O
pen
A
rchive
T
OULOUSE
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
This is an author-deposited version published in :
http://oatao.univ-toulouse.fr/
Eprints ID : 16786
To link to this article : DOI : 10.1016/j.colsurfa.2015.01.015
URL :
http://dx.doi.org/10.1016/j.colsurfa.2015.01.015
To cite this version :
Raiah, Kenza and Djalab, Abdelkader and
Hadj-Ziane-Zafour, Amel and Soula, Brigitte and Galibert,
Anne-Marie and Flahaut, Emmanuel Influence of the hydrocarbon chain
length of imidazolium-based ionic liquid on the dispersion and
stabilization of double-walled carbon nanotubes in water. (2015)
Colloids and Surfaces A: Physicochemical and Engineering Aspects,
vol. 469. pp. 107-116. ISSN 0927-7757
Any correspondence concerning this service should be sent to the repository
administrator:
staff-oatao@listes-diff.inp-toulouse.fr
Influence
of
the
hydrocarbon
chain
length
of
imidazolium-based
ionic
liquid
on
the
dispersion
and
stabilization
of
double-walled
carbon
nanotubes
in
water
Kenza
Raiah
a,
Abdelkader
Djalab
a,
Amel
Hadj-Ziane-Zafour
a,
Brigitte
Soula
b,
Anne-Marie
Galibert
b,
Emmanuel
Flahaut
b,c,∗aLaboratoiredeGénieChimique,UniversitéSaadDahlab,RoutedeSoumaa,B.P.270Blida,Algeria
bUniversitédeToulouse,UPS,INP,InstitutCarnotCirimat,118,RoutedeNarbonne,F-31062Toulousecedex9,France cCNRS,InstitutCarnotCirimat,F-31062Toulouse,France
h
i
g
h
l
i
g
h
t
s
•Synthesisofimidazolium-basedionic
liquids from natural compounds
(fattyacids).
•Stabilization of 50mg/L double
walledcarbonnanotubesinwaterup
to20days.
•Dispersibilityincreasedwith
increas-ing the length of the hydrocarbon
chain.
g
r
a
p
h
i
c
a
l
a
b
s
t
r
a
c
t
Keywords:
Carbonnanotubes
Imidazolium-basedionicliquids Aqueoussuspensions Stabilization
a
b
s
t
r
a
c
t
Imidazolium-based ionic liquids with a long hydrocarbon chain
1-methyl-1-ethanol-2-alkyl-imidazoliumiodide([M-E-Cn-Im]I,n=13,15and17)wereusedforthedispersionofDWCNTsinwater.
DWCNTssuspensionsobtainedwerestableformorethanamonth,andnosedimentationwasobserved.
Thestabilityofthesuspensionswasinvestigated(measurementofopticaldensity,zetapotential,
parti-clesize,viscosity,andTEMimages).MonitoringoftheabsorbancebyUV/visspectrophotometryfor20
daysshowedthatatlowconcentration(1mM),thebestsuspensionwasobtainedwiththeionicliquid
([M-E-C15-Im]I).Athigherconcentration(10mM),thedispersionefficiencyincreasedwiththelengthof
thehydrocarbonchain.Thiscouldbeexplainedbythehydrophobicinteractionbetweenthehydrophobic
moietiesoftheionicliquidandtheCNTs.Therefore,wewereabletostabilizeDWCNTsusingalow
con-centration(1mM)ofimidazolium-basedionicliquidssynthesizedfromnaturalcompounds.Thiswork
highlightsthepotentialofimidazolium-basedionicliquidsforthepreparationofaqueoussuspensions
ofDWCNTsathighconcentrationwithalimitedamountofaddedsurfactant(50mg/LofDWCNTswith
50mg/Lofionicliquid).
∗ Correspondingauthorat:CNRS,InstitutCarnotCirimat,F-31062Toulouse, France.Tel.:+33561556280.
E-mailaddress:flahaut@chimie.ups-tlse.fr(E.Flahaut).
1. Introduction
Carbonnanotubes(CNTs),describedassuchforthefirsttime in 1991, are still of great interest for thescientific community duetotheirexceptionalintrinsicproperties(electrical,mechanical andthermal),openingaverywidefieldofapplicationsforthese
nanomaterials [1–3]. Though carbonnanotubes have promising applications, their insolubility in water and most organic sol-ventsischallenging.Thisinsolubilityisexplainedbybothintrinsic hydrophobicityandtheirstrongagglomerationcausedbyvander Waalsinteractionsandentanglement[4].Severalstudieshavetried tosolvethisproblembycovalentfunctionalizationmethods[5–7]
orbyadsorptionofamphiphilicagents[8–12].Covalent function-alizationusingoxidizingacidsathightemperaturecausesdefects onthewallsofthecarbonnanotubesandtheirlengthdecreases, thereforetheirelectricalandmechanicalpropertiesaredegraded
[13,14].Non-covalentfunctionalizationbyadsorptionofsurfactant orpolymersanduseofmildultrasonictreatments,centrifugation andfiltrationdoesnotdisturbthep–pstackingorthelengthsof CNTs,thus,maintainingintrinsicpropertiesoftheCNTs[15–17].
