© Delphine Hardy, 2018
The role of different subtypes of granule cells in the
adult olfactory bulb
Thèse
Delphine Hardy
Doctorat en neurobiologie
Philosophiæ doctor (Ph. D.)
The role of different subtypes of granule cells in the
adult olfactory bulb
Thèse
Delphine Hardy
Sous la direction de :
iii
R
ESUME
Le bulbe olfactif (BO) est un réseau neuronal complexe qui traite et transfert les informations olfactives aux structures corticales supérieures. Le réseau bulbaire est composé d’une large population de cellules granulaires (CGs) GABAergiques qui sont continuellement renouvelées tout au long de la vie de l’animal. Cette population peut exprimer différents marqueurs neuronaux comme la calretinine (CR+) et la CaMKIIα (CaMKIIα+), mais jusqu’à présent les études ayant eues pour but de comprendre le rôle des CGs dans le BO n’ont pas pris en considération cette hétérogénéité. Cependant, il est possible que différentes sous-populations de CGs présentent des propriétés morpho-fonctionnelles différentes et jouent un rôle spécifique dans le comportement olfactif. Ici nous comparons les caractéristiques morphologiques et eléctrophysiologiques des cellules CR+ générées chez l’adulte versus les cellules qui ne n’expriment pas la calretinine (CR−), ainsi que des cellules CaMKIIα+ générées chez l’adulte versus les cellules qui ne l’expriment pas (CaMKIIα−). Nous démontrons que les CGs CR+ et les CGs CaMKIIα+ présentent des morphologies similaires mais reçoivent moins de courants inhibiteurs que les cellules négatives. Nous révélons également qu’au sein d’une même sous-population, des cellules générées pendant le développement ou bien générées chez l’adulte ont les mêmes propriétés morpho-fonctionnelles. De plus, nous démontrons par quantification de l’expression de gènes à expression précoce immédiate ainsi que par l’inhibition de sous-populations de CGs à l’aide d’outils pharmacogénétiques combiné à des tests de comportement l’implication spécifique des cellules CR+ et des cellules CaMKIIα+ dans la discrimination olfactive spontanée et suite à un apprentissage associatif.
Dans le dernier chapitre de la section Résultat, nous révélons l’existence d’une nouvelle forme de plasticité structurelle présente uniquement sur les CGs générées chez l’adulte, permettant la formation de nouvelles synapses dans un lapse de temps très court. Nous montrons que les épines disposent de fins filopodes sur leurs têtes qui scrutent l’environnement et induisent une relocalisation
iv
des épines dépendamment de l’activité. Nous montrons également que ce phénomène dépend de l’activation des récepteurs AMPA et TrkB se trouvant sur les CGs par le glutamate et le BDNF libéré par les cellules mitrales.
iii
A
BSTRACT
The olfactory bulb (OB) is a complex neuronal network which processes and transfers olfactory information to higher cortical structures. The bulbar network is composed of a large population of GABAergic granule cells (GCs) which is continuously renewed throughout animal lifetime. This population can express diverse neurochemical markers such as calretinin (CR) and CaMKIIα, but so far most of the studies that aimed to unveil the role of GCs in the OB and odor behaviors didn’t take into consideration this heterogeneity. However, it is possible that different subpopulations of GCs display different morpho-functional properties and play specific roles in olfactory behaviors. Here we compared morphological and electrophysiological characteristics of adult-born CR-expressing cells versus CR-non expressing cells, as well as expressing cells versus CaMKIIα-non expressing cells. We showed that CR-expressing and CaMKIIα-expressing GCs display similar morphological properties but receive less inhibitory inputs than their respective negative counterparts. I also revealed that among the same subpopulation, cells generated during brain development or adulthood, display the same morpho-functional properties. In addition, we demonstrated by immunostaining for early-immediate gene markers as a proxy of neuronal activity and pharmacogenetic inhibition of GC subpopulations combined with behavioral tasks, the specific implication of CR- and CaMKIIα-expressing cells in spontaneous and odor-associative learning discrimination.
In the last chapter in Result section, we revealed the existence of a new form of structural plasticity occurring in adult-born, but not early-born GCs, in a very short time. We showed that spines display thin filopodia on their heads which scrutinize the microenvironment and induce spine relocation in an activity-dependent manner. We also revealed that this phenomenon depends on activation of AMPA and TrkB receptors located on GCs by glutamate and BDNF released by active mitral cells.
iv
TABLE OF CONTENTS
RESUME ... III ABSTRACT ... III LIST OF ABBREVIATIONS ... VIII LIST OF FIGURES ... XI LIST OF TABLES ... XIII REMERCIEMENTS ... XV AVANT-PROPOS ... XVII
I. INTRODUCTION ... 1
I.1.ADULT NEUROGENESIS ... 1
A. General Overview of adult neurogenesis ... 1
a. In rodents and other vertebrates ... 1
b. In humans ... 2
B. Different processes of adult neurogenesis in the rodents OB. ... 3
a. NSCs maintenance and progenitors proliferation ... 3
b. Migration ... 7
c. Adult-born neurons morphological maturation and integration ... 9
I.2.THE OB IN RODENTS ... 10
A. Role of olfaction in rodents ... 10
B. Structural and synaptic organization of the OB. ... 11
a. Cellular organization ... 11
b. Olfactory signal transduction ... 13
c. Synaptic organization ... 14
I.3.MORPHO-FUNCTIONAL PROPERTIES OF OBGCS ... 16
A. survival, synaptogenesis and plasticity in the OB. ... 16
a. Cell survival and distribution ... 16
b Synaptogenesis ... 19
c. Activity-dependent structural plasticity ... 24
B. Electrophysiological properties of GCs in the OB. ... 27
I.4.FUNCTIONAL ROLE OF GCS IN THE BULBAR NETWORK AND OLFACTORY BEHAVIOR 28 A. Functional role of adult-born GCs. ... 28
B. Different subpopulations of GCs. ... 35
I.5.HYPOTHESIS AND OBJECTIVES ... 37
II. RESULTS: THE ROLE OF CALRETININ-EXPRESSING GRANULE CELLS IN OLFACTORY BULB FUNCTIONS AND ODOR BEHAVIOR ... 39
II.1.RESUME ... 40
II.2.ABSTRACT ... 41
II.3.INTRODUCTION... 42
II.4.RESULTS ... 43
A. CR+ and CR− GCs display similar morphological characteristics ... 43
B. CR+ and CR− GCs receive similar excitatory but different inhibitory inputs. 45 C. Spontaneous odor discrimination increases the activation of CR+ GCs ... 48
v
D. The go/no-go olfactory learning task induces the activation of CR+ GCs ... 50
E. The pharmacogenetic inhibition of CR+ GCs diminishes the fine olfactory discrimination ability of mice ... 51
II.5.DISCUSSION ... 53
II.6.MATERIAL AND METHODS ... 56
A. Animals ... 56
B. Stereotaxic injections ... 57
C. Immunohistochemistry ... 58
D. Patch-clamp recordings ... 59
E. Morphological analysis ... 61
F. Analysis of gephyrin immunolabeling... 61
G. Behavioral procedures ... 62
a. Odor discrimination ... 62
b. Go/no-go olfactory discrimination learning... 62
c. Pharmacogenetic inactivation of CR-expressing GCs ... 64
H. Statistical analysis ... 65
II.7.REFERENCES... 66
II.8.FIGURES ... 70
II.9.TABLES ... 82
