ContentslistsavailableatSciVerseScienceDirect
Lung
Cancer
jo u rn al h om epa g e :w w w . e l s e v i e r . c o m / l o c a t e / l u n g c a n
Case
report
Clinical
activity
of
afatinib
(BIBW
2992)
in
patients
with
lung
adenocarcinoma
with
mutations
in
the
kinase
domain
of
HER2/neu
夽,夽夽
J.
De
Grève
a,∗,
E.
Teugels
a,
C.
Geers
a,
L.
Decoster
a,
D.
Galdermans
b,
J.
De
Mey
a,
H.
Everaert
a,
I.
Umelo
a,
P.
In’t
Veld
a,
D.
Schallier
aaOncologischCentrumUZBrussel,Brussels,Belgium
bZNAMiddelheim,Antwerp,Belgium
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received13May2011
Receivedinrevisedform13January2012
Accepted15January2012
Keywords:
Non-smallcelllungcancer(NSCLC)
Epidermalgrowthfactorreceptor(EGFR)
Humanepidermalgrowthfactorreceptor2
(HER2) Afatinib Adenocarcinoma Mutation
a
b
s
t
r
a
c
t
Humanepidermalgrowthfactorreceptor(HER)2/neukinasedomainmutationsarefoundin approxi-mately1–4%oflungadenocarcinomaswithasimilarphenotypetotumorswithepidermalgrowthfactor receptor(EGFR)mutations.AfatinibisapotentirreversibleErbBfamilyblocker.Wedeterminedthe tumorgenomicstatusoftheEGFRandHER2genesinnon-orlightsmokerswithlungadenocarcinoma inpatientswhowereenteredintoanexploratoryPhaseIIstudywithafatinib.Fivepatientswitha non-smokinghistoryandmetastaticlungadenocarcinomasbearingmutationsinthekinasedomainofHER2 genewereidentified,threeofwhichwereevaluableforresponse.Objectiveresponsewasobservedin allthreepatients,evenafterfailureofotherEGFR-and/orHER2-targetedtreatments;thecasehistories ofthesepatientsaredescribedinthisreport.Thesefindingssuggestthatafatinibisapotentialnovel treatmentoptionforthissubgroupofpatients,evenwhenotherEGFRandHER2targetingtreatments havefailed.
© 2012 Elsevier Ireland Ltd.
1. Introduction
The presence of activatingmutations in the tyrosinekinase domain of the human epidermal growth factor receptor 1 (EGFR/HER1/erbB1) in non-small cell lung cancer (NSCLC) cor-relates with a clinical phenotype of adenocarcinoma in never orlightsmokers,andrendersthetumorexquisitelysensitiveto EGFRtyrosinekinaseinhibitors(TKIs)[1–3].Theintroductionof targeted drugs for the treatmentof NSCLCwith EGFR-directed small-moleculeTKIs [3] and monoclonal antibodies[4] has led toasignificantbutrelativelysmalloverallimprovementin clini-caloutcomeofunselectedpatientswithadvanceddisease.EGFR mutations and increased EGFR copy number by fluorescence
夽 Previouspublications:Inabstractandposterformatthe4thLatinAmerican
ConferenceonLungCancer(LALCA)2010,inabstractandposterformat2nd
Euro-peanLungCancerConference(ELCC)2010,inabstractformattheWorldConference
onLungCancer(WCLC)2009andtheCongressoftheEuropeanCancer
Organisa-tionand34thCongressoftheEuropeanSocietyforMedicalOncology(ECCO-ESMO)
2009.
夽夽 Clinical trial details:Registry name: Single-arm Trialof BIBW 2992 in
DemographicallyandGenotypicallySelectedNSCLCPatients.Registrationnumber:
NCT00730925.
∗ Correspondingauthorat:MedicalOncology,OncologischCentrum,UZBrussel,
Laarbeeklaan101,1090Brussels,Belgium.Tel.:+3224776415;fax:+3224776210.
E-mailaddress:Jacques.degreve@uzbrussel.be(J.DeGrève).
insituhybridization(FISH)arepredictivebiomarkersthatidentify patientswhoaremostsensitivetoTKIs[5,6].
