Contribution to the development of a process for color
improvement of low grade dark maple syrup by
adsorption on activated carbon
Mémoire
Bita Sadeghi-Tabatabai
Maîtrise en génie agroalimentaire
Maître ès sciences (M.Sc.)
Québec, Canada
Résumé
Dans ce travail, nous avons utilisé le charbon actif (trois types) pour décolorer le sirop d'érable foncé, classé dans la catégorie ``Non retenu`` de la classification du Québec du sirop d’érable. Nous avons testé l'efficacité de la rétention des pigments colorés du sirop d'érable sur le charbon actif en grains et en poudre. Aussi, nous avons comparé les différentes efficacités de ces matériaux. La première étape de nos essais expérimentaux a eu pour but de suivre les cinétiques d'adsorption des pigments. Dans cette partie, plusieurs paramètres expérimentaux ont été optimisés par utilisation d'un plan factoriel complet; à savoir : le temps d'agitation (20,40, 60 min), la masse du charbon actif utilisée par 100 ml de sirop (0.1, 0.3, 0.5 g), le type du charbon actif (I, II, III), le diamètre moyen des particules du charbon (25, 50, 75 µm), la vitesse d'agitation (200, 400, 600 rpm) et la température (40,60, 80 °C). La variable dépendante que nous avons considérée dans ce projet est la transmittance de la lumière du sirop mesurée à 560 nm. La deuxième étape a été consacrée sur l'étude du pouvoir adsorbant des trois types de charbons actifs utilisés avec l’application des modèles de Langmuir, Freundlich et de Langmuir-Freundlich pour décrire les cinétiques d’adsorption. Les résultats obtenus ont montré que la décoloration du sirop d’érable foncé est optimale avec les paramètres suivants : temps d'agitation t = 40 min, masse du charbon actif ajouté m = 0.3 g et charbon actif de type III. Ces paramètres ont donné une valeur de la transmittance de la lumière à 560 nm de 83.7 ± 0.2 %; et qui classe le sirop dans la catégorie Extra claire. Ensuite, ce travail a montré que seul le charbon actif de type III vérifiait à la fois une cinétique d’adsorption qui se décrit par les isothermes de sorption de Langmuir, Freundlich et Langmuir-Freundlich. Finalement, un autre plan d'expérience avait complété dans ce travail et qui portait sur l'optimisation de la taille des particules du charbon et qui a montré qu’une granulométrie moyenne d = 25 µm (mésopores) était la plus optimale pour décolore le sirop d’érable avec une agitation de 200 rpm à une température T = 80°C.
Abstract
Low grade dark maple syrup was successfully discolored on activated carbon. Several experimental parameters were tested, namely the mixing time (20, 40, 60 min), concentration of the activated carbon (0.1, 0.3, 0.5 g/100 mL), type of activated carbon (I, II, III), particle size (25, 50, 75 μm), stirring speed (200, 400, 600 rpm), and temperature (40, 60, 80°C). The obtained results showed that the discoloration is optimal by applying the parameters such as an agitation time of 40 min with an activated carbon of type III at a concentration of 0.3 g/100 mL. These parameters yielded a light transmittance of 83.70 ± 0.21%, which ranks the syrup in the extra clear class. The results showed that among the tested carbons, the adsorption on the type carbon of III followed the Langmuir, Freundlich and Langmuir-Freundlich isotherms. Regarding the effect of the particle size, the obtained results showed that a mean size of 25 µm with a stirring speed of 200 rpm and a working temperature of 80°C was the most effective. The optimized conditions showed a good adequacy with the Langmuir and Freundlich models. The discoloration process by using the activated carbon of type III followed pseudo-second order kinetics.
Table of contents
Résumé ... iii
Abstract ... v
Table of contents ... vii
List of tables ... ix List of figures ... xi Dédicaces ... xiii Remerciements ... xv Avant-propos ... xvii INTRODUCTION ... 1
CHAPTER 1: LITRATURE REVIEW ... 3
1.1. Maple syrup ... 3
1.1.1. Brief history of maple syrup and its production ... 3
1.1.2. Composition of the maple syrup ... 3
1.1.3. Economic importance of the maple syrup ... 4
1.1.4. Classification of the maple syrup ... 4
1.1.5. Causes of the color darkening ... 7
1.1.5.1. Microorganism activity ... 7
1.1.5.2. Boiling ... 7
1.1.5.2.1. Millard reaction (browning non-enzymatic reaction) ... 7
1.1.5.2.2. Reaction of caramelization ... 13
1.1.5.3. Type of storage containers ... 14
1.1.6. Possible ways for commercial use of dark maple syrup ... 14
1.2. Activated carbon ... 15
1.2.1. Activated carbon ... 15
1.2.2. The application of activated carbon ... 17
1.2.2.1. Air treatment ... 17
1.2.2.2. Hydrogen sulfide (H2S) and odor control ... 17
1.2.2.3. Gaseous waste incinerators and biogas purification ... 17
1.2.2.4. Wastewater treatment ... 18
1.2.2.5. Food industry and pharmaceutical applications ... 18
viii
2.1. Research Hypothesis ... 21
2.2. General objective ... 21
2.2.1. Specific objectives ... 22
CHAPTER 3: MATERIALS, METHODS, RESULTS AND DISCUSSION ... 23
Abstract ... 26
Résumé ... 27
3.1. Introduction ... 28
3.2. Materials and Methods ... 30
3.2.1. Reagents and Samples ... 30
3.2.2. Characterization of the activated carbon ... 30
3.2.2.1. Mean particle size ... 30
3.2.2.2. Scanning electron microscopy (SEM) and Energy dispersive X-ray spectrometer (EDS) ... 30
3.2.2.3. Bulk density ... 31
3.2.3. Experimental procedure ... 31
3.2.4. Statistical modeling and adsorption isotherms ... 31
3.2.5. Experimental design and statistical analysis ... 32
3.3. Results and Discussion ... 33
3.3.1. Characteristics of the used activated carbons ... 33
3.3.2. Effect of the concentration and type of the added activated carbon ... 34
3.3.3. Effect of the mixing stirring time ... 36
3.3.4. Effect of the temperature, particle size and mixing speed ... 37
3.3.5. Statistical modeling of the discoloration process ... 38
3.3.5.1. Adsorption isotherm models ... 39
3.3.5.2. Dark maple syrup discoloration kinetic order ... 41
Conclusion ... 42
Acknowledgements ... 42
Conflict of Interest Statement ... 43
CONCLUSION GÉNÉRALE ET PERSPÉCTIVES ... 45
List of tables
Table 1: Federal classification of maple syrup. ... 5 Table 2: Classification of the maple syrup according to the Quebec standard. ... 6 Table 3: Color classification of the maple syrup (Québec office Standard, 2011). ... 6 Table 4: Adsorption isotherm models used to describe the color removal from dark maple syrup. .. 47 Table 5: Variation of transmittances at different stirring time and different mass of the activated carbon (type III). ... 48 Table 6: Pseudo-first and pseudo-second order equations used to describe the adsorption kinetic models of maple syrup discoloration. ... 49
List of figures
Figure 1: Maillard reaction scheme adapted (Hodge, 1953)... 9
Figure 2: Representation of the first stage of the Maillard reaction corresponding to the condensation phase (Çelebi, 2006). ... 10
Figure 3: Stage of the Amadori rearrangement of the Millard reaction (Hodge, 1953). ... 12
Figure 4: Strecker degradation occuring during the Maillard reaction (Yaylayan, 2003). ... 13
Figure 5: Different materials used in activated carbon production. ... 15
Figure 6: Activated carbon in granular, crushed and powder form. ... 15
Figure 7: Schematic representation of the experimental set-up. ... 52
Figure 8: Activated carbon particle size distribution. ... 53
Figure 9: Scanning electron micrographs of the surface activated carbon particles used in maple syrup discoloration. ... 54
Figure 10: Energy dispersive X-ray spectrometer of different type of activated carbon. ... 55
Figure 11: Bulk density comparison of different activated carbon. ... 56
Figure 12: Transmittance as a function of time at different activated carbon concentrations and different activated carbons: (a) Type I, (b) Type II, (c) Type III, (d) Type IV. ... 57
Figure 13: Transmittance as a function of activated carbon mass at different activated carbon and different agitation time: (a) 20 min, (b) 40 min, (c) 60 min. ... 58
Figure 14: Transmittance as a function of activated carbon mass at different stirring time and different activated carbon. (a) Type I, (b) Type II, (c) Type III, (d) Type IV. ... 59
Figure 15: Transmittance as a function of particle diameters of activated carbon type III at different working temperatures and different stirring speed: (a) 200 rpm, (b) 400 rpm, (c) 600 rpm. ... 60
Figure 16: Transmittance as a function of stirring speed at different particle size of activated carbon III and different working temperatures: (a) 40°C, (b) 60°C, (c) 80°C. ... 61
Figure 17: Transmittance as a function of particle size of activated carbon III at different stirring speed and different working temperatures: (a) 40°C, (b) 60°C, (c) 80°C... 62
Figure 18: Response surface at X1 = 0, X2 (-1, 0, +1), and X3 (-1, 0, +1). ... 63
Figure 19: Iso-response curve at different level of X1 (-1, 0, +1), X2 (-1, 0, +1) and X3 (-1, 0, +1). ... 64
Figure 20: Coloration concentration of the maple syrup solution as a function of coloration concentration adsorbed by activated carbon at different adsorption isotherm type. (a) type I, (b) type II, and (c) type III. ... 65
Figure 21: Kinetic models of the adsorption by using activated carbon type III. (a) Pseudo-first order; (b) Pseudo-second order. ... 66
Dédicaces
À mes chers parents : merci à mes parents Sariyeh Alizadeh et Ahmad Sadeghi Tabatabai pour leur amour inconditionnel et leur soutien.
