Biodiesel production under ultrasound and homogeneous catalysts
Thèse
Kiran Shinde
Doctorat en génie chimique
Philosophiae Doctor (Ph.D.)
Québec, Canada
ii
Résumé
Le biodiesel est obtenu par une réaction de transestérification de triglycérides d’huiles végétales ou des graisses par un monoalcool comme le méthanol. Cette réaction est aussi connue sous la désignation d’alcoholyse. La technique de production de biodiesel sous ultrasons est une nouvelle technologie prometteuse pour cette alternative aux combustibles fossiles. La production de biodiesel sous ultrasons est basée sur l’utilisation de sondes ultrasoniques. En utilisant cette technique, le biodiesel peut être produit à grande échelle. Des techniques d’ultrasonification continue peuvent causer une forte émulsion des phases de l’alcool et d’huile rapidement. Pour un temps de résidence faible, de fortes conversions sont obtenues en présence de différents catalyseurs homogènes. Par conséquent, il est nécessaire de régler les défis restants de la production de biodiesel, en termes de conception de réacteur, de récupération des catalyseurs, de coûts et d’enjeux environnementaux, pour que cette méthode de production de biodiesel devienne une technologie industrielle viable.
Les technologies de production de biodiesel étudiées précédemment comportent encore certains défis comme : le problème de récupération du méthanol, la séparation des catalyseurs, le temps de réaction, la température de réaction et les impuretés dans les produits. Donc, il y a toujours un besoin continu pour le développement et la modification des technologies de production du biodiesel.
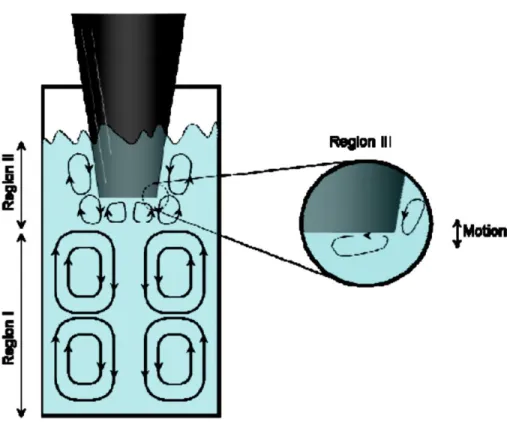
Ce travail abordera le sujet du développement de la production de biodiesel sous ultrasons. L’aspect original des conclusions du travail est la vision par laquelle les ondes ultrasonores affectent la vitesse des réactions de transestérification. Les ultrasons génèrent de fines émulsions du système biphasique dans tout le volume du réacteur. Ceci va évidemment affecter le transfert de masse interphase. Le volume catalytiquement actif est toutefois restreint a
iii
une petite zone de réaction située à proximité de la sonde sonotrode. Dans cette fraction du volume, une vitesse de réaction extrêmement élevée est fort probablement associée à des effets de cavitation.
Pour augmenter la production de biodiesel par l’éthanol sous ultrasons, nous avons testé les effets possibles d’une addition de méthanol ou d’autres composantes à basse tension de vapeur sur le phénomène accélérant dans les réactions de transestérification des triglycérides, du aux ultrasons.
Dans la dernière partie de ce travail, nous avons étudié la réaction de transestérification de l’huile de canola avec du méthanol sur différents types de catalyseurs utilisant à la fois une agitation mécanique et les ultrasons. L’efficacité du transfert de masse dans le champ ultrasonore a amélioré la conversion maximale de transestérification comparativement aux conditions d’agitation mécanique. Dans le cas du propyl-2, 3 dicyclohexylguanidine et 1, 3- dicyclohexyl 2 n-octylguanidine (DCOG) utilisés comme catalyseurs sous ultrasons, les réactions de transestérification que nous avons obtenues ont causé une augmentation notable de la vitesse de conversion des triglycérides. Dams ce cas plus de 80% de récupération de la guanidine dans le mélange réactionnel a été possible en utilisant une colonne d’échange cationique à base de silice.
Mots clés: ultrason, transestérification, huile de canola, FAME, méthoxyde de sodium, hydroxyde de sodium, l'hydroxyde de potassium, tétraméthyle d’hydroxyde d’ammonium, Guanidine, colonnes d'échange de cation de silice.
iv
Abstract
Biodiesel is obtained by transesterification reaction of triglycerides from vegetable oils or fats and a mono alcohol like methanol. This reaction is also known as alcoholysis. Ultrasound biodiesel production technique has recently emerged as a promising technology for synthesis of this alternative for fossil fuels. Ultrasound biodiesel production is based on the use of ultrasonic probes. By using this technique biodiesel production can be made on a large scale. Continuous ultrasonication technique can induce strong emulsion of alcohol and oil phases in a short time. Within very small residence time, high conversions are obtained in presence of different homogeneous catalysts. Therefore, it is necessary to solve the remaining challenges of biodiesel production, in terms of reactor design, catalyst recovery, cost and environment issues, in order to address the biodiesel production as a viable industrial technology.
The previously studied biodiesel production technologies still show some challenges such as: methanol recovery issue, catalyst separation, reaction time, reaction temperature and oxide impurities in products. Therefore, there is still need to develop and modify the continuous biodiesel production technology.
This work deals with the development of ultrasound biodiesel production. The original aspect of the present work conclusions is a vision of how ultrasound waves affect the transesterification reactions rates. Ultrasounds generate a fine emulsion of the biphasic system in the entire reactor volume. This will obviously affect interphase mass transfer. The catalytically active volume is however restricted to a small part of the reaction medium located in the immediate vicinity of the sonotrode probe. Within this volume fraction the extremely high reaction rate is very likely associated with the effects of cavitation.
v
To increase the biodiesel production in presence of ethanol under ultrasound we tested the possible effects of minor methanol or other low vapor tension component additions on the accelerating phenomenon in triglycerides transesterification reactions due to ultrasounds.
In the last part of the work we studied the transesterification reaction of canola oil with methanol and different types of catalysts using both mechanical stirring and ultrasonication reaction. The efficiency of mass transfer in the ultrasound field enhanced the higher rate of transesterification reaction as compared to stirring conditions. In case of propyl-2, 3 dicyclohexylguanidine and 1, 3- dicyclohexyl 2 n-octylguanidine (DCOG) as catalysts under ultrasound transesterification reaction we got noticeable TG conversion where as more than 80% regeneration of guanidine is possible from the reaction mixture by using silica cation exchanger columns.
Keywords: ultrasound, transesterification, canola oil, FAME, sodium methoxide, sodium hydroxide, potassium hydroxide, Tetramethyl ammonium hydroxide, Guanidine, silica cation exchanger columns.
