ContentslistsavailableatScienceDirect
Mammalian
Biology
j o ur na l ho me p ag e :w w w . e l s e v i e r . c o m / l o c a t e / m a m b i o
Original
investigation
Comparison
of
diet
and
prey
selectivity
of
the
Pyrenean
desman
and
the
Eurasian
water
shrew
using
next-generation
sequencing
methods
Marjorie
Biffi
a,∗,
Pascal
Laffaille
a,
Jérémy
Jabiol
a,
Adrien
André
b,
Franc¸
ois
Gillet
b,
Sylvain
Lamothe
a,
Johan
R.
Michaux
b,c,
Laëtitia
Buisson
aaEcoLab,UniversitédeToulouse,CNRS,INPT,UPS,118routedeNarbonne,31062Toulousecedex9,France
bLaboratoiredeBiologieEvolutive,UnitédeGénétiquedelaConservation,UniversitédeLiège,InstitutdeBotaniqueB22,QuartierVallée1,Chemindela
Vallée4,4000Liège,Belgium
cCIRAD,AgirsUnit,TAC-22/E-CampusinternationaldeBaillarguet,34398MontpellierCedex5,France
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received9December2016 Accepted12September2017 HandledbyLauraIacolina Availableonline14September2017 Keywords: COI Dietaryoverlap Foragingstrategy Scatanalyses Semi-aquaticmammal
a
b
s
t
r
a
c
t
Inthisstudy,theinteractionsbetweentwosemi-aquaticmammals,theendangeredPyreneandesman GalemyspyrenaicusandtheEurasianwatershrewNeomysfodiens,wereinvestigatedthroughtheanalysis oftheirsummerdietusingnext-generationsequencingmethods,combinedwithanalysesofprey selec-tivityandtrophicoverlap.Thedietofthesepredatorswashighlydiverseincluding194and205genera forG.pyrenaicusandN.fodiensrespectively.Overall,bothspeciesexhibitedrathernon-selectiveforaging strategiesasthemostfrequentlyconsumedinvertebrateswerealsothemostfrequentandabundantin thestreams.ThissupportedageneralistforagingbehaviourforG.pyrenaicusandN.fodiensinthestudy area.ThePiankaindex(0.4)indicatedasignificantbutmoderatedietaryoverlapasG.pyrenaicusmostly reliedonpreywithaquaticstageswhereaspreyofN.fodiensweremainlyterrestrial.Moreover,no dif-ferenceinG.pyrenaicuspreyconsumptionwasfoundinpresenceorabsenceofN.fodiens.Adifferential useoftrophicresourcesthroughmechanismssuchasplasticfeedingbehaviourordifferencesinforaging micro-habitatarelikelytofacilitatethecoexistencebetweenthesetwomammalspecies.
©2017DeutscheGesellschaftf ¨urS ¨augetierkunde.PublishedbyElsevierGmbH.Allrightsreserved.
Introduction
Biodiversityconservationrequiresathoroughknowledgeofthe
complexnatureofinteractions betweenspeciesand their
envi-ronment.Accordingtotheecologicalnichetheory(Hutchinson,
1957),sympatricspeciesareexpectedtoexhibitsomeniche
dif-ferentiationinpreyorhabitatusetocoexist(Pianka,1974).Under
limitingconditions,strongnicheoverlapbetweenspeciesmaylead
tostrongcompetitiveinteractions,andmayultimatelyresultinthe
competitiveexclusionoftheweakestcompetitorimplying
conse-quencesonitslocalandregionaldistribution(Wiszetal.,2013).The
studyofresourceuseandpotentialnicheoverlapwith
competi-torsseemsthuscrucialtoassessthevulnerabilityofspecieswith
Abbreviations:FOdiet,frequencyofoccurrenceofinvertebratetaxainthe
preda-tordiet(numberoffaecescontainingthetaxondividedbythetotalnumberof predatorfaeces); FOstream,frequencyofoccurrenceofinvertebratetaxainthe
streams(numberofSurbersampleswiththetaxadividedbythetotalnumberof Surbersamplescollectedinthestudysites).
∗ Correspondingauthor.
E-mailaddress:m.biffi@live.fr(M.Biffi).
conservationconcern.Thisisparticularlytruewhenfocusingon
specieslivinginecosystemsthatarevulnerabletoanthropogenic
alterations,suchasfreshwaterecosystems(Dudgeonetal.,2006).
Anyquantitativeor qualitativeshiftin theresource availability
and/ordiversity(e.g.preycommunity)followingdisturbance(e.g.
aquaticpollution,alterationofriverflow)mayexacerbatethe
com-petitiveinteractionsbetweenconsumers.
ThePyreneandesman,Galemyspyrenaicus(E.Geoffroy
Saint-Hilaire,1811,Talpidae)isasmallsemi-aquaticmammal,endemic
tothePyreneesMountainsandtheIberian Peninsula(northern
and central Spain, northern Portugal). The species is listed as
vulnerable by the IUCN (Fernandes et al., 2008)and is legally
protectedinallthecountriesencompassingitsdistributionarea.
ThealarmingdeclineofG.pyrenaicuspopulationsovertherecent
decadesacrossitswholerange(Charbonneletal.,2016;Gisbert
andGarcía-Perea,2014)hasencouragedlocal,nationaland
Euro-peanconservationinitiatives(e.g.,inFrance:Life+Desman,2013;
Némozetal.,2011).Yet,inspiteofanincreasingnumberof
stud-iesfocusingonthisspecies(e.g.,AymerichandGosàlbez,2015;Biffi
etal.,2016;Charbonneletal.,2016;Escodaetal.,2017),the
respec-tiveinfluenceofprey,competitorsandpredatorsonitssurvivaland
distributionstillremainstobeexplored.
http://dx.doi.org/10.1016/j.mambio.2017.09.001
Modernadvancesinmoleculargeneticswererecentlyapplied
toinvestigate the diet of G. pyrenaicus in the French Pyrenees
(Gillet, 2015; Gilletet al.,2015).Theystressed thewide
diver-sityof prey(156genera) withhalfof them beingfoundinthe
dietofG.pyrenaicusforthefirsttime.Besides,thesefirststudies
confirmedthedietarypreferencesof G.pyrenaicustowards
Tri-choptera,EphemeropteraandPlecoptera(Insecta),aspreviously
knownfromtraditionalmethodsbasedongutcontentanalysisor
visualinspectionoffaecesremains(e.g.,Bertrand1994;Castiénand
Gosálbez1995;Santamarina1993;SantamarinaandGuitian1988).
Inaddition,Gillet(2015)emphasizedasubstantialconsumptionof
terrestrialprey.
Several aquatic or semi-aquatic animals are knownto prey
and use similar food and habitat resources as G. pyrenaicus
and act as potential competitors for resource acquisition: the
browntroutSalmotruttaandthedipperCincluscinclus(Bertrand,
1994; Santamarina, 1993; Santamarina and Guitian, 1988), the
EurasianwatershrewNeomysfodiens(CastiénandGosálbez1999;
Morueta-Holmeetal.,2010;SantamarinaandGuitian1988),the
MediterraneanwatershrewNeomysanomalus(Santamarina,1993)
and thePyreneanbrook newt Calotritonasper(Bertrand, 1994).
AmongthesespeciesN.fodiensexhibitssimilarhabitatpreferences
asG.pyrenaicus,i.e.swiftly-flowingstreamswithnumerous
shel-ters(e.g.cavities)ontheriverbeds(Greenwoodetal.,2002;Keckel
etal.,2014).N.fodiensisalsoknownasanopportunisticfeeder
consumingbothaquatic(e.g.,crustaceans,insectlarvae)and
ter-restrial(e.g,coleopterans,gastropods,spiders,earthworms)prey
(CastiénandGosálbez,1999;Churchfield,1985;Churchfieldand Rychlik,2006;Haberl,2002).Moreover,N.fodiensexhibitsa
simi-larpolyphasicactivitypatternasG.pyrenaicus(Meleroetal.,2014),
withactivityphases varyingacross seasons(Churchfield, 1984;
Greenwoodetal.,2002;Keckeletal.,2014;Rychlik,2000).Despite
importantsimilaritiesintheirhabitat,resourcepreferencesandlife
style,fewstudieshavefocusedonthetrophicoverlapbetweenN.
fodiensandG.pyrenaicustodate.Thesestudies,limitedtosmall
samplessizes(e.g.,onlysixG.pyrenaicussamplesinSantamarina,
1993)andrelyingsolelyonthevisualinspectionofpreyremainsin
faecesorgutcontent,concludedthatcoexistenceanddiet
differ-entiationwerelikelytheresultofadifferentuseofmicro-habitats
(CastiénandGosálbez,1999).
