Any correspondence concerning this service should be sent to the repository administrator:
staff-oatao@inp-toulouse.fr
To link to this article:
DOI: 10.1016/j.jeurceramsoc.2012.08.005
Official URL:
http://dx.doi.org/10.1016/j.jeurceramsoc.2012.08.005
This is an author-deposited version published in:
http://oatao.univ-toulouse.fr/
Eprints ID: 8787
To cite this version:
Duluard, Sandrine and Paillassa, Aude and Puech, Laurent and Vinatier,
Philippe and Turq, Viviane and Rozier, Patrick and Lenormand, Pascal and
Taberna, Pierre-Louis and Simon, Patrice and Ansart, Florence Lithium
conducting solid electrolyte Li1.3Al0.3Ti1.7(PO4)3 obtained via solution
chemistry. (2013) Journal of the European Ceramic Society, vol. 33 (n° 6). pp.
1145-1153. ISSN 0955-2219
O
pen
A
rchive
T
oulouse
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
Lithium
conducting
solid
electrolyte
Li
1.3
Al
0.3
Ti
1.7
(PO
4
)
3
obtained
via
solution
chemistry
Sandrine
Duluard
a,∗,
Aude
Paillassa
a,
Laurent
Puech
b,
Philippe
Vinatier
b,
Viviane
Turq
a,
Patrick
Rozier
a,
Pascal
Lenormand
a,
Pierre-Louis
Taberna
a,
Patrice
Simon
a,
Florence
Ansart
aaInstitutCarnotCIRIMAT(CNRS/UniversitéPaulSabatier-UMR5085),118routedeNarbonne,F-31602ToulouseCedex9,France bICMCB,CNRS,UniversitédeBordeaux,sitedel’ENSCBP-IPB(CNRS-UPR9048),87avenueduDr.Schweitzer,F-33608Pessac,France
Abstract
NaSICON-typelithiumconductorLi1.3Al0.3Ti1.7(PO4)3(LATP)issynthesizedwithcontrolledgrainsizeandcompositionusingsolutionchemistry.
Afterthermaltreatmentat850◦C,sub-microniccrystallizedpowderswithhighpurityareobtained.TheyareconvertedintoceramicthroughSpark
PlasmaSinteringat850–1000◦C.Byvaryingtheprocessingparameters,pelletwithconductivitiesupto1.6×10−4S/cmwithdensityof97%of
thetheoreticaldensityhavebeenobtained.XRD,FEG-SEM,ac-impedanceandVickersindentationwereusedtocharacterizetheproducts.The
influenceofsinteringparametersonpelletcomposition,microstructureandconductivityisdiscussedinadditiontotheanalysisofthemechanical
behaviorofthegrainsinterfaces.
Keywords:Powders-chemicalpreparation;Sintering;Microstructure;Ionicconductivity;Batteries
1. Introduction
Batteriesarekeysystemsforthedevelopmentof technolo-giessuchasportabledevicesandtransportationsystems.One of themost appealingchallenges isthe increaseof their spe-cificmassenergyforapplicationsrequiringlargeenergystorage capacitye.g.electricvehicleorgridstorage.1,2 Inthisrespect, reversible lithium-air batterieswith an expected energy den-sity of more than 500Wh/kg i.e. a potential range of more than800kmhaveshownpromisingresults.3–5Sofar,twomain solvent-basedtechnologieshavebeen studied:aqueous6,7 and non aqueous lithium-air devices.8,9 Other concepts are also beinginvestigated,e.g.anall-solidstatedevicewasdescribed recently by Kumar et al.10 In water-based devices, the main electrolyteisaqueous-saturatedlithia.Inordertoprevent reac-tions of the lithium anode with the aqueous electrolyte, an ionicconductingseparatorisnecessary.Accordingly,a lithium-conducting solid electrolyte is necessary for both all-solid state andwater-basedtechnologies. Solidelectrolytessuch as
∗Correspondingauthor.Tel.:+33561557292;fax:+33561556163.
E-mailaddress:duluard@chimie.ups-tlse.fr(S.Duluard).
