Thalamic modulation of the cortical slow oscillation
Mémoire
Anastasiia Ozur
Maîtrise en neurobiologie
Maître ès sciences (M. Sc.)
Québec, Canada
© Anastasiia Ozur, 2016
Thalamic modulation of the cortical slow oscillation
Mémoire
Anastasiia Ozur
Sous la direction de: Igor Timofeev
Igor Timofeev, directeur de recherche
iii
Résumé
Il est bien établi que le thalamus joue un rôle crucial dans la génération de l'oscillation lente synchrone dans le cortex pendant le sommeil lent. La puissance des ondes lente / delta (0.2-4 Hz) est un indicateur quantifiable de la qualité du sommeil. La contribution des différents noyaux thalamiques dans la génération de l’activité à ondes lentes et dans sa synchronisation n'est pas connue. Nous émettons l'hypothèse que les noyaux thalamiques de premier ordre (spécifiques) influencent localement l’activité à ondes lentes dans les zones corticales primaires, tandis que les noyaux thalamiques d'ordre supérieur (non spécifiques) synchronisent globalement les activités à ondes lentes à travers de larges régions corticales. Nous avons analysé les potentiels de champ locaux et les activités de décharges de différentes régions corticales et thalamiques de souris anesthésiées alors qu'un noyau thalamique était inactivé par du muscimol, un agoniste des récepteurs GABA. Les enregistrements extracellulaires multi-unitaires dans les noyaux thalamiques de premier ordre (VPM) et d'ordre supérieur (CL) montrent des activités de décharges considérablement diminuées et les décharges par bouffées de potentiels d'action sont fortement réduites après inactivation. Nous concluons que l'injection de muscimol réduit fortement les activités de décharges et ne potentialise pas la génération de bouffées de potentiel d'action à seuil bas. L'inactivation des noyaux thalamiques spécifiques avec du muscimol a diminué la puissance lente / delta dans la zone corticale primaire correspondante. L'inactivation d'un noyau non spécifique avec le muscimol a significativement réduit la puissance delta dans l'ensemble du cortex étudié. Nos expériences démontrent que le thalamus a un rôle crucial dans la génération de l'oscillation lente corticale.
iv
Abstract
It is well established that thalamus plays a crucial role in the generation of the synchronous slow oscillation in the cortex during non-REM sleep. The slow/delta power (0.2-4 Hz) is the main measured factor of the quality of sleep. However, the contribution of different thalamic nuclei to the generation of the slow wave activities and its synchronization is not known. We hypothesized that the first-order (specific) thalamic nuclei provide a control of slow waves in primary cortical areas, while higher-order (non-specific) thalamic nuclei may synchronize the slow-wave activities across wide cortical regions. We analyzed local field potentials and spiking activities from different cortical and thalamic areas of anesthetized mice while a thalamic nucleus was inactivated by the GABA-agonist muscimol. Extracellular multiunit recordings in first-order (VPM) and higher-order (CL) thalamic nuclei show dramatically decreased spiking activity and strongly reduced burst firing after inactivation with muscimol. We conclude that the injection of muscimol strongly reduced the spiking activity and does not potentiate the generation of low-threshold spike mediated bursts. Inactivation of specific thalamic nuclei with muscimol decreased the slow/delta power in the corresponding primary cortical area. The inactivation of a non-specific nucleus with muscimol significantly reduced the delta power in all investigated cortical areas. Our experiments demonstrate that the thalamus is required for the fine tuning of the cortical slow oscillation.
v
Table of contents
Résumé ... iii
Abstract ... iv
Table of contents ... v
List of figures ... vii
List of abbreviations ... viii
Acknowledgments ... x
Chapter I ... 1
General Introduction ... 1
1.1 Architecture of thalamocortical system ... 2
1.1.1 Thalamus ... 3
1.1.2 Thalamic reticular nucleus ... 4
1.1.3 Neocortex ... 5
1.1.3.1 Pyramidal neurons ... 6
1.1.3.2 Interneurons ... 6
1.1.3.3 Electrophysiological types of cortical neurons ... 7
1.1.3.4 Laminar and columnar organisation of cortex ... 8
1.1.3.5 Synaptic connectivity in neocortex ... 9
1.2 States of vigilance ... 10
1.2.1 Wakefulness ... 11
1.2.2 Rapid eye movement sleep ... 12
1.2.3 NREM sleep (non-rapid eye movement sleep) ... 12
1.3. Types of oscillatory activities during SWS sleep ... 13
1.3.1 Slow oscillation ... 13
1.3.2 Delta oscillation... 15
1.4 Thalamic contribution to the generation and modulation of the slow wave activity .. 16
1.4.1 VPM nucleus projects to the primary somatosensory cortex ... 16
1.4.2 CL nucleus has widespread projections across all cortical areas ... 17
vi
Chapter II ... 19
Materials and Methods ... 19
2.1 Experiments on anesthetized mice ... 20
2.2 Inactivation of thalamic nuclei... 20
2.3 Electrophysiological recordings ... 21
2.4 Histology ... 22
2.5 Data analysis ... 23
Chapter III... 24
Results ... 24
3.1 Muscimol injection in VPM and CL nuclei abolishes spiking activity of neurons and does not trigger bursting ... 25
3.2 Effect of saline injection in the VPM nucleus on slow-wave activities during anesthesia ... 26
3.3 Effect of thalamic inactivation of the VPM nucleus on slow-wave activities during anesthesia ... 26
3.4 Effect of thalamic inactivation of the CL nucleus on slow-wave activities during anesthesia ... 27
3.5 Intracellular recordings ... 27
Chapter IV ... 29
Discussion ... 29
vii
List of figures
Figure 1. Thalamocortical system. ... 33 Figure 2. Experimental protocol. ... 34 Figure 3. Effects of saline and muscimol injection on spiking activity in VPM and CL thalamic nuclei. ... 36 Figure 4. Saline injection in VPM nucleus does not change firing in VPM neurons and does not affect cortical LFP activities. ... 38 Figure 5. Thalamic inactivation of the VPM nucleus with muscimol abolishes firing of VPM neurons and produces local cortical reduction of slow-wave activities. ... 40 Figure 6. Thalamic inactivation of the CL nucleus with muscimol abolishes firing within these nuclei and produces global cortical reduction of slow-wave activities. ... 42 Figure 7. Intracellular and LFP recordings of neurons within frontal cortex during slow wave oscillation in anesthetized mouse. ... 44 Figure 8. Intracellular and LFP recordings of neurons within somatosensory cortex during slow wave oscillation in anesthetized mouse. ... 45
viii
List of abbreviations
AC Accelerating AD Adapting CC Corticocortical
CL Central lateral thalamic nucleus EEG Electroencephalogram
EPSP Excitatory postsynaptic potential FFT Fast Fourier Transformation FO First-order
FRB Fast rhythmic bursting FS Fast Spiking
GABA Gamma-amino-butyric acid HO Higher-order
IB Intrinsically-Bursting
Ih Hyperpolarisation-activated cation current INa(p) Persistent sodium current
It Low-threshold calcium current IPSP Inhibitory postsynaptic potential IS Irregular spiking
KAc Potassium acetate LFP Local Field Potential
LTCP Low-threshold Ca2+ potential
LTD Long-term depression LTS Low-threshold spike NFS Non-fast spiking
NREM Non-rapid eye movement PO Posterior nucleus
PPT Pedunculopontine nuclei PBS Phosphate buffer saline RS Regular Spiking
ix
SD Standard deviation SW Slow waves
SWS Slow-wave sleep
TRN Thalamic reticular nucleus Vm Membrane potential
VPL Ventral posterolateral thalamic nucleus VPM Ventral posteromedial thalamic nucleus ZI Zona incerta
x
Acknowledgments
I would like to express the greatest appreciation to my supervisor Igor Timofeev for the opportunity to work in his laboratory and for his limitless patience and genuine support in my beginning to live in Canada.
I thank Sylvain Chauvette, an incredible scientist, invaluable research assistant and simply good friend for his being wherever I need him to be.
Thanks to Josée Seigneur and Sara Soltani for their priceless advices and help with experimental techniques.
A special thank to Elvira Surina for her energy and positivity which covered me as a warm blanket in the hardest moments in my life here.
Also I would like to thank my best friend forever Maria for her optimism and gift to go ahead in spite of what would happen in future and what you left behind.
