Impact des pratiques agricoles intensives sur la
structure du sol: paramètres physiques et
biologiques
Thèse
Yan Xu
Doctorat en sols et environnement
Philosophiæ doctor (Ph. D.)
Québec, Canada
Impact des pratiques agricoles intensives sur la
structure du sol: paramètres physiques et biologiques
Thèse de doctorat
Yan Xu
Sous la direction de :
Léon-Etienne Parent, Ph.D., directeur de recherche
Noura Ziadi, Ph.D., codirectrice de recherche
Doctorat en Sols et Environnement
ii
Résumé
Les pratiques agricoles intensives produisent un compactage du sol qui influence la plupart des propriétés et des processus physiques, chimiques et biologiques du sol qui favorisent l'agrégation. Les microorganismes du sol jouent un rôle majeurs dans la structure physique du sol. Ce travail visait à (1) construire des modèles de prédiction des indices de compactage du sol, (2) mesurer l'impact des pratiques de travail du sol sur l’agrégation, (3) construire des modèles de prédiction des indices d’agrégation, et (4) mesurer la réponse de la diversité bactérienne du sol à l'agrégation et au compactage. L'ensemble de données comprend la teneur en eau gravimétrique, la masse volumique apparente, la distribution des particules, la répartition granulométrique, la masse volumique maximale, la teneur critique en eau, le carbone organique total, l'azote total, les concentrations de Si, Fe, Al, Mn, Mg et Ca dans des échantillons du sol prélevés sur 49 sites au Québec en 2014 pour élaborer les modèles de compactage et d’agrégation. Les données de 94 essais comparant les systèmes de labour conventionnel et de semis direct provenaient de 20 articles. Des échantillons de sol prélevés sur 16 fermes au Québec en 2014 ont été analysés pour diverses mesures de la diversité bactérienne et reliées au compactage et à l’agrégation. Les interactions entre les composantes impliquées dans la structure du sol ont été prises en compte dans l'analyse des données compositionnelles.
Les indices de compactage du sol ont été prédits à partir de la teneur en eau du sol, de la matière organique, des particules minérales du sol et des agents de cimentation minéraux. Les résultats ont démontré que les agents de cimentation minéraux étaient les principaux contributeurs aux indices de compactage du sol. Les oxydes de Si, Al, Fe et Ca ont fait augmenter la masse volumétrique apparente, le degré de compacité et la teneur en eau critique, mais ont réduit la densité apparente maximale. La méta-analyse a démontré les avantages de la culture sous semis direct par rapport aux pratiques conventionnelles dans la couche superficielle de 0 à 5 cm, qui augmentaient 10 à 16 ans après la mise en œuvre du semis direct. L'effet du semis direct était plus important dans les sols dont le pH était supérieur à 7 et avec des cultures annuelles continues. Les sols dont les agrégats montraient une perturbation maximale inférieure à 1,27 par rapport à la déstructuration bénéficiaient davantage du semis direct. L’analyse de redondance a montré que la macro-agrégation était étroitement liée aux agents de cimentation minéraux, et légèrement liée à l'argile et à la matière organique. Le limon et le sable fin étaient positivement reliés aux micro-agrégats. Nous avons construit des modèles reliant les agents de cimentation à la distance d’Aitchison et au log-ratios isométriques, qui pourraient servir d’indices pour la stabilité des agrégats. L’agrégation des sols dans les deux couches supérieures de sol dépendait de la matière organique et des ciments minéraux. L'agrégation du sol dans la couche inférieure a été stabilisée principalement par les particules de sol et les ciments minéraux. La richesse bactérienne était liée positivement aux micro-agrégats. L'indice de diversité phylogénétique présentait une corrélation positive avec les fractions 1-0,5 mm et 2-1 mm. En plus de la richesse et de la diversité bactériennes, la structure de la
iii
communauté bactérienne a été significativement affectée par le compactage du sol. Il y avait une richesse plus importante et une diversité bactériennes plus abondante dans les sites très compactés.
Mots-clés: Analyse de données compositionnelles; labour conventionnel; semis direct; modèles de prédiction;
iv
Abstract
Intensive agricultural practices cause soil compaction which influences most soil physical, chemical and biological properties and processes promoting soil aggregation. Soil microorganisms play a decisive role in soil structure. This work aims to (1) build models to predict soil compaction indices, (2) study the impact of tillage practices on soil aggregate, (3) build models to predict soil aggregation indices, and (4) analyze the response of soil bacterial diversity to aggregation and compaction. Gravimetric water content, bulk density, particle-size distribution, aggregate-size distribution, maximum bulk density, critical water content, organic carbon, total nitrogen, Si, Fe, Al, Mn, Mg and Ca analyzed from soil samples collected on 49 farm sites in Quebec in 2014 were used to elaborate compaction and aggregation models. The dataset on the impact of tillage practices on soil aggregate consisted of 94 paired conventional tillage and no-till experiments retrieved from 20 peer-reviewed papers. The dataset for the last objective includes bacterial diversity variables, soil compaction degree and aggregate size distribution analyzed in soil samples taken from 16 farm sites in Quebec in 2014. The interactions among soil components involved in soil structure were accounted for using compositional data analysis.
Compaction indices were predicted from soil water content, organic matter, soil mineral particles and mineral cementing agents. Mineral cementing agents were shown to be the major contributors to soil compaction. The Si, Al, Fe and Ca oxides increased bulk density, degree of compactness and critical water content, but reduced maximum bulk density. Meta-analysis showed that the benefit of no-till over conventional practices predominated in the surface 0-5 cm layer and increased up to 10-16 years after implementing tillage. The effect size of no-till was larger in soils of pH > 7 and under continuous annual cropping systems. The soil group highly responsive to no-till (soil aggregation value from maximum disruption structure below 1.27) largely benefited from no-till in the long run. Redundancy analyses indicated that macro-aggregation was closely related to mineral cementing agents and slightly related to clay and organic matter contents. Silt and fine sand were strongly and positively related to micro-aggregates. We built models relating cementing agents to the Aitchison distance and isometric log-ratio which could be used as indices of aggregate stability. Soil aggregation in the upper two layers depended on organic matter and mineral cementing agents. Soil aggregation in the lower layer was stabilized mainly by soil particles and polyvalent cations. Bacterial species richness was positively related to micro-aggregation. The phylogenetic diversity index showed a positive correlation with the 1-0.5mm and 2-1 mm fractions. Not only bacterial richness and diversity but also bacterial community structure was significantly affected by soil compaction. Larger bacterial richness and diversity were found in soils showing the highest degree of compaction.
