T
T
H
H
È
È
S
S
E
E
En vue de l'obtention du
D
D
O
O
C
C
T
T
O
O
R
R
A
A
T
T
D
D
E
E
L
L
’
’
U
U
N
N
I
I
V
V
E
E
R
R
S
S
I
I
T
T
É
É
D
D
E
E
T
T
O
O
U
U
L
L
O
O
U
U
S
S
E
E
Délivré par l'Université Toulouse III - Paul Sabatier Discipline ou spécialité : Biologie
JURY
Prof. Georges DELSOL, Président Dr. Dominique WEIL, Rapporteur
Dr. Serge ROCHE, Rapporteur Dr. Edouard BERTRAND, Examinateur
Dr. Dominique MORELLO
Ecole doctorale : Biologie-santé-biotechnologie Unité de recherche : UMR 5547
Directeur(s) de Thèse : MORELLO Dominique Rapporteurs : Serge ROCHE et Dominique WEIL
Présentée et soutenue par Mohamad-ALI FAWAL Le 17 septembre 2009
Comme le veut la tradition, je vais tenter de satisfaire au difficile exercice de la page
des remerciements, peut-être la tâche la plus ardue de ces années de thèse. Non qu'exprimer
ma gratitude envers les personnes en qui j'ai trouvé un soutien soit contre ma nature, bien au
contraire. La difficulté tient plutôt dans le fait de n'oublier personne. Ces remerciements
sont rédigés dans un moment de doux relâchement intellectuel, sans véritable rigueur ni souci
taxinomique.
J'exprime mes profonds remerciements à ma directrice de thèse, Dominique
MORELLO, pour l'aide compétente qu'elle m'a apportée, pour sa patience et son
encouragement à finir un travail commencé il y a longtemps. Elle a su me laisser la liberté
nécessaire à l'accomplissement de mes travaux, tout en y gardant un œil critique et avisé.
Merci du temps qu’elle m’a consacré à redonner un peu de rigueur à mon français très
approximatif et à ma plume qui a tendance quelques fois à déraper... Pour tout cela, sa
confiance et son soutien financier en fin de thèse, je la remercie vivement.
Je remercie Serge ROCHE et Dominique WEIL de m'avoir fait l'honneur en
acceptant de juger ce travail et d’en être les rapporteurs, d'autant que je dois reconnaître que
je ne leur ai pas facilité la tâche. Je vous remercie pour vos conseils et vos suggestions qui ont
permis l’amélioration de ce manuscrit tant sur la forme que sur son fond.
Je remercie Georges DELSOL et Edouard BERTRAND. Je suis très sensible à
l’honneur que vous me faites en acceptant de participer à ce jury de thèse. Soyez assurés,
Messieurs, de mon plus profond respect.
Je remercie tout particulièrement Nathalie VANZO pour nos fréquentes discussions,
pour tout ce que tu as fait pour moi pendant mon DEA et puis ma thèse. Je tiens enfin à te
remercier pour ton implication dans ce travail de thèse, pour ton aide, ta disponibilité, tes
nombreux conseils et ton soutien sans faille à des moments critiques où je manquais de
repères. Merci…
Je pense particulièrement à Henri DUPONT, pour la finesse de ses attitudes sur le
plan aussi bien humain que scientifique. Tu m’as tout d’abord enseigné les techniques de
biologie moléculaire ainsi que les techniques de biochimies que tu connais. C’est à cette
occasion que j’ai pu apprécier ta rigueur scientifique mais aussi ton humour. J'espère que
cette thèse sera un remerciement suffisant au soutien et à la confiance dont tu as fait preuve
en mon égard.
J’ai eu également le plaisir de collaborer avec Olivier JEAN-JEAN. Sa manière bien
à lui de pousser continuellement à la remise en question m'a été d'une aide précieuse.
«Faire une thèse» est une échappée solitaire au milieu de compagnons de fortune (ou
d'infortune). On ne se rend pas toujours compte à quel point ils peuvent être importants dans
le travail et dans la vie, jusqu'au jour où nos chemins se séparent. Il y a cependant des
personnes qui ne m’entendront certes jamais et qui n’auront plus jamais l’occasion de me lire.
Et c’est le lieu pour leur rendre un vibrant hommage. Je remercie en vrac « On a besoin de
plus de pluie » Jacques, « google » Thomas, « le savent fou » Rami, « l’inséparable » Dani,
« peace and love » Magalie, « le Respect » Aicha, « Nemesis » Mai, l’équipe de foot , mes
potes de danse ainsi que
la promo M2R 2004/5 avec sa troupe de shagasses et ses soirées
rhum arrangé
et surtout les filles de 2ème étage. Merci pour leur soutien et leur confiance
renouvelée lors des derniers mois de ma thèse.
En plus de me supporter dans des conditions «normales», ce qui n'est déjà pas une
mince affaire je le conçois, ils ont du subir mon humeur de barbu en fin de thèse. Pour leur
témoignage d’amitié et de complicité, je remercie « Mollets » Math, « Frotman» Romain,
« Frotwoman» Caro, « Poussin » Cricri et «Cosmopolitan » Nath. De même, je remercie et
exprime toute mon amitié doublée de reconnaissance à Florent « mon sauveur à plusieurs
reprise», sa grand-mère (parce qu’il m’a demandé de la mettre) et « pas de prise de tete »
Adrien, l’escapade au Portugal c’était génial, on la refera, et cette fois si, sans passer par la
case hôpital ! Ah Adrien !
La qualité des données présentées ici sont le fruit d’un travail d’équipe sur le terrain
et de maintenance soutenue des plateformes d’imagerie IFR-109, confocal (« Brice
RONSIN » et « Aurelie LeRue ») , électronique (« Nacer BERNARDIE » et « Stephanie
BALOR ») ainsi que la plateforme protéomique IPBS ( « Bernard MONSORRAT » et
« Carine FROMENT ») et la plateforme bioinformatique IFR- 150 « Jason S.
JACOVONI ». Pour la patience et la disponibilité dont ils ont fait preuve pour discuter
certains des aspects scientifiques et techniques du sujet, mais aussi, pour résoudre les
difficultés que j'ai rencontré avec l'utilisation des outils et du matériel informatique, mes
dettes de reconnaissance sont, à ce point de vue, énormes à leur égard.
Merci aux membres du Centre de Biologie du développement de Toulouse pour leur
accueil, leur soutien et leur confiance.
Cela va de soi, je remercie évidemment ma famille pour son irremplaçable et
inconditionnel soutien. Ils ont été présents pour écarter les doutes, soigner les blessures et
partager les joies. Cette thèse est un peu la leur.