vi
Table of contents
Résumé………...ii Abstract………..iv List of tables………x List of figures……….xi Ackowledgements……….xv Foreward……….xvii Chapter 1 Introduction ... 1 1.1 Biodiesel ... 21.2 Historical developments of biodiesel production ... 5
1.3 Transesterification reaction ... 6
1.4 Alcohols and catalysts commonly used in biodiesel production... 7
1.5 Main feed stocks for biodiesel fuel ... 9
1.6 New technologies for biodiesel production ... 14
1.7 International biodiesel regulations ... 16
1.8 Annual biodiesel production worldwide ... 20
1.9 Thesis structure ... 24
1.10 References ... 25
Chapter 2. ... 32
2.1 Biodiesel production under ultrasound ... 33
2.1.1 Homogeneous base catalyzed transesterification ... 38
2.1.2 Homogeneous acid catalyzed transesterification ... 41
2.2 Engineering of ultrasound reactors ... 45
vii
2.2.2 Cavitation ... 48
2.2.3 Acoustic streaming ... 49
2.2.4 Tooling design ... 50
2.2.5 Transesterification reaction using ultrasounds ... 53
2.2.6 Objectives ... 58 2.3 References ... 59 Chapter 3. ... 69 3.1 Introduction ... 72 3.2 Experimental ... 75 3.2.1 Materials ... 75 3.2.2 Catalyst preparation... 75 3.2.3 Apparatus ... 76
3.2.4 Transesterification reaction tests ... 76
3.2.5 UHPLC analysis ... 77
3.3 Results and discussion ... 78
3.4 Conclusions ... 88 3.5 References ... 89 Chapter 4. ... 96 4.1 Introduction ... 99 4.2 Results ... 100 4.2.1 Glycerolysis ... 100
4.2.2 FAME transesterification by glycerol ... 101
4.2.3 FAME transesterification by ethanol ... 102
viii
4.3 Discussion and conclusion ... 104
4.4 Supporting Information ... 105 4.5 References ... 107 Chapter 5. ... 109 5.1 Introduction ... 112 5.2 Experimental ... 115 5.2.1 Materials ... 115 5.2.2 Catalyst preparation... 116
5.2.3 Ultrasonic Irradiation Unit ... 116
5.2.4 Transesterification reaction ... 117
5.2.5 Methyl ester analysis ... 118
5.3 Result and discussion ... 118
5.3.1 Experimental data of biodiesel production... 118
5.3.2 Catalyst concentration and the effect of methanol to oil ratio ... 119
5.3.3 Comparison between ultrasound and mechanical stirring in presence of CH3ONa catalyst. ... 120
5.3.4 Comparison between ultrasound and mechanical stirring in presence of KOH catalyst……….. ... 122
5.3.5 Comparison between ultrasound and mechanical stirring in presence of NaOH catalyst. ... 124
5.3.6 Comparison between ultrasound and mechanical stirring in presence of Tetramethyl ammonium hydroxide catalyst. ... 125
5.3.7 Comparison between ultrasound and mechanical stirring in presence of catalyst Guanidines. ... 127
ix
5.4 Conclusions ... 135
5.5 References ... 136
Chapter 6. Conclusion and future work ... 140
6.1 Conclusions ... 141
6.2 Future research ... 143
x
List of tables
Table 1-1. Average density and heating value of diesel, biodiesel and blends………....3
Table 1-2. Properties of B100 biodiesel and diesel………...4
Table 1-3. Fatty acid composition of oils……….11
Table 1-4. World biodiesel projections in average for the period from 2013-2025………….…22
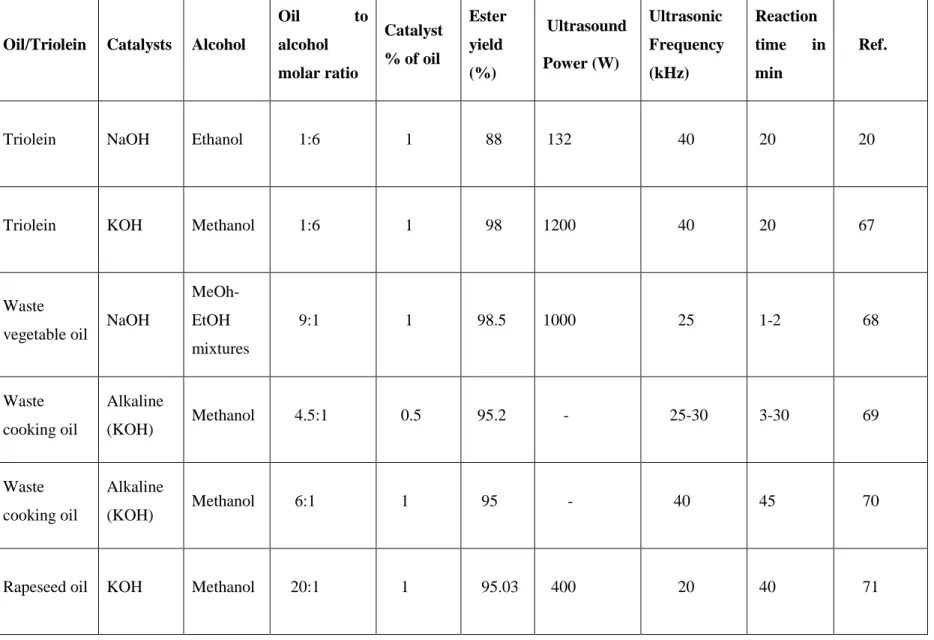
Table 2-1. A comparison among the various techniques used in the biodiesel production. ………...37 Table 2-2. Biodiesel production from various feedstocks under different conditions using ultrasound irradiation……….56 Table 5-1. Sequence of operations in the catch and release technique………….……….135
xi
List of figures
Figure 1-1. Transesterification reaction………..7
Figure 1-2. World biodiesel production and trade………....23
Figure 1-3. (a)-(b) Regional distributions of world biodiesel production and use in 2025……..23
Figure 2-1. Classification of biodiesel production techniques………...35
Figure 2-2. Growth and collapse of cavitation bubble in a liquid medium when ultrasonic waves are applied………..46
Figure 2-3. Streaming observed in a liquid after ultrasonication……….49
Figure 2-4. Ultrasonic probe……….50
Figure 2-5. Different shapes of Converter………...51
Figure 2-6. Titanium Ultrasonic Booster………..52
Figure 2-7. Different types of Ultrasonic Horn………52
Figure 3-1. Reaction setup………....77
Figure 3-2. UHPLC Chromatograms for A = Canola oil, B = Non-polar phase at 60 % TG conversion, C= Non-polar phase at 100% TG conversion………80
Figure 3-3. Effect of reaction time on methyl ester production with 0.5 wt % catalyst (CH3ONa) Methanol/Oil ratio 4:1, ultrasound amplitude 60%. Temperature 35°C………...80
Figure 3-4. Effect of catalyst concentration on methyl ester production. Catalyst (CH3ONa) methanol/oil ratio 4:1, ultrasound amplitude 60 %, Residence time 20 s. Temperature 35 °C………...81
xii
Figure 3-5. Mole fraction of TG, FAME, DG and MG, Reaction conditions 4:1 CH3ONa:Canola
oil, Amplitude 60 %, Residence time 20 s. CH3ONa 0.5 wt% with canola oil. Temperature 35
°C………...82 Figure 3-6. Steady state mole fraction of TG, FAME, DG and MG with different wt % of catalyst, Reaction conditions 4:1 CH3ONa:Canola oil, Amplitude 60%, Residence time 20 s,
CH3ONa 0.5 wt % with canola oil. Temperature 35°C……….83
Figure 3-7. Temperature and power change during reaction………...83
Figure 3-8. Effect of amplitude on methyl ester production with 0.5 wt % catalyst (CH3ONa)
methanol/Oil ratio 4:1, residence time 20 second. Temperature 35°C………..84 Figure 3-9. Effect of temperature on TG conversion: a= 35°C, b= 45°C, C=55°C. 0.5 wt % catalyst (CH3ONa) Methanol/Oil ratio 4:1, ultrasound amplitude 60%...85
Figure 3-10. Effect of mole ratio on continuous methyl ester production with 0.5 wt % catalyst (CH3ONa), ultrasound amplitude 60%, residence time 20 s. Temperature 35°C………..85
Figure 4-1. Glycerolysis of Canola oil at 140 °C A-Stirring without US; B-US without solvent addition; C-US with dropwise addition of THF; D- US with 0.33 wt % octane; E- US with 0.33 wt % nonane (with respect to oil)………101 Figure 4-2. FAME conversion by reaction with glycerol at 140 °C A-US and F:G*= 1:1; B-US and F:G=1:2; C- stirring no US, F:G=1:1; D- stirring no US, F:G=1:2; E- US with 0.33 wt % octane; F:G=1:1, F- US with 0.33 wt % octane F:G=1:2. F:G*=FAME to glycerol molar ratio………..102
xiii
Figure 4-3. Transesterification of triglycerides by ethanol. Catalyst KOH 0.5 wt %; ethanol/oil molar ratio 4:1; residence time 75 s; Temperature 35°C; ultrasound amplitude 60%...103 Figure 4-4. Transesterification of triglycerides by butanol. Catalyst KOH 0.5 wt %; butanol/oil molar ratio 4:1; residence time 75 s; Temperature 35 ºC; ultrasound amplitude 60%...104 Figure 5-1. Effect of catalyst concentration on triglyceride conversion. Catalyst (CH3ONa),
methanol:oil ratio ( 6:1, 4:1, 3:1 ) ultrasound amplitude 60%, temperature 35 ºC……….121 Figure 5-2. Ultrasound biodiesel production batch reaction, catalyst (CH3ONa), methanol:oil
ratio ( 6:1, 4:1, 3:1 ) ultrasound amplitude 60%, 0.5 wt %, temperature 35 ºC……….122 Figure 5-3. Mechanical stirring biodiesel production batch reaction, catalyst (CH3ONa),
methanol:oil ratio ( 6:1, 4:1, 3:1 ) 0.5 wt %, temperature 65 ºC……….123 Figure 5-4. Ultrasound biodiesel production batch reaction, catalyst (KOH), methanol:oil ratio ( 6:1, 4:1, 3:1 ) ultrasound amplitude 60%, 0.5 wt %, temperature 35 ºC………124 Figure 5-5. Mechanical stirring biodiesel production batch reaction, catalyst (KOH), methanol:oil ratio ( 6:1, 4:1, 3:1) 0.5 wt %, temperature 65 ºC………..124 Figure 5-6. Ultrasound biodiesel production batch reaction, catalyst (NaOH), methanol:oil ratio ( 6:1, 4:1, 3:1 ) ultrasound amplitude 60%, 0.5 wt %, temperature 35 ºC………125 Figure 5-7. Mechanical stirring biodiesel production batch reaction, catalyst (NaOH), methanol:oil ratio ( 6:1, 4:1, 3:1 ) 0.5 wt %, temperature 65 ºC………..…126
xiv