1
Chapter I
2
Sleep slow oscillation is generated by neocortical network interactions. Recent studies demonstrated that thalamus actively contributes to reshaping this cortical rhythm. Thalamocortical neurons from first vs. higher order nuclei have multiple different features including very different patterns of cortical projections. The main subject of the proposed study is to investigate how inactivation of first vs. higher order thalamic nuclei influences cortical slow oscillation.
The first section of the introduction is a general description of the architecture of the thalamocortical system, which is composed of the dorsal thalamic nuclei, the thalamic reticular nucleus, and the neocortex. The second section is dedicated to the electrographic activities during the three states of vigilance (wake, rapid-eye-movement (REM) or paradoxical sleep, and non-rapid-eye-movement NREM-sleep) focused on describing the thalamocortical oscillations occurring during slow wave sleep, or SWS sleep.
1.1 Architecture of thalamocortical system
The thalamus and cerebral cortex are tightly interconnected through topologically well-organized projections (Fig. 1). Previous studies have long revealed that the thalamus acts as the “gateway” for almost all extrinsic and intrinsic information before they reach the cortex via thalamocortical connections (Sherman and Guillery, 2002). Furthermore, the function of thalamus is crucial to generation and maintenance of the states of vigilance, including wakefulness, sleep and consciousness (Alkire et al., 2008; Poulet et al., 2012). Thalamocortical connectivity is essential for the establishment of oscillatory brain waves (Jones, 2007). Importantly, abnormalities in the thalamocortical system have been observed in multiple brain disorders like schizophrenia (Welsh et al., 2010; Woodward et al., 2012), suggesting its vital role in brain function (Liang et al., 2013).
The thalamocortical (TC) system generates many different types of oscillatory activities with distinct mechanisms, which frequencies range from as slow as 0.02 Hz to as fast as 500 Hz.
Three structures are implicated in the generation of TC network oscillations: the dorsal thalamic nuclei, the inhibitory thalamic reticular nucleus (TRN) and zona incerta (ZI), as well as the neocortex.
3
1.1.1 Thalamus
The dorsal thalamus receives information from ascending pathways and gives glutamatergic projections to both the neocortex and the TRN. It comprises roughly 15 nuclei with relay cells, specific (first-order) and non-specific (higher-order) nuclei. Relay TC neurons are also considered as the thalamic core neurons and non-specific TC neurons as the thalamic matrix neurons (Jones, 1998). The traditional role of the sensory thalamus is
to relay current sensory information to cortex via first-order thalamic nuclei. Higher-order nuclei, however, subserve different, frequently more complex, functions
including attention, perception and memory formation (Kinomura et al., 1996; Komura et al., 2001; Bartho et al., 2002). First-order thalamic nuclei project to the primary cortical areas in a specific manner, while higher-order thalamic nuclei provide widespread influence and innervate multiple cortical areas (Sherman and Guillery, 2002).
Large number of studies shows that cortical active state onsets propagate in the anteroposterior direction as well as demonstrate simultaneous termination of active states across neocortex (Massimini et al., 2004; Volgushev et al., 2006; Ruiz-Mejias et al., 2011; Sheroziya and Timofeev, 2014). Simultaneous termination of active states implies the presence of active synchronous inhibitory process at the onset of active cortical states (Lemieux et al., 2015). In the mouse thalamus, sensory nuclei are dominated by the inhibition during active phases of the slow oscillation, whereas higher-order thalamic nuclei show the prevalence of the excitation (Sheroziya and Timofeev, 2014). The first-order thalamic nuclei fire following the corresponding cortical areas during the slow oscillation; onsets of silent states in the higher-order thalamic neurons show synchronous termination with the slow-wave silent states in the cortex. Ascending sensory pathways drive specific thalamic nuclei, form large synapses on proximal dendrites with multiple release sites, whereas pyramidal cells from layer V provide ‘driver mode’ excitation to the higher-order nuclei arriving on distal dendrites with small synapses (Groh et al., 2008; Sheroziya and Timofeev, 2014) and serve by themselves as the neurons which generate the slow oscillation (Sanchez-Vives and McCormick, 2000; Chauvette et al., 2010; Beltramo et al., 2013). According to the known relative numbers of synapses, excitatory synapses in different thalamic nuclei (Van Horn and Sherman, 2007), it was determined that cortical
4
modulation of the thalamic relay in the cortico-thalamo-cortical higher order pathway is much more prominent than in first order relays.
1.1.2 Thalamic reticular nucleus
The thalamic reticular nucleus (TRN, or nucleus reticularis thalami) is a thin shell of GABA (γ-amino-butyric acid)-ergic neurons adjoining the lateral aspect of the dorsal thalamus and provide inhibitory input to thalamic relay cells and generate synchronized activity during sleep (Landisman et al., 2002; Pinault, 2004). Evidence suggests that TRN neurons in major sensory sectors are composed of thin slab-like domains that are further organized into sublaminae that preserve the topography of associated relay nuclei (Crabtree, 1992; Bourassa et al., 1995; Pinault et al., 1995). The thalamoreticular pathway is organized in a topographic manner. For example, the somatosensory region of the TRN can be roughly organized into three-tiers. From the inner (thalamoreticular) border to the outer, in a manner reciprocal to the reticulothalamic pathway, each of these tiers receives its input from one of the somatosensory relays of the thalamus, posterior (PO), ventral posteromedial (VPM) and ventral posterolateral (VPL) nuclei, respectively (Lam and Sherman, 2011).
GABAergic neurons in the thalamic reticular nucleus are electrically coupled (Fuentealba et al., 2004). Experimental evidence and modeling studies support a synchronizing role for electrical coupling in the cat thalamic reticular neurons in vivo (Landisman et al., 2002). In rodents these connections form two types of gap-junction-coupled (GJ) clusters with distinctive patterns and axonal projections. GJs form two distinct types of inhibitory networks that correlate activity either within or across functional modules of the thalamus (Lee et al., 2014).
Spatially restricted synchronous inhibition could be responsible for a correlated switch in firing mode in neighboring thalamic neurons, allowing for the generation of burst firing in local clusters of thalamic neurons but preventing widespread synchronous burst firing and the generation of epileptiform activity (Pita-Almenar et al., 2014). More generally, thalamic circuits formed by TRN and ventrobasal complex (VB, consisting of ventral posteromedial nucleus, VPM, and ventral posterolateral nucleus, VPL) neurons, long assumed to be involved in maintaining synchronous oscillations can in fact play a
5
critical role in decorrelating afferent synaptic inputs, with important consequences for information processing.
Upon natural patterns of stimulation, inhibitory GABAergic synapses between TRN and TC neurons of the rat somatosensory nucleus develop a long-term depression (I-LTD) in experiments conducted in vitro. The mechanism underlying this synaptic plasticity presents unique features, being both heterosynaptic and homosynaptic and requiring calcium entry specifically guided through T-type Ca2+ channels (Pigeat et al., 2015). T-type
calcium channels (T-channels) are critically important in the control of cellular excitability and in the generation of rhythmic oscillations between mutually interconnected cortical and thalamocortical relay neurons in the VB complex and TRN, a main inhibitory structure in the thalamus (DiGruccio et al., 2015).
TRN neurons are the pacemaker of spindle oscillations (waxing-and-waving field potentials of 7-14 Hz) (Bazhenov et al., 1999; Fuentealba and Steriade, 2005). Inhibitory neurons of TRN fire a spike burst that evokes IPSP in thalamocortical relay neurons which generate rebound spike-burst and excite back reticular neurons and initiate new spindle oscillation cycle (Timofeev and Chauvette, 2011).
In addition to reciprocal corticothalamic projections ending in dorsal thalamic nuclei from which a given cortical field receives inputs, distinct neocortical areas have access to multiple thalamic nuclei through indirect projections relayed in GABAergic reticular thalamic neurons (Steriade et al., 1993c).
It appears that the TRN provides a stronger inhibition into sensory thalamic nuclei during cortical active states than the ZI onto higher-order nuclei because a majority of TRN neurons fire bursts of action potentials (Avanzini et al., 1989; Contreras et al., 1992; Fuentealba and Steriade, 2005), but ZI neurons fire tonically (Trageser et al., 2006). Therefore, the postsynaptic inhibitory action of TRN neurons is stronger compared with ZI neurons (Sheroziya and Timofeev, 2014).