HER2kinasedomainmutationsarerareinNSCLC,andarefound inapproximately1–4%oflungadenocarcinomaswithasimilar phe-notypeastumorswithEGFRmutations[7–9].In229patientswith adenocarcinomaofthelung,withalittleornosmokinghistory,we identifiedaHER2mutationinthetumortissueoffivepatients(2%), whichis10-foldrarerthanthefrequencyofEGFRmutationsinthe samecohortofpatients[10].Inothercohortswithpotentially dif-feringphenotypicselectioncriteria,theHER2mutationratewas evenlower:intumorsfrom830patientsanalyzedwithintheNCI’s LungCancerMutationConsortium(LCMC)[11]aHER2mutation wasfoundinonlythreecases(1%)comparedto98caseswithan EGFRmutation.In552samplesanalyzedatMassachusettsGeneral Hospital,onlyonepatientwitha HER2mutationwasidentified
[12].TheHER2mutationsfoundinclinicalsamplessofarareallin exon20.
Afatinibisapotent,irreversibleErbBfamilyblockerwith pre-clinicalactivityinBa/F3cellsexpressinganartificialHER2mutant andinahumanlungcancercelllinewithaninsertionalmutation atcodon776[13].
We determinedthe tumor genomic status of theEGFR and HER2genesinnon-orlightsmokerswithlungadenocarcinoma bydenaturinggradientgelelectrophoresis(DGGE)/DNA sequenc-ingofNSCLCtumortissueorincreasedcopynumberoftheEGFR gene,asdeterminedbyFISHanalysis.HER2FISHwasnotrequired
0169-5002© 2012 Elsevier Ireland Ltd.
doi:10.1016/j.lungcan.2012.01.008
Open access under CC BY-NC-ND license.
124 J.DeGrèveetal./LungCancer76 (2012) 123–127
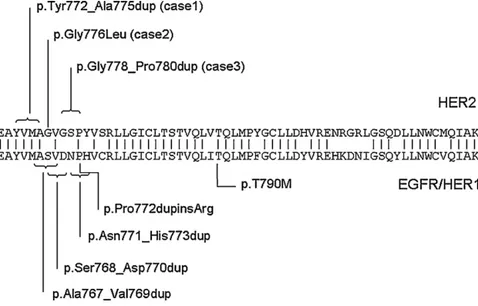
Fig.1.ExamplesofHER2exon20mutations.
forentryintothestudyandthereforenotsystematically under-taken.InCase2,HER2FISHwasperformedlongbeforeinclusion intothecurrentstudy.Patientswereenteredintothisexploratory PhaseIIstudywithafatinib,which,amongothers,includedacohort ofpatientswithHER2kinasedomainmutations[14].Therewere norestrictionsinpriortherapyforpatientswithHER2mutations, althoughpatientshadtohaveatleastonemeasurabletumorlesion thatcouldbeaccuratelymeasuredbycomputedtomography(CT) scanormagneticresonanceimaging[14].Here,wereportthefirst therapeuticactivityofafatinibinthreepatientswithlung adeno-carcinomaanda non-smoking history,whose tumors exhibited activatingHER2 mutationsin exon 20 (Fig.1). Treatmentwith afatinibresultedin anobjectiveremission in allthree patients, evenafterfailureofotherEGFR-and/orHER2-targetedtreatments. Followingdiseaseprogression,therewasanoptiontocombinea lowerlevelofafatinibwithweeklypaclitaxelat80mg/m2 ona
3/4-weekschedule.Fivepatientsweretreatedinthisstudy;two patientswerenotevaluableduetoearlytreatmentdiscontinuation. ThestudywasapprovedbytheEthicalCommitteeofthe Univer-sitairZiekenhuis Brussel and participatingcentersand patients provided informedconsent. Here we reportonthree evaluable patients.
2. Case1
A72-year-old,non-smokingfemalewasdiagnosedwithastage IIIlungadenocarcinoma (rightlower lobe)in May2007. Treat-ment withfour cyclesof carboplatin/gemcitabine resultedin a partialremission. Followingprogressive disease(PD)inJanuary 2008,administrationofanadditionalfourcyclesofreduceddose carboplatin/gemcitabineresulted in stable disease(SD).In May 2008,thepatientwasfoundtohavePDinthelung,with symp-toms of mildly productive cough. An exon 20 HER2 mutation (p.Tyr772Ala775dup;Fig.1)wasfoundinthetumorDNAextracted fromtheoriginaldiagnosticbiopsyinMay2007.