Figure 5-8. Ultrasound biodiesel production batch reaction, catalyst (Tetramethyl ammonium hydroxide) 3 wt %, methanol:oil ratio ( 6:1, 4:1) ultrasound amplitude 60%, temperature 35 ºC……….127 Figure 5-9. Stirring biodiesel production batch reaction, catalyst (Tetramethyl ammonium hydroxide) 3 wt %, methanol:oil ratio ( 6:1, 4:1), temperature 35 ºC………127 Figure 5-10. Ultrasound biodiesel production batch reaction, catalyst (Guanidine A) 3 % mol, 4:1 and 3:1 (Methanol: Canola oil) 3 % catalyst 60% amplitude, 35 ºC………128 Figure 5-11. Ultrasound batch reaction 4:1 (Methanol : Canola oil ), Catalyst (Guanidine A) 3 and 5 % mol, 60% amplitude, temperature 35 ºC………...129
Figure 5-12. Ultrasound batch reaction 4:1 (Methanol : Canola oil ) 3 % mol catalyst, 60% amplitude, temperature 35 ºC………..130
Figure 5-13. Mechanical stirring batch reaction 4:1 (Methanol : Canola oil ) 3 % mol catalyst, temperature 65 ºC………130 Figure.5-14. Guanidine catch and release technique………..134
xv ACKNOWLEDGEMENTS
I want to begin by thanking my advisor (Guru), Professor Serge Kaliaguine. Without his inputs, it wouldn’t have been possible to accomplish my doctoral degree. Every scientific discussion with him enhanced my knowledge in the field of research and helped me to think and act independently. I really value his guidance and support, and the independence that he gives us in lab to explore our research interests. My time in his lab has been very educational and enjoyable. I also deeply appreciate that he has been very supportive of my career goals and choices, and has really done a lot to help me reach them. I have learnt lots of valuable spiritual wealth, which is definitely priceless. I would also like to acknowledge my Co-supervisor: François Béland for his motivation and for allowing me to use his Silicycle lab facilities. I would be especially grateful to Madam Guoying Xu for the kindness and support since the first day.
Additionally, I want to acknowledge my doctoral general examination committee members Prof. Frej Mighri, Prof. Trong-On Do, Dr. Bendaoud Nohair for their thoughtful comments and suggestions. I want to thank chemical engineering program director Prof. Alain Garnier for his valuable guidance. I am very thankful for all of their support over the years.
The Kaliaguine’s group has been a great group to work with, and I especially want to thank our wonderful research assistants Dr. Bendaoud Nohair and Mr. Gilles Lemay for their help in my experiments. Special thanks to my lab members Luc Charbonneau, Lin Chen, Zheng Fang, Arsia Afshar Taromi, Thanh Binh Nguyen, Tien Binh Nguyen, Valerica Pandarus, Neyra Mighri, Chi Cong Tran, Rouholamin Biriaei, Xavier Foster and Sara Madadi. I also wish to thank my past lab members Dr. Vinh Thang Hoang, Dr. Zhen Kun Sun and Dr. Foroughazam Afsahi.
xvi
I am also greatly indebted to many teachers in the past: Dr. B. D. Kulkarni, Dr. S. Mayadevi, Dr. Venkat Panchagnula, Dr. A.G. Gaikwad and Dr. P.P. Wadgaonkar, at National Chemical Laboratory, Pune, India, for the motivation and for getting me interested in research and coming to Canada. Thanks to working group Dr. B. D Kulkarni and CEPD for making my stay comfortable at NCL.
My sincere thanks and best wishes were also extended to my close Indian friends Dr. Aniruddha Joshi, Dr. Tushar Borase and Dr. Sagar Mohan for their friendship, encouragement, and support.
Finally, I want to thank my family for supporting me and being in my life. I always know how important you are in my life. Thanks for making me feel loved and always being supportive of me.
Dedicated to my Family.
I love you!
xvii
Forweword
This dissertation is composed of seven chapters. The first chapter is an introduction of the field of biodiesel production. It contains sections on Biodiesel, Historical developments of biodiesel, Transesterification reaction, Catalysts and Alcohols commonly used in biodiesel production, Oilseed crops as raw materials, International biodiesel regulations, Annual biodiesel production worldwide and New technologies for biodiesel production. The second chapter introduces the biodiesel production under ultrasounds using homogeneous alkali catalyzed and homogeneous acid catalyzed transesterification. In this chapter, Engineering of ultrasound reactors, Ultrasonication, Cavitation, Acoustic streaming and tooling design are also discussed. This second chapter constitutes a complete review of different ultrasound technologies for biodiesel production.
Chapters three, four and five report the results of this dissertation in the form of three scientific articles, two of which are already published whereas the third one is not yet submitted. The list of articles relevant to each chapter is as follows. The first author for the three articles is the author of this Ph D thesis.
Chapter 3
xviii
Published in Int. J. Chem. React. Eng., 15(1), 117–125, 2017.
Kiran Shinde, Bendaoud Nohair and Serge Kaliaguine
Department of Chemical Engineering Laval University,Quebec, G1 V 0 A6, Canada
In this chapter we showed a systematic experimental analysis of ultrasound assisted continuous biodiesel production using canola oil in the presence of methanol and sodium methoxide as catalysts. The effects of various reaction parameters such as residence time, catalyst concentration, reaction temperature, ultrasounds amplitude and power, methanol/oil molar ratio were established by the first author of the paper who is the principal author of the paper.
Chapter 4
Triglycerides Transesterification Reactions under Ultrasounds
Published in ChemistrySelect, 1(18), 6008-6010, 2016.
Kiran Shinde and Serge Kaliaguine
xix
In this chapter, we studied possible effects of minor methanol or other low vapor tension component additions on the accelerating phenomenon in triglycerides transesterification reactions with alcohols due to ultrasounds. The most important effect of ultrasound on the rate of triglyceride transesterification is due to cavitation and the mass transfer enhancement in this biphasic reaction due to high dispersion of the polar phase.
Chapter 5
Ultrasound biodiesel production using various homogeneous catalysts and their separation over silica cation exchanger columns
Kiran Shinde1, François Béland2 and Serge Kaliaguine1
1Department of Chemical Engineering Laval University, Quebec, G1 V 0 A6, Canada 2SiliCycle Inc., 2500, Boul. du Parc-Technologique, Québec City, Québec G1P 4S6, Canada
In this chapter, NaOH, KOH, CH3ONa, tetramethyl ammonium hydroxide and two
guanidines are tested for transesterification reaction in a batch reactor both under ultrasound and mechanical stirring. The synthesis of different guanidines and separation of guanidines from reaction medium using silica cation exchanger columns are described. This manuscript will soon be submitted for publication. The first author of the paper and principal investigator is the author of this thesis.
Chapter 6, gives the general conclusions and some recommendations for future work.
2
1.1 Biodiesel
Biodiesel is a term designating fatty acid alkyl esters produced as a result of transesterification reaction between triglycerides and any alkyl alcohol. It is widely recognized in the alternative fuels industry as well as by the Department of Energy (DOE), the American Society of Testing and Materials (ASTM) and the Environmental Protection Agency (EPA). Biodiesel can be produced from virgin oil feedstock (such as rapeseed or soyabean), animal oil/fats, tallow and waste cooking oil. The National Soy Diesel Development Board (presently National Bio-diesel Board) is pioneer in the commercialization of bio-diesel in the USA since 1992 [1]. Biodiesel has properties similar to diesel fuel, but has many following advantages over diesel fuel.
