The transcriptomic profile and synaptic excitability of
vasoactive intestinal peptide-expressing interneurons in
the mouse hippocampus
Thèse
Xiao Luo
Doctorat en biochimie
Philosophiæ doctor (Ph. D.)
Québec, Canada
© Xiao Luo, 2018
The transcriptomic profile and synaptic excitability of
vasoactive intestinal peptide-expressing interneurons in
the mouse hippocampus
Thèse
Xiao Luo
Sous la direction de :
iii
Résumé
Les neurones sont les éléments constitutifs du système nerveux. Dans le cortex, les neurones peuvent être divisés en cellules principales qui effectuent des calculs excitateurs via des connexions synaptiques locales et à longue distance et des interneurones qui contrôlent tous les domaines subcellulaires des cellules principales. Les interneurones inhibent les cellules principales en hyperpolarisant la membrane postsynaptique via les récepteurs GABA. En plus de contrôler le niveau d’excitabilité de cellules isolées via une inhibition transitoire ou à long terme, elles coordonnent le déclenchement des ensembles cellulaires principaux pour générer des oscillations de réseau qui traversent les zones du cerveau. Le dysfonctionnement des interneurones entraîne des troubles cérébraux comme la schizophrénie, l'autisme et l'épilepsie. Contrairement aux cellules principales, les interneurones présentent un haut niveau de diversité, cohérent avec leurs différents rôles fonctionnels dans les circuits cérébraux. Pour comprendre leurs fonctions de réseau, les neuroscientifiques ont développé plusieurs critères pour classer les interneurones, notamment la cytomorphologie, la connectivité, les propriétés électrophysiologiques et les marqueurs moléculaires. En général, trois types d’interneurones représentent la majorité des interneurones corticaux: les cellules somatostatine (SOM)+ ciblant la dendrite, les cellules parvalbumine (PV)+ ciblant le soma, et les cellules l'interneurone specifique peptide vasoactif intestinal (VIP)+. Dans l'hippocampe, les cellules VIP+ (hormis les cellules VIP+) jouent un rôle unique dans le réseau, car elles innervent de préférence les interneurones mais évitent les cellules principales. Cependant, leur taxonomie et leurs propriétés physiologiques sont moins claires que celles des autres types d’interneurones. Mon projet de thèse a porté sur deux sous-types de cellules VIP: la cellule VIP+ à cellule longue et la cellule à interneurone de type 3 (IS3).
Une nouvelle cellule VIP+ à longue projection (VIP-LRP) a été identifiée dans l'hippocampe CA1 Oriens/Alveus (Francavilla et al., 2018). Ces cellules ciblent de manière sélective les interneurones dans la zone hippocampique CA1, mais se projettent également dans le subiculum de la zone voisine. En outre, ils sont plus actifs pendant la période stationnaire d'éveil et silencieux pendant les oscillations thêta ou d'ondulation. Cependant, les marqueurs moléculaires qu'ils expriment n'étaient pas clairs. Pour examiner leurs marqueurs moléculaires et développer des lignées de souris spécifiques de type cellulaire en utilisant une approche génétique combinatoire, j'ai d'abord procédé à une immunohistochimie pour déterminer les marqueurs couramment exprimés, notamment le récepteur muscarinique 2, la cholécystokinine, la calbidine nNOS), calrétinine (CR) et SOM dans les cellules VIP dans les striatum oriens (SO) de CA1. Nous avons constaté que les cellules VIP-LRP étaient négatives pour SOM et nNOS, mais que la moitié d'entre elles exprimaient M2R. De plus, une petite fraction des cellules GFP expriment CCK, CB et CR. La proportion de cellules M2R + VIP-LRP était différente entre différentes souches de souris.
Ensuite, nous avons effectué le profilage transcriptomique de VIP-LRP identifiés anatomiquement en utilisant le séquençage d'ARN à cellule unique. J'ai identifié plusieurs
iv
marqueurs moléculaires, tels que la proenképhaline, le neuropeptide Y et la nétrine G1, ainsi que de nombreux autres gènes appartenant à plusieurs familles de gènes importantes: canaux ioniques, récepteurs de neurotransmetteurs, neuromodulateurs, molécules d'adhésion cellulaire et de myélinisation. De plus, les LRP VIP partagent des gènes communs lors de la comparaison avec les types de cellules VIP, CR et VIP, CCK dans le néocortex. Ensemble, ces données suggèrent que même si les LRP-VIP représentent un groupe intermédiaire dans le sous-type VIP, ils peuvent exprimer des gènes liés à des caractéristiques spécifiques permettant une coordination à longue distance des activités neuronales dans le CA1 et le subiculum.
Après cela, j'ai examiné les propriétés synaptiques excitatrices d'un autre sous-type VIP +, la cellule IS3. Des études antérieures ont montré qu’ils fabriquaient des synapses sur les interneurones dans le SO, qui contrôlent à leur tour l’intégration des apports excitateurs reçus par les dendrites proximales et distales des cellules pyramidales. Cependant, les propriétés des entrées excitatrices véhiculant les cellules IS3 restent inconnues. En utilisant l'enregistrement par patch clamp et le recalage du glutamate à deux photons, nous avons évalué les propriétés synaptiques de deux entrées excitatrices formées sur les cellules IS3 par les collatérales de Schaffer (CA) et la voie temporoammonique (TA) du cortex entorhinal. Les résultats ont montré que les courants postsynaptiques excitateurs (EPSC) évoqués dans les cellules IS3 par stimulation électrique de la voie TA avaient une amplitude, une élévation et un temps de décroissance inférieurs à ceux des synapses SC. De plus, la transmission synaptique TA-IS3 était médiée par les récepteurs AMPA et NMDA. En outre, les deux AT et SC-EPSC ont montré une facilitation synaptique à court terme en réponse à une stimulation répétitive. Enfin, les voies TA et SC ont montré un degré similaire d'intégration spatiale. Lorsque ces propriétés synaptiques ont été incorporées dans le modèle de calcul IS3 in
vivo (Guet-McCreight et al., 2016), l'activation des cellules IS3 peut être provoquée par
ces entrées excitatrices au cours des oscillations du thêta et de l'ondulation hippocampique. L'imagerie in vivo à deux photons chez des souris éveillées a montré que le déclenchement des cellules IS3 augmentait pendant le rythme thêta, alors que leurs activités n'étaient pas associées
v
Summary
Neurons are the building blocks of nervous system. In the cortex, neurons can be divided into principal cells that perform excitatory computations through local and long-range synaptic connections and interneurons that controls all subcellular domains of principal cells. Interneurons inhibit principal cells by hyperpolarizing the postsynaptic membrane via GABA receptors. In addition to controlling the level of excitability of single cells via transient or long-lasting inhibition, they coordinate the firing of principal cell ensembles to generate network oscillations that travel across brain areas. The malfunction of interneurons leads to severe brain disorders such as schizophrenia, autism and epilepsy. In contrast to principal cells, interneurons display high level of diversity, consistant with their various functional roles in the brain circuitry. To understand their network functions, neuroscientists have developed several criteria to classify interneurons, including cytomorphology, connectivity, electrophysiological properties, and molecular markers. In general, three interneuron types account for the majority of cortical interneurons: dendrite-targeting somatostatin (SOM)+ cells, soma-dendrite-targeting parvalbumin (PV)+ cells, and interneuron-specific vasoactive intestinal peptide (VIP)+ cells. In the hippocampus, VIP+ cells (excluding VIP+ basket cell) play a unique role in the network, since they preferentially innervate interneurons but avoid principal cells. However, their taxonomy and physiological properties are less clear compared to other interneuron types. My PhD project focused on two subtype of VIP cells: VIP+ long-projecting cell and type 3 interneuron-specific (IS3) cell.