Enfin, une pensée émue pour tous les étudiants avec qui j’ai partagé une salle, un
café, un repas ou une console d’ordinateur pendant ces quatre années... et toute la troupe du
très convivial Laboratoire CBD…
2
TABLE OF ILLUSTRATIONS ...5
TABLE OF ABBREVIATIONS...9
I. Introduction ...14
I.1. Anaplastic large cell lymphomas... 17
I.1.1. ALK function in normal cells ... 17
I.1.2. ALK function in cancer ... 19
I.1.2.1. ALK fusion proteins ... 20
I.1.2.2. Contribution of ALK or its N-terminal partner to oncogenesis... 21
I.1.2.3. ALK mediated oncogenic transformation ... 22
I.1.3. ALCLs therapeutic approaches ... 24
I.2. mRNA metabolism and Cancer... 25
I.2.1. mRNA decay ... 26
I.2.2. mRNA cis-regulatory elements ... 27
I.2.3. Trans-acting factors ... 29
I.2.3.1. sncRNAs... 29
I.2.3.2. mRNA binding proteins... 30
I.2.3.2.1. AUBPs and ARE-mediated decay (AMD) ... 31
I.2.3.2.2. AUF1 ... 33
I.2.3.2.3. HuR... 35
I.2.3.2.4. TIA/TIAR... 37
I.2.3.2.5. Tristetraprolin (TTP) and BRF proteins ... 39
I.2.3.2.6. K homology-type splicing regulatory protein (KSRP) ... 40
I.3. Cytoplasmic granules ... 42
I.3.1. Stress granules ... 42
I.3.1.1. Stress-activated signaling cascades ... 43
I.3.1.2. SG protein composition ... 44
I.3.1.3. SG associated-mRNAs and triage ... 45
I.3.2. Processing bodies... 46
I.3.2.1. PBs discovery and composition... 47
I.3.2.2. Role of PBs... 49
I.3.3. PBs and SGs revisited ... 51
I.4. Working hypothesis... 53
II. Results...55
II.1. A "liaison dangereuse" between AUF1/hnRNPD and the
oncogenic tyrosine kinase NPM-ALK ... 56
II.2. Interplay between processing bodies and specialized cytoplasmic
mRNA-Containing granules in ALK-Tumorigenic cells ... 57
II.3.1. AGs size and number vary under different cellular conditions ... 59
II.3.1.1. AGs and cell proliferation ... 59
II.3.1.2. Microtubule-dependent movement of AGs... 60
II.3.1.3. AGs and translation... 61
II.3.2. AUBP post-translational modifications and partners ... 62
II.3.2.1. HuR ... 62
II.3.2.2. AUF1... 63
II.3.3. AGs purification and protein composition ... 65
II.3.3.1. Protein candidate approach ... 65
II.3.1.2. Global approach ... 66
II.3.4. AGs and cell stress ... 68
II.3.4.1. AGs are different form SGs ... 68
II.3.4.2. ALK expression alleviates cell responses ... 69
II.3.4.3. AUF1 and HuR sucrose gradient profile during stress ... 70
III. Discussion and perspectives ...71
III.1. NPM-ALK and mRNA stability... 73
III.2. AUBPs post-translational modifications... 74
III.3. AGs components... 77
III.3.1. Protein content... 78
III.3.2. mRNA content ... 79
III.4. Role of AGs ... 80
III.4.1. Interplay between AGs and PBs ... 80
III.4.2. Interplay between Ags and polysomes ... 80
III.3.1. Interplay between Ags and SGs ... 81
IV. Materials and methods...83
IV.1. Cloning of the Halo-ALK, GFP-ALK and TAPc-ALK vectors .. 84
IV.1.1. Polymerase chain reaction (PCR) ... 84
IV.1.2. Purification of PCR fragments for cloning ... 84
IV.1.3. Plasmid extraction ... 84
IV.1.4. DNA purification phenol extraction and ethanol precipitation ... 85
IV.1.5. Ligation... 85
IV.1.6. Transformation of competent cells ... 85
IV.2. DNA transfections ... 86
IV.3. Protein extraction ... 86
IV.3.1. Total cell extracts... 86
IV.3.2. Cytoplasmic and nuclear cell extracts ... 87
IV.3.3. Cytoplasmic cell extracts (cold lysis) ... 87
IV.4. Sucrose gradient ... 87
4
IV.5.1. Native gel ... 88
IV.5.2. Electro-elution... 89
IV.6. Protein electrophoresis and staining ... 89
IV.7. Immunoprecepitation and Western Blot analysis... 91
IV.8. Immunofluorescence and time-lapse analysis ... 91
IV.9. Electron microscopy (EM) ... 91
IV.9.1. Grids preparation... 91
IV.9.2. Cell fixation for EM observation... 92
IV.9.3. Negative staining... 92
IV.9.4. Positive staining ... 93
IV.9.5. Cell fixation for EM observation... 93
V. ANNEXES ...94
5
TABLE OF ILLUSTRATIONS
FIGURES
Introduction
Figure 1: Timeline of the major events in the characterization of ALCL Figure 2: Diagram of the human ALK receptor tyrosine kinase
Figure 3: Diagram of the full-length ALK receptor tyrosine kinase and the most common oncogenic ALK fusion protein, NPM-ALK
Figure 4: Molecular network interacting with NPM-ALK
Figure 5: Mitogenic signalling dependent on NPM-ALK is mainly due to the activation of the Ras–extracellular signal-regulated kinase (ERK) pathway
Figure 6: The key player in the survival mechanisms triggered by NPM–is signal transducer and activator of transcription 3 (STAT3)
Figure 7: The contribution of phosphatidylinositol 3-kinase (PI3K) in NPM-ALK fusion protein-driven oncogenic process consists of a crucial anti-apoptotic signal Figure 8: The mRNA biogenesis pathway and posttranscriptional gene regulation Figure 9: Mechanism of normal mRNA degradation
Figure 10: NMD, NSD and NGD pathways
Figure 11: The generic structure of an eukaryotic mRNA, illustrating some post-transcriptional regulatory elements that affect gene expression
Figure 12: CRD of c-fos and c-myc
Figure 13: Biogenesis of miRNAs and assembly into miRISC in mammals Figure 14: Possible Mechanisms of miRISC-Mediated Repression
Figure 15: The three major pathways by which AMD is executed
Figure 16: Mechanisms of post-transcriptional regulation and their alteration in cancer Figure 17: AUF1/hnRNP D protein isoforms and conserved motifs
Figure 18: Schematic diagram of posttranslational modification sites within the HuR protein
Figure 19: Influence of cytoplasmic HuR levels and HuR-regulated S and G2/M cyclins on cell cycle progression
Figure 20: Schematic representation of subsets of transcripts (blue) found to be targets of association with HuR
Figure 21: Structural domain organization of TIA-1 and the related protein TIAR Figure 22: Schematic representation of TTP, BRF-1, and BRF-2
Figure 23: A model for coordinated regulation of BRF1 activity and protein stability Figure 24: Control of ARE-mediated mRNA degradation by TTP
Figure 25: Domain organization of KSRP. KH: K-homology domain
Figure 26: A model proposed for stabilization of labile mRNAs by the Wnt/ß-Catenin? Pitx2 Pathway
Figure 27: Sequence and structure of the predicted HuR, AUF1 and TIA-1 signature motif, as identified among their associated transcripts
Figure 28: The dynamic interplay among AUBPs
Figure 29: Assembly and disassembly of arsenite -induced SGs Figure 30: Translational initiation in the absence or presence of stress Figure 31: Model of stress granule assembly
7 Figure 32: mRNA decay factors in the processing bodies
Figure 33: Model for predominant cytoplasmic flow of mRNAs through PBs SGs mRNP states
Figure 34: Hypothetical model of the relationship between SGs and PBs
Results
Figure 35: AGs and cell cycle
Figure 36: AGs and culture conditions
Figure 37: AG size increases when cultivated in “Biomedia” FCS
Figure 38: Sucrose gradient analysis of AGs in different culture conditions Figure 39: AGs cruising along microtubules highways
Figure 40: Expression level of different proteins contained in AGs as well as those implicated in translation
Figure 41: Sucrose gradient analysis of general mR NA profile Figure 42: HuR profile in 15-50% sucrose gradient
Figure 43: Analysis of HuR phosphorylation status in NIH3T3 cells expressing or not NPM-ALK
Figure 44: Code colour representation of AUF1 unique partners involved in different biological processes
Figure 45: Gene candidate search for proteins found in AGs Figure 46: Isolation of AGs on native gel
Figure 47: AGs composition analysis by mass spectrometry Figure 48: AGs and SGs share several components
Figure 49: SG formation in cells expressing or not NPM-ALK
Figure 50: Correlation between reduced stress response in ALK expressing cells and absence of phsophorylation of eIF2a
Figure 51: AUF1 and HuR profile in stressed cells
Discussion
Figure 52: Altered RNA binding protein expression in CML
Figure 53: Schematic representation of AUF1 predicted phosphorylation sites Figure 54: The trans-activating ability of hnRNP D is regulated by hierarchical
phosphorylation
Figure 55: Schematic representation of HuR predicted phosphorylation sites
TABLES
Introduction
Table 2: Classification of ARE motifs and examples of some mRNAs containing such motifs
Table 3: The major ARE-binding proteins and their effect on their target mRNAs Table 4: Survey of modulators of signaling pathways effects on subcellular localization