Enfin, je dédicace ce mémoire à la communauté scientifique internationale et à l’Université Laval qui m’a fourni tout le nécessaire pour réaliser ce travail et en garder de nombreux bons souvenirs qui resteront avec moi à jamais.
Remerciements
Tout d'abord, je remercie le professeur Mohammed Aïder qui m'a accueillie dans son équipe de recherche. Qu'il trouve ici l'expression de ma profonde gratitude pour m'avoir fait bénéficier de sa compétence et de ses connaissances scientifiques.
Mes sincères remerciements se portent vers Dr Amara Aït-Aissa pour ses conseils et sa disponibilité. Je lui exprime toute ma gratitude pour l'intérêt constant qu'il a porté à mon travail.
Je tiens à remercier sincèrement les membres du jury d'avoir accepté de participer à corriger ce mémoire, mais aussi pour l'attention qu'ils ont témoignée à mon travail.
Mes remerciements les plus sincères vont aussi à madame Diane Gagnon et madame Jocelyne Giasson. Je leur exprime toute ma gratitude pour leur aide concernant les analyses avec le spectrophotomètre et les analyses de couleurs.
Je ne saurais oublier de remercier tous mes ami(e)s avec qui j'ai partagé le laboratoire et le bureau. Merci à Ourdia, Alexey, Nata'lia, Mabrouk, Zeinabou.
Je profite de ce moment pour adresser ma gratitude et mes remerciements à toute ma famille, surtout à mes très chers parents, Sariyeh et Ahmad qui ont toujours tout sacrifié pour moi. Je remercie ma sœur Gita et mes frères Babak et Barmak pour leur encouragement.
Avant-propos
Ce mémoire se compose de trois parties principales. Une introduction générale, une revue de littérature qui porte sur deux éléments principaux; à savoir, la coloration du sirop d’érable et la décoloration par du charbon actif et une partie ``Résultats et discussion`` sous forme d'un article scientifique rédigé en anglais soumis pour publication dans Food Bioscience, un journal international avec comité de lecture. Les participants à la rédaction de l’article sont : Bita Sadeghi-Tabatabai, candidate à la maîtrise avec mémoire en génie agroalimentaire, Dr Amara Aït Aissa, chercheur postdoctoral et Dr Mohammed Aider, professeur et responsable du projet. L’article s’intitule ``Improvement of the color of low grade dark maple syrup by adsorption on activated carbon and study of the adsorption kinetics``.
INTRODUCTION
Ce projet se veut une contribution à la valorisation des sirops d'érable des catégories
non conforme (NC) et retenu (RE) via une décoloration par adsorption sur du charbon actif
en utilisant des conditions opératoires facilement reproductible par l’industrie du sirop d’érable du Québec. Le but final étant de produire du sirop commercialisable de haute qualité. Le présent projet a été entrepris suite à une réflexion sur la problématique entourant l’absence de valorisation efficace du sirop d’érable foncé et le manque d’études scientifiques, tant académiques qu’industrielles sur ce sujet. En effet, selon les statistiques de la Fédération des producteurs acéricoles du Québec, en 2013, la production des sirops d'érable des catégories non conforme (NC) et retenu (RE) était de 0.6 et 0.2 millions de livres, respectivement. Elle était de 1.8 et 0.3 millions de livres en 2012. Comme ces sirops ne sont pas commercialisables, ils s'accumulent d'année en année et occupent inutilement des espaces importants dans les entrepôts. Par conséquent, il est important de trouver des moyens efficaces et peu coûteux pour donner de la valeur à ces sirops. Cela permettra de générer des revenus supplémentaires aux producteurs et de libérer des espaces d'entreposage inutilement occupés. L'amélioration de la couleur et du goût des sirops des catégories NC et RE permettra de leur trouver un marché dans l'industrie de la transformation des aliments, mais surtout en exportation, car, la législation nationale par rapport aux produits de l'érable ne s'applique pas de la même façon pour les produits destinés à l’exportation. Comme la couleur du sirop sera nettement améliorée et comme ce paramètre est d’une grande importance technologique, il est attendu à ce qu’il serait possible et technologiquement faisable de fabriquer du sucre d'érable granulé de haute qualité à partir des sirops obtenus suite à la décoloration des sirops de grade NC et RE. Conséquemment, l'application de la technologie de cristallisation par refroidissement aux sirops générés suite à la décoloration sur du charbon actif permettra de produire des produits dérivés d’une apparence et attributs organoleptiques semblables à ceux faits à partir de sirop d'érable des catégories A ou B.
L'objectif principal de ce projet de recherche est d'appliquer la technologie d’adsorption sur du charbon actif de grade alimentaire pour une amélioration substantielle de la couleur sirops d'érable des catégories non conforme (NC) et retenu (RE). Pour
2
atteindre l’objectif final, un dispositif expérimental de type factoriel sera utilisé pour générer un maximum de données qui nous permettront de bien comprendre l’effet de chaque paramètre expérimental sur la variable dépendante principale qui est la couleur exprimée par la valeur de la transmittance de lumière mesurée à 560 nm. Le temps d'agitation du mélange sirop avec du charbon, la masse du charbon actif utilisée par volume de sirop, le type du charbon actif et sa granulométrie, ainsi que la vitesse d'agitation et la température sont des variables indépendantes qui seront mises à l’étude dans le présent projet.
CHAPTER 1: LITRATURE REVIEW
1.1. Maple syrup
1.1.1. Brief history of maple syrup and its production
Maple syrup production is attributed to indigenous inhabitants of North America and is produced from the sap collected from the sugar maple tree Acer saccharum. Evaporation of water to obtain concentrated maple sap was done by the natives inside clay pots by placing hot stones in the bucket. Also, the sap was frozen overnight and a layer of the formed ice was removed so as more concentrated sap was obtained. Iron and copper kettles were introduced to the process by the first white settlers. The use of wooden containers hung from the trees for the collection of sap had a good efficiency. In 1872, an evaporator with two pan as well as a metal arch was developed for reducing boiling time of the maple sap. During the 1970, tubing systems were equipped with vacuum pumps and the sap was transferred from the tree to the evaporator. Later, reverse osmosis filters was used to eliminate part of water of the sap before it was boiled. Today, processing technology of maple sap has been improved for enhancing the rate and quality of production by using stainless steel evaporators for boiling the maple sap, new tubing systems as well as new filtering techniques such as more effective reverse osmosis (Wood et al., 2012). Maple syrup is produced traditionally by boiling sap collected from various maple tree in the sugarhouse by evaporating water and concentrating the sugar content up to 66–67° brix. It has a special flavour and smell (Kallio et al., 1987). The syrup is produced during short season consisting of four to eight weeks in late winter and early spring (Clément et al., 2010; Filteau et al., 2012; Koffi et al., 2014; Yuan et al., 2013).