A novel long-projecting VIP+ cell (VIP-LRP) has been identified in the hippocampal CA1 Oriens/Alveus (Francavilla et al., 2018). These cells selectively target interneurons in the hippocampal CA1 area, but also project to the neighbouring area subiculum. In addition, they are more active during the stationary period of wakefulness, and silent during theta or ripple oscillations. However, the molecular markers they express were unclear. To examine their molecular markers and develop cell-type specific mouse lines using combinatorial genetic approach, I first performed immunohistochemistry to profile commonly expressed markers including muscarinic receptor 2 (M2R), cholecystokinin (CCK), calbidin (CB), neuronal nitric oxide synthase (nNOS), calretinin (CR), and SOM in VIP cells in the striatum oriens (SO) of CA1. We found that VIP-LRP cells were negative for SOM and nNOS but half of them expressed M2R. Moreover, a small fraction of GFP cells express CCK, CB, and CR. The proportion of M2R+ VIP-LRP cells was different between different mouse strains.
Next, we performed transcriptomic profiling of anatomically identified VIP-LRPs using single-cell RNA sequencing. I identified several molecular markers, such as proenkephalin, neuropeptide Y and netrin G1, as well as many other genes that belong to several important gene families including ion channels, neurotransmitter receptors, neuromodulators, cell adhesion and myelination molecules. In addition, VIP-LRPs share common genes when comparing with VIP;CR and VIP;CCK cell types in the neocortex. Together, these data suggest that although VIP-LRPs represent an intermediate group
vi
within the VIP subtype, they may express genes related to specific features that allow for long-distance coordination of neuronal activities in the CA1 and the subiculum.
After that, I examined the excitatory synaptic properties of another VIP+ subtype-the IS3 cell. Previous studies showed that they make synapses on interneurons in SO, which in turn control the integration of excitatory inputs received by the proximal and distal dendrites of pyramidal cells. However, the properties of excitatory inputs conveying on IS3 cells remain unknown. Using patch clamp recording and two-photon glutamate uncaging, we evaluated the synaptic properties of two excitatory inputs formed on the IS3 cells by the Schaffer collaterals (SC) from CA3 and the Temporoammonic (TA) pathway from entorhinal cortex. The results showed that the excitatory postsynaptic currents (EPSCs) evoked in IS3 cells by electrical stimulation of the TA pathway had a smaller amplitude, slower rise and decay time compared to that of the SC synapses. In addition, TA-IS3 synaptic transmission was mediated by AMPA and NMDA receptors. Furthermore, both TA and SC-EPSCs showed short-term synaptic facilitation in response to repetitive stimulation. Finally, TA and SC pathways displayed similar degree of spatial integration. When these synaptic properties were incorporated into the in vivo-like IS3 computational model (Guet-McCreight et al., 2016), The activation of IS3 cells can be driven by these excitatory inputs during hippocampal theta and ripple oscillations. In vivo two-photon imaging in awake mice showed that the firing of IS3 cells increased during theta rhythm, whereas their activites were not associated with ripples. Together, these data shows that while excitatory inputs are able to drive the firing of IS3 cells during theta, additional mechanisms, such as local inhibition and subcortical modulation may account for the silence of IS3 cells during ripples.
vii
Table of Contents
Résumé ... iii
Summary ... v
List of figures and tables ... xiii
Abbreviations ... xiv
Acknowledgements ... xvi
Preface ... xvii
Chapter 1: Introduction ... 1
1.The anatomy of the hippocampus ... 1
1.1 Connectivity ... 1
1.2 Cellular architecture of the hippocampal CA1 area ... 10
1.2.1 Distal dendrite targeting interneurons ... 11
1.2.2 Proximal and basal dendrite targeting interneurons ... 12
1.2.3 Soma targeting cells ... 14
1.2.4 Axonal initial segment targeting interneurons ... 15
1.2.5 Interneuron-specific interneurons ... 16
1.2.6 IS3 cells ... 17
2. Single-cell RNA sequencing and its application in identifying neuronal markers ... 19
2.1 Neurochemical properties of hippocampal interneurons ... 19
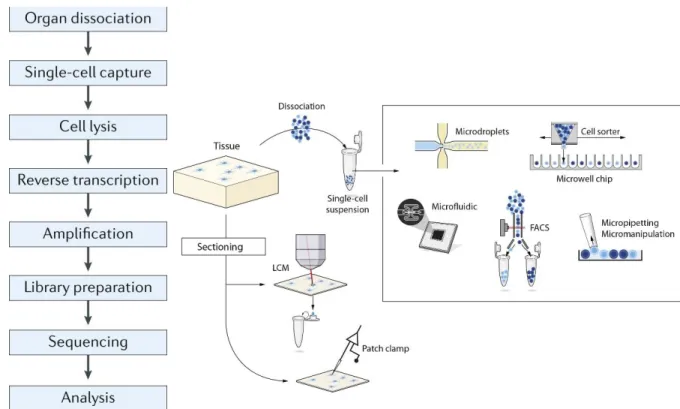
2.2 Pipeline of scRNA-seq ... 21
2.2.1 Single cell harvesting ... 22
2.2.2 cDNA library construction and sequencing ... 24
viii
2.3 Cell-type specific neuronal markers identified with scRNA-seq ... 26
2.3.1 scRNA-seq in cortical neuron classification ... 27
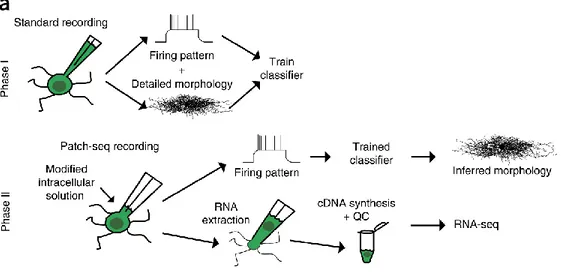
2.3.2 The application of patch-seq ... 32
3. Glutamatergic synaptic transmission in hippocampal interneurons ... 34
3.1 Glutamate receptors in hippocampal interneurons ... 35
3.1.1 AMPA receptors ... 35
3.1.2 NMDA receptors ... 36
3.1.3 Kainate and metabotropic glutamate receptors ... 39
3.2 Comparison of TA and SC synapses in the CA1 region ... 40
4. Temporal and spatial summation on dendrites of interneurons... 44
4.1 Short-term plasticity at excitatory synapses of interneurons ... 44
4.2 Dendritic spatial integration in PCs and interneurons ... 48
4.2.1 Dendritic properties in PCs ... 48
4.2.2 Dendritic integration in interneurons ... 52
General hypothesis 1 ... 59
General hypothesis 2 ... 60
Chapter 2 Integrated article 1: Connectivity and network state-dependent recruitment of long-range VIP-GABAergic neurons in the mouse hippocampus ... 61
Résumé... 61
Abstract ... 62
Introduction ... 62
Results ... 64
VIP-LRP neuron in the CA1 hippocampus ... 64
Local connectivity of VIP-LRP cells ... 66
ix
Activity of VIP-LRP cells in awake mice ... 69
Diversity of subiculum-projecting VIP-LRPs ... 72
Discussion... 74
Methods ... 77
Mouse lines ... 77
Viral constructs ... 78
Slice preparation and patch-clamp recordings ... 78
Two-photon laser scanning photostimulation by glutamate uncaging ... 80
ChR2-based mapping of VIP-LRP targets ... 81
In vitro patch-clamp data analysis ... 82
Cell reconstruction and immunohistochemistry ... 83
Retrograde labeling ... 84
Electron microscopy ... 85
Two-photon imaging in awake mice ... 86
Analysis of two-photon Ca2+ imaging data ... 88
Statistics ... 90
References ... 90
Figure Legends ... 95
Figures ... 101
Chapter 3 Integrated article 2: Transcriptomic profile of hippocampal long-range VIP-GABAergic neurons... 108
Résumé... 108
Abstract ... 109
Introduction ... 110
Materials and Methods ... 111
x
Discussion... 120
References ... 122
Figures and tables ... 126
Chapter 4 Integrated article 3: Synaptic properties and the network state-dependent recruitment of the VIP interneuron-specific interneurons in the CA1 hippocampus ... 132
Résumé... 132
Abstract ... 133
Introduction ... 134
Results ... 136
Synaptic properties of IS3 interneurons ... 136
Synaptic properties of IS3 cells predict that they fire during rising/peak phases of theta oscillations and during SWRs in vivo ... 139
IS3 cells are preferentially activated during locomotion ... 142
IS3 cells prefer to fire near the rising/peak phases of theta oscillations ... 145
IS3 cells are silent during ripples ... 147
Discussion... 147
Methods and Materials ... 152
Animals ... 152
Whole cell patch-clamp recordings in hippocampal slices in vitro... 152
Two-photon glutamate uncaging... 154
Computational model ... 154
Stereotaxic injections ... 159
In vivo two-photon Ca2+-imaging in awake mice ... 160
Immunohistochemistry and morphological reconstructions ... 161