1.1.3 Neocortex
The neocortex of human is a thin, extended, convoluted sheet of tissue with a surface area of ~2600 cm2, and thickness 3–4 mm. It contains up to 28*109 neurons and
6
from the environment and internal organs flows to neocortical neurons which integrate these signals and transmit the obtained information to the subcortical structures. All neocortical neurons contain two major types of cells: excitatory pyramidal cells (~80%) and inhibitory interneurons (~20%).
1.1.3.1 Pyramidal neurons
Pyramidal neurons have a pyramidal shape in fixed tissue; and look like ovoid in the tissue which is not fixed. All pyramidal neurons have long axons and they are located in all cortical layers except layer I (DeFelipe and Farinas, 1992). A pyramidal neuron usually has numerous dendrites that arise from the upper pole of the neuronal body and could form large dendritic trees (Elston, 2003). The span of the dendritic tree depends on the laminar localization of the cell body, but it may, as in giant pyramidal neurons, spread over several millimeters covering the entire cortical thickness. In addition, pyramidal neurons gives rise to several basal dendrites oriented either horizontally or downward. The axon of pyramidal cells is oriented downward; it originates from the base of neuronal body or from the very proximal part of basal dendrites.
Generally dendrites of pyramidal neurons are covered with enormous number of dendritic spines varied in their size and shape and highly plastic. Most excitatory glutamatergic inputs form synapses with dendritic spines and their extent is considerably varied among different regions and species (Spruston, 2008). In contrast, the soma and the axon hillock receive inhibitory GABAergic inputs.
1.1.3.2 Interneurons
Unlike a large population of pyramidal neurons which constitutes approximately 80% of the entire brain and are mainly glutamatergic, non-pyramidal neurons (also called interneurons) are much smaller population of local circuit neurones, oftenly using γ-aminobutyric acid as a neurotransmitter to inhibit their targets (Somogyi et al., 1998; Markram et al., 2004).
Neocortical interneurons have short axons arborizing within neocortex either in vertical or horizontal direction. The axonal arborization can reveal the anatomical identity of an interneuron because interneurons seem to be particularly specialized to target
7
different domains of neurons, different layers of a column and different columns. On the basis of their axon targets, the interneurons can be functionally divided into axon-targeting, soma- and proximal dendrite-targeting, dendrite-targeting, and dendrite- and tuft-targeting interneurons, and also into intralaminar–intracolumnar, interlaminar–intracolumnar, intralaminar–intercolumnar and interlaminar–intercolumnar interneurons. The relative percentages of each interneuron type vary in different species, brain regions and layers (Markram et al., 2004).
Among interneuronal cells, there are the spiny stellate cells in layer IV, which have a star-shaped dendritic arbor with spines and act as glutamatergic excitatory cells. Mostly they receive inputs from axons of first-order (specific) thalamic nuclei and send projections to cortical layers II-III.
According to the Petilla’s classification interneurons could be identified by their physiological properties in six main types: fast-spiking (FS) neurons (brief spikes and large fast after-hyperpolarizations), non-fast spiking (NFS) neurons (no apparent increase in the interspike interval at steady-state), adapting (AD) neurons (increase in the interspike interval at steady-state), accelerating (AC) neurons (a decrease in the interspike interval at steady-state), irregular spiking (IS) neurons (an irregular interspike interval), intrinsic bursting (IB) neurons which produce a stereotypical burst of two or more spikes followed by a slow afterhyperpolarization potential (DeFelipe et al., 2013).
1.1.3.3 Electrophysiological types of cortical neurons
Neurons composing the neocortex are dramatically diversified, but can be distinguished in four major groups by their intrinsic firing patterns and somatic and dendritic morphology (Connors and Gutnick, 1990; Gray and McCormick, 1996; Steriade et al., 1998; Steriade et al., 2001).
The first group of Regular-Spiking (RS) neurons, which have relatively small cell body and simple dendritic arbors, is considered as a principal type of corticocortical cells (Jacob et al., 2012). RS cells are the most common type of pyramidal cells recorded in the neocortex and fire individual regular spike in response to steady current injection followed by afterhyperpolarization. Increase in stimulus amplitude will cause the decrease in first interspike interval (Connors and Gutnick, 1990). Their axons mainly contribute to callosal
8
connections to the sensory cortex in the other hemisphere (Winer and Prieto, 2001) and also to corticostriatal projections (Sun et al., 2013).
The second type of neocortical cells produce burst of spikes to steady somatic current injection and is called Intrinsically-Bursting (IB) cells. Morphologically they could be recognized by complex dendritic arbors that branch deeper in cortex and large somata (McCormick et al., 1985; Greenhill et al., 2015).
The most apparent characteristic of FS neurons is a firing fast action potentials with a uniquely rapid rate of fall and a prominent postspike undershoot (fast-spiking neurons) and could be found in all layers (McCormick et al., 1985). Interestingly, fast spiking cells (putative inhibitory interneurons) tend to show transient responses and increased their firing rates following stimulus presentation (Baeg et al., 2001).
The last group is represented by specific neurons which intrinsic properties alone are able to generate outputs within the gamma frequency range in the absence of influences from local networks. These neurons display fast rhythmic bursting (FRB) with two to five spikes per burst, with interburst frequencies of 20 Hz and upward, in response to suprathreshold depolarizing current pulse (Gray and McCormick, 1996).
1.1.3.4 Laminar and columnar organisation of cortex
There is the well-known notion of mammalian neocortex as a six-layered structure with stereotyped structure (Douglas and Martin, 2004). The canonical cytoarchitecture of normal cortex is remarkably uniform and has been organized into six laminae (layers) horizontally and into minicolumns of synaptically linked cells vertically. Minicolumns are fundamental blocks of the adult neocortex and they are further interconnected by short-range horizontal links into cortical columns (Mountcastle, 1997; Kaas, 2012).
The first layer of the neocortex located below the pia mater, Layer1(L1), comprises a sparse density of mostly GABAergic neurons and glia along with extensions of apical dendrites from pyramidal neurons of the underlying layers, and horizontally oriented axon collaterals from other neocortical areas and the thalamus (Winer and Larue, 1989; Muralidhar et al., 2013).
Superficial layers 2/3 consist of small pyramidal neurons and numerous interneurons and receive projections from other cortical structures, from the posterior thalamic nuclear
9
group, and from the lateral amygdala (Shrestha et al., 2015). Layers 2/3 display strong inhibitory synaptic transmission between intracolumnar L2/3 local-inhibitor-to-L2/3 pyramidal neuron pairs and receive feed-forward excitatory projections that originate from spiny neurons in layer 4 (Chisum et al., 2003; Hoffmann et al., 2015). Layer 3 pyramidal neurons provide major contribution to interhemispheric dialogue via corpus callosum (Porter and White, 1986; Barbaresi et al., 1989; Barbaresi et al., 1994; Cisse et al., 2003; Cisse et al., 2007).
Layer 4 neurons are the primary recipients for thalamic sensory inputs and integrate the information from intra-hemispheric corticocortical afferents (Miquelajauregui et al., 2015).
Layer 5 consists of two classes of neurons such as large pyramidal cells (including regular-spiking cells and intrinsically bursting cells) and interneurons (McCormick et al., 1985; Hattox and Nelson, 2007). Physiologically, there is a rich diversity among layer 5 neurons. Different subgroups with their own properties have various cortical and subcortical projection targets. Layer 5 is the main output layer of neocortex including cortico-thalamic fibers to higher order thalamic nuclei. It also contributes to the callosal projections between two hemispheres (Fame et al., 2011).
Pyramidal cells in layer 6 receive direct thalamocortical input, establishing layer 6 along with layer 4 as a sensory input layer. However, it is also an output layer which sends large descending projections to various thalamic nuclei (primarily first order nuclei). Corticothalamic neurons of this layer constitute only some 30–50% of the pyramidal cells while corticocortical (CC) cells send long horizontal axons which form connections across cortical columns and cortical areas (Thomson, 2010).
1.1.3.5 Synaptic connectivity in neocortex
There are two main morphological types of synapses in the cerebral cortex:
asymmetric and symmetric synapses, which correspond, respectively, to Gray's type I and II
(DeFelipe and Farinas, 1992). Asymmetric synapses are characterized by a prominent postsynaptic density and symmetric synapses by a very thin postsynaptic density. The number of synapses receiving by each pyramidal cell can reach 5,000 to 60,000 (Mountcastle, 1997; Somogyi et al., 1998).