Treatmentwithafatinib(50mg/day)startedinJuly2008.After8 days,positronemissiontomography-CT(PET-CT)imagingshowed a radiological partial response (PR) and a metabolic complete responsethatwasmaintainedfor3months(Fig.2A).Treatment wasinterruptedthreetimesduetosideeffects(diarrhea, dysgeu-siaandskinadverseevents[AEs];allCommonTerminologyCriteria forAdverseEvents[CTCAE]Grade2)andpromptedsuccessivedose reductionsto30mg/day. Thepatientwasdeemed tohave pro-gressionafter3monthsbasedonanapproximate20%increasein
targetlesionsabovethenadir,althoughthetotaltumorburdenwas belowbaselineandthepatientcontinuedtoreceivemonotherapy withafatinib.FollowingfurtherprogressioninMay2009,afatinib wascombinedwithpaclitaxel,butthepatientshowedprogression solelyduetotheoccurrenceofbrainmetastasesshortlyafterwards anddiedonemonthaftergoingoffstudywithouthavingreceived anysubsequenttherapy.Thepatientwastreatedwithafatinibfor atotalof9monthsandsurvivedoneyearfromstudyentry.
3. Case2
A62-year-old,non-smokingfemalewithadenocarcinomaofthe rightlung wasinitially diagnosedin 2002.Hertumorcells had increasedEGFR/HER1copynumber,asassessedbyFISH,aswell asmutationsintheEGFRkinasedomain(exon21:p.Ala859Thr) andinHER2(exon20:p.Gly776Leu).Sheunderwentalobectomy forapT2N1adenocarcinomaandreceivedadjuvantchemotherapy withcisplatin/gemcitabine,followedbyradiotherapy.Arelapsein thelung andmediastinallymphnodesinJuly2003wastreated withfourcyclesofthesamechemotherapy,resultinginSD.From 2004through2008,PDwastreated sequentiallywithdocetaxel (sixcycles;SD),gefitinib(PD),trastuzumabwithpaclitaxel(PR), lapatinib,gemcitabineandvinorelbine.
Atinclusioninthecurrentstudy,thispatientsufferedfrom dys-pneaandretrosternalandrightchestwallpainrequiringnarcotic painrelief,aswellasfacialandcervicalsoft-tissuecongestion.Her EasternCooperativeOncologyGroup(ECOG)performancestatus (PS)was2.
From July 2008, this patient was treated with afatinib (50mg/day). Within 2 weeks, the cervical soft-tissue swelling decreased withmarked improvement in her general condition (ECOG PS: 1). On Day 15, a metabolic response was observed ina PET-CT scan (Fig.2B). Treatment-relatedAEsincludedskin reactions,diarrhea,intermittentnauseaandvomiting,pyrosisand epigastricpain,fatigue,mucositis,sialorrhea, hairthinning,nail changesandfissuresofthenailbedandfingertip.After2monthsof treatment(August2008),aPRwasobservedbyCTscan.Treatment wasinterruptedduetotheassociateddiarrhea,andthedosewas reducedsuccessivelyto40mg/dayand30mg/day(October2008). Atthattime,thepatientwasprogressivecomparedtothenadir ofresponse,butstillhadatumorburdenreduction(20%decrease intargetlesions)byCTscan,comparedtobaseline.Thetimeto progressiononsingle-agentafatinibwas4months;inDecember 2008,shedevelopedfurtherPDintheliverandmediastinallymph
Fig.2.(A)Case1– responsetosingle-agentafatinib.PanelsA1andA3arethebaselinePET-CTandCTscans,respectively.PanelsA2andA4arethepost-treatmentPET-CTand
CTscansshowingtheearlyresponsetoafatinib.(B)Case2–responsetosingle-agentafatinib.PanelB1isabaselinePET-CTimage.PanelsB2andB3arethepost-treatment
PET-CTscansshowingmetabolicresponseonDay15oftreatmentandpartialremissionafter2months,withdiseaseprogressionat4months(PanelB4).(C)Case3–response
tosingle-agentafatinibandincombinationwithpaclitaxel.PanelC1isabaselinePET-CTimage,panelC2showstheimportantresponseinpleuralandliverdisease.Panel
126 J.DeGrèveetal./LungCancer76 (2012) 123–127 nodes.Weeklypaclitaxelwasaddedandthedoseofafatinibwas
reducedto20mg.ThepatienthadSDoverall,butwithametabolic andradiologicalresponseintheliverfor9monthsuntilApril2009, afterwhichsheprogressed.Thetimetoprogressionafterpaclitaxel wasaddedtoafatinibwas4months.ThepatientdiedinSeptember 2009,atotalof14monthsfromstudyentry.