II.10.ACKNOWLEDGEMENTS ... 86
III. RESULTS: CAMKIIΑ EXPRESSION DEFINES TWO FUNCTIONALLY DISTINCT POPULATIONS OF GRANULE CELLS INVOLVED IN DIFFERENT TYPES OF ODOR BEHAVIOR. ... 87
III.1.RESUME ... 88
III.2.ABSTRACT ... 89
III.3.HIGHLIGHTS ... 90
III.4.INTRODUCTION... 91
III.5.RESULTS ... 92
A. Functionally heterogeneous populations of GCs in the adult OB ... 92
B. GCs can be divided into two functionally different subtypes based on the expression of CaMKIIα ... 93
C. The population of CaMKIIα+ GCs is sensitive to olfactory experience ... 95
D. Structuro-functional properties of CaMKIIα+ and CaMKIIα− GCs... 97
E. Perceptual learning activates CaMKIIα− GCs ... 98
F. CaMKIIα+ GCs are essential for spontaneous and go/no-go odor discrimination ... 100
III.6.DISCUSSION ... 103
III.7.STAR METHODS ... 105
A. Contact for reagent and resource sharing ... 105
B. Experimental models and subject details ... 106
III.8.METHOD DETAILS ... 107
A. Stereotaxic injections ... 107
B. Cranial window surgery and in vivo two-photon calcium imaging ... 108
C. Immunohistochemistry ... 109
D. Morphological analysis ... 110
vi
F. Novel odor stimulation ... 111
G. Sensory deprivation ... 111
H. Behavioral procedures ... 112
a. Odor discrimination ... 112
b. Perceptual learning ... 112
c. Go/no-go olfactory discrimination learning ... 113
d. Long-term associative memory ... 114
e. Pharmacogenetic inactivation of CaMKIIα+ GCs ... 114
I. Quantification and statistical analysis ... 115
J. Key resource table ... 117
III.9.REFERENCES... 120
III.10.FIGURES ... 126
III.11.ACKNOWLEDGEMENTS ... 142
III.12.SUPPLEMENTARY MOVIE LEGENDS ... 142
IV. RESULTS: PRINCIPAL CELL ACTIVITY INDUCES SPINE RELOCATION OF ADULT-BORN BULBAR INTERNEURONS ... 143
IV.1.RESUME ... 144
IV.2.ABSTRACT ... 145
IV.3.INTRODUCTION ... 146
IV.4.RESULTS ... 147
A. Dendritic spines of adult-born GC relocate in the OB network ... 147
B. Spine relocation is preceded by spine head filopodia growth ... 148
C. Relocated spines are maintained in the OB network ... 150
D. MC activity induces SHF directional growth and spine relocation ... 151
E. Glutamate released from MC controls the motility of SHF ... 153
F. MC-derived BDNF induces the spine relocation of adult-born GC ... 154
G. Spines with SHF are maintained after sensory deprivation ... 155
H. Spine relocation is involved in odor information processing ... 156
IV.5.DISCUSSION ... 157
IV.6.METHODS ... 160
A. Animals ... 160
B. Stereotaxic injection ... 160
C. Time-lapse two-photon imaging in vivo ... 161
D. Time-lapse two-photon imaging in vitro ... 162
E. Image analysis ... 163
F. Stimulation of mitral cells ... 165
G. Iontophoresis and puff application ... 166
H. Unilateral nostril occlusion ... 167
I. Immunohistochemistry ... 167 J. In situ hybridization ... 168 K. OB network model ... 169 L. Statistical analysis... 170 IV.7.REFERENCES ... 170 IV.8.FIGURES ... 174 IV.9.ACKNOWLEDGEMENTS ... 200
vii
V. CONCLUSION ... 201
VI. DISCUSSION AND PERSPECTIVES ... 203
VI.1.MORPHO-FUNCTIONAL PROPERTIES OF GC SUBPOPULATIONS. ... 203
VI.2.STRUCTURAL PLASTICITY IN GC SUBPOPULATIONS ... 206
VII. REFERENCES ... 211
ANNEX A. DIFFERENT FORMS OF STRUCTURAL PLASTICITY IN THE ADULT OLFACTORY BULB. ... 224
A.1.ABSTRACT ... 225
A.2.INTRODUCTION ... 226
A. Different forms of structural plasticity of GCs in the OB... 226
B. Activity-dependent regulation of different forms of structural plasticity ... 228
C. Cellular and molecular mechanisms underlying different forms of structural plasticity ... 230
A.3.CONCLUSION ... 231
A.4.ACKNOWLEDGEMENTS ... 232
A.5.REFERENCES ... 232
viii
LIST
OF
ABBREVIATIONS
AMPA 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid
AAV Adeno-associated virus
aCSF Artificial cerebrospinal fluid
AMPAR 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid receptor
AON Anterior olfactory nucleus
APV 4-Aminopyridine
AraC Cytosine-beta-D-arabinofuranoside
ATP Adenosine triphosphate
BDNF Brain-derived neurotrophic factor
BL Baseline
BMI Bicucullin methiodide
BMP Bone morphogenic protein
BrdU 5-bromo-2-deoxiuridine
BV Blood vessel
CaMKIIα Ca2+/calmodulin-dependent protein kinase IIα CaMKIIβ Ca2+/calmodulin-dependent protein kinase IIβ CaMKIV Ca2+/calmodulin-dependent protein kinase IV
cAMP Cyclic adenosine monophosphate
CB Calbindin
CBA Chicken β actin
Cc Corpus callosum
CdK5 Cyclin-dependent kinase 5
cFos Cellular oncogene response
ChR2 Channel-rhodopsin 2
CR Calretinin
Dcx Doublecortin
DG Dentate gyrus
DlX2 Distal-less homeobox 2
DNA Deoxyribonucleic acid
Dpi Days post injection
DREADDs Designer receptors exclusively activated by designer drugs EGFR Epidermal growth factor receptor
EPL External plexiform layer
EPSC Excitatory post synaptic current
GABA Gamma aminobutyric acid
GAD Glutamic acid decarboxylase
GC Granule cell
GCL Granule cell layer
GFAP Glial fibrillaric acidic protein
GFP Green fluorescent protein
GL Glomerular layer
ix
GTP Guanosine-5’-triphosphate
Igf Insulin-like growth factor IPL Internal plexiform layer
IPSC Inhibitory post synaptic current
Kyn Kynurenic acid
LOT Lateral olfactory tract
LTP Long-term potential
LV Lateral ventricle
MC Mitral cell
MCL Mitral cell layer
mEPSC Miniature excitatory post synaptic current mGluR2 metabotropic glutamate receptors 2 mIPSC Miniature inhibitory post synaptic current NaChBac Na+-selective channel of bacteria
NBQX 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline -7-sulfonamide
NeuN Neuronal nuclei
NMDA N-Methyl-D-Aspartate
NMDAR N-Methyl-D-Aspartate receptor
NSC Neural stem cell
OB Olfactory bulb
OE Olfactory epithelium
ONL Olfactory nerve layer;
OR Olfactory receptor
OSN Olfactory sensory neurons
P75 NTR p75 neurotrophin receptors
Pax6 Paired box gene 6
PFA Paraformadehyde
PGC Periglomerula cell
PSA-NCAM Polysialated neural cell adhesion molecule PSD95 Postsynaptic density protein 95
PSTHs Post-stimulus time histograms
PV Parvalbumin
RFP Red fluorescent protein
RMS Rostral migratory stream
RNA Ribonucleic acid
SAC Short-axon cell
sEPSC Spontaneous excitatory post synaptic current
SGZ Subgranular layer
SHF Spine head filopodia
SHH Sonic hedgehog
sIPSC Spontaneous inhibitory post synaptic current Sp8 specificity protein-8
Stim Stimulation
SVZ Subventricular zone
TC Tufted cell
x TrkB Tropomyosin-related kinase B
TTX Tetrodotoxin
VEGF Vascular endothelial growth factor
Wpi Week post-injection
xi
LIST
OF
FIGURES
Figure 1.1: Adult-born cells are constantly arriving into the OB. ... 2
Figure 1.2: Cellular composition of the ventricular–subventricular zone (V-SVZ). ... 5
Figure 1.3: Morphogenesis of adult-born GCs in the OB. ... 10
Figure 1.4: Cellular organization of the OB... 13
Figure 1.5: Synapses between MCs and GCs in the EPL providing recurrent and lateral inhibition on MCs. ... 15
Figure 1.6: Unilateral olfactory deprivation reduces the number of BrdU+ cells in the main OB. ... 18
Figure 1.7: Spinogenesis on adult-born GCs in the OB. ... 22
Figure 1.8: Long-term spine dynamic of adult-born GCs. ... 23
Figure 1.9: Sensory deprivation and intrinsic excitability differently control synaptic development in the dendritic domains of adult-born neurons. ... 26