Duringthepastyears,moleculargeneticmethodsbasedon
fae-cesanalyseswereincreasinglyusedinsteadof‘traditional’methods
basedongutcontentanalysisorvisualinspectionoffaecesremains
(Pompanonetal.,2012).Theirmainadvantagesarethat(i)they
donot requestanimal sacrificecomparedtogutcontent
analy-sis,(ii)theyidentifypreywithhightaxonomicresolution(genus
andspecieslevels),(iii)includinghighlydegradedorsoft-bodied
species(molluscs,earthworms)thatcannotbeidentified
morpho-logically,(iv)theyarelesstime consuming,and (v)theydonot
requireanytaxonomicalexpertiseoftherangeofpotentialpreyas
longastaxaarepresentingeneticdatabases(seePompanonetal.,
2012forareview).Todate,thoughthedietofG.pyrenaicushasbeen
recentlydescribed usingmolecularmethods(Gillet,2015;Gillet
etal.,2015;Biffietal.,2017),suchdataaboutN.fodienshavenever
beengathered,makinganycomparisonoftherespectivedietsof
thesetwospeciesratherspeculative.
Inthisstudy,weaimedatdescribingthesummerdietofthese
twomammals,usingrecentmoleculargeneticmethods,inapart
oftheFrenchPyrenees(i.e.,theAriègedepartment)where they
areknowntoco-occur.Moreover,wecomparedthepreyofthese
predatorspecieswithstreambenthicinvertebratecommunities,on
whichG.pyrenaicusmostlyfeeds.Thisallowedusassessingtheprey
selectivityofthesetwomammalspeciesandthepotentialtrophic
overlapduringsummerinordertodiscussmechanismsthatcould
facilitatetheircoexistence.
Materialandmethods
Studyareaandsamplingsites
Samplingwas conducted in 65 sites spread over the Ariège
department,aFrenchadministrativeregioninthePyrenees
Moun-tains(Fig.1).ThisareaexhibitsrelativelyhighoccurrenceofG.
pyrenaicus(Biffietal.,2016;Charbonneletal.,2015,2016),and
the presence of N. fodiens was recently reported (Charbonnel
et al., 2015). The mean elevation of the 65 sampling sites is
757.9±259.3mandvariesbetween375.2mand1755.6m.Mean
monthly streamflow equals to1.1±2.0m3/s witha maximum
of13.2m3/s(Charbonneletal.,2016).Naturalzoneswith
herba-ceous or shrubby vegetation (52.1±36.1%), agricultural lands
(43.9±34.7%)andforests(40.6±32.5%;CorineLandCover©DB
2012)dominatethelandcover surroundingthe65sites.Inthis
mountainous area, the climate is cold (mean annual air
tem-perature=10.4±1.3◦C, SAFRAN © DB) and wet (mean annual
rainfall=1141.0±110.9mm,SAFRAN©DB).
Faecessamplingandmoleculargeneticsanalyses
FaecescollectionofG.pyrenaicusandN.fodienswereconducted
twicebetweenJuneandSeptember2015inthe65selectedsites
(Fig.1).Skilledobserversmeticulouslyinspectedeachemergent
item(i.e.,rock,treerootorbranch)alonga250mriverbed
tran-sect.Thislengthisacompromisebetweenthehomerange(HR)size
andtheaveragedistancetravelledalongstreamchannels(ADTS)
during24hforN.fodiens(narrowandlinearHRalongastream:
106–509m2,ADTS:49±25m;Cantoni,1993;Lardet, 1988)and
G. pyrenaicus (linear HR: ≈500m; ADTSbetween resting sites:
≈250m;Meleroetal.,2012,2014).Oursamplingprotocolmeets
therecommendationsofParryetal.(2013)whofoundthat
repeat-ingvisitsinasinglesiteratherthanenlargingthesampledarea
allowedbetterdetectionprobabilitiesfortheEurasianotter.Itis
alsoinagreementwithCharbonneletal.(2014)whoobtained
rea-sonabledetectionprobabilitiesforG.pyrenaicus(0.58)inasurvey
of 100m-longsectionsof riversusingtemporal replicates.The
searchforfaecesisastandardandeffectiveprotocolfor
detect-ingthepresenceofG.pyrenaicus(Charbonneletal.,2014)andhas
alsoprovenefficiencyforN.fodiens(AymerichandGosálbez,2004).
AllputativeG.pyrenaicusorN.fodiensfaeces basedontheir
colour,size,smellandposition,werecollectedandanalysedwith
moleculargenetictoolsbothtoconfirmthespeciesidentityofthe
consumerandthepreyconsumed.Followingthemanufacturer’s
instructions, DNA wasextracted from faecal samplesusing the
StoolMiniKit(Qiagen Inc.,Hilden,Germany).PCRamplification
wasduplicated for each sample ona portionof the
mitochon-drialcytochromeoxydaseIgene(COI;fordetails,seeGilletetal.,
2015).NegativeDNAextractionandnegativePCRcontrolswere
includedintheprocedure.AgencourtAMPureXPbeads(Beckman
CoulterLifeSciences,IN,USA)and thenQuant-iTTM PicoGreen®
dsDNAAssayKit(ThermoScientific,MA,USA)wereusedto
puri-fiedPCRproductsandtoquantifiedpurifiedampliconsrespectively.
Afterthequantificationstep,productswerepooledat
equimolar-ityandsenttotheGIGAGenomicsplatform(UniversityofLiège,
Belgium) for sequencing on an ILLUMINA MiSeq V2 benchtop
sequencer.Rawsequencesweresortedandfilteredusingascript
mixingFASTXToolkit(http://hannonlab.cshl.edu/fastxtoolkit;
23-09-16) and USEARCH (Edgar,2010)functions (seeAndré etal.,
2017fordetailsonbioinformatics).Sequencesoriginatinglikely
fromextractionorPCRcontaminantswereexcludedfromfurther
analyses.Theremainingsequenceswerethencomparedwith
pub-lished sequencesavailable intheonline BOLDdatabaseforCOI
(Ratnasinghamand Hebert,2007).Sequencesthat hadaunique
Fig.1. Locationofthestudyareaandsamplingsites(blackdots)inFrance.
score(i)higherorequalto90%fortheidentificationofpreytaxaat
thegenuslevel(whenpossible),and(ii)higherorequalto99%for
theidentificationofthepredatoratthespecieslevel.
PreytaxawerevalidatedaspotentiallyoccurringinFranceand
inthePyreneesusing theFrenchNationalInventoryof Natural
Heritage(Muséumnationald’Histoirenaturelle,2003–2017)and
theFrenchOfficeforInsectsandtheirEnvironment(OPIE-Benthos,
2017)onlinedatabases.Taxaidentifiedasendemicofotherpartsof
theworldwerekeptintheanalysisanddesignatedbyanasterisk
hereafter(*)astheymorelikelycorrespondtoageneticallysimilar
taxonpresentinthePyreneesbutabsentinthegeneticdatabases.
Whenpreytaxacouldnotbeidentifiedatthegenuslevel,theywere
groupedtogetheratahighertaxonomiclevel.
Aquaticmacroinvertebratessampling
Aquaticmacroinvertebrateswerecollectedin27outofthe65
sites,amongwhich N.fodiensandG.pyrenaicuswerepresentin
19and25sites,respectively(called“Neomyssites”and“Galemys
sites”,respectively).G.pyrenaicuswasdetectedaloneineightsites
(“Galemysalonesites”)andbothspecieswerefoundtoco-occurin
17sites(“Galemys+Neomyssites”).N.fodienswasdetectedalonein
twosites(“Neomysalonesites”).
The available habitats for aquatic macroinvertebrates were
describedaccordingto12categoriesofsubstratetype:bryophytes,
hydrophytes, helophytes, litter, twigs roots, algae,large stones
(>25cm),cobbles/pebbles(2.5–25cm),gravel(0.2–2.5cm),mud,
sand,bedrock;andfourcategoriesofcurrentvelocity:null,slow,
medium,fast.Thepercentagecoverofeachcombinationof
sub-stratetype/currentvelocitywasvisuallyestimatedineachsite.
Six Surber net samples (mesh size: 500m, sampled area:
0.04m2)wereconductedineachsiteaccordingtoastratified
sam-plinginthedominanthabitats(e.g.mainlycoarsemineralsubstrate
inhigh-flowfacies)coveringmorethan5%ofeachstreamtransect
inordertoberepresentativeofthesite.Thesampled
macroinver-tebrateswerefrozenbeforebeingsorted,countedandidentifiedat
thefamilylevel(exceptforOligochaetaandHydracarina)following
Tachetetal.(2000)atthelaboratory.Themeandensity(numberof
individuals/m2),andthefrequencyofoccurrence(FO
stream:relative
numberofSurbersampleswiththetaxon)werethencalculatedfor
eachsiteandinvertebratetaxon.