1.1.2. Composition of the maple syrup
Maple syrup is a sugary product and contains is mainly composed of sacccharose with a content of 60-67%, reducing sugars (glucose, fructose, hexose) with content of 0.1-10%, oligosaccharides and polysaccharides (0.1-4%), organic acids (0.6% of malic acid, 0.06% of succinic acid, 0.006% of formic acid) and traces of tartaric, citric, and oxalic acids. It contains also flavouring compounds resulting from the Maillard reaction between
4
the amino acids and the reducing sugars, flavonoides and minerals such as potassium (˷2000mg/kg), calcium (˷1000 mg/kg), magnesium (˷200 mg/kg), manganese (˷40mg/kg),
zinc (˷10 mg/kg) and sodium (˷10 mg/kg) (Khalf et al., 2010; Perkins and van den Berg,
2009). Maple syrup contains also some phytochemicals as phenolics, and more than fifty phenolics and phenolic derivatives (Wild and Yanai, 2015).
1.1.3. Economic importance of the maple syrup
The industry of maple sap transformation plays very important role in the economics of Canada and the Unites States which are the only maple syrup producing countries in the world (Rapp and Crone, 2015). In 2006, Canada was the largest maple syrup producer with 82% of that production with a production of 33,745 metric tonnes (MT) valued at CAN $177,9 million, while the United States produced 7,24 MT of maple syrup valued at US $ 45.3 million (AAC, 2007). In 2011, there were 10,000 maple farms in Canada with over 44 million taps, approximately 4,000 taps per farm. Quebec is the largest maple syrup producing province with 92.39% of domestic production, Ontario with 2.86% and New Brunswick with 0.32% in 2012 (Agriculture and Agri-Food Canada, 2014). From commercial point of view, maple syrup production plays an important economic role. In fact, Canada with exports of $ 249 million is the largest exporter of maple products (85%) in the world. Quebec has 94% of the Canadian maple product exports to the countries such as United States, Japan and Germany (AAC, 2014).
1.1.4. Classification of the maple syrup
Maple syrup is categorized based on its light transmission measured at a wavelength of 560 nm as specified by the International Maple Syrup Institute (IMSI). It is classified into four categories: light (>75 %), amber (50–75 %), dark (25–50 %), and very dark (<25 %) (Perkins and van den Berg, 2009). From commercial point of view, the browning in the syrup has a negative impact. Moreover, it is usually accompanied by some sensorial defects. The maple season is between a late winter and early spring. The amber syrup (light) is resulted from sap which runs of the early season. The syrup becomes darker in color when the season warms up. The maple syrups are variable in color and vary from pale golden to dark brown. Maple syrup is graded base on the color, clearness and flavor. It must contain at least 65% solid (sugar) (ACIA, 2015). In Canada, the Canadian Food
Inspection Agency (CFIA) is the regulatory agency and responsible for the quality requirements for maple syrup. The CFIA approves standards and regulatory framework for food safety requirements for maple syrup which is classified based on its colour and flavour (Clément et al., 2010; Perkins and van den Berg, 2009). There are two types of classification for syrup maple in Canada: the federal and provincial classification. Federal classification standard consists of three classes and five grades of syrup: Canada 1, (Extra light, Light, Medium), Canada 2 (Amber), Canada 3 (Dark) and syrups from any other category with flavour flaws (ACIA, 2015; Québec, 2015) (Table 1).
Table 1: Federal classification of maple syrup.
Canada No.1 Extra light Light Medium
Canada No.2 Amber
Canada.No.3 Dark, or any
other ungraded category
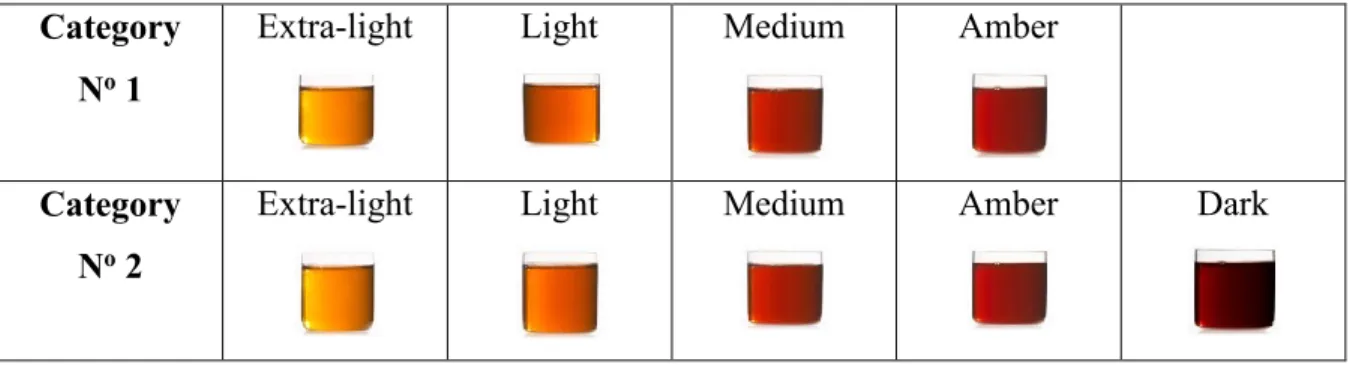
The provincial classification of Québec consists of two categories and five colour classes: AA (extra Light), A (Light), B (Medium), C (amber) and D (dark amber and strong taste and is used for industrial purpose) (Québec Maple Syrup Producers Federation) (Tables 2
and 3). According to this a regulation, the color shall be determined optically by a
6
Table 2: Classification of the maple syrup according to the Quebec standard. Category
No 1
Extra-light Light Medium Amber
Category No 2
Extra-light Light Medium Amber Dark
Table 3: Color classification of the maple syrup (Québec office Standard, 2011). Item Color class Percentage of Light Transmission
1. Extra Light 75% or more
2. Light 60.5% or more, but less than 75% 3. Medium 44% or more, but less than 60.5% 4. Amber 27% or more, but less than 44% 5. Dark Less than 27%
In the Unites States of America (USA), maple syrup is categorized into two grade by color and flavour. Grade A and Grade B under the authority of the U.S. Department of Agriculture (Standards for Grades of Maple Syrup) (Houston, 1980).
Grade ``A``: This maple syrup has a bright color and have a moderate flavour than Grad B
that is of dark grade with rich flavour.
Grade A light amber: This type of maple syrup is lighter than the USDA light Amber Glass
Color standard in color.
Grade A medium amber: This maple syrup is darken than light amber but it is no darker
than the USDA light Amber Glass Color standard in color.
Grade A dark amber: This Grade of maple syrup is darker than medium amber, but is no
Grade ``B``: It is characterized by a fairly good color and this kind of maple syrup is
darker than the USDA Dark Amber Glass Color Standard, but is not off-color for any reason with a fairly good flavor.
1.1.5. Causes of the color darkening
1.1.5.1. Microorganism activity
In late winter, sap is clear with a weak sweet taste. The biochemistry occurred in the sap results in different changes of color and flavor of the maple syrup produced. Maple syrup of dark grades is generally produced from sap with high concentration of microorganisms while a sap which is not affected by microorganism activity gives possibility of making a lighter grades of syrup (Filteau et al., 2012). Color changes occurring in the sap during boiling and those resulted from the microorganism activity in the maple syrup is considered as the main cause of color darkening and caramelization (Lagacé et al., 2006). Microorganisms in the maple syrup are a factor of the enzymatic hydrolysis of sucrose-producing sugars (glucose and fructose) and those are responsible of the intense caramel flavor and dark color (Sy, 1908). Microorganisms such as
Pseudomonas fluorescens and Guehomyces Pullulans were found in several production
sites in different regions of Quebec. Moreover, different yeasts (Candida) were related with fructose and glucose concentrations in maple syrup (Filteau et al., 2011). P.
fluorescens group bacteria and some yeast were associated to vanilla attributes of maple
syrup when the maple syrup was produced at the end of the harvesting season (Filteau et al., 2010, 2011, 2012).