4. Case3
InMarch2006,a49-year-oldCaucasian,non-smokingwoman wasdiagnosedwithstageIVrightupper-lobelungadenocarcinoma withdiffusepleural,liverand soft-tissuemetastases.Thetumor cells hadan increasedEGFRgene copynumber, asassessed by FISH,witha wild-typesequence.Thispatientreceivedfirst-line treatmentwitherlotinibat150mg/day,butclinicaland radiolog-icalprogressionoccurredwithin3months.FromJune2006,she wastreatedwithcisplatin/gemcitabine,withanobjectivetumor response,buttreatmentwasinterruptedduetocumulative toxic-ity.Shethenreceived,sequentially,gemcitabine(PD),carboplatin (transient response, but hematological intolerance), vinorelbine (PD),pemetrexed(transientresponse)andweeklycisplatin (symp-tomatic and objective response; treatment stopped because of intolerance).Additionalgenomicanalysisrevealedaninsertional duplication(p.Gly778Pro780dup)in exon20 oftheHER2 gene (Fig.1).AtinclusioninthecurrentstudyinJune2008[14],the patientwasseverelysymptomatic,withpainintherightchest, righthypochondriumandrightshoulder,andanorexiaandfatigue. Shehadalsodevelopedasymptomaticbonemetastasesandhadan ECOGPSof1.
Within2 weeks of starting afatinib(50mg/day), thepatient hadarapid clinicaland symptomaticresponse,with disappear-anceofalldisease-relatedsymptoms,aswellasoverallSDwith aradiologicalresponseinliverandpleura,whichwasmaintained for3months(Fig.2C).Treatmentwithafatinib(50mg/day)was associated withskin-relatedAEs, diarrhea and mucosal inflam-mationwithintermittentepistaxis, aphthousstomatitisand dry eyes.
Thetimetoprogressiononsingle-agentafatinibwas4months; following PD in October 2008, the patient received afatinib (40mg/day)combinedwithweekly paclitaxel(80mg/m2).After
onecycle,disease-relatedsymptomsdisappearedandadramatic partialremissionwasseen.AsofJuly2009,thispatienthadanECOG PSof0,adiseasevolumeoflessthanthatatherremissionafter first-linecisplatin-basedchemotherapy2.5yearsearlier(Fig.2C). Sustainedcontrolofcarcinoembryonicantigen(CEA)tumormarker levelswasalsoachievedduringafatinibtreatment.Therewasan increaseinCEAlevelsduringineffectivepriorchemotherapy treat-mentandCEAlevelsdeclinedrapidlytonormalaftercombination ofafatinibandweeklypaclitaxel.Afatinibtreatmentwascontinued foratotalof15months,11ofwhichwereincombinationwith pacli-taxel,afterwhichtimethepatientdevelopedabrainmetastasis withoutconcurrentprogressionattheotherdiseasesites.Adverse eventswithafatinibandweeklypaclitaxelweremildandincluded skinreaction,diarrhea,fatigueandhematologicalAEs.
AftergoingoffstudyinSeptember2009,thepatientreceived trastuzumabsequentiallycombinedwithweeklypaclitaxelfor6 months(CEAmarkerstabilizationfor3months),liposomal dox-orubicinfor4months(markerstabilizationfor2months),weekly cisplatinforthreeadministrations,andoraletoposidefor3months withnofurtherclinicalbenefit. Inaddition, shedeveloped lep-tomeningealdiseasein June2010,whichwastreated withfour intrathecaladministrationsofdepocyteleadingtoadurable com-pletecytologicalandsymptomaticresponseofherleptomeningeal disease.ThepatientdiedinMarch2011,withanoverallsurvivalof 32monthsafterinclusioninthestudy.