1) High oxygen content: The high oxygen content in biodiesel facilitates its complete combustion that leads to the complete utilization of the fuel without producing any harmful by-products.
2) Reduction of particulate matter emissions: Particulate matter is a mixture of complex organic and inorganic compounds, such as carbon residues, lubricating oil components etc. The suspension of these particulate matters in the environment leads to many adverse effects such as pollution, intoxication of air, climate imbalance etc.
3) Reduction of carbon dioxide emissions: It is known that the carbon dioxide is a greenhouse gas and a major contributor of global warming. The use of biodiesel significantly reduces the carbon dioxide atmospheric balance since the carbon in vegetable materials is borrowed from the atmosphere.
3
4) Reduction of carbon monoxide emissions: carbon monoxide causes serious health hazards by blocking oxygen intake in humans and animals. It is reported that the use of 100% biodiesel [B100] reduces carbon monoxide emissions by 35%.
5) Reduction of sulfur oxides emissions: Sulfur based compounds are also identified to be among the potential harms for the environment. For example, the sulfur dioxide causes respiratory tract irritation in humans. Biodiesel is generally sulfur-free, as long as sulfuric acid is not used in the biodiesel production process.
6) High flash point: Flash point is the temperature at which a fuel becomes flammable. As the biodiesel has higher flash point than diesel, it sufficiently avoids any sort of fire accidents. The following table (Table 1-1) gives average heating and density values of biodiesel in comparison with diesel.
Table 1-1. Average density and heating value of diesel, biodiesel and blends [2].
Fuel Net heating value Avg. (MJ/L) Density (g/cm3)
Diesel 36.09 0.85
Biodiesel (B 100) 32.97 0.88
B 20 Blend 35.04 0.85
4
Table 1-2 Comparison of the properties of biodiesel and diesel [3].
Fuel property Biodiesel (B 100) Diesel
Lower heating value Btu/gal 118,170 129,050
Fuel standard ASTM D6751 ASTM D975
Specific gravity kg/L@150C 0.88 0.85
Kinematic viscosity cSt@600C 4-6 1.3-4.1
Density Ib/gal @ 150C 7.32 7.07
Carbon, wt. % 77 87
Hydrogen, wt. % 12 13
Water and sediment, vol. % 0.05 max 0.05 max
Sulfur, wt. % 0.0 to 0.0024 15-50 ppm Flash point, 0C 100 to 170 60 to 80 Boiling point, 0C 315 to 350 180 to 340 Pour point 0C -15 to 10 - 35 to -15 Cloud point, 0C -3 to 12 -15 to 5 Oxygen, by dif. wt.% 11 0
5
1.2 Historical developments of biodiesel production
In the historical development of biodiesel productions, the vegetable oils have been used as fuel more than one hundred years ago as per the report by Knothe [4,5]. It is a noteworthy historical event that Rudolf Diesel conducted engine tests using peanut oil at the Paris show 1900 under the French government. However, the interest in vegetable oils-based biodiesel production diminished as the fossil fuels soon became available in much higher quantity and lower cost as compared to the biodiesel productions. It was established that the high fuel viscosity in compression ignition is one of the major problems associated in use of vegetable oils as a fuel. It should be noted that the viscosity of vegetable oil is around 10-20 times higher than that of diesel [6,7]. Therefore, the use of such oil directly in engines is limited because of high viscosity and low volatility.
Alternatively, it was proposed to use the mixture of fossil fuel and vegetable oil as fuels. However, it was found, according to the high end point of the distillation curve, coupled with poor fuel atomization that this mixed use of fuels led to incomplete evaporation and mixing processes and poor combustion (formation of small particles and carbonaceous deposits) [8,9]. Therefore, long-term operation on mixture of vegetable oil and fossil fuel resulted in engine damage [10]. As a result the alternative approach proposed to overcome this problem was preheating the oil [11] and using oil mixed in very low proportions with fossil fuel [12].
Since the use of mixtures of fossil fuel and vegetable oil in modern engines can present similar difficulties as reported above, the “transformation” of the oil directly into fuels, is recommended as the products are expected to exhibit properties similar to fossil fuels.
One of the major drawbacks of vegetable oils is their high viscosity. In order to reduce/control the viscosity, there are four major techniques such as microemulsion, dilution,
6
pyrolysis and transesterification as well as direct dose of the oil, which were employed essentially to reduce the viscosity of these vegetable oils.
It is found that microemulsions with different alcohols overcome the problem of high viscosity of vegetable oils. Similarly, pyrolysis, which is defined as the cleavage to smaller molecules by thermal energy, of vegetable oils over catalysts has been investigated [7,13]. The transesterification process has also been shown to reduce the viscosity of triglycerides [14].
Biodiesel has been prepared as the mixture of monoalkyl esters of fatty acids derived from vegetable oil or animal fat [8]. Therefore, biodiesel is biodegradable, lacks toxic aromatics, lowers automobile emissions and is carbon neutral. Compared to fossil fuel, biodiesel produces around 75-90% less particulate matters [15], unburned hydrocarbons, CO and sulphates. Monoalkyl esters of fatty acids appeared as a fuel in Belgium in 1937 [16]. After the Belgium patent two more patents are recorded in 1980 one from Germany and a second one from Brazil.
Today, biodiesel production is commercialized in many countries such as Austria, Italy, Argentina, Spain, USA, Brazil, Indonesia, Germany and France [8]. Currently, there are number of large scale biodiesel production plants under operation and they produce more than 5000 million gallons of biodiesel per year worldwide. Only in America, there are more than 90 biodiesel-production plants under operation [17].
1.3 Transesterification reaction
Transesterification reactions were first reported in 1852 [18] for high quality soap and water free glycerol production. Transesterification is the process of modifying esters. There are two transesterification biodiesel production methods: a) without catalyst b) with catalyst. In more detail, one mole of triglyceride reacts with three moles of alcohols to form one mole of glycerol
7
and three moles of esters. This process includes three reversible reactions in which the triglyceride molecule is converted step by step into diglycerides, monoglycerides and glycerol. In every step, one mole of alcohol is consumed and one mole of ester is liberated. In order to shift the equilibrium to the right, alcohol is added in excess in most of biodiesel production plants.
Fig.1-1. Transesterification reaction
1.4 Alcohols and catalysts commonly used in biodiesel production
The most commonly used primary alcohols in biodiesel production are methanol, ethanol, straight chain high carbon alcohols, and other kinds of alcohols available to date [19]. Increasing the length of alcohol chain can greatly increase the difficulty of separation after the reaction. [20]. An important factor to choose the primary alcohol is the water content. Water interferes with biodiesel production reactions when using alkaline catalysts which results in poor biodiesel yield, with high level of soap, free fatty acid and triglycerides. Lower alcohols are hygroscopic and may absorb water from the atmosphere. After transesterification, methanol is considerably easier to recover than ethanol, as the latter forms an azeotrope with water so that it is expensive to purify. If the water is not removed then it interferes with biodiesel production [8]. Methanol can be recycled more easily because it does not form an azeotrope. For this reason, the use of anhydrous alcohol is needed. Since chemical grade ethanol is typically denatured with poisonous materials to prevent its intake, to find undenatured ethanol is difficult [19]. Nevertheless, ethanol
8
has a positive impact for biodiesel production as it can be considered as a more sustainable reactant than methanol [19, 21]. Other advantage of methanolysis is that both products, FAME and glycerol, are immiscible, thus producing separate phases. FAME yields can be increased by minimizing the excess methanol and carrying out the reaction in two or three steps [19, 22].
In addition, the ultrasound-assisted biodiesel production exhibits a certain relationship at different chain lengths of alcohols [23-25]. Hanh et al. [26] reported the effect of different alcohols. In this study they showed that the reaction rate was the fastest with methanol and ethanol which gave good yields among the different kinds of alcohols. However, the straight chain of high carbon alcohols, such as 1-octanol, 1-hexanol and 1 decanol showed relatively slow reaction rate on yielding the biodiesel.