Keywords: Compositional data analysis; Conventional tillage; No-till; Prediction models; Soil aggregation; Soil
v
Table des matières
Résumé ... ii
Abstract ... iv
Table des matières ... v
Liste des figures ... ix
Liste des tableaux ... xii
Liste des abréviations ... xiii
Remerciements ... xv
Avant-propos ... xix
General introduction ... 1
Chapter 1 Literature Review ... 10
1.1 Soil structure ... 11
1.1.1 Basic concepts of aggregation ... 11
1.1.2 Methods of expressing the size distribution of soil aggregates ... 12
1.1.3 Mechanisms of aggregation ... 12
1.1.4 Agents of aggregation ... 14
1.1.5 Soil aggregates and microorganisms ... 17
1.2 Soil compaction ... 19
1.2.1 Effects of compaction on soil physical properties and soil biodiversity ... 19
1.2.2 Assessment of soil compaction ... 20
1.3 Compositional data analysis ... 22
Research priorities ... 22
Hypotheses and objectives ... 23
1.4 References ... 25
Chapter 2 Compaction of coarse-textured soils: balance models across mineral and organic compositions ... 40
2.1 Résumé ... 41
2.2 Abstract ... 42
2.3 Introduction ... 43
2.4 Materials and methods ... 45
2.4.1 Materials ... 45
2.4.2 Log-ratio transformation ... 45
vi
2.5 Results ... 48
2.5.1 Variables related to soil bulk density and degree of compactness ... 48
2.5.2 Factors affecting soil maximum bulk density and critical water content ... 50
2.5.3 Predictive models for compaction indices ... 54
2.6 Discussion ... 57
2.6.1 Effects of sampling depth and soil components on bulk density and degree of compactness ... 57
2.6.2 Effects of soil components on maximum bulk density and critical water content ... 58
2.6.3 Effects of mineral cementing agents on compaction indices ... 59
2.6.4 Remediation techniques supported by the predictive models ... 60
2.7 Conclusions ... 61
2.8 References ... 62
2.9 Appendices ... 66
Chapter 3 Log-ratio indicator for assessing the impact of tillage practices on soil aggregation and agricultural soil recovery: a meta-analysis ... 67
3.1 Résumé ... 68
3.2 Abstract ... 69
3.3 Introduction ... 70
3.4 Materials and methods ... 71
3.4.1 Dataset ... 71
3.4.2 Methods ... 72
3.5 Results ... 75
3.5.1 Soil properties and aggregation state ... 75
3.5.2 The magnitude of the effect of tillage practices on soil aggregation at depth ... 75
3.5.3 The magnitude of the effect of experiments duration on soil aggregation ... 76
3.5.4 The magnitude of the effect of tillage practices on soil aggregation in various cropping systems ... 77
3.5.5 The magnitude of the effect of tillage practices on soil aggregation in different pH soils ... 78
3.5.6 Influence of the initial aggregation index on response to NT ... 79
3.6 Discussion ... 80
3.6.1 Soil properties ... 80
3.6.2 Tillage effect ... 81
vii 3.7 Conclusions ... 82 3.8 Acknowledgements ... 82 3.9 References ... 84 3.10 Appendices ... 91 3.11 Appendices References ... 98
Chapter 4 Water-stable aggregates and cementing agents: Balances between mineral and organic constituents ... 100
4.1 Résumé ... 101
4.2 Abstract ... 102
4.3 Introduction ... 103
4.4 Materials and methods ... 104
4.4.1 Materials ... 104
4.4.2 Data transformation ... 104
4.4.3 Statistical analysis and model development ... 106
4.5 Results ... 108
4.5.1 Soil properties ... 108
4.5.2 Soil aggregate-size distribution across soil horizons ... 110
4.5.3 Redundancy analyses among soil aggregates and soil properties ... 110
4.5.4 Predictive models for soil aggregate stability ... 112
4.6 Discussion ... 114
4.6.1 The relationship between soil physicochemical properties and aggregate-size distribution ... 114
4.6.2 Soil structure improvement supported by the predictive models ... 116
4.7 Conclusions ... 117
4.8 References ... 118
Chapter 5 Soil bacterial community richness and structure associated with aggregate-size distribution and soil compaction in potato cropping systems ... 124
5.1 Résumé ... 125
5.2 Abstract ... 126
5.3 Introduction ... 127
5.4 Materials and Methods ... 128
5.4.1 Soil sampling and determination of the physical and chemical properties ... 128
5.4.2 High throughput sequencing data analysis of bacterial community ... 130
viii
5.5 Results ... 134
5.5.1 Soil properties and bacterial community richness and structure ... 134
5.5.2 Soil properties as drivers of bacterial richness and communities ... 140
5.5.3 Correlation of soil bacterial richness and community structure to soil aggregate-size distribution ... 141
5.5.4 Soil compaction effects on bacterial community structure ... 144
5.6 Discussion ... 145
5.6.1 The correlation of bacterial richness and community structure to soil properties and cropping systems ... 145
5.6.2 The correlation of bacterial diversity to soil aggregation and compaction ... 146
5.6.3 The correlation of bacterial community structure with soil aggregation and compaction ... 147
5.7 Conclusions ... 149
5.8 References ... 150
General discussion ... 161
1 The relationship between soil structure and soil physical, chemical, and microbial properties ... 161
2 Unbiased statistic method for soil compositional data ... 162
General conclusion ... 164
Perspectives ... 166
ix
Liste des figures
Figure 1 - 1 Hierarchy of soil aggregates. (Brady and Weil, 2008). ... 13 Figure 1 - 2 Possible scenarios of aggregation. Organic matter (OM), particulate organic matter (POM), clay
(Cl), particle (P) (Bronick and Lal, 2005). ... 14
Figure 1 - 3 An overview of the role of bacterial and fungal inocula in the formation of soil aggregates (Rashid
et al., 2016). ... 18
Figure 2 - 1 Sequential binary partition (SBP) of soil components to compute isometric log ratios for bulk
density and degree of compactness. SWC: Soil water content; Fs: Fine sand; Ms: Medium sand; Cs: Coarse sand. ... 46
Figure 2 - 2 Sequential binary partition (SBP) of soil components to compute isometric log ratios for maximum
bulk density and critical water content. CWC: Critical water content; Fs: Fine sand; Ms: Medium sand; Cs: Coarse sand. ... 47
Figure 2 - 3 Model prediction of (A)BD and (B)DC. BD: bulk density; DC: degree of compactness; AIC: Akaike
information criterion; MPE: mean prediction error; RMSE: root mean squared error. ... 55
Figure 2 - 4 Relationship between maximum bulk density (MBD g cm − 3 ) and critical water content (CWC, 100 kg kg − 1). Open and closed symbols refer to horizon A and horizon B, respectively. ... 57
Figure 3 - 1 Difference in aggregation state (Δ[Micro | Macro]) between no-till and conventional tillage in three
soil layers (0-5cm, 5-10cm, 10-20cm). The horizontal bars are the 95% confidence intervals of Δ[Micro | Macro]. ... 76
Figure 3 - 2 Difference in soil aggregation state (Δ[Macro | Micro]) between no-till and conventional tillage as
driven by duration of experiments: a) < 4years; b) 4 - 8 years; c) 10 - 16 years; d) >20 years. The horizontal bars are the 95% confidence intervals of Δ[Micro | Macro]. ... 77
Figure 3 - 3 Difference in soil aggregation state (Δ[Micro | Macro]) between no-till and conventional tillage as
driven by cropping systems. The horizontal bars are the 95% confidence intervals of Δ[Micro | Macro]. ... 78
Figure 3 - 4 Difference in soil aggregation state (Δ[Micro | Macro]) between no-till and conventional tillage as
driven by pH class: a) pH < 7; b) pH > 7. The horizontal bars are the 95% confidence intervals of Δ[Micro | Macro]. ... 78
Figure 3 - 5 Binary classification of Aitchison distances ()) between no-till and conventional tillage ()*+ − ,+) related to the ) between conventional tillage and maximum disruption (),+ − -./). TN = true negative; TP = true positive; FN = false negative; FP = false positive; acc.=accuracy. ... 79
Figure 3 - A. 1 Meta-analysis of the effect of tillage practices (no-till vs. conventional) on the isometric log ratio
between macro-aggregates (>0.25 mm) and micro-aggregates (<0.25 mm) in different soil layers (0–5 cm, 5– 10 cm, and 10–20 cm). SD, standard deviation; MD, mean difference; CI, confidence interval. ... 92
x
Figure 3 - A. 2 Meta-analysis of the effect of tillage practices (no-till vs. conventional) on the isometric log ratio
between macro-aggregates (>0.25 mm) and micro-aggregates (<0.25 mm) depending on the duration of the experiment in different soil layers (0–5 cm and >5 cm): (a) less than 4 years; (b) 4 to 8 years; (c) 10 to
16 years; and (d) >20 years. SD, standard deviation; MD, mean difference; CI, confidence interval. ... 94
Figure 3 - A. 3 Meta-analysis of the effect of tillage practices (no-till vs. conventional) on the isometric log ratio
between macro-aggregates (>0.25 mm) and micro-aggregates (<0.25 mm) with varied cropping systems (continuous cropping, crop rotation, and sequential cropping). SD, standard deviation; MD, mean difference; CI, confidence interval. ... 96
Figure 3 - A. 4 Meta-analysis of the effect of tillage practices (no-till vs. conventional) on the isometric log-ratio
between macro-aggregates (>0.25 mm) and micro-aggregates (<0.25 mm) in two pH categories (pH ≥ 7 and pH < 7) in different soil layers: (a) 0 to 5 cm; and (b) greater than 5 cm. ... 97
Figure 4 - 1 Basic physico-chemical properties of soils. Similar lowercase letters indicate non-significant
differences between soil horizons (P < 0.05) by Wilcoxon rank-sum test. ... 109
Figure 4 - 2 (a) Scatterplot for three horizons (horizon Ap1, horizon Ap2 and horizon B), (b) Bi-plot for five
aggregate-size distribution variables of principal components analysis (PCA) for all the soil samples, (c) Distribution of water-stable aggregates in three horizons. Different lowercase letters above each horizon within an aggregate class indicated significant differences among horizons according to Tukey’s HSD test (P < 0.05). ilr1.agg: [(> 2 mm, 2 − 1 mm, 1 − 0.5 mm, 0.5 − 0.25 mm)/(0.25 − 0.1 mm, < 0.1 mm)], ilr2.agg: [(> 2 mm, 2 − 1 mm, 1 − 0.5 mm)/ (0.5 − 0.25 mm)], ilr3.agg: [(> 2 mm, 2 −
1 mm)/ (1 − 0.5 mm)], ilr4.agg: [(> 2 mm)/(2 − 1 mm)], ilr5.agg: [(0.25 − 0.1 mm)/(< 0.1 mm)]. ... 110
Figure 4 - 3 Redundancy analysis of soil mineral and organic cementing agents and soil aggregation variables
(n=87, ilr1: [organic matter / (soil particle, polyvalent cations)] ; ilr2: [C/N]; ilr3: [soil particle/ polyvalent cations]; ilr4: [(CS, MS, FS, silt)/clay]; ilr5 : [(CS, MS, FS)/Silt]; ilr6 : [(CS, MS)/FS]; ilr7: [CS/MS]; ilr8: [Si/(Al, Fe, Mn, Mg, Ca)]; ilr9: [Al, Fe, Mn/(Mg, Ca)]; ilr10: [(Al, Fe)/Mn]; ilr11:[Al/Fe]; ilr12:[Ca/Mg]; ilr1.agg: [(> 2 mm, 2 − 1 mm, 1 − 0.5 mm, 0.5 − 0.25 mm)/(0.25 − 0.1 mm, < 0.1 mm)], ilr2.agg: [(> 2 mm, 2 − 1 mm, 1 − 0.5 mm)/ (0.5 − 0.25 mm)], ilr3.agg: [(>
2 mm, 2 − 1 mm)/ (1 − 0.5 mm)], ilr4.agg: [(> 2 mm)/(2 − 1 mm)], ilr5.agg: [(0.25 −
0.1 mm) < (0.1 mm)]. ... 111
Figure 4 - 4 Diagrams describing the partitions of variation of the size distribution of soil aggregates by soil
properties, site and depth. ... 112
Figure 4 - 5 Coefficients of variables that significantly contributed in the LME (linear mixed effect) models for
ilr_aggregation and Aitchision distance in horizons Ap1, Ap2 and B. *,**, *** indicates significance at P < 0.05, 0.01 and 0.001, respectively in the LME models. RMSE: root mean squared error. ilr2: [C/N]; ilr3:
[Soil particle/polyvalent cations]; ilr5: [(CS, MS, FS)/silt] ; ilr6: [(CS, MS)/FS]; ilr9:
[ (Al, Fe, Mn)/(Mg, Ca)]. ... 113
Figure 5 - 1 Average distribution of 16S rRNA sequence classified at the class level for bacteria of 16 sites.