10
As a rule, neurons contain excitatory synapses on dendrites and dendtritic spines and most of inhibitory synapses are located in the perisomatic region (Szentagothai, 1975; DeFelipe and Farinas, 1992). As action potential usually generates in axon hillock (in some cases in dendrites) synapses that are located closer to this place have a stronger influence on action potential generation than synapses located farther (DeFelipe and Farinas, 1992).
However, synapses distant from the soma might be able to compensate for their distance through the activation of various dendritic voltage-gated channels simultaneously with activation of several synapses (Williams and Stuart, 2003).
The neuronal composition of the neocortex includes both excitatory neurons and GABAergic inhibitory interneurons as well, which form ~20% of the whole number of neurons (Wozny and Williams, 2011). The soma and the axon of pyramidal cell receive inhibitory GABAergic inputs (Spruston, 2008).
The strength of synaptic connections within neocortex and between neocortex and subcortical structures, such as hippocampus, basal ganglia and cerebellum, are modified during different states of vigilance and support the restructure of neuronal circuits.
Up-states in SWs are thought to be initiated by activity in layer 5 (L5) pyramidal cells (Sanchez-Vives and McCormick, 2000; Chauvette et al., 2010; Beltramo et al., 2013; Stroh et al., 2013; Lorincz et al., 2015), but thalamic activity may also contribute to the SW generation (Crunelli and Hughes, 2010; David et al., 2013; Lemieux et al., 2014). Within gamma cycles nested in the slow-wave depolarization, cortical pyramidal cells fired earlier than did interneurons. At the start of slow-wave depolarizations, activity in thalamic neurons receiving inhibition from the basal ganglia occurred earlier than activity in cortical neurons (Ushimaru and Kawaguchi, 2015).
1.2 States of vigilance
All functions and activities of normal brain occur during the three states of vigilance. These are waking state, slow-wave sleep (SWS, sometimes called non-REM sleep), and REM (rapid-eye-movement) sleep (sometimes called paradoxical sleep) (Timofeev and Chauvette, 2011). States of vigilance can be well distinguished by the evaluation of three criteria: electroencephalogram (EEG) or local field potential recordings (LFP), electromyogram (EMG), and electrooculogram (EOG). During wakefulness and REM sleep
11
thalamocortical neurons are relatively depolarized (around -62 mV); during SWS activities, thalamocortical neurons alternate between depolarized (mean around -62 mV) and hyperpolarized (mean around -70 mV) states (Steriade et al., 2001; Timofeev et al., 2001). The available data show that LTS bursts are a rare occurrence in waking (≈1% of action potentials in background activity), whereas they appear during the hyperpolarized state of TC and TRN neurons during slow-wave sleep (Llinas and Steriade, 2006). Burst firing of thalamocortical cells is common in awake but inactive rabbits (Bezdudnaya et al., 2006). 1.2.1 Wakefulness
The electrographic activities of waking state are characterized by activated cortical LFP and/or EEG recordings (high frequency, low amplitude waves), by the presence of a muscle tone (usually irregular), and might show eye movements. The membrane potential of neurons remain relatively stable, displays continuous trains of synaptic events and neurons usually fire action potentials (Timofeev et al., 2000a; Steriade et al., 2001; Timofeev et al., 2001). During wakefulness, animals perform a variety of behavioural patterns, inattentive, quiet or highly reactive, sometimes with quick changes from one to another. For maintaining waking state two pathways are critical: the ascending reticular activating system in brainstem send projections to upper systems (thalamus, hypothalamus and cortex) and the descending pathway to spinal cord is important for muscle activity (Lee and Dan, 2012). The activating system is composed of several neuromodulatory systems such as cholinergic, serotoninergic, and norepinephrinergic, etc. (Wright et al., 2012). Cholinergic neurons are clustered in the pedunculopontine (PPT) and laterodorsal tegmental nuclei (LDT), and they project widely to the thalamus, hypothalamus and basal forebrain causing behavioural arousal and are highly active not only during wakefulness, but also during REM sleep, unlike monoaminergic neurons (McCormick and Bal, 1997). Activation of dopaminergic neurons located primarily in the substantia nigra and in the ventral tegmental areas which project to the striatum and frontal cortex, are important for behavioral arousal, reward seeking, and movement (reviewed in (Wright et al., 2012)). The locus coeruleus consists of small amount of norepinephrine (noradrenergic) neurons that project almost to the entire central nervous system and are involved in attention, enhancing cortical activation, and behavioral and emotional arousal (Berridge, 2008; Greene et al.,
12
2009; Tully and Bolshakov, 2010). Optogenetic stimulation of the noradrenergic neurons can cause an immediate transition from sleep to wakefulness (Carter et al., 2010). Serotonin neurons mostly from the dorsal raphe nuclei project widely to the cortex and subcortical areas and involve in sleep-wake transition (Hale and Lowry, 2011). Importantly, all monoaminergic neurons fire at high rates during wake, but almost inactive during NREM sleep. However, a subset of noradrenergic neurons fire in phase of sleep slow oscillation (Eschenko et al., 2012).
1.2.2 Rapid eye movement sleep
Rapid eye movement sleep, also known as paradoxical sleep, is characterized by cortical activation, rapid eye movements, skeletal muscle paralysis and vivid dreaming. Cholinergic neurons in the pons are activated during this state of vigilance by the glutamatergic sublaterodorsal nucleus and controlled by brainstem serotonergic and noradrenergic systems which promote inhibitory effect on REM sleep (Siegel, 2005; McCarley, 2007; Fraigne et al., 2015). The glutamatergic pathway excites glycinergic and GABAergic neurons, inducing the inhibition of motoneurons and causing muscle atonia. Similar to waking state, the membrane potential of cortical neurons remain relatively stable, displays continuous trains of synaptic events and neurons fire action potentials (Steriade et al., 2001; Timofeev et al., 2001).
1.2.3 NREM sleep (non-rapid eye movement sleep)
The main EEG hallmark of non-rapid eye movement (non-REM) sleep is slow wave oscillation characterized by large amplitude low frequency waves in cortical LFP and/or EEG recordings, a steady muscle tone, and the absence of eye movements (Crunelli and Hughes, 2010; Nir et al., 2011; Brown et al., 2012). This oscillation alters between active (depolarized, Up) and silent (hyperpolarized, Down) states (Steriade et al., 1993b; Contreras and Steriade, 1995; Timofeev et al., 2000a; Steriade et al., 2001; Timofeev et al., 2001; Crunelli et al., 2012). Slow wave activities can be recorded in both cortical and thalamic neurons, in isolated cortical slabs, but not in deafferentated thalamus that points to the cortical origin of slow wave oscillation (Timofeev and Steriade, 1996; Sanchez-Vives and McCormick, 2000; Timofeev et al., 2000b).
13
NREM sleep episodes are usually longer and are often followed by a shorter episode of REM sleep. The NREM – REM sleep cycle repeats itself several times before arousal (Vyazovskiy and Tobler, 2012).
NREM and REM sleep have distinct and complementary contributions to the overall function of sleep. Specifically, cortical slow oscillations, occurring within specific functionally interconnected neuronal networks during NREM sleep, enable information processing, synaptic plasticity, and prophylactic cellular maintenance (“recovery process”). In turn, periodic excursions into an activated brain state—REM sleep—appear to be ideally placed to perform “selection” of brain networks, which have benefited from the process of “recovery,” based on their offline performance. Such two-stage modus operandi of the sleep process would ensure that its functions are fulfilled according to the current need and in the shortest time possible (Vyazovskiy and Delogu, 2014).
1.3. Types of oscillatory activities during SWS sleep
There are many different types of oscillations which are generated within thalamocortical network, however, they could be grouped in several frequency bands. Slow rhythms include the spindle (7-15 Hz), theta (4-7 Hz), delta (1-4 Hz) and slow (<1 Hz) and infra-slow (<0.1 Hz) oscillations. Whereas fast rhythms refer to beta (15-30 Hz), gamma rhythms (30-100 Hz) and ripples (>100 Hz) (Timofeev and Chauvette, 2011). Present brief description is focused on oscillatory activities within slow frequency range which occur during slow-wave sleep.
It is widely accepted that two different phenomena are present during SWS: slow wave oscillation and delta oscillations (Achermann and Borbely, 1997). Spindle activities can be present during SWS, but they represent essential feature of rather stage 2 of NREM– sleep and not SWS.