of HuR
Table 5: protein partners interacting with TTP and their function Table 6: Treatments that induce SGs and PBs
Table 7: Selected and newly identified SG-associated proteins Table 8: Protein components of processing bodies
Results
Table 9: AUF1 partners in different cellular contexts
Table 10: Mass spectrometry analysis of the proteins containing in the High band after NPM-ALK immunoprecepitation
Discussion
Table 11: Up- and down-regulated genes in NPM-ALK expressing NIH 3T3 cells Table 12: Common up-and down regulated genes found in both ALCLs and NPM-ALK
expressing NIH 3T3 cells
Materiel and methods
Table 13: Primers list Table 14: Antibodies list
ANNEXES
Annexe 1: AUF1 unique partners involved in protein modification Annexe 2: AUF1 unique partners involved in mRNA metabolism Annexe 3: AUF1 unique partners involved in transport
Annexe 4: AUF1 unique partners involved in translation Annexe 5: AUF1 unique partners involved in proteolysis
Annexe 6: L30 YFP-MS2NLS vector map (generous gift of Bertrand’s lab) Annexe 7: RSV-LacZ-MS2 24 vector map (generous gift of Bertrand’s lab) Annexe 8: NPM-ALK-Halo vector map
Annexe 9: NPM-ALK-GFP vector map
Annexe 10: NPM-ALK-TAPC vector map (pCDNA3-TAPC vector was a generous gift of Gorospe’s lab)
Annexe 11: RFP-hDcp1a vector map (generous gift of Weil’s lab) Annexe 12: RFP-hp54 vector map (generous gift of Weil’s lab)
9
TABLE OF ABBREVIATIONS
Abbreviations list
aa: amino acids Ago: Argonaute AGs: ALK granules
ALCLs: Anaplastic large cell lymphomas ALK: Anaplastic lymphoma kinase AMD: ARE-mediated decay AMPK: AMP-activated kinase APRIL: Acidic protein rich in leucine ARE: AU-rich element
AUBPs: ARE-binding proteins AUF1: AU-binding factor 1 BP: Biological process BPB: Bromophenol blue BRF: Butyrate response factor BSA: Bovine Serum Albumin C5H8O2: Glutaraldehyde
CAT-1: Cationic amino acid transporter 1 (CH3)2AsOONa-3H2O: Sodium cacodylate CIP: Calf intestinal phosphatase
CPEB: Cytoplasmic polyadenylation element-binding protein CPEB: Cytoplasmic polyadenylation-binding protein
CRD: Coding region determinants
CRM1: Chromosome maintenance region 1 CTGF: Connective tissue growth factor DC: Petergents compatible
DDSA: Dodecenyl Succinic Anhydride DMEM: Dulbecco's Modified Eagle Medium DTT: Di-thio-threitol
ECL: Enhanced chemiluminescence
EDTA: Trypsin- Ethylene diamine tetraacetic acid EGF: Epidermal growth factor
EGTA: Ethylene glycol tetraacetic acid ELAV: Embryonic-lethal-abnormal-vision EM: electron microscopy
ERK: Extra-cellular signal-regulated kinase ePAB: Embryonic poly(A)-binding protein ER: Reticulum
FasL: Fas ligand
FAST K: Fas activated threonine kinase FCCP: p-(trifluoromethoxy)phenylhydrazone FCS: Foetal Calf Serum
FP: fused pyrrolocarbazole
11 G3BP: Ras-gap SH3-binding protein
GADPH: Glyceraldehyde-3-phosphate dehydrogenase GAM: Goat anti-mouse
GAR: Goat anti-rabit
GFP: Green fluorescent protein
GM-CSF: Granulocyte-macrophage-colony-stimulating factor Hcs24: Hypertrophic chondrocyte -specific gene product 24 HIF-1a: Hypoxia Inducible Factor
HSGs: Heat shock granules HSP: Heat-shock protein HuR: Human (hu) antigen R IF: Immuno-fluorescence IFN: Interferon
IGF-1R: Type 1 insulin-like growth factor receptor IGF-II: Insulin-like growth factor II
IL-9: Interleukin 9
IMT: Inflammatory myofibroblastic tumor IP: Immunopreceptiation
IP: Immunopreceptitation IRE: Iron response element IRS1: Insulin receptor substrate JAK3: Janus kinase 3
Jeb: Jelly belly
JNK: JUN N-terminal kinase KCl: Potassium chloride
KSRP: K homology-type splicing regulatory protein LPS: Lipo Poly Saccharides
LRP: Lipoprotein receptor-related protein mABs: Monoclonal antibodies
MAPK: Mitogen activated protein Kinase MEF: Mouse embryonic fibroblasts Mg(CH3COO)2: Magnesium acetate MgCl2: Magnesium chloride
miRNA: MicroRNAs MK: Midkine
MK2: Mitogen-activated protein kinase-activated protein kinase 2 mRNA: Messenger ribonucleic acid
MS: Mass spectrometry
MS-MS: Tandem mass spectrometry MSN: Moesin
MW: Molecular weight MYH9: Myosin heavy chain NaCl: Sodium Chloride NaOH: Sodium hydroxide
NGD: No-go Mediated mRNA decay pathway NH4Cl: Ammonium chloride
NH4HCO3: Ammonium bicarbonate NHL: Non-Hodgkin’s lymphoma NK: Natural killer
NMA: Nadic Methyl anhydride
NMD: Nonsense Mediated mRNA decay pathway NPM: Nucleophosmin
NSD: Nonstop mediated mRNA decay pathways nt: Nucleotide
OD: Optic density
ORF: Open reading frame OsO4: Osmium tetroxyde
p130cas: p130 Crk-associated substrate PABP: Poly(A)-binding protein
PAGE: Polyacrylamide gel electrophoresis PARN: Poly(A) ribonuclease
PBS: Phosphate Buffered Saline PBs: Processing bodies
PC: pCDNA3
PI3K: Phosphatidylinositol 3-kinase PKO: Colorectal carcinoma cells PKR: Protein kinase R
PMSF: Phenyl-methyl-sulphonyl-fluoride PP2A: Phosphatase 2A
PRD: Prion related domain
ProTa: Anti-apoptotic prothymosin a PS: Penecillin-Streptamycin
pSer: Phosphorylated serine
PTB: Polypyrimidine tract binding protein PTC: Premature termination codons PTN: Pleiotrophin
pTyr: Phosphorylated tyrosine RBPs: RNA binding proteins
RIPA: Radio Immunoprecipitation Assay RISC: RNA-Induced Silencing Complex RPTP: Receptor protein tyrosine phosphatase RRM: RNA Recognition Motifs
RTK: Receptor tyrosine kinase
RT-PCR: Reverse transcriptionpolymerase chain reaction S: Sedimentation coefficient
SDS: Sodium dodecylsulfate SG: Stress granules
SH2: Src homology 2
SHC: SH2 domain containing transforming protein SHH: Sonic hedgehog
siRNAs: Small interfering RNAs SMN: Survival motor neurone
13 sncRNAs: Small non-coding RNAs
Syndecan 3: Heparan sulphate proteoglycan N-syndecan TAE: Acetate Ethylene Diamine Tetra-acetic Acid TEMED: Tetramethylethylenediamine
TFR: Transferring receptor
TGF-ß: Transforming growth factor ß Thr: Threonine
TIA-1: T-cell intracellular antigen-1
TIAR: T-cell intracellular antigen related protein TNF a: Tumor necrosis factor a
TPM3: Tropmyosine 3 TPM3: Tropomysine 3 Trn2: Transportin-2 TTP: Tristetraprolin
TZF: Tandem CCCH zinc finger UrAc: Uranyl Acetate
UTR: Untranslated region UV: Ultraviolet
VEGF: Vascular endothelial growth factor WB: Western blot
15
I. Introduction
Receptor tyrosine kinases (RTKs) are the high affinity cell surface receptors for many polypeptide growth factors, cytoki nes and hormones and function to detect, amplify, filter and process a variety of environmental and intercellular cues. Thus, RTKs are central components of cell signaling networks and play crucial roles in normal physiological processes, such as embryogenesis, cell proliferation and cell death (apoptosis).
All RTKs share a similar structure. They are comprised of an extracellular ligand -binding domain, a single hydrophobic transmembrane a helix, and a cytosolic domain that includes a region with protein-tyrosine kinase activity, as well as regulatory sequences. This kinase activity consists of transfering phosphate groups from high-energy donor molecules, such as ATP to specific target molecules (substrates), in a process called phosphorylation. The ligands for RTKs are soluble or membrane-bound peptide/protein hormones including nerve growth factor (NGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), and insulin. Binding of ligand causes most RTKs to dimerize which in turn stimulates the receptor's intrinsic protein-tyrosine kinase. This occurs in two stages. First, the phosphorylation in trans by the dimeric partner in a region known as the activation loop, which in the resting state occludes the active site of the kinase in cis and prevents substrate and/or ATP access. This phosphorylation leads to a conformational change with the repositioning of the activation loop away from the active site and allows substrate to gain access. Second, The receptor kinase activity then phosphorylates other sites in the cytosolic domain and the resulting phosphotyrosines serve as docking sites for other proteins involved in RTK-mediated signal transduction. This triggers a cascade of events through phosphorylation of intracellular proteins that ultimately transmit ("transduce") the extracellular signal to the nucleus leading to changes in cellular physiology and/or patterns of gene expression (Johnson, 2009; Paul and Mukhopadhyay, 2004; Robinson et al., 2000; Schlessinger, 2000).