... 138
Figure 5 - 2 Nonmetric multidimensional scaling plots of soil bacterial communities at the class level, showing
xi
Corn/ryegrass/clover/mustard-Canola/oat-Potato; C-P-C: Corn-Corn/canola; P-C-P:
Potato-Corn/canola-Potato. ... 140
Figure 5 - 3 Relationships between soil aggregates balances and soil bacterial alpha diversity indices (n=48).
ilr1_agg: [(> 2 mm, 2 − 1 mm, 1 − 0.5 mm, 0.5 − 0.25 mm) / (0.25 − 0.1 mm, < 0.1 mm)], ilr2_agg: [(> 2 mm, 2 − 1 mm, 1 − 0.5 mm) / (0.5 − 0.25 mm)], ilr3_agg: [(> 2 mm, 2 − 1 mm) / (1 − 0.5 mm)], ilr4_agg: [(> 2 mm) / (2 − 1 mm)], ilr5_agg: [(0.25 − 0.1 mm)/ (< 0.1 mm)], OTU_Richness: OUT counts, PD_index: Faith’s PD index of bacteria. The relatively light blue or orange and small circles indicate a relatively weak positive or negative correlation; the dark blue or red and large circles indicate a relatively strong positive or negative correlation. ... 142
Figure 5 - 4 showing the relationships between soil aggregate balances and dominant soil bacterial classes (P
= 0.001, N = 48, ilr1_agg: [(> 2 mm, 2 − 1 mm, 1 − 0.5 mm, 0.5 − 0.25 mm)/(0.25 − 0.1 mm, < 0.1 mm)], ilr2_agg: [(> 2 mm, 2 − 1 mm, 1 − 0.5 mm)/(0.5 − 0.25 mm)], ilr3_agg: [(> 2 mm, 2 − 1 mm)/(1 − 0.5 mm)], ilr4_agg:[(> 2 mm)/ (2 − 1 mm)], ilr5_agg: [(0.25 − 0.1 mm)/(<
0.1 mm)]. ... 143
Figure 5 - 5 Nonmetric multidimensional scaling plots of soil bacterial communities at the class level, showing
the relative differences in community composition. DC: degree of compactness. ... 144
Figure 5 - 6 Relative abundance of seventeen bacterial classes that were significantly different among
compaction groups. a) the relative abundances of classes were the highest in the low or moderate compaction group; b) the relative abundances of classes were the highest in the high compaction group. DC: degree of compactness. ... 145
xii
Liste des tableaux
Table 1-1 Soil functions and sub-functions that are directly affected by soil compaction and soil parameters as
possible indicators (Lebert et al. 2007) ... 21
Table 2 - 1 Results of principal component analysis and loadings of the component for soil bulk density and the degree of compaction ... 50
Table 2 - 2 Results of principal component analysis and loadings for soil properties related to the maximum bulk density ... 51
Table 2 - 3 Principal component analysis and loadings for soil properties related to the critical water content 53 Table 2 - 4 Linear mixed effects models for soil bulk density and degree of compactness ... 54
Table 2 - A. 1 Descriptive statistics of soil chemical and physical properties in horizon A and B ... 66
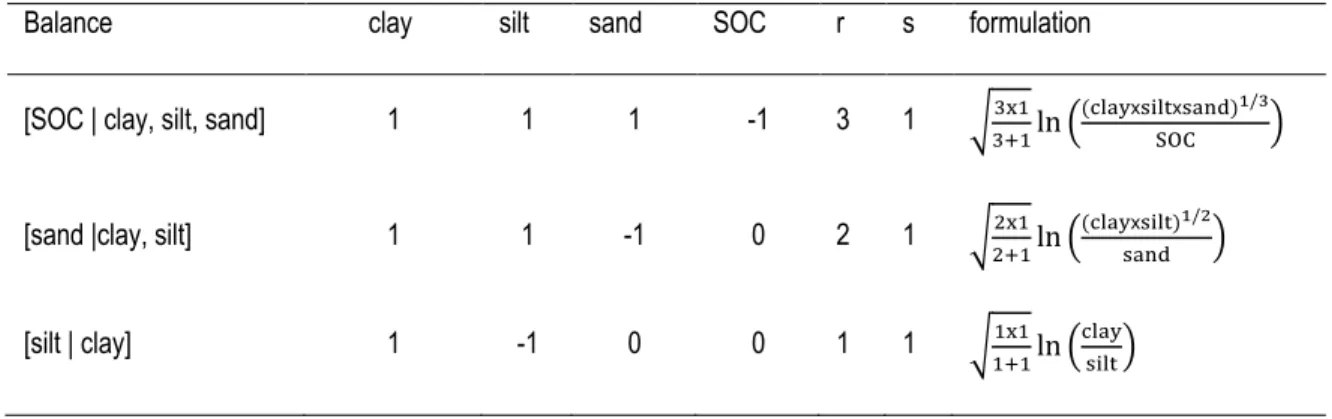
Table 3 - 1 Sequential binary partition among soil mineral components and soil organic carbon (SOC) ... 73
Table 3 - 2 Cate-Nelson partitioning of aggregation states ... 75
Table 3 - 3 Correlations between soil particles balances and soil aggregation (n = 82) ... 75
Table 3 - A. 1 List of the 20 studies retained for analysis of the effect size of tillage practices on soil aggregation. ... 91
Table 4 - 1 Sequential binary partition of soil variables promoting aggregation to compute isometric log-ratios ... 105
Table 4 - 2 Sequential binary partition among six sieve sizes for wet-sieved soil samples ... 105
Table 4 - 3 Predicting models for aggregate stability indices obtained by different methods (LME, PLSR, Stepwise Regression) ... 113
Table 5 - 1 Characteristics of sixteen sampling sites ... 129
Table 5 - 2 Sequential binary partition among six sieve sizes for wet-sieved soil samples ... 132
Table 5 - 3 Sequential binary partition among soil textural components (clay, silt and sand) ... 133
Table 5 - 4 Soil bacterial alpha diversity indices and soil physicochemical properties at each field site ... 135
Table 5 - 5 Averaged bacterial OTU richness, Faith’s PD index and soil physicochemical properties in three cropping systems ... 139
Table 5 - 6 Averaged bacterial OTU richness and Faith’s PD index of the same current crop from different cropping systems and different current crops in the same cropping system ... 139
Table 5 - 7 Variance partitioning of bacterial richness and community structure by selected soil properties set ... 141
Table 5 - 8 Pearson correlation between the soil aggregate-size fractions and eight typical bacterial classes (n=48) ... 143
xiii
Liste des abréviations
); Aitchison distance Acc.; accuracy
AIC: Akaike information criterion
alr: additive log ratio
BD: bulk density C: carbon
CEC: cation exchange capacity Cl: clay
clr: centred log ratios
COS: organique du sol CS, coarse sand CT: conventional tillage CWC: critical water content
DC: degré de compacité; degree of compactness DOC: dissolved organic carbon
DP: diversité phylogénétique FA: fulvic acid
FN: false negative FP: false positive FS, fine sand
GMD: geometric mean diameter HA: humic acid
HS: humic substance HU: humin
ICP: inductively coupled plasma
ilr: isometric log ratio
LF: light fraction LME: linear mixed effect MBC: microbial biomass carbon MBD: maximum bulk density MPE: mean prediction error MS, medium sand
xiv
MVA: masse volumique apparente
MVAM: masse volumique apparente maximale MWD: mean weight diameter
NMDS: Nonmetric multidimensional scaling NT: no-till
OM, organic molecules
RMSE: root mean squared error RT: ridge tillage
SBP: sequential binary partition SOC: soil organic carbon SOM: soil organic matter TEC: teneur en eau critique TN: true negative
TP: true positive OC: organic carbon ODR: oxygen diffusion rate ORD: oxygen diffusion rate OTU: operational taxonomic units OUT: operational taxonomic unit PCA, principal component analysis PD: phylogenetic diversity
PLSA, partial least square regression POC: paticulate organic carbon POM: particulate organic matter RDA: redundancy analysis RMSE, root mean squared error RT: ridge tillage
TN: total nitrogen
VIF: variance inflation factor VIP: variable importance plot; WSA: water-stable aggregate ZT: zero tillage
xv
Remerciements
Completing a Ph.D. is a journey that requires enthusiasm, passion for science, determination, confidence, hard work as well as help and support from professors, friends and family. Some of my friends think Ph.D. is only dealing with experiments and paper, however in my opinion, besides research, the most important things are will and perseverance. Although this Ph.D. took more time than I expected, I still feel happy and grateful when I finish the thesis. The completion of the thesis is attributed to many peoples’ support and encouragement.
