راتخم يجاب ةعماج
ةبانع
UNIVERSITE BADJI MOKHTAR
ANNABA
Columba livia
©BOUAOUICHE.A,2009
1
1
2
15
1.2
15
2.2
15
1.2.2
15
2.2.2
16
3.2
17
4.2
18
1.4.2
11
2.4.2
11
5.2
11
6.2
12
1.6.2
12
1.1.6.2
12
2.1.6.2
13
3.1.6.2
13
4.1.6.2
13
5.1.6.2
14
6.1.6.2
14
7.1.6.2
14
8.1.6.2
15
9.1.6.2
15
2.6.2
15
1.2.6.2
15
2.2.6.2
16
3.2.6.2
17
3.6.2
18
1.3.6.2
18
2.3.6.2
23
3.3.6.2
23
4.3.6.2
30
5.3.6.2
23
4.6.2
Biuret
21
1.4.6.2
21
2.4.6.2
21
3.4.6.2
21
4.4.6.2
22
5.4.6.2
22
6.4.6.2
22
7.4.6.2
23
8.4.6.2
23
9.4.6.2
23
5.6.2
Trinder
35
1.5.6.2
24
2.5.6.2
24
3.5.6.2
25
4.5.6.2
25
5.5.6.2
26
6.5.6.2
26
7.5.6.2
26
8.5.6.2
27
9.5.6.2
27
7.2
27
1.7.2
28
2.7.2
28
8.2
33
9.2
31
1.3
31
2.3
Testosterone
33
3.3
Thyroxine
46
4.3
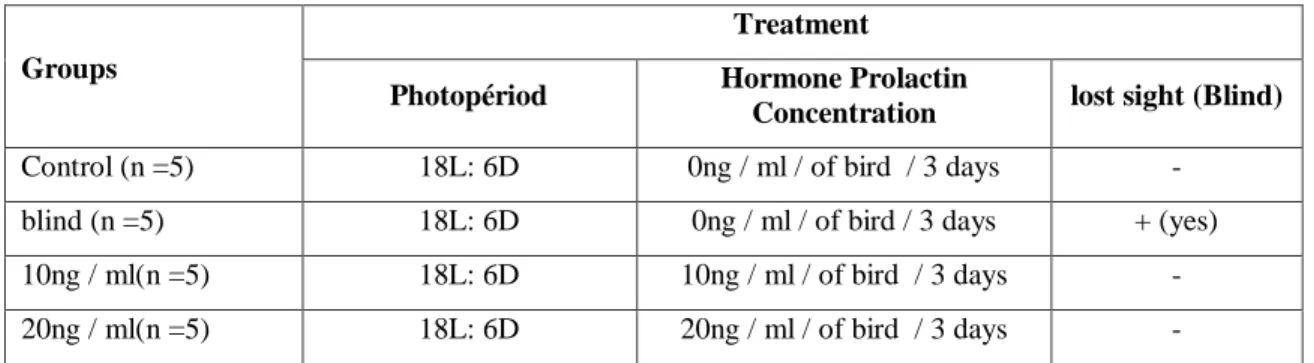
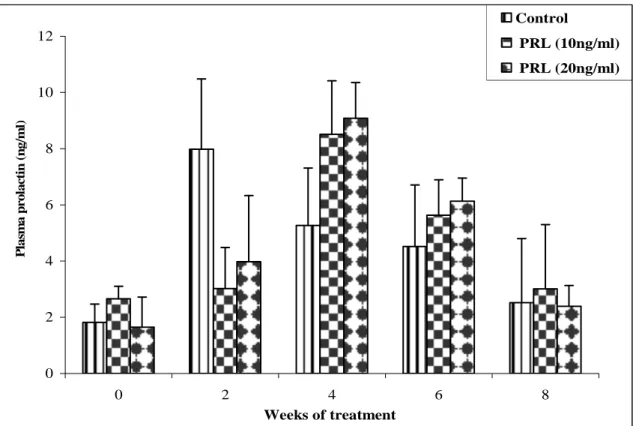
Prolactine:
48
5.3
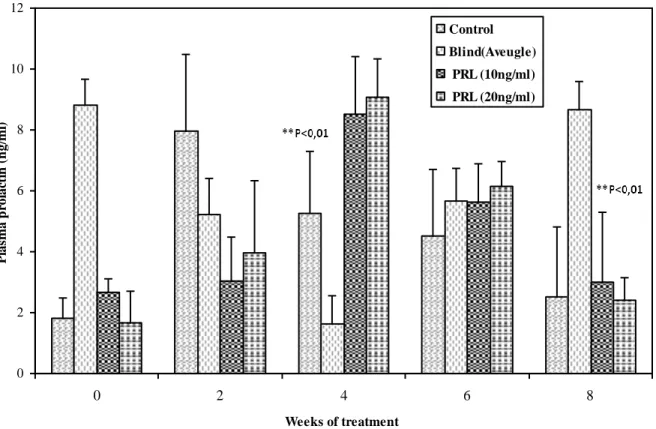
Protéine
43
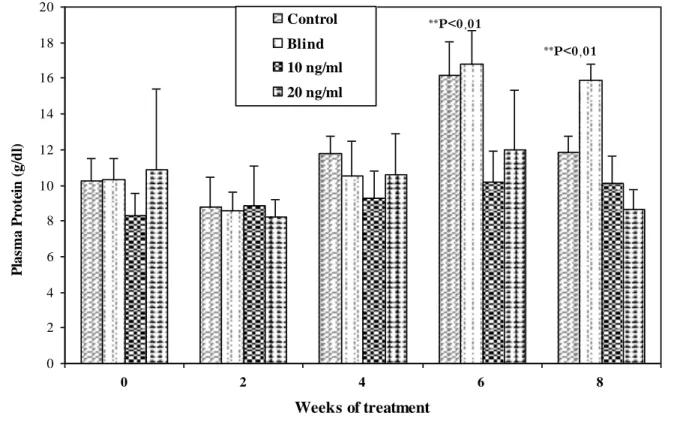
6.3
Glucose
41
7.3
43
8.3
45
9.3
47
1..3
53
4
53
68
71
73
75
1
17
2
13
1.2
14
2.2
14
3
16
1.3
17
4
18
1.4
21
5
21
1.5
22
2.5
22
6
24
1.6
26
2.6
26
7
27
1
x
13
2
pM
17
3
21
)
4
(
)
2
(
Columba livia
)
( 18 L:6D