Differentkindsofsurfactantshavebeenusedsuccessfullyfor thedispersionofCNTs,suchastheanionicSDS(sodiumdodecyl sulphate)[18,19],cationicCTAB(cetyltrimethylammonium bro-mide)[20,21]andnon-ionicTritonX-100[22].Howeverthenature ofthesurfactantanditsconcentrationhaveanimportantroleinthe dispersionofCNTsinwaterandorganicmedia[23,24].Previous workhasshownthationicliquidshaveabetterdispersing effi-ciencycomparedtoconventionalsurfactants[25–28].Somestudies have been conducted on imidazolium-based ionic liquids: Kim etal.[29]preparedstablesuspensionsusinganimidazolium-based ionicliquid(1-butyl-3-methylimidazoliumtetrafluoroborate)and SWCNTs.SWCNTssuspendedbyimidazolium-basedionicliquid formagel,calledBuckygel[30].Non-covalentfunctionalization ofoxidizedsingle-walledcarbonnanotubesbypoly imidazolium-basedionicliquidswasalsousedfor thesynthesis ofgels[31]. SWCNTsdispersedin imidazolium-basedionicliquids[32]have beenusedascathodeinhigh-powerlithiumbatteries.AMWCNTs suspensionwith1-ethyl-3methylimidazoliumtetrafluoroboratein dimethlformamidewasusedas animmunosensorof myeloper-oxidasedetectioninhumanserum[33].Amagneticallysensitive materialwaspreparedwithSWCNTsandamagneticionicliquid (1-butyl-3-methylimdazolium[FeCl4]),whichfindsapplicationsin
biomedicalresearch(suchastargeteddrugdeliveryandcontrolled release,proteinseparation,cancertreatment)[34].Amethodwas developedtodecorateMWCNTswithnoblemetalparticles(gold) by themeansof ionicliquid 1-hexadecyl-3-methylimidazolium bromide[27].Yang etal.[35] performeda quantitative charac-terizationbyUV/visspectrophotometryofSWCNTssuspensions prepared inionic liquid 1-N-butylmethylimidazolium hexafluo-rophosphate.
DiCrescenzoetal.[26]haveshownthattheionicliquidwith alonghydrocarbonchain1-hexadecyl-3-vinyl-imidazolium bro-mide formed a stable homogeneous aqueous dispersions with SWCNTsabove its critical micelleconcentration. Liu et al. [25]
reportedthattheyobtainedstableaqueoussuspensionsof MWC-NTswithionicliquid-typeGeminiimidazolium([Cn-C4-Cn-Im]Br2, n=12,14)evenatlowconcentration(1mM).Thebinarymixture of 1-tetradecyl-3-methylimidazolium chloride/ethylammonium nitratesuccessfullydispersedMWCNTs[36].Theionicliquidwith polycyclicaromaticfractionandalonghydrocarbonchain– [n-(N-carbazole)alkyl]-3-methylimidazoliumbromideyieldedgood CNTssuspensionsinwater(10months,at1mM),showingthe com-binedeffectsofbothahydrocarbonchainandacarbazolemoiety
[37].Theadsorptionoftheimidazolium-basedionicliquidonto SWCNTswasexplainedbytheirinteractionwithlowvanderWalls forces. Therefore,the electronicpropertiesof SWCNTremained unmodified[38].Madriaetal.[39]haveshownthat 1-methyl-3-alkoxyalkyland1-methyl-3-fluoroalkylimidazoliumarerelatively nontoxictohumanhealth.Riduanetal.[40]investigatedtheuse ofimidazoliumsaltsinseveralareasofbio-applications,including antitumor, antimicrobial,antioxidant and bioengineering. Imid-azolium ionic liquids can be regarded as chemical compounds
exhibitingapotential,slighttoxicitytothegrowthand develop-mentoftheearlydevelopmentalstagesofhigherlandplants[41]. Ionicliquidsproducedfromthebiocompoundscholineandamino acidswerefoundtohavelowtoxicitytohumansandtothe envi-ronment[42].
Inthiscontext,thegoalofthisworkwastodisperseand sta-bilize DWCNTsin water, using imidazolium-basedionic liquids synthesizedfromnaturalmaterialnamelyfattyacids.We investi-gatedtheabilityof1-methyl-1-ethanol-2-alkyl-imidazolineiodide todispersetheDWCNT,aswellastheeffectofthelengthofthe hydrocarbonchainandconcentration.Inordertocomparethe effi-ciencyof thethree synthesizedionic liquidswe monitoredthe concentrationofCNTsversustime(opticaldensitymeasurements); wemeasuredthevaluesofzetapotentialandviscosityandalso used staticlight diffusiontoestimatethesize ofagglomerates. Finally,transmissionelectronmicroscopyobservationswerealso performedinordertoevidencede-bundlinginthepresenceof sur-factant.
2. Materialsandmethods 2.1. Materials
Double-walledcarbonnanotubes(DWCNT)weresynthesized attheCIRIMATby catalyticchemical vapourdeposition(CCVD)
[43].AfterextractionbytreatmentwithaconcentratedHCl aque-oussolution(removalofcatalystsupportandunprotectedmetal nanoparticles),theCNTwererecovered,washedandkeptinasmall volumeofdeionizedwater.TheDWCNTthuspreparedarecalled “rawwettubes”.Myristicacid(99%),palmiticacid(99%)andstearic acid(99%),N(2-hydroxyethyl)ethylenediamine,ethyliodide,were purchasedfromSigma–Aldrich.
2.2. Synthesisandcharacterizationofionicliquids
Imidazolium-basedionicliquids([M-E-Cn-Im]I,n=13,15or17)
weresynthesizedbythereactionbetweenthecorresponding nat-uralfattyacidwithN(2-hydroxyethyl)ethylenediamine(Fig.1). Allthereagentswereinthesamemolarratio.Themixturewas heatedintoluenefor8hwithstirringandthewaterformedwas removedazeotropicallyusingaDean–Starkapparatus.Resulting productwascooledandfilteredandthesolventevaporated.The solidwasprecipitatedbyadding1-butanolandthenrecrystallized fromethanol.Theprecipitatedsolidandethyliodidewereheated byrefluxinginisopropanolfor4h,theresultingproductwas fil-teredandthesolventevaporated.Thesolid wasprecipitatedby addingpetroleumether[44,45].Weobtainedagoodyield(91.5%)in agreementwiththosealreadypublished((89%)[46],(92.1%)[44]). Thecharacterizationofthesynthesizedproductswascarriedoutby FT-IR,GC–MS,1HNMRand13CNMRanalysis,andbymeasurement
ofCMC(criticalmicelleconcentration).