First and foremost, I want to extend my heartfelt gratitude to my supervisors Dr. Leon- Étienne Parent and Dr. Noura Ziadi, whose patient guidance, valuable suggestions and constant encouragement make me successfully complete this thesis.
In 2012, Leon accepted me as a visit Ph.D. student in his team. I still remember how Leon picked me up from the airport, took me to the registration office and introduced everything in our department to me. At that moment, I knew nothing about the research environment in Canada. Leon gave me many chances to get familiar with their projects and researchers in Quebec. Thanks for introducing me to lots of professors, giving me the chances to attend the conferences, and most importantly, opening the gate of composition data analysis to me. I admire Leon’s enthusiasm, passion, creativity and profound knowledge of soil science. That is the most important reason why I came to Laval. Thank you, Leon, for giving me the opportunity to work in soil science, a subject area outside my previous experience and expertise. I want to thank you for your direction in the past six years. You always answered my questions even when they were just basic. You have given me many sources to make my knowledge of soil quality enriched since I changed my area from land resources management to soil science. You have given me many opportunities for attending academic conferences, presenting myself and getting in touch with other researchers. Thanks for your hard working on reviewing my paper and thesis. I am so grateful for the countless times that we discussed and thought-provoking comments you gave to my thesis. Lastly, I am deeply indebted to your patience. You have given me lots of help and support when I need to postpone my defense date because of my family issue.
I want to express my deepest gratitude to my co-supervisor, Noura. Thanks for providing the scholarship. Without this scholarship, I could not come to Canada and achieve my dream. She has supervised me about science and life, and always stood behind me. Her conscientious academic spirit and open-minded personality inspire me. As a female scientist, she can balance her work and family very well which I think is very hard after I became a mother. She is helpful and thoughtful. She knows what we were going through as international students. She cares about my studies as well as my life. She always tried her best to solve my problems and give me helpful advice. I value the discussions with Noura which gave me valuable suggestions for each step of my work.
xvi
The discussions with her also helped me a lot in improving my understanding of my research and related topics. As a supervisor, Noura is more like a friend, and this kind of friendship made me feel confident in my studies.
Also, I would like to express my sincere gratitude to all the professors who have taught me at Laval University. Their instructions have helped broaden my horizon and their enlightening teaching has provided me with a solid foundation to accomplish this paper and will always be of great value for my future career and academic research. Especially, I would like to thank Serge-Étienne Parent. Serge is like my supervisor in R. He is a very smart and patient professor. He has the strong background knowledge and innovative insights on statistical analysis. He provided me with many books, articles, and scripts to enlarge my knowledge on R. Thanks to the discussions with him that helped me find solutions when I had difficulties in the data analysis. Thanks for your contribution to my thesis.
Next, I would like to thank the following persons who contributed a lot to my soil samples collecting and lab tests. Without your contributions, there would not have been any thesis.
Nicolas Samson: He is a kind, easy-going and enthusiastic person. Before going to the sampling sites, I know few about how to proceed with the sampling work. He taught me everything from preparing the tools to printing the labels. Nicolas always tried his best helping me out every time I had questions. He is very thoughtful. Because I don't speak French (it's a pity), he sometimes even worked as a translator for me when we went to the fields. The sampling work was full of fun because of his sense of humor.
Michael Leblanc: Michael is a reliable person. The first characteristic I learned from him is rigorous. He gave me many directions on how to conduct the sampling work and experiments accurately. Michael went to all the 49 sites with me, which I deeply appreciate. I am also greatly indebted to his contribution to this thesis. The discussions with him gave me a lot of ideas in processing compositional data analysis.
Marie-Hélène Lamontagne and Elizabeth Parent: They have tested 653 samples in total for me. I really appreciate their hard work.
Daniel Marcotte: He kindly provided me with the facilities in the lab. He is a nice and kind person. I enjoy chatting with him.
Maikel Abreu Jimenez: He is a visiting student from CUBA, coming to Laval to help me doing the compaction test. It was a very hard and exhausting test. Without him, I could not finish that experiment by myself. Although he cannot speak English, just one week after working together, we had nothing difficulties in communication. Our work was efficient and quick, which was attributed to his hard work.
xvii
Then I want to express my thanks to some teachers in our department. Firstly, Dr. Antoine Karam. Thank you for your help on every issue regarding my enrollment. Secondly, Marie-Andrée Ouellet. I had troubled her many times for the registration. Every time she was kind and helpful. Thirdly, Nadine Lemay in the office of our department. She is a very nice and lovely lady. I like her a lot.
Sincerest thanks to Dr. Richard Hogue and Thomas Jeanne of IRDA. Thank Dr. Hogue for giving me the opportunity of participating project of IRDA on soil biodiversity and his advice on my paper. Thank Thomas for your well-organized data set. The discussions we had really addressed many of my concerns about bacteria.
Now it comes to all my dear friends. Shi Yichao is the first one I want to thank. She is truly a big sister of mine. I am so lucky I could have her as a best friend. We had a similar life path and so much in common. So, she understands me very well. Yichao is really a kind and warm-hearted girl. She took care of me as a sister. Besides, I admire her research ability. She has a broad knowledge scope of soil quality. I am so thankful that she had given me many pieces of advice on my project and my paper.
Qiuying is like my sister. We always lived close to each other, so we could eat and study together. Qiuqing’s major is different from mine. But she could give me many pieces of advice when I had confusion no matter with life or study. We shared feelings and secrets with each other. Thanks for every chat we had and every encouragement you gave me.
Haixiao is a legendary person to us. He is always full of energy and thirst for knowledge. I am happy that I had him as comrades-in-arms. I remember those times we did our homework together; I remember those conferences we attend together; I remember all the help he gave to me because I don’t speak French. He has been extremely supportive through some difficult times. Thank you for always being there for me.
Thank you Guoqi and his family, Xiaojin and Nancy. When I was in Quebec by myself, they treated me as their family member. Thanks for every meal they invited me. I didn’t feel lonely anymore with them. After I moved to Ottawa, Guoqi dealt with all my registration issues. Thank you from the bottom of my heart.
Then I want to thank Reza Jamaly. His optimism, sense of humor and life attitude always infected me. He is a great guy and my comrades-in-arms as well.
Besides, I would like to thank Feng Shiting and Ju Bing. Thank you for spending so much time on the talk of Ph.D. study and soil quality with me.
xviii
Last but not least, I should thank Mervin St. Luce, Dalel Abdi, Hada Damar. Although we didn’t have much time working together, the conversations with them broadened my knowledge in related research areas, and I also admire their passions for both work and life. I am glad I could know them.