m±SD,n=5
SD
m
n
32
لكش
)
5
(
:
mg/dl
(18L:6D)
(m±SD, n=5 )
SD
m
n
34
6
)
pM/L
(
)
18L:6D
(
)
m±SD, n=5
(
SD
m
n
36
)
7
(
)
ng/ml
(
18 L:6D)
(
)
m±SD, n=5
(
SD
m
n
38
)
8
(
)
g/dl
(
L:6D)
17
(
m±SD, n=5)
(
SD
m
n
41
9
)
mg/dl
(
Columba
livia
18 L:6D)
(
(m±SD,
n=5)
SD
m
n
42
10
Columba livia
18L:6D)
(
m± SD, n=5)
(
SD
m
n
44
11
Columba livia
18L:6D)
(
m± SD, n=5)
(
SD
m
n
12
Columba livia
18L:6D)
(
m± SD, n=5)
(
SD
m
n
46
48
1.13
IT
(Tissu
interstitiel)
Leydge
×
333
51
2.13
IT
(Tissu Interstitiel)
Leydge
×
333
51
3.13
TA
(Tunica albuginea) :S
(sperm)
×
333
51
4.13
IT
(Tissu interstitiel)
Leydge
×
4..
51
5.13
13
2
spermatogonia
×
333
52
6
13
2
spermatogonia
×
333
52
0
ةماعلا ةمذمملا
1
1
(Rowan,1925;Bissonnette,1931;Burger et al.,1977)
.
(Passériformes)
.
2
(Woitkewitsch, 1940 ; Goldsmith&Nicholls, 1984)
; Rowan, 1925)
1931
.(Bissonette,
Farner et al.,1988)
(
12
)
1980
Follet & Robinson,
3
.,1989)
et al
(Foster
)
1978
Reiter,
(
(Groos,1982 )
(Nicholls et
al.,1988)
Hypothalamus)
(
GnRH
FSH
LH
)
,1998
( Sharp& Dawson
4
(Woitkewitsch,1940)
.(Woitkewitsch, 1940 ; Goldsmith&Nicholls, 1984a)
Sturnus vulgaris
.