1.1.5.2. Boiling
Dark color appears from a browning reaction when the sap is boiled further during the evaporation process and putting too much sap in evaporation are the main factors of the formation of a darker colored syrup during the stages of boiling of maple sap (Asadi, 2007). 1.1.5.2.1. Millard reaction (browning non-enzymatic reaction)
The most important change during processing of maple syrup is the browning reaction known as non-enzymatic browning which is associated with the Millard reaction. This reaction is complex and involves different the low molecular weight compounds
8
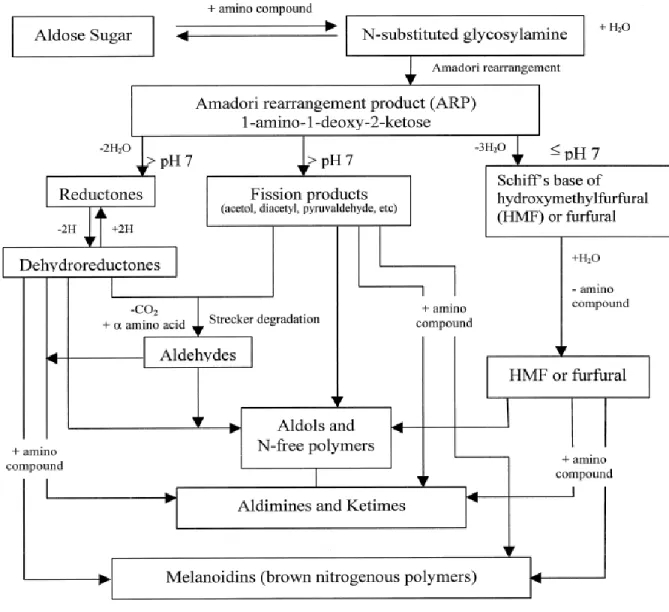
(Raisi and Aroujalian, 2007). This chemical reaction takes place between the reducing sugars (glucose and fructose) (Ames, 2009) which are carbonyl compounds produced by the decomposition of sucrose via bacterial activity in the maple sap and the amino acids (amino groups of proteins) present in the sap at high temperatures (Carabasa-Giribet and Ibarz-Ribas, 2000). The formation of Brown-colored (Melanoidins) is the consequence of polymerisation reactions of highly reactive intermediates formed within the Maillard reaction (Ekasari et al., 1986). Three major steps of the Maillard reaction are observed: a condensation reaction, Amadori rearrangement and polymerisation or formation of the brown color (high molecular weight melanoidins) (Figure 1) (Frazier, 2004). However, most of formed colorant agents can be removed by purification (Eskin, 1990; Manley, 2000).
Figure 1: Maillard reaction scheme adapted (Hodge, 1953).
First stage (Condensation): Glucose syrups with high DE could have good reaction with
proteins (amino acids) (Dziedzic and Kearsley, 1984). A condensation product (N-substituted glycosylamine) is derived from a reaction between a reducing sugar (glucose and fructose) with an amino acid or protein of sap. N-substituted glycosilamine is obtained as a result of a nucleophilic attack (NH2) of an amino acid on the electrophilic carbonyl
group of the sugar (Çelebi, 2006). Aldosylamine (when the reducing sugar is an aldose) or ketosylamine (when the reducing sugar is a ketose) are formed rapidly as Schiff base after loss of water (Figure 2).
10
Figure 2: Representation of the first stage of the Maillard reaction corresponding to the
condensation phase (Çelebi, 2006).
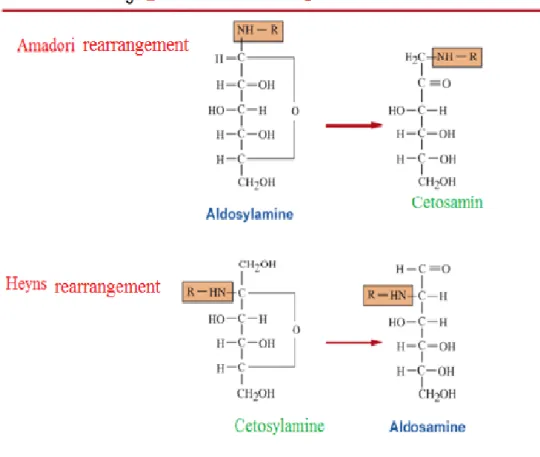
Second stage (Amadori rearrangement or Heyns): The Amadori rearrangement is the
second step in the Maillard reaction corresponding to the phase of glycosylamines formation by the Amadori arrangement (Bristow and S. Isaacs, 1999). It leads to the formation of heterocyclics by the Amadori rearrangement that are responsible for the flavor and odor of the maple syrup. Aldosylamines and ketosylamines could have various transformation. Aldosylamines lead to ketosamines and ketosylamines lead to aldosamines by Heyns rearrangement. These reactions are catalysed by carboxyl function of the amino acids. Thereafter, aldosamines and ketosamines obtained from the Amadori rearrangement have various decomposition pathways involving cleavage and dehydration reaction. Thus, the creation of small molecules, aromatic substances or the carbonyl compounds (such as reductones) and polycarbonyles is very unstable. The reductones are dicetonics substances
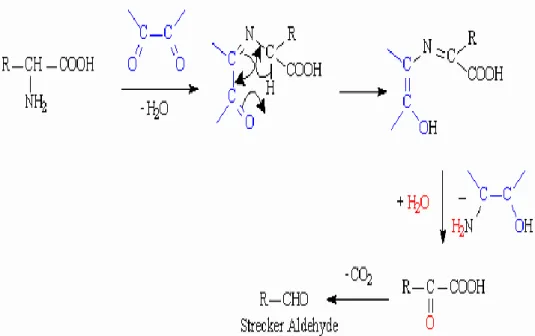
(R-CO-CO-R) are extremely reactive. The Strecker degradation is autocatalytic and it involves destruction of α-amino acids and formation of aromatic molecules like aldehydes. Morselli and Whalen (1986) reported that, in the end of season, there is a trend to diversification of amino acids in the sap with an increasing concentrations. This degradation is characterized by a production of CO2. At pH = 7 or blow, furfural is formed
from an enolization of the Amadori product. At the pH of sap (syrup) above 7, hydroxyethyl furfural (HMF) is formed HMF (Çelebi, 2006) (Figures 3, 4).
Third stage (polymerization and browning): The third stage is the polymerization of
various substances formed during the previous step. Moreover, some low molecular weight molecules, volatile compounds and odorants are also formed together with the brown or black pigments. These pigments called melanoidines and they are complex molecules with high molecular weight. Temperature, the reaction time, water content, concentration and the nature of the precursors influence this stage of the Mailllard reaction (Hodge, 1953).
12
Figure 4: Strecker degradation occuring during the Maillard reaction (Yaylayan, 2003).
1.1.5.2.2. Reaction of caramelization
This brown color is produced from heating of sucrose (sugar) solution in the evaporator during boiling by the breakdown of sucrose to glucose and fructose (Asadi, 2006). This thermal degradation doesn't involve presence of amino acids and proteins. Three main product are of dehydration are formed: Caramelan (C12H18O9) and two
polymers which are caramelen (C36H50O25)and caramelin (C125H188O80). An intense heat
equal or higher than 200 C leads to the pyrolyse (degradation) and this degradation involves break of the osidic links and intermolecular rearrangement (Ramı́rez-Jiménez et al., 2000). A smell of burn of the syrup is the quality change caused when containers are filled with very hot syrup and these containers are closely stacked together. Intensive heat coupled to a high cooling time enhances the formation of this sensory defect. Burn in the syrup causes the change of the syrup grade from Amber grade to the Dark (Purlis, 2010). Thus, to avoid darkening, hot syrup inside of containers should be cooled previously the containers are packed together. Moreover, the containers must be separated to facilitate air circulation and the cooling of the syrup.
14
1.1.5.3. Type of storage containers
The type of storage containers can affect the color of the maple syrup during storage. It has been reported that plastic containers are the main factor influencing the color darkening after three months in storage (Morselli, 1980). The syrup packed in tinned steel containers produces light grade of the maple syrup due to increased leached tin and develops a flavour of high iron after the tin coating dissolves. Glass container was also reported to be responsible for the darkening of maple syrup but not such as plastic containers (ACIA, 2015).
1.1.6. Possible ways for commercial use of dark maple syrup
As it has been highlighted above, the use of dark maple syrup is quite limited because of the non-attractive color, strong smell and presence of odor of caramelization. All these compounds are formed as a result of different chemical reactions such as condensation, polymerization and transformation. To make such syrup usable for food applications, these compounds must be removed. Theoretically, different processes can be applied to achieve this objective. For example, the odor can be improved following deodorization under vacuum and the color can be improved by adsorption. The first objective is quite difficult to be attained. Indeed, the evaporation will not remove the strong smell because the responsible molecules are difficult to evaporate. Moreover, it is technologically a huge process.