Finally, I must thank my dear family. Thank my parents for supporting and believing in me all these years. Thanks, father, without your encouragement ten years ago, I couldn’t make the decision of pursuing Ph.D. Sorry mother, I spent much more time on study than you expected, which made you worried. I appreciate everything you did for me and my family. You are the best mom in the world. Thank you, Zhou Yan, we have known each other since 2012, and I have been a student since then. Thank you so much for taking on the responsibility of our family. Thank you so much for your love, support and trust. Thanks, Evan, my son, thank you for being a good and healthy baby. I am so grateful I could have you.
xix
Avant-propos
This thesis contained a general introduction, literature review which summarizes the previous research in the thesis research field and includes the overall objectives and hypotheses, four chapters related to the research topic, followed by a general discussion and conclusion. Chapter 2, 4 and 5 are experimental results based on original experiments. Chapter 3 is a review of the published papers.
The chapter 2 is a study relating soil compaction indices to the basic components of coarse-textured soils using unbiased numerical techniques. This chapter was published in Frontiers Ecology and Evolution in 2017. The candidate was the senior author and co-authors included Maikel Abreu Jimenez, Serge-Étienne Parent, Michael Leblanc, Noura Ziadi and Leon-Étienne Parent. The chapter 3 is a meta-analysis of published researches assessing the effect of soil tillage practices on aggregation and submitted to Pedosphere with the title: Log-ratio indicator for assessing the impact of tillage practices on soil aggregation and agricultural soil recovery: a meta-analysis. The candidate was the senior author and co-authors included Serge-Étienne Parent, Haixiao Li, Leon-Étienne Parent and Noura Ziadi.
The chapter 4 is a study predicting soil aggregate stability from cementing agents using unbiased numerical techniques. It is in preparation for submission to Catena. The candidate was the senior author and co-authors included Haixiao Li, Leon-Étienne Parent and Noura Ziadi.
The chapter 5 associates soil biological quality with soil aggregation or compaction resulting from agricultural practices. It is in preparation for submission to Applied Soil Ecology. The candidate was the senior author and co-authors included Thomas Jeanne, Richard Hogue, Yichao Shi, Noura Ziadi and Leon-Étienne Parent.
The candidate was fully responsible for protocol design, data collection and laboratory analysis (except the bacterial diversity and richness data, which were done by Richard Hogue, Thomas Jeanne and their team). The candidate was main responsible for statistical analyses, data interpretation and writing the manuscript.
The results of these studies were presented in 2017 Canadian Society of Soil Science Conference (Peterborough, Canada), in 2015 during the 20th International soil tillage research organization conference (Nanjing, China), in 2015 during the Joint meeting: Commission of the International Union of Soil Science (IUSS), Canadian Society of Soil Science (CSSS), Association québécoise des spécialistesen sciences du sol (AQSSS) (Montreal, Canada), and in 2014 during the 28th conference of l’Association Québécoise de Spécialistes en Sciences du Sol (AQSSS) (Victoriaville, Canada) for a total of one oral communication and four posters.
1
General introduction
Soil structure is one of the key soil physical attributes. It supports plant and animal life and moderate environmental quality, with particular emphasis on soil carbon (C) sequestration and water quality (Bronick and Lal, 2005). The influence of soil structure on crust formation, root penetration, soil water and air movement, CO! emission, erosion, nutrient retention, and biological activity is well known. On the other hand, soil structure
depends on the interaction between soil type, cementing agents, crops, fertilization, tillage, agricultural machinery, traffic drainage and environmental conditions (Bronick and Lal, 2005; Kibblewhite et al., 2008). Aggregate stability is an indicator of soil structure (Six et al., 2000). Soil aggregation is very sensitive to mechanical stress and organic matter transformation and is thus a key soil structure attribute as modified by soil tillage and crop management. Soil compaction caused by overuse of machinery, intensive cropping, short crop rotations, intensive grazing, and inappropriate soil management results in soil structure deterioration, both in the topsoil and the subsoil (Horn et al., 1995; Hamza and Anderson, 2005). The decline in soil structure influences many soil characteristics including species biodiversity. Generally, greater soil microbial biomass and functional diversity were reported under Zero tillage (ZT) than under CT (Munyanziza et al., 1997; Lupwayi et al., 2001). Roots and microorganisms, in turn, enhance soil structure by enmeshing soil particles and providing extracellular compounds that bind particles together.
Soil aggregation, as defined by Martin et al. (1955), is the natural occurring cluster or group of soil particles in which the forces holding the particles together are much stronger than the forces between adjacent aggregates. Soil aggregation is the result of aggregate formation and stabilization (Allison, 1968). Aggregation results from the rearrangement of particles, flocculation, and cementation (Duiker et al., 2003). It is mediated by soil organic carbon (SOC), biota, ionic bridging, clay, and carbonates (Bronick and Lal, 2005). Soil management, specifically the use of different tillage systems, affects soil aggregation directly by physical disruption of macro-aggregates, and indirectly through alteration of biological and chemical factors (Barto et al., 2010). CT mechanically breaks macro-aggregates (Borie et al., 2006) and decreases the content of soil organic matter (SOM), microbial biomass and faunal activities (Mikha and Rice, 2004; Sainju et al., 2009; Curaqueo et al., 2011). The decrease in soil aggregate stability results in soil nutrient depletion and erosion (Borie et al., 2006; Chaudhary et al., 2009; Zhang and Horn, 2001). In contrast, conservation tillage, such as no-till (NT) and ridge tillage (RT), have positive effects on aggregates and their binding agents (Bronick and Lal, 2005; Curaqueo et al., 2011). Several studies have shown that NT lead to higher SOC stocks compared to CT (Six et al., 2002; West and Post, 2002; Ogle et al., 2003) and also favor the formation of macro-aggregates (Six et al., 2006).
Aggregate-size distribution following the application of mechanical stress using either dry or wet-sieving methods is a useful way of expressing structure quantitatively. The need to characterize soil aggregate-size distribution
2
with a single parameter has long been recognized. Aggregate-size distribution is generally synthesized into mean weight diameter (MWD) (Van Bavel, 1950), geometric mean diameter (GMD) (Mazurak, 1950) or fractal dimensions (Anderson et al., 1997). In most studies, MWD or GMD measurements showed an important variation under different cropping and tillage practices (Abid and Lal, 2008; Zhang et al., 2012). Several investigators have used fractal parameters, such as the number-based fractal dimension, the mass-size fractal dimension or the non-linear fractal dimensions, as indices to evaluate the influence of tillage practices on the size distribution and found significant tillage effects on fractal parameters (Perfect and Blevins, 1997; Sepaskhah et al., 2000). However, such indices are inherently biased and dimensionally difficult to tackle and have severe limitations when attempting to compare studies on soil aggregation. For example, information is lost after integrating aggregate-size fractions into MWD biased towards the largest sieve-size fractions (Cambardella, 2006; Caruso et al., 2011). Difficulties to interpret scaling factors as fractal dimensions occur due to weak or invalid assumptions that produce large uncertainties in particle counts (Young and Crawford, 2004).
Since the state of compactness is an important soil structural attribute, there is a need to find a parameter for its characterization. Bulk density, relative bulk density (Håkansson and Lipiec, 2000), soil strength (Panayiotopoulos et al., 1994; Hamza et al., 2001; Hamza and Anderson, 2003) and water infiltration rate (Hamza and Anderson, 2002) are used as measures of soil compaction. To better monitor and predict soil compaction and determine the best soil condition for reducing the compaction process, it is important to document the relationship between soil components and compaction state. Soil compaction indices were usually predicted from soil physical and chemical properties, such as particle size distribution, organic matter, pH and water content (Nhantumbo and Cambule, 2006; Zhao et al., 2008; Martin et al., 2009; Jalabert et al., 2010; Suuster et al., 2011; Brahim et al., 2012). Besides, cementing agents also affect the resistance of compaction. Bivalent Ca!" and Mg!" cations improve soil structure through cationic bridging with clay particles and SOC
(Bronick and Lal, 2005). Polyvalent Al#" and Fe#" cations benefit soil structure from cationic bridging and
formation of organo-metallic compounds and gels (Amezketa, 1999). The Na" and K" are highly dispersive
agents resulting directly in the breakup of aggregates and indirectly affecting soil resistance to compaction (Haynes and Naidu, 1998).