Li3Ncompoundsandsulfide-basedglassesaregoodcandidates
withconductivityashighas6×10−3S/cm.11However,being
hygroscopic, their fabrication in inert atmosphere is manda-tory.OxideelectrolytessuchasLiSICONelectrolytearemore suited due to their ease of preparation. Even though LiSI-CON typecompound Li2+2xZn1−xGeO4 (0.45<x<0.55) was
reportedasbeinghighlyconductive,itsconductivityisstilltoo low atroomtemperature (10−6S/cm).12 With conductivityin the rangeof 10−4S/cm, NaSICONtypeLi1+xAlxTi2−x(PO4)3
(LATP) compounds with x=0.3–0.4 benefitfrom oneof the best lithium conductivity amongst air and moisture stable compounds.13 In stronglyreducing environment (e.g.lithium metalelectrode),LATPmaterialsarecombinedwitha protec-tivelayer(e.g.LiPON)toavoidTi4+reduction.14Thesynthesis of LATP via solid-state chemistry is much documented.15,16 Thanks to the large choice in the raw material nature and synthesis parameters, solution chemistry favors the prepara-tionofpowderswithcontrolledcompositionandmorphology. In this work, we propose a method for the preparation of high-purity LATP powder by co-precipitation. By optimiza-tion of the sintering parameters, pellets with conductivity as high as 1.6×10−4S/cm at room temperature were pre-pared.
Fig.1.ProjectionofthestructureofLi1+xAlxTi2−x(PO4)3.TheMIandMIIintercalationsitescorrespondrespectivelytothemainoccupiedandexcess(x)Li+sites.
2. Experimental
Crystallographicphasesanalysis wascarried out byX-ray diffractionwithaBruckerD4Endeavordiffractometerusinga Cu Karadiationsource(Ka=0.15418nm).Elementary anal-yses were performed by SCA – CNRS. Inductively couple plasma (ICP) analyses were used for quantity determination oflithium,aluminium,titaniumandphosphorus. Thermogravi-metric analyses were performed on a Setaram TG-DTA 92 microbalance with 30mg of sample and alumina as a refer-ence.ScanningelectronmicroscopewithFieldEmissionGun (FEG-SEM)JEOLJSM6700Fwitha5kVacceleratingvoltage wasusedformorphologicalandmicrostructuralinvestigations. Agglomeratessizesweredeterminedbylasergranulometry.The densityofthepelletswasdeterminedviaArchimede’smethod on a KERN ARJ 220-4M balance. A density of 2.92g/cm3 is taken as the theoreticalvalue of bulk LATP(x=0.3). Sur-face roughness of the pellets was studied by optical surface profilometry with an optical interferometer ZygoNew View 100withMetroProTMsoftware.Impedancespectroscopywas used to determine the conductivity of the pellets. Gold elec-trodesweredepositedonthetwofacesofthepelletsbyagold sputter-coater. Impedance measurements at frequencies from 1Hzto107Hzwithvaryingtemperaturewereperformedusing aSolartron1260impedanceanalyserandafurnaceequipped withaEurothermtemperaturecontroller.Microhardnesstests wereperformedonapolishedsurfacewithaVickersindenter HMV-2000Shimadzu.
3. LATPpowderssynthesisandcharacterization
The powder synthesis is based on the co-precipitation of alkoxideandmetallicsaltsadaptedfromaprocedureofCretin etal.17Thewater/ethanolratio,hydrolysisratioandannealing temperaturewereoptimized.
3.1. LATPpowdersynthesis
Liacetatedihydrate(CH3CO2Li·2H2O,≥99%),ammonium
phosphatemonobasic(NH4H2PO4,99.999%pure),aluminium
tri-sec butoxide (Al(tri-sec-OBu), ≥97%) and titanium iso-propoxide (Ti(OiPr)4, ≥97%) were used as raw precursors
and purchased from Sigma–Aldrich. A solution v/v 80/20 water/ethanol with a concentration of 0.4mol/L of metallic saltsandstoichiometricproportionsof precursorsis obtained byadditionofawaterbasedsolutionwithappropriateamounts of lithium acetate and ammonium phosphate monobasic in theethanolsolutioncontainingtitaniumandaluminium alkox-ides.Thehydrolysisratio(h=[H2O]/([Ti(OiPr)4]+
[Al(tri-sec-OBu)])washigh(h=200),sothatpolycondensationoccurred andaprecipitatewasformed.Solutionwasstirredfor2hatroom temperature.Aftersolventevaporationat80◦Cfor48hawhite powder was obtained. The resulting powder wasannealed at temperaturefrom450◦Cto1100◦Cfor150minwitha100◦C/h temperaturerampinheatingand300◦C/hincooling.