Mean macroinvertebrates densities were compared with
Wilcoxonsign-rankedtestsandP-valuesadjustedwiththe
Bon-ferronicorrection,betweencategoriesofsites(i.e.,Galemysalone
sites,NeomysalonesitesandGalemys+Neomyssites).
Dietcompositionandcomparisonbetweenmammals
Presenceor absenceof prey in each faeces of G. pyrenaicus
andN.fodienswereusedtodescribethedietcompositionofboth
species.Thefrequencyofoccurrence(FOdiet,i.e.,thenumberof
fae-cescontainingthetaxondividedbythetotalnumberoffaeces)inG.
pyrenaicusandN.fodiensdietwascalculatedforeachorder,family
andgenusofinvertebratesandforthedifferenttypesofprey’s
habi-tat(i.e.,exclusivelyaquatic,exclusivelyterrestrialorwithaquatic
andterrestrialstages).Tocomparethefaecescompositionbetween
thetwo species,aCorrespondenceAnalysis(CA)wasappliedto
presence-absencedataofpreyinfaecesatthegenuslevel.The
coor-dinatesofeachfaecesonthefirstandsecondaxesoftheCAwere
comparedbetweenthetwospecieswithaStudenttestfor
homo-geneousvariances.Rarepreytaxa,withFOdietbelow5%bothinG.
pyrenaicusandN.fodiensfaeces,werenotincludedinthisanalysis.
ToassesspreyselectivityofG.pyrenaicusandN.fodiens,the
pres-enceofinvertebratesinthefaeceswereconsideredatthesitescale.
Whenaninvertebratetaxonwaspresentinatleastonefaecesof
themammalspeciesinasite,itwasconsideredasbelongingto
themammaldietinthissite.PreyFOdietinthefaeceswerethen
calculatedforthe19Neomyssitesandthe25Galemyssites.Data
onpreyavailabilityinthestreams(i.e.,FOstreampersitefromthe
Surbersamples)andpreyconsumedbythepredatorinthesite
(i.e.,FOdietpersiteinthesampledfaeces)atthefamilylevelwere
comparedusingtheIvlev’selectivityindex(Ivlev,1961).Thisindex
rangesfrom−1(i.e.,avoidanceofprey)indicatingthatthetaxon
isfrequentinstreamsbutneverfoundinfaeces,to1(i.e.,active
selection)whenthetaxonisrareinstreamsbutfoundinavery
highnumberoffaeces.Azerovalueindicatesthatconsumptionis
proportionaltotheamountofinvertebratesavailableinstreams.
5%inboththeSurbersamples(FOstream)andthefaecesofG.
pyre-naicusandN.fodiens(FOdiet)werenotconsideredinthisanalysis.
Trophicoverlapbetweenmammals
TodeterminethedegreeoftrophicoverlapbetweenG.
pyre-naicusandN.fodiensduringsummer,thePianka’sdietaryniche
overlapindex(Pianka,1973)wascalculatedfromthepreyFOdietin
thefaecesatthegenusandfamilylevels.Thisindexrangesfrom0
(notrophicresourceusedincommon)to1(fulldietaryoverlap).
Moreover,totestiftheco-occurrenceofbothmammalspecies
inasitecouldmodifythedietofG.pyrenaicus,thefaeces
composi-tioninpreywascomparedbetweentheGalemysalonesites(8)and
theGalemys+Neomyssites(17).Themeanfrequencyofoccurrence
ofeachpreytaxonfoundinthefaecesofG.pyrenaicus(atthefamily
level)werecomparedwithaWilcoxonsign-rankedtestbetween
thetwocategoriesofsites.ThedietofN.fodienscouldnotbe
com-paredbetweenNeomysalonesitesandGalemys+Neomyssitesdue
toaverysmallnumberofNeomysalonesites(2).
AllstatisticalanalyseswereconductedinR3.3.1(RCoreTeam,
2014)usingtheade4andspaapackages.
Results
Molecularidentificationofpredatorsproducingthefaecesand
preycontents
Atotalof464faeceswerecollectedfromthe65sampledsites
(7±2faecespersite)andanalysedusingmoleculargeneticstools.
AfterthetwoPCRamplifications,atotalof2,160,447readswere
obtained.1,348,331readswerecorrectlyassignedto199faeces
(42.9%)thatbelongedtoG.pyrenaicus(3±2faecespersite)and
whosepresencewasconfirmedin58sites.Amongthem,itwas
possibletoidentifypreyin184faeces.Seventy-ninefaeces(17%
offaeces–463,706reads),including78faeceswithdiet
informa-tion,belongedtoN.fodiens(2±1faecespersite)whichconfirmed
itspresencein39sites.Fromtheremainingfaeces,51(11.0%)were
assignedto14otherhostspecies(mammalssuchasNeomys
anoma-lus,Apodemussp.,Sorexsp.;batsorbirds).Molecularidentification
ofpredatorsandpreyfailedfor29.1%ofthesamplesdueto
insuf-ficientDNAquantityordegradedsamples.
Overalldiversityofpreyinthepredatorfaeces
ThefaecesofG.pyrenaicusandN.fodienscontainedalmost
exclu-sivelyinvertebrate preyexcept oneamphibian foundinone G.
pyrenaicusfaeces.Preydiversitywashighwith10and9classes,
30and33orders(Table1),111and117families,and194and205
genera(AppendixAintheSupplementarymaterial)forG.
pyre-naicusandN.fodiens,respectively.Intotal,309differentgenera
wereidentifiedwhose178wereconfirmedtooccurinthe
Pyre-nees,95inFranceand9wereendemicofotherpartsoftheworld
(e.g.,Australia,America,Asia).Theremaining27taxacouldnotbe
identifiedatthegenuslevelinthedatabasesandtheirdistribution
rangeisunknown.
Forthetwomammalspecies,frequentlyconsumedprey(i.e.,
presentinmorethan5%ofthefaeces)representedasmall
propor-tionofthetotalpoolofpreyconsumed(12.9%and20.5%ofgenera
eatenbyG.pyrenaicusandN.fodiens,respectively).Thedominant
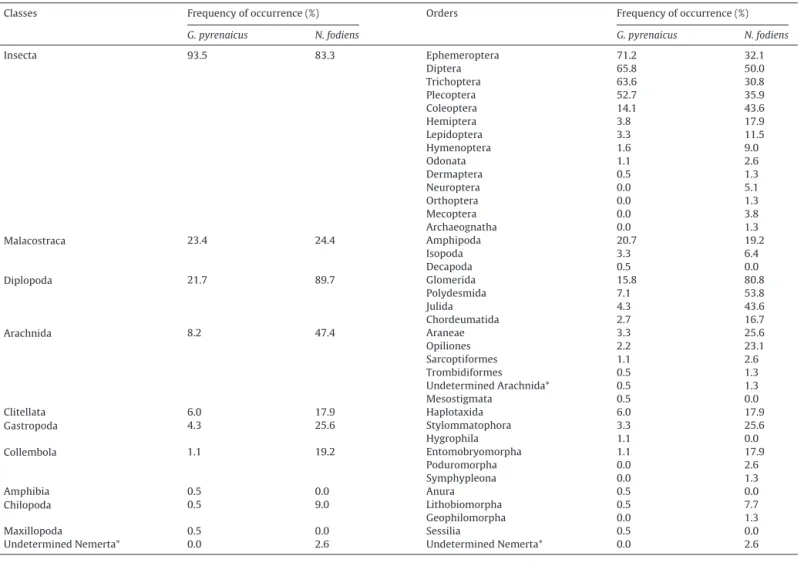
preyfoundinthefaecesofG.pyrenaicuswereInsecta(93.5%of
thefaeces),Malacostraca(23.4%)andDiplopoda(21.7%).Among
insects,G.pyrenaicuspreyedmorefrequentlyonEphemeroptera
(71.2%)whichincludedthemostfrequentfamily(Heptageniidae:
61.4%)butnotthemostfrequentgeneraHydropsyche(Insecta
Tri-choptera Hydropsychidae: 52.7%). N. fodiens seemed to have a
morediversedietwithDiplopoda(89.7%offaeces),Insecta(83.3%),
Arachnida(47.4%),Gastropoda(25.6%) andMalacostraca(24.3%)
occurringfrequentlyinthecollectedfaeces.Thepreyfoundthe
mostfrequentlyfor thisspeciesbelongedtothegenusGlomeris
(75.6%).