Receptor tyrosine kinases have been shown to be not only key regulators of normal cellular processes but also to have a critical role in the development and progression of many types of cancer (Bache et al., 2004; Muller-Tidow et al., 2004; Paul and Mukhopadhyay, 2004). Indeed, regulation of the kinase activity of RTK and the subsequent Phosphorylation of tyrosine residues in target proteins is essential for maintaining cellular homeostasis, yet this post-translational modification also provides the means by which a number of cellular oncogenes deregulate various signaling pathways and induce transformation. There are several mechanisms by which RTK might become constituvaly active and acquire transforming abilities; first, by the mutation of receptor tyrosine kinases resulting in their constitutive activation (e.g. EGFR (Lemmon, 2003)); second as a component of fusion genes resulting from chromosomal translocation (for example BCR-ABL in chronic Myeloid Leukemia (CML) (Vardiman, 2009)and NPM-ALK in Anaplastic Large Cell Lymphoma (ALCLs) (Chiarle et al., 2008); and third, tyrosine kinases may be activated simply by overexpression or defective downregulation; and finally by autocrine or paracrine loops (e.g. IGFR (Cardillo et al., 2003)). In every case, the result is a hyperactive kinase, that confers an aberrant, ligand -independent, non-regulated growth stimulus to the cancer cells.
During my PhD, I was interested in understanding the malignant transformation of lymphoid cells in ALCLs. These lymphomas are characterized by the expression of a fusion protein (X-ALK), in which the N-terminal partner (in most cases the nucleophosmin NPM) is fused to the cytoplasmic portion of the ALK protein (Anaplastic Lymphoma Kinase) containing a tyrosine kinase domain. Several lines of evidence indicate that tumorigenesis in humans is a multistep process and that these steps reflect a succession of alterations conferring one or another type of growth advantage leading to the progressive conversion of normal cells into cancer cells. Indeed, in ALCLs, the alterations of several signalling pathways have been implicated in the malignant transformation. The finding that AUF1, a RNA binding protein (RBP), is a partner of X-ALK and the growing body of evidence implicating aberrant regulation or RNA in cancer led us to hypothesise that impaired post-transcriptional regulation of gene expression was an intrinsic part of the malignant transformation of lymphoid cells into ALCLs. In the
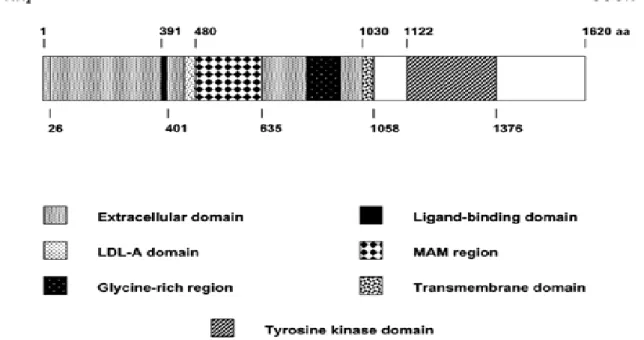
Figure 2: Diagram of the human ALK receptor tyrosine kinase. The formation of ALK
fusion proteins by oncogenic chromosomal translocations results in the loss of the entire extracellular portion of ALK (aa 1-1030) containing the signal peptide sequence (aa 1-26), the PTN and MK ligand-binding site (aa 391-401), the LDL-A region, the MAM (Meprin/A5/protein tyrosine phosphatase Mu) region (aa 480-635), the glycine-rich region, as well as the 28-aa transmembrane segment (aa 1030-1058). The intracellular region (aa 1058-1620) containing the tyrosine kinase domain (aa 1122-1376) forms the ALK-portion of the oncogenic tyrosine kinase fusion proteins (Pulford et al., 2004b).
Figure 1: Timeline of the major events in the characterization of ALCL (Chiarle et al., 2008).
following section of this manuscript, an up-to-date introduction to ALCLs as well as mRNA metabolism and its implication in cancer is presented.
I.1. Anaplastic large cell lymphoma
Anaplastic large cell lymphoma, first described in 1985 by (Stein et al., 1985), is a category of T/natural killer (NK) lymphoma with characteristic neoplastic cells, referred to as “hallmark” cells, that express CD30 (Fig. 1). It is a distinct clinicopathological entity of non-Hodgkin’s lymphoma (NHL), which has been included in the recent World Health Organization (WHO) classification as a T-cell neoplasm (Harris et al., 1999). ALCL is a rare disease representing around <5% of all cases of NHL (Kadin and Morris, 1998) and a male/female ratio of 2.5:1, with a peak incidence in childhood and accounts for approximately 40% of NHL cases diagnosed in pediatric populations (Burkhardt et al., 2005; Chiarle et al., 2008).
I.1.1. ALK function in normal cells:
Although ALK was originally identified as an oncogene activated in ALCLs, full length ALK is a member of the insulin receptor super-family of receptor tyrosine kinase (RTK). RTKs are involved in cell proliferation, differentiation and malignant trans formation. The stimulation of these receptors by their ligands leads to their dimerisation and activation through trans -phophorylation. In common to this RTK family, ALK has an extracellular ligand binding region, a transmembrane -spanningdomain and a cytoplasmic kinase catalytic domain (Fig 2). ALK is highly conserved across species, namely 85% identity between the human ALK and murine ALK proteins, and 34% identity between human ALK and its drosophila counterpart (DAlk) (Pulford et al., 2004a; Pulford et al., 2004b).
In human, two secretory heparin-binding growth and differentiation factors, pleiotrophin (PTN) and midkine (MK), have been proposed to bind and activate ALK on
18 outgrowth and has mitogenic activity in a wide range of cell lineages and MK is implicated in the direction of neurite connections as well as the migration of neurons (Li and Morris, 2008; Pulford et al., 2004b). However, the receptor protein tyrosine phosphatase (RPTP) beta/zeta and the heparan sulphate proteoglycan N-syndecan (syndecan 3) have also been described as PTN receptors. Like wise, MK receptors other than ALK have been described, namely neuroglycan C, low -density lipoprotein receptor-related protein (LRP) and alpha4beta1 and alphabeta1-integrins (Pulford et al., 2004a). Therefore, a clear role for PTN- and MK-mediated activation of ALK in the regulation of normal physiological cell responses remains to be established, as other receptor proteins may transduce some of the effects of PTN and MK.
When no known ligand is described, the use of monoclonal antibodies (mAbs) to study the function of a receptor has proven to be a valuable tool. Indeed, incubation of human neuroblastoma SK-N-SH cells, which strongly express the ALK receptor, with the monoclonal antibody 16-39 (mAb13-69) directed against the extra-cellular domain of ALK resulted in the phosphorylation of ALK and induced cell growth and neurite outgrowth. These effects are totally blocked by a MEK inhibitor suggesting an essential role of the ERK (extra-cellular signal-regulated kinase)/MAPK (mitogen activated protein Kinase) pathway in mediating both mitogenic and differentiation signals in ALK-expressing neural cells (Motegi et al., 2004). In Drosophila melanogaster, DAlk is activated by Jelly belly (jeb) protein and the jeb/DAlk pathway appears to be important for the activation of RAS/MAPK pathway and the normal specification of visceral gut muscle development (Amin and Lai, 2007; Li and Morris, 2008). Northern blot analysis revealed that murine ALK mRNA is widely expressed in the nervous system of mouse embryos and greatly decreased after birth. In human, ALK is only limited to rare neural cells and scattered pericytes and endothelial cells after birth. Finally, DAlk mRNA transcripts were identified in the brain and ventral nerves as well as in the musculature of the digestive tract throughout Drosophila embryonic development (Li and Morris, 2008).
These observations suggested that ALK plays an important role in normal development and function of these systems. However, even though studies of DAlk
Figure 3: Diagram of the full-length ALK receptor tyrosine kinase and the most common oncogenic ALK fusion protein, NPM-ALK. As a result of the t(2;5)(p23;q35) the entire
extracellular region of ALK is lost and is replaced by the amino-terminus of NPM. This region of NPM contains an oligomerisation domain whose presence permits the formation of NPM-ALK homodimers and NPM/NPM-NPM-ALK heterodimers. The formation of NPM-NPM-ALK homodimers results in dysregulation of the tyrosine kinase domain of ALK with consequent activation of downstream signalling pathways. The binding sites of various important molecules in these signalling pathways are indicated (Pulford et al., 2004a).