5. Additionalcases
TwootherpatientswithHER2mutationswereenrolledintothe study,butbothcaseswereconsideredtobenon-evaluable.One patientwasa51-year-oldwomanwitha4pack-yearsmoking his-tory(whostoppedsmoking29yearsbeforestudyentry).Shewas treatedwithafatinibmonotherapyfor7weeksanddiscontinued treatmentduetotheoccurrenceofGrade3rash.Stabledisease wasobservedatthistime.Thepatientreceivedsubsequent peme-trexedtherapywithdiseaseprogressionaftertwocycles,followed bydocetaxelwithdiseasestabilizationfor5months,afterwhich thepatientwaslosttofollow-up.
Thesecondpatientwasa62-year-oldfemale,never smoker, whoreceivedafatinibforonly2weeksandwasdiscontinueddue toGrade3diarrheaanddeteriorationofhergeneralcondition.No tumorassessmentswereundertakenwithinthestudyafter base-line.Thepatientwassubsequentlylosttofollow-up.
6. Discussion
Wedescribe thefirstevidenceofclinicalbenefit from treat-mentwithafatinibinpatientswithanexon20HER2-mutantlung adenocarcinomawhohavepreviouslyfailedvarious chemother-apy regimens and the EGFR and/or HER2 inhibitors erlotinib, trastuzumaband lapatinib.Fivepatientswereidentified witha HER2mutation,althoughonlythreewereevaluableforresponse; mutationsinallthreepatientswereinexon20(twoinsertional duplications and one single amino-acid mutation). Analogous mutationsinEGFRinexon20arerelativelyinsensitiveto inhibi-tionbythereversibleinhibitorgefitinib[15].Intwopatients,arapid metabolicresponsewasobservedwithin1–2weeks.Twopatients hadgenomicactivationofbothEGFRandHER2.
The most striking response to single-agent afatinib was observed in Case 1, with a p.Tyr772Ala775dup mutation in HER2.Comparedwiththeothertwopatients,thispatientshowed genomicactivationofHER2only.Thismutationcausesanamino acidchange identicalto a mutation studiedin a recently pub-lishedpreclinicalmodelofmutantHER2-drivenlungcancer[16]. Inthismousemodel,theforcedexpressionofthemutantallele iscapableofinducinginvasiveadenosquamouscarcinomasthat arerestrictedtotheproximalanddistalbronchioles.Thesecancers werecompletelydependentonthepresenceofthismutationand regressedcompletelywhentheexpressionofthemutantgenewas reversed.Treatmentwithafatinibledtosignificanttumor regres-sion in this preclinical model. In two of our clinical cases,the additionofpaclitaxeltoafatinibledtoadditionaldiseasecontrol, withprolongedremissioninonepatientdespiteashortresponse tosingle-agentafatinib,raisingthepossibilityofsynergism.Ina xenograftoftheHER2mutantlungcancercelllineH1781,which containsahomozygoussingleamino-acidinsertioninexon20[8], administrationofafatinibresultedindiseasestabilization,in con-trasttothetumorregressionobservedinthepreclinicalmouse model.Takentogetherwithourclinicalexperience,thisindicates thatthehumanHER2-drivenlungcancermayhaveamorecomplex molecularpathogenesisthanthepreclinicalHER2-drivenmouse model.
ThetherapeuticeffectobservedinCase2wasalsoof consid-erableinterest,asthetumorshowedgenomicactivationofboth EGFRandHER2,andwaspreviouslytreatedwith,andhadbecome clinicallyresistantto,erlotinib,trastuzumabandlapatinib[17].
Althoughwe cannotexcludethat thesecondresponse, with addedpaclitaxel, resultsfromtheactivityof single-agent pacli-taxel,themagnitudeanddurationoftheresponseinpatientswith diseaseresistanttomultipleotherchemotherapiessuggeststhat
theresponsewastosomeextentachievedbythecombinationof afatinibwithpaclitaxel.