Figure 1.10: Conditioning odor-reward association task (go/no-go). ... 30
Figure 1.11: Stimulation of adult-born neurons accelerates learning. ... 33
Figure 1.12: Olfactory bulb interneurons diversity. ... 36
Figure 2.1: Morphological characterization of early-born and adult-born CR+ GCs and adult-born CR− GCs ... 71
Figure 2. 2: The excitatory postsynaptic inputs of CR+ and CR− GCs are indistinguishable ... 73
Figure 2.3: CR+ GCs receive weaker inhibitory inputs than CR− GCs and have fewer gephyrin+ puncta on their primary dendrites ... 75
Figure 2.4: Spontaneous odor discrimination induces the activation of CR+ GCs 77 Figure 2.5: Odor discrimination learning activates CR+ GCs ... 79
Figure 2.6: The pharmacogenetic inhibition of CR+ GCs reduces the olfactory discriminatory ability of mice... 81
Figure 3.1: In vivo Ca2+ imaging of GC activity under baseline and odor stimulation conditions ... 127
Figure 3.2: Expression of CaMKIIα in the adult olfactory bulb ... 129
Figure 3.3: Sensory stimulation induces higher Ca2+ activity in CaMKIIα+ GCs .. 131
Figure 3.4: CaMKIIα+ cells are more responsive to sensory activity ... 133
Figure 3.5: Morphological and functional characteristics of CaMKIIα+ and CaMKIIα−GCs ... 135
Figure 3.6: CaMKIIα+ GCs are not required for perceptual learning ... 137
Figure 3.7: CaMKIIα+ GCs are required for go/no-go odor discrimination learning and spontaneous odor discrimination ... 139
Figure S3.1: GCs subtypes activation during long-term associative memory, ... 141
Movie 3.1: In vivo Ca2+ imaging of GCs during odor stimulation, Related to Figure 1 ... 142
Movie 3.2: In vivo Ca2+ imaging of two populations of GCs under baseline conditions, Related to Figure 2 ... 142
Figure 4.1: Relocation of mature spines of adult-born GC in the OB ... 175
Figure 4.2: SHF determine the relocation of the spines of adult-born but not early-born GC ... 177
xii
Figure 4.3: Relocated spines are stabilized in the OB network and are part of the
dendro-dendritic synapses ... 179
Figure 4.4: Olfactory sensory activity stabilizes SHF and promotes spine relocation ... 181
Figure 4.5: Glutamate released from MC stabilizes spine head filopodia ... 183
Figure 4.6: BDNF application promotes spine relocation ... 185
Figure 4.7: The activity of BDNF-lacking MC does not induce spine relocation .. 187
Figure 4.8: Spines with SHF are selectively maintained after sensory deprivation ... 189
Figure 4.9: Spine relocation promotes fast synchronization of MC with functional consequences for odor information processing ... 191
Figure S4.1: Direction vector amplitudes of GC spines and dendrites ... 193
Figure S4.2: SHF dynamic at different maturational stages ... 195
Figure S4.3: Random stimulation pattern of MC does not induce GC spine relocation ... 197
Figure S4.4: Activation of AMPARs is required for SHF motility. ... 199
Supplementary Movie 4.1: Spine relocation of 70 dpi adult-born GC in vivo. ... 200
Supplementary Movie 4.2 : Spine relocation of adult-born GC in vitro. ... 200
Figure A.1: Schematic representation of the mouse forebrain and of OB neurogenesis. ... 238
xiii
LIST
OF
TABLES
Table 2.1: Morphological properties of CR+ and CR− GCs ... 82 Table 2.2: Intrinsic electrophysiological properties of early- and adult-born CR+ GCs and adult-born CR− GCs. ... 83 Table 2.3: EPSC amplitudes and frequencies of early-born and adult-born CR+ GCs and adult-born CR− GCs ... 84 Table 2.4: IPSC amplitudes and frequencies of early- and adult-born CR+ GCs and adult-born CR− GCs, and number of gephyrin immunopuncta on adult-born CR+ and CR− GCs ... 85 Table 3.1: Key resource table ... 117
xiv
xv
R
EMERCIEMENTS
Je remercie mon directeur de recherche le Dr Armen Saghatelyan pour m’avoir accompagné et soutenu tout au long de ces six années et demie de thèse. Il m’a offert l’opportunité de réaliser un projet ambitieux tout en me permettant de contribuer à d’autres travaux. Il m’a aidé à améliorer mes compétences scientifiques et à développer mon esprit critique. Il a été à l’écoute de mes attentes et de mes problèmes et a toujours su me prodiguer de bons conseils.
Je remercie mon comité le Dr Igor Timofeev, la Dre Lisa Topolnik et le Dr Paul De Koninck d’avoir suivi l’avancement de mon projet de thèse ainsi que pour leurs conseils avisés. Je remercie aussi la Dre Graziella Di Cristo d’avoir accepter d’examiner mon travail de thèse.
Je remercie également mes collègues Archana, Karen, Sarah et Cédric pour avoir partagé mes joies et mes peines au cours de ces dernières années. Notre complicité et nos fous rires me manqueront. Votre soutien dans les moments les plus difficiles m’a aidé à aller de l’avant, et Archana ton humour involontaire m’a bien des fois redonné le sourire. Pour tout ça, merci beaucoup. Je remercie également tous les autres membres de l’équipe, passé et présent, Vincent, Linda, Arthur, Rodrigo, Qian, Alessandra, Tiziano, et Maxim, pour tous les bons moments partagés ensemble.
Je remercie Caroline pour son aide précieuse, ainsi que Marina pour tous ses judicieux conseils.
Je remercie ma famille pour leur soutien, mon père Dominique, ma sœur Aurélie et mon frère Mathéo. Depuis le tout début de mes études ils ont toujours cru en moi et m’ont donné les moyens de réussir. Leur confiance en moi m’a donné la force de persévérer. Durant toutes ces années d’études j’ai eu la chance de pouvoir compter sur l’immense soutien de mari Flavien qui a dû supporter toutes mes périodes de stress mais a toujours su trouvé les mots réconfortants dont j’avais besoin. Merci d’être là, à mes côtés, tous les jours.
xvi
Merci mon fils, mon Bastien, merci de me donner autant d’amour et d’égailler ma vie. Merci de me rappeler quotidiennement que les petites choses les plus simples font les plus grands bonheurs. Tu es ma force et ma fierté.
Enfin j’envoie le plus gros des mercis à ma maman Florence qui a été mon meilleur soutien et ma plus fidèle admiratrice. Si j’en suis là aujourd’hui c’est grâce à toi et à ton éducation. Depuis ma plus tendre enfance tu m’as répété que je devais faire un travail que j’aime et être épanouie, mon plus grand échec aurait été de te décevoir. J’ai suivi tes conseils maman je suis allée jusqu’au bout même si je voulais tout lâcher quand tu es partie. Tant de fois nous avons imaginé ensemble le jour de ma soutenance, nous en rêvions autant l’une que l’autre. Mais la vie a fait que je vais devoir vivre cette journée sans toi mais sache que ce diplôme c’est à toi que je le dois.
xvii
A
VANT
-
PROPOS
La première étude présentée dans ce mémoire a été en grande partie réalisée par moi-même, a été soumis en décembre 2017 et est en cours de révision dans le journal Scientific Reports. J’ai effectué l’ensemble des enregistrements électrophysiologiques, l’étude morphologique ainsi que toutes les analyses immunohistochimiques. Concernant les expériences de comportements olfactifs j’ai réalisé les tests de discrimination associée à une récompense (go/no-go) et l’étudiante Sarah Malvaut a réalisé le test de discrimination olfactif spontanée. Vincent Breton-Provencher a aidé à la réalisation des expériences lors des premières étapes du projet. Le Dr Saghatelyan a supervisé le projet.