One of the promising ways to enhance the use of such syrup by lightening its color and diminishing the intensity of the smell consists of a treatment on active surface that can absorb the pigments giving the dark color and strong smell of burn (caramelization). Moreover, such technology must be easy to use in the real conditions of maple syrup industry. Adsorption on food grade active carbon offers the possibility of achieving this objective while making the process feasible from technological point of view.
1.2. Activated carbon
1.2.1. Activated carbon
Activated carbon is manufactured from carbon containing materials such as wood, charcoal, peat, bituminous coal, coconut shells, lignite, pecan shells, bones, pulp mill black, ash saw dust, plastic residuals by activation processes (Worch, 1991). It find several applications in different fields and processes, including the food industry. To obtain activated carbon, different steps are required. The first one consist of carbonization which is necessary to transform the raw material into carbonized carbon which is the first step to make activated carbon (Bandosz and Kante, 2015). The carbon from carbonaceous materials is a material that has an infinity pore (a few Angstroms) clogged with organic matter (Figure 5). To be converted into activated carbon, the carbonaceous material must be free of all organic material. For this, the high-temperature used for heating in a rotary or vertical oven is 700 ° C and more (Strachowski and Bystrzejewski, 2015).
16
The second step consists of activation and there are two activation methods: (a) the physical process gas in which the coal is mixed with water vapor and nitrogen (Angın et al., 2013). Organic matter is destroyed and then a carbon skeleton that has it special properties is obtained. The whole is heated at 700-1000 ° C according to the equation:
2 2
C + H OCO + H
(b) the chemical process in which the carbonaceous material is mixed with sulfuric acid, phosphoric acid or zinc chloride and the whole is heated to 400-800 °C. This type of activation is used for the activated carbon in powder or granular forms (Figure 6) (Angın et al., 2013).
The large surface area of activated carbon provides a high adsorptive capacity to this material. It is this great adsorptive capacity which is the basis for numerous industrial as well as medical uses (Gao et al.; Sun and Webley, 2010). There are different kinds of activated carbon adsorption with different characteristics. The adsorptive characteristics are determined by the configuration of the surface area of activated carbon. Moreover, activated carbon is widely used in the treatment of acute poisoning with substances such as acetaminophen, salicylates, barbiturates and tricyclic antidepressants. The activated carbon absorbs strongly aromatic substances such as the foregoing, reducing their absorption from the gastrointestinal tract. At the other hand, most inorganic poisons are not significantly absorbed by the charcoal. Industrially, the activated carbon is produced in three main types: carbon grain, powder and extruded. These three categories of activated carbon can have properties tailored depending on the type of application (Danish et al., 2014).
Activated carbon has a specific surface area between 800 to 1500 m2/g and that is
why all ions of activated carbon are available for the absorption process (Asadi, 2007). Activated carbon mainly contains micropores with diameters smaller than 2 nm. Thus, high microporous structure and a high degree of surface reactivity make activated carbons suitable for different adsorption process. This specific surface area is used for decolorizing and removing impurities from fluids (Kumar, 2003).
1.2.2. The application of activated carbon
1.2.2.1. Air treatment
It is used for volatile organic compounds (VOCs) removal from the air. Indeed, activated carbon is the leading technology in cutting VOCs in the air or any other gas. It is used in particular cases for the elimination of siloxanes biogas. In many cases, a compound may be removed below the detection level. Therefore, the more stringent rules on air quality and safety can be met. The technology is reliable, robust and has proven effective over the years (Baléo and Subrenat, 2000; Bañuelos et al., 2014; Guo et al., 2014).
1.2.2.2. Hydrogen sulfide (H
2S) and odor control
The greater environmental awareness and improving the working conditions have focused the concentration on the reduction of odors from sewage treatment plants, municipal and industrial effluents. Harmful compounds such as H2S and mercaptans are
readily captured by the adsorption and removed by chemisorption or by catalytic conversion, while others organic contaminants are readily adsorbed by the coal (Masuda et al., 1999; Sitthikhankaew et al., 2011; Xiao et al., 2008a, b).
1.2.2.3. Gaseous waste incinerators and biogas purification
The incineration of household waste, hazardous industrial waste, medical waste, sewage sludge and crematoria lead to the formation of a flue gas containing a wide range of pollutants. Dioxins and heavy metals such as mercury and cadmium are not normally removed at sufficiently lowconcentrationsby conventionaltreatment.The use ofpowdered activated carbonis an effective methodto reduce concentrationsof these substancesbelow the levels specified by legislation. For biogas, it is produced by anaerobic digestion of organic matter. It is used locally for the production of green energy. Activated carbon is needed in biogas plants to remove impurities and to protect cogeneration engines from corrosion or damage from silica deposits. It is also essential when it comes to bring purity biomethane at a level sufficient to allow its injection into the network of use (Esteves et al., 2008; Hernández et al., 2011a; Hernández et al., 2011b; Pipatmanomai et al., 2009).
18
1.2.2.4. Wastewater treatment
Activated carbon is one of the most effective ways to eliminate a wide range of contaminants and it is highly effective for wastewater treatment generated from different industries. It is also used for the treatment of landfill leachate or contaminated soils. As strong adsorbent, it is capable of processing a large number of contaminants from such water. Activated carbon can be used to treat all or a portion of the contaminants directly or in combination with other steps. At the moment when the environmental protection is a necessity, recycling of process water in the industrial setting is a new economic path. Indeed, this type of treatment will reduce the impact on the environment, reduce disposal costs of waste water and reduce raw water samples of the natural environment. Here are some of the water contaminants that activated carbon is able to remove: non-biodegradable organics, absorbable organic halogenated, organic halogens, toxic molecules and coloring pigments (Altmann et al., 2014; Guo et al., 2014; Hadi et al., 2015b; Mailler et al.; Margot et al., 2013; Ruhl et al., 2014; Tammaro et al., 2014).
1.2.2.5. Food industry and pharmaceutical applications
In the food industry, the granular activated carbon is used for different applications such as for the:
Discoloration of cane and beet sugar syrups as well as purification of the glucose, fructose and sweeteners (Mudoga et al., 2008; Pendyal et al., 1999b).
Discoloration and purification of organic acids and amino acids from fermentation processes (Ayranci and Duman, 2006; Bayram and Ayranci, 2012).
Removal of chlorine in the industries using this chemical for the production of beer, soft drinks and other food products (Marsh and Rodríguez-Reinoso, 2006; Razvigorova et al., 1998).
Purification of the carbon dioxide used in soft drinks (Holden, 1998).
Decaffeination of tea and coffee (Clarke, 2003; Dong et al., 2011; Marsh and Rodríguez-Reinoso, 2006; Przepiórski, 2006; Vuong et al., 2013).
Removal of unwanted natural compounds and harmful anthropogenic compounds from edible oils such as polycyclic aromatic hydrocarbons (Hadi et al., 2015a; Sardella et al., 2015; Updyke et al., 2012).
Elimination of undesirable odoriferous compounds and dyes from glycerin.
Removal of off-tastes, odor or undesirable compounds dyes from alcoholic beverages such as wine, vodka, vermouth, beer (Rodriguez-Illera et al.; Siebert, 2013; Vanderhaegen et al., 2006).
Purification of fruit juices by eliminating undesirable dyes or compounds such as mycotoxins patulin in apple juice (Angin, 2014; Diban et al., 2007; Laksameethanasana et al., 2012; Soto et al., 2011; Suárez-Quiroz et al., 2014).
Debittering of intermediate food or flavorings (Saha and Hayashi, 2001).
Get value-added molecules of agricultural products (AlOthman et al., 2014; Galiatsatou et al., 2002; Pendyal et al., 1999a; Sardella et al., 2015).
In the pharmaceutical industry, activated carbons are used in many industrial process for the discoloration, purification, catalysis and cleanup (Kyzas and Deliyanni). Moreover, activated carbon is a gastrointestinal adsorbent. The large surface area of activated carbon provides a high absorbent capacity to this material. It is effective in adsorbing aromatic or benzenoid-like substances, fatty acids and fatty alcohols. Aromatics, such as acetaminophen, salicylates, barbiturates and tricyclic antidepressants, are strongly absorbed by the charcoal (Baccar et al., 2012; Calisto et al., 2015; Margot et al., 2013; Mestre et al., 2014; Snyder et al., 2007; Wang et al.).