TheFouriertransmissioninfra-red(FT-IR)measurementswere madeonPerkinElmerSpectrometeroperatingwithUATR (Univer-salattenuationtotalreflectance),spectralrangebetween4000and 650cm−1,at4cm−1ofresolution.Thegaschromatographycoupled
withmassspectrometry(GC–MS)analysiswasperformedwitha PerkinElmerClarus500,RTX-5MSequippedwithacapillaryGC column(60m,0.25mm,0.25mm),andthesystemwasheatedfrom 100to290◦Cwithaheatingrateof8◦C/minandkeptat290◦Cfor
10min.Thetemperatureoftheinjectoranddetectorwere250◦C
and200◦C,respectively.Thecharacterizationsby1HNMRand13C
NMRwerecarriedoutusingaBrükeradvance(500MHz)equipped with a TCI probe type (Triple CryoprobeInverse); the samples weredissolvedinCDCl3.Thedeterminationofthecriticalmicellar
Fig.1. Generalschemeforthesynthesisreactionsoftheionicliquids.
concentration (CMC) was performed by conductivity measure-ments(Hannainstruments,EC215).
TheFT-IRspectraofthethreeionicliquidsareshowninFig.2. Thepresenceofthebandat2922cm−1 correspondstoCH3 and
CH2groups.Thepresenceofastrongbandat3392cm−1indicates
theformationofprimaryamine.Inaddition,theappearanceofa bandat1642cm−1indicatestheformationoftheamide(C N)and
isanevidenceoftheformationoftheimidazolinering[46].Mass spectradatawereanalysedusingtheNISTMSSearchlibrary,and thefollowingspectralprofileswereobtainedwithMS(m/z%):74 (99),84(92),87(77),43(61),56(58),44(45),128(43),41(39),55 (32)and141(26)(Fig.3)[46].
Thecharacterizationby1H NMRhasgiventhespectrashow
in Fig. 4. Identification of the peaks is: ı 3.04ppm (t, 2H, C N C CH2 N ),ı3.38ppm(t,2H, CH2 C OH),ı3.71ppm
(t,2H, C N CH2 ),ı2.21ppm(t,2H, N C CH2 ).InFig.5are
shown the13CNMR spectra,indicatingthepresenceofpeaksı
173.80ppm( N C N )andı60.48ppm( C OH).Themain dif-ferencebetweenthethreespectraisaround3ppmandcorresponds toimpurities(residuesofreactants);thesignalsexpectedforour compoundsarewellidentified,although1HNMRdoesnotgiveany
informationaboutthealiphaticchainlength.
Fig.6showsthevariationoftheelectricalconductivityasa func-tionoftheconcentrationforthethreeionicliquids.Thesecurves allowedustodeterminethevaluesoftheCMCforeachionicliquid. TheCMCwascloseto0.8mMforthethreecompounds(theCMC istheconcentrationatwhichtheslopechanges,itwasdetermined usingthefirstderivative).
2.3. Dispersionofcarbonnanotubes
DWCNTsuspensionswerepreparedindeionizedwateralone. Foreachionicliquid,astocksolutionwasalsopreparedin deion-izedwater,undermagneticstirringfor4h,inordertoensurethe dissolutionofeachsurfactant.Themixtureofthetwostock dis-persion/solution(DWCNTandsurfactant)wasdesignedtoobtain 10mlsuspensionsofDWCNTataconcentrationof50mg/L,and thethreeionicliquidsatconcentrationsequalto1,1.5and10mM. DWCNTsuspensions obtainedweremixedinanultrasonicbath (BioblockT570,35KHz,160W)for10min,thenusinganultrasonic probe(VibraCellModel75042,20kHz,500W)for30minat25% amplitudewithapulseof5sonand5soff,inordertoavoid over-heating.Ourchoiceofahighconcentrationof50mg/Lisjustified byfurtherworkrelatedtotheenvironmentalimpactassessmentof suchsuspensionswhichwillberequiredinordertocomparethe performanceoftheseImidazolium-baseddispersingagentswith
ourearlierworkwithDWCNTsinvolvingcellulosederivativessuch ascarboxymethylcellulose[47,48]orxanthanderivatives[17]. 2.4. Characterizationofsuspensions
DWCNTsuspensionswerecharacterizedbyUV/visabsorption (Varian,Cary300).Theabsorbancemeasurementswereperformed from400to900nm,immediatelyafterpreparationandfollowing 20-day sedimentation. Zetapotential measurements were per-formedwithzetasizer (ModelZEN3691,MalvernInstruments), usingsuspensionscentrifugedat1600rpmfor30min.The super-natant wasseparated andthen lefttosettledown for24h.No sedimentationcouldbeobservedwithin24h.Therateofviscosity versusshearratewasdeterminedwitharheometer(AntonPaar Physica,Germany),operatingwithaconcentriccylinder,and con-nectedtoawaterbathinordertoensureaconstanttemperature of25◦C.Sizemeasurementsofdispersedcolloidswereperformed
bythetechnique ofstaticlight diffusion(SZ-100,Horiba Scien-tific),thesizesweremeasuredfor48hatregulartimeintervals. DWCNTsuspensionswerealsocharacterizedbytransmission elec-tronmicroscopy(JEOL1400TEM),operatedat120kV.Thesamples werepreparedbypipettinganddroppingafewdropsof suspen-sionon400meshLaceyCarboncoppergrids,purchasedfromAgar Scientific.
3. Resultsanddiscussion
Characterization of DWCNT suspensions was performed by UV/vis spectrophotometry. Although individual CNTs may be UV–visactiveinthemeasurementrange(400–900nm),thereis noabsorptionbandforbundledCNTs[12,25].Fig.7representsthe absorbanceoftheDWCNTsuspensionsat50mg/Lwiththethree ionicliquids([M-E-Cn-Im]I,n=13,15and17)ataconcentrationof
10mM,aswellaspreparedindeionizedwateronly(nosurfactant). Althoughwedidnotdeserveanymaximumabsorbanceband cor-respondingtoCNTs,theeffectoftheadditionofasurfactantwas ratherclearintermsofincreasedabsorbance.Astheionicliquids donothaveanyabsorptionbandwithintheanalysedrange,the absorptioncanonlybeattributedtoCNTs.