3.2. Structuredescriptionandphaseanalysis
Li1+xAlxTi2−x(PO4)3 (LATP, x=0–0.5) belongs to the
NaSICON-type (Na1+xZr2SixP3−xO12, 0<x<3) structure. It
crystallizesintherhombohedralsystem(spacegroupR3¯c)with cellparametersa=8.48(2) ˚A,c=20.76(2) ˚A.16Thestructureis builtupwithTiO6octahedra andPO4 tetrahedrasharing
cor-nerstoforma3-DopenframeworkasschematizedinFig.1.Li cationsarelocatedintotwositeslabeledMIandMII.Themain
one(MI),whichisidenticaltothatofun-substitutedLTP
par-entstructure,correspondstoadistortedoctahedraloxygenated environment.ThesubstitutionofAl3+toTi4+leads,forcharge compensation,toextra lithium ionslocated inMII sites with
irregulareightcoordinatedsites.18,19
Byanalogy withNaSICON,themigrationpathway of Li+ ionsinLTPisspeculatedtooccurviabottlenecksalongapath
Fig.2.X-raydiffractiondiagramsmeasuredatroomtemperatureforLATP powderannealedattemperaturefrom450◦Cto1100◦C.
MI-MII-MI.19,20Itwasalsoshownthatthenarrowestplacesof
thebottleneckshapedchannelare largeenoughforLi+ ionto migratewithoutanydistortionoftheLTPnetwork.TiIV substitu-tionbyAlIIIcontributestoopenthebottlenecksizebyincreasing theoccupationoftheMIIsites.21Thiseffectaddedtotheincrease
inLi+concentrationfavorsLi+diffusioninLATPascompared to LTP. This results in conductivity at room temperature of 10−4S/cmfor LATPfor x=0.3–0.4versus2×10−6S/cmfor LTP.22
X-raydiffractionpatternsofsamplesannealedattemperature rangingfrom450◦Cto1100◦CarereportedinFig.2.The crys-tallizationofLATPoccursat700◦C.PureLATPisobtainedfor annealingtemperaturesfrom750◦Cto850◦C.Above850◦C, decomposition occurs leading to the formation of secondary phasesAlPO4,TiO2andLi4P2O7.Forfurtherexperiments,the
annealingtemperatureisthensetat850◦C.
The composition of this powder was checked by induc-tivelycoupledplasma(ICP)elementaryanalyses.Acomposition of Li1.21±0.01Al0.32Ti1.72±0.02P3O12 was then obtained which
impliesanevaporationof0.2%w/woflithiumascomparedto thetheoreticalstoichiometryLi1+xAlxTi2−x(PO4)3withx=0.3.
Thiscorrespondstoamaximumof 2–4w/w% of impurities (AlPO4orLi4P2O7by-productconsidered)presentinthe
pow-der.
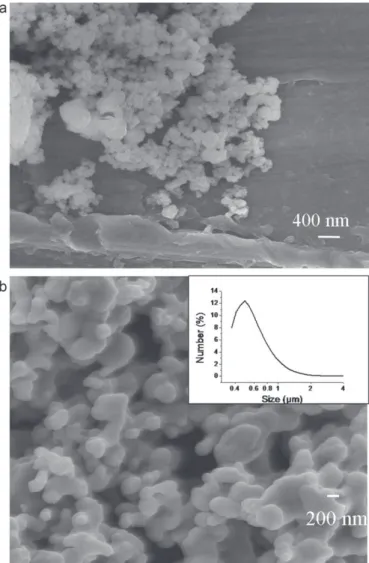
3.3. Powdermorphology
Scanning electron micrographs of LATP powder are pre-sentedinFig.3.Thenonannealedpowderismostlycomposed of particlesof 50–100nm insize withadditionallarger parti-clesof200–400nm.Theannealingat850◦Cleadstoparticles largerthan100nmwithalargedistributioninshapes.Theresults of the 850◦C annealed powder are confirmed by laser gran-ulometry, as presented in Fig. 3(b), with a calculated mean
Fig.3.ImagesofLATPpowderobtainedbyscanningelectronmicroscopyand distributioninsizeofparticlesobtainedbylasergranulometry:(a)powderbefore thermaltreatment,(b)powderannealedat850◦C.