Data analysis ... 162
xi
Acknowledgments ... 167
Reference ... 167
Figures and legends ... 179
Chapter 5: Discussion ... 198
1. VIP-LRPs as an intermediate subpopulation of VIP interneurons ... 198
2. Molecular profiling of VIP+ cells in CA1 SO ... 202
3. Input-specific synaptic transmission in IS3 cells ... 202
4. Short-term facilitation at excitatory synapses to IS3 cells ... 204
5. Spatial summation of excitatory inputs in IS3s ... 205
6. Comparison of IS3s with PV-BCs ... 206
7. The potential role of IS3 cells as network gate-keepers in behavior ... 209
8. Limitations and perspectives ... 212
Chapter 6: Conclusion ... 214
Bibliography ... 217
Appendices ... 231
Article 4: Coordination of dendritic inhibition through local disinhibitory circuits ... 231
Résumé ... 231
Abstract: ... 231
Introduction ... 232
Properties and connectivity of is3 cells ... 232
Morphological and neurochemical features ... 232
Physiological properties ... 233
Connectivity ... 233
Properties of IS3 synapses ... 233
xii
Functional role of disinhibitory circuits ... 234
Acknowledgments ... 235
References ... 236
Figures and legends ... 239
Tables in “Transcriptomic profile of hippocampal long-range VIP-GABAergic neurons” ... 240
xiii
List of figures and tables
Figure 1 Comparative views of the hippocampal system for the human (left), monkey
(middle), and rat (right). ... 3
Figure 2 Drawing of the circuitry and major excitatory inputs to the hippocampal formation. ... 9
Figure 3 Three types of pyramidal cell are accompanied by at least 21 classes of interneuron in the hippocampal CA1 area. ... 10
Figure 4 Dendrite targeting interneurons and interneuron selective interneurons (ISIs). ... 13
Figure 5 Perisomatic targeting interneurons ... 15
Figure 6 VIP-positive interneurons at the PYR/RAD border target O–LM interneurons. 15 Figure 7 Immunohistochemical markers primarily associated with CGE-derived interneurons. ... 20
Figure 8 Work flow of scRNA-seq. ... 20
Figure 9 Neuron subclasses in the somatosensory cortex ... 29
Figure 10 Schematic of patch-seq approach. ... 33
Figure 11 MGE- and CGE-dependent expression of synaptic glutamate receptors. ... 38
Figure 12 Similar current–voltage curves for isolated AMPA EPSCs and different voltage dependence of the isolated NMDA response in the SC and PP (equal to TA) inputs. .. 42
Figure 13 Short-term plasticity at synapses with different I–V properties.……….………46
Figure 14 Figure 14 Dendritic excitability of pyramidal neurons..……….……..……51
Figure 15 Biophysical properties of PV and O-LM neuron dendrites..………..57
Figure 16 Simplified disinhibitory network in the hippocampal CA1 area mediated by IS3 and VIPLRPs...………..……….…………211
xiv
Abbreviations
5-HT3AR 5-hydroxytryptamine receptor 3A AHP Afterhyperpolarization
AMPAR α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor AP Action potential
AUROC Mean area under the receiver operator characteristic curve bAP back-propagating action potential
BC Basket cell BIS Bistratified cell
CA Cornu ammonis CAM Cell adhesion molecule CB Calbindin
CB1R Cannabinoid receptor 1
CCK Cholecystokinin
cDNA Complementary DNA CGE Caudal ganglionic eminence
CNV Gene copy number variants COUPTF2 COUP transcription factor 2 DG Dentate gyrus
DNQX 6,7-dinitroquinoxaline-2,3(1H,4H)-dione EC Entorhinal cortex
EPSC Excitatory postsynaptic current EPSP Excitatory postsynaptic potential FACS Fluorescence-activated cell sorting
FPKM Fragments per kilobase per million mapped reads GABA Gamma-aminobutyric acid
GAD Glutamate decarboxylase GSEA Gene set enrichment analysis gw Gestational week 63
HCN Hyperpolarization-activated cation channel IPSP Inhibitory postsynaptic potentials
IS Interneuron specific KR Kainate receptor
Kv Voltage-gated potassium channel LCM Laser capture microdissection LEC Lateral entorhinal cortex LRP Long-range projecting LTP Long-term potentiation
M2R Muscarinic receptor type 2 MACS Magnetic-activated cell sorting MEC Medial entorhinal cortex
MGE Medial ganglionic eminence mGluR Metabotropic glutamate receptor mRNA Messenger RNA
xv
NGFC Neurogliaform cell NGL-1 Human netrin-G1 ligand NMDAR N-methyl-D-aspartate receptor nNOS Neuronal nitric oxide synthase NPY Neuropeptide Y
O-LM Oriens lacunosum-moleculare interneuron PC Pyramidal cell
PCA Principle component analysis
RT-PCR Real-time polymerase chain reaction PPA Perforant path-associated cell
ProMMT Probabilistic Mixture Modeling for Transcriptomics PV Parvalbumin
Rin Input resistance
RPKM Reads per kilobase per million mapped reads S1 Primary somatosensory cortex
SC Schaffer collaterals
SCA Schaffer collateral-associated cell scRNA-seq Single-cell RNA sequencing SLM Stratum lacunosum-moleculare SO Stratum oriens SOM Somatostatin SP Stratum pyramidale SR Stratum radiatum STD Short-term depression STF Short-term facilitation STP Short-term plasticity SVZ Subventricular zone TA Temporoammonic
TPM Transcripts per million mapped reads
t-SNE T-distributed stochastic neighbor embedding UMI Unique molecular identifiers
VGCC Voltage-gated calcium channels VGIC Voltage-gated ion channels VGLUT Vesicular glutamate transporter VIP Vesoactive intestinal peptide Vm Resting membrane potential
xvi
Acknowledgements
First, I would like to express my sincere gratitude to my advisor Lisa Topolnik, she helped me to build the knowledge base in the field of interneuron study and made a detailed plan for my project. She showed me some experimental skills in person, so that I can quickly adapt to the laboratory work. She is always patient and providing useful advices whenever I run into trouble or have a question about my experiment. Finally, she guided me earnestly in writing thesis and research ariticles. Without her help, I couldn’t have achieved all this.
Next, I would like to offer my great thank my former and current lab collegues including Ruggiero Francavilla, Olivier Camiré, Sona Amalyan, Einer Munoz Pino, Linda Suzanne David, Vincent Villette, Ekaterina Martianova, Elise Magnin, Étienne Gervais, and Alfonsa Zamora Moratalla. In particular, Ruggiero, Olivier and I have worked in collaboration for the longest time in the lab. We published many articles as co-authors. I would like to thank former and current lab technicians Sarah Coté, Stéphanie Racine, Émilie Pic and Juliette Tremblay. All of them helped me a lot in doing experiment, data processing and other lab works. They provided support and suggestions during ups and downs of my life. I become good friends with many of them. I’m grateful for the proofreading of the thesis performed by Qian Li.
I also appreciate the efforts made by external collaborators, including Alexandre Guet-McCreight, Frances Skinner, Peter Somogyi, Maxime Vallée, Arnaud Droit, Chin Wai Hui and Marie-Eve Tremblay. We have achieved high quality research with sincere cooperation.
Last but not least, I would like to thank my parents for their spiritual support far away from China and their good advices for my life and health. I wish them long lives full of happiness.
xvii
Preface
I included four articles in my thesis. The first three articles represent my major works in the lab. They are integrated in the thesis as chapter 2, 3 and 4. I also contributed to the review articles (article 4) attached to the appendix. The information for each article is listed below:
Integrated article 1: Connectivity and network state-dependent recruitment of long-range VIP-GABAergic neurons in the mouse hippocampus
Publication status: Published online in the journal “Nature communication” on
November the 28th, 2018.
