely dynamic (Dailey and Smith, 1996; Lendvai et al., 2000; Niell et al., 2004; Ziv and Smith, 1996). They function as spine precursors by actively exploring the extracellular environment of the cells in order to find axonal partners, and, afterward, they stabilize to form a synapse (Fiala et al., 1998; Goda and Davis, 2003; Ziv and Smith, 1996). Similar to OB adult-neurogenesis, the number of filopodia is decreasing with cell maturation in the developing cortex (Goda and Davis, 2003; Lendvai et al., 2000; Miller and Peters, 1981), leaving room for stable spines (Alvarez and Sabatini, 2007).

![Figure 1.10: Labeled cells in the GCL of the olfactory bulb at different survival points after [3H]thymidine injection](https://thumb-eu.123doks.com/thumbv2/123doknet/6662673.182413/53.918.135.630.629.942/figure-labeled-olfactory-different-survival-points-thymidine-injection.webp)