AbouthalfofG.pyrenaicuspreybelongedtoEphemeroptera,
Ple-copteraandTrichopteraorderswhileonly16.6%ofpreybelongedto
theseordersforN.fodiens.Theproportionofexclusivelyterrestrial
preywashigherforN.fodiens(70.8±29.4%)thanforG.pyrenaicus
(20.6±30.5%),whiletheproportionofpreywithaquaticand
ter-restrialstageswashigherforG.pyrenaicus(74.6±31.4%)thanforN.
fodiens(26.1±28.3%).Exclusivelyaquaticpreyweremarginalfor
bothspecies.
Amongthe309identifiedgenerainthefaecesofbothmammals,
only90generawereconsumedbybothG.pyrenaicusandN.fodiens.
Thepreytaxaconsumedbyonlyoneofthetwospeciesweremostly
exclusivelyterrestrialgenera(52and80%inG.pyrenaicusandN.
fodiensfaeces,respectively).
Preycompositionoffaeceswassignificantlydifferentbetween
G.pyrenaicusandN.fodiensonthefirstaxisoftheCA(Student:
t=14.2,df=149.2,p<0.001).Thisdifferencecouldbeduetothe
majorityofterrestrialpreyinN.fodiensfaecesincontrastwithprey
withbothaquaticandterrestrialstagesfoundmorefrequentlyin
G.pyrenaicusfaeces(Fig.2).
Aquaticmacroinvertebratesavailability
Inthe27 siteswherestreaminvertebratesweresampled,G.
pyrenaicusandN.fodiensconsumedrespectively81and88
inver-tebratefamiliesintotaloutofwhich40and25familiesincluded
invertebrateswithatleastoneaquaticstageintheirlifecycle.
Fromstreaminvertebratessamples,atotalof51different
inver-tebratefamilieswereidentified(27±5persite)amongwhich42
familiesofInsecta,1Malacostracafamily,1Hydracarinafamily,2
Gastropodafamilies,1Bivalviafamily,1flatwormfamily,2
Clitel-latafamiliesand1Nematodesfamily.Insectataxahadthehighest
meandensitiesintheSurbersamples(Fig.3a).
Nineinvertebratefamilies(Rhagionidae,Caenidae,
Mermithi-dae, Planariidae, Leptoceridae, Gyrinidae, Calopterygidae,
Hydrophilidae,Psephenidae)wereexcludedfromfurtheranalyses
as they were found in the Surber samples and in faeces with
FOstream and FOdiet below 5%. Conversely, one-quarter of the
invertebratefamilieswithatleastoneaquaticstagefoundinthe
faecesofbothG.pyrenaicusandN.fodienswerenotfoundinthe
invertebratessamples.Thisconcerned9familiesoutof40forG.
pyrenaicusand5outof25familiesforN.fodiens.However,these
familieshada FOdietbelow10%inbothdiets,exceptthefamily
Anthomyiidae(20%inG.pyrenaicusdietand10.5% inN.fodiens
diet)andThaumaleidae(10.5%inN.fodiensdiet),andwerethus
alsoexcludedfromfurtheranalyses.
Densitiesofavailableinvertebrateswerenotsignificantly
differ-entbetweentheGalemysalonesites,theNeomysalonesitesandthe
Galemys+Neomyssites(Wilcoxonsign-rankedtests:alladjusted
p>0.05)allowingthecomparisonofinvertebratescontentin
fae-cescollectedinthedifferentcategoriesofsites.Forty-sixfamilies
and 50familieswereavailablein Galemysalone sitesand
Gale-mys+Neomyssitesrespectively,including45familiescommonto
bothtypesofsites.
Preyselectivitybetweenthetwomammals
Overall,themostfrequentlyconsumedpreybyG.pyrenaicus
corresponded to the most frequent and abundant invertebrate
taxa in streams (Fig. 3a and b). Seven out of the nine most
abundantpreywereconsumedinaccordancewiththefrequency
of occurrence and density estimated in the streams
Table1
Preytaxaidentifiedwithpositivematches(≥80%)from184faecesofG.pyrenaicusand78faecesofN.fodienscollectedinthestudyarea.Thefrequencyofoccurrence(%of faecescontainingtheprey)isdisplayed.SeeAppendixAinthesupplementarymaterialforthefulllistoftaxaatthefamilyandgenuslevel.*indicatesmisidentifiedtaxaby geneticdatabases.
Classes Frequencyofoccurrence(%) Orders Frequencyofoccurrence(%)
G.pyrenaicus N.fodiens G.pyrenaicus N.fodiens
Insecta 93.5 83.3 Ephemeroptera 71.2 32.1 Diptera 65.8 50.0 Trichoptera 63.6 30.8 Plecoptera 52.7 35.9 Coleoptera 14.1 43.6 Hemiptera 3.8 17.9 Lepidoptera 3.3 11.5 Hymenoptera 1.6 9.0 Odonata 1.1 2.6 Dermaptera 0.5 1.3 Neuroptera 0.0 5.1 Orthoptera 0.0 1.3 Mecoptera 0.0 3.8 Archaeognatha 0.0 1.3 Malacostraca 23.4 24.4 Amphipoda 20.7 19.2 Isopoda 3.3 6.4 Decapoda 0.5 0.0 Diplopoda 21.7 89.7 Glomerida 15.8 80.8 Polydesmida 7.1 53.8 Julida 4.3 43.6 Chordeumatida 2.7 16.7 Arachnida 8.2 47.4 Araneae 3.3 25.6 Opiliones 2.2 23.1 Sarcoptiformes 1.1 2.6 Trombidiformes 0.5 1.3 UndeterminedArachnida* 0.5 1.3 Mesostigmata 0.5 0.0 Clitellata 6.0 17.9 Haplotaxida 6.0 17.9 Gastropoda 4.3 25.6 Stylommatophora 3.3 25.6 Hygrophila 1.1 0.0 Collembola 1.1 19.2 Entomobryomorpha 1.1 17.9 Poduromorpha 0.0 2.6 Symphypleona 0.0 1.3 Amphibia 0.5 0.0 Anura 0.5 0.0 Chilopoda 0.5 9.0 Lithobiomorpha 0.5 7.7 Geophilomorpha 0.0 1.3 Maxillopoda 0.5 0.0 Sessilia 0.5 0.0
UndeterminedNemerta* 0.0 2.6 UndeterminedNemerta* 0.0 2.6
Fig.2.CorrespondenceAnalysis(CA)computedonthefrequentlyusedpreytaxa(FOdiet>5%)derivedfrom184G.pyrenaicusand78N.fodiensfaeces.(a)Projectionsofprey taxaonthefirst(8.4%)andsecond(4.2%)axisoftheCA:preytaxaaredepicteddifferentlyaccordingtothetypeofhabitatduringtheirlifecycle.(b)ProjectionsofG.pyrenaicus (“Galemys”)andN.fodiens(“Neomys”)faecesonthefirstfactorialplane.
Fig.3. PreyselectivityforG.pyrenaicus(Galemys;darkbarsfor25Galemyssites)andN.fodiens(Neomys,lightbarsfor19Neomyssites).(a)Meandensitiesofinvertebrate preyintheSurbersamplesforeachsiteinGalemysandNeomyssites.(b)Frequencyofoccurrenceofprey(FOdiet)incollectedfaeces.(c)Ivlev’selectivityindex.Theelectivity indexisbasedonthefrequencyofpreyinfaecesrelativetopreyavailableinstreamswherethefaeceswerecollected.Blacktrianglesidentifyexclusivelyaquaticinvertebrate familiesasopposedtofamilieswithouttrianglesthatincludeinvertebrateswithatleastoneaquaticstageandoneterrestrialstageintheirlifecycle.
Simulidae,Nemouridae;Fig.3c). N.fodiensconsumed
Gammari-daeaccordingtotheirrelativeamountinthestreamsandavoided
theothermostabundantfamilies.
However,mostinvertebratefamiliessampledinstreamswere
avoidedbyG.pyrenaicusandN.fodienswithnegativeIvlev’svalues
below −0.25 for 22 and 31 available taxa out of 41,
respec-tively(Fig.3c).BothG.pyrenaicusandN.fodiensseemedtoavoid
Brachycentridae and Elmidae prey in spite of their high
den-sities in streams. An active selection of invertebrate prey was
highlightedforsevenfamiliesforG.pyrenaicus(Cordulegastridae,
Blephariceridae,Tipulidae,Scirtidae,Limnephilidae,Psychodidae,
Ephemeridae)andfivefamiliesforN.fodiens(Cordulegastridae
Dix-idae,Limnephilidae,Scirtidae,Tipulidae).