diameterof520nmand90%oftheagglomeratesintherange of400–1000nm.
3.4. Powderconductivity
Toourknowledge,whereasLATPstructurewithx=0.3has been demonstrated as convenient for lithium conduction, no mentionof thepercolationbehaviorof suchpowder hasbeen reportedintheliterature.Inordertodeterminewhethera perco-lationnetworkcouldbeformedatroomtemperaturebetweenthe LATPgrains,theconductivityofapressedpellethasbeen mea-sured by impedancespectroscopy.The chosen LATP powder (annealed850◦C,2h30)waspressedinbetweentwobrassdisc
electrodes. The maximum compaction, whichcorresponds to theminimuminsamplethickness,isreachedatanapplied pres-sureof110MPa.Nyquistimpedanceplotsforseveralapplied pressurepresentthesamebehavior.Onesemicircleisobserved at highfrequencyandan inclinedtail isobservedatlow fre-quencies.Theresistancewasmeasuredbyfittingthesemi-circle
Fig.4.Impedancespectraofthepressedpowderdependingonthepressure appliedandcorrespondingconductivity.
with R1 the electrical resistance of the measurement system,
R2 the resistance of the electrolyte andCPE the
correspond-ing constant phase element.23 The Nyquist plots normalized insurfaceandthicknessaregiveninFig.4(a).No difference of resistivityis measured as compactionincreases and thick-nessdecreasessothatthemeasuredphenomenonarisesdirectly fromthe resistancebeingproportionaltothepelletthickness. Whateverthecompactionratio,alowconductivity(intherange of 3.1–3.6×10−7S/cm)isobtained. Thecontactbetweenthe
grainsisnot efficientenoughtocreateapercolation network thatwouldenhancelithiumconductivity.
4. Ceramicsintering
Sincephysicalbindingisnotsufficientenoughforallowing lithiumconduction withinthepellets,sintering ofthepowder wasperformedtopromotechemicalbondingbetweenthegrains. LATP powders annealed at 850◦C were pressed into pellets betweentwosheetsofgraphitepapers(Papyex®)andsintered under vacuum using the SparkPlasma Sintering (SPS) tech-nique inaSPS 2080Sumitomo apparatus. Apulsesequence (12pulses,2idletime,3.3ms/cycle)withadjustedcurrentwas applied.Thediewasheateduptothesinteringtemperaturewitha 100◦C/minramp.Anisostaticpressureof100MPawasapplied beforeheating.Thebeginningofthesinteringoccursat750◦C
withamaximumintheshrinkagerateat800–850◦Casobserved
bydilatometricstudies.
4.1. Sinteredpelletsandprocessingparameters
Asummaryofthe varioussinteringconditionsisindicated inTable1.Temperature from850◦Cto1000◦Candduration
Table1
Sinteringconditionsforthepreparationofpellets.
Ceramicpellets Sinteringtemperature(◦C) Sinteringduration(min)
A1-850-5 850 5 B1-900-5 900 5 C1-950-5 950 5 D1-1000-5 1000 5 A2-850-10 850 10 A3-850-15 850 15 A4-850-20 850 20
Fig.5.X-raydiffractiondiagramsatroomtemperatureforpelletssinteredby SPSunder100MPaappliedpressure,vacuumatmosphere.Sinteringduration: 5min.Sinteringtemperatures:850◦C,900◦C,950◦C,1000◦C.
from5min to20min wereused.Aftersintering, thesamples werepolishedinordertoremovethegraphitepaper.