Considering the aforementioned information about the adsorptive capacity of the activated carbon in different applications, it seems possible to apply this material to adsorb coloring pigments and other molecules responsible of the strong smell in the dark maple syrup.
CHAPTER 2: HYPOTHESIS AND OBJECTIVES
2.1. Research Hypothesis
Considering that:
1) The dark maple syrup contains pigments and coloring products that are originated from different browning reactions and that these products are easily absorbable on active sites contained in matrices such as activated carbon;
2) The activated carbon of food grade is able to adsorb coloring pigments and products which are similar to those found in dark maple syrup;
3) The use of activated carbon as a bleaching agent is easy to introduce in the food industry, in general, as well as in the industry of maple products, in particular;
Thus:
It will be possible to use food grade activated carbon for bleaching unmarketable dark maple syrup in order to make a product that falls into a category of maple syrups with a high commercial value.
2.2. General objective
The general objective of the present research project is to discolor unmarketable low grade dark maple syrup with activated carbon in order to make a product with light transmittance similar to a marketable maple syrup.
22
2.2.1. Specific objectives
1) To achieve the general objective of this work, the first specific objective was aimed
to study the effect of different independent variables on the light transmittance of the dark maple syrup measured at 560 nm:
Effect of the mixing time;
Effect of the concentration of the activated carbon; Effect of the type of activated carbon;
Effect of the activated carbon mean particle size; Effect of the stirring speed;
Effect of the working temperature.
2) A second specific objective consisted of applying the Langmuir, Freundlich and
Langmuir-Freundlich adsorption isotherms to describe the discoloration kinetics of the dark maple syrup on activated carbon according to the best results obtained in the first specific objective.
CHAPTER 3: MATERIALS, METHODS, RESULTS
AND DISCUSSION
Le présent mémoire de maîtrise est rédigé par insertion d’un article scientifique soumis pour publication dans Food Bioscience, un journal international avec comité de lecture. Par conséquent, la partie ``Matériels et méthodes`` est incluse dans ce chapitre.
Improvement of the color of low grade dark maple syrup
by adsorption on activated carbon and study of the
adsorption kinetics
Running title: Activated carbon to improvement color of low grade dark maple syrup
Bita Sadeghi-Tabatabai1,2, Amara Aït-Aissa1, 2, Mohammed Aïder*1, 2
1Department of Soil Sciences and Agro-Food Engineering, Université Laval, Quebec, Qc,
G1V 0A6, Canada
2Institute of Nutrition and Functional Foods (INAF), Université Laval, Quebec, Qc, G1V
0A6, Canada.
* Corresponding author
E-mail: mohammed.aider@fsaa.ulaval.ca
Tel: (418) 656-2131 # 4051 Fax: (418) 656-3723
26
Abstract
Low grade dark maple syrup was successfully discolored on activated carbon. Several experimental parameters were tested, namely the mixing time (20, 40, 60 min), concentration of the activated carbon (0.1, 0.3, 0.5 g/100 mL), type of activated carbon (I, II, III), particle size (25, 50, 75 μm), stirring speed (200, 400, 600 rpm), and temperature (40, 60, 80°C). The obtained results showed that the discoloration is optimal by applying the parameters such as an agitation time of 40 min with an activated carbon of type III at a concentration of 0.3 g/100 mL. These parameters yielded a light transmittance of 83.70 ± 0.21%, which ranks the syrup in the extra clear class. The results showed that among the tested carbons, the adsorption on the type carbon of III followed the Langmuir, Freundlich and Langmuir-Freundlich isotherms. Regarding the effect of the particle size, the obtained results showed that a mean size of 25 µm with a stirring speed of 200 rpm and a working temperature of 80°C was the most effective. The optimized conditions showed a good adequacy with the Langmuir and Freundlich models. The discoloration process by using the activated carbon of type III followed pseudo-second order kinetics.
Résumé
Dans ce travail, nous avons utilisé le charbon actif (trois types) pour décolorer le sirop d'érable foncé, classé dans la catégorie ``Non retenu`` de la classification du Québec du sirop d’érable. Nous avons testé l'efficacité de la rétention des pigments colorés du sirop d'érable sur le charbon actif en grains et en poudre. Aussi, nous avons comparé les différentes efficacités de ces matériaux. La première étape de nos essais expérimentaux a eu pour but de suivre les cinétiques d'adsorption des pigments. Dans cette partie, plusieurs paramètres expérimentaux ont été optimisés par utilisation d'un plan factoriel complet; à savoir : le temps d'agitation (20,40, 60 min), la masse du charbon actif utilisée par 100 ml de sirop (0.1, 0.3, 0.5 g), le type du charbon actif (I, II, III), le diamètre moyen des particules du charbon (25, 50, 75 µm), la vitesse d'agitation (200, 400, 600 rpm) et la température (40,60, 80 °C). La variable dépendante que nous avons considérée dans ce projet est la transmittance de la lumière du sirop mesurée à 560 nm. La deuxième étape a été consacrée sur l'étude du pouvoir adsorbant des trois types de charbons actifs utilisés avec l’application des modèles de Langmuir, Freundlich et de Langmuir-Freundlich pour décrire les cinétiques d’adsorption. Les résultats obtenus ont montré que la décoloration du sirop d’érable foncé est optimale avec les paramètres suivants : temps d'agitation t = 40 mn, masse du charbon actif ajouté m = 0.3 g et charbon actif de type III. Ces paramètres ont donné une valeur de la transmittance la transmittance de la lumière à 560 nm de 83.7 ± 0.2 %; et qui classe le sirop dans la catégorie Extra clair. Ensuite, ce travail a montré que seul le charbon actif de type III vérifiait à la fois une cinétique d’adsorption qui se décrit par les isothermes de sorption de Langmuir, Freundlich et Langmuir-Freundlich. Finalement, un autre plan d'expérience avait complété dans ce travail et qui portait sur l'optimisation de la taille des particules du charbon et qui a montré qu’une granulométrie moyenne d = 25 µm (mésopores) était la plus optimale pour décolore le sirop d’érable avec une agitation de 200 rpm à une température T = 80°C.
28
3.1. Introduction
Maple syrup is largely produced in North America by heat evaporation of maple sap collected from maple sugar trees (Acer saccharum) during the early spring season (Clément et al., 2010). Canada is the largest exporter of maple products in the world with 249 million $ in 2012 and Quebec accounted for 94% of the Canadian maple products exported. In Canada, the maple syrup is mainly classified according to the color by measuring its light transmittance at 560 nm (Perkins and van den Berg, 2009). There are two types of classification for maple syrup: the federal government classification and that of the provincial governments. The Canadian Food Inspection Agency governs the quality of maple products in Canada and is responsible for the federal classification of maple syrup which can be classified as extra light, light, medium, amber and dark. The latter is generally associated with a strong burnt caramel flavor and taste. Moreover, huge quantities of maple syrup said ``unclassified`` are produced each year. This product is stored and large quantities are accumulate from year to year, creating serious problems of management and marketing. Indeed, gradually, as the season progresses, the fructose and glucose content increases in the sap, while the sucrose content decreases slightly. Moreover, the content of other natural compounds found in the sap is also changing during the season (amino acids and minerals). These changes in the composition of the sap cause a change in the color and flavor of maple syrup, mainly because of the occurred reaction between the reducing sugars and amino acids of the sap (Danehy, 1986). Early in the season, the syrup is generally clear with good sweet taste, corresponding to the syrup of the best quality. However, as the season progresses, the syrup becomes darker and tastes caramelized sugar with less refined flavor.
To increase the profitability of the maple syrup industry, it is important to commercialize all the produced products, including the dark syrup called unclassified. To achieve this objective, it is necessary to improve the product color and make it at least amber or light. Moreover, the material or process used to achieve the color improvement must also affect positively the sensor quality of the end product, namely its smell and taste. Since maple syrup is a food product, the used material for color correction must be safe and
economically affordable. In this context, activated carbon (charcoal) seems to be an appropriate decolorizing and taste/smell improving agent. However, the choice of the most appropriate type of activated carbon for a particular application must be technologically feasible because the achievement of the targeted objective will depend on different factors such as the physical and chemical properties of the adsorbed material, proper functional properties of the activated carbon and other experimental conditions that can play an important role in the adsorption process (Agueda et al., 2011).