La seconde partie des résultats présentés a fait l’objet d’une publication récente dans le journal Current Biology (2017) et dont je suis deuxième auteure à contribution égale. J’ai effectué une partie de l’analyse morphologique ainsi que l’analyse du recrutement des cellules dans les comportements olfactifs. Sarah Malvaut a réalisé les expériences d’imagerie calcique in vivo, et la majorité des expériences de comportement. Linda David a réalisé les enregistrements électrophysiologiques et l’équipe du Dr Isabelle Caillé (Simona Gribaudo, Laura Daroles, Alain Trembleau et Isabelle Caille) a été impliquée dans l’étude morphologique et une partie de l’analyse du recrutement des cellules dans les comportements olfactifs. Le Dr. Holzenberger et Zyana Chaker ont fourni les cerveaux de souris CamKIICre-TdTomato. Le Dr Daniel Côté a fourni le microscope 2-photon. Marie-Anne Lebel-Cormier, Simon Labrecque et le Dr Paul De Koninck ont contribué à l’élaboration de programmes Matlab nécessaires à l’analyse de l’imagerie in vivo. Le Dr Saghatelyan a supervisé le projet.
La dernière partie des résultats a été publiée dans le journal Nature Communication en 2016, dans lequel je suis deuxième auteure à contribution égale. Dans ce projet j’ai participé aux expériences d’enregistrements électrophysiologiques combinés à l’imagerie time lapse en 2-photon des épines dendritiques in vitro, ainsi qu’aux expériences de privation sensorielle. Vincent
xviii
Breton-Provencher a réalisé la majorité des expériences d’électrophysiologie et d’imagerie. Karen Bakhshetyan et le Dr. Rodrigo Roberto Bammann ont participé aux expériences d’imagerie in vivo et d’imagerie calcique in vitro respectivement. La Dre Marina Snapyan a réalisé les expériences de biologie moléculaire. Le Dr Daniel Côté a fourni le microscope in vivo 2-photon et le Dr Michele Migliore et Francesco Cavarretta ont réalisé l’étude de modélisation. Le Dr Saghatelyan a participé aux expériences d’imagerie in vivo et a supervisé le projet.
1
I.
INTRODUCTION
I.1.
A
DULT NEUROGENESIS
A. General Overview of adult neurogenesis
a. In rodents and other vertebrates
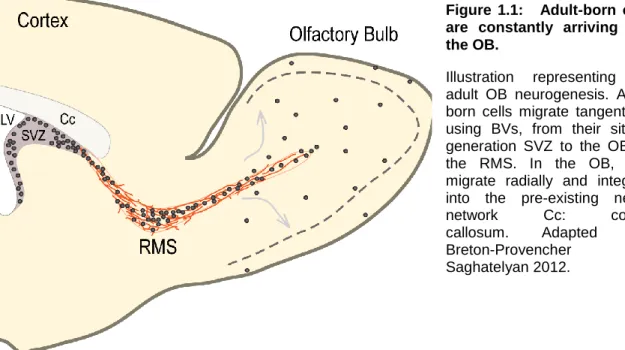
For decades neurogenesis was considered to occur only during embryogenesis. While in 1965, Altman and Das reported for the very first time the presence of adult-born neurons in rat hippocampus (Altman and Das, 1965), these findings were largely ignored (Rakic 1974; Rakic 1985). It is only at the end of the 20th century that research on adult neural stem cells extensively increased due to the development of new tools allowing to document the presence of newborn neurons in the adult brain. The existence of neural stem cells has been demonstrated, first, in the subventricular zone (SVZ) (Reynolds and Weiss, 1992; Richards et al., 1992), and later on, in the subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus (Gage et al., 1995; Palmer et al., 1997) of rodents. Neuroblasts emerging from SVZ migrate along the rostral migratory stream (RMS) to integrate the olfactory bulb (OB), and differentiate into two types of inhibitory interneurons: the granule cells (GCs) and the periglomerular cells (PGCs), which have distinct bulbar localizations, the granule cell layer (GCL) and the glomerular layer (GL), respectively (Figure 1.1). Cells from the SGZ do not migrate much to integrate the GCL of the dentate gyrus of the hippocampus, and differentiate into excitatory glutamatergic interneurons.
About 30 000 to 40 000 adult-born cells reach the OB every day, and only half of them differentiate and integrate the bulbar network (Petreanu and Alvarez-Buylla, 2002; Yamaguchi and Mori, 2005). The vast majority of adult-born neurons (95%) turns into GCs, and the rest becomes PGCs. Throughout the animal life, it has been evaluated that 10-15% of the GCs, and 30 % of the PGCs are renewed (Lagace et al., 2007; Ninkovic et al., 2007). Thus, in rodents, the OB represents the
2
largest neurogenic brain region exposed continuously to cell replacement and network rewiring, making it a very interesting model for studying the adult neurogenesis.
Neurogenesis also occurs in other animal species like birds and fish, where the phenomenon is less area-restricted. The proliferative regions are, like in mammals, found close to the ventricle but progenitors migrate to several areas of the telencephalon (Alvarez-Buylla et al., 1994; Ekstrom et al., 2001). In birds, adult-born cells integrate brain regions implicated in song control (higher vocal center and X area) (Goldman and Nottebohm, 1983; Lipkind et al., 2002), auditory processing (nidopallidum caudale) (Lipkind et al., 2002) and spatial learning (hippocampus) (Barnea and Nottebohm, 1996). In fish, the proliferative cells are found in a widespread manner along the rostrocaudal axis (Zupanc and Horschke, 1995; Ekstrom et al., 2001), and integrate several nuclei of the telencephalon, the OB and the cerebellum (Zupanc and Horschke, 1995; Zupanc et al., 2005; Grandel et al., 2006).
b. In humans
Figure 1.1: Adult-born cells are constantly arriving into the OB.
Illustration representing the adult OB neurogenesis. Adult-born cells migrate tangentially, using BVs, from their site of generation SVZ to the OB via the RMS. In the OB, they migrate radially and integrate into the pre-existing neural network Cc: corpus callosum. Adapted from Breton-Provencher and Saghatelyan 2012.
3
Although adult neurogenesis has been extensively studied in rodents, studies on humans are less abundant and are often controversial. Postnatal neurogenesis in human was described for the first time in 1998 by Eriksson and colleagues who demonstrated the existence of adult-born cells in human hippocampus and SVZ (Eriksson et al., 1998). These observations were confirmed by another study that took advantage of elevated concentration of atmospheric radioactive carbon atoms during the Cold War. The (14)C incorporate into DNA of proliferating cells allowing to birthdate neurons in humans and thus assess the presence of adult neurogenesis (Spalding et al., 2013). Although, stem cells are present in the SVZ, which is a neurogenic niche providing adult-born cells in rodents OB, the existence of adult-born neurons in human OB seems to be very low, or inexistent (Sanai et al., 2004; Bergmann et al., 2012). The presence of a migratory pathway leading to the OB is also controversial (Sanai et al., 2004; Kam et al., 2009). It seems conceivable that postnatal neurogenesis into the OB is decreasing with time, as studies reported the presence of a migrating corridor for neuroblasts in very young children (less than 6 month-old) and its disappearance in older children (from 2 year-old) and adult (Sanai et al., 2011). It is possible that adult neurogenesis in human OB vanished due to the fact that olfaction is not as crucial for survival as it is in other species like rodents. Interestingly, Ernst and colleagues demonstrated that neuronal precursors in the SVZ migrate into the adult striatum instead of OB (Ernst et al., 2014). Recently, the presence of adult neurogenesis in human DG was also challenged (Sorrells et al., 2018), and currently there is an intense debate about the existence of adult neurogenesis in humans (Andreae, 2018; Boldrini et al., 2018; Kempermann et al., 2018; Snyder, 2018) Thus, while it is generally agreed that stem cells are present in the adult brain, it is still debated whether these stem cells divide to produce new neurons and what is their functional implication.
B. Different processes of adult neurogenesis in the rodents
OB.
4
The SVZ is composed of different type of cells: the neural stem cells (NSCs), also called type B cells, which can be found in a quiescent or activated states, the transit-amplifying C type cells, the neuroblasts or type A cells, the ependymal cells, the astrocytes, and the blood vessels (BVs).