1.3.1 Slow oscillation
During slow-wave sleep (SWS) and some types of anesthesia the presence of a low frequency (0.3 – 1 Hz) oscillatory activity pattern occurs within the entire cortical network. This activity alternates between silent (Hyperpolarizing, or Down) and active (Depolarizing, or Up) states, each lasting 0.2–1 s. During depolarizing states synaptic
14
activity of cortical neurons is strong and they fire at fast frequencies (Mukovski et al., 2007), throughout silent periods synaptic activity is absent and is mediated by leak potassium current (Timofeev et al., 2001; Chauvette et al., 2011). Slow waves usually start from frontal and midline part of the cortex and then propagate to posterior and lateral areas, however slow wave activity can originate in other regions (Massimini et al., 2004; Volgushev et al., 2006; Nir et al., 2011; Stroh et al., 2013; Sheroziya and Timofeev, 2014). The onset of silent states occurs more or less synchronously across different cortical areas (Volgushev et al., 2006; Sheroziya and Timofeev, 2014).
During active periods of slow wave oscillation in the cortex neurons in reticular thalamic nucleus are depolarized and fire either in tonic or bursting mode with low-threshold Ca2+ spikes (Contreras and Steriade, 1995; Fuentealba and Steriade, 2005), while
thalamocortical neurons display rhythmic IPSPs and occasional rebound LTS with bursts (Timofeev and Steriade, 1996).
Animal studies demonstrated that spontaneous active states of cortical slow oscillation start can start in any layer, but more often layer 5 neurons are the first to demonstrate transition to active states (Sanchez-Vives and McCormick, 2000; Chauvette et al., 2010). Optogenetic stimulation of layer 5 but not layer 3 pyramidal cells was sufficient to trigger network wide active sates in mice (Beltramo et al., 2013). This low-frequency bursting remains stable after block of GABA and ionotropic glutamate receptors and this putative driver fire in synchrony with LFP fluctuations at the time point of slow waves onset (Lorincz et al., 2015). However, intracranial recordings on epileptic patients suggested, that in human, at least in these pathological conditions, the active states might start in layer 3 (Cash et al., 2009; Csercsa et al., 2010)due to increase of the excitability in upper layers.
The full expression of the slow (<1 Hz) sleep rhythm in the EEG requires the fine interplay of cortical and thalamic oscillators with the low-threshold Ca2+ potential (LTCP)
mediated burst at the onset of the TC neuron UP states providing a robust signal for the start of a new UP state in somatotopically linked cortical territory and for a global increase in intracellular Ca2+across the entire TC neuron dendritic tree (Crunelli et al., 2011).
15
Slow oscillations phase topographically orchestrate (1) spindle and gamma power, (2) spindle phase synchrony, and (3) local and interregional phase amplitude coupling between spindles and beta activity (Steriade et al., 1993a; Cox et al., 2014).
It was hypothesized that slow oscillations might help synaptic consolidation or produce synaptic downscaling and increase signal-to-noise ratios in relevant neural circuits (Huber et al., 2004). However, later studies directly demonstrated that sleep slow oscillation induces long-term potentiation of cortical synapses (Chauvette et al., 2012).
Slow wave sleep may be essential for memory consolidation and memory formation. It has been proposed that synaptic plasticity associated with slow and delta oscillations could contribute to the consolidation of memory traces acquired during wakefulness.
1.3.2 Delta oscillation
Delta oscillations (1-4 Hz) are present on field potential recordings from neocortex during the deepest stages of sleep. Two distinct mechanisms are supposed to be rhythmogenic for delta oscillations: the first one originates in the neocortex in layer 5, and the other is relying on intrinsic properties of the thalamocortical cells.
Interactions between hyperpolarization activated cation current (h-current) and low-threshold Ca2+ current (T-current) of thalamic relay neurons produce delta activity. Thus,
delta oscillation can be observed during NREM sleep, when thalamic neurons are hyperpolarized enough to activate Ih (McCormick and Pape, 1990).
The ionic basis of cortical generation of delta oscillation is poor understood. It may include the activation of intrinsic bursting pyramidal neurons in layer 5 and subsequent activation of potassium leak current. Cortical delta activity is increased upon large thalamic lesions (Villablanca and Salinas-Zeballos, 1972).
Comparison of cortical slow-wave activity between SWS and anesthesia showed higher power in the slow/delta (0.1-4 Hz) and spindle (8-14 Hz) frequency range during sleep, while under anesthesia the power in the gamma band (30-100 Hz) was higher. During anesthesia, slow waves were more rhythmic and more synchronous across the cortex. Intracellular recordings revealed that silent states were longer and the amplitude of membrane potential around transition between active and silent states was bigger under anesthesia. Slow waves were largely uniform across cortical areas under anesthesia. By
16
contrast, in SWS they were most pronounced in associative and visual areas, but smaller and less regular in somatosensory and motor cortices. Although the main features of the slow oscillation in sleep and anesthesia appear similar, multiple cellular and network features are differently expressed during natural SWS as compared to ketamine-xylazine anesthesia (Chauvette et al., 2011).
1.4 Thalamic contribution to the generation and modulation of the slow wave activity Thalamic relay neurons have been proposed to participate in two types of thalamocortical circuits (Sherman and Guillery, 1996). The first-order (FO) relays transmit peripheral information to the cortex, while the higher order (HO) relays mediate transthalamic-cortico-cortical flow. The ventrobasal complex in the dorsal thalamus is composed of the ventral posterior medial (VPM) and the ventral posterior lateral (VPL) nuclei of the thalamus with face representation in the VPM nucleus and body representation in the VPL nucleus (Vazquez-Garcia et al., 2014). The midline and intralaminar thalamic nuclei represent non-specific (matrix) relays, which innervate numerous targets including the neocortex, striatum, reticular thalamic nucleus, amygdala, subthalamic nuclei etc.
1.4.1 VPM nucleus projects to the primary somatosensory cortex
Certain thalamic neurons have been shown to participate in only one of these circuits. For instance, in rats the ventral posteromedial nucleus (VPM) consists of neurons that are contacted by driving inputs originating solely in the somatosensory trigeminal nuclei, forming lemniscal pathway, and thus VPM is referred to as the FO somatosensory nucleus (Land et al., 1995; Landisman and Connors, 2007; Jones, 2009). Ventral basal complex cells (including VPM) fired intrinsic bursts when activated from relatively hyperpolarized membrane potentials (more negative than – 70 mV) and tonic spikes when activated from more depolarized potentials (more positive than − 55 mV) (Turner et al., 1997; Rosanova and Timofeev, 2005). TC cells in VPM nucleus fire only a single burst following a depolarizing step from hyperpolarized holding potentials. If a long (several hundred milliseconds), strong depolarizing step was delivered from a holding potential equal to or more hyperpolarized than − 70 mV, neurons within VPM fired a fast burst of action potentials followed by tonic spikes (Landisman and Connors, 2007).
17
In rodents VPM neurons from each barreloid respond to a different feature, but of one whisker (Timofeeva et al., 2003).
1.4.2 CL nucleus has widespread projections across all cortical areas
Anterograde and retrograde tracing studies have demonstrated that numerous passing fibres extending from intralaminar thalamic nuclei (including central lateral nucleus) go to the ipsilateral cerebral cortex predominantly.
The central lateral nucleus is considered to be a source of input to the frontal and parietal cortical fields and striatum (Deschenes et al., 1996). Its projections include the agranular fields of the frontal cortex, caudal parts of the parietal cortex, and medial agranular field of the motor cortex. A sparse projection from the central lateral nucleus to the ventrolateral orbital cortex was also observed with retrograde tracing methods (Berendse and Groenewegen, 1991).
18
1.5 General objective and hypothesis
The thalamus is essentially required for complete establishment of cortical slow oscillation, but the role of different thalamic nuclei remains poorly investigated. We hypothesized that the first-order (specific) thalamic nuclei provide a control of slow waves in primary cortical areas, while higher-order (non-specific) thalamic nuclei may synchronize the slow-wave activities across wide cortical regions. To examine the contribution of first-order and higher-order thalamic nuclei, we have injected the selective GABAA-agonist muscimol in the given nucleus in order to inactivate the neuronal activity of thalamocortical afferents and evaluated the alternation of cortical activity.