19 mutant Drosophila strains revealed the absence of the normal gut musculature development, no detectable gross histological defects have been observed in ALK knock out mice that exhibited a normal life span (Amin and Lai, 2007; Pulford et al., 2004a). Thus, the exact role of ALK (versus other receptors) throughout embryonic development is a critical topic that requires additional study for clarification. Besides its function in embryonic neural development, the full length ALK receptor protein was also detected in rhabdomysarcoma and neuroblastoma. Subsequently, it was detected in cell lines derived from tumors such as neuroectodermal tumors, glioblastomas as well as breast carcinoma and melanoma lines. In addition to non-hematopoietic tumors, full length ALK protein expression was also reported in a rare subtype of B-cell lymphoma (Delsol et al., 1997). The biological significance and the contribution of full length ALK to oncogenesis in these tumors are still not clearly defined, but it seems that the degree of ALK expression is a rate-limiting factor in apoptosis and tumor growth (Li and Morris, 2008). More work is needed to firmly establish the connection between the endogenous ALK and ALK+ malignancies.
I.1.2. ALK functions in cancer:
RTKs have been implicated in oncogenesis due to gene abnormalities where point mutations, gene amplification or fusion to a hetereologous gene resulted in the deregulation of their kinase catalytic domain (Paul and Mukhopadhyay, 2004). Even though to date, several point mutations and amplification of the ALK gene locus have been reported only in neuroblastomas (Janoueix-Lerosey et al., 2008), the most common mechanism of constitutive ALK activation known to occur in cancer involves chromosomal rearrangements that interrupt the ALK gene and fuse it with another gene. Indeed, the observation of a chromosomal translocation involving a breakpoint on the long arm of chromosome 5 at position q35 was first made in 1989 (Kaneko et al., 1989; Rimokh et al., 1989). However it was not till 1994 that the translocation t(2;5)(p23;q35) was formally associated with ALCLs (Fig. 3) (Morris et al., 1994).
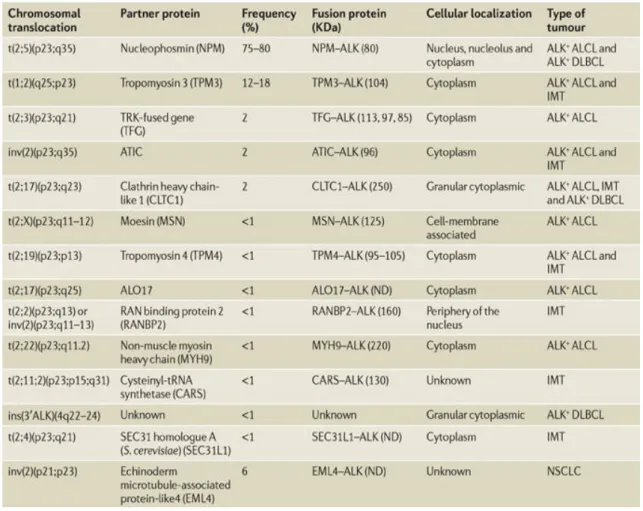
Table 1: Recurrent chromosomal translocations involving ALK in cancers. ALCL, anaplastic
large cell lymphoma; ALK, anaplastic lymphoma kinase; ALO17, ALK lymphoma oligomerization partner on chromosome 17; ATIC, 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/IMP cyclohydrolase; DLBCL, diffuse large B-cell lymphoma; IMT, inflammatory myofibroblastic tumours; ND, not determined; NSCLC, non-small-cell lung cancer (Chiarle et al., 2008).
20
I.1.2.1. ALK fusion proteins:
This translocation causes the NPM gene located at 5q35 to fuse with a gene at 2p23 encoding the receptor tyrosine kinase, ALK. As a consequence, the ALK gene coding for a tyrosine kinase domain comes under the control of the NPM promoter, which induces a constitutive transcription of the NPM-ALK hybrid gene, resulting in the production of an 80-kd chimeric protein, NPM-ALK or p80.
Even though NPM/ALK fusion protein was identified in more than 80% of ALCL cases, other variant ALK translocations have been reported (Duyster et al., 2001; Falini et al., 1999; Niitsu et al., 2009; Pulford et al., 2004b) (Table 1). Recent studies have determined that ALK fusion proteins not only contribute to the development of human lymphoma but also a mesenchymal tumor known as inflammatory myofibroblastic tumor (IMT) and rare cases of B-cell lymphomas, making ALK one of the rare RTKs implicated in both haematopoietic and non-haematopoietic tumors (Pulford et al., 2004a; Pulford et al., 2004b). Eventhough the presence of NPM-ALK protein has yet to be identified in normal tissues, reverse transcription-polymerase chain reaction (RT-PCR) revealed the presence of NPM-ALK mRNA in samples of normal peripheral blood and reactive lymphoid tissue. Thus, additional factors are required for the chromosomal rearrangement to become apparent clinically (Li and Morris, 2008).
Immunohistochemical staining has shown that NPM-ALK expression is both cytoplasmic and nuclear/nucelolar whereas strictly cytoplasmic in most of the other variants (Table 1). All ALK fusion proteins share certain characteristics. With the apparent exception of moesin (MSN)-ALK and non-muscle myosin heavy chain (MYH9)-ALK, all of the fusion proteins contain the same region of the full length receptor ALK, namely residues 1058-1620. This portion of ALK comprises the complete intracytoplasmic segment including the tyrosine kinase catalytic domain. Moreover, all N-terminal partners of ALK are proteins widely expressed in normal cells, the promoter of which controls the aberrant transcription of ALK chimeric mRNA. They possess an oligomerisation domain that mediates constitutive self-association of the ALK fusion
causing the constant activation of ALK kinase domain(Li and Morris, 2008; Pulford et al., 2004a; Pulford et al., 2004b).
Several models such as retroviral transduction models, transgenic mice and xenograft models have been established to study in vivo ALK-mediated tumorigenesis (Li and Morris, 2008). Even though none of the above models mimic precisely ALK+ human lymphoma, they have nonetheless provided an unequivocal proof that these fusions are at the origin of these tumors. In addition, transgenic mice have shown that the enforced expression of NPM-ALK leads to the development of B-cell malignancies, even when NPM-ALK expression was driven by T-cell associated antigen promoter (Li and Morris, 2008; Pulford et al., 2004a; Pulford et al., 2004b) . Thus, despite the universally accepted role of NPM-ALK in promoting lymphomagenesis, these data suggest that it is not the only determinant factor in the development of ALK+ ALCLs. This conclusion is in agreement with data that show certain biochemical differences even between 2 different cell lines derived from two different patients with the same type of ALCLs (Turturro et al., 2001).
I.1.2.2. Contribution of ALK or its N-terminal partner to oncogenesis:
There is no clear difference in clinical presentation and prognosis between NPM-ALK positive tumors versus tumors associated with other X-NPM-ALK variants. However, when testing the NIH3T3 cell-transforming potential of X-ALK fusion proteins in regards to proliferation rate, colony formation in soft agar, invasion, migration through the endothelial barrier and tumorigenesis, certain disperancies were found (ARMSTRONG, 2004). In agreement with what is observed in tumors, all X-ALK variants are phosphorylated and their subcellar distribution is as expected. The higher the level of X-ALK protein, the more proliferative the cells are with the exception of tropomyosine 3 TPM3 (tropomyosine 3)-ALK (Armstrong et al., 2004). These results suggest that the N-terminal partner may serve additional function for oncogenesis other than homo-oligomerization.
22 For instance, NPM also called B23, nutramin and NO38, is a ubiquitously expressed phosphoprotein involved in many cellular processes including ribosome assembly/transport, cytoplasmic/nuclear trafficking and regulation of p53 (Armstrong et al., 2004). NPM is mainly located in the nucleolus, but continuously shuttles between the nucleus and cytoplasm. A recent role for NPM in driving ribosome nuclear export has been established (Maggi et al., 2008). Thereby, NPM by amplifying the export of newly synthesized rRNAs results in the increase rates of protein synthesis, providing a consensus function for NPM in regulating cell growth (Maggi et al., 2008). Moreover, NPM is deposited selectively on mRNA undergoing polyadenylation (Palaniswamy et al., 2006) and has also been implicated in the control of CCN2 (connective tissue growth factor [CTGF]/hypertrophic chondrocyte-specific gene product 24 [Hcs24]) stability in chondrocytes by binding its 3’ UTR (Leask, 2009). Thus, NPM could also act at the post-transcriptional level to regulate and mediate some of the oncogenic features of NPM-ALK.