Biologic influences on soil structure can be demonstrated in the formation and stabilization of aggregates. Previous research has focused on the role of specific soil microorganisms in the aggregation of soil particles (Amellal et al., 1998). The abundance of specific microbial species such as Rhizobium sp., Chlamydomonas sajao and Azotobacter vinelandii increase aggregate stability through the production of extracellular compounds or polysaccharides such as microbial mucilage (Chaney and Swift, 1986; Metting, 1986; Alami et al., 2000). In turn, soil structural attributes, such as aggregate size and pore size distribution affect microbial activity and distribution. Kihara (2012) evaluated how different tillage, cropping systems, and residue management affect
3
soil structure and related microbial diversity. Their results show bacterial diversity and community composition were influenced by any means of reduced tillage management, while fungal diversity even partially declined when macro-aggregates were restored, likely due to the dominance of selected fast-growing species. Soil compaction increases soil bulk density and decreases porosity and macro-pore continuity that may create less favorable conditions for soil microorganisms, which subsequently alters microbial distribution and activity and modifies soil microbial community structure (Grigal, 2000). Compacting soil may also alter the spatial distribution and availability of organic substrates and nutrients and decrease soil water availability and aeration (Breland and Hansen, 1996; Chen et al., 2003). Bacteria are often associated with clay and polysaccharides in micro-aggregates (Tisdall and Oades, 1982). However, the effect of aggregate size on microbial activity depends on numerous factors. Soil microbial biomass (per unit soil mass) has been found to be greater in macro-aggregates than in micro-aggregates (Franzluebbers and Arshad, 1997). Lupwayi (2001) reported bacterial diversity tended to increase with increasing aggregate size. Besides, bacterial activity may dominate in micro-aggregates while fungal activity dominates in macro-aggregates (Tisdall and Oades, 1982; Schutter and Dick, 2002).
Furthermore, little attention has been paid to the nature of soil compositional data. As proportions of a whole, soil components data, such as particle-size distribution, aggregate-size distribution, soil mineral, and organic components, are compositional data. Soil components are subject to methodological bias if they are not handled as compositional data (Parent et al., 2012), due to systematic negative bias, sub-compositional incoherence, redundancy of information and non-normal distribution (Van den Boogaart et al., 2006). To appropriately conduct multivariate analysis on compositional data, compositional data analysis is useful for handling soil and plant compositions (Parent et al., 2012, 2013).
4
References
Abid, M., Lal, R., 2008. Tillage and drainage impact on soil quality: I. Aggregate stability, carbon and nitrogen pools. Soil Till. Res. 100, 89–98. https://doi.org/10.1016/j.still.2008.04.012.
Alami, Y., Achouak, W., Marol, C., Heulin, T., 2000. Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobiumsp. Strain isolated from sunflower roots. Appl. Environ. Microbiol. 66, 3393–3398. https://doi.org/10.1128/AEM.66.8.3393-3398.2000.
Allison, F.E., 1968. Soil aggregation-some facts and fallacies as seen by a microbiologist. Soil Sci. 106, 136– 143.
Amellal, N., Burtin, G., Bartoli, F., Heulin, T., 1998. Colonization of Wheat Roots by an Exopolysaccharide-Producing Pantoea agglomerans Strain and Its Effect on Rhizosphere Soil Aggregation. Appl. Environ. Microbiol. 64, 3740–3747.
Amezketa, E., 1999. Soil aggregate stability: a review. J. Sustain. Agric. 14(2-3), 83-151.
Anderson, A.N., McBratney, A.B., Crawford, J.W., 1997. Applications of fractals to soil studies. Adv. Agron. 63, 1–76. https://doi.org/10.1016/S0065-2113(08)60241-2.
Barto, E.K., Alt, F., Oelmann, Y., Wilcke, W., Rillig, M.C., 2010. Contributions of biotic and abiotic factors to soil aggregation across a land use gradient. Soil Biol. Biochem. 42, 2316–2324. https://doi.org/10.1016/j.soilbio.2010.09.008.
Borie, F., Rubio, R., Rouanet, J.L., Morales, A., Borie, G., Rojas, C., 2006. Effects of tillage systems on soil characteristics, glomalin and mycorrhizal propagules in a Chilean Ultisol. Soil Till. Res. 88, 253–261. https://doi.org/10.1016/j.still.2005.06.004.
Brahim, N., Bernoux, M., Gallali, T., 2012. Pedotransfer functions to estimate soil bulk density for Northern Africa: Tunisia case. J. Arid Environ. 81, 77–83. https://doi.org/10.1016/j.jaridenv.2012.01.012.
Breland, T.A., Hansen, S., 1996. Nitrogen mineralization and microbial biomass as affected by soil compaction. Soil Biol. Biochem. 28, 655–663. https://doi.org/10.1016/0038-0717(95)00154-9.
Bronick, C. J., Lal, R., 2005. Soil structure and management: a review. Geoderma 124(1), 3-22. https://doi.org/10.1016/j.geoderma.2004.03.005.
5
Cambardella, C.A., 2006. Aggregation and organic matter, in: Encyclopedia of Soil Science. Boca Raton, FL. pp. 52–55.
Caruso, T., Barto, E.K., Siddiky, M.R.K., Smigelski, J., Rillig, M.C., 2011. Are power laws that estimate fractal dimension a good descriptor of soil structure and its link to soil biological properties? Soil Biol. Biochem. 43, 359–366. https://doi.org/10.1016/j.soilbio.2010.11.001.
Chaney, K., Swift, R.S., 1986. Studies on aggregate stability. I. Re-formation of soil aggregates. Eur. J. Soil Sci. 37, 329–335. https://doi.org/10.1111/j.1365-2389.1986.tb00035.x.
Chaudhary, V., Bowker, M., O’ dell, T., Grace, J., Redman, A., Rillig, M., Johnson, N., 2009. Untangling the biological contributions to soil stability in semiarid shrublands. Ecological Applications 19: 110-122. https://doi.org/10.1890/07-2076.1.
Chen, C.R., Xu, Z.H., Blumfield, T.J., Hughes, J.M., 2003. Soil microbial biomass during the early establishment of hoop pine plantation: seasonal variation and impacts of site preparation. For. Ecol. Manag. 186, 213–225. https://doi.org/10.1016/S0378-1127(03)00275-5.
Curaqueo, G., Barea, J.M., Acevedo, E., Rubio, R., Cornejo, P., Borie, F., 2011. Effects of different tillage system on arbuscular mycorrhizal fungal propagules and physical properties in a Mediterranean agroecosystem in central Chile. Soil Till. Res. 113, 11–18. https://doi.org/10.1016/j.still.2011.02.004.
Duiker, S. W., Rhoton, F. E., Torrent, J., Smeck, N. E., Lal, R., 2003. Iron (hydr) oxide crystallinity effects on soil aggregation. Soil Sci. Soc. Am. J. 67, 606–611. https://doi.org/0.2136/sssaj2003.6060.
Franzluebbers, A.J., Arshad, M.A., 1997. Soil microbial biomass and mineralizable carbon of water-stable aggregates. Soil Sci. Soc. Am. J. 61, 1090–1097. http://doi.org/10.2136/sssaj1997.03615995006100040015x.
Grigal, D.F., 2000. Effects of extensive forest management on soil productivity. For. Ecol. Manag. 138, 167– 185. https://doi.org/10.1016/S0378-1127(00)00395-9.
Håkansson, I., Lipiec, J., 2000. A review of the usefulness of relative bulk density values in studies of soil structure and compaction. Soil Till. Res. 53, 71–85. https://doi.org/10.1016/S0167-1987(99)00095-1.
Hamza, M.A., Anderson, S.H., Aylmore, L.A.G., 2001. Studies of soil water drawdowns by single radish roots at decreasing soil water content using computer-assisted tomography. Soil Res. 39, 1387–1396. https://doi.org/10.1071/SR98057.
6
Hamza, M.A., Anderson, W.K., 2002. Improving soil physical fertility and crop yield on a clay soil in Western Australia. Aust. J. Agric. Res. 53, 615–620. https://doi.org/10.1071/AR01099.
Hamza, M.A., Anderson, W.K., 2003. Responses of soil properties and grain yields to deep ripping and gypsum application in a compacted loamy sand soil contrasted with a sandy clay loam soil in Western Australia. Aust. J. Agric. Res. 54, 273–282. https://doi.org/10.1071/AR02102.
Hamza MA, Anderson WK. 2005. Soil compaction in cropping systems: A review of the nature, causes and possible solutions. Soil Till. Res. 82:121–145. https://doi.org/10.1016/j.still.2004.08.009.
Haynes, R.J., Naidu, R., 1998. Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: a review. Nutr. Cycl. Agroecosystems 51, 123–137. https://doi.org/10.1023/A:1009738307837.
Horn, R., Domżżał, H., Słowińska-Jurkiewicz, A., Van Ouwerkerk, C., 1995. Soil compaction processes and their effects on the structure of arable soils and the environment. Soil Till. Res. 35(1-2), 23-36. https://doi.org/10.1016/0167-1987(95)00479-C.