4.2. Crystallographicphasesanalysisofthepellets
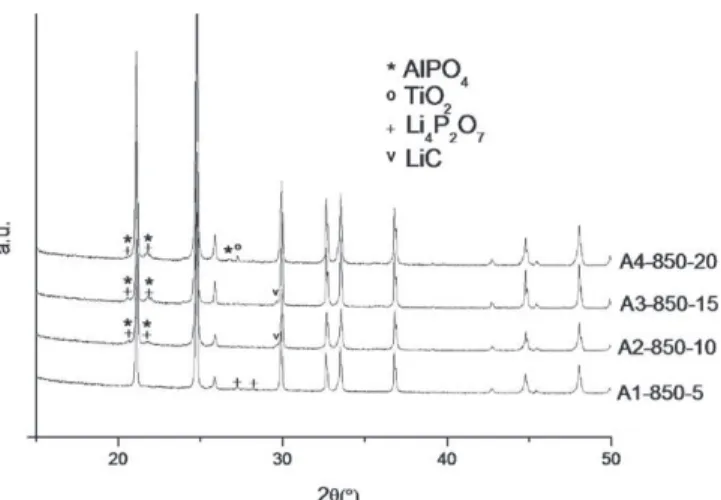
XRD patterns for the sintered pellets versus the sin-tering temperature and sintering duration are presented in Figs. 5 and6.All the majorpeaks areindexed inthecrystal systemof the LATPpowder. Nochange incellparametersis observed(a=8.48±0.02 ˚Aandc=20.76±0.03 ˚A).Side prod-uctsAlPO4appearinallthepellets,exceptforthepelletsintered
atthelowesttemperatureforshorttime(850◦C,5min)inwhich
onlyasmallamountofLi4P2O7ispresent.Li4P2O7couldbe
presentinallthesamples,howeverinsomecasesonlythemajor peak(2θ=20.5◦)thatisacommonpeakwithAlPO4phasesis
visible.Inaddition,aLi2C2phase isdetectedfor pellets
sin-teredfor morethan 5min.A sharpeningof the peakson the XRDpatternsisobservedforsinteringtemperaturefrom850◦C to950◦C, so that the primary particles increasein size with increasingsinteringtemperature.
Fig.6.X-raydiffractiondiagramsatroomtemperatureforpelletssinteredby SPSunder100MPaappliedpressure,vacuumatmosphere.Sintering tempera-ture:850◦C.Sinteringduration:5,10,15,20min.
Fig.7.DensitymeasuredbyArchimede’smethodandconductivitymeasured byelectrochemicalimpedancespectroscopy.PelletsA1-850-5,B1-900-5, C1-950-5,D1-1000-5.Durationofsintering:5min.
5. Resultsanddiscussions
5.1. Densityandconductivity
Figs.7 and8show theevolutionof thedensity(measured atroomtemperature)oftheSPS-preparedpelletsversus sinter-ingtemperatureandduration.Whateverthesinteringconditions, pelletswithdensityrangingfrom2.82g/cm3to2.84g/cm3(i.e. 97%ofthetheoreticaldensity)wereobtained.Itshouldbenoted thatsinceallthesampleshavethesamedensity,thedirect com-parisonoftheconductivityoftheLATPpelletsisaccurate.
Roomtemperatureconductivitiesofthepelletsexhibita sim-ilarbehaviortotheonedescribedforLATPpowderinSection 3.4. Only one semi-circle, representative of the total resis-tanceoftheelectrolyte,isobserved.Valuesfrom1×10−4S/cm
to 1.6×10−4S/cm are obtained for pellets sintered between
850◦Cand950◦C.Thesevalues,similartothosereportedin the literature,1,3,24 indicate in addition that the conductivity increaseswithincreasingtemperatures.However,while sinter-ingattemperature above1000◦Cleadstoalmostfullydense pellets,theirconductivitiesdropdownto1×10−5S/cmwhich fullycontradicttheexpectedevolution.