Author status: Second author.
Authors: Ruggiero Francavilla1, 2, 4, Vincent Villette1, 2, 4, Xiao Luo1, 2, Simon Chamberland2, Einer Muñoz-Pino1, 2, Olivier Camiré1, 2, Kristina Wagner3, Viktor Kis3, Peter Somogyi3, Lisa Topolnik1, 2, *
Affiliations of each author:
1 Neuroscience Axis, CHU de Québec Research Center – Université Laval; Québec, PQ, G1V 4G2, Canada
2 Dept. Biochemistry, Microbiology and Bio-informatics, Université Laval, Québec, PQ,
G1V 0A6, Canada
3 Dept. Pharmacology, Oxford University, Oxford, OX1 3QT, UK 4 Co-first author
Author Contributions:
L.T. and P.S. supervised the whole study; R.F., X.L., S.C., E.M.-P., O.C. and L.T. performed in vitro recordings; V.V., R.F. and L.T. performed in vivo recordings; K.W., V.K. and P.S. conducted EM studies; R.F. and L.T. wrote the manuscript with comments from V.V., X.L., S.C., E.M.-P., O.C. and P.S.; R.F., L.T. and X.L. prepared the figures.
The authors declare no conflict of interest.
Correspondence should be addressed to:
xviii
Integrated article 2: Transcriptomic profile of hippocampal long-range VIP-GABAergic neurons
Publication status: Submitted to the journal “Scientific Reports” in November of 2018. Author status: First author.
Authors: Xiao Luo1, 2, Einer Muñoz-Pino1, 2, Ruggiero Francavilla1, 2, Maxime Vallée3, Arnaud Droit3, Lisa Topolnik1, 2
Affiliations of each author:
1
Dept. Biochemistry, Microbiology and Bio-informatics, Université Laval, Québec, PQ,
G1V 0A6, Canada 2 Neuroscience Axis, CHU de Québec Research Center – Université Laval; Québec, PQ, G1V 4G2, Canada 3CHU de Québec Research Center – Université Laval; Dept. Molecular Medicine, Laval University, Québec, PQ, G1V 4G2, Canada 4 Co-first author.
Contribution: I developed the experimental protocol for single cell harvesting. I collected
three single cell samples for RNA sequencing. I also contributed to the data anaylsis and writing of the manuscripts and provided suggestions for the data processing.
Correspondence: Dr. Lisa Topolnik Lisa.topolnik@bcm.ulaval.ca
Integrated article 3: Synaptic properties and the network state-dependent recruitment of the VIP interneuron-speci1c interneurons in the CA1 hippocampus Publication status: Submitted to the journal “eLife” in August of 2018, currently under
revision.
Authors: Xiao Luo1,2 *, Alexandre Guet-McCreight3,4 *, Ruggiero Francavilla1,2, Vincent Villette1,2, Frances K Skinner3,5, Lisa Topolnik1,2
Affiliations of each author: 1Dept. Biochemistry, Microbiology and Bio-informatics, Université Laval, Québec, PQ, G1V 0A6, Canada; 2Neuroscience Axis, CHU de Québec Research Center – Université Laval; Québec, PQ, G1V 4G2, Canada; 3Krembil Research Institute, University Health Network, Toronto, ON, Canada; 4Department of Physiology, University of Toronto, Toronto, ON, Canada; 5Departments of Medicine (Neurology) and Physiology, University of Toronto, Toronto, ON, Canada
*the two authors contribute equally to this work
xix
Contribution: I conducted experiments for examining synaptic transmissions,
pharmacology, short-term plasticity at SC-IS3 and TA-IS3 synapses, as well as TA and SC stimulation induced AP firing in IS3 cells. I also performed VIP cell molecular profiling in the CA1, and data analysis for the experiments mentioned above. I contributed to the analysis and writing of the major chapters of the manuscript and provided suggestions for
in vivo data processing and analysis.
Correspondence: Dr. Lisa Topolnik Lisa.Topolnik@bcm.ulaval.ca
Attached article 4: Coordination of dendritic inhibition through local disinhibitory circuits
Publication status: Received: 28 November 2014; accepted: 11 February 2015;
published online: 26 February 2015 in the journal Frontiers in Synaptic Neuroscience.
Author status: Second author.
Authors: Ruggiero Francavilla, Xiao Luo, Elise Magnin, Leonid Tyanand Lisa Topolnik* Affiliations of each author: Department of Biochemistry, Microbiology and
Bio-informatics, Université Laval; Axis of Cellular and Molecular Neuroscience, IUSMQ, Québec, PQ, Canada
Contribution: I wrote the chapter on functional role of disinhibitory circuits. *Correspondence:
Lisa Topolnik, Department of Biochemistry, Microbiology and Bio-informatics, Université Laval; Axis of Cellular and Molecular Neuroscience, IUSMQ, 2601 Ch. De La Canardière, CRULRG, Québec, PQ G1J 2G3, Canada
E-mail: Lisa.Topolnik@bcm.ulaval.ca
This article is published in the journal Frontiers in Synaptic Neuroscience. Copyright © 2015 Francavilla, Luo, Magnin, Tyan and Topolnik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
1
Chapter 1: Introduction
1.The anatomy of the hippocampus
In 1957, Scoville and Milner reported memory deficit following the medial temporal lobe exsection from a patient HM (Scoville and Milner 1957). Since then, the critical role of the hippocampus and neighbouring areas in memory formation has been well established. The progress made in the last 50 years in the connectivity, neuroanatomy, cellular architectures, and physiology of the hippocampus have deepened our understanding of this simple yet complicated structure. In this chapter, we will focus on the anatomy and connectivity of parahippocampal regions and the hippocampal formation, as well as the cellular diversity in the CA1 area.
1.1 Connectivity
1.1.1 Parahippocampal regions
Despite the controversies in terminology, the hippocampal region can be divided into the hippocampal formation and parahippocampal regions. It is generally accepted that the hippocampal region including the hippocampal formation and neighbouring cortical areas should be considered as an integrated system involved in episodic memory. Parahippocampal (or retrohippocampal) regions include perirhinal, postrhinal, entorhinal, presubicullar, and parasubicular cortices. The hippocampal formation consists of the dentate gyrus (DG), Cornu Ammonis (CA) areas: CA3, CA2, CA1, and subiculum. In rodents, the hippocampal region occupies a large proportion of the brain, indicating a crucial role of this structure. In primates, this region is located in the medial temporal lobe and accounts for a small part of the brain, although its absolute size is 100 times larger in human than in the rats. Despite the size difference, the fundamental structure of the hippocampal region is essentially similar across species. The most significant species differences are reported for the entorhinal cortex (EC). In rodents, EC can be segregated into two cytoarchitectonically different areas: the medial and lateral area (MEC and LEC),
2
whereas in monkeys, 7 subregions are found, and in human, there are 9 subregions (Hevner and Wong-Riley, 1992; Krimer et al., 1997).
The postrhinal cortex is a six-layer cortical area located near the caudal pole of the brain (Figure 1). According to cellular architecture, this area can be divided into two subregions. The major afferents of this cortex arise from visual-association areas, such as the posterior parietal cortex and the dorsal retrosplenial cortex. Besides, ventrolateral orbital frontal cortex, caudal, and ventral temporal areas also contribute to the cortical afferents. Other inputs include the hippocampus and the subcortical nuclei such as the thalamus. Postrhinal cortex projects to another cortical area reciprocally, with strong innervation to the perirhinal cortex and MEC. In addition, there are strong inter-connections between CA1, subiculum and postrhinal cortex (Burwell and Amaral, 1998).
The perirhinal cortex is bordered rostrally by the insular cortex and is associated with the fundus and both banks of the rhinal sulcus (Figure 1). Similarly, the perirhinal cortex includes two subareas: Brodmann areas 35 and 36. Brodmann area 35 is characterized by the soma size: big, heart-shaped pyramidal cells (PC) are located in layer V, small cells are located in superficial layers. In contrast, Area 36 has a narrow granular layer IV and smaller cells located in layer V. Area 35 receives major input from the piriform cortex; the other inputs are from the entorhinal, and insular cortex, temporal association, and frontal regions. Area 36 receives afferents mainly from the ventral temporal association cortex as well as insular, entorhinal, and frontal areas. Area 36 also receives subcortical inputs from the amygdala and the thalamus, whereas area 35 receives inputs from the endopiriform nucleus and piriform transition area. In particular, area 35 projects to temporal CA1, temporal subiculum, and presubiculum (Burwell and Amaral, 1998).