A limited number of studies in NSCLC have attempted to evaluate the activity of HER2-targeting agents, and have been summarizedbyKellyetal.[18].Thesestudiescouldnotreveala significantbenefitfromtrastuzumaborlapatinib.However,these studieswereperformedinNSCLCpatientpopulationsunselected forHER2status(HER2copynumberormutation)and primarily incombinationwithchemotherapeuticagents,andthereforewere notapttodetectclinicalbenefitinpatientswithagenomic acti-vationofHER2.Therewas,however,areportofonepatientwith aHER2FISHpositivetumor,butnoHER2orEGFRmutation,who achievedashort-livedresponse(4weeks)toapan-HERinhibitor (dacomitinib;PF-00299804)andsubsequentlyprogressed follow-ingadditionaltreatmentwithtrastuzumab,butwho responded aftervinorelbinewasadded.Furthermore,anadditionalpatient withaHER2mutationrespondedtotrastuzumabplusvinorelbine afterfailureofplatinum-basedchemotherapyandgefitinib. How-ever,thiscasedoesnotallowfortheassessmentoftheindependent activityoftrastuzumab[19].
ThisreportsuggeststhatthepresenceofHER2mutationsmay characterizeasubgroupofNSCLCthatisconstitutivelydependent ontheHER2pathway.Afatinibisapotentialnoveltreatmentoption forthissubgroupofpatients,evenwhenotherEGFRandHER2 tar-getingtreatmentshavefailed.Therateanddurationofresponse associatedwithafatinibandthecombinedactivityofafatiniband paclitaxelshouldbefurtherassessedinearlierlinesoftreatment inthisgenomicallydefinedpopulation.
Funding
ThisworkwassupportedbyBoehringerIngelheimandgrants fromtheNationalCancerPlan,Action29(grantPNC/KNP-29-011), Belgium;andtheStichtingtegenKanker,Belgium(grantnumber: SCIE2004-45).
Conflictofintereststatement
JacquesDeGrèvereceivedhonorariaandaresearchgrantfrom BoehringerIngelheim.IjeomaUmelodeclaredVUBOZRfellowship; andacollaboratorintheHER2trial.Nootherconflictsofinterestto disclose.CarolineGeers,ErikTeugels,DenisSchallier,Henrik Ever-aert,DaniellaGaldermans,LoreDecoster,JohanDeMeyandPeter In’tVeldhavenodisclosurestodeclare.
Acknowledgements
Thefirstdraftofthismanuscriptandfinalamendswere writ-tenbyJacquesDeGrève.Editorialsupportforthepreparationof thismanuscriptwasprovidedbyOgilvyHealthworldMedical Edu-cation;fundingwasprovidedbyBoehringerIngelheim.Wethank DrFedericoCappuzzoforreferralofapatientforinclusioninthis study.
References
[1] LynchTJ,BellDW,SordellaR,GurubhagavatulaS,OkimotoRA,BranniganBW,
etal.Activatingmutationsintheepidermalgrowthfactorreceptor
underly-ingresponsivenessofnon-small-celllungcancertogefitinib.NEnglJMed
2004;350:2129–39.
[2] Mok TS,Wu YL, Thongprasert S,YangCH, ChuDT, SaijoN,et al.
Gefi-tiniborcarboplatin-paclitaxelinpulmonaryadenocarcinoma.NEnglJMed
2009;361:947–57.
[3] ShepherdFA,RodriguesPereiraJ,CiuleanuT,TanEH,HirshV,ThongprasertS,
etal.Erlotinibinpreviouslytreatednon-small-celllungcancer.NEnglJMed
2005;353:123–32.
[4] Pirker R, Pereira JR,SzczesnaA, vonPawel J, Krzakowski M,RamlauR,
etal.Cetuximabpluschemotherapyinpatientswithadvanced
non-small-celllung cancer(FLEX):an open-labelrandomisedphaseIII trial. Lancet
2009;373:1525–31.