Thevariationoftheabsorbanceasafunctionoftime(20days) fortheDWCNTsuspensionspreparedwiththethreeionicliquids atamolarconcentrationof1mM,1.5mMand10mMisshownin
Fig.8.Theabsorbancemeasurementswereperformedat500nm
[22,49].Accordingtoourdata,at1and1.5mMconcentrations,we obtainedanalmostidenticalabsorbancewiththetwoionicliquids [M-E-C15-Im]Iand[M-E-C17-Im]I.Thelowconcentrations(1mM
Fig.2.FT-IRspectraof(A)[M-E-C13-Im]I,(B)[M-E-C15-Im]Iand(C)[M-E-C17-Im]I.
Fig.3.Massspectraof(a)[M-E-C15-Im]I,comparedto(b)datafromNISTMSSearchlibrary(asanexample).
Fig.5.13CNMRspectraof[M-E-C
15-Im]I(asanexample).
Fig.6. Variationoftheelectricalconductivityasafunctionoftheconcentrationofionicliquid.
Fig.7.UV/visspectraofionicliquids[M-E-C13-Im]I,[M-E-C15-Im]I,[M-E-C17-Im]Iat10mMandDWCNTsuspensionswithionicliquids[M-E-C13-Im]I,[M-E-C15-Im]I,
Fig.8. UV/visabsorbanceintensityversustimeofDWCNTs(50mg/L)inionicliquids(a)[M-E-C13-Im]I,(b)[M-E-C15-Im]I,(c)[M-E-C17-Im]Iand(d)deionizedwateralone
ataconcentrationof(A)10mM,(B)1.5mMand(C)1mM,upto20days.
and1.5mM)arejust abovethecritical micelleconcentrationof thethreeionicliquids(CMCisequaltoca.0.8mM,evaluatedfrom electricalconductivitymeasurements)anddidnotleadtogood dis-persionandstability.Thisphenomenoncanbeexplainedbythefact thatthedispersionofthenanotubeswiththeamphiphilesisonly
possibleabovethelimitoftheCMC[50,51].Thus,thedispersing poweroftheionicliquidsclosetotheCMCwaslowcomparedto theconcentrationof10mM(10timesmore).
Attheconcentrationof10mMofionicliquid,theabsorbance of suspensions increased with increasing the length of the
Fig.9. Variationoftheviscosityversusshearrateforthethreeionicliquidsof1mMandDWCNT(50mg/L)suspensionswiththethreeionicliquids.
hydrophobicchain. Thebestvalue wasobtainedwiththeionic liquid[M-E-C17-Im]I.Similarlytheabsorbanceofthesuspension
preparedwith[ME-C15-Im]Iwashigherthantheoneprepared with[ME-C13-Im]I.Thethree ionicliquidshavea homologous structure.Theonlydifferenceliesinthelengthofthehydrocarbon chain(C=13,15and17).Theincreaseindispersingefficiencywith increasingthelengthofthehydrocarbonchaincanbeexplained bytheincreaseinhydrophobicinteractionbetweenthe hydrocar-bonchainsofionicliquidsandDWCNTs[25,52](generally,because CNTshaveaveryhydrophobicsurface,thehydrocarbonchainstend tobestronglyadsorbed[53,54]).Abetterinterfacebetweenthe hydrophobicchainandthenanotubesmeansahigherstabilityof theadsorptionandlesspossibleexchangeswiththesurrounding solvent.Inthiscomparison,thereisprobablynomodificationof thesterichindranceduetothesmalldifferencesintermsofcarbon atomsinthealkylchain.Takingintoaccountthefactthatthecharge iscomingfromexactlythesamechemicalgroupfortheseriesof ionicliquids,theelectrostaticrepulsionisnotexpectedtobe differ-ent.Ifthedifferenceinchainlengthwasenoughtoleadtodifferent ratesofcoverageontothenanotubes,thisshouldfinallyleadtoa
differentnumberofchargesandthiswouldfinallybeinfavourof theshorteralkylchainionicliquid(C13).Consequently,weassume thatthekeyparameteristhestabilityoftheinteractionbetween theadsorbedionicliquidandthenanotubes,whichisinfavourof thelongestalkylchain.
Themeasurementofzetapotentialisimportanttoexplainthe stabilityofthecolloidalparticlesinaqueoussuspension[28,55].WE measuredthezetapotentialofDWCNTsuspensionusingthethree ionicliquidsattheconcentrationof1mMandobtainedzeta poten-tialvalues largerthan40mV.AccordingtoDong etal.[37] the particleswithazetapotentiallargerthan15mVinabsolutevalue areconsideredtobestabilizedbyelectrostaticrepulsions.Ahigh positivevalueofzetapotentialindicatesthestrongadsorptionof theionicliquidtothesurfaceofDWCNTs,leadingtostable suspen-sionsduetoCoulombforcebetweentheCNTs[25].Therefore,ahigh stabilityofDWCNTsuspensionswasevidenced.Table1illustrates thevaluesofzetapotentialoftheDWCNTsuspensionswiththe threeionicliquids.Thelargestvalueofzetapotentialwasobtained withtheDWCNTssuspensionpreparedwiththeionicliquid [M-E-C15-Im]I,althoughitisquitesimilartothevalueobtainedfor
Fig.11.TEMmicrographofDWCNTsuspensionsindeionizedwaterat50mg/Lwithionicliquidat1mM(a)[M-E-C13-Im]I,(b)[M-E-C15-Im]I,(c)[M-E-C17-Im]Iand(d)
deionizedwateralone.Thescalebar(50nm)isidenticalforallfourimages(samemagnification).
Table1
ZetapotentialofDWCNTsuspensionsforthethreeionicliquids(1mM).