Jalabert, S.S.M., Martin, M.P., Renaud, J.-P., Boulonne, L., Jolivet, C., Montanarella, L., Arrouays, D., 2010. Estimating forest soil bulk density using boosted regression modelling. Soil Use Manag. 26, 516–528. https://doi.org/10.1111/j.1475-2743.2010.00305.x.
Kibblewhite, M.G., Ritz, K., Swift, M.J., 2008. Soil health in agricultural systems. Philos. Trans. R. Soc. B Biol. Sci. 363, 685–701. https://doi.org/10.1098/rstb.2007.2178.
Kihara, J., Martius, C., Bationo, A., Thuita, M., Lesueur, D., Herrmann, L., Amelung, W., Vlek, P.L., 2012. Soil aggregation and total diversity of bacteria and fungi in various tillage systems of sub-humid and semi-arid Kenya. Appl. Soil Ecol. 58, 12–20. https://doi.org/10.1016/j.apsoil.2012.03.004.
Lupwayi, N.Z., Arshad, M.A., Rice, W.A., Clayton, G.W., 2001. Bacterial diversity in water-stable aggregates of soils under conventional and zero tillage management. Appl. Soil Ecol. 16, 251–261. https://doi.org/10.1016/S0929-1393(00)00123-2.
Martin, J.P., Martin, W.P., Page, J.B., Raney, W.A., De Ment, J.D., 1955. Soil aggregation, in: Norman A.G. (Eds.), Advances in Agronomy. Elsevier, New York, 1–37. https://doi.org/10.1016/S0065-2113(08)60333-8.
7
Martin, M. P., Lo Seen, D., Boulonne, L., Jolivet, C., Nair, K. M., Bourgeon, G., Arrouay, D., 2009. Optimizing pedotransfer functions for estimating soil bulk density using boosted regression trees. Soil Sci. Soc. Am. J. 73, 485–493. https://doi.org/10.2136/sssaj2007.0241.
Mazurak, A.P., 1950. Effect of gaseous phase on water-stable synthetic aggregates. Soil Sci. 69, 135–148.
Metting, B., 1986. Population dynamics of Chlamydomonas sajao and its influence on soil aggregate stabilization in the field. Appl. Environ. Microbiol. 51, 1161–1164.
Mikha, M.M., Rice, C.W., 2004. Tillage and manure effects on soil and aggregate-associated carbon and nitrogen. Soil Sci. Soc. Am. J. 68, 809–816. doi:10.2136/sssaj2004.8090.
Munyanziza, E., Kehri, H.K., Bagyaraj, D.J., 1997. Agricultural intensification, soil biodiversity and agro-ecosystem function in the tropics: the role of mycorrhiza in crops and trees. Appl. Soil Ecol. 6, 77–85. https://doi.org/10.1016/S0929-1393(96)00152-7.
Nhantumbo, A.B., Cambule, A.H., 2006. Bulk density by Proctor test as a function of texture for agricultural soils in Maputo province of Mozambique. Soil Till. Res. 87, 231–239. https://doi.org/10.1016/j.still.2005.04.001.
Ogle, S.M., Jay Breidt, F., Eve, M.D., Paustian, K., 2003. Uncertainty in estimating land use and management impacts on soil organic carbon storage for US agricultural lands between 1982 and 1997. Glob. Change Biol. 9, 1521–1542. https://doi.org/10.1046/j.1365-2486.2003.00683.x.
Panayiotopoulos, K.P., Papadopoulou, C.P., Hatjiioannidou, A., 1994. Compaction and penetration resistance of an Alfisol and Entisol and their influence on root growth of maize seedlings. Soil Till. Res. 31, 323–337. https://doi.org/10.1016/0167-1987(94)90039-6.
Parent, L.E., de Almeida, C.X., Hernandes, A., Egozcue, J.J., Gülser, C., Bolinder, M.A., Kätterer, T., Andrén, O., Parent, S.-É., Anctil, F., Centurion, J.F., Natale, W., 2012. Compositional analysis for an unbiased measure of soil aggregation. Geoderma 179–180, 123–131. https://doi.org/10.1016/j.geoderma.2012.02.022.
Parent, L. E., Parent, S.-É., Hébert-Gentile, V., Naess, K., Lapointe, L., 2013. Mineral balance plasticity of cloudberry (Rubus chamaemorus) in Quebec-Labrador bogs. Am. J. Plant Sci. 4, 1508. doi:10.4236/ajps.2013.47183.
Perfect, E., Blevins, R.L., 1997. Fractal characterization of soil aggregation and fragmentation as influenced by tillage treatment. Soil Sci. Soc. Am. J. 61, 896–900. doi:10.2136/sssaj1997.03615995006100030026x.
8
Sainju, U.M., Caesar-TonThat, T., Jabro, J.D., 2009. Carbon and nitrogen fractions in dryland soil aggregates affected by long-term tillage and cropping sequence. Soil Sci. Soc. Am. J. 73, 1488–1495. doi:10.2136/sssaj2008.0405.
Sepaskhah, A., Mosavi, A., Borzma, L., 2000. Evaluation of fractal dimensions for analysis of aggregate stability. Iran Agric. Res. 19, 99–114. doi: 10.22099/IAR.2000.4311.
Schutter, M.E., Dick, R.P., 2002. Microbial community profiles and activities among aggregates of winter fallow and cover-cropped soil. Soil Sci. Soc. Am. J. 66, 142–153. doi:10.2136/sssaj2002.1420.
Six, J., Elliott, E.T., Paustian, K., 2000. Soil structure and soil organic matter II. A normalized stability index and the effect of mineralogy. Soil Sci. Soc. Am. J. 64, 1042–1049. doi:10.2136/sssaj2000.6431042x.
Six, J., Feller, C., Denef, K., Ogle, S., de Moraes Sa, J.C., Albrecht, A., 2002. Soil organic matter, biota and aggregation in temperate and tropical soils-Effects of no-tillage. Agronomie 22, 755–775. https://doi.org/10.1051/agro:2002043.
Six, J., Frey, S.D., Thiet, R.K., Batten, K.M., 2006. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 70, 555–569. doi:10.2136/sssaj2004.0347.
Suuster, E., Ritz, C., Roostalu, H., Reintam, E., Kõlli, R., Astover, A., 2011. Soil bulk density pedotransfer
functions of the humus horizon in arable soils. Geoderma 163, 74–82.
https://doi.org/10.1016/j.geoderma.2011.04.005.
Tisdall, J.M., Oades, J., 1982. Organic matter and water stable aggregates in soils. Eur. J. Soil Sci. 33, 141– 163. https://doi.org/10.1111/j.1365-2389.1982.tb01755.x.
Van Bavel, C.H.M., 1950. Mean Weight Diameter of Soil Aggregates as a Statistical Index of Aggregation. Soil Sci. Soc. Am. J. 14, 20–23. http://dx.doi.org/10.2136/sssaj1950.036159950014000C0005x.
Van den Boogaart, K.G., Tolosana-Delgado, R., Bren, M., 2006. Concepts for handling zeroes and missing values in compositional data, in: Proceedings of IAMG.
West, T.O., Post, W.M., 2002. Soil organic carbon sequestration rates by tillage and crop rotation. Soil Sci. Soc. Am. J. 66, 1930–1946. doi:10.2136/sssaj2002.1930.
Young, I.M., Crawford, J.W., 2004. Interactions and self-organization in the soil-microbe complex. Science 304, 1634–1637. doi: 10.1126/science.1097394.
9
Zhang, B., Horn, R., 2001. Mechanisms of aggregate stabilization in Ultisols from subtropical China. Geoderma 99, 123–145. https://doi.org/10.1016/S0016-7061(00)00069-0.
Zhang, S., Li, Q., Zhang, X., Wei, K., Chen, L., Liang, W., 2012. Effects of conservation tillage on soil aggregation and aggregate binding agents in black soil of Northeast China. Soil Till. Res. 124, 196–202. https://doi.org/10.1016/j.still.2012.06.007.
Zhao, Y., Krzic, M., Bulmer, C.E., Schmidt, M.G., 2008. Maximum bulk density of British Columbia forest soils from the Proctor test: Relationships with selected physical and chemical properties. Soil Sci. Soc. Am. J. 72, 442–452. doi:10.2136/sssaj2007.0075.
10
Chapter 1 Literature Review
Chapter one is a literature review discussing soil aggregation and soil compaction. The review examines the basic concepts, mechanisms and binding agents of aggregation, and the relationship between soil aggregates and microorganisms. It also discusses the effects of soil compaction on soil properties, as well as the assessment of soil compaction.