Transesterification reactions can occur in the absence of catalysts [8] however, it requires high temperature, long reaction time and pressure. There are different types of catalyst reported, such as homogeneous or heterogeneous (including enzymes). The most commonly used homogeneous catalysts in biodiesel production are potassium hydroxide and sodium hydroxide [19]. Alkaline catalysts are highly hygroscopic and form chemical water. That absorbed chemical water affects biodiesel production yield. Alkaline catalysts give good results when raw material with high quality (FFA<1 % w/w and less moisture) are used [27]. Acid catalysts were also reported for biodiesel production, but they are very slow for industrial process and commonly used for the esterification of free fatty acid, only in the case of high free fatty acid oils [19, 28]. Heterogeneous catalysis involves the use of insoluble compounds in either ethanol or methanol that reduce the problems arising from employment of homogeneous catalysts, such as contamination and washing steps. This leads to a decrease of both economic and environmental costs [29]. Heterogeneous catalysts consist of a large number of compounds of different
9
chemical nature such as transition metal oxides, mesoporous silica, alkaline earth oxides, alkali doped materials, acidic polymers, heteropolyacids, waste carbon-derived solid acids and miscellaneous solid acid [30]. Commercially used enzymes in the biodiesel production are Pseudomonas cepacia, Rhizomucor miehei, Candida Antarctica, Pseudomonas fluorescens [31].
1.5 Main feed stocks for biodiesel fuel
The main feedstocks for biodiesel production are listed below: (i) Waste vegetable oil:
This includes the use of spent frying oil that considerably reduces the cost of biodiesel. The waste vegetable oil from food industries is getting popular as a possible source of feedstock. However, the presence of free fatty acids or water in waste oil to be used as feedstock results in changes in the reaction procedure, which is a limitation in use of waste vegetable oils.
(ii) Non-Edible oils:
Non-edible oils such as those of Jatropha, Pongamia, Madhuca and Azhadirachta are used to produce biodiesel. The fatty acids composition of the Jatropha oil is similar to other oils. The presence of some toxic material in kernel renders the oil inedible. Jatropha is being actively investigated as a promising source of feed stocks for biodiesel production in development in developing countries of Asia.
(iii) Animal fats:
Waste animal fat is a cheap source of for biodiesel production and its utilization also serves environmental benefits.
10
Sunflower, Canola, Palmoils and Soybean are the most commonly used virgin oil based raw materials for biodiesel fuel. Their production quantity governs their selection for biodiesel production. The other commonly used feedstock vegetable oils are castor, peanut, cottonseed, rapeseed oils, due to their content of triglycerides.
(v) Algaes:
Algaes offer many advantages in the search for their sustainable, renewable bioenergy feedstocks. They have been recognized as a potentially good source for biodiesel production for a long period of time because of their high lipid content and rapid biomass production.
Composition of different vegetable oils
Vegetable oils are extracted from different plants and their combustion yields completely recycled carbon, since the plants assimilate atmospheric carbon dioxide.
The fatty acid compositions of different origins are reported in Table 1-3[32].
Table 1-3. Fatty acid composition of oils [32] (a) Vegetable Origin
12 (b) Animal Origin
13
It should be noted that the biodiesel has higher cloud and pour points compared to diesel fuels so it is not convenient to use in winter [33, 34]. The cetane number of vegetable oils is very high hence reducing the ignition delay [35]. Vegetable oil has high iodine value and therefore increased oxidation rate. Therefore the long time storage is not possible or recommended for these kinds of fuels [36].
Natural fat oils are esters of glycerol and fatty acids. There are two types of fatty acids, saturated fatty acid and unsaturated fatty acids. Saturated fatty acids contain single carbon-carbon bonds, while the unsaturated fatty acids contain one or more double bonds. The common fatty acids are stearic (18:0), linoleic (18:2), oleic (18:1) and palmitic (16:0).
The schemes below show the chemical structures of triglycerides, diglycerides and monoglycerides.
a) Triglycerides
14 c) Monoglycerides
The chemical structures of fatty acids are described below: Palmitic acid/Hexadecanoic acid R-(CH2)14CH3
Stearic acid/Octadecanoic acid R-(CH2)16CH3
Oleic acid/9(Z)-octadecenoic acid R-(CH2)7CH=CH-(CH2)7CH3
Linoleic acid/9(Z), 12(Z) -octadecadienoic acid R- (CH2)7CH=CH-CH2-CH=CH-(CH2)4CH3
Linolenic acid/ R-(CH2)7-(CH=CH-CH2)3-CH3
1.6 New technologies for biodiesel production
Human beings have always been dependant on the use of energy in every sphere of life such as industry, agriculture, transportation, food, etc. [37,38]. With the increase in population, the requirement of energy has also increased. Especially, the fuels play major role in the above said fields. Therefore, producing energy from biodiesel is the best way to meet out the energy requirements without affecting the ecological balance of the environment. In this context, there is a rapid growing interest on the production of biodiesel. The growing biodiesel production has made the scientific community and private sector to seek new efficient and economical technology for the energy requirements. This is the reason why the biodiesel production has
15
undergone numerous technological developments. All of them are intended to make the reaction rate faster by using lower quantity of raw materials and avoiding significant energy consumption. In the context of biodiesel production, the vigorous mixing of the reactant is most important. For instance, the conventional transesterification reaction requires a temperature of 40-65oC and vigorous stirring of the reaction mixture as to establish a maximum contact between alcohol and oil [39, 40]. Based on the above requirements, the new technologies in biodiesel production involve the use of different kind of techniques in order to optimize different reaction parameters. This kind of new technologies involves the use of auxiliary energy to mix the reactants by replacing heating. In such strategies, the hydrodynamic cavitation, ultrasound, microwaves and radio frequencies are employed [41-46]. Consequently, research and the use of these technologies have been expanded significantly in the last couple of decades, which could be evidenced by the number of papers published.
In order to improve the transesterification process, solvents are used to control the physical properties especially the viscosity of the oil. These solvents include tetrahydrofuran, hexane, diethyl ether, dibutyl ether, tert-butyl methyl ether, diisopropyl ether, etc [47-49]. It has been shown that the use of solvents improves the process conversion. However, the use of new substances can make the process even more complex and expensive. Another approach in the transesterification process involves performing the reaction under supercritical and subcritical conditions [50, 51]. In such cases, there are some advantages such as the enhancement of reaction rate, enhanced yields and improved purity of the resulting products, etc. Nevertheless, the production of biodiesel through supercritical and subcritical reactions has some disadvantages such as high energy consumption and sophisticated equipment as high temperature
16
and pressure has to be developed in the system. These requirements are relatively not feasible for the industrial scale applications [52-53].
1.7 International biodiesel regulations
The consistent global growth of biodiesel production required standardization in the quality of biodiesel. It is known that the introduction of any new product for day-to-day applications demands the recognition and surpassing of technical, economic, social and legislative hurdles. It is vital to establish rules and standards in order to define the quality of the product according to its usages in the everyday life. The postulation of quality rules must be the outcome of the sharing of information, discussion and accordance among the people involved in the production and distribution. Accordingly, the standards of biodiesel are of importance for their producers, suppliers and consumers. Therefore, the authorities should require the approved standards for the assessment of safety risks and other issues such as environmental pollution. Similarly, standards are also necessary for the vehicles that are operated using biodiesel and therefore, they are becoming the essential prerequisites for the introduction and commercialization of biodiesel in the market. The quality of biodiesel requires the inclusion of its physical and chemical properties into the requirements of the adequate standard for the utilization of biodiesel. Accordingly the quality standards of biodiesel are also continuously updated because of the evolution of the factors such as compression ignition engines, ever stricter emission standards, re-evaluation of the eligibility of feed-stocks used for biodiesel production, etc. The specifications of biodiesel technology are having a direct control over the selection of raw materials and production strategies. Regulation of the biodiesel standards started in the 1990s, as to mainly support the increasing use of alkyl esters-based biodiesel and its mixtures as automotive fuels. In the development of quality standards for biodiesel, Austria was
17
found to be pioneer in all levels. Consequently, the Austrian Standards Institute published the first quality standard for FAME from rapeseed oil (ONORM C1190) and its subsequent amendment ONORM C1191 (1996) [54]. However, this standard was not allowed for either diesel fuel-biodiesel blends or using sunflower oil as feedstock. In Germany, a pre-standard norm was developed and revealed in 1992 (DIN V 51606 for FAME, animal fats and vegetable oils). Despite this, it was only until 1997 that, the DIN E 51605 for rapeseed methyl esters and vegetable oil methyl esters was set and also limits were established for the density, kinematic viscosity and cold filter plugging point. A mandate was also given to CEN (European Committee for Standardization) by the EC to develop standards and methods applied for biodiesel production and utilization concerns [55]. In Europe, EN 14214 BD standard (based on former DIN 51606) commenced in October 2003. Previously, in November 2001, the EC released a draft proposal for a Directive of the European Parliament and of the Council on the promotion of the use of biofuels for transport [56], with a specific objective to provide the Community with a scope that would promote the use of biofuels exclusively for transport within the EU. Later, a proposal has been put forth with a commitment on Member States in 2005 to make sure that there should be a minimum of share of transport fuel sold on their territory which should be biofuels, with permission for the Member States to decide how to meet this at their best. As a result, a share of minimum 2% was proposed in 2005, which was increased by 0.75% per year up to 5.75% in 2010. The ASTM International (formerly American Society for Testing and
Materials) followed a provisional specification PS121 for biodiesel in 1999 and the first ASTM
standard (ASTM D6751) was taken up in 2002.