DWCNTsuspensionswithionicliquid Zetapotential(mV)
[M-E-C17-Im]I 60.6
[M-E-C15-Im]I 66.0
[M-E-C13-Im]I 45.0
[M-E-C17-Im]I.Thesevaluesarehoweversignificantlyhigherthan thezetapotentialmeasuredfor[M-E-C13-Im]Iinthesame
exper-imentalconditions.
Wewereabletostabilize50mg/LofDWCNTsusinga50mg/L ofimidazolium-basedionicliquidscomparedtousing1000mg/L ofionicliquid-typeGeminiimidazoliumforsuspending200mg/L ofMWCNTs[25].
Thevariationof theviscosityversus shearrateforthethree ionicliquidsandtheDWCNTsuspensionswiththethreeionic liq-uidsisshowninFig.9.Wehaveobservedalowviscosityofthe DWCNTsuspensionswiththethreeionicliquids;thislowviscosity canbeexplainedbybetterchargerepulsionbetweenhydrophobic groups(thehydrocarbonchains)orientedalongCNTs,whenthe hydrophilicheadsaredirectedtowardstheaqueousphase,which preventstheagglomerationoftheCNT[17,56,57].
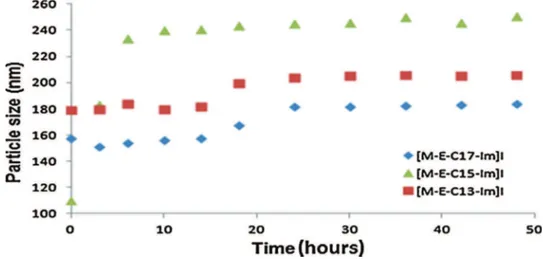
InordertodemonstratethestabilitythroughtimeoftheDWCNT suspensions,sizemeasurementsofdispersedcolloidswere per-formedbythetechniqueofstaticlightdiffusion.Forthispurpose, suspensionsof carbonnanotubeswerepreparedwiththethree
ionicliquidsat1mMasstabilizers.Thesizesweremeasuredfor 48hatregulartimeintervals(Fig.10).
Measurementsofparticlesizeforthethreesuspensionsof DWC-NTswitheachionicliquidclearlyshowthatCNTsareintheform ofverysmallagglomerateswithanaveragediameterbetween150 and250nm.Wemeasuredmonomodalparticlesizedistributions andapolydispersityindexof0.068(thevalueofthisindexgives information about monodisperse systems, for which the index shouldbebetween0.05 and0.08).Thesizeoftheagglomerates inthesuspensions ofDWCNTswaspracticallystableduringthe 48hofmeasurementandespeciallyafter20h.
Transmissionelectronmicroscope(JEOL1400)imagesof DWC-NTssuspensionspreparedwiththreeionicliquids,aswellasthe onespreparedwithdeionizedwateralone,areshowninFig.11. These images showthat thethree DWCNTs samplesstabilized byionicliquidsformedsmallerbundlescomparedtoDWCNTsin deionizedwateralone.Therefore,theuseofionicliquidslimited theagglomerationofDWCNTs.However,it isimportanttonote thatTEMobservationswereperformedafterdryingthesamples onTEMgrids,sotheimagesareprobablynotshowingthereal sit-uationinthesuspensionsinthepresenceofliquid(agglomeration upondryingduringsamplepreparation).
Finally,DWCNTshaveamorphologyveryclosetoSWCNTsand sharewiththemtheabilitytofromlongandflexiblebundles.In ourDWCNTssamples,thebundles are notvery large(typically 20–30nmmaximum)andthesamplescontainmanyindividual(or atleastindividualpartoftheirlength).However,theyarethefirst
multi-walledcarbonnanotubesoftheseriesandthusalsoshare withlargerMWCNTsthefactthattheouterwallprotectsallthe innerones.Astheinteractionwithasurfactantmoleculeistaking placeonlyattheinterface,theouterwallistheonlytobeconcerned. DWCNTsarethusatthefrontierbetweenSWCNTsandMWCNTs ingeneralandforthisreasonwecanexpectthattheresultsofthis studycanextrapolatedtoallotherkindsofCNTs,aslongasthey frombundles(whichisstillthecaseformanykindsofMWCNTs). However,itisclearthatresultsmaynotbeextrapolatedtomuch shorterandindividualCNTs,whichcouldoccureitherintentionally ifcuttingproceduresareapplied(SWCNTs)orevenunintentionally inthecaseoftoostrongsonicationconditionsformostMWCNTs. 4. Conclusion
Imidazolium-basedionicliquids 1-methyl-(1-ethanol)-2-alkyl-imidazoline([M-E-Cn-Im]I,n=13,15and 17)weresynthesized
fromthecorrespondingnaturalfattyacidsandshowntobeable toeffectively suspend carbonnanotubes inwater, even atvery low concentration (1mM), for a period ofmore than a month. Quality of suspension was evidenced by optical density mea-surement,ofzetapotential,particlesizeandTEM.Theresultsof these analyses evidenced that we obtained stable suspensions, andalsomanagedtopartiallyde-bundletheDWCNTs. Compar-ingthedispersing strengthof thethreeionicliquids,we found thatthedispersibilityincreasedwithincreasingthelengthofthe hydrocarbonchain,whichisexplainedbytheincreased hydropho-bicinteractionsbetweenthehydrocarbonchainandDWCNTs.In addition,thesuspensionsofDWCNTswithionicliquidshada vis-cositysimilartothatof water,which is suitablefor interaction withbiologicalenvironments.Moreover,theseresultsencourage the suspension of DWCNTs using imidazolium-basedionic liq-uids,forpossibleapplicationinthestudyof toxicity/ecotoxicity ofcarbonnanotubes,thankstotheirexpectedlowintrinsic toxic-ity(whichishoweverstilltobemeasuredforourseriesofnew compounds).Acknowledgments
TheauthorsexpresstheirsincerethankstotheMinistryofHigh EducationandScientificResearch(Algeria)andtheFrenchMinistry ofForeignandEuropeanAffairsforfinancialsupportofPHC-TASSILI 10MDU797project.