(13D : 11L)
.(Goldsmith et al.,
5
(13D : 11L)
.(Hamner,1971)
et al.,1989)
(Goldsmith
Couturnix
couturnix japonica
(16D :8L)
.(Follet et al ., 1988)
et al .,1985)
Haase
(
FSH
LH
LH
Buntin et al.,1987)
(
6
(El-Halawani et
al., 1991a)
12
Farner et al.1988)
(
( Boulakoud et
al., 1991)
(Hour Glasse)
12
(Saiovici et
al.,1987)
(Circannual
7
Rhythms)
365
(Gwinner,1971)
(Endogenous Rhythms)
)
12
(
(Scatophase)
(Photophase)
12
(Endogenous)
.( Hamner, 1971; Bunning, 1936)
. (Rétinales )
.GnRH
8
GnRH
(Gln8)
GnRH-I
II
(His5 , Trp7 , Tyr8 )
GnRH-.(King & Millar ,1982; Miyamota et
al., 1984)
GnRH-I
LH
FSH
(Mikami et al.,1988;
Katz et al., 1990; Sharp et al., 1988
LH
Interstitiel Cells
LH
Androgen
FSH
Abp(Androgen binding protien)
Gonocytes
primordiaux
.(Follett & Maung, 1978)
9
)
(
)
12
(
.(Dawson, 1989; Boulakoud & Goldsmith,
1994)
(Lofts& Murton,1965)
:
:
(Hamner, 1968)
(Nicholls et al., 1988)
)
10
GnRH
LH
FSH
.( Sharp et al., 1988)
)
(
GnRH
LH
FSH
8
)
(
LH
(Wilson & Follett,1974; Goldsmith & Nicholls,1984a,b
; Wilson,1985)
11
LH
FSH
.(Stetson et
al., 1973; Nicholls et al.,1983)
LH
FSH
GnRH
LH
FSH
.(Wingfield et al., 1979; Nicholls et al., 1984)
GnRH
12
.(Haase et al.,1985; Mauro et
al.,1989)
GnRH
FSH ,LH
.(Silverin et
al., 1989; Silverin,1994)
LH
.(Buntin et al., 1988; El Halawani et al.,
1991a,b)
. (Silver et al.1992;Wilson & Reinert, 1993;
13
(Dawson & Goldsmith, 1982; Goldsmith & Nicholls,
1984)
.(Juss &
Goldsmith, 1992; Juss,1993)
(Thyroxine, T3)
.
(Wilson & Follett ,1974)
.(Dawson,
1989a, b)
.(Wilson & Reinert,
1995a, 1996b; Reinert & Wilson, 1996a,b)
Spizella arborea
Sturnus vulgaris
(Wieselthier&
14
Passer domesticus
.(Wilson & Reinert,
1993, 1995a, b)
. (Moenter
et al ., 1991; Dahl et al., 1994; Parkinson & Follett, 1994; Parkinson
Follett, 1995)
(Dawson et al., 2001;
Bichops et al , 1996)
Coturnix
Coturnix Quail
Passer domesticus
.(Wilson &
Reinert, 1996)
.(Goldsmith & Nicholls., 1984)
.(Goldsmith & Nicholls,
1984a,b; Dawson, 1989a,b; Boulakoud et al., 1991)
Spizella arborea
Coturnix
Coturnix
Rouge-gorge
Embriza bruniceps
15
(Chaturvedi &Thapliyal 1983;
Thapliyal & Gupta, 1984)
12
(Farner& Wingfield, 1980)
.(Boulakoud
et al., 1991)
(18L:6D)
10
20
3
Columba livia
16
قرطلاو داوملا
لصفلا
يناثلا
17
2
1.2
Columba livia
10
15
8
)
130
346
(
5
)
60
54
52
(
36D)
(18L
15
)
21
1
°
(
70
75
4
10
3
20
3
1
2.2
1.2.2
5
)
60
54
52
(
318
)
21
1
°
(
70
75
18
6
6D)
(18L
15
(8L :16D)
05
05
05
10
3
05
20
3
1
8330
10330
2.2.2
5
19
.Xylocaine visquese
3.2
1
20
05
1
5
n
18L: 6D
0
3
5
n
18L: 6D
0
3
11
5
n
18L: 6D
10
3
21
5
n
18L: 6D
20
3
20
4.2
Xylocaine
visquese
2
035
1
2
1
(Dawson
et
al.,1986 ;
Wilson&Reinert,
1993 ;
21
1
3