Among the developed standards, the European and USA standards possessed international recognition as they are conventionally the beginning point for biodiesel
18
specifications developed in other countries. In this context, there are two major specifications established the quality requirements for alkyl ester-based biodiesel; they are the ASTM D6751 in USA and the EN 14214 in Europe.
European biodiesel standards
The European standard EN 14214 is accepted and followed by all 31 member states involved in CEN. These member states are Austria, Belgium, Greece, Bulgaria, Finland, Croatia, Cyprus, Czech Republic, Denmark, Norway, Estonia, France, Germany, Hungary, Malta, Iceland, Ireland, Italy, Latvia, Slovenia, Lithuania, Luxembourg, Netherlands, Poland, Spain, Portugal, Romania, Slovakia, Sweden, Switzerland and Britain [8]. The European biodiesel specification is even more restrictive and is implemented only to mono-alkyl esters made with methanol (FAME) [57]. As per their standards, the addition of components that are not FAME (excluding the additives) is not permitted. In Europe, EN 14214 developed the specifications for FAME used as fuel for diesel engines. European standard could be used ‘unblends’ in a diesel engine (if the engine has been adapted to operate on B100) or blended with diesel fuel to produce a blend as per the EN 590, which is the European diesel fuel specification. Later, EN 14214:2012 introduced a number of modifications that includes an extension of the scope to cover heating oil applications and to cover blends up to B10. Further, an auxiliary set of climatic classes that are based on monoglycerides contents were also developed. Biodiesel/diesel fuel blends are essentially covered by EN 590. The EN 590:2004 allowed the blends up to 5% of FAME in diesel fuel, while EN 590:2009 increased the allowable FAME content up to 7%. The authentic EN 590:2013 standard does not limit the blending ratio of the paraffinic bio-component in diesel fuels. Eventually, these products obtained by the catalytic hydrogenation of vegetable oils can be
19
blended into gasoil by up to 10 % or even more as to satisfy the above EU requirements with respect to the renewable fuels utilization.
American biodiesel standards
A Task Force was formed in June 1994 within the American Society for Testing and Materials to initiate the development of “standards” for biodiesel. The first step adopted by the Task Force was the resolution of the philosophy for the standard. As per the resolution, various options were considered that included the addition of a section into the existing ASTM diesel standards (ASTM D975), development of a standard for a blend of biodiesel with petro-diesel, and even a ‘stand-alone’ standard. As a result, the following was approved by Biodiesel Task Force and subsequently by the membership of ASTM in the mid1990s.
1. To work closely and cooperatively with petroleum, engine manufacturing and biodiesel interests.
2. To establish a ‘stand-alone’ specification for straight biodiesel, B100.
3. To start with existing D975 petro-diesel specifications and the removal of items that are not applicable to biodiesel.
4. To focus the development of the standard on the end-products’ physical and chemical attributes that are needed for satisfactory operation and not either on the source of biodiesel or the manufacturing process. (This is the same philosophy adopted for the development of the USA petro-diesel requirement, ASTM D 975.)
5. To broaden it to address the biodiesel specific properties that are needed for the satisfactory engine operations.
20
Finally, ASTM D6751-03 standard specification for biodiesel fuel blend stock for distillate fuels was approved. This norm defined the biodiesel as “mono-alkyl esters of long chain fatty acids derived from vegetable oils and animal fats”. In this norm the type of alcohol used was not specified and thereby mono-alkyl esters could be produced with any alcohol (methanol, ethanol, etc.) as far as it meets the detailed needs that are outlined in the fuel specifications. Then, the ASTM D6751 standard defines two grades of biodiesel since 2012. They are (i) grade 2-B (identical to biodiesel defined by earlier versions of the standard) and (ii) grade 1-B with tighter controls on monoglycerides and cold soak filterability. In addition to this, there are two more automotive standards for biodiesel/diesel fuel blends also published by ASTM: The ASTM standard specification for diesel oil ASTM D975, which was modified in 2008, is allowing up to 5% biodiesel to be blended with the fuel and the ASTM D7467 is a specification for biodiesel blends in the range from 6% BD (B6) to 20% (B20).
1.8 Annual biodiesel production worldwide
Currently, worldwide there are number of large scale biodiesel production plants under operation. Only in America there are more than 90 biodiesel production plants in operation [15]. “Pacific Biodiesel” is one of the first biodiesel production plants in the USA in 1996 by recycling the used cooking oil in Hawaii. In 2005, worldwide biodiesel production reached around 1 million gallons and the major contributor was the European Union, (EU). Biodiesel production is increased because of two reasons (i) global warming and (ii) rising of crude oil prices [58]. It is unfortunate that the reliable data on biodiesel production is not documented until the early 90’s. However, in mid 2000s, a significant production of biodiesel was reported, where the global biodiesel production is also increasing yearly. It should be noted that the biodiesel is taking up a significant role in the modern renewable energy production and consumption. In
21
connection with the raw materials for the production of biodiesel, the rapeseed, soybean and palm oils are the preferred raw materials. According to predictions of Oil World Information Service (OWIS), which is about the increasing global production and consumption of biodiesel in 2014, reported that one-third of around 30 million t of biodiesel production comes from palm oil, followed by soybean and rapeseed oils [59].
The EU is reportedly the world topper in importing the palm oil for biodiesel production. According to the report [59], for instance, in 2013, the EU attained a record of 6.9 million t of palm oil of which 3.7 million were spent for energy production that includes biodiesel production which is 2.5 million. The data according to the OWIS report, confirmed that the major target of palm oil that enters into EU is for the energy needs. OWIS estimated nearly 9.6 million t of palm oil used for biodiesel consumption in 2014 globally [59].
In another recent report by OECD Agriculture [60], statistical data were given on the yearly production of biodiesel from vegetable oil, waste based oil and biomasses as shown in Table 1-4. Estimations of the production, consumption and price of biodiesel up to the year 2025 were also given.
22
Table 1-4. World biodiesel yearly incremental increases in average for the period from 2013-2025 [60]. Avera ge 2013-15 2016 2017 2018 2019 2020 2021 2022 2023 2 2024 2025 BD world production (bIn L) 31.1 33.2 34.5 35.3 36.7 37.9 38.8 39.6 40.2 40.8 41.4 Vegetable oil based (bIn L) 25.2 2 26.3 2 26.6 2 26.9 2 27.5 28.4 29.0 29.3 29.5 29.8 30.1 Waste oil based (bIn L) 2.4 2.9 3.4 3.7 4.2 4.4 4.7 5.1 5.4 5.8 6.0 Consumpti on(bIn L) 30.3 33.5 34.7 35.5 36.9 38.1 39.0 39.8 40.4 41.0 41.6 Price1 (USD/t) 93.9 72.1 71.9 73.7 76.8 81.5 85.9 87.3 87.1 88.4 88.4
[Note: Average 2013-15est: Data for 2015 are estimated
23
Further, Fig.1- 2 shows the world biodiesel production and international trade from 2008 to 2025, and Fig. 1-3(a)-(b) shows the prediction over the regional distributions of world biodiesel production and use in 2025, respectively.