References
[1]S.Iijima,Helicalmicrotubulesofgraphiticcarbon,Nature354(1991)56–58.
[2]A.Peigney,C.Laurent,E.Flahaut,R.R.Bacsa,A.Rousset,Specificsurfacearea ofcarbon nanotubesandbundlesofcarbonnanotubes,Carbon39(2001) 507–514.
[3]S.Roche,Carbonnanotubes:exceptionalmechanicalandelectronicproperties, Ann.Chim.Sci.Matér.25(2000)529–532.
[4]L.A.Girifalco,M.Hodak,R.S.Lee,Carbonnanotubes,buckyballs,ropes,anda universalgraphiticpotential,Phys.Rev.B62(2000)13104–13110.
[5]E.V.Basiuk,V.A.Basiuk,V.Meza-Laguna,F.F.Contreras-Torres,M.Martínez,A. Rojas-Aguilar,M.Salerno,G.Zavala,A.Falqui,R.Brescia,Solvent-freecovalent functionalizationofmulti-walledcarbonnanotubesandnanodiamondwith diamines:lookingforcross-linkingeffects,Appl.Surf.Sci.259(2012)465–476.
[6]B.A.Kakade,V.K.Pillai,Anefficientroutetowardsthecovalentfunctionalization ofsinglewalledcarbonnanotubes,Appl.Surf.Sci.254(2008)4936–4943.
[7]G.Xu,B.Zhu,Y.Han,Z.Bo,Covalentfunctionalizationofmulti-walledcarbon nanotubesurfacesbyconjugatedpolyfluorenes,Polymer48(2007)7510–7515.
[8]C.Hu,H.Liao,F.Li,J.Xiang,W.Li,S.Duo,M.Li,Noncovalentfunctionalization ofmulti-walledcarbonnanotubeswithsiloxanepolyethercopolymer,Mater. Lett.62(2008)2585–2588.
[9]A.Zhang,M.Tang,J.Luan,J.Li,Noncovalentfunctionalizationofmulti-walled carbonnanotubeswithamphiphilicpolymerscontainingpyrenependants, Mater.Lett.67(2012)283–285.
[10]L.Vaisman,H.D.Wagner,G.Marom,Theroleofsurfactantsindispersionof carbonnanotubes,Adv.ColloidInterfaceSci.128–130(2006)37–46.
[11]M.Zhang,L.Su,L.Mao,Surfactantfunctionalizationofcarbonnanotubes(CNTs) forlayer-by-layerassemblingofCNTmulti-layerfilmsandfabricationofgold nanoparticle/CNTnanohybrid,Carbon44(2006)276–283.
[12]A.L.Alpatova,W.Shan,P.Babica,B.L.Upham,A.R.Rogensues,S.J.Masten,E. Drown,A.K.Mohanty,E.C.Alocilja,V.V.Tarabara,Single-walledcarbon nano-tubesdispersedinaqueousmediavianon-covalentfunctionalization:effect
ofdispersantonthestability,cytotoxicity,andepigenetictoxicityofnanotube suspensions,WaterRes.44(2010)505–520.
[13]D.Tasis,N.Tagmatarchis,V.Georgakilas,M.Prato,Solublecarbonnanotubes, Chem.Eur.J.9(2003)4001–4008.
[14]L.Liu,K.C.Etika,K.-S.Liao,L.A.Hess,D.E.Bergbreiter,J.C.Grunlan,Comparison ofcovalentlyandnoncovalentlyfunctionalizedcarbonnanotubesinepoxy, Macromol.RapidCommun.30(2009)627–632.
[15]H.Wang,Dispersingcarbonnanotubesusingsurfactants,Curr.Opin.Colloid InterfaceSci.14(2009)364–371.
[16]M.Bassiouk,V.A.Basiuk,E.V.Basiuk,E.Álvarez-Zauco,M.Martínez-Herrera, A.Rojas-Aguilar,I.Puente-Lee,Noncovalentfunctionalizationofsingle-walled carbonnanotubeswithporphyrins,Appl.Surf.Sci.275(2013)168–177.
[17]A.Skender,A.Hadj-Ziane-Zafour,E.Flahaut,Chemicalfunctionalizationof Xan-thangumforthedispersionofdouble-walledcarbonnanotubesinwater, Carbon62(2013)149–156.
[18]H.Sato,M.Sano,Characteristicsofultrasonicdispersionofcarbonnanotubes aidedbyantifoam,ColloidsSurf.A:Physicochem.Eng.Asp.322(2008)103–107.
[19]I.Schwyzer,R.Kaegi,L.Sigg,A.Magrez,B.Nowack,Influenceoftheinitial stateofcarbonnanotubesontheircolloidalstabilityundernaturalconditions, Environ.Pollut.159(2011)1641–1648.
[20]C.Hu,C.Yang,S.Hu,Hydrophobicadsorptionofsurfactantsonwater-soluble carbonnanotubes:asimpleapproachtoimprovesensitivityand antifoul-ingcapacityofcarbonnanotubes-basedelectrochemicalsensors,Electrochem. Commun.9(2007)128–134.
[21]P.S.Goh,B.C.Ng,A.F.Ismail,M.Aziz,S.M.Sanip,Surfactantdispersed multi-walled carbon nanotube/polyetherimidenanocomposite membrane, Solid StateSci.12(2010)2155–2162.
[22]R.Rastogi,R.Kaushal,S.K.Tripathi,A.L.Sharma,I.Kaur,L.M.Bharadwaj, Com-parativestudyofcarbonnanotubedispersionusingsurfactants,J. Colloid InterfaceSci.328(2008)421–428.
[23]H.T.Ham,Y.S.Choi,I.J.Chung,Anexplanationofdispersionstatesof single-walledcarbonnanotubesinsolventsandaqueoussurfactantsolutionsusing solubilityparameters,J.ColloidInterfaceSci.286(2005)216–223.