Adult NSCs are derived from radial glial cells. They are bipolar cells with basal process contacting the BVs and apical process bearing a primary cilia contacting the ventricle. They can switch from quiescent to activated, proliferative, state to give rise to the transit-amplifying type C cells, which in turn, divide to give rise to neuroblasts (Figure 1.2). Each of these different cell types can be identified by the expression of several protein markers:
- Quiescent type B cells, or quiescent NSCs, express the glial fibrillaric acidic protein (GFAP) and the transmembrane glycoprotein CD133 (prominin);
- Activated type B cells, or activated NSCs, express GFAP, prominin and the epidermal growth factor receptor (EGFR);
- Transit-amplifying type C cells, or progenitors, express EGFR but not GFAP;
- Type A cells, or neuroblasts, express the CD24, polysialated neural cell adhesion molecule (PSA-NCAM) or doublecortin (Dcx) proteins.
The microenvironment of the SVZ is crucial for the proliferation of NSCs. Alvarez-Buylla and Lim have demonstrated that transplantation of NSCs in the SVZ of another animal preserve the proliferative properties of NSCs, but not when the transplantation is done into a non-neurogenic region (Alvarez-Buylla and Lim, 2004). This microenvironment is regulated by the different cell types that compose the niche, and secrete several factors necessary for maintaining the balance between NSCs proliferation and quiescence, as well as progenitor proliferation.
Ependymal cells are multicilliated cells that compose the wall of the lateral ventricle (LV), and form a pin-wheel like structure by surrounding the primary cilia of NSCs (Mirzadeh et al., 2008; Shen et al., 2008). Ependymal cells secrete
5
Figure 1.2: Cellular
composition of the ventricular–subventricular zone (V-SVZ).
Coronal section of adult mouse brain is shown in the upper right. The V-SVZ region indicated by the black arrow is shown enlarged in the lower left. Type B1 cells (blue) are the astrocytes that serve as the V-SVZ stem cell. These can divide and produce type C cells (green), which are rapidly dividing, transit amplifying cells. Type C cells give rise to type A cells (red), the migratory neuroblasts. A BV is shown
neurogenic factors necessary for cell proliferation, such as bone morphogenic protein (BMP) inhibitor Noggin.
BMP activation promotes glia differentiation, and Noggin-mediated BMP inhibition induces progenitor proliferation (Lim et al., 2000; Peretto et al., 2004). However, it has been also shown that BMP activation favors neurogenesis and its inhibition, oligodendrogenesis (Colak et al., 2008). In addition to BMP several other soluble molecules such as insulin-like growth factor (Igf1, Igf2) (Lehtinen et al., 2011) or sonic hedgehog (SHH) (Palma et al., 2005) signalling pathways have also been demonstrated to be implicated in NSCs proliferation. The role of Wnt signalling pathway has been studied in mice neocortex development (Zhou et al., 2006), in hippocampal adult neurogenesis (Lie et al., 2005) and in the development and
regeneration of the olfactory epithelium (OE) (Wang et al., 2011). However, its potential role in SVZ NSCs proliferation remains unknown. The BVs are known to release soluble molecular factors, activating the Notch signalling pathway,
at the right. The apical surface of type B1 cells has a primary cilium and makes contact with the ventricle, which is at the left. These apical surfaces are found at the center of a “pin-wheel” composed of multiciliated ependymal cells (yellow). The V-SVZ can be subdivided into three domains based on the structure and spatial arrangement of type B1 cells: Domain I (apical) contains the type B1 cells apical process and the body of ependymal cells; domain II (intermediate) contains the cell body of most type B1 cells, which are in contact with the type C and A cells; and domain III (basal) contains the B1 cell’s basal process with end-feet on BVs. (Lim and Alvarez-Buylla, 2016)
6
necessary for promoting self-renewal cells abilities (Imayoshi et al., 2010) and inhibiting cell differentiation (Shen et al., 2004).
Neurotransmitters can also affect adult neurogenesis. For example, neuroblasts-derived γ-aminobutyric acid (GABA) regulates negatively cell proliferation (Liu et al., 2005; Fernando et al., 2011; Giachino et al., 2014), although dopamine (Hoglinger et al., 2004; Kim et al., 2010; Lennington et al., 2011), serotonine (Tong et al., 2014) and acetylcholine (Paez-Gonzalez et al., 2014) promote it.
The adult NSCs give rise to different types of interneurons in the OB, that express different markers (Calretinin (CR), Calbindin (CB), Ca2+/calmodulin-dependent protein kinase (CaMKIIα, CaMKIIβ, CaMKIV), Tyrosine hydroxylase (TH), metabotrophic gluatame receptors (mGluR2), etc) and have distinct localisations in the OB layers (GL, superficial or deep GCL). It has been demonstrated that the fate of the progenitors is determined very early during cell genesis, as it is controlled by transcriptional/morphogen codes in the different sub-regions of SVZ (Merkle et al., 2007) and the age of the animal (Batista-Brito et al., 2008). Merkle and colleagues showed that NSCs from ventral SVZ will preferentially give rise to GCs localized in the deep GCL and CB+ PGCs, while dorsal SVZ will be more prone to produce superficial GCs and TH+ PGCs. CR+ GCs and PGCs seem to originate from more anterior SVZ regions (RMS and the septal wall of the LV), (Merkle et al., 2007). In 2011, Ihrie and colleagues reported the prominent role of SHH in the cellular fate regulation of ventrally derived NSCs (Ihrie et al., 2011). The localized expression of some transcription factors in the SVZ, such as distal-less homeobox 2 (Dlx2), specificity protein-8 (Sp8) and paired box protein 6 (Pax6) seems to be as well decisive in the cellular fate (Hack et al., 2005; Waclaw et al., 2006; Brill et al., 2008; de Chevigny et al., 2012). NSCs fate is also temporally determined. TH+ and CB+ PGCs are mostly provided during early and late embryogenesis, respectively, although CR+ PGCs and GCs are preferentially generated postnatally (Batista-Brito et al., 2008). Even thought studies aiming at characterization of fate determination of the different subpopulations of OB interneurons have been published, the role of different subtypes of interneurons in
7
the bulbar network and odor behavior remains poorly documented (Murata et al., 2011; Malvaut and Saghatelyan, 2016; Takahashi et al., 2016).
b. Migration
Once neuroblasts are generated and exit the SVZ, they have to travel a 5 mm long journey to reach their final destination. They first migrate tangentially, and then radially once they are in the RMS layer of the OB (RMSOB). During cortical development, cells migrate by using the radial glial processes as physical substrate. In the adult brain, another migrating strategy is used. Neuroblasts make cell-cell contacts to form a chain of migratory cells ensheated by astrocytes, and follow very closely the BVs organized in parallel to the migratory stream (Lois et al., 1996; Bovetti et al., 2007b; Saghatelyan, 2009; Snapyan et al., 2009). In addition, all along their journey from SVZ to the OB, the migration occurs in a saltatory fashion, cells alternating migratory and stationary phases (Wichterle et al., 1997; Bovetti et al., 2007a; Snapyan et al., 2009).
Neuroblasts morphology is very different from the mature interneurons. These are very small cells with a leading process tipped with a dynamic growth cone, which is, most of the time, oriented toward the OB. During the migratory phase, the cell movement can be dissociated in three steps: (1) the neuroblast extends its leading process in the direction of migration, (2) a cytoplasmic dilatation is formed in front of the nucleus, and (3) the nucleus translocate to the leading process (Schaar and McConnell, 2005; Lalli, 2014). The cellular displacement relies on intracellular mechanisms regulating the actin filaments, responsible for cellular repolarisation, and the microtubules polymerisation-depolymerisation, responsible for maintenance and growth of leading process (Koizumi et al., 2006). Dcx, cyclin-dependent kinase 5 (CdK5) and dynein regulate these processes (Tanaka et al., 2004a; Tanaka et al., 2004b; Koizumi et al., 2006; Hirota et al., 2007).