Trophicoverlapbetweenmammals
ThedietaryoverlapbetweenG.pyrenaicusandN.fodienswas
moderateatthegenus(Piankaindex=0.4)andfamily(0.5)levels.
NodifferenceintheFOdietofthepreyfoundinG.pyrenaicus
faeces wasdetectedat thefamily level betweenthesites with
orwithoutthepresenceofN.fodiens(Wilcoxonsign-rankedtest:
V=2748,p=0.3).Whenconsideringallconsumedpreyfamilies,72
wereconsumedbyG.pyrenaicusinGalemysalonesites(38with
FOdiet>5%)and86inGalemys+Neomyssites(44withFOdiet>5%).
Only47 taxawereconsumed inboth categoriesof sites.Ratios
betweennumbersofrareprey(i.e.,FOdiet<5%)andfrequentprey
(i.e.,FOdiet>5%)inG.pyrenaicusdietweresimilar(around50%)in
GalemysalonesitesandinGalemys+Neomyssites.
Discussion
NovelinsightsintothedietofG.pyrenaicusandN.fodiensduring
summer
This study provides additional support to the usefulness of
moleculargenetictoolsindietanalyses,asitprovidesanenhanced
identificationofpreycontentfromindirectsignsofpresence(i.e.,
faeces)ofpredators.Theproportionoffaecesmisidentificationin
thefield(i.e.,belongingtootherhostspecies:11%)orunidentified
faecesinthelaboratory(29%)areconsistentwithpreviousstudies
ofG.pyrenaicusdiet(Gillet,2015)confirmingtheissuesarisingfrom
visualidentificationoffaecesandDNAextractionfromdegraded
faeces.
Fromthe194invertebrategeneraidentifiedinthefaecesofG.
pyrenaicusinthepresentstudy,only61werecommonwiththe156
generaidentifiedinapreviousstudyusingsimilargeneticmethods
butconductedinthewholeFrenchPyrenees(Gillet,2015;Biffietal.,
2017).Newlyidentifiedpreyincludedanamphibian,aquaticand
terrestrialsnails,leeches,earwigs,Hydracarina,Chilopoda,
Maxil-lopodaandCollembola.
Thisstudyalsoprovidesunprecedentedinsightsintothe
sum-mertrophicnicheofN.fodiens,byidentifyingpreyidentityatthe
familyandgenuslevelsforthefirsttime(butseeattheorderlevel:
Castiénand Gosálbez,1999; Churchfield,1985;Churchfield and Rychlik,2006).Inaccordancewithpreviousstudiesconductedat
ahighertaxonomiclevel,wefoundthatN.fodiensfedon
terres-trialpreyatalargerextentthanG.pyrenaicus,withadominance
Ephemeroptera,PlecopteraandTrichopterawaslow(about17%)
comparedtoG.pyrenaicus(55%).
Thedensity and species composition of streaminvertebrate
communitiesdescribedinthisstudyareconsistentwithprevious
reportsoffreshwaterinvertebratefaunaoftheFrenchPyrenees
(e.g.,Brownetal.,2006;Finnetal.,2013).BothG.pyrenaicusand
N.fodiensexhibitedrathernon-selectiveforagingstrategiesasthe
mostfrequentlyconsumedinvertebrateswerealsothemost
fre-quentandabundantinthestreams.Thissupportedageneralistdiet
forbothspeciesinthestudyareaalthoughasignificantnumberof
taxawereconsumedinlowerorhigherfrequenciesthanexpected
(Fig.3).
N.fodiensavoidedahighernumberofpreyandactivelyselected
asmallernumberofpreythanG.pyrenaicusintheaquatic
envi-ronment.Thissupportsthedifferenceintrophicnichesbetween
thetwospecies,withmoreterrestrialtaxainN.fodiensdiet
com-paredwith G.pyrenaicus. This latter species hasmorphological
features adapted to livein aquatic environments (e.g.,webbed
feet,Palmeirim,1983;Puisségur,1935;highdivingskills:1–4min,
Richard and Micheau, 1975 compared to 3–24s for N. fodiens,
Lardet,1988;Mendes-SoaresandRychlik,2009)thatlikelyresult
inabetterefficiencyinthecaptureofaquaticpreythanN.fodiens.
Foragingefficiencymaydeterminepreyselectivityinorderto
maximiseindividualsuccess.Itdependsonthebalancebetween
theenergyprovidedbypreyconsumptionandtheenergeticcosts
offoragingunderwater. Themostvaluableresourcesmaythus
correspond to easy-to-catch prey with low mobility (e.g.,
Tri-choptera)and/orhighabundance(e.g.,Gammaridae),soft-bodied
preythatcanbecompletelydigested(e.g.,incontrastwithsmall
and chitinous Hydracarina or Coleoptera,Costa et al., 2015) or
largeprey(Bertrand,1994).Inthisstudy,invertebrateswithhard
andchitinousbodies(e.g.Elmidae,HydraenidaeHydracarina)and
hard casesmadeof wood(Brachycentridae) or mineral
materi-als(Glossossomatidae,Sericostomatidae)wereavoidedbybothG.
pyrenaicusandN.fodiens.
SomeofourresultsaboutpreyselectivitybyG.pyrenaicus
con-tradictpreviousobservationsbyBertrand(1994)andSantamarina
(1993).Forinstance,Rhyacophilidaewereavoidedand
Limnephil-idaeactivelyselectedbyG.pyrenaicusinourstudywhereasthe
oppositewas observedby Bertrand(1994). Chironomidaewere
avoided and Sericostomatidae activelyselected in Santamarina
(1993)whereaswefoundtheoppositepatternforthesefamilies.
Thesedifferencesmaybeduetodifferentsamplingmethods
(stom-achanalysesvs.digestedremainsinfaecesvs.molecularanalyses)
aswellassamplesizes.Theymayalsoreflectthegeneralistdietof
G.pyrenaicusthatmayvarybetweenregions(i.e.,acatchmentof
Spainvs.thewholeFrenchPyreneesvs.asub-regionoftheFrench
Pyrenees)andseasons(Santamarina,1993).Finally,the
variabil-ityinlife-historytraitswithinandbetweeninvertebratespeciesof
thesamefamilyorgenus(e.g.,developmentstage,bodysize,local
adaptations)mayinfluencetheirexposuretopredation.
Trophicoverlapbetweenmammals
Thefaecesandaquaticinvertebratessamplingswereconducted
duringsummerwhenmany late instarsof aquaticinsects have
emergedfrommountainstreamsandlefttheaquaticenvironment
(Fürederet al.,2004).Invertebratecommunities are dominated
bysmall-sizedinvertebratesforthisperiod.Thisimpoverishment
inpreydiversityanddensitymayexacerbatethetrophicoverlap
betweentheirpredators,suchasG.pyrenaicusandN.fodiens,
com-paredtotherestoftheyear.Inspiteofthis,thePianka’sindex
oftrophicoverlapbetweenthetwospeciesequalled0.4
indicat-ingasignificantbutratherlowoverlapinthesummerdiets.This
valueisconsistentwithapreviousestimateofoverlap(Castiénand
Gosálbez,1999).
G.pyrenaicusconsumedalargernumberofdifferentpreyinthe
siteswhereN.fodienswasalsopresent.Thisincreasewasdrivenby
ahigherconsumptionofterrestrialtaxabutwasnotlinkedtoany
increaseinrarepreyconsumptionasratiosofrareprey/frequent
preyweresimilarinsiteswithorwithoutN.fodiens.However,no
significantdifferencewasfoundindietcompositionofG.pyrenaicus
betweensiteswhereN.fodienswasdetectedornot.Alltheseresults
suggestnoevidenceofashiftofG.pyrenaicusdiettowards
sub-optimalpreyinthepresenceofanotherinsectivorespecieswith
similarfeedingstrategies.
Theabsenceof shiftin G.pyrenaicusdiet inthepresence of
N.fodienscouldresultfromthenon-limitingtrophicresourcesin
streamsand/oronterrestrialhabitatsduringsummer.Actually,the
measureofnicheoverlapmaymostlyreflectthepotential
compe-titioninthecaseoflimitingresources(Abrams,1980).
ItcouldalsobeduetoresourcepartitioningbetweenG.
pyre-naicusandN.fodiens.First,asynchronyofseasonalordailyactivity
periodsmayplayanimportantroleinresourcepartitioningand
facilitatecohabitation(e.g.,Harringtonet al.,2009).A temporal
shiftofnichehasbeenobservedinherbivorestoreducethelength
ofthetrophic overlapatwaterholesduringaridseasons(Valeix
etal.,2007).RadiotrackingconductedonafewindividualsofG.
pyrenaicusandN.fodiensseemstoindicatepolyphasicactivity
pat-terns(Lardet,1988;Meleroetal.,2014;Stone,1987)withasimilar
timingofactivityduringdayandnightperiods.Thissuggeststhat
thismechanismisprobablynotinvolvedintheresource
partition-ingbetweenthesetwospecies.