Fig. 1- 2 World biodiesel production and international trade [61].
24
1.9 Thesis structure
This PhD thesis is divided into six chapters:
Chapter 1 describes biodiesel, production mechanisms, historical developments, regulations, worldwide production and finally thesis structure.
Chapter 2 is a literature review of ultrasound biodiesel production and objectives of the work. Chapter 3 is a publication published in International Journal of Chemical Reactor Engineering, entitled “A Parametric Study of Biodiesel Production Under Ultrasounds”.
Chapter 4 is a journal paper published in ChemistrySelect, entitled “Triglycerides Transesterification Reactions under Ultrasounds”.
Chapter 5 is a journal paper, this manuscript is will soon be submitted for publication, entitled “Ultrasound biodiesel production using various homogeneous catalysts and their separation over silica cation exchanger columns”.
Chapter 6 gives some conclusions and recommandations for future work. Chapter 7 is a list of scientific contributions by the author of this thesis.
25
1.10 References
[1] A. S. Ramadhas, S. Jayaraj, C. Muraleedharan. Use of vegetable oils as I. C.engine fuels-a review. Renew. Energy, 2004, 29,727-42.
[2] J. Sheehan, K. S, Tyson, J. Duffield, H. Shapouri, M. Graboski, V. Cambobreco, R. Conway, J. Ferrell, M. Voorhies. Life Cycle Inventory of Biodiesel and Petroleum Diesel for Use in an Urban Bus. USDA and USDOE, 1998.
[3] U. S Department of Energy, Biodiesel Handling and Use guidelines, Oak Ridge, USA, 2006. [4] G. Knothe. Historical perspectives in vegetable oils-based diesel fuels. Inform, 12, 11, 2001, 1103-1107.
[5] G. Knothe, J. V. Gerpen, J. Krahl. The Biodiesel Handbook. AOCS Publication, Peoria, Illinois, USA, 2005.
[6] A. Demirbas. Biodiesel production via rapid transesterification. Energy Sources Part A, 2008, 30, 1830-4.
[7] M. Balat. Production of biodiesel from vegetable oils: a survey. Energy Sources Part A, 2007, 29, 895-913
[8] M. Mittelbach, C. Remschmidt. Biodiesel: The comprenhensive handbook. Boersedruk Ges. M.B.H,Vienna, 2004.
[9] S. Pinzi, P. Rounce, J. M. Herreros, A. Toslakis, M. P. Dorado, The effect of biodiesel fatty acid composition on combustion and diesel engine exhaust emissions. Fuel, 2013, 104, 170-182. [10] A. Srivastava, R. Prasad. Triglycerides-based diesel fuels. Renewable & Sustainable Energy Reviews, 2000, 4, 111-133.
26
[11] H. Masjuki, M. Z. Abdulmuin, H. S. Sii. Investigations on preheated palm oil methyl esters in the diesel engine. Proceedings of the Institution of Mechanical Engineers Part a-Journal of Power and Energy, 1996, 210, 131-138.
[12] M. P. Dorado, J. M. Arnal, J. Gomez, A. Gil, F. J. Lopez. The effect of a waste vegetable oil blend with diesel fuel on engine performance. Transactions of the Asae, 2002, 45, 519-523. [13] M. Giridhar, K. Chandana, K. Rajnish. Synthesis of biodiesel in supercritical fluids. Fuel, 2004, 83, 2029-33.
[14] L. C. Meher, D. Vidya Sagar, S. N. Naik. Technical aspects of biodiesel production by transesterification - a review. Renew. Sust. Energy Rev, 2006, 10, 248-68.
[15] G. Antolin, F. V. Tinaut, Y. Briceno, Optimization of biodiesel production by sunflower oil transesterification. Bioresource technology, 2002, 83, 111-114.
[16] G. Chavanne. Procédé de Transformation d’Huiles Végétales en Vue de Leur Utilisation comme Carburants. Patent number: 422877. Universty of Brussels, Brussels, 1937.
[17] http://biodiesel.org/production/plants/plants-listing. (last accessed on 20 June 2017) [18] P. Duffy. On the Constitution of Stearine. J. Chem. Soc. 1852, 5, 303.
[19] J. Van Gerpen, B. Shanks, R. Pruzsko, D. Clements, G. Knothe. Biodiesel production technology. National Renewable Energy Laboratory, 2004.
[20] M. Takase, W. Feng, W. Wang. Silybum marianum oil as a new potential non-edible feedstock conventional and ultrasonic assisted method. Fuel Processing Technology, 2014, 123, 19-26.
27
[21] C. Brunschwig, W. Moussavou, J. Blin. Use of bioethanol for biodiesel production. Progress in Energy and Combustion Science, 2012, 38, 283-301.
[22] M. P. Dorado, E. Ballesteros, J. A. De Almeida, C. Schellert, H. P. Lohrlein, R. Krause. An alkali-catalyzed transesterification process for high free fatty acid waste oils. Transactions of the ASAE, 2002, 45, 525-529.
[23] B. H. Samani, H. Zareiforoush, Z. Lorigooini. Ultrasonic-assisted production of biodiesel from Pistacia Atlantica Desf.oil, Fuel, 2016, 168, 22-26.
[24] A. A. Koutsoiki, E. Tegou, S. Kontakos. In situ transesterification of Cynara Cardunculus L. seed oil via direct ultrasonication for the production of biodiesel. Fuel Processing Technology, 2015, 134,122-129.
[25] M. Mostafaei, B. Ghobadian, M. Barzegar. Optimization of ultrasonic assisted continuous production of biodiesel using response surface methodology. Ultrasonics Sonochemistry, 2015, 27, 54-61.
[26] H. D. Hanh, N. T. Dong, K. Okitsu. Biodiesel production by esterification of oleic acid with short-chain alcohols under ultrasonic irradiation condition. Renewable Energy, 2009, 34, (3) 780-783.
[27] T. Le Tu, K. Okitsu, B. Luu Van, Y. Maeda. Catalytic Technologies for Biodiesel Fuel Production and Utilization of Glycerol: A Review. Catalysts, 2012, 2, 191-222.
[28] M. Farooq, A. Ramli. Biodiesel production from low FFA waste cooking oil using heterogeneous catalyst derived from chicken bones. Renewable Energy, 2015, 76, 362-368.
28
[29] S. M. Coman, V. I. Parvulescu. Chapter 4-Heterogeneous Catalysis for Biodiesel Production. The Role of Catalysis for the Sustainable Production of Bio-fuels and Bio-chemicals. Elsevier; Amsterdam, 2013.
[30] A. F. Lee, J. A. Bennett, J. C. Manayil, K. Wilson. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chemical Society Reviews, 2014, 43, 7887-7916.
[31] A. Bajaj, P. Lohan, P. N. Jha, R. Mehrotra. Biodiesel production through lipase catalyzed transesterification: An overview. Journal of Molecular Catalysis B: Enzymatic, 2010, 62, 9-14. [32] F. Gunstone. Fatty Acid and Lipid Chemistry. Blackie: London, 1996.
[33] N. U. Soriano, V. P. Migo, K Sato, M. Matsumura. Crystallization behavior of neat biodiesel and biodiesel treated with ozonized vegetable oil. Eur J Lipid Sci Technol., 2005, 107, 689–96.
[34] R. M Joshi, M. Pegg. Flow properties of biodiesel fuel blends at low temperatures. Fuel, 2007, 86, 143–51.
[35] M. Ahmad, S. Rashid, A. K. Ajab, M. Zafar, S. Sultana, S. Gulzar. Optimization of base catalyzed transesterification of peanut oil biodiesel. Afr J Biotechnol, 2009, 8, 441–6.
[36] M. Lapuerta, J. R. Fernandez, E. F. de Mora. Correlation for the estimation of the cetane number of biodiesel fuels and implications on the iodine number. Energy Policy, 2009, 37; 4337–44.
[37] C. C. Enweremadu, M. M. Marques. Technical aspects of production and analysis of biodiesel from used cooking oil-A Review. Renewable Sustainable Energy Rev., 2009, 13, 2205-2224.