The EC has drawn more attention due to its unique structural and functional connection with the hippocampal formation. As mentioned before, EC is divided into MEC and LEC in rodents. Dorsal LEC is bordered by perirhinal and postrhinal
3
cortices, rostral LEC is located closely to the piriform cortex, and the caudal portion of LEC is next to the dorsal MEC. The MEC is relatively smaller and narrower than the LEC, rostral MEC is bordered by ventral LEC, and caudal MEC is next to the ventral occipital pole (see figure 1). The cytoarchitectonical study has shown that Lamina dissecans of layer IV is considered as the hallmark of EC, which is more
Figure 1 Comparative views of the hippocampal system for the human (left), monkey (middle), and rat (right).
The upper panel shows the relevant structures in lateral views of the human brain (a), the monkey brain (b), and the rodent brain (c). The lower panel shows unfolded maps of the relevant cortical structures for the human brain (d), the monkey brain (e), and the rodent brain (f). Shown for the human and monkey brain are unfolded layer IV maps of the perirhinal (PER) areas 35 and 36, parahippocampal (PH) areas TF and TH, and entorhinal cortex (EC). Figures adapted from Burwell RD, Witter MP, and Amaral DG (1995) The perirhinal and postrhinal cortices of the rat: A review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus 5: 390–408; Insausti R, Tuñón T, Sobreviela T, Insausti AM, and Gonsalo LM (1995) The human entorhinal cortex: Acytoarchitectonic analysis. J. Comp. Neurol. 355: 171–198. Shown for the rodent brain are unfolded surface maps of the PER areas 35 and 36, the postrhinal cortex (POR), and the lateral and medial entorhinal areas (LEA and MEA). The rodent POR is the homolog of the primate PH (see text for details). In the monkey and the rat brain, the parasubiculum (ParaS) is interposed between the entorhinal and POR/PH (arrows). The pre- and parasubiculum, which are components of the parahippocampal region, are not shown. Abbreviations: cs, collateral sulcus; rs, rhinal sulcus; DG, dentate gyrus; D, dorsal; L, lateral; M, medial; ParaS, parasubiculum; PreS, presubiculum; S, septal; Sub, subiculum; T, temporal; V, ventral. Adapted from Byrne (2008).
4
significant in MEC (Figure 1). Moreover, LEC has a narrower layer II compared to MEC (Dolorfo and Amaral, 1998). EC receives inputs from various cortical and subcortical areas. Thus, it is considered as a node linking the sensory information with the hippocampus. The most prominent inputs of EC come from parahippocampal regions. In turn, EC provides strong feedback projections to these cortices. Other strong afferents of EC are from pre- and parasubiculum. However, the feedback projection form EC is less significant (Witter and Amaral, 2004). The neocortical inputs of LEC come from piriform, agranular insular, medial and orbital frontal regions. MEC also receives inputs from piriform, frontal, cingulate, parietal, and occipital cortices. Subcortical inputs of EC include claustrum, olfactory structures, the amygdala, septal nuclei and dorsal thalamus (Pereira et al., 2016). EC projects to all regions of the hippocampal formation, including DG, CA3, CA2, CA1, and subiculum. In general, the lateral EC innervates dorsal, septal hippocampus, whereas medial EC project to ventral, temporal hippocampus. The details of these projections will be discussed in chapter 1.1.2.
The dorsal parasubiculum is bordered by the presubiculum, and the ventral parasubiculum is located near the MEC. The layer I and II/III of this cortex can be stained by acetylcholinesterase. The strongest input to this region is from MEC. The superficial layers of parasubiculum receive inputs from subiculum, while projecting back to the hippocampal formation. The major output region is the postrhinal cortex, followed by the layer II of MEC (Witter and Amaral, 2004).
Dorsoventrally, the presubiculum is located in between retrosplenial cortex and parasubiculum. Layers can be differentially stained by acetylcholinesterase. Presubiculum projects intensively to the contralateral part, and is interconnected with hippocampal formation, especially the subiculum. Besides, it is reciprocally connected with MEC, parasubiculum, and postrhinal cortex (Witter and Amaral, 2004).
5
1.1.2 Hippocampal formation
The hippocampal formation is a banana-shaped structure in rodents. Its rostrodorsal end is near the septal nuclei, and the caudalventral end is located next to the temporal lobe. DG and CA fields are present along the septotemporal axis, whereas subiculum is present at ventral two-third of the axis. Growing evidence has shown that dorsal and ventral hippocampi are anatomically and functionally distinct compartments. Dorsal hippocampus is involved in cognition related processes such as spatial navigation and episodic memory, whereas ventral hippocampus is responsible for stress, mood, emotion and motivation(Fanselow and Dong, 2010). Here we focus on the dorsal portion of the rodent hippocampus.
The DG consist of three layers (Figure 2). The innermost layer is called polymorphic layer. The beginning of CA3 principal cell layer extends to this layer. It contains various interneuron cell types and the axons of dentate principal cells. One of the major cell types is the mossy cell, characterized by their large dendritic spines that receive inputs from dentate principal cell. Other interneurons include hilar perforant-path associated cells, hilar commissural-associational perforant-pathway related cells, and molecular layer perforant-path associated cells (Amaral, 1978). The granule layer contains the somas of dentate excitatory principal cells, thus also named the granule cells. These cells have small and densely packed somas. Their spiny dendrites extend to the molecular layer, the outermost layer close to the fissure. Their axons target interneurons in the polymorphic layer and project all the way to principal cells in the CA3, forming the mossy fiber pathway. In addition to granule cell dendrites, some interneurons are located in the molecular layer, such as molecular layer perforant path-associated cells (Han et al., 1993) and axo-axonic cell (Soriano and Frotscher, 1989). The EC is the only cortical region that projects to DG through the perforant path (Figure 2). This pathway originates predominately form the EC layer II. In particular, the EC-DG projection is organized topographically through septotemporal and transversal axis. LEC projects to septal DG, whereas MEC terminates on temporal DG. Moreover, LEC fibers innervate the outer third of the
6
molecular layer, whereas MEC fibers target the middle third of this layer (Steward, 1976;Wyss, 1981). Other major inputs of DG come from subcortical areas. The medial septal and diagonal band of Broca area provide cholinergic input to septal DG, and temporal DG receives lateral septal projection. The supramamillary body of hypothalamus innervates the inner molecular layer next to the granule layer. Noradrenergic input comes from the pontine nucleus of the locus coeruleus and serotoninergic input is from raphe nuclei innervate polymorphic layer. CA3 is the only region that DG sends outputs to. The mossy fibers formed by granule cell axons pass through the polymorphic layer and terminate in CA3 PC layer and stratum
lucidum.
The hippocampus proper is also called Ammon’s horn. This region can be divided into CA3, CA2, and CA1 based on their cellular architecture and connectivity (Figure 2). The principal cells in this region have pyramid-like somas. All subfields show a similar laminar structure. The superficial layer next to the neocortex is called stratum
oriens (SO) which contains the basal dendrites of PCs. The axonal bundles of PCs
form alveus in the outer SO. Deep to SO is the stratum pyramidale (SP), where the densely packed somas of PCs are located. Stratum radiatum (SR) contains the proximal part of the PC apical dendrites. Stratum lacunosum-moleculare (SLM) is located next to DG. Distal dendrites of PCs reside in this layer. CA3 has an additional layer stratum lucidum between SP and SR.