19
Chapter II
20
2.1 Experiments on anesthetized mice
Experiments were carried out in accordance with the guideline of the Canadian Council on Animal Care and approved by the Université Laval Committee on Ethics and Animal Research. Young male and female C57BL/6 mice of the same age and weight were used in experiments. Animals were housed in a humidity-controlled facility maintained on a 12-h light/dark cycle (lights on at 7:00 a.m.). All animals were kept with food and water available ad libitum. The mice were purchased from Charles River Laboratories. First, mice were anesthetized with ketamine and xylazine (100 and 10 mg/kg, i.p.) and placed in a Kopf stereotaxic frame. We chose this type of anesthesia because under ketamine-xylazine anesthesia, the EEG displays a slow oscillation that is similar to the slow oscillation occurring during natural slow-wave sleep (Chauvette et al., 2010; Sheroziya and Timofeev, 2015). Lidocaine (0.5%) was injected for local anesthesia subcutaneously before skin dissection. Two screws (stainless steel, 1 mm diameter) were placed in the bone over the somatosensory cortex (S1, lateral: 2.5 mm; anteroposterior: -1.5 mm from bregma (Paxinos and Franklin, 2001)) and cerebellum (reference electrode) in the right hemisphere to monitor the EEG. Additional anesthesia was administered when required (pinch withdrawal reflex is active or whisker twitching is observed) or when EEG slow waves started weakening. The temperature of the platform in the stereotaxic frame where the mouse was placed on was maintained at 37°C. To avoid cortical surface drying, a mineral oil (Sigma) or saline (0.9%) were applied over the exposed area.
2.2 Inactivation of thalamic nuclei
Thalamic nuclei were inactivated by injection of 0.5 µl muscimol (1%). Muscimol, a constituent and psychoactive ingredient of the mushroom Amanita muscaria, is regarded as a potent selective agonist at ionotropic receptors for GABA (gamma-aminobutyric acid) which is classified as an inhibitory neurotransmitter (Krogsgaard-Larsen et al., 1979; Johnston, 2014).It is described as universal agonist for all γ-aminobutyric acid type A receptor (GABAA-R) subtypes, with preferential efficacy and selectivity, but not specificity, to high-affinity receptor subtypes (Chandra et al., 2010).
Blue Coomassie dye (0.5%) was added to the solution to confirm histologically sites of the injection.
21
The injection of muscimol was achieved via micro-pipette pulled from 1.5mm borosilicate. The tip of the pipette was broken to about 10 m and it was back-filled with 1% muscimol, 0.5 %Blue Coomassie diluted in saline. Then two thin copper wires were inserted in the pipette base and it was sealed with epoxy. To inject drugs 5 current pulses of 75 mA were given via copper wires that released gas into the pipette, which pushed out about 0.5 l of solution.
2.3 Electrophysiological recordings
In acute conditions we used several experimental techniques:
1. In order to investigate cortical slow-wave activity in control and after thalamic inactivation we performed local field potentials (LFP) recordings from different cortical areas (Fig. 1D). A six tungsten electrode recording array was positioned along the anterior-posterior axe and lowered from the cortical surface in 0.5-0.6 mm depth. The distance between electrodes of array was 1.5 mm and virtually divided the cortex into 6 cortical zones such as frontal (lateral: 1.3 mm, anteroposterior: 3.2 mm), motor 1 (lateral: 1.3 mm, anteroposterior: 1.7 mm), motor 2 (lateral: 1.3 mm, anteroposterior: 0.2 mm), somatosensory 1 (lateral: 1.3 mm, anteroposterior: -1.3 mm), somatosensory 2 (lateral: 1.3 mm, anteroposterior: -2.8 mm), visual (lateral: 1.3 mm, anteroposterior: -4.3 mm). We recorded slow-wave activities with monopolar LFP electrodes before and after injection of muscimol in a given thalamic nucleus. Slow wave activities in the slow-delta frequency range (0.2 – 4 Hz) are characteristic of the EEG during slow-wave sleep (SWS) or anesthesia (Fig. 1 E, upper traces). Activities in the delta frequency range can be quantified by computing delta power (Franken et al., 2001). To the end we performed Fast Fourier Transform analysis which shows a power of activities in all recorded range of frequencies (Fig. 1 E, lower histograms) and computed area under frequency range of 0.2-4 Hz (Fig. 1 E, lower histograms, filled bars). A five-minutes recording before muscimol injection and after muscimol injection from all channels were cut in 2 sec sliding window with 1 sec overlap. The Fast Fourier Transform analysis was performed on each of these segments. Then we calculated the area covered by resulting Fast Fourier Transform curve in the frequency range of delta waves. The results of each of these calculations are shown as individual points on plots in figures 3-5.
22
2. Extracellular recordings in thalamic nuclei in control and after injection of muscimol. In order to investigate multiunit activities of neurons within the thalamic nucleus we developed recording tool comprising two or four platinum-iridium electrodes (stereotrode) (12 or 25 µm in diameter). Then we filled glass micropipette with drug solution (muscimol in 0.9% saline) and glued it to the electrodes thus the tips of both were placed very close (Fig. 1A and C). Multiunit activities of first-order thalamic nucleus were recorded in ventral posterior medial nucleus (VPM, lateral: 1.5 mm, anteroposterior: - 1.7 mm, depth: 3.6 mm), MUA of higher-order thalamic nucleus were recorded in central lateral nucleus (CL, lateral: 0.7 mm, anteroposterior: - 1.6 mm, depth: 2.5 mm). In order to increase a number of identified neurons in VPM nucleus we applied airpuff stimulation to whisker system of mouse in order to identify neurons within this sensory nucleus. The neuronal firing was recorded before and after muscimol injection.
3. Intracellular recordings. Intracellular recordings from the given cortical areas were performed by using glass micropipettes pulled from borosilicate glass capillaries (WPI; P-97, Sutter Instrument) and filled with a solution of 2 M potassium acetate (DC resistance of 50–70 MΩ). In each experiment dura was removed after applying a small drop of 3% peroxide on the dura as it was described previously (Sheroziya and Timofeev, 2014). A high-impedance amplifier (Neurodata IR-283 amplifiers; Cygnus Technology) with active bridge circuitry was used to record the membrane potential and to inject current into the neurons. Simultaneously, LFP in frontal and somatosensory cortical areas and EEG recordings were obtained. To stabilize the intracellular recordings the fourth ventricle was opened and agar (4% in saline 0.9%) was applied filling the hole made in the skull.
In all cases we put mineral oil (Sigma) or saline on the brain surface to prevent it from drying.
The intracellular recordings from the same neurons were performed within somatosensory and frontal cortices before and after thalamic administration of muscimol. 2.4 Histology
To verify the site of muscimol injection immediately after completion of experiment, the animal was deeply anesthetized with either ketamine-xylazine or penthobarbital and then perfused transcardially with saline followed by 4% paraformaldehyde solution. After
23
the perfusion brains were kept in 4% paraformaldehyde overnight. Serial slices of 70 µm of the required region were cut with a vibratome and prepared for rouge neutrine staining. The brain slices were analysed under a microscope to determine the injection sites. The brain areas were identified by comparison with a mouse brain atlas (Paxinos and Franklin, 2001). 2.5 Data analysis
Raw signal was filtered (0.1-10 kHz), amplified via AM 3000 amplifiers (A-M Systems) with custom modifications, and provided into LabChart with 20 kHz sampling rate. Further analysis was performed offline on a personal computer using IgorPro (WaveMetrics) software. Spikes were detected using a threshold. Data are expressed as mean± SD or SEM when indicated. Statistical analyses were performed in Prism 6 software (GraphPad). Depending on the normality of data and the equality of variances we used either parametric two-tailed t-test or non-parametric tests (Mann–Whitney sum rank test). Spiking activity of given thalamic nuclei before and after injection of muscimol was evaluated by peristimulus time histogram (bin width 1 ms) or autocorrelograms. To measure delta power (0.2-4 Hz) Fast Fourier transform analysis was used for local field potential (LFP) recordings for all investigated cortical areas as described above.
24
Chapter III
25
Our experiments were designed to study thalamic mechanisms in the generation and modulation of the cortical slow oscillation. First, we investigated the ability of GABA agonist muscimol to induce inactivation of neurons in the thalamic nuclei. Then we compared the effects of inactivation of different thalamic nuclei (first-order vs. higher-order) onto slow wave activities in the neocortex. The further question was what features of individual neuronal activities were responsible for the reduction in delta power after inactivation of higher-order thalamic nucleus. The last group of experiments were performed using intracellular and LFP recordings in frontal and somatosensory cortices before and after inactivation of non-specific thalamic nucleus.