At the end of the 18th century, the adsorption properties of activated carbon were
observed. Then, the activated carbon was used for the first time in England in the sugar industry in 1794 to improve the process efficiency and product quality (Harris, 1942). However, the modern industrial production and the use of activated carbon were reported for the first time in a patent deposed by Ostrejko in 1901 (Ostrejko, 1901). Today, the activated carbon is used in several industrial applications; including gas and air cleaning, purification and recovery of different materials and substances, environmental protection for the removal of hydrocarbons and solvents (Xu et al., 2014). Furthermore, the activated carbon is also used increasingly in the treatment of water, including drinking water, groundwater, and wastewater. Its main role is to absorb dissolved organic impurities and eliminate all substances that affect the smell, taste and color of the treated material (Dubinin and Serpinsky, 1981; Liu et al., 2010; Ugurlu et al., 2008; Wood, 2002). Furthermore, the activated carbon is widely applied for liquid discoloration, which is particularly important in the pharmaceutical and food industry; including the maple syrup industry (Li et al., 2013b; Senthil Kumar et al., 2010).
This paper presents both experimental and theoretical interpretation of the mechanisms involved in maple syrup discoloration by activated carbon. The goal of this study was to use food grade activated carbon as adsorption material to improve the color and smell/taste of dark maple syrup in order to produce lighter syrup corresponding to syrup of light or at least amber grade. Specifically, the effect of many experimental factors such as the agitation time (X1), activated carbon mass in the sample (X2), and activated
carbon type (X3) on the syrup transmittance was studied by using a three level full factorial
experiment design. The kinetics of adsorption of the maple syrup pigments were interpreted (discussed) by using two kinetic models: the pseudo-first-order and the
pseudo-second-30
order models. Kinetic parameters and correlation coefficients were also determined. Moreover, the adsorption isotherm models of Langmuir, Freundlich and Langmuir-Freundlich were used to evaluate the effect of the experimental parameters on the performance of the activated carbon to adsorb the maple syrup coloration pigments.
3.2. Materials and Methods
3.2.1. Reagents and Samples
Dark, low grade (unclassified) maple syrup was purchase from a local maple syrup farm, Quebec City, Canada. The activated carbon type I, II and IV were purchased from Sigma Aldrich (Sigma-Aldrich, St. Louis, MO, USA). The activated carbon type III was purchased from Fisher Scientific (Fisher Scientific, Waltham, MA, USA).
3.2.2. Characterization of the activated carbon
3.2.2.1. Mean particle size
The mean particle size of each activated carbon used in this study was determined by the sieving method by using a series of sieves from the Canadian Standard Sieve Series (W. S. Tyler Company, ON, Canada) according to the ASTM procedure (ASTM, 2010).
3.2.2.2. Scanning electron microscopy (SEM) and Energy
dispersive X-ray spectrometer (EDS)
Scanning electron microscopy (SEM) images of the activated carbon were taken by using an electron microscope (Joel, JSM-840A, North Billerica, MA, USA) equipped with an EDS energy dispersive X-ray spectrometer (PGT Instrument, model Avalon, Princeton, NJ, USA). The EDS condition was set at 15 kV. The samples were first metalized by coating with a thin gold / palladium layer in order to make the surface conductive and allow the free flow of the excess electrons. This procedure is necessary to prevent the sample to be charged when it is exposed to the SEM electron probe (Chou et al., 2008).
3.2.2.3. Bulk density
The apparent density of the activated carbon used in this work is calculated by the tube method as adapted from the ASTM method D2854-09 (ASTM, 2014). A known amount of activated carbon sample was weighed and poured into a test tube which was taped with 10 strokes. Then, the resulted volume of the activated carbon in the sample was read. Knowing the mass and the volume, the apparent density of each analyzed activated carbon was determined.
3.2.3. Experimental procedure
The discoloration process used in this work is illustrated in Figure 7. To carry out the experiments, three amounts of activated carbon (0.01, 0.03 or 0.05 g) were weighed and introduced into a sample of 10 mL of maple syrup to yield a final concentration of 0.1, 0.3 and 0.5 g/100 mL. Then, the mixture was placed in a thermostatic bath (ThermoFisher Scientific, model 1534258, NH, USA) at a temperature of 25°C with a low agitation of 200 rpm on a magnetic stirrer (IKA, model RW20DSI, Wilmington, NC, USA). The stirring (mixing) time was set to 20, 40 and 60 min. At the end of the mixing time, the mixture was first filtered by using a simple paper filter with a goal of removing the large particles of the used activated carbon. The second filtration was carried out by using two syringe filters with mesh size of 45 and 20 microns, respectively. Finally, the light transmittance of the recovered samples was analyzed by a UV-Visible spectrophotometer (HP S/N 4582901633, USA) at 560 nm (Corbella and Cozzolino, 2005).
3.2.4. Statistical modeling and adsorption isotherms
In this work, two blocks of experiments were carried out and each one was a three level full factorial experimental design (3k) with k = 3 in triplicate. The polynomial
equation (Eq. 3.1) was used to model the response variable
ˆy
as a function of the input factors X’i.2 2 2
1 1 3 1 2 3
ˆy = 11.61+ 4.55 X - 4.406 X X + 23.19 X + 23.16 X + 27.46 X (Eq. 3.1) A second order regression equation was used to calculate the optimal values of all parameters which can allow giving a maximal color improvement of the maple syrup. For
32
achieve this objective, it is necessary to solve the system of equations (Eqs. 3.2) by deriving the predictive equation at each variable X1, X2 and X3.
1 1 2 2 3 3 ˆy 0 X = 40 min X ˆy 0 X = 0.03g X ˆy 0 X = type III X (Eqs. 3.2)
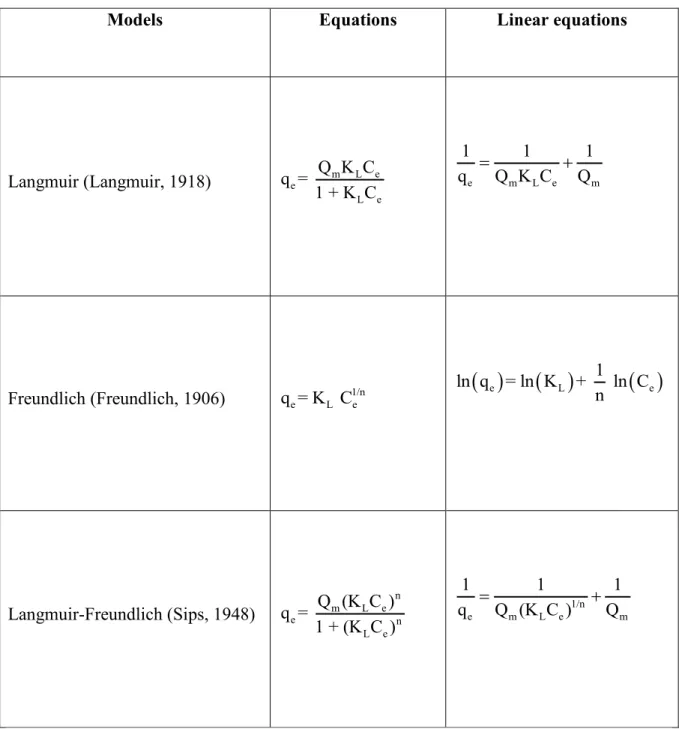
In the present study, it has been hypothesized that the discoloration of the maple syrup resulted from an adsorption mechanism of the coloring pigments on the surface of the activated carbon. Thus, in order to obtain an adequate interpretation of the observed color improvement of the maple syrup, the intensity of coloration of the syrup solution was plotted as a function of adsorbed coloration. The data were plotted against the Langmuir, Freundlich, and Langmuir-Freundlich adsorption isotherms, as shown in Table 4.