The CA3 contains PCs with larger somas and dendritic arborisation. The axons of CA3 PCs are highly collateralized, which project to other ipsilateral CA fields as well as the contralateral hippocampus. The axonal collaterals that project to CA1 are called the Schaffer Collaterals (SCs). This projection is organized topographically, with the proximal part of CA3 next to DG innervating septal CA1, and distal CA3 next to CA2 projecting to temporal CA1. Moreover, proximal CA3 cells preferentially target superficial CA1 SP close to SR, whereas distal CA3 cells target deep CA1 SP and SO. The CA3-CA3/CA2 projection is termed as the associational projections. The CA3-contralateral projection is termed commissural projection. Unlike SCs,
7
these projections travel along the septotemporal axis and form complex topographical connections (Witter and Amaral, 2004). Similar to DG, CA3 receives input from EC, medial septum, diagonal band of Broca area, locus coeruleus, and raphe nucleus. Additionally, CA3 receives inputs from the basal nucleus of the amygdala.
The connectivity of CA2, a relatively small region has only been fully characterized recently (Kohara et al., 2014). CA2 PCs have larger somas similar to CA3, but lack complex spines on apical dendrites. Proximal apical dendrites of CA2 PC receive strong mossy fiber innervation (Häussler et al., 2016), whereas CA3 innervation terminates on distal parts of apical dendrites. The cortical input of CA2 derives from EC layer II stellate cells. CA2 also receives strong subcortical input from the supramammillary body of the hypothalamus (Haglund et al., 1984). CA2 axons selectively target the basal dendrites of deep CA1 PCs in SO.
Unlike CA3, CA1 field lacks internal and contralateral connections. However, efferent projections are substantial. CA1 is innervated by all parahippocampal regions, including perirhinal, postrhinal, and entorhinal cortices. In particular, EC inputs to CA1 region mainly arise bilaterally from layer III, with minor projections also from layer II and deep layers are also found. These projections form the temporoammonic (TA) pathway (also called perforant path in some literatures), which terminates in SLM, with intensive projections located in the deeper part (close to the fissure). EC-CA1 projections are topographically organized along the transversal axis. LEC preferentially target lateral CA1 close to subiculum, whereas MEC selectively innervates medial CA1 close to CA2. Moreover, a recent study suggests that MEC projection is biased toward deep PCs and LEC tends to excite superficial PCs (Masurkar et al., 2017).
Subcortical inputs to the CA1 region are similar to those to CA3. Septal inputs terminate in SO and exclusively target interneurons. Basal and accessory basal nuclei of amygdala target CA1 intensively. Substantial inputs from the nucleus reuniens of thalamus innervate SLM. The noradrenergic input from the locus
8
coeruleus, serotonergic input from the raphe nucleus, and dopaminergic input from the ventral tegmental area and Substantia nigra are relatively weaker.
The major target region of CA1 axons is the subiculum. The distal CA1 projects to the proximal subiculum, whereas the proximal CA1 projects to distal subiculum. In addition to mutual connections with parahippocampal areas, the CA1 also project to the retrosplenial, preinfralimbic, and medial prefrontal cortices. Subcortical output regions are the anterior olfactory nucleus, the hypothalamus, nucleus accumbens, and the basal nucleus of the amygdala.
The subiculum is considered as the output region of the hippocampus. It can be divided into three layers: the polymorphic layer, the pyramidal layer, and the molecular layer. The extensive afferents come from CA1. Parahippocampal regions are reciprocally connected with the subiculum. The major neocortical targets of subiculum are retrosplenial and prefrontal cortices. The subcortical efferent targets of subiculum include the nucleus accumbens, the amygdala, septal complex, the hypothalamus, and the thalamus.
9
Figure 2 Drawing of the circuitry and major excitatory inputs to the hippocampal formation. Left: Cajal proposed that the nervous system is made up of countless separate units, or nerve cells composed of dendrites, soma, and axons, each of which is a conductive de vice. He further proposed that information is received on the cell bodies and dendrites and conducted to distant locations through axons. Abbreviations: A, retrosplenial area; B, subiculum; C, Ammon’s horn; D, dentate gyrus; E, fimbria; F, cingulum; G, angular bundle; H, corpus callosum; K, recurrent collaterals; a, axon entering the cingulum; b, cingulum fibers; c-e, perforant path fibers; g, subicular cell; h, CA1 pyramidal cells; i, Schaffer collaterals; collaterals of alvear fibers. Adapted from Byrne (2008). Right: a: An illustration of the hippocampal circuitry. b: Diagram of the hippocampal neural network. The traditional excitatory trisynaptic pathway (entorhinal cortex (EC)–dentate gyrus–CA3–CA1–EC) is depicted by solid arrows. The axons of layer II neurons in the entorhinal cortex project to the dentate gyrus through the perforant pathway (PP), including the lateral perforant pathway (LPP) and medial perforant pathway (MPP). The dentate gyrus sends projections to the pyramidal cells in CA3 through mossy fibres. CA3 pyramidal neurons relay the information to CA1 pyramidal neurons through Schaffer collaterals. CA1 pyramidal neurons send back-projections into deep-layer neurons of the EC. CA3 also receives direct projections from EC layer II neurons through the PP. CA1 receives direct input from EC layer III neurons through the temporoammonic pathway (TA). The dentate granule cells also project to the mossy cells in the hilus and hilar interneurons, which send excitatory and inhibitory projections, respectively, back to the granule cells. Adapted from Deng et al. (2010).
10
1.2 Cellular architecture of the hippocampal CA1 area
As mentioned previously, cortical neurons can be divided into excitatory principal cells and inhibitory interneurons (Ramón y Cajal, 1893; Andersen et al., 1963). Compared to principal cells, interneurons have a variety of morphologies and connectivity patterns, allowing them to implement complex tasks. Experimental data and computer simulation results have shown that the inhibition is crucial for controlling the excitatory information flow along the dendrites of PCs during
Figure 3 Three types of pyramidal cell are accompanied by at least 21 classes of interneuron in the hippocampal CA1 area.
The main termination of five glutamatergic inputs are indicated on the left. The somata and dendrites of interneurons innervating pyramidal cells (blue) are orange, and those innervating mainly other interneurons are pink. Axons are purple; the main synaptic terminations are yellow. Note the association of the output synapses of different interneuron types with the perisomatic region (left) and either the Schaffer collateral/commissural or the entorhinal pathway termination zones (right), respectively. VIP, vasoactive intestinal polypeptide; VGLUT, vesicular glutamate transporter; O-LM, oriens lacunosum moleculare. Adapted from Klausberger and Somogyi, (2008).
11
behavioral tasks (Constantinidis et al., 2002; Yizhar et al., 2011). Moreover, interneurons can coordinate the activation of large cell ensembles and modulate network oscillations (Traub et al., 1996). Thus, it is essential to understand the classification of interneurons in terms of cellular anatomy and connectivity.
Interneurons can be categorized according to different criteria, such as morphology, connectivity, molecular markers, electrophysiological properties etc. Here, we classify hippocampal CA1 interneurons according to their postsynaptic targets. Different compartments of CA1 PCs are targeted by distinct populations of interneurons (Figure 3).
1.2.1 Distal dendrite targeting interneurons
The neurogliaform cells (NGFCs) have small and round somas located mainly in SLM. Their dendrites ramify around the soma, forming a dense cloud, which resembles the morphology of glial cells (Figure 4D) (Elfant et al., 2008). The axons are highly collateralized in SLM, forming dense axonal plexus, but also project to the molecular layer of DG, as well as SR. The dense axonal cloud of NGFCs is believed to be responsible for slow inhibitory synaptic transmission mediated by gamma-aminobutyric acid (GABA)A and GABAB receptors (Price et al., 2005). The neurochemical markers expressed by NGFCs include reelin, neuronal nitric oxide synthase (nNOS), neuropeptide Y (NPY), actinin2, and COUP transcription factor 2 (COUPTF2). Interestingly, NGFCs originate from different embryonic structures during development. nNOS-expressing NGFCs derive from the medial ganglionic eminence (MGE), whereas nNOS-lacking NGFCs originate from the caudal ganglionic eminence (CGE).
The somas and dendrites of the perforant path-associated cells (PPAs) reside in SR and SLM. Their axons are densely ramified in SLM, but also target the dendrites of DG granule cells by crossing the fissure. These cells belong to CGE-derived interneurons that express cholecystokinin (CCK) (Pawelzik et al., 2002). Together,
12
PPAs and NGFCs receive excitatory input mainly from EC and reuniens nucleus while providing feed-forward inhibitory control to the distal dendrites of PCs.