[24]I.Madni,C.-Y.Hwang,S.-D.Park,Y.-H.Choa,H.-T.Kim,Mixedsurfactant sys-temforstablesuspensionofmultiwalledcarbonnanotubes,ColloidsSurf.A: Physicochem.Eng.Asp.358(2010)101–107.
[25]Y.Liu,L.Yu,S.Zhang,J.Yuan,L.Shi,L.Zheng,Dispersionofmultiwalled car-bonnanotubesbyionicliquid-typeGeminiimidazoliumsurfactantsinaqueous solution,ColloidsSurf.A:Physicochem.Eng.Asp.359(2010)66–70.
[26]A.DiCrescenzo,D.Demurtas,A.Renzetti,G.Siani,P.DeMaria,M.Meneghetti, M. Prato,A. Fontana, Disaggregation of single-walled carbon nanotubes (SWNTs)promotedbytheionicliquid-basedsurfactant 1-hexadecyl-3-vinyl-imidazoliumbromideinaqueoussolution,SoftMatter5(2009)62–66.
[27]J.Jiao,H.Zhang,L.Yu,X.Wang,R.Wang,Decoratingmulti-walledcarbon nano-tubeswithAunanoparticlesbyamphiphilicionicliquidself-assembly,Colloids Surf.A:Physicochem.Eng.Asp.408(2012)1–7.
[28]F.Lu,S.Zhang,L.Zheng,Dispersionofmulti-walledcarbonnanotubes (MWC-NTs)byionicliquid-basedphosphoniumsurfactantsinaqueoussolution,J.Mol. Liq.173(2012)42–46.
[29]H.B.Kim,J.S.Choi,S.T.Lim,H.J.Choi,H.S.Kim,Preparationandnanoscopic inter-nalstructureofsingle-walledcarbonnanotube-ionicliquidgel,Synth.Met.154 (2005)189–192.
[30]T.Aida,T.Fukushima,Softmaterialswithgraphiticnanostructures,Philos. Trans.R.Soc.A:Math.Phys.Eng.Sci.365(2007)1539–1552.
[31]S.H.Hong,T.T.Tung,L.K.H.Trang,T.Y.Kim,K.S.Suh,Preparationofsingle-walled carbonnanotube(SWNT)gelcompositesusingpoly(ionicliquids),Colloid Polym.Sci.288(2010)1013–1018.
[32]C.Li,L.Gu,J.Tong,J.Maier,Carbonnanotubewiringofelectrodesfor high-ratelithiumbatteriesusinganimidazolium-basedionicliquidprecursoras dispersantandbinder:acasestudyonironfluoridenanoparticles,ACSNano5 (2011)2930–2938.
[33]L.Lu,B.Liu,C.Liu,G.Xie,Amperometricimmunosensorformyeloperoxidase inhumanserumbasedonamulti-wallcarbonnanotubes-ionicliquid-cerium dioxidefilm-modifiedelectrode,Bull.Kor.Chem.Soc.31(2010)3259–3264.
[34]X.Pei,Y.H.Yan,L.Yan,P.Yang,J.Wang,R.Xu,M.B.Chan-Park,A magnet-icallyresponsivematerialofsingle-walledcarbonnanotubesfunctionalized withmagneticionicliquid,Carbon48(2010)2501–2505.
[35]J.Yang,Z.Zhang,D.Zhang,Y.Li,Quantitativeanalysisofthe(n,m)abundanceof single-walledcarbonnanotubesdispersedinionicliquidsbyopticalabsorption spectra,Mater.Chem.Phys.139(2013)233–240.
[36]M.Zhao,Y.Gao,L.Zheng,Lyotropicliquidcrystallinephasesformedinbinary mixture of 1-tetradecyl-3-methylimidazolium chloride/ethylammonium nitrateanditsapplicationinthedispersionofmulti-walledcarbonnanotubes, ColloidsSurf.A:Physicochem.Eng.Asp.369(2010)95–100.
[37]B.Dong,Y.Su,Y.Liu,J.Yuan,J.Xu,L.Zheng,Dispersionofcarbonnanotubesby carbazole-tailedamphiphilicimidazoliumionicliquidsinaqueoussolutions,J. ColloidInterfaceSci.356(2011)190–195.
[38]J.Wang,H.Chu,Y.Li,Whysingle-walledcarbonnanotubescanbedispersedin imidazolium-basedionicliquids,ACSNano2(2008)2540–2546.
[39]N.Madria,T.A.Arunkumar,N.G.Nair,A.Vadapalli,Y.-W.Huang,S.C.Jones,V.P. Reddy,Ionicliquidelectrolytesforlithiumbatteries:synthesis, electrochemi-cal,andcytotoxicitystudies,J.PowerSources234(2013)277–284.
[40]S.N.Riduan,Y.Zhang,Imidazoliumsaltsandtheirpolymericmaterialsfor bio-logicalapplications,Chem.Soc.Rev.42(2013)9055–9070.
[41]R.Biczak,B.Pawłowska,P.Bałczewski,P.Rychter,Theroleoftheanioninthe toxicityofimidazoliumionicliquids,J.Hazard.Mater.274(2014)181–190.
[42]W.Gouveia,T.F.Jorge,S.Martins,M.Meireles,M.Carolino,C.Cruz,T.V.Almeida, M.E.M.Araújo,Toxicityofionicliquidspreparedfrombiomaterials, Chemo-sphere104(2014)51–56.
[43]E.Flahaut,R.Bacsa,A.Peigney,C.Laurent, Gram-scaleCCVDsynthesisof double-walledcarbonnanotubes,Chem.Commun.9(2003)1442–1443.
[44]S.-H.Yoo,Y.-W.Kim,K.Chung,S.-Y.Baik,J.-S.Kim,Synthesisandcorrosion inhibitionbehaviorofimidazolinederivativesbasedonvegetableoil,Corros. Sci.59(2012)42–54.