The tangential migration relies on the presence of several molecular cues, acting at different levels of the migratory process, such as PSA-NCAM, brain derived
8
neurotrophic factor (BDNF), GABA, slit/robo and integrins/laminin pathways. The PSA-NCAM and the integrins/laminin pathway have been demonstrated to be implicated in the cell-to-cell contact (Cremer et al., 1994; Hu et al., 1996; Lois et al., 1996; Belvindrah et al., 2007). The slit/robo pathway acts as repellent cue and is, at least in part, responsible for the maintenance of the neuroblasts inside the tube-like structure formed by astrocytes (Kaneko et al., 2010). Slit1 is released by neuroblasts and binds to robo2 receptors present on ensheating astrocytes (Kaneko et al., 2010). BDNF is secreted by endothelial cells of BVs and promotes neuronal migration via low affinity p75 neurotrophin receptors (p75NTR) localized on neuroblasts plasma membrane (Snapyan et al., 2009). The GABA released by neurobasts appears to be also important for postnatal cell migration, as increasing GABA concentration leads to the reduction of the rate of chain migration via the activation of GABAA receptors, and, on the contrary, antagonizing GABAA receptors increased the migration rate (Bolteus and Bordey, 2004). GABA released by neuroblasts also activates astrocytic GABAA receptors leading to the recruitment of high affinity tropomyosin-related kinase B (TrkB) receptors in a Ca2+-dependent manner, which in turn binds to the BDNF (Bolteus and Bordey, 2004; Saghatelyan, 2009; Snapyan et al., 2009). It is thus possible that the reduced BDNF concentration in the microenvironment available for neuroblasts induces their entry in a stationary phase (Bolteus and Bordey, 2004; Saghatelyan, 2009; Snapyan et al., 2009). In addition, it has been reported, that the activation of a Ca2+-dependent K+ channel (KCa3.1) present on neuroblasts in the SVZ and RMS, prolonged the stationary phases, and, consequently, regulates negatively neuroblasts migration (Turner and Sontheimer, 2014).
Once the migrating neurons enter the RMSOB, they have to reach the different layers of the OB (Lledo and Saghatelyan, 2005). To do so, they switch from tangential to radial migration that requires the implication of other molecules. Two glycoproteins have been shown to be essential for radial migration: the extracellular matrix glycoprotein tenascin-R, which is expressed in the GCL (Saghatelyan et al., 2004), and, reelin, which is expressed in the MCL (Hack et al., 2002; Kim et al., 2002). Both proteins play an important role in the detachment of
9
neuroblasts from the migrating chain as well as in their final migration to the OB cellular layers (Hack et al., 2002; Kim et al., 2002; Saghatelyan et al., 2004; David et al., 2013).
c. Adult-born neurons morphological maturation and
integration
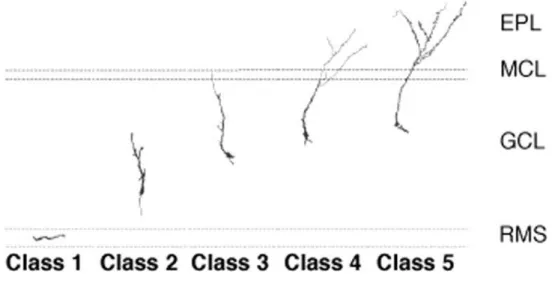
When adult-born cells reach their final destination they have to functionally integrate into the bulbar network. To do so, they undergo morphological and electrophysiological metamorphosis to become either GCs or PGCs. The maturation of neuroblasts into GCs has been described as a succession of 5 stages (Figure 1.3) (Petreanu and Alvarez-Buylla, 2002; Carleton et al., 2003). The stage 1, occurring between 2 to 7 days after cell birth, corresponds to tangentially migrating neurons in the RMS and RMSOB, that display a round or elongated cell body prolonged with a leading process tipped with a dynamic growth cone, and occasionally a trailing process (Figure 1.3). The stage 2 corresponds to radially migrating cells (Figure 1.3) that have been described as neurons with longer trailing and leading processes where the latter can sometimes be bifurcated (7 to 11 days after cell generation). From the age of 9 day-old, cells have reached their final destination (stage 3, Figure 1.3). They present a larger and rounder cell body, and develop basal dendrites, as well as a preliminary draft of the future primary dendrite. They also start to display varicosities on the cell body and basal dendrites. The 4th stage begins to be observed since day 11 and is mainly characterized by apical dendritic development (Figure 1.3). The primary dendrite extends toward the external layers of the OB and is ramified in aspiny secondary and tertiary dendrites. On the 5th and last stage the cells are fully developed with dense spiny apical dendrites (Figure 1.3), and are morphologically indistinguishable from pre-existing neurons. However, adult-born and pre-existing GCs have different preferential localisation into the GCL. The deep layers are mainly colonized by adult-born neurons while the superficial layers preferentially contain pre-existing GCs (Lemasson et al., 2005; Imayoshi et al., 2008), suggesting that the two populations have different cellular targets and distinct role
10
in the bulbar network and olfactory behavior (Orona et al., 1983; Koyano et al., 2005).
A minor proportion of neuroblasts give rise to PGCs which integrate the most external layer of the OB. They can have varying morphological properties, displaying several primary dendrites (Whitman and Greer, 2007b; Kiyokage et al., 2010). As GCs, PGCs reach their final destination around 10 days after generation (Mizrahi, 2007; Kovalchuk et al., 2015), implying a faster migration speed and/or a more rostral progenitors origin (Hack et al., 2005). 10 day-old PCGs display essentially ramified non-spiny dendrites (Mizrahi, 2007) and acquire a fully mature morphology only after 6 weeks (Whitman and Greer, 2007b).
I.2.
T
HE
OB
IN RODENTS
A. Role of olfaction in rodents
The main OB is crucial for odor detection and discrimination and plays a central role in rodents’ social interactions and survival. It conveys and processes odors information from the OE to cortical structures. Olfaction is essential for many
Figure 1.3: Morphogenesis of adult-born GCs in the OB.
Representative morphological evolution of adult-born GCs from their arrival in the RMSOB (class
11
animal species including rodents as it allows them to locate food (Yang and Crawley, 2009), and identify predators (Wallace and Rosen, 2000). Olfactory deprivation suppresses sexual behavior in mice (Rowe and Edwards, 1972) and abolishes the preference for opposite-sex olfactory cues in both sex (Keller et al., 2006) and reduces lordosis behavior in female, representative of sexual receptivity (Thompson and Edwards, 1972; Edwards and Burge, 1973). The role of olfaction in rodents sexual behavior is controversial depending on the method used to abolish/impair olfaction (Edwards and Burge, 1973; Mandiyan et al., 2005; Keller et al., 2006) which can affect or not the vomeronasal organ known to be important for pheromones detection, and thus for sexual behavior (Pfeiffer and Johnston, 1994; Boehm et al., 2005). Maternal behavior that includes pups retrieval, nesting and ano-genital licking, highly relies on specific odors and pheromones detection. Indeed, olfactory impairments in mothers lead to the loss of maternal behavior in rodents (Gandelman et al., 1971; Seegal and Denenberg, 1974; Dickinson and Keverne, 1988; Fraser and Shah, 2014). Anosmic animals are also used as depression model since severe anosmia leads to anxiety and depressive-like behavior (Zueger et al., 2005; Hellweg et al., 2007).
B. Structural and synaptic organization of the OB.
a. Cellular organization
The main OB displays a very structured cellular organization divided in several concentric circles of cell layers (Shepherd, 2004). The most external layer is the olfactory nerve layer (ONL) followed by the GL, mostly composed of PGCs and short-axon cells (SACs) organized in small glomeruli (Figure 1.4). PGCs are numerous, intrinsic interneurons mostly anaxonic and GABAergic, displaying several phenotypes and morphologies with dendrites extending through 1 or 2 glomeruli. It has been shown that PGCs can express the glutamate decarboxylase (GAD), TH, CB, CR, parvalbumine (PV) (Kosaka et al., 1995; Bagley et al., 2007; Whitman and Greer, 2007b; Batista-Brito et al., 2008; Brill et al., 2008; Kiyokage et al., 2010; Fogli Iseppe et al., 2016). In contrast, SACs are glutamatergic
12
interneurons, present in small number, and contact several adjacent glomeruli (Brill et al., 2009). The external plexiform layer (EPL) is located after GL. The EPL mainly contains the primary and lateral dendrites of the mitral cells (MCs), secondary dendrites of GCs (Figure 1.4), but also some GABAergic and glutamatergic interneurons, and tufted cells (TCs). TCs are glutamatergic principal neurons which can display different morphological characteristics. Some TCs present lateral dendrites extending through the EPL in a symmetric or asymmetric manner with respect to the soma, and a primary dendrite with apical ramification contacting one glomerulus (Figure 1.4). TCs project axons to higher cortical layers (Macrides and Schneider, 1982; Antal et al., 2006). The EPL is delimited by a monolayer of MCs somi called mitral cell layer (MCL, Figure 1.4).