Theeffectofsinteringdurationhasalsobeenstudiedand plot-tedinFig.8forasinteringtemperatureof850◦C.Thedensities
Fig.8.DensitymeasuredbyArchimede’smethodandconductivitymeasured byelectrochemicalimpedancespectroscopy.PelletsA1-850-5,A2-850-10, A3-850-15,A4-850-20.Temperatureofsintering:850◦C.
andconductivitiesremainthesameindependentoftheduration ofsinteringfrom5minto20min.Then,thesecondaryphase for-mationdetectedbyXRD(cf.Section4.2)onthesamplesintered at10–20minisnotdetrimentaltodensityandconductivity.
5.2. Microstructuralanalysisofthepellets
Inordertodeterminewhetherthedifferenceinconductivity couldberelatedtoachangeinmicrostructure,SEMmicrographs analysisofthecross-sectionofthesampleshasbeenperformed. Themicrostructureofthepelletsstronglydependsonthe sinter-ingtemperature.Whereasthepelletsinteredat1000◦Cpresenta uniformmicrostructurewithlargegrains(6–30mmindiameter),
forpelletssinteredat850◦Candupto950◦C,aheterogeneous microstructureappears.AspresentedinFig.9,partswithonly small grainsof200–300nmindiameterwerepresentcloseto zoneswithlargegrainsoftensofmicrometres.Astemperature increases,thesurfaceareacoveredbylargegrainsbecomes pre-dominant. On thecontrary,the durationof sinteringdoes not mainlyinfluence themicrostructure. Thesame structurewith largeandsmallgrainsisobtainedforpelletssinteredat850◦C for 5, 10, 15 and20min (micrographsnot shown here). For LATPmaterials(x=0.3–0.4),bulkconductivityisreportedto be higherthan grainboundariesconductivity.17 The presence oflargergrainsisthenexpectedtobebeneficialto conductiv-itywhichfully agreeswiththeobservedevolutionfor pellets sinteredbetween850◦Cand950◦C.However,thedropdown inconductivityobservedat1000◦C,whereasonlyapopulation oflargegrainsisobserved,contradictsthisexpectedevolution. TheaggregationofanonLi+conductivesecondaryphaseatthe grainboundariescouldexplainthisphenomenon.Howeverno significantdifferenceingrainboundariescompositionshasbeen observedbyEDXanalysesonconductiveandnonconductive samples.Acarefulobservationofpelletssinteredabove1000◦C indicatesthatthecohesionbetweenlargegrainsisweakasshown inFig.10.Thenthelackofcohesionbetweenthegrainscanbe suspectedtobeattheoriginofthedropinconductivity.
5.3. Mechanicalproperties
Inordertostudythemechanicalbehaviorofthezoneswith small grainsandthe zones withlarge grains, Vickers micro-hardnessmeasurementsundervariousnormalloads(2N,2.9N, 4.9Nand9.8N)wereperformed.OpticalmicroscopyandSEM analysesoftheindentedsurfaceshavebeencarriedout.
AVickershardnessdecreasefrom700–800HVforthezones withsmallgrainstoonly300–400HVinthezonesconsisting oflargegrainsisobserved.Thedecreaseofplasticyieldstress withincreasinggrainsizehasbeendescribedbytheHall–Petch law:σy=σ∞+kd−1/2,withdthegrainsize,kaconstant,σ∞
yieldstressof thesinglecrystal,σyplasticyieldstressthatis
consideredasproportionaltothehardnessforsuchceramics.25 Thislawisrelevantforgrainsfromseveraltensof nanome-ters to several tens of microns such as the size of grainsin the materials studied in thiswork. Moreover,fractures surg-ingfromthesidesoftheVickersindentationareonlyvisiblein
Fig.9.SEMimagesofthefracturesurfaceofpelletssinteredat(a)850◦C,(b)900◦Cand(c)950◦C.
Fig.11.OpticalmicrographsofVickershardnessindentationmarksinsinteredpelletsforseveralappliedloads.(a)Zoneswithsmallgrainsand(b)zoneswithlarge grains.
thezoneswithsmallgrainsduetothehigherhardnessofthis zone.