29
[38] A. S. Silitonga, A. E. Atabani, T. M. I. Mahlia, H. H. Masjuki, I. A. Badruddin and S. Mekhilef. A Review on Prospect of jatropha curcas for biodiesel in Indonesia. Renewable Sustainable Energy Rev., 2011, 15, 3733-3756.
[39] M. P. Dorado, E. Ballesteros, M. Mittelbach, F. J. Lopez. Kinetic parameters affecting the alkali-catalyzed transesterification process of used olive oil. Energy and Fuels, 2004, 18, 1457-1462.
[40] D. E. Leiva-Candia, M. F. Ruz-Ruiz, S. Pinzi, J. R. Garcia-Ruiz, J. Dominguez, I. L. Garcia, M. P. Dorado. Influence of nitrogen fertilization on physical and chemical properties of fatty acid methyl esters from Brassica napus oil. Fuel, 2013, 111,865-871.
[41] R. Gordon, I. Gorodnitsky, V. Grichko, (Cavitation Technologies, Inc., Chatsworth, CA, (US)), 8, 981, 135 B2, 2015.
[42] J. Hernando, P. Leton, M. P. Matia, J. L. Novella, J. Alvarez-Builla. Biodiesel and FAME synthesis assisted by microwaves: Homogeneous batch and flow processes. Fuel, 2007, 86, 1641-1644.
[43] S. Liu, Y. Wang, T. Mcdonald, S. E. Taylor. Efficient production of biodiesel using radio frequency heating. Energy & Fuels, 2008, 22, 2116-2120.
[44] N. Azcan, A. Danisman. Microwave assisted transesterification of rapeseed oil. Fuel, 2008, 87, 1781-1788.
[45] F. Motasemi, F. N. Ani. Microwave irradiation biodiesel processing of waste cooking oil.
30
[46] V. B. Veljkovic, J. M. Avramovic, O. S. Stamenkovic. Biodiesel production by ultrasound-assisted transesterification: State of the art and the perspectives. Renewable & Sustainable Energy Reviews, 2012, 16, 1193-1209.
[47] R. Sawangkeaw, K. Bunyakiat, S. Ngamprasersith. Effect of co-solvents on production of biodiesel via transesterification in supercritical methanol. Green Chemistry, 2007, 9, 679-685. [48] Y. Alhassan, N. Kumar, IM. Bugaje, H. S. Pali, P. Kathkar. Co-solvents transesterification of cotton seed oil into biodiesel: Effects of reaction conditions on quality of fatty acids methyl esters. Energy Conversion and Management, 2014, 84, 640-648.
[49] J. M. Encinar, J. F. Gonzalez, A. Pardal, G. Martinez. Transesterification of rapeseed oil with methanol in the presence of various co-solvents. Third International Symposium on Energy from Biomass and Waste. 2010.
[50] S. Saka, Y. Isayama, Z. Ilham, J. Y. Xin. New process for catalyst-free biodiesel production using subcritical acetic acid and supercritical methanol. Fuel, 2010, 89, 1442-1446.
[51] S. A. Biktashev, R. A. Usmanov, R. R. Gabitov, R. A. Gazizov, F. M. Gumerov, F. R. Gabitov, I. M. Abulagatov, R. S. Yarullin, I. A. Yakushev. Transesterification of rapeseed and palm oils in supercritical methanol and ethanol. Biomass & Bioenergy, 2011,35, 2999- 3011. [52] J. M. Bernal, P. Lozano, E. Garcia-Verdugo, M. Isabel Burguete, G. Sanchezgomez, G. Lopez-Lopez, M. Pucheault, M. Vaultier, S. V. Luis. Supercritical Synthesis of Biodiesel. Molecules, 2012, 17, 8696-8719.
[53] S. Glisic, D. Skala. The problems in design and detailed analyses of energy consumption for biodiesel synthesis at supercritical conditions. The Journal of Supercritical Fluids, 2009, 49 293-301.
31
[54] W. Korbitz. Biodiesel production in Europe and North America, an encouraging prospect. Renewable Energy, 1999, 16, 1078-1083.
[55] M/245 Mandate to CEN for the elaboration and adoption of standards concerning minimum requirement specifications including test methods for fatty acid methyl esters (FAME) as fuel for diesel engines and for space heating.1997.
[56] Directive of the European Parliament and of the Council on the promotion of the use of biofuels for transport COM 547 final. 2001.
[57] G. Knothe. Analyzing biodiesel: Standards and other methods. Journal of the American Oil Chemists Society, 2006, 83, 823-833.
[58] T. Serra, J. M. Gil. Biodiesel as a motor fuel price stabilization mechanism. Energy Policy, 2012, 50, 689-698.
[59] http://www.oilworld.biz (last accessed on 2015)
[60] http://dx.doi.org/10.1787/888933382219 (last accessed on 20 June 2017) [61] http://dx.doi.org/10.1787/888933382050 (last accessed on 20 June 2017) [62] http://dx.doi.org/10.1787/888933382060 (last accessed on 20 June 2017)
32
33
2.1 Biodiesel production under ultrasound
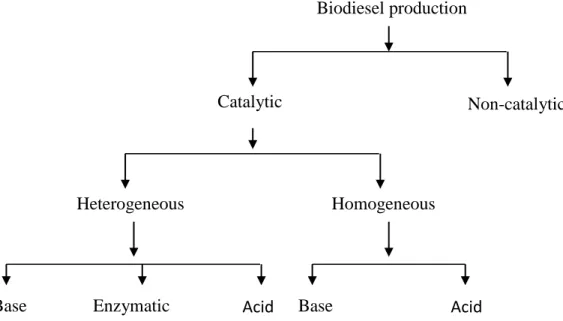
The most common method for biodiesel production is the transesterification process, where the triglycerides (TG) are gradually converted through two intermediates i.e diglycerides and monoglycerides, into three molecules of fatty acid methyl ester (FAME), which is known as biodiesel, and one molecule of glycerol [1]. For FAME production proper mixing is very much important to establish the sufficient contact between the two phases of oil or animal fat and alcohol. For this, ultrasonication helps to increase the liquid–liquid interfacial area through emulsification, which is important for the formation of vapor bubbles and cavitation bubbles in viscous liquids, such as plant oils and animal fats. Vapor bubbles within the liquid, such as methanol bubbles generated mechanically or ultrasonically in liquid oils or fats, oscillate and move with the steady currents in the bulk liquid caused by the high frequency acoustic oscillations or acoustic streaming. This phenomenon enhances the mass transfer across the interfaces of the bubbles and, thus, accelerates the chemical reaction rates under diffusion limited conditions such as the early stage of transesterification of oils and fats in biodiesel production. Therefore, it is more crucial to explore this technique for large scale biodiesel production.
Consequently, there are several publications on FAME production available in the current literature. Most of this works are focused on the laboratory scale [2]. However, few articles describe the production of FAME in a large scale. Carlini et al. [3] in their pilot study investigated the operating conditions for biodiesel production from waste cooking oils obtained from households with an acid value of 2.12 mg KOH g-1. Their work was focused on the comparison of catalyst type i.e H2SO4 and NaOH, at different concentrations. The best reaction
conditions with the highest FAME yield (94.3 %) were obtained using 0.5 % of NaOH and excess methanol. Da Cunha et al [4] reported the biodiesel production from a pilot plant using
![Table 1-2 Comparison of the properties of biodiesel and diesel [3].](https://thumb-eu.123doks.com/thumbv2/123doknet/5530742.132193/23.918.205.714.149.1047/table-comparison-properties-biodiesel-diesel.webp)
![Table 1-4. World biodiesel yearly incremental increases in average for the period from 2013-2025 [60]](https://thumb-eu.123doks.com/thumbv2/123doknet/5530742.132193/41.918.104.823.195.875/table-world-biodiesel-yearly-incremental-increases-average-period.webp)
![Fig. 1- 2 World biodiesel production and international trade [61].](https://thumb-eu.123doks.com/thumbv2/123doknet/5530742.132193/42.918.118.764.246.528/fig-world-biodiesel-production-international-trade.webp)
![Fig. 2-2 Growth and collapse of cavitation bubble in a liquid medium when ultrasonic waves are applied [52]](https://thumb-eu.123doks.com/thumbv2/123doknet/5530742.132193/65.918.239.738.120.442/growth-collapse-cavitation-bubble-liquid-medium-ultrasonic-applied.webp)