MCs represent the second type of glutamatergic principal neurons in the OB and also send afferences to cortical structures. They have lateral dendrites extending in opposite direction into the EPL, and a primary dendrite extending to the GL. The very distal part of the MC primary dendrite ramifies in several small dendrites contacting a single glomerulus (Figure 1.4). Afferent TCs and MCs axons bundle altogether to form the lateral olfactory tract (LOT) and converge to several brain regions such as piriform cortex, olfactory tubercle (Nagayama et al., 2010), anterior olfactory nucleus (AON), hippocampus, enthorinal cortex and amygdala (Haberly and Price, 1977; Neville and Haberly, 2003). The MCL layer is followed by a narrow internal plexiform layer (IPL) and the GCL. The GCL is the largest cell layer of the OB mostly represented by GCs. GCs have small cellular bodies, they display a primary dendrite giving rise to ramified secondary dendrites extending to the EPL (Figure 1.4). These cells have also small basal dendrites and are anaxonic. The output synapses of GCs are dendrodendritic reciprocal synapses and are located on the secondary dendrites. The most internal layer is the RMSOB containing neuroblasts which will further differentiate into interneurons.
13 b. Olfactory signal transduction
Volatile odorant molecules present in the air enter the nasal cavity and get trapped in the mucosa of the OE. These molecules will be detected by odor receptors (ORs) located on cilia of apical dendrites of olfactory sensory neurons (OSNs), and the signal will be transduced from chemical to electric, and transferred to OB neurons via OSN axons. Each OSN expresses only one type of OR and all OSNs expressing the same OR converge their axons to the same glomerulus in the OB. Mice display about 1000 different ORs (Godfrey et al., 2004), which are seven-transmembrane domains G-protein (Golf) coupled receptors. The liaison of odorant molecules to the receptor induces a signaling cascade starting with the activation of the Golf protein which binds to an adelynate cyclase leading to an increase of the Figure 1.4: Cellular organization of the OB.
Odor inputs coming from OSN innervate glomeruli of GL. They make synaptic contact with the tufted apical extremity of MCs/TCs primary dendrite and some PGCs. The EPL is mainly composed of TCs cell bodies and dendrites of principal and GCs. The narrow MCL contains MCs somi. The GCL is composed of GCs somi, which can be located in the superficial (1) or deep (2) layers, and MCs/TCs axons.
14
intracellular cyclic adenosine monophosphate (cAMP) concentration (Breer et al., 1990). cAMP activates a Na+/Ca2+ permeable channel increasing the intracellular cationic concentration contributing to cell depolarisation. In turn, Ca2+ present in the cell activates a Ca2+-gated Cl- channel extruding the anion which is largely responsible for cell depolarisation (Menini, 1999). Finally, to stop the transduction signal the receptor gets phosphorylated, cAMP is hydrolysed by phosphodiesterases and intracellular Ca2+ concentration is reduced via Na+/Ca2+ exchanger (Breer, 1994, 2003).
c. Synaptic organization
The transfer of olfactory information from the OE to the cortex is operated by the 2 principal cells of the OB (MCs/TCs). Apical dendrites of MCs/TCs make monosynaptic contacts with OSN axons and convey the signal to higher cortical structure. PGCs of the GL form dendrodendritic synapses with MCs/TCs. In addition, distal dendrites of GABAergic/dopaminergic TH+ PGCs mainly receive excitatory inputs from OSN axons expressing the dopamine receptor D2 which activation leads to decreasing glutamate release. TH+ PGCs also receive excitatory inputs from serotoninergic centrifugal fibers (Ennis et al., 2001; Kiyokage et al., 2017). This suggests that TH+ GCs play an important role in the early modulation of odor processing by MCs/TCs. In addition it has been demonstrated that presynaptic OSNs inputs are highly regulated by intraglomerular feedback inhibition (McGann et al., 2005). Glutamatergic short-axon cells of GL form intraglomerular synapses with the primary dendrites of MCs/TCs and interglomerular connexions with PGCs. MCs/TCs lateral dendrites synapse with GCs secondary dendrites in the EPL (Figure 1.5).
15
The dendrodendritic connexions between GCs/PGCs and MCs/TCs are called reciprocal synapses (Jahr and Nicoll, 1982a, b; Isaacson and Strowbridge, 1998). MCs/TCs activation leads to dendritic glutamate release on GCs/PGCs dendrite which in turn inhibits the MCs/TCs due to GABA release at the same synapse (recurrent inhibition, Figure 1.5) (Whitman and Greer, 2007a). In addition, since GCs are connected with several MCs/TCs, the excitation of one principal cell leads to lateral inhibition of the surrounding MCs/TCs (Urban, 2002) (Figure 1.5). This GCs/PGCs mediated inhibition enables firing synchronisation of MCs/TCs (Lagier et al., 2007; Lepousez and Lledo, 2013) which is critical for fine spatio-temporal tuning of MCs/TCs responses to odors (Yokoi et al., 1995; Urban, 2002; Urban and Sakmann, 2002; Arevian et al., 2008; Fukunaga et al., 2014; Grier et al., 2016). The reciprocal MCs/TCs inhibition mainly relies on the activation of
N-methyl-D-Figure 1.5: Synapses between MCs and GCs in the EPL providing recurrent and lateral inhibition on MCs.
Electron micrographs of synapses in the EPL. A, A dendrodendritic synapse in the EPL between a MC dendrite and a GC spine, unlabeled, for reference. The mitral to granule synapse is defined by a collection of small spherical vesicles closely apposed to the presynaptic membrane of the MC secondary dendrite, and an asymmetrically thick membrane specialization in the GC spine head. The reciprocal granule to mitral synapse is defined by the elliptical cluster of vesicles in the spine head and symmetrical thickenings in the presynaptic and postsynaptic membranes. B, C, Examples of mitral to granule excitatory synapses on labeled spines at 42 days post injection (dpi). Virally labeled cells were marked by GFP immunohistochemistry and DAB to form an electron dense product, so spines of new GCs are darkly stained. D, An example of a bidirectional dendrodendritic synapse on the same spine head. On the right, the mitral to granule synapse can be seen, and on the left, the granule to mitral inhibitory synapse. Arrows indicate the direction of the synapse. Scale bar, 1 μm. Adapted from Whitman and Greer, 2007a. E. Schematic representation of recurrent (red arrows) and lateral (blue arrows) inhibition providing on MCs by GCs.
16
aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-isoxazolepropionic (AMPA) receptors (on GCs/PGCs) and the ionotropic GABAA receptors (on MCs/TCs lateral dendrites). The AON and the piriform cortex send glutamatergic top-down inputs onto both MCs/TCs and interneurons of the OB, leading to, respectively, MCs/TCs direct excitation and feedforward inhibition mediated by metabotropic GABAB receptors (Haberly and Price, 1978; Mazo et al., 2016). In addition, neuromodulatory brain regions such as locus coeruleus (noradrenergic) (Shea et al., 2008), raphe nucleus (serotoninergic) (McLean and Shipley, 1987; Petzold et al., 2009) and the horizontal limb of the diagonal band of Broca (cholinergic) (Wenk et al., 1977; Shipley and Ennis, 1996) also send fibers onto OB interneurons. These cortical feedbacks modulate OB network response to olfactory stimulation (Boyd et al., 2012; Ma and Luo, 2012; Markopoulos et al., 2012; Nunez-Parra et al., 2013).
I.
3.