Second,successfulresourcepartitioningmayberelatedtohow
thepredatorsaccesstofoodresourcesaccordingtotheirforaging
strategies. Thesegregationof trophic nichesbased on
differen-tiation of foraging modes and foraging micro-habitats is well
documentedamongshrews(e.g.,ChurchfieldandRychlik,2006;
ChurchfieldandSheftel,199).First,G.pyrenaicusandN.fodienshave
differentbodysize(i.e.,10–15cmvs.7–9cmwithoutthetailforG.
pyrenaicusandN.fodiens,respectively)andmass(i.e.,50–60gvs.
8–17gforG.pyrenaicusandN.fodiens,respectively).Duetothis
differenceofsize,thetwospeciescouldtargetcontrasted
develop-mentstageandpreysizewithinagiventaxa,asitisknowntoallow
resource partitioning among shrew species (Churchfield et al.,
1999).Second,theuseofdifferenthabitatstratawasalsoidentified
insmallmammalcommunitiestomodulatetheintensityof
inter-specificcompetition(CastiénandGosálbez,1999;Churchfieldand
Sheftel,1994;Rychlik,1997).Specializationonparticular
micro-habitatsinstreamsisalsoknownforothersemi-aquaticmammals.
Forinstance,theplatypusOrnithorhynchusanatinusdoesnot
allo-cateanequalforagingefforttobenthicmacroinvertebratesacross
allhabitats,itspreferencegoingtopoolsandlittoralmarginsrather
thanriffles(McLachlan-Troupetal.,2010).Theoveralllow
selec-tivity,activeselectionoravoidanceofdifferenttaxa,togetherwith
thedistincttrophicnicheweobservedinthewholestudyareain
G.pyrenaicusandN.fodiens,suggestthattheyusedistinctforaging
micro-habitats(CastiénandGosálbez,1999)withinthestreamand
riparianmosaic.Otheraquaticpredatorssuchasthebrowntrout
S.truttacouldhavehigherdietoverlapwithG.pyrenaicusdueto
theirbroaduseofhabitatswithinstreamsandhighlyopportunistic
feedingstrategies(GillerandGreenberg,2015).
Methodologicalconsiderationsandperspectives
Whilemolecularmethodsprovideanenhancedidentificationof
preycomparedtotraditionalmethods(i.e.,visualinspectionofprey
remainsingutsorfaeces),theyalsohavesomeshortcomings.First,
identifyingmanyadditionalpreytaxawithmoleculartools
com-paredtotraditionalmethodsisconsistentwithClareetal.(2014)
who suggest that preyoccurrence data obtainedfrom
preyandoverestimaterareprey(Clareetal.,2014;Krügeretal.,
2014).Nevertheless,20taxaidentified,suchasCollembolaor
sev-eralgenusoftheTachinidaefamily,areunlikelytobedirectpreyof
G.pyrenaicusorN.fodiens,astheyarepartofthesoilmicrofaunaor
areinvertebrateparasites.Othertaxa,suchassomeAnthomyiidae
(e.g.Polietes)orsmallterrestrialColeoptera,mayhavebeen
col-lectedwiththefaecesastheymaydevelopatthelarvalstageorfeed
onscat.Finally,taxaidentifiedaspreycouldhavebeenpassively
ingestedwiththeconsumptionofpredatororscavenger
inverte-brates(e.g.Trichoptera,Plecoptera,Odonata).Thiscontributesto
thedebateaboutthehighsensitivityofnext-generation
sequenc-ingmethodsandthedetectionofsecondarypredation(Sheppard
etal.,2005).
Second,moleculardatadonotgiveinformationonthestage
orsizeatwhichpreyareconsumed.Thisisaparticularlystrong
limitationwhen(i)feedingstrategiesareadapted tothemouth
morphologyandsizeofthepredatoror(ii)preyexhibit
impor-tantvariationinhabitatuseduringtheirlifecyclesuchasmany
aquaticinvertebratespecies.Giventherelativeshortnessofthe
terrestrialstageincomparison withtheaquaticstagefor many
invertebratespecies,theprobabilitythat invertebratesfoundin
thefaeceswereconsumedattheaquaticstage(oratthetimeof
emergence)remainshigh,whichissupportedbyprevious
morpho-logicalidentificationofpreyitemsinfaecalsamples(Trichoptera;
e.g.,Bertrand,1994;DuPasquierandCantoni,1992).The
combi-nationofmolecularandtraditionalmethodsofpreyidentification
seemsthusimportanttobringdetailedinformationontheidentity,
sizeandstageofeatenprey.
Third,themoleculardataweuseddonotallowaquantitative
assessmentofthepreyconsumed(e.g.relativeandabsolute
abun-dance/biomass ofdifferenttaxa). Klareetal. (2011)stated that
suchqualitativeestimates(i.e.,presence-absenceandfrequencyof
occurrenceofprey)tendtooverestimatenichebreadthanddietary
overlapsbetweenspeciesleadingpotentiallytounreliable
conclu-sions.However,theyalsopointedoutthatsuchbiasremainslow
whenthedietsofthecomparedspeciesarecomposedofsimilar
taxonomicgroupsofprey,whichisthecaseforG.pyrenaicusandN.
fodiens.Moreover,thelikelybiasindietestimationduetotheuse
offrequenciesofoccurrenceissimilarbetweenG.pyrenaicusand
N.fodienswhichenablesareliablequalitativecomparisonofdiet
overlapandpreyselectionpatternsintermsofpreyidentity.Thisis
supportedbythePiankaindexoftrophicnicheoverlapquantified
inthisstudywhichisconsistentwithpreviousobservationsbased
onquantitativemethodsofpreyestimates.
Despitethoselimitations,molecularmethodsprovedefficiency
intheidentificationofahighlydiversedietforG.pyrenaicusand
N.fodiens that werehighlighted toexhibit a generalist feeding
behaviour.Thissuggeststhattheidentityofpreytaxaisnotthe
most important criteria for both mammals prey selection and
thattheymayexhibitsometolerancetovariationin prey
com-munitycomposition.Hence,stream communitychanges dueto
anthropogenicimpactsonriverecosystemsshouldhavemoderate
consequences(Costaetal.,2015)aslongasfoodresourcesremain
abundantenoughintheirrespectiveforaginghabitats.However,
pressuresonpreycommunitiescouldincreasethetrophicoverlap
betweenthetwospeciesaswellaswithotherspotential
competi-tors.
ThisstudyalsostressedthatthepresenceofN.fodiensdidnot
affectG.pyrenaicuspreyselection.However,thehigherproportion
ofexclusivelyterrestrialpreyinN.fodiensdietfoundhere
com-paredtootherdietarystudies(e.g.Churchfield,1984;DuPasquier
andCantoni, 1992)maysuggesta shiftinthedietofN.fodiens
towardsamoreterrestrialnicheinpresenceofG.pyrenaicus.The
verysmallnumberofsiteshostingN.fodienswithoutG.pyrenaicus
didunfortunatelypreventustotestforthispotentialcompetitive
interaction.
Identifyingmorefinelytheforagingmicro-habitatsofG.
pyre-naicusandN.fodiens,comparingthedietofN.fodiensinpresence
andabsenceofG.pyrenaicusandobservingtheirbehavioural
inter-actionsinthefieldalongtheyearshouldbefurtherinvestigatedin
thelightofterrestrialinvertebratessamplingandtheuseofdietary
quantitative data (e.g.abundance or biomass of prey in scats).
Thiswouldimproveourknowledgeaboutmechanisms
facilitat-ingtheircohabitationorcausingpotentialcompetitiveinteractions
andtheirvulnerabilitytohabitatandtrophicresourcealterations.
Acknowledgements
Wearegratefultoallpeoplewhohelpedcollectingdatainthe
field:C.Dupuyds,M.Alvarez.,C.Lauzeral,F.Colas,F.JulienandV.
Lacaze.Wealsothankthe“Conservatoired’EspacesNaturels
Midi-Pyrénées”(CEN-MP),especiallyM.NémozandF.Blanc,fortheir
preciousadviceatmanystepsofthestudy.Thisstudywasfunded
byANRT(Cifren◦2011/1018),EDF(ElectricitédeFrance)andthe
EuropeanUnion(FEDER)inthecontextoftheLIFE+Nature
pro-grammedevotedtoG.pyrenaicus(LIFE13NAT/FR/000092).M.Biffi
wassupportedbyaPhDfellowshipgrantedbythe“EcoleDoctorale
Sciencesdel’Univers,del’Environnementetdel’Espace”(SDU2E)
attheUniversityofToulouse.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in
theonlineversion,athttp://dx.doi.org/10.1016/j.mambio.2017.09.