[5]ChangJW,LiuHP,HsiehMH,FangYF,HsiehMS,HsiehJJ,etal.Increased
epidermalgrowthfactorreceptor(EGFR)genecopynumberisstrongly
asso-ciatedwith EGFRmutations andadenocarcinoma innon-small celllung
cancers:achromogenicinsituhybridizationstudyof182patients.LungCancer
2008;61:328–39.
[6] CappuzzoF,HirschFR,RossiE,BartoliniS,CeresoliGL,BemisL,etal.Epidermal
growthfactorreceptorgeneandproteinandgefitinibsensitivityin
non-small-celllungcancer.JNatlCancerInst2005;97:643–55.
[7]ButtittaF,BarassiF,FresuG,FelicioniL,ChellaA,PaolizziD,etal.Mutational
analysisoftheHER2geneinlungtumorsfromCaucasianpatients:mutations
aremainlypresentinadenocarcinomaswithbronchioloalveolarfeatures.IntJ
Cancer2006;119:2586–91.
[8]ShigematsuH,TakahashiT,NomuraM,MajmudarK,SuzukiM,LeeH,etal.
SomaticmutationsoftheHER2kinasedomaininlungadenocarcinomas.Cancer
Res2005;65:1642–6.
[9]StephensP,HunterC,BignellG,EdkinsS,DaviesH,TeagueJ,etal.Lungcancer:
intragenicERBB2kinasemutationsintumours.Nature2004;431:525–6.
[10] DeGreveJ,VanMeerbeeckJP,VansteenkisteJF,TeugelsE,GeersC,MeertA,etal.
First-lineerlotinibinadvancednon-smallcelllungcancer(NSCLC)carryingan
activatingEGFRmutation:amulticenteracademicphaseIIstudyinCaucasian
patients(pts)(NCT00339586)–FIELTstudygroup.JClinOncol2011;29(Suppl.;
abstr7597).
[11]KrisMG,JohnsonBE,KwiatkowskiDJ,IafrateAJ,WistubaII,AronsonSL,etal.
Identificationofdrivermutationsintumorspecimensfrom1,000patientswith
lungadenocarcinoma:TheNCI’sLungCancerMutationConsortium(LCMC).
JClinOncol2011;29(Suppl.;abstrCRA7506).
[12] SequistLV,HeistRS,ShawAT,FidiasP,TemelJS,LennesIT,etal.SNaPshot
genotypingofnon-smallcelllungcancers(NSCLC)inclinicalpractice.JClin
Oncol2011;29(Suppl.;abstr7518).
[13] LiD,AmbrogioL, ShimamuraT,KuboS,TakahashiM,Chirieac LR,etal.
BIBW2992,anirreversibleEGFR/HER2inhibitorhighlyeffectiveinpreclinical
lungcancermodels.Oncogene2008;27:4702–11.
[14]Single-ArmTrialofBIBW2992inDemographicallyandGenotypicallySelected
NSCLCPatients;2009,ClinicalTrials.govidentifier:NCT00730925.
[15] WangSE,NarasannaA,Perez-TorresM,XiangB,WuFY,YangS,etal.HER2
kinasedomainmutationresultsinconstitutivephosphorylationandactivation
ofHER2andEGFRandresistancetoEGFRtyrosinekinaseinhibitors.CancerCell
2006;10:25–38.
[16]PereraSA,LiD,ShimamuraT,RasoMG,JiH,ChenL,etal.HER2YVMAdrives
rapiddevelopmentofadenosquamouslungtumorsinmicethataresensitive
toBIBW2992andrapamycincombinationtherapy.ProcNatlAcadSciUSA
2009;106:474–9.
[17]Cappuzzo F, Bemis L, Varella-Garcia M. HER2 mutation and response
to trastuzumab therapy in non-small-cell lung cancer. N Engl J Med
2006;354:2619–21.
[18] KellyRJ,CarterC,GiacconeG.Personalizingtherapyinanepidermalgrowth
factorreceptor-tyrosinekinaseinhibitor-resistantnon-small-celllungcancer
usingPF-00299804andtrastuzumab.JClinOncol2010;28:e507–10.
[19]TomizawaK,SudaK,OnozatoR,KosakaT,EndohH,SekidoY,etal.
Prognos-ticandpredictiveimplicationsofHER2/ERBB2/neugenemutationsinlung