Optical micrographsafter indentation on zones withlarge grains andzones with small grainsare shownin Fig. 11. A
deformationaroundtheindentationisvisibleinbothcaseseven forthelowestload(2N).Thezoneswithlargegrainsaremore subject to largedeformations at the edge of the indentation. Underaloadfrom2Nto9.8N,themarkleftinthiszoneis1.5
Fig.12.MicrographsobtainedbySEMonsamplesurfacesafterindentationat9.8Nload(a)inthezoneswithsmallgrains(b)inthezoneswithlargegrains.
largerindiagonalthaninthezonewithsmallgrains.Moreover, broken partsoflargegrainsareobservedinFig.12,bySEM micrographicanalysisattheedgeoftheindentation.
Thistendencyof largegrainstobetaken offthe matrixis alsosupportedbysurfaceroughnessmeasurementsafter polish-ingofthesamples.Whereaspartswithsmallgrainsaresmooth with apeak-to-valley height of 0.4mm, aresidual roughness
afterpolishingismeasuredonthezoneswithlargegrainswitha peak-to-valleyvalueof10mm(whichistheaveragesizeoflarge
grains).Moreover,theskewnessoftheroughnessprofileis neg-ative withvaluesfrom−1to−3.Theselargenegativevalues aretypicalofasymmetricalroughnessprofileswith preponder-anceofholesascomparedtohills(thebulkofthematerialbeing abovethemeanline).
Hence,lackofcohesionbetweenthelargegrainscouldbe thelimitingparametertolithiummigrationandtheexplanation of the lowertotalionicconductivity ofthe pelletssintered at 1000◦C.Fortheotherpellets,thepresenceofzoneswithlarge grainsisnotdetrimentaltotheglobalconductivity.Wecanthen inferthatinthesecasesthezoneswithsmallgrainspromotethe mechanicalcohesionbetweenthelargeparticles.
6. Conclusion
Powders with high purity have been prepared by co-precipitationmethod.LATPpelletssintered bySpark Plasma Sinteringwithconductivitiesamongstthehighestpublishedare obtainedwith1.6×10−4S/cmatroomtemperature.Veryhigh densitiesarealsoobtained(97% ofthetheoreticaldensity)so thatthesemembranescanbereadilyusedasseparatorfor aque-ouslithium-airbatteries.Moreover,thesedenseandconductive pelletsweresynthesizedattemperaturesaslowas850◦Candfor treatmentdurationof5minwhereasbyclassicalsinteringat tem-peratureof1000◦Cfor1hwouldbenecessary.Anewfeature
thatcouldbebroughttotheknowledgeoftheresearchfieldisthe correlationofthedropinconductivitywiththelossofcohesion ofgrainsasSPSsinteringtemperaturereaches1000◦C. Investi-gationsaregoingontodeterminetheoriginofthisphenomenon, wherethepresenceofamorphousphasesatthegrainboundaries isbeinghypothesized.
Acknowledgements
ThisworkwassupportedbytheFrenchNationalResearch Agency(projectANRLiO2).TheSPSsinteringwasperformed
attheSPSFrenchNationalPlatform(PNF2/CNRS)atToulouse. WeespeciallythankGeoffroyChevallierandClaudeEstournes fortheirtechnicalsupport.
References
1.TarasconJM,ArmandM.Issuesandchallengesfacingrechargeablelithium batteries.Nature2001;414:359–67.
2.ArmandM,TarasconJM.Buildingbetterbatteries.Nature2008;451:652–7. 3. ViscoSJ,NimonE,DeJongheLC.Secondarybatteries:metal-airsystems
|lithium-air.In:JürgenG,editor.Encyclopediaofelectrochemicalpower sources.Amsterdam:Elsevier;2009.p.376–83.
4.JiDX,TaeLK,NazarLF.Challengesoflithium-sulfurandlithium-aircells: oldchemistry,newadvances.On:scalableenergystorage:beyond lithium-ion.SanJose,USA:AlmadenInstitute;2009.Aug.26.
5.BrucePG,FreunbergerSA,HardwickLJ,TarasconJ-M.LiO2 andLiS
batterieswithhighenergystorage.NatMater2012;11:19–29.