On the other hand, two separate studies have shown that ALK kinase activity is essential for the survival and proliferation of ALK positive ALCL cells in culture (Wan et al., 2006) and the functionally important role of NPM in NPM-ALK is restricted to the formation of kinase-active oligomers and does not involve alteration of normal NPM function (Bischof et al., 1997). In addition, tandem mass spectrometry analysis of NPM-ALK partners revealed that, while NPM and NPM-ALK have specific and unique partners, they also bind common protein such as Grb2 and centromere protein F (Crockett et al., 2004; Cussac et al., 2006). Collectively, these data stress out the importance of the cellular context with the different molecular factors able to interact with X-ALK in multimeric complexes through which ALK-mediated events promote and maintain tumorigenesis.
I.1.2.3. ALK mediated oncogenic transformation:
It is a generally acknowledged idea that ALK mediates its oncogenic trans formation through interaction with downstream molecules that can activate cellular signalling cascades. Indeed, there are 21 putative auto -phosphorylation sites, each serving
Figure 4: Molecular network interacting with NPM-ALK. A complex network of protein
kinases, protein phosphatases, transcription factors, apoptosis and cell-cycle regulators, adaptor proteins, and other molecules has been proposed to interact with NPM-ALK (Amin and Lai, 2007).
Figure 5: Mitogenic signalling dependent on NPM-ALK is mainly due to the activation of the Ras–extracellular signal-regulated kinase (ERK) pathway. the direct binding of
insulin receptor substrate 1 (IRS1), SRC homology 2 domain-containing (SHC) and SRC to specific tyrosine residues of ALK. The SHP2–GRB2 (growth factor receptor-bound protein 2) complex interacts with ALK and SHC to enhance phosphorylation of ERK1 and ERK2 through SRC. Phospholipase C-δ (PLCδ) is directly bound and activated by ALK to trigger mitogenic stimuli by the generation of diacylglycerol (DAG) and inositol triphosphate (IP3), which, in turn, mobilize calcium stores from the endoplasmic reticulum and activate protein kinase C (PKC). ALK-induced mammalian target of rapamycin (mTOR) activation is transduced through the ERK signalling pathway and ends in the phosphorylation of the mTOR targets ribosomal protein S6 kinase (p70S6K) and S6 ribosomal protein (S6RP), which in turn stimulate protein translation and ribosome biogenesis. Activation of mTOR also leads to phosphorylation and inactivation of the eukaryotic initiation factor 4E-binding protein 1 (EIF4EBP1), dissociating EIF4EBP1 from the RNA cap-binding protein EIF4E, thus promoting cap-dependent translation of mRNA. Potential therapeutic targets (indicated by red inhibitory arrows) already being validated in clinical trials, could be Ras and mTOR, to be treated with farnesyl and geranyl transferase inhibitors and rapamycin, respectively. Specific inhibitors of the ALK tyrosine kinase (TK) domain are also being validated. Note that the NPM–ALK fusion protein is not drawn to scale. JNK, JUN N-terminal kinase; SOS, son of sevenless (Chiarle et al., 2008).
Figure 6: The key player in the survival mechanisms triggered by NPM–is signal transducer and activator of transcription 3 (STAT3). STAT3 is activated by ALK either
directly or through Janus kinase 3 (JAK3). SHP1 is reported to act as a negative regulator by dephosphorylating ALK and/or ALK receptor-associated kinases such as SRC and JAK family kinases. In anaplastic large cell lymphoma, SHP1 expression is lost owing to gene methylation, resulting in the enhancement of STAT3 signalling. The downstream effectors of STAT3 are the B-cell leukaemia/lymphoma 2 (BCL2) family of apoptosis-regulating proteins BCL2, BCL-XL and myeloid cell leukaemia sequence 1 (MCL1). Other targets are the transcription factor C/EBPβ and survivin (a member of the inhibitor of apoptosis protein family that inhibits apoptosis and also mediates the spindle assembly checkpoint and cytokinesis). A role for STAT5 has been suggested but remains poorly defined. The available inhibitors to impair the ALK-related survival pathway (red inhibitory arrows) are the selective inhibitors for the ALK tyrosine kinase (TK) domain, STAT3 DNA-binding activity, JAK3, and BCL2 and BCL-XL(Chiarle et al., 2008).
Figure 7: The contribution of phosphatidylinositol 3-kinase (PI3K) in NPM-ALK fusion protein-driven oncogenic process consists of a crucial anti-apoptotic signal. PI3K
phosphorylates AKT1 and AKT2, which in turn enhance the survival of cells by blocking the function of pro-apoptotic proteins, such as B-cell leukaemia/lymphoma 2 (BCL2)-antagonist of cell death (BAD). AKT1 and AKT2 phosphorylate forkhead box O3A (FOXO3A) on Tyr24, Ser256 and Ser319, thus blocking FOXO3A-mediated transcription of target genes that promote apoptosis, cell-cycle arrest and metabolic processes, such as BIM, which stimulates cell death in haematopoietic lineages after cytokine withdrawal and p27, leading to its cytosolic localization and so attenuating its cell-cycle inhibitory effects. FOXO3A inhibition also results in the upregulation of cyclin D2, which promotes the G1–S-phase cell-cycle transition. The survival pathway is synergistically driven by mammalian target of rapamycin (mTOR), through the activation of AKT1 and AKT2. Potential therapeutic targets (red inhibitory arrows) could be ALK, mTOR and Akt. The nucleophosmin (NPM)–ALK fusion protein is depicted as the example ALK fusion in the figure. EIF4EBP1, eukaryotic initiation factor 4E-binding protein 1; TK, tyrosine kinase (Chiarle et al., 2008).
as a potential docking site for downstream factors and to date, a wide range of oncogenic molecules have been shown to interact with ALK and more precisely with NPM-ALK (Fig. 4). ALK is thought to act through 3 major signalling pathways: the ERK pathway, the janus kinase 3 (JAK3)-STAT3 pathway and the phosphatidylinositol 3-kinase (PI3K)-Akt pathway and probably involves functional redundancy due to common substrates of ALK among the different signaling pathways.
The increased growth of ALK+ ALCL cells is mainly attributable to the activation of the Ras-ERK pathway (Fig. 5). Recent evidence suggests that the sonic hedgehog (SHH) signalling pathway activation in ALCLs also contributes to cell proliferation and cell survival (Singh et al., 2009). A multitude of molecules implicated in regulating cell proliferation and promoting mitogenic stimuli have been found to associate with NPM-ALK. For instance, adaptor molecules such as Src homology 2 (SH2) domain-containing proteins (e.g. SH2 domain containing transforming protein (SHC) and insulin receptor substrate (IRS-1)), as well as downstream effectors (pp60c-src, PLC?, SHP2, JUN N-terminal kinase (JNK) and CD30 and NIPA)(Amin and Lai, 2007; Chiarle et al., 2008; Pulford et al., 2004b). Finally, a negative regulator of NPM-ALK has been identified, SHP1, whose loss is caused by hypermethylation of the SHP1 promotor and usually correlates with uncontrolled cell growth and increased proliferation of ALK+ ALCLs (Honorat et al., 2006).
Another aspect of tumoregenesis is the enhanced survival through inhibition of apoptosis. This is mostly done by the activation of the JAK-STAT and PI3K-Akt pathways (Fig. 6). In fact, inhibition of both pathways in ALK+ ALCL cells induces apoptosis (Amin and Lai, 2007; Chiarle et al., 2008). A novel association and reciprocal functional collaboration between the type 1 insulin-like growth factor receptor (IGF-1R) and NPMALK has been revealed (Shi et al., 2009). IGF1R can activate multiple anti -apoptotic pathways such as Phosphatidylinositol 3-kinase (PI3K), Akt and Protein kinase B (Fig. 7) (Peruzzi et al., 1999). Thus, NPM-ALK via this association can mediate cell protection from apoptosis.
24 Finally, ALK mediated transformation is associated with peculiar histo -morphological features and implicates cell migration and changes in cell shape. Regulation of actin filament assembly and cytoskeletal rearrangements is mediated by p130cas (for p130 Crk-associated substrate), which is bound and phosphorylated by NPM-ALK (Ambrogio et al., 2005). SRC is also probably implicated in ALK-mediated transformation. In fact, in colon carcinoma cells, SRC is found implicated in the promotion of cellular invasion, as its newly identified substrates include proteins with signalling, cytoskeleton and vesicular trafficki ng (Leroy et al., 2009) Recent data indicates that ALK is capable of increasing migration and invasion by modulating the activity of Rho family GTPases and the resulting RAC1 activation (Chiarle et al., 2008).