11
1.1 Soil structure
Soil structure represents the organization and arrangement of soil particles and pore networks in the soil (Ghezzehei, 2012). Soil structure influences soil water movement and retention, erosion, crusting, nutrient recycling, root penetration and crop yield (Bronick and Lal, 2005). A well-structured soil has stable aggregates and a network of soil pores that can allow air and water entry into the soil for healthy plant growth, improved drainage and reduced soil erosion caused by excess surface run-off. Aggregate stability is often used as an indicator of soil structure (Six et al., 2000). Favourable soil structure and high aggregate stability are important to improve soil fertility, increasing agronomic productivity, enhancing porosity and decrease erodibility (Bronick and Lal, 2005). Soil management, such as tillage and amendments could influence aggregate stability (Blanco-Canqui et al., 2009; Blanco-Moure et al., 2012) and aggregate-associated C and N (Mikha and Rice, 2004; Zibilske and Bradford, 2007; Mikha et al., 2013). Previous studies indicated that intensive tillage practices increased SOM decomposition (Six et al., 2002; Blanco-Moure et al., 2012) and reduced the “life cycle” of soil macro-aggregates (Six et al., 2000). Traditionally, most farmers in Quebec utilized conventional tillage. Intensive tillage in Quebec resulted in the reduction in the organic matter content, compaction and deterioration of the structure (Inventory of Québec Agricultural Soil Degradation Problems,1990). In recent decades farmers have increasingly substituted conventional tillage with no-till seeding techniques or conservation tillage, such as reduced tillage. Conservation practices enhances soil aggregation by minimizing soil mixing and disturbance, allowing SOM accumulation and microbial activities (Six et al., 2000; Busari et al., 2015). Adding organic amendments such as manure was found to enhance the formation and stabilization of soil macro-aggregates, increase macro-aggregate-associated C and N (Aoyama et al., 1999; Mikha and Rice, 2004; Wortmann and Shapiro, 2008), reduce soil crust formation and decrease water runoff (Pagliai et al., 2004).
1.1.1 Basic concepts of aggregation
Soil aggregate is a group of primary soil particles that cohere to each other by soil physical, chemical and biological influences (Ahamad et al., 2012). It formed through the combination of mineral particles with organic and inorganic substances. Soil aggregation includes the processes of formation and stabilization (Amézketa, 1999). Formation of soil aggregates happens mainly as a result of physical forces, while the stabilization of soil aggregates is produced by the interaction of many factors, in particular the quantity and quality of inorganic and organic stabilizing agents, including mainly clays, polyvalent cations and organic matter (Lynch and Bragg, 1985; Oades, 1993; Dalal and Bridge, 1996).The physical forces include alternate wetting and drying (semi-arid and sub-humid regions), freezing and thawing (temperate regions), the compressive as well as drying action of roots, and the mechanical action of soil fauna, mainly earthworms, termites and ants (Amézketa, 1999). Aggregates occur in a variety of forms and sizes. These are often grouped as macro-aggregates (>250 µm) and
micro-12
aggregates (<250 µm) that are further subdivided by size (Tisdall and Oades, 1982). Different size groups differ in properties such as binding agents and carbon and nitrogen distribution.
1.1.2 Methods of expressing the size distribution of soil aggregates
To evaluate treatments or rank soils, an aggregation index is necessary. Mean weight diameter (MWD) (Van Bavel, 1950), geometric mean diameter (GMD) (Mazurak, 1950) and fragmental fractal dimensions (Perfect and Kay, 1991; Rieu and Sposito, 1991; Tyler and Wheatcraft, 1992) have been frequently used. However, Parent et al. (2012) pointed out that MWD is biased because (1) proportions have an inherently non-normal distribution; (2) one proportion is redundant but not omitted; (3) the size of the index largely depends on sieve number and size since greater sieve size provides more weight to large-size aggregate and (4) differences in measurement scales, such as including or not the solid sand fraction, generates spurious correlations. Fragmentation fractal analysis is also biased since (1) particle counts are based on weak or invalid assumptions on particle diameter, shape and bulk density and (2) the fractal domains are chosen arbitrarily (Parent et al., 2012). Since aggregate size fractions are compositional data, compositional data analysis provides tools to project the aggregate data into the Euclidean space and avoid biases (Aitchison, 1986; Buccianti et al., 2006; Pawlowsky-Glahn and Egozcue, 2006; Pawlowsky-Glahn and Buccianti, 2011). Parent et al. (2012) proposed using isometric log-ratios (ilrs) and an Aitchison distance between any two aggregation states to characterize soil aggregation and to implement as unbiased index of soil aggregation.
1.1.3 Mechanisms of aggregation
There are several models of aggregation. Emerson (1959) postulated a simplified model, which indicates that organic substances bridge sand and clay crystals to form and stabilize aggregates, called “soil crumbs”. Greenland (1965) proposed that aggregates are formed and stabilized by microbial polysaccharides derived from fresh plant residues. Edwards and Bremner (1967) indicated that micro-aggregates join together to form macro-aggregates and the bonds within micro-aggregates formed through bridges of polyvalent cations (such as Ca!", Fe#", Al#", aluminosilicates and hydroxyaluminium) are stronger than the bonds between
micro-aggregates. In their model, micro-aggregates (<250 µm) are formed from organic molecules (OM) attached to clay (Cl) and polyvalent cations (P) to form compound particles (Cl–P–OM) that can join together and then form macro-aggregates [(Cl– P– OM)$]% (Edwards and Bremner, 1967; Tisdall, 1996). The (Cl– P– OM)$ represent compound particles of clay size (<2 µm in diameter) and x and y are finite whole numbers with limits dictated by the size of the primary clay particles. The aggregate hierarchical model of Tisdall and Oades (1982) outlines four stages of macro- and micro-aggregation. Primary particles and then micro-aggregates are linked together to form macro-aggregates (Figure 1-1). Oades (1984) revised this model of aggregate hierarchy and
13
indicated that macro-aggregates (>250 µm) burst into micro-aggregates (20 to 250 µm) as a prior step in forming micro-aggregates.
Alternatively, macro-aggregates can form around particulate organic matter (POM). Macro-aggregates become more stable and micro-aggregates form inside macro-aggregates when POM is decomposed, and microbial exudates are released (Beare et al., 1994; Jastrow et al., 1998; Six et al., 1999; Plante and McGill, 2002). Another conceptual model of interactions between aggregation and biota activity was given by Six et al. (2002). Aggregates physically protect SOM by (1) forming a physical barrier between microorganisms plus microbial enzymes and their substrates, (2) controlling food web interactions, and (3) influencing microbial turnover.
Figure 1 - 1 Hierarchy of soil aggregates. (Brady and Weil, 2008).
In conclusion, aggregates could form through a combination of processes (Figure 1-2). Macro-aggregates may form through the accumulation of micro-aggregates or around POM or bacterial cores. They may also decompose or break down later into micro-aggregates. On the other hand, micro-aggregates may initially form through the compound particles of clay, SOM, and cations, or as turnover products from macro-aggregates (Bronick and Lal, 2005).
14
Figure 1 - 2 Possible scenarios of aggregation. Organic matter (OM), particulate organic matter (POM), clay
(Cl), particle (P) (Bronick and Lal, 2005).
1.1.4 Agents of aggregation
1.1.4.1 CarbonThe source of C, whether it is SOC or soil inorganic carbon (SIC), influences its composition and concentration in soil, which in turn influences its effectiveness in aggregation through associations with cations and soil particles (Bronick and Lal, 2005). Increased SOC is related to increasing aggregate-size classes because large aggregate-size classes are composed of small aggregate-size classes plus organic binding agents (Elliott, 1986). SOM is closely related to SOC dynamics in the soils because it constitutes the largest terrestrial reservoir of SOC. SOM can either directly act as a binding agent (Piccolo and Mbagwu, 1999; Spaccini et al., 2002) or indirectly promote soil microbial activity, enhancing aggregate formation and stability (Murphy, 2015). In soils where SOM is the major binding agent, plant growth and the decomposition of organic inputs lead to the development of a hierarchy aggregate structure (Tisdall and Oades, 1982; Oades and Waters, 1991). The SOM is expected to be the primary binding agent in 2:1 clay-dominated soils because polyvalent-organic matter complexes form bridges between the negatively charged clay platelets. In contrast, SOM is not the only binding agent in oxide- and 1:1 clay-dominated soils (Six. et al., 2000). The chemical properties of SOC determine their charge and complexation capacities and influence decomposition rates which have direct effects on aggregation (Schulten and Leinweber, 2000).
(1) Particulate organic matter
The POM is comprised of large particles of organic matter (250-2000 µm) that exist as free POM light fraction (LF) or encrusted with soil particles, which in turn offers physical protection from decomposition (Plante and McGill, 2002). The LF in the soil is generally associated with clay and polyvalent cations to form aggregates.