6.Visco,S.,Nimon,Y.,inventors;PolyPlusBatteryCompany,assignee.Li/Air nonaqueousbatteries.UnitedStatespatentUS20070117007.2007May24. 7.Stevens,P.,Toussaint,G.,inventors;ElectricitédeFrance,assignee. Dis-positifélectrochimiqueàélectrolytesolideconducteurd’ionsalcalinsetà électrolyteaqueux.WO2011051597.2011May5.
8. Abraham KM, Jiang Z. A polymer electrolyte-based rechargeable lithium/oxygenbattery.JElectrochemSoc1996;143:1–5.
9. OgasawaraT,DebartA,HolzapfelM,NovakP,BrucePG.Rechargeable Li2O2electrodeforlithiumbatteries.JAmChemSoc2006;128:1390–3.
10.KumarB,KumarJ,LeeseR,FellnerJP,RodriguesSJ,AbrahamKM.A solid-state,rechargeable,longcyclelifelithium-airbattery.JElectrochem Soc2010;157:A50–4.
11.LappT,SkaarupS,HooperA.IonicconductivityofpureanddopedLi3N.
SolidStateIonics1983;11:97–103.
12.Bruce PG, WestAR. The AC conductivity of polycrystalline Lisicon Li2+2xZn1−xGeO4,andamodelforintergranularconstrictionresistances.
JElectrochemSoc1983;130:662–9.
13. AonoH,SugimotoE,SadaokaY,ImanakaN,AdachiG.Ionicconductivity ofsolidelectrolytesbasedonlithiumtitaniumphosphate.JElectrochemSoc
1990;137:1023–7.
14. WestWC,WhitacreJF,LimJR.Chemicalstabilityenhancementoflithium conductingsolidelectrolyteplatesusingsputteredLiPONthinfilms.JPower Sources2004;126:134–8.
15.Fu J. Superionic conductivity of glass-ceramics in the system Li2O–Al2O3–TiO2–P2O5.SolidStateIonics1997;96:195–200.
16.ArbiK,MandalS,RojoJM,SanzJ.Dependenceofionicconductivityon compositionoffastionicconductorsLi1+xTi2−xAlx(PO4)3,0≤x≤0.7.A
parallelNMRandelectricimpedancestudy.ChemMater2002;14:1091–7. 17.CretinM,FabryP.Comparativestudyoflithiumionconductorsinthesystem Li1−xAlxAIV2−x(PO4)3withAIV=TiorGeand0≤x≤0.7foruseasLi+
sensitivemembranes.JEurCeramSoc1999;19:2931–40.
18.AatiqA,MenetrierM,CroguennecL,SuardE,DelmasC.Onthestructure ofLi3Ti2(PO4)3.JMaterChem2002;12:2971–8.
19.NusplG,TakeuchiT,WeissA,KageyamaH,YoshizawaK,YamabeT. LithiumionmigrationpathwaysinLiTi2(PO4)3andrelatedmaterials.J
ApplPhys1999;86:5484–90.
20.BarjM,PerthuisH,ColombanP.Existencedomains,structuraldistortions andvibrationmodesofconductingionsinNasiconhostlattices.SolidState Ionics1983;11:157–77.
21. Martinez-JuarezA,PecharromanC,IglesiasJE,RojoJM. Relationship betweenactivationenergyandbottlenecksizeforLi+ionconductionin NASICONmaterialsofcompositionLiMM′(PO
4)3;M,M′=Ge,Ti,Sn,
Hf.JPhysChemB1998;102:372–5.
22.AonoH,SugimotoE,SadaokaY,ImanakaN,AdachiG.Ionicconductivity andsinterabilityoflithiumtitaniumphosphatesystem.SolidStateIonics
1990;40–1:38–42.
23.RandlesJEB.Kineticsofrapidelectrodereactions.DiscussFaradaySoc
1947;1:11–9.
24.Ando Y, Hirose N, Kuwano J, Kato M, Otsuka H. Ceramics today-tomorrow’sceramics.Amsterdam:Elsevier;1991.
25.Louchet F, Weiss J, Richeton T. Hall–Petch law revisited in terms of collective dislocation dynamics. Phys Rev Lett 2006;97, 075504-1-4.