I.1.3. ALCLs therapeutic approaches:
Despite the significant progress in understanding the molecular mechanisms underlying ALK+ ALCLs, the therapeutic approaches to this lymphoma have not changed drastically through the last decade. ALCLs are currently treated with doxorubicin-containing combination chemotherapy as well as innovative therapies that use CD30-agonist antibodies such as SGN-30 (Amin and Lai, 2007; Li and Morris, 2008; Pulford et al., 2004a). This treatment induces complete remission in up to 95% of the patients, but relapse and resistance occur in more than 40% of the cases (Amin and Lai, 2007). The fact that NPM-ALK acts as the central cause of ALK+ ALCL makes it an interesting therapeutic target. Over the past decade, the study of NPM-ALK tumorigenicity has led to the generation of animal models, through retroviral transduction and transgenic approaches, which provide a setting to test novel ALK-targeted therapeutic approaches in the context of the whole animal. They revolve around 4 major axes: (1) small molecules that inhibit ALK activity (Li and Morris, 2008), notably fused pyrrolocarbazole (FP) (Wan et al., 2006), (2) gene therapy with the adenovirus delivery of cyclin dependent kinase inhibitors such as p27Kip1, p21Waf1 and p16INK4A as well as SHP1, the negative regulator of ALK, (3) immunotherapy which takes advantage of the fact that ALK, in adult, is only expressed in rare cells and represents a form of vaccination against lymphoma with ALK specific antigen and finally (4) cleavage of
Figure 8: The mRNA biogenesis pathway and posttranscriptional gene regulation.
Overview of the dynamic interactions of the major RNPs involved in pre-mRNA processing, mRNA biogenesis, and regulation of gene expression. The nascent transcript (pre-mRNA), capped with a 7-methyl guanosine (m7G) cap, is bound by hnRNP proteins and SR (serine-arginine-rich domain-containing) proteins. Small nuclear ribonucleoprotein particles (snRNPs) bind to the splice sites at the 5' and 3' ends of introns. The spliced introns are excised as lariats, which are then debranched and degraded. The exon junction complex (EJC) assembles on spliced mRNA 20 nucleotides upstream of joined exons, followed by the export of the mRNA to the cytoplasm. MicroRNAs (miRNAs) in the form of ribonucleoprotein complexes (miRNPs) bind to target mRNAs to regulate translation and mRNA stability. The poly(A)-binding protein (PABP) binds to the poly(A) tail of cytoplasmic mRNAs. The SR domains are indicated with a star. cEJC indicates the remaining stable EJC on mRNA in the cytoplasm, which is removed by translating ribosomes (Cooper et al., 2009).
25 NPM-ALK fusion protein with the use of hammerhead ribozymes or targeting ALK and its downstream effectors through RNAi (Amin and Lai, 2007; Chiarle et al., 2008; Pulford et al., 2004a).
Despite the central role of NPM-ALK in ALK+ ALCLs, the model that emerges from the current studies indicates that its oncogenecity is mediated though the collaboration of several members of a molecular network rather than individual culprits. Therefore, the use of combination therapy to specifically disrupt multiple targets to eliminate the synergistic effects of the various members of the molecular network or combining small ALK inhibitor molecules followed by vaccination against ALK could increase the rate of therapeutic success among ALK+ ALCL patients.
I.2. mRNA metabolism and cancer
Messenger ribonucleic acid (mRNA) is a molecule that serves as a “blueprint” for a protein product. mRNA is transcribed from a DNA template in the nucleus, exported to the cytoplasm and carried into the sites of protein synthesis: the ribosomes. Historically, attention has been focused on the regulation of RNA synthesis (transcription). However, during its brief existence, an mRNA molecule is also subject to regulatory mechanisms such as processing, nucleo-cytoplasmic export, localization, stabilization and translational regulation (Fig. 8) (Cooper et al., 2009). To date, a handful of regulatory factors discussed below has been identified and appears to control a large pool of target mRNAs. This suggests that a slight perturbation in post-transcriptional control mechanisms may generate wide ranging effects that could contribute to the development of a complex disorder such as cancer.
In mammals, up to 50% of changes in gene expression in response to cellular signals are due to changes in mRNA stability (Cheadle et al., 2005; Fan et al., 2002), underscoring the importance of mRNA turnover control as a gene expression regulatory mechanism. This fact is supported by the increasing number of reports on the modified
Figure 9: Mechanism of normal mRNA degradation. (a)
Most mRNAs undergo
decay by the deadenylation-dependent pathway. The poly(A) tail is removed by a
deadenylase activity, shown here as either CCR4–NOT or PARN. Following
deadenylation, two mechanisms can degrade the mRNA: either decapping followed by
5' 3' decay or 3' 5' decay. In the decapping pathway, the Lsm1–7 complex associates
with the 3’ end of the mRNA transcript and induces decapping by the Dcp1–Dcp2
complex, in which Dcp2 is the catalytic subunit
(Van Dijk et al. 2002, Liu and Kiledjian
2006)
. This leaves the mRNA susceptible to decay by the 5' 3' exoribonuclease Xrn1.
Alternatively, the deadenylated mRNA can be degraded in the 3' 5' direction by the
exosome, with the remaining cap structure being hydrolysed by the
scavenger-decapping enzyme DcpS. (
b)In
Saccharomyces cerevisiae, deadenylation-independentpathways require recruitment of the decapping machinery. Here, Rps28B interacts with
enhancer of decapping-3 (Edc3) to engage the decapping enzyme. Following decapping,
the mRNA is degraded by Xrn1. (
c) Endonuclease-mediated mRNA decay initiates with
internal cleavage of the mRNA, which generates two fragments each with one
unprotected end. The fragments are degraded by Xrn1 and the exosome (Garneau et al.,
2007).
26 mRNA stability and/or translational efficiency in ca ncer (reviewed in (Hollams et al., 2002)). Numerous mRNAs encoding proto-oncogenes, such as c-myc, c-fos and c-jun, cytokines and cell cycle regulators are stabilized in cancer cells leading to increased proliferation. For example, the development of malignant skin tumors is impaired in c-fos null mice (Saez et al., 1995) and conversely ectopic expression of this proto-oncogene in transgenic mice induces formation of chondrogenic tumors (Wang et al., 1991). Other proto-oncogene mRNAs, such as c-myc and c-jun, are stabilized in human T-cell leukemia and myeloma, hodgkin’s lymphoma and breast carcinoma as well as a number of pathological conditions including ulcers, arthritis and wound repair (reviewed in (Audic and Hartley, 2004).
The driving force for the recognition of post-transcriptional implication in such pathogenesis relies on the identification of (a) cis-acting elements present on mRNAs that regulate their turnover as well as their translability, (b) RBPs recognizing these elements and thus enabling modulation of their target mRNA expression, (c) the discovery that extra-cellular signals via activation of signaling pathways modulate RBPs activity, localisation and consequently modify mRNAs expression and (d) the ongoing characterization of the mRNA degradation pathways/machineries and their coupling with translation.
I.2.1. mRNA decay:
The decay of mRNA is not a random process and can proceed via 3 different pathways. In yeast, mRNAs are degraded mainly through the 5’-3’ decay pathway, which is initiated by shortening of the 3’ poly(A) tail (deadenylation) followed by the cleavage of the 5’ m7GpppN cap (decapping) and finally 5’ to 3’ exonucleolytic degradation (Fig. 9A). On the other hand, the 3’-5’ pathway depends on the exosome complex, a multi-protein complex endowed with an exoribonucleolytic function enabling it to degrade various types of RNAs. “Deadenylation-independent mRNA decay” and endonuclease-mediated mRNA decay” can also occur (see legend Fig. 9 for further
Figure 10: NMD, NSD and NGD pathways. (a) NMD. Following splicing in the nucleus,
the exon junction complex (EJC), which contains UPF3 (a core protein of the NMD pathway), is associated with the transcript, and the resulting messenger ribonucleoprotein is exported to the cytoplasm. In the cytoplasm, a second NMD core protein, UPF2, binds to UPF3. Ribosomes associate and translate the mRNA, but are stalled on encountering a PTC. This results in binding of the SURF complex (comprising SMG1, UPF1 and the peptide-release factors eRF1 and eRF3) to the ribosome. UPF1 also binds UPF2, thereby linking the EJC to the PTC. Phosphorylation of UPF1 by SMG1 leads to dissociation of eRF1 and eRF3 and binding of the SMG7 adaptor protein. Subsequent steps that are still being elucidated lead to mRNA decay by various pathways. (b) NSD. Translation of an mRNA that lacks a stop codon results in ribosomes traversing the poly(A) tail, displacing poly(A)-binding protein (PABP) and stalling at the 3' end of the mRNA. One model proposes that, in yeast and mammalian cells, Ski7, an adaptor protein that functions as a molecular mimic of tRNA, binds to the A site on the stalled ribosome to release the transcript, and then recruits the exosome. The exosome degrades the poly(A) tail and mRNA body. In another pathway described in Saccharomyces cerevisiae, in the absence of Ski7, the displacement of PABP by the translating ribosome renders the mRNA susceptible to decapping and 5'-3' decay by the 5'-3' exoribonuclease Xrn1. (c) NGD. Ribosomes can stall within the ORF, for example, on encountering a strong secondary RNA structure. The Dom34 and Hbs1 proteins bind the transcript near the stalled ribosome and initiate an endonucleolytic cleavage event near the
Figure 11: The generic structure of an eukaryotic mRNA, illustrating some post-transcriptional regulatory elements that affect gene expression. Abbreviations (from 5' to
3'): UTR, untranslated region; m7G, 7-methyl-guanosine cap; hairpin, hairpin-like secondary structures; uORF, upstream open reading frame; IRES, internal ribosome entry site; ARE, AU rich element; CPE, cytoplasmic polyadenylation element; AAUAAA, polyadenylation signal (adpted from (Mignone et al., 2002)).