3.2.5. Experimental design and statistical analysis
In the first experimental block, a full factorial experimental design (33) was used
and all the experiments were carried out at ambient temperature (25 C). The independent variables were as follow: agitation time (X1) (20, 40, 60 min); activated carbon
concentration (X2) (0.1, 0.3, 0.5%); and activated carbon type, (X3) (I, II, III). The second
experimental block was also carried out as a full factorial design with three independent variables: temperature (X1) (40, 60, 80 C), type activated carbon mean size (X2) (25, 50,
75 microns) and agitation speed (X3) (200, 400, 600 rpm). The analysis of variance
(ANOVA) and normality Test (Shapiro-Wilk) were used to investigate the differences between the mean values of the compared treatments at a 95% significant level by using the SigmaPlot v. 11 software (Systat Software Inc, San Jose, Ca, USA) and Maple Software v.14 (Maplesoft, Waterloo, ON, Canada). The isotherm model constants (Langmuir, Freundlich and Langmuir-Freundlich), average relative errors (%), and coefficient of determination (R2) based on the actual deviation between the experimental points and the
predicted values were estimated by using SigmaPlot v.11. All experiments were carried out at least in triplicate and mean values were used for different calculations and comparisons.
3.3. Results and Discussion
3.3.1. Characteristics of the used activated carbons
The particle size analysis served to determine the size distribution of the particles constituting the different used activated carbons. The total carbon mass which was sifted was 200 g and the particle size distribution was in the range up to 300 μm. The particle size distribution was dependent of the form of the carbon (powder or grain), and of the particle shape (spherical or heterogeneous shape). It has been found that the used carbons did not present a uniform size distribution and have heterogeneous shapes including some spherical form. Moreover, the particle size of the activated carbon of type III have particle size diameter up to 150 µm (Figure 8).
The different activated carbons used in this work were inert carbonaceous materials. Some of them have highly developed intrinsic porosity which gives them good adsorbing properties facilitating the fixation on their surface of the colored pigments present in the dark maple syrup used (Wang and Yuan, 2014). This feature is due to the micro pores present in these carbons. The number and distribution of these micro pores can significantly affect the adsorption capacity of these materials. Unlike to the activated carbon type IV, the micro pores distribution is much pronounced on the surfaces of the activated carbon of type I, II and III (Figure 9). The structure of the activated carbon of type III seems to be highly developed then the other types of the used carbons. The pore structure increases the specific surface of the activated carbon which can reach approximate values of 1.500 m²/g of carbon. This specific surface has an effect of increasing the carbon adsorptive properties. For the texture, the active carbons of type I, II and IV have an amorphous texture which is made of graphite microcrystal in different interconnected form. The activated carbon of type III has a crystalline structure with sites which are favorable for good adsorptive properties. In addition, each microcrystal comprises a stack of several crystalline layers with a high degree of porosity (Figure 9).
The structural analysis showed also that the different activated carbons used contain different types of elements in different amounts. They are mainly consisted of carbon. However, besides carbon, the activated carbon of type I consists of some volatile matters, in particular oxygen. The other composition is represented by ash material composed of different minerals such as calcium, which was found in the activated carbon of type III. The
34
presence of Ca can affect the adsorption properties of this activated carbon, as reported in (Li et al., 2013a). The observed the gold and palladium were from the metalized samples that were used as coating to form the thin gold/palladium layer in order to make the carbon surface conductive and allow the free flow of the excess electrons during the Energy dispersive X-ray spectrometric analysis (Figure 10).
The results obtained for the bulk density showed that the values corresponding to the activated carbons of type I, II, and III are almost similar. These carbons are used in the form of powder to discolor the dark maple syrup. The activated carbon of type IV was different from the other carbons and was characterized by a high bulk density which may be due to its initial granular form. In addition, the observed differences of the bulk densities can be attributed to the differences observed in the particle shape of the used activated carbons. Applied to the targeted adsorption application of this study, the spherical form of the activated carbon particles will be more favorable because this form present low bulk density and punctual inter particle contact (Bhargavi et al., 2011) (Figure 11).
3.3.2. Effect of the concentration and type of the added activated
carbon
The evolution of the maple syrup light transmittance at 560 nm as a function of the mixing time at different concentrations of the activated carbon added is shown in Figure
12. Statistical analysis of the obtained data showed a significant effect (p < 0.001) of this
independent variable and the plotted values of the dependent variable (light transmittance) followed a behavior that can be described by a second order polynomial function. It has been observed that the light transmittance of the treated maple syrup increased by increasing the concentration of the activated carbon added. This can be explained by the increasing of the contact surface between the adsorbent and the adsorbate which yielded a high amount of adsorption of the colored pigments. In fact, it has been already reported that the higher the contact area is, the higher the absorption reaction is (Hall et al., 1966). In
Figure 12a which corresponds to the data obtained by using the activated carbon of type I,
it can be seen that the light transmittance increased from 11.11 ± 0.25% up to 31.39 ± 0.30% in the case of using 0.1% of activated carbon; and up to 64 ± 0.3% in the case of using 0.3% g. However, addition of 0.5% of activated carbon resulted in final syrup with light transmittance of 56 ± 0.32% which is lower than the value obtained with 0.3%. At the
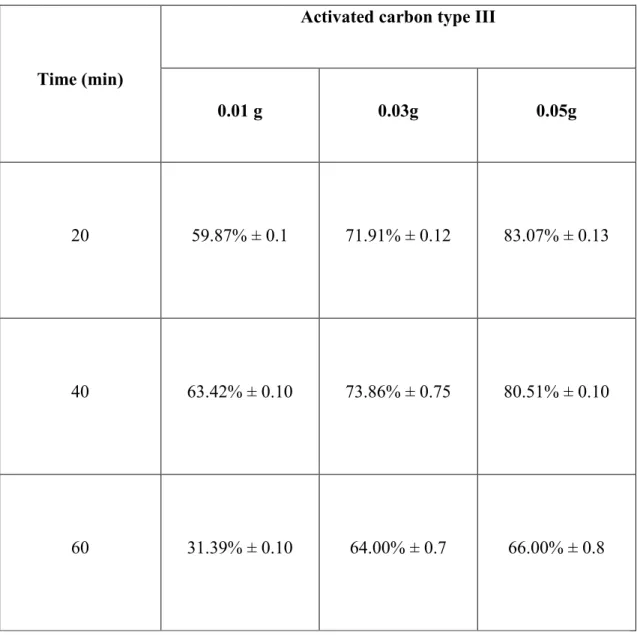
other hand, the Figure 12b shows that the transmission range varies between 11 and 17% in the case of using activated carbon of type II. At the end of the mixing time of 60 min, the light transmittance values of the syrup were 15.11 ± 0.25%, 15.65 ± 0.3% and 16.63 ± 0.25%, for activated concentration added of 0.1, 0.3 and 0.5%, respectively. This result showed the non-significant effect (p > 0.05) of the activated carbon of type II concentration. Finally, the Figure 12c shows that the transmittance range varies between 23.42 and 61.51% in the case of using the activated carbon of type III. The final syrup light transmittances obtained after 60 min treatment were 23.42 ± 0.31, 83.86 ± 1.15, and 61.51 ± 0.51% for a concentration of the added activated carbon of 0.1, 0.3 and 0.5%, respectively. This type of activated carbon gave the highest light transmittance of the final maple syrup, which is probably due to its large surface area (Figure 9). The results obtained with the activated carbon of type IV (Figure 12d) were not significantly different from those obtained with the activated carbon of type II.
The optimized results showed that the optimal activated carbon concentration to be added to the dark maple syrup in order to obtain a final product with significantly improved color is 0.3%. Thus, the use of 0.05% activated carbon is not necessary since the occurred adsorption phenomenon was saturated at 0.03%. This saturation can be explained by the chemisorption process which is more dominant in the present case. Indeed, it has been reported that chemisorption is a common process in the adsorption of dyes on different adsorbents (Ho et al., 2005; Ho and McKay, 1998; Ofomaja, 2007). Finally, it has been found that the use of the activated carbon type I gives a syrup which can be classified as amber with light transmittance ranging between 27-43.9% or as medium with a light transmittance of 44-61%. The activated carbon of type II and IV did not improve the color of the syrup gives. The use of the activated carbon of type III did not improve the color of the syrup when it was used in a concentration of 0.1%, but it yielded syrup that can be classified as light when the carbon concentration was 0.5% or extra clear light when the active carbon concentration was 0.3%.
The evolution of the light transmittance of the treated maple syrup as a function of different activated carbon types is shown in Figure 13. The lines represent the regression polynomial of second order. In Figures 13(a, b, c), it is observed that the transmittances increased with the increasing of the mass of the activated carbons according to their