Oriens lacunosum-moleculare interneurons (O-LMs) are named by their morphologies (Figure 4C). Their horizontally oriented somas and spiny dendrites are exclusively located in SO and alveus, indicating that they may receive excitatory inputs from axonal collaterals of CA1 PCs. Their axons extend into SP and SR with few branches and ramify intensively when they reach SLM (Gulyas et al., 1993). Thus, O-LMs are thought to be responsible for the feedback control of PC distal dendrites, gating the information coming from EC. O-LMs express somatostatin (SOM), parvalbumin (PV) and reelin. This cell type has dual origins, which is similar to NGFCs, with the serotonin 3A receptor (5-HT3AR)-expressing subgroup deriving from CGE, whereas the 5-HT3A-lacking subgroup is derived from MGE (Chittajallu et al., 2013).
1.2.2 Proximal and basal dendrite targeting interneurons
Schaffer collateral-associated cells (SCAs) have somas and dendrites mainly located in SR but with a smaller extent in SLM (Figure 4B). Their axons target the PC oblique dendrites that receive CA3 inputs in SR and SO. They are a part of the CCK-expressing interneuron groups mediating the feed-forward inhibition of PCs (Pawelzik et al., 2002).
Bistratified cells (BISs) have horizontally oriented somas exclusively located in SO and alveus. Their aspiny multipolar dendrites branch in all layers except in SLM (Figure 4A). Their axons selectively arborize in SO and the proximal part of SR but avoid SP. They co-express the markers SOM, NPY and calbindin (CB). Trilaminar cells have similar soma and dendrite locations with BISs. However, their dendrites extend to SLM. Unlike BISs, the axons of trilaminar cells also target SP. In addition, they have long-projecting axonal collaterals innervating the medial septum and subiculum (Tóth and Freund, 1992; Ferraguti et al., 2005).
13
The somas of ivy cells are mainly located in SP, but also in SO and SR. They represent the largest interneuron group in CA1. They have aspiny multipolar dendrites extending to SO and SR (Figure 4E). The word “ivy” is used to describe their dense fine axons targeting the proximal and basal dendrites of PCs. Ivy cells represent the largest interneuron subtype originated in MGE (Bezaire and Soltesz, 2013). They express the markers nNOS, NPY, and COUPTF2. Together, these cells with different embryonic origins are positioned in controlling the CA3 input arriving at the proximal and base dendrites of CA1 PCs.
Figure 4 Dendrite targeting interneurons and interneuron selective interneurons (ISIs).
A: morphological reconstruction of a representative bistratified cell (BiC). At right, the reconstructed cell is illustrated to be immunopositive for SST and NPY while a different BiC highlights PV expression in this interneuron subtype. B: morphological reconstruction of a representative SCA with top inset showing that the cell is CCK immunopositive. Also shown at right (bottom) are STORM images illustrating strong CB1R immunolabeling within terminals of a separate dendrite targeting CCK interneuron. (Bottom right panel modified with permission from Dudok et al. (2015). C: morphological reconstruction of a representative O-LM with insets illustrating SST and mGluR1 immunoreactivity in
14
the soma and along a dendritic segment, respectively. D–F: Morphological reconstructions of representative NGFC (D), IvC (E), and ISI3 (F) cells along with single cell RT-PCR profiles probing for mRNA expression of the indicated markers. [A modified with permission from Klausberger et al. (2004). B modified with permission from Lee et al. (2010). C modified with permission from Katona et al. (2014). D–F modified with permission from Tricoire et al. (2010) Adapted from Pelkey et al. (2017).
1.2.3 Soma targeting cells
Basket cells (BCs) are named by their “basket-like” axonal boutons formed on somas and proximal dendrites of PCs (Figure 5B). BCs can be further classified by their developmental origins. MGE-derived BCs express PV (PV-BCs). These cells preferentially innervate deeply located PCs near to SO (Lee et al., 2014). They have large pyramidal-like somas located predominately in SP, but also in SO. Their aspiny dendrites extend to all layers of CA1, allowing them to integrate excitatory inputs in different layers. Their densely arborized axons are mainly distributed in SP, but also in SO and SR to a smaller degree. Beside chemical synapses formed on PCs, they are highly interconnected via gap junctions (Galarreta et al., 1999). PV-BCs are the members of MGE-derived interneurons expressing the transcription factor Nkx2.1 (Butt et al., 2005).
Another subtype of BCs expresses CCK (CCK-BCs) (Figure 5C). They differ from PV-BCs in several aspects. Although their somas are mainly located in SR, other layers such as SO, SP and SR/SLM border are also populated by this cell type. Compared to PV-BC, CCK-BCs innervate a smaller number of PCs, and a small proportion of them target interneurons, including other CCK interneurons and PV-BCs. Moreover, CCK-BCs can be further divided into two subtypes according to their neurochemical markers: vasoactive intestinal peptide (VIP)-expressing and vesicular glutamate transporter 3 (VGLUT3)-expressing subgroups. Besides, CCK-BC terminals are decorated with cannabinoid type 1 receptors (CB1Rs), which suppress the GABA release from the terminals of these cells.
15
1.2.4 Axonal initial segment targeting interneurons
The axo-axonic cells are also called chandelier cells. Their somas are largely found in SP, SO, and SR areas adjacent to SP. A subtype of these cells possesses dendritic tufts extending to all layers of CA1 (Figure 5A). Another subtype has horizontally oriented dendritic trees dominantly occupying SO (Ganter et al., 2004). Their axonal arborisation is selectively found within SP and proximal SO. Their axonal branches travel horizontally along the SP and either vertically or obliquely penetrate the SP, forming bouton rows exclusively targeting initial segments of PCs. Each row contains 5-12 boutons, which resemble the shape of a chandelier.
Figure 5 Perisomatic targeting interneurons
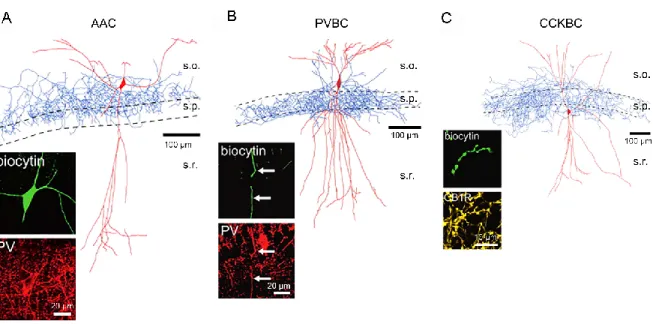
A: morphological reconstruction of a representative axo-axonic cell (AAC). Inset shows that the dye-filled AAC is immunopositive for parvalbumin (PV). B: morphological reconstruction of a representative PV-BC with inset confirming PV immunoreactivity within a dendritic segment of the dye filled PV-BC. C: morphological reconstruction of a representative CCK-BC with inset showing CB1R immunolabeling within a segment of dye filled axon. Adapted from Nissen et al. (2010).
.
Figure 6 VIP-positive interneurons at the PYR/RAD border target O–LM interneurons.Figure 7 Perisomatic targeting interneurons
A: morphological reconstruction of a representative axo-axonic cell (AAC). Inset shows that the dye-filled AAC is immunopositive for parvalbumin (PV). B: morphological reconstruction of a representative PV-BC with inset confirming PV immunoreactivity within a dendritic segment of the dye filled PV-BC. C: morphological reconstruction of a representative CCK-BC with inset showing CB1R immunolabeling within a segment of dye filled axon. Adapted from Nissen et al. (2010).
16
Approximately 1200 PCs are targeted by a single axo-axonic cell. This cell type express PV. Like other MGE-derived cells, they also express Nkx2.1 but lack the transcription factor SATB1, which is expressed in most MGE-derived cell types (Close et al., 2012).