M
ORPHO
-
FUNCTIONAL PROPERTIES OF
OB
GC
S
A. Survival, synaptogenesis and plasticity in the OB.
a. Cell survival and distribution
Apoptosis represents an important cellular process that regulates the number of neurons during both embryogenesis and adulthood. However, the survival rate of interneurons produced during early life is higher than adult-born generated interneurons (Petreanu and Alvarez-Buylla, 2002; Lemasson et al., 2005; Yamaguchi and Mori, 2005). Indeed, in rodents, out of ≈40 000 adult-born cells generated every day only 50% are going to mature and functionally integrate the OB. In contrast, the majority of cells generated during embryogenesis or early post-natal days (hereafter called early-born cells) persist throughout life (Petreanu and Alvarez-Buylla, 2002; Lemasson et al., 2005; Yamaguchi and Mori, 2005). While both populations are found uniformly distributed across the rostro-caudal OB axis, they have distinct dorso-ventral distribution (Lemasson et al., 2005).
17
Early-born GCs appear to be more prone to invade the superficial layers of the GCL while adult-born GCs are more present in the deepest layers (Lemasson et al., 2005). Nonetheless, GABAA receptors composition seems to differ between superficial and deep GCs as the decay time of inhibitory events from superficial GCs is slower than deeper cells (Carleton et al., 2003). Importantly, cells in the deep or superficial layers of GCL have similar dendritic arborisation but the different localisations suggest that they could make synapses with different type of cells (Orona et al., 1983; Koyano et al., 2005). Cells found in the deepest regions of GCL extend the secondary dendrites into the EPL close to the MCL. In contrast, cells located in the superficial layers extend their dendrites into the EPL close to the GL, and are thus, probably more prone to form reciprocal synapses with the external TCs. External TCs connect iso-functional odor columns via intrabulbar projections (Belluscio et al., 2002; Lodovichi et al., 2003; Zhou and Belluscio, 2008). It is thus possible that superficial GCs-mediated inhibition plays a role in the synchronization of iso-functional odor columns within the same OB and thus facilitates odor discrimination. This different localization can underlie different role of GCs in the bulbar network and olfactory behavior. Sensory activity can modulate the survival rate of both early- and adult-born neurons. For example, sensory deprivation reduces the number of, not only adult-born neurons (Figure 1.6) (Petreanu and Alvarez-Buylla, 2002; Saghatelyan et al., 2005; Mandairon et al., 2006c; Bovetti et al., 2009; Bastien-Dionne et al., 2010; Denizet et al., 2017), but also the number of early-born GCs and PGCs (Bastien-Dionne et al., 2010) leading to a global reduction of OB size (Figure 1.6), and specifically impairs development and expression of dopaminergic PGCs (Bovetti et al., 2009; Bastien-Dionne et al., 2010). Alteration of adult-born cells integration by sensory deprivation is effective only during the critical period of cell development lasting from 2 to 4 weeks after cell birth (Yamaguchi and Mori, 2005; Mandairon et al., 2006c).
18
In opposition to sensory deprivation, adult-born neurons survival rate is temporary promoted by odor-enriched environment (Rochefort et al., 2002; Rochefort and Lledo, 2005; Bovetti et al., 2009; Moreno et al., 2009), and return to normal values approximately 1 month after odor exposure (Rochefort and Lledo, 2005; Bovetti et al., 2009). In line with these observations, olfactory behaviors such as olfactory discrimination or associative learning tasks, happening during the critical period (Mouret et al., 2008), improve specifically survival of adult-born cells located in the bulbar regions responding to odors (Alonso et al., 2006; Mandairon et al., 2011; Sultan et al., 2011b). Interestingly, adult-born neurons recruited during a session of
Figure 1.6: Unilateral olfactory deprivation reduces the number of BrdU+ cells in the main OB.
A. Photomicrographs of coronal sections displaying the different layers of the OB. Note the size difference between the control (Open) and the occluded (Occl.) bulb sections of the same animal taken at the same rostrocaudal position. B. BrdU+ cells in the GCL of the control (Open) and odor-deprived (Occl.) bulbs. C and D. Decrease in the number of adult-born neurons (C) and GCL area (D) following odor deprivation. E. Density of BrdU-labeled cells in the control and odor-deprived bulbs. BrdU counting was performed 21 days following BrdU injections and 33 days following unilateral nostril occlusion. *p < 0.05 with a Student’s t test. Adapted from Saghatelyan et al., 2005.
19
odor-reward association task are submitted to apoptosis after a second conditioning session in order to break the odor-reward association, and thus erasing the odor memory trace from OB (Sultan et al., 2011a).
It is also important to take into consideration the diversity of NMDA receptors subunit composition that can be found on different dendritic regions, and evolve over time. The alteration of NMDA receptors composition can affect their response to glutamate release and affect adult-born cells integration and/or survival rate (Platel et al., 2010; Kelsch et al., 2012). Notably, GluN2b-containing NMDA receptors are mainly expressed in immature brain in rats (Monyer et al., 1994), but the deletion of this subunit doesn’t affect GCs synaptogenesis, dendrites formation and maturation of inhibitory synaptic inputs. However, the density of postsynaptic density protein 95 (PSD95) clusters on primary dendrite is reduced leading to a negative alteration of glutamatergic inputs which impairs cell survival (Kelsch et al., 2012). These results are in line with previous data showing the implication of GluN1-containing NMDA receptors in neuroblasts survival rate in the RMS (Platel et al., 2010).
b Synaptogenesis
Although the OB is continuously supplied with new GCs throughout animal’s life, the vast majority of GCs are early-born GCs (Lagace et al., 2007; Ninkovic et al., 2007). Early- and adult-born GCs integrate the OB network under very different environmental conditions since in the former case the neuronal network is immature, whereas in the latter case it is fully functional, and has already been subjected to olfactory stimulations and memory. Analysis of GCs inputs and outputs maturational profiles based on quantification of presynaptic and postsynaptic proteins, synaptophysin and PSD95, respectively, showed that early-born GCs develop glutamatergic synaptic inputs (starting between 10 to 14 days after cell birth on proximal and apical dendrites, and 14 to 17 days on basal dendrites) and GABAergic outputs synapses (starting 14 days after cell birth) simultaneously. On the contrary, adult-born GCs display a sequential synaptic
20
development. They first develop excitatory and GABAergic inputs synapses on the primary dendrite very rapidly after their arrival in the bulb (Whitman and Greer, 2007a; Kelsch et al., 2008; Panzanelli et al., 2009; Pallotto et al., 2012). Later on, axo- and dendrodendric glutamatergic inputs are formed on basal and secondary dendrites respectively, as well as inhibitory outputs on secondary dendrites (Kelsch et al., 2008). During maturation PSD95 cluster proteins are present on immature adult-born GCs dendrites, suggesting a possible role of glutamate release by MCs/TCs in the maturation of adult-born interneurons (Whitman and Greer, 2007a; Panzanelli et al., 2009; Breton-Provencher et al., 2014).
Both inhibitory and excitatory spontaneous inputs increase as the interneuron matures, but analysis of gephyrin and PSD95 clusters revealed that their formation and distribution across the different cell compartments and time evolve differently (Carleton et al., 2003; Whitman and Greer, 2007a; Kelsch et al., 2008; Panzanelli et al., 2009; Pallotto et al., 2012). It has been shown that gephyrin clusters densities remain stable on soma, basal and primary dendrites, while PSD95 densities increase during the last maturational stages (class 4 to class 5). On immature adult-born GCs soma, gephyrin density is higher than PSD95, but this repartition is inverted on mature GCs. On basal dendrites, GABAergic and glutamatergic synapses are first equally represented, but the number of PSD95 clusters increases and exceeds gephyrin clusters. On the primary dendrite, gephyrin clusters are predominant on immature cells, but glutamatergic synapses increase with time to finally be equally represented (Panzanelli et al., 2009). In the EPL, the dendrodendritic synapses between GCs and MCs/TCs dendrites are formed progressively from stage 3 to stage 5. The reciprocal synapses of the OB are identifiable by the direct apposition of GABAARα1/α3 on MCs/TCs lateral dendrites and PDS95 clusters on GCs dendrites (Panzanelli et al., 2009). Viral labelling of adult-born GCs coupled with immunostaining against PSD95 and GABAA receptors during maturation revealed that both PSD95 and GABAA receptor clusters “alone” decrease progressively and their direct apposition increases from class 3 to class 5 neurons (Whitman and Greer, 2007a; Panzanelli