001.
References
Abrams,P.,1980.Somecommentsonmeasuringnicheoverlap.Ecology61,44–49. André,A.,Mouton,A.,Millien,V.,Michaux,J.,2017.Livermicrobiomeof
Peromyscusleucopus,akeyreservoirhostspeciesforemerginginfectious diseasesinNorthAmerica.Infect.Genet.Evol.52,10–18.
Aymerich,P.,Gosàlbez,J.,2015.Evidenciasderegresiónlocaldeldesmánibérico (Galemyspyrenaicus)enlosPirineosmeridionales.Galemys27,31–40. Aymerich,P.,Gosálbez,J.,2004.Laprospeccióndeexcrementoscomometodología
paraelestudiodeladistribucióndelosmusga ˜nos(Neomyssp.).Galemys16, 83–90.
Bertrand,A.,1994.Répartitiongéographiqueetécologiealimentairedudesman desPyrénées,Galemyspyrenaicus(Geoffroy,1811)danslesPyrénées franc¸aises.Thèsededoctorat.UniversitéPaulSabatierdeToulouse(50pp). Biffi,M.,Charbonnel,A.,Buisson,L.,Blanc,F.,Némoz,M.,Laffaille,P.,2016.Spatial
differencesacrosstheFrenchPyreneesintheuseoflocalhabitatbythe endangeredsemi-aquaticPyreneandesman(Galemyspyrenaicus).Aquat. Conserv.Mar.Freshw.Ecosyst.26,761–774.
Biffi,M.,Gillet,F.,Laffaille,P.,Colas,F.,Aulagnier,A.,Blanc,F.,Galan,M., Tiouchichine,M.-L.,Némoz,M.,Buisson,L.,Michaux,J.R.,2017.Novelinsights intothedietofthePyreneandesman(Galemyspyrenaicus)using
next-generationsequencingmolecularanalyses.J.Mammal.,http://dx.doi.org/ 10.1093/jmammal/gyx070.
Brown,L.E.,Milner,A.M.,Hannah,D.M.,2006.Stabilityandpersistenceofalpine streammacroinvertebratecommunitiesandtheroleofphysicochemical habitatvariables.Hydrobiologia560,159–173.
Cantoni,D.,1993.Socialandspatialorganizationoffree-rangingshrews,Sorex coronatusandNeomysfodiens(Insectivora,Mammalia).Anim.Behav.45, 975–995.
Castién,E.,Gosálbez,J.,1995.DietofGalemyspyrenaicus(Geoffroy,1811)inthe NorthoftheIberianpeninsula.Neth.J.Zool.45,422–430.
Castién,E.,Gosálbez,J.,1999.Habitatandfoodpreferencesinaguildof insectivorousmammalsintheWesternPyrenees.ActaTheriol.44,1–13. Charbonnel,A.,D’Amico,F.,Besnard,A.,Blanc,F.,Buisson,L.,Némoz,M.,Laffaille,
P.,2014.Spatialreplicatesasanalternativetotemporalreplicatesfor occupancymodellingwhensurveysarebasedonlinearfeaturesofthe landscape.J.Appl.Ecol.51,1425–1433.
Charbonnel,A.,Buisson,L.,Biffi,M.,D’Amico,F.,Besnard,A.,Aulagnier,S.,Blanc,F., Gillet,F.,Lacaze,V.,Michaux,J.R.,Némoz,M.,Pagé,C.,Sanchez-Perez,J.M., Sauvage,S.,Laffaille,P.,2015.Integratinghydrologicalfeaturesandgenetically validatedoccurrencedatainoccupancymodellingofanendemicand endangeredsemi-aquaticmammal,Galemyspyrenaicus,inaPyrenean catchment.Biol.Conserv.184,182–192.
Charbonnel,A.,Laffaille,P.,Biffi,M.,Blanc,F.,Maire,A.,Némoz,M.,Sanchez-Perez, J.M.,Sauvage,S.,Buisson,L.,2016.Canrecentglobalchangesexplainthe
dramaticrangecontractionofanendangeredsemi-aquaticmammalspeciesin theFrenchPyrenees?PLoSOne11,e0159941.
Churchfield,S.,Rychlik,L.,2006.DietsandcoexistenceinNeomysandSorexshrews inBiałowie ˙zaforest,easternPoland.J.Zool.269,381–390.
Churchfield,S.,Sheftel,B.I.,1994.Foodnicheoverlapandecologicalseparationina multi-speciescommunityofshrewsintheSiberiantaiga.J.Zool.234,105–124. Churchfield,S.,Nesterenko,V.A.,Shvarts,E.A.,1999.Foodnicheoverlapand
ecologicalseparationamongstsixspeciesofcoexistingforestshrews (Insectivora:soricidae)intheRussianFarEast.J.Zool.248,349–359. Churchfield,S.,1984.Aninvestigationofthepopulationecologyofsyntopic
shrewsinhabitingwater-cressbeds.J.Zool.Lond.204,229–240.
Churchfield,S.,1985.ThefeedingecologyoftheEuropeanwatershrew.Mammal Rev.15,13–21.
Clare,E.L.,Symondson,W.O.C.,Broders,H.,Fabianek,F.,Fraser,E.E.,MacKenzie,A., Boughen,A.,Hamilton,R.,Willis,C.K.R.,Martinez-Nu ˜nez,F.,Menzies,A.K., Norquay,K.J.O.,Brigham,M.,Poissant,J.,Rintoul,J.,Barclay,R.M.R.,Reimer,J.P., 2014.ThedietofMyotislucifugusacrossCanada:assessingforagingqualityand dietvariability.Mol.Ecol.23,3618–3632.
Costa,A.,Salvidio,S.,Posillico,M.,Matteucci,G.,DeCinti,B.,Romano,A.,2015. Generalisationwithinspecialization:inter-individualdietvariationintheonly specializedsalamanderintheworld.Sci.Rep.5,13260.
DuPasquier,A.,Cantoni,D.,1992.Shiftsinbenthicmacroinvertebratecommunity andfoodhabitsofwatershrew,Neomysfodiens(Soricidae,Insectivora).Acta OEcologicaOEcal.Gener.13,81–99.
Dudgeon,D.,Arthington,A.H.,Gessner,M.O.,Kawabata,Z.-I.,Knowler,D.J., Lévêque,C.,Naiman,R.J.,Prieur-Richard,A.-H.,Soto,D.,Stiassny,M.L.J., Sullivan,C.A.,2006.Freshwaterbiodiversity:importance,threats,statusand conservationchallenges.Biol.Rev.81,163–182.
Edgar,R.C.,2010.SearchandclusteringordersofmagnitudefasterthanBLAST. Bioinforma.Oxf.Engl.26,2460–2461.
Escoda,L.,González-Esteban,J.,Gómez,A.,Castresana,J.,2017.Usingrelatedness networkstoinfercontemporarydispersal:applicationtotheendangered mammalGalemyspyrenaicus.Mol.Ecol.,http://dx.doi.org/10.1111/mec. 14133.
Füreder,L.,Wallinger,M.,Burger,R.,2004.Longitudinalandseasonalpatternof insectemergenceinalpinestreams.Aquat.Ecol.39,67–78.
Fernandes,M.,Herrero,J.,Aulagnier,S.,Amori,G.,2008.GalemysPyrenaicus.The IUCNRedListofThreatenedSpecies.Version2014.2(<www.iucnredlist.org> Downloadedon1June2014)http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS. T8826A12934876.en.
Finn,D.S.,Khamis,K.,Milner,A.M.,2013.Lossofsmallglacierswilldiminishbeta diversityinPyreneanstreamsattwolevelsofbiologicalorganization.Glob. Ecol.Biogeogr.22,40–51.
Giller,P.,Greenberg,L.,2015.Therelationshipbetweenindividualhabitatuseand dietinbrowntrout.Freshw.Biol.60,256–266.
Gillet,F.,Tiouchichine,M.-L.,Galan,M.,Blanc,F.,Némoz,M.,Aulagnier,S., Michaux,J.R.,2015.AnewmethodtoidentifytheendangeredPyrenean desman(Galemyspyrenaicus)andtostudyitsdiet,usingnextgeneration sequencingfromfaeces.Mamm.Biol.80,505–509.
Gillet,F.,2015.GénétiqueetbiologiedelaconservationdudesmandesPyrénées (Galemyspyrenaicus)enFrance.Thèsededoctorat,UniversitéPaulSabatierde Toulouse(France)/UniversitédeLiège(Belgique),228pp.