1.2.5 Interneuron-specific interneurons
PCs are not the only target of interneurons. Several interneuron subtypes are contacting each other as well as other subtypes (Sik et al., 1995). For instance, O-LMs innervate other dendritic targeting interneurons such as NGFCs, BCs, PPAs and SCAs (Elfant et al., 2008). Besides, there are interneuron-specific (IS) interneurons that specialize in controlling other interneurons but avoid PCs. These cells account for 20% of total interneurons in the CA1 and express the marker calretinin (CR) and/or VIP. Early studies showed that VIP cells in the rat hippocampus are heterogeneous in morphology, and form symmetrical synapses onto PCs (Léránth et al., 1984). Detailed morphological characterization and immunostaining have confirmed the GABAergic nature of VIP and CR cell projections. Despite a subgroup of VIP+ basket cells that target the perisomatic area of PCs, the rest of them are subdivided into three subtypes based on their soma, dendrite, and axon locations (IS1-3, Acsády et al., 1996a, b; Gulyás et al., 1996). The somas of IS1s are evenly distributed in all layers of CA1. Their multipolar spine-free dendrites extend to all layers but appear denser in SR. An interesting feature of IS1 is that several IS1s often form dendro-dendritic connections, such that 2-7 dendrites approach each other and travel in parallel for more than 100 µm. Their axons spread in SP and SR, and rarely in SO. The axonal boutons are unevenly distributed; high-density segments are separated by long bouton-free segments. The postsynaptic targets of IS1s include calbindin (CB)-expressing interneurons, such as SCAs PPAs and CCK-BCs, as well as other, IS1 interneurons expressing CR. IS2s have somas residing at the border between SLM and SR. Their sparsely spiny dendritic arbors are restricted in SLM. The axons of IS2s travel vertically in SR,
17
innervating CB+, CR+ and VIP+ interneurons in this layer. However, another subtype of IS2s have somas in SP or SR with their dendrites spreading to all layers. Their axons arborize vertically in SR, but sparsely in SP and SO (Ascády et al., 1996a). The first type of IS2 mentioned above express VIP but not CR, whereas the second type co-express VIP and CR. As IS3 cells were studied in details, here in the following chapter I will present their properties in details.
1.2.6 IS3 cells
IS3s have round or oval somas located in SP and proximal SR. Their dendrites are aspiny, with all synapses forming directly on the shaft. They form 2-4 primary bidirectional or single directional dendrites extending to all layers, with dense collaterals residing in SLM, indicating that they may receive EC excitatory input. Their axons originate from somas or primary dendrites, then go directly down to SO and the alveus border and branches horizontally in this region, indicating that they target interneurons in this layer (Figure 4F). Immunostaining shows that IS3 cells co-express VIP and CR (Figure 6B).
CR+ cells are also found in the hippocampal formation of humans and monkeys. In the CA1 area, these cells have small bipolar or fusiform somas frequently located in SLM and SP but rarely in SO and SP. Their dendrites are sparsely spiny and vertically oriented towards the hippocampal fissure, which resembles the morphology of IS3 cells in rodents. However, a striking difference is that CR+ cells in primate hippocampus target both principal cells and interneurons, as shown by electron microscopy (Seress et al., 1993).
What are the postsynaptic targets of IS3s? Using two photon uncaging and patch-clamp recording techniques, Chamberland et al (2010) studied the local and long-range inhibitory inputs of O-LMs located in SO. Focal uncaging on the somas of SP/SR interneurons evoked slow IPSCs with large amplitude and low failure rate in LMs. Post hoc reconstruction showed that one of the major inhibitory inputs of
O-18
LMs were from IS3s. In addition, local inhibitory inputs exhibited short-term depression, but no long-term plasticity was observed (Chamberland et al 2010).
Tyan et al. (2014) further examined the postsynaptic targets of IS3s through paired recording and optogenetics. Consistent with previous study, O-LMs represent the major targets of IS3s (Figure 6D). The IS3-O-LM synapses have low release probability and form multiple release sites. Moreover, the inhibition provided by IS3s is able to control the firing of O-LMs through rebound spikes following after-hyperpolarizations. IS3s innervate other SO interneurons, such as BIS, BC and
Figure 6 VIP-positive interneurons at the PYR/RAD border target O–LM interneurons.
(A) Maximal projection of a two-photon z-stack acquired in the CA1 region of the hippocampus of a VIP-eGFP mouse, showing bipolarly oriented VIP-positive cell bodies located at the PYR/RAD border and a dense axonal arborization in the O/A. (B) Immunofluorescence images of neurons located in PYR positive for calretinin (top) and VIP (middle) as well as their superimposition (bottom). Scale bar: 20 μ m. (C) Reconstruction of a bipolarly oriented VIP-positive cell, showing anatomical features of IS-IIIs (soma and dendrites are shown in black and axon is shown in red) and its irregularly spiking firing pattern typical for these cells. (D) Neurolucida reconstruction of a connected pair of interneurons: presynaptic IS-III (soma and dendrites are in black and axon is in red) and postsynaptic O–LM (soma and dendrites are in green, axon is in blue) and examples of unitary IPSCs evoked by two-photon glutamate uncaging (bottom left) and presynaptic spikes during paired recordings (bottom right). Black arrows indicate three putative contact sites onto O– LM dendrites. Modified from Chamberland et al. (2010).
Figure 8 Immunohistochemical markers primarily associated with CGE-derived interneurons.Figure 9 VIP-positive interneurons at the PYR/RAD border target O–LM interneurons.
(A) Maximal projection of a two-photon z-stack acquired in the CA1 region of the hippocampus of a VIP-eGFP mouse, showing bipolarly oriented VIP-positive cell bodies located at the PYR/RAD border and a dense axonal arborization in the O/A. (B) Immunofluorescence images of neurons located in PYR positive for calretinin (top) and VIP (middle) as well as their superimposition (bottom). Scale bar: 20 μ m. (C) Reconstruction of a bipolarly oriented VIP-positive cell, showing anatomical features of IS-IIIs (soma and dendrites are shown in black and axon is shown in red) and its irregularly spiking firing pattern typical for these cells. (D) Neurolucida reconstruction of a connected pair of interneurons: presynaptic IS-III (soma and dendrites are in black and axon is in red) and postsynaptic O–LM (soma and dendrites are in green, axon is in blue) and examples of unitary IPSCs evoked by two-photon glutamate uncaging (bottom left) and presynaptic spikes during paired recordings (bottom right). Black arrows indicate three putative contact sites onto O– LM dendrites. Modified from Chamberland et al. (2010).
19
Oriens-Oriens (O-O) cells to a smaller degree. In addition, IS3 cells exhibit high input resistance, low membrane capacitance, and irregular firing pattern. Apart from the target cells of IS3s, their excitatory inputs remain unknown. The morphology of their dendrites indicates that they may receive excitatory inputs located in SR and SLM, including SC from CA3 PCs and TA from EC principal cells. In this study, we compared the synaptic properties, along with the spatial and temporal summation of these two inputs in IS3 cells.
2. Single-cell RNA sequencing and its application in identifying
neuronal markers
2.1 Neurochemical properties of hippocampal interneurons
Cortical interneurons can be classified based on the expression of molecular markers identified by immunostaining. The Petilla terminology classified these specifically expressed proteins into several functional groups including transcription factors, neurotransmitters or their synthesizing enzymes, neuropeptides, Ca2+ -binding proteins, neurotransmitter receptors, structural proteins, ion channels, connexins, pannexins and membrane transporters (The Petilla interneuron nomenclature Group, 2008). Like neocortical interneurons, hippocampal interneurons derive from their precursor cells in two embryonic structures: MGE and CGE in the basal telencephalon. Cell type differentiation is regulated by various transcription factors. Accordingly, MGE-derived cells can be immunolabeled by the Thyroid transcription factor Nkx2.1, which is specifically expressed in MGE. MGE-derived interneurons account for 60% of total hippocampal interneurons, and can be labelled by several markers including PV, SOM, nNOS and NPY. For example, PV-BCs, BISs, and AACs express the marker PV, while SOM is present in O-LMs, BISs and a subset of long-projecting cells (Tricoire et al., 2011). On the other hand, no common transcription factor has been identified in CGE-derived cells, but they are often labelled with 5-HT3AR. The molecular markers for CGE-derived hippocampal cells include CCK, COUPTF2, Muscarinic receptor type 2 (M2R), CR and VIP