3.1 Muscimol injection in VPM and CL nuclei abolishes spiking activity of neurons and does not trigger bursting
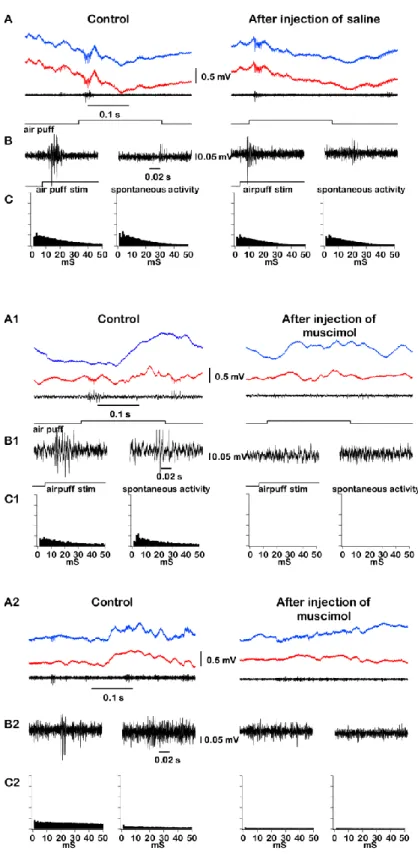
Muscimol is known as a strong GABAa agonist and produces inhibitory effect on neuronal activities. In turn, thalamocortical cells are known for their ability to fire bursts of spikes when a neuron is going from hyperpolarized to depolarized state (Fig. 2B, (Jahnsen and Llinas, 1984)). Therefore, muscimol can either silence neurons or promote burst firing. In order to study the possibility that muscimol would cause the burst triggering in thalamic relay neurons or not we performed several experiments on anesthetized mice. Animals were anesthetized with ketamine-xylazine and placed in stereotaxic frame (Fig. 2A). We used stereotrode or tetrode (made of platinum-iridium microwires of 25 or 12 µm diameters respectively) to obtain extracellular multiunit recordings from either first-order (VPM) or higher-order (CL) thalamic nuclei (Fig. 2C). Also we characterized the firing properties of VPM neurons applying air-puffs of 0.2 s duration at 0.5 Hz to whisker system of mouse (Fig. 3A, B and 3A1, B1). In intact brain high-pass filtered waves showed multiunit activity with a typical burst of spikes of thalamocortical cells recorded within VPM and CL nuclei (Fig. 3B1 and 3B2 respectively). Peri-event histograms of spikes in control conditions (Fig. 3C1 and 3C2, left panel) and after muscimol injection (Fig. 3C1 and 3C2, right panel) revealed a dramatically decreased spiking activity of neurons within both nuclei. We concluded that the injection of muscimol strongly reduced the spiking activity and did not potentiate generation of low-threshold spike mediated bursts.
26
3.2 Effect of saline injection in the VPM nucleus on slow-wave activities during anesthesia
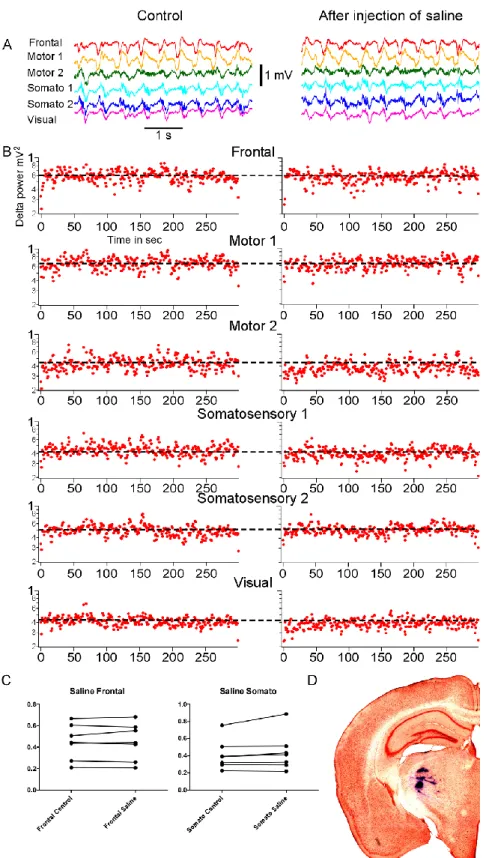
Before investigating the effect of thalamic inactivation on cortical slow oscillation we decided to measure the impact of biologically neutral solution delivery into the place of recording through glass micropipette. Whether the method of liquid delivery via micropipette with current applying would affect activities of neurons or not. Extracellularly recorded activities of cells in VPM nucleus were filtered at 500 – 20000 Hz and displayed trains of action potentials. Saline was injected in VPM nucleus. The site of injection was verified histologically (Fig. 4D). The high-pass filtered LFP signal displayed multiunit activities with a typical burst of spikes of thalamocortical cells recorded within VPM (Fig. 3A, B). Peri-event histograms of spikes in control conditions (Fig. 3C, left panel) and after saline injection (Fig. 3C, right panel) demonstrated that injection of saline within VPM thalamic nucleus didn’t cause any changes in spiking activity of neurons. Then we analysed LFP recordings obtained from different cortical areas before and after saline injection (Fig. 4A, left and right panel respectively) and delta power was measured for each electrode (Fig. 4B, left panel showing results for control conditions, right panel for recordings after saline administration). Each dot on the plots represents the area between 0.2 and 4 Hz for a two second segment (sliding time window of 1 sec) of delta power. After saline injection we did not observe any change in cortical slow wave activities (t test, Wilcoxon matched pairs-signed rank test p>0.05, Fig. 4C).
3.3 Effect of thalamic inactivation of the VPM nucleus on slow-wave activities during anesthesia
VPM nucleus is a first-order thalamic nucleus and projects to the primary somatosensory cortex (Sherman and Guillery, 1996; Jones, 1998). At the beginning we performed multiunit recordings in VPM nucleus with microwire stereotrode with airpuff whiskers stimulation (Fig. 3 A1, B1, C1). Glass micropipette with drug solution was glued to the microwire electrode so that tips were located very close and we could be assured that the site of recordings coincides with the site of injection (Fig. 5D). Simultaneously we recorded local field potentials from different cortical areas (frontal, motor1, motor2, somatosensory1, somatosensory2, visual, see Methods section for coordinates) which
27
demonstrated the presence of a robust and synchronized slow oscillation (Fig. 5A, left panel, control). When we abolished the thalamic output to the somatosensory cortex by the inactivation VPM nucleus, the slow wave activities recorded from the electrode located in this corresponding somatosensory cortical area displayed dramatic reduction, while delta power in other cortical regions remained stable (Fig. 5A, B, right panel, after inactivation). Sometimes slight recovery of delta power was observed, but still remained lower than in the intact cortex. The further statistical analysis confirmed these findings (t test, Wilcoxon matched pairs-signed rank test p<0.05, Fig. 5C). For statistical analysis only 5 minutes before and after inactivation recording samples were used.
As it was hypothesized the inactivation of the VPM nucleus by muscimol had a local effect, a reduction of the delta power occurred only in one electrode located in the somatosensory cortex.
3.4 Effect of thalamic inactivation of the CL nucleus on slow-wave activities during anesthesia
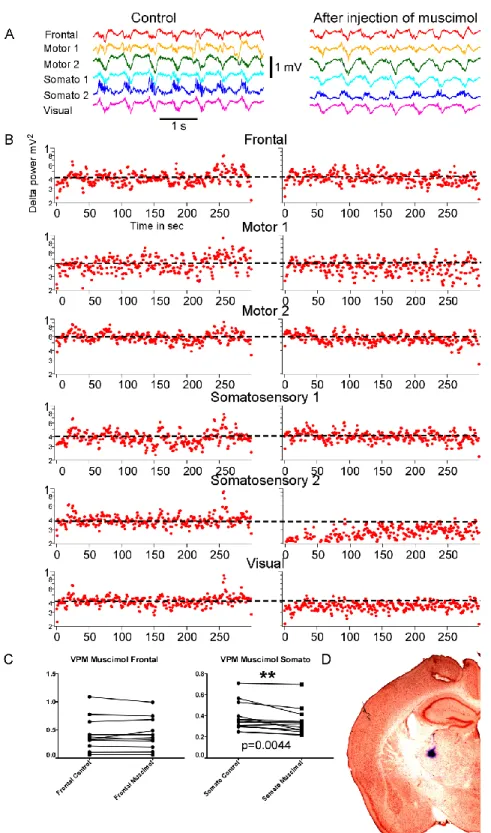
CL nucleus is a higher-order thalamic nucleus from intralaminar group of thalamic nuclei and projects to the different cortical areas (Van der Werf et al., 2002). To quantify changes in slow wave activities induced by thalamic inactivation, we performed the LFP recordings using six-electrode array in control and after muscimol injection in the CL nucleus (Fig. 6A, left and right panels, respectively). The sites of injection were identified in each experiment by histological analysis (Fig. 6D). Visual inspection suggested a strong reduction of slow waves after muscimol injection (Fig. 6A, right panel). Our observations were confirmed by analysis of delta power dynamic, which in response to CL inactivation revealed significant decrease in delta power in all investigated cortical areas (Fig. 6B, t test, Wilcoxon matched pairs-signed rank test p<0.05 and p<0.005 for frontal and somatosensory cortices, respectively, Fig. 6C).