[45]S.F. Wang, T. Furuno, Z. Cheng, Synthesis of 1-hydroxyethyl-2-alkyl-2-imidazolineanditsderivativesulfonateamphotericsurfactantfromtalloil fattyacid,J.WoodSci.49(2003)371–376.
[46]S. Sharma,S. Gangal,A. Rauf,Convenient one-pot synthesisof novel 2-substitutedbenzimidazoles,tetrahydrobenzimidazoles andimidazolesand evaluationoftheirinvitroantibacterialandantifungalactivities,Eur.J.Med. Chem.44(2009)1751–1757.
[47]F.Mouchet,P.Landois,E.Sarremejean,G.Bernard,P.Puech,E.Pinelli,E.Flahaut, L.Gauthier,Characterisationandinvivoecotoxicityevaluationofdouble-wall carbonnanotubesinlarvaeoftheamphibianXenopuslaevis,Aquat.Toxicol.87 (2008)127–137.
[48]F.Bourdiol,F.Mouchet,A.Perrault,I.Fourquaux,L.Datas,C.Gancet,J.-C. Boutonnet,E.Pinelli,L.Gauthier,E.Flahaut,Biocompatiblepolymer-assisted dispersionofmultiwalledcarbonnanotubesinwater,applicationtothe inves-tigationoftheirecotoxicityusingXenopuslaevisamphibianlarvae,Carbon54 (2013)175–191.
[49]S.Azoubel,S.Magdassi,Theformationofcarbonnanotubedispersionsbyhigh pressurehomogenizationandtheirrapidcharacterizationbyanalytical cen-trifuge,Carbon48(2010)3346–3352.
[50]C.Richard,F.Balavoine,P.Schultz,T.W.Ebbesen,C.Mioskowski, Supramolecu-larself-assemblyoflipidderivativesoncarbonnanotubes,Science300(2003) 775–778.
[51]S.Utsumi,M.Kanamaru,H.Honda,H.Kanoh,H.Tanaka,T.Ohkubo,H.Sakai, M.Abe,K.Kaneko,RBMbandshift-evidenceddispersionmechanismof single-wallcarbonnanotubebundleswithNaDDBS,J.ColloidInterfaceSci.308(2007) 276–284.
[52]Y.Bai,D.Lin,F.Wu,Z.Wang,B.Xing,AdsorptionofTritonX-series surfac-tantsanditsroleinstabilizingmulti-walledcarbonnanotubesuspensions, Chemosphere79(2010)362–367.
[53]M.F.Islam,E.Rojas,D.M.Bergey,A.T.Johnson,A.G.Yodh,Highweightfraction surfactantsolubilizationofsingle-wallcarbonnanotubesinwater,NanoLett. 3(2003)269–273.
[54]B.Yu,F.Zhou,G.Liu,Y.Liang,W.T.S.Huck,W.Liu,Theelectrolyteswitchable solubilityofmulti-walledcarbonnanotube/ionicliquid(MWCNT/IL)hybrids, Chem.Commun.(2006)2356–2358.
[55]J.Rausch,R.-C.Zhuang,E.Mäder,Surfactantassisteddispersionof functional-izedmulti-walledcarbonnanotubesinaqueousmedia,Compos.A:Appl.Sci. Manuf.41(2010)1038–1046.
[56]O.Osazuwa,K.Petrie,M.Kontopoulou,P.Xiang,Z.Ye,A.Docoslis, Charac-terizationofnon-covalently,non-specificallyfunctionalizedmulti-wallcarbon nanotubesandtheirmeltcompoundedcompositeswithanethylene–octene copolymer,Compos.Sci.Technol.73(2012)27–33.
[57]F.-C.Li,J.-C.Yang,W.-W.Zhou,Y.-R.He,Y.-M.Huang,B.-C.Jiang, Experimen-talstudyonthecharacteristicsofthermalconductivityandshearviscosityof viscoelastic-fluid-basednanofluidscontainingmultiwalledcarbonnanotubes, Thermochim.Acta556(2013)47–53.

![Fig. 2. FT-IR spectra of (A) [M-E-C 13 -Im] I, (B) [M-E-C 15 -Im] I and (C) [M-E-C 17 -Im] I.](https://thumb-eu.123doks.com/thumbv2/123doknet/3211504.91813/5.892.169.709.77.458/fig-ft-ir-spectra-im-im-and-im.webp)
![Fig. 7. UV/vis spectra of ionic liquids [M-E-C 13 -Im] I, [M-E-C 15 -Im] I, [M-E-C 17 -Im] I at 10 mM and DWCNT suspensions with ionic liquids [M-E-C 13 -Im] I, [M-E-C 15 -Im] I, [M-E-C 17 -Im] I and water only (immediately after preparation).](https://thumb-eu.123doks.com/thumbv2/123doknet/3211504.91813/6.892.208.706.441.745/spectra-ionic-liquids-dwcnt-suspensions-liquids-immediately-preparation.webp)
![Fig. 8. UV/vis absorbance intensity versus time of DWCNTs (50 mg/L) in ionic liquids (a) [M-E-C 13 -Im] I, (b) [M-E-C 15 -Im] I, (c) [M-E-C 17 -Im] I and (d) deionized water alone at a concentration of (A) 10 mM, (B) 1.5 mM and (C) 1 mM, up to 20 days.](https://thumb-eu.123doks.com/thumbv2/123doknet/3211504.91813/7.892.164.711.74.971/absorbance-intensity-versus-dwcnts-ionic-liquids-deionized-concentration.webp)
![Fig. 11. TEM micrograph of DWCNT suspensions in deionized water at 50 mg/L with ionic liquid at 1 mM (a) [M-E-C 13 -Im] I, (b) [M-E-C 15 -Im] I, (c) [M-E-C 17 -Im] I and (d) deionized water alone](https://thumb-eu.123doks.com/thumbv2/123doknet/3211504.91813/9.892.167.712.78.621/micrograph-dwcnt-suspensions-deionized-water-ionic-liquid-deionized.webp)