Gisbert,J.,García-Perea,R.,2014.Historiadelaregresióndeldesmánibérico Galemyspyrenaicus(É.GeoffroySaint-Hilaire,1811)enelSistemaCentral (PenínsulaIbérica).Conservationandmanagementofsemi-aquaticmammals ofsouthwesternEurope.MunibeMonogr.Nat.Ser.,3.,pp.19–35.
Greenwood,A.,Churchfield,S.,Hickey,C.,2002.Geographicaldistributionand habitatoccurrenceofthewatershrew(Neomysfodiens)intheWealdof South-EastEngland.MammalRev.32,40–50.
Haberl,W.,2002.Foodstorage,preyremainsandnotesonoccasionalvertebrates inthedietoftheEurasianwatershrew,Neomysfodiens.FoliaZool.51,93–102. Harrington,L.A.,Harrington,A.L.,Yamaguchi,N.,Thom,M.D.,Ferreras,P.,
Windham,T.R.,Macdonald,D.W.,2009.Theimpactofnativecompetitorsonan alieninvasive:temporalnicheshiftstoavoidinterspecificaggression?Ecology 90,1207–1216.
Hutchinson,G.E.,1957.Concludingremarks.ColdSpringHarb.Symp.Quant.Biol. 22,415–427.
Ivlev,V.S.,1961.ExperimentalEcologyofFeedingFishes.YaleUniversityPress, NewHaven,Connecticut(302pp).
Keckel,M.R.,Ansorge,H.,Stefen,C.,2014.Differencesinthemicrohabitat preferencesofNeomysfodiens(Pennant1771)andNeomysanomalusCabrera 1907inSaxony.Germany.ActaTheriol.59,485–494.
Klare,U.,Kamler,J.F.,Macdonald,D.W.,2011.Acomparisonandcritiqueof differentscat-analysismethodsfordeterminingcarnivorediet.Mammal Review41,294–312.
Krüger,F.,Clare,E.L.,Greif,S.,Siemers,B.M.,Symondson,W.O.C.,Sommer,R.S., 2014.Anintegrativeapproachtodetectsubtletrophicnichedifferentiationin thesympatrictrawlingbatspeciesMyotisdasycnemeandMyotisdaubentonii. Mol.Ecol.23,3657–3671.
Lardet,J.-P.,1988.Spatialbehaviourandactivitypatternsofthewatershrew Neomysfodiensinthefield.ActaTheriol.33,293–303.
Life+Desman,2013.TechnicalApplicationForms–ConservationoftheFrench PopulationsofGalemysPyrenaicusandItsPopulationsontheFrenchPyrénées (LIFE13NAT/FR/000092).274p.
McLachlan-Troup,T.A.,Dickman,C.R.,Grant,T.R.,2010.Dietanddietaryselectivity oftheplatypusinrelationtoseason,sexandmacroinvertebrateassemblages.J. Zool.280,237–246.
Melero,Y.,Aymerich,P.,Luque-Larena,J.J.,Gosálbez,J.,2012.Newinsightsinto socialandspaceusebehaviouroftheendangeredPyreneandesman(Galemys pyrenaicus).Eur.J.Wildl.Res.58,185–193.
Melero,Y.,Aymerich,P.,Santulli,G.,Gosálbez,J.,2014.Activityandspacepatterns ofPyreneandesman(Galemyspyrenaicus)suggestnon-aggressiveand non-territorialbehaviour.Eur.J.Wildl.Res.60,707–715.
Mendes-Soares,H.,Rychlik,L.,2009.Differencesinswimminganddivingabilities betweentwosympatricspeciesofwatershrews:Neomysanomalusand Neomysfodiens(Soricidae).J.Ethol.27,317–325.
Morueta-Holme,N.,Fløjgaard,C.,Svenning,J.-C.,2010.Climatechangerisksand conservationimplicationsforathreatenedsmall-rangemammalspecies.PLoS One5,e10360.
Muséumnationald’Histoirenaturelle[Ed].2003–2017.InventaireNationaldu PatrimoineNaturel.https://inpn.mnhn.fr.Accessed15May2016. Némoz,M.,Bertrand,A.,Sourie,M.,Arlot,P.,2011.Afrenchconservationaction
planforthepyreneandesmanGalemyspyrenaicus.Galemys23,47–50. OPIE-Benthos,2017.OfficePourlesInsectesetleurEnvironnement
(http://www.opie-benthos.fr/opie/insecte.php.Accessed15May2016). Palmeirim,J.M.,1983.Galemyspyrenaicus.Mamm.Species207,1–5. Parry,G.S.,Bodger,O.,McDonald,R.A.,Forman,D.W.,2013.Asystematic
re-samplingapproachtoassesstheprobabilityofdetectingottersLutralutra usingspraintsurveysonsmalllowlandrivers.Ecol.Inform.14,64–70(The analysisandapplicationofspatialecologicaldatatosupporttheconservation ofbiodiversity).
Pianka,E.R.,1973.Thestructureoflizardcommunities.Annu.Rev.Ecol.Syst.4, 53–74.
Pianka,E.R.,1974.Nicheoverlapanddiffusecompetition.Proc.Natl.Acad.Sci.71, 2141–2145.
Pompanon,F.,Deagle,B.E.,Symondson,W.O.C.,Brown,D.S.,Jarman,S.N.,Taberlet, P.,2012.Whoiseatingwhat:dietassessmentusingnextgeneration sequencing.Mol.Ecol.21,1931–1950.
Puisségur,C.,1935.RecherchessurleDesmandesPyrénées.Bull.Soc.Hist.Nat. Toulouse67,163–227.
RCoreTeam,2014.R:ALanguageandEnvironmentforStatisticalComputing.R FoundationforStatisticalComputing,Vienna,Austriahttp://www.R-project. org/.
Ratnasingham,S.,Hebert,P.D.N.,2007.BOLD:thebarcodeoflifedatasystem (www.barcodinglife.org).Mol.Ecol.Notes7,355–364.
Richard,P.B.,Micheau,C.,1975.Lecarrefourtrachéendansl’adaptationdudesman desPyrénées(Galemyspyrenaicus)àlaviedulc¸aquicole.Mammalia39, 467–478.
Rychlik,L.,1997.Differencesinforagingbehaviourbetweenwatershrews:Neomys anomalusandNeomysfodiens.ActaTheriol.42,351–386.
Rychlik,L.,2000.Habitatpreferencesoffoursympatricspeciesofshrews.Acta Theriol.45(Suppl.1),173–190.
Santamarina,J.,Guitian,J.,1988.Quelquesdonnéessurlerégimealimentairedu desman(Galemyspyrenaicus)danslenord-ouestdel’Espagne.Mammalia52, 302–307.
Santamarina,J.,1993.Feedingecologyofavertebrateassemblageinhabitinga streamofNWSpain(Riobo;Ullabasin).Hydrobiologia252,175–191. Sheppard,S.K.,Bell,J.,Sunderland,K.D.,Fenlon,J.,Skervin,D.,Symondson,W.O.C.,
2005.DetectionofsecondarypredationbyPCRanalysesofthegutcontentsof invertebrategeneralistpredators.Mol.Ecol.14,4461–4468.
Stone,R.D.,1987.TheactivitypatternsofthePyreneandesman(Galemys pyrenaicus)(Insectivora:talpidae),asdeterminedundernaturalconditions.J. Zool.213,95–106.
Tachet,H.,Richoux,P.,Bournaud,M.,Usseglio-Polatera,P.,2000.Invertébrésd’eau douce.Systématique,Biologie,Ecologie,CNRSEditions,Paris.
Valeix,M.,Chamaillé-Jammes,S.,Fritz,H.,2007.Interferencecompetitionand temporalnicheshifts:elephantsandherbivorecommunitiesatwaterholes. Oecologia153,739–748.
Wisz,M.S.,Pottier,J.,Kissling,W.D.,Pellissier,L.,Lenoir,J.,Damgaard,C.F., Dormann,C.F.,Forchhammer,M.C.,Grytnes,J.-A.,Guisan,A.,Heikkinen,R.K., Høye,T.T.,Kühn,I.,Luoto,M.,Maiorano,L.,Nilsson,M.-C.,Normand,S., Öckinger,E.,Schmidt,N.M.,Termansen,M.,Timmermann,A.,Wardle,D.A., Aastrup,P.,Svenning,J.-C.,2013.Theroleofbioticinteractionsinshaping distributionsandrealisedassemblagesofspecies:implicationsforspecies distributionmodelling.Biol.Rev.88,15–30.