3.5 Intracellular recordings
When muscimol was applied to higher-order thalamic nuclei the delta power was reduced across wide cortical regions. In order to study the intracellular mechanisms of this reduction we presumed two possibilities: either a reduction of amplitude of slow waves in
28
individual neurons or a reduction in synchronization among local neuronal populations. While the second possibility remains the matter of further study, we investigated the first one using single cell intracellular recordings from neurons in the frontal and somatosensory cortices before and after injection of muscimol in the CL nucleus (Fig. 7 and 8). We recorded intracellularly eight cells (frontal n=5, somatosensory n=3). The resting membrane potential (Vm) was – 60 ± 2.5 mV (mean + SE), spike amplitude was - 65 ± 9
mV. The intracellular recordings were performed simultaneously with EEG recorded in the contralateral hemisphere, LFPs recorded in frontal and somatosensory areas of the cortex ipsilaterally.
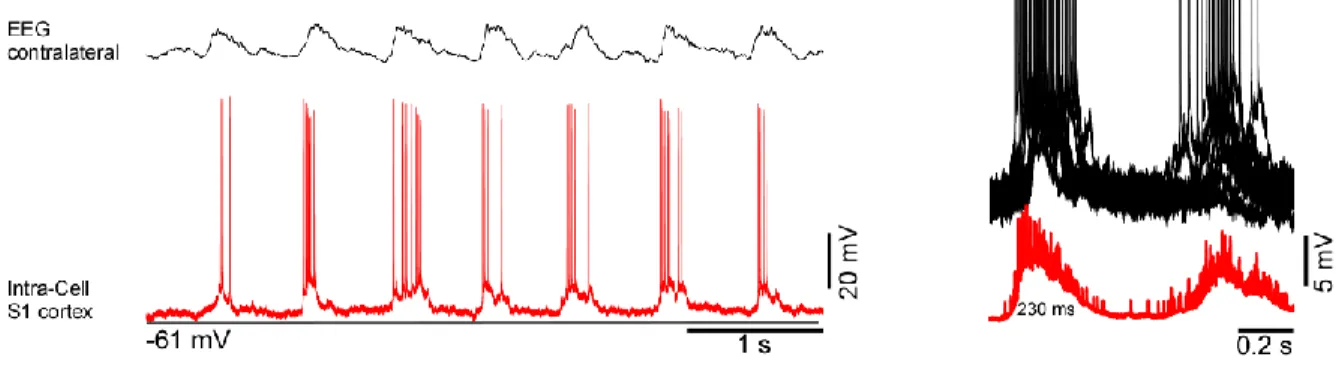
Superpositions of segments of intracellular activities of recorded cells in the frontal cortex before and after inactivation of CL nucleus showed that duration of active states measured at half-amplitude in control was 632 ms, and after injection of muscimol became 193 ms (Fig. 7).
The duration of active states in the somatosensory cortex was also decreased after CL nucleus inactivation (Fig. 8, control: 230 ms; injection: 124 ms).
29
Chapter IV
30
In this study we investigated thalamic contribution to the generation and modulation of the cortical slow oscillation. The sufficiency of the cortex for the generation of slow oscillation is proven by several studies including experiments with thalamic lesions, cortical slabs, cortical slices, cortical cell cultures (Timofeev and Steriade, 1996; Sanchez-Vives and McCormick, 2000; Timofeev et al., 2000b; Cossart et al., 2003; Hinard et al., 2012). Thus, oscillations fluctuating at a lower frequency between UP depolarized state and DOWN hyperpolarized state can be recorded from relatively small cortical circuits, but not from the thalamus of decorticated animals.
Although slow oscillation has cortical origin, it is already well known that thalamus is absolutely required for complete emergence of EEG slow waves during non-REM sleep (Crunelli and Hughes, 2010; David et al., 2013; Lemieux et al., 2014; Sheroziya and Timofeev, 2014). During DOWN state thalamocortical relay cells are hyperpolarized and fire a post-inhibitory rebound spike-burst under inhibitory processes providing by TRN, which is followed by depolarized state in the cortex (Sheroziya and Timofeev, 2014). It would seem that thalamocortical projections are important in the initiation of UP states and the termination of the slow wave activity.
However, the role of different thalamic nuclei involving in this behavioural process is still poorly explored. First-order (specific) thalamic nuclei send their projections to the primary cortical areas, whereas higher-order (non-specific) thalamic nuclei have widespread projections and receive corticofugal inputs from different cortical areas. We hypothesized that 1) specific nuclei control slow wave activities in targeted cortical areas (VPM to the somatosensory cortex), 2) while non-specific nuclei might influence the synchronization of slow wave activities across wide cortical regions.
We decided to address this issue by using the GABAA agonist muscimol in order to inactivate either first-order thalamic nuclei (VPM) or higher-order thalamic nuclei (CL) and analysing the deafferentation influence on slow wave dynamics in the cortex. We conducted experiments on mice anesthetized with ketamine-xylazine, which mimics electrographic brain activities recorded during SWS.
Previous studies demonstrated that inactivation of thalamic inputs to the cortex reduced the occurrence of active states of the slow oscillation and showed dramatic decrease in slow waves (David et al., 2013; Lemieux et al., 2014).
31
In control conditions, LFP recordings in the cortex (6-electrode array or 2 tungsten electrodes in frontal and somatosensory cortex) under ketamine-xylazine anesthesia demonstrated the presence of the robust and synchronized slow oscillation (Fig. 1). Airpuff applied as sensory stimulation during recordings in VPM nucleus of the thalamus at 2 Hz evoked strong response of spiking activity of neurons in this nucleus also provided confirmation of location, in addition to stereotaxic and morphological physiological identification of this nucleus. The CL nucleus was identified by morphological location. As expected, in both conditions (before and after injection of muscimol) all cortical neurons displayed depolarized UP states and hyperpolarized DOWN states and thalamic neurons fired spikes. In a previous study (Lemieux et al., 2014) when LP nucleus of thalamus in cats was inactivated by QX-314 (sodium channel blocker), the slow waves in the targeted parietal cortical regions were reduced. Here too, after injection of GABAA-agonist muscimol in VPM an airpuff stimulation applied to whiskers did not elicit any response indicating that this thalamic site was effectively inactivated. The slow wave activities in corresponding cortical area, somatosensory cortex, were decreased after inactivation of VPM nucleus.
Inactivation of specific thalamic nuclei (VPM) decreased the power of slow/delta band in primary cortical areas. We could identify that the injection of muscimol in ventral posterior medial nucleus which sends projections to the somatosensory cortex has local effect and reduces the slow wave activities only in the corresponding area. Delta power recorded from the other electrodes remained stable after the inactivation.
The inactivation of a non-specific nucleus (CL) with muscimol, affected all cortical areas. The increased inhibition of the thalamocortical cells, which innervate different cortical regions, induced the reduction of slow/delta activity across all cortical regions. It is well known that the efficacy of thalamocortical connections from intralaminar nuclei is low (Morison and Dempsey, 1941). However, an inhibition of CL nucleus induces major behavioral arrests and induces sleep-like cortical LFP activity (Giber et al., 2015). Here we started from sleep-like state, and we demonstrated that inactivation of CL nucleus leads in further decreases of cortical activation, which is primarily reflected in a decrease in the duration of cortical active states of slow oscillation.