Figure 12: CRD of c-fos and c-myc. (A) Ribonucleoprotein complex formation on the c-fos
CRD and (B) general model of the interactions between the 5' and the 3' end of the mRNA via PABP1. The proteins that are part of the CRD bridging complex are in green. PAIP1 bridges PABP1 to eIF4A. UnR interacts directly with the CRD, while PABP1 interacts with the poly(A) tail. The interactions between the other proteins of the CRD bridging complex are unknown. (C) Model showing competition between the coding region determinant-binding protein (CRD-BP) and a nuclease for the c-myc mRNA. The nuclease is shown as a lightening bolt and two circles depict the ribosome. Once translation is initiated, the ribosome brings both the nuclease and CRD-BP to the CRD. Competition between CRD-BP and the nuclease ensues for binding to the CRD. If the nuclease associates first, the RNA is cleaved (1), otherwise, CRD-BP protects the mRNA and the ribosome can continue scanning (2). The RNA can thus be degraded at each cycle of translation (Audic and Hartley, 2004).
details) (Fillman and Lykke-Andersen, 2005; Garneau et al., 2007; Houseley and Tollervey, 2009; Liu and Kiledjian, 2006; van Dijk et al., 2002).
The mRNA surveillance process functions in both the nucleus and cytoplasm (Houseley and Tollervey, 2009; Wagner and Lykke-Andersen, 2002). Fidelity checks of mRNA molecules in the nucleus result in the degradation of improperly processed transcripts before export into the cytoplasm. Cytoplasmic surveillance mechanisms assess mRNA transcripts for the absence of or presence of premature stop codons. Three cytoplasmic surveillance mechanisms are currently known to function within cells: the Nonsense Mediated mRNA Decay pathway (NMD) involved in detection and decay of mRNA transcripts that contain premature termination codons (PTCs) (Fig. 10A); the Nonstop Mediated mRNA Decay pathways (NSD) involved in the detection and decay of mRNA transcripts which lack a stop codon (Fig. 10B); and the No-go Mediated mRNA decay pathway (NGD) which targets mRNA transcripts which have caused the ribosome to stall part way down the mRNA during translation (Fg. 10C) (see legend of Fig. 10 for further details).
I.2.2. mRNA cis-regulatory elements:
mRNA cis-regulatory elements often correspond to binding sites for one or more trans-acting factors such small non coding RNAs (sncRNAs) and proteins that regulate mRNA cellul ar fate. These cis-regulatory elements may be located in the 5’ untranslated region (UTR), the open reading frame (ORF) or the 3’UTR (Fig. 11) (Mignone et al., 2002). Control at the 5’ UTR level involves RNA secondary structure such as internal ribosome entry site, upstream open reading frames, 5’ terminal polypyrimidine-rich sequences, and the methylation state of the cap structure. In the ORF, a synthesized peptide may affect translation efficiency of its own mRNA, while the presence of rare codons may cause ribosome pausing during translation. Coding region determinants (CRDs) of mRNA instability can also be found in the ORF and target mRNAs for rapid degradation by endonucleolytic cleavage (c-myc) or rapid dedadenylation and degradation (c-fos) (Fig. 12) (Audic and Hartley, 2004; Hosoda et al., 2003; Lopez de
Class Motif mRNA
I Dispersed AUUUA and a U-rich
region c-fos, c-myc, p21, Cyclin D1
II WWWWAUUUAWWWW GM-CSF, TNFa, Cox-2, IL-2, Bcl-2
III U-rich, GUUUG repeats c-jun, p53
Table 2: Classification of ARE motifs and examples of some mRNAs containing such motifs (Barreau et al., 2005).
from a mechanistic point of view the 3’ UTR is a place of choice for such elements. Indeed it is likely that the 3’UTR is not scanned by the ribosomes (Poyry et al., 2004). Therefore, any RNA/protein interaction taking place in this region persists through translation, enabling regulation to take place at any time. Indeed, numerous 3’ UTRs contain regulatory sequences that bind RBPs and sncRNAs, resulting in the stabilization or destabilization of a given mRNA or its translational activation or repression. Example of such sequences are: (a) the iron response element (IRE) that stabilises transferring receptor (TFR) mRNA when the intracellular iron concentration is low, resulting in iron uptake and (b) the most commonly found AU-rich element (ARE), on which we are going to focus in this manuscript (Lopez de Silanes et al., 2007). ARE was first described within the 3’ UTR of mRNAs encoding several cytokines or lymphokines which are inflammatory mediators (Caput et al., 1986). Their direct role in mRNA turnover was demonstrated through a fusion gene experiment, in which the ARE found in the 3’UTR of human granulocyte-macrophage-colony-stimulating factor (GM-CSF) mRNA was inserted into the 3’UTR of a globin reporter mRNA. This caused the otherwise stable ß-globin mRNA to become highly unstable ex vivo (Shaw and Kamen, 1986).
AREs range in size from 50 to 150 nucleotides and based on their sequence features and functional properties, these elements have been experimentally grouped into three classes: class I and class II AREs, containing several copies of scattered or grouped AUUUA motif respectively, and class III AREs lacking this motif but containing a long continuous U-rich region (Barreau et al., 2005; Bevilacqua et al., 2003; Chen et al., 1995). However since ARE discovery, the list of mRNAs that contain such motifs has considerably lengthened. A computational analysis, using the motif WWWUAUUUAUWW where W is A or U and with mismatch= -1 (ARED 2, (Bakheet et al., 2003)) led to the estimation that 8-12% of human genes code for ARE-containing mRNAs. Although encoding functionally diverse proteins, numerous ARE-mRNAs encode proteins involved in cell growth, proliferation and the inflammatory and stress response (Table 2) (Bakheet et al., 2001; Bakheet et al., 2003).
Figure 14: Possible Mechanisms of miRISC-Mediated Repression. Nonrepressed mRNAs
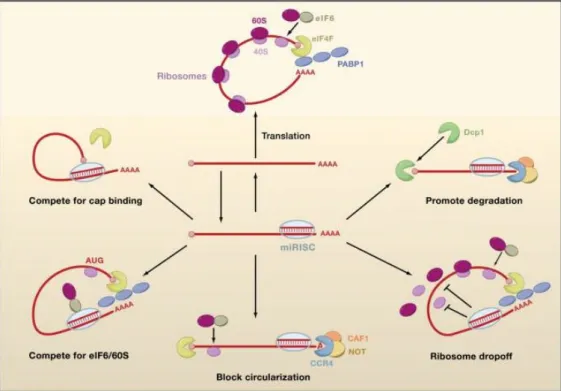
recruit initiation factors and ribosomal subunits and form circularized structures that enhance translation (top). When miRISCs bind to mRNAs, they can repress initiation at the cap recognition stage (upper left) or the 60S recruitment stage (lower left). Alternatively, they can induce deadenylation of the mRNA and thereby inhibit circularization of the mRNA (bottom). They can also repress a postinitiation stage of translation by inducing ribosome s to drop off prematurely (lower right). Finally, they can promote mRNA degradation by inducing deadenylation followed by decapping (upper right) (Carthew and Sontheimer, 2009).
Figure 13: Biogenesis of miRNAs and assembly into miRISC in mammals. Drosha
processes the pri-miRNA with the aid of DGCR8 to generate a pre-miRNA species. This is exported from the nucleus and processed by Dicer to form the mature miRNA/miRNA* duplex. After processing, miRNAs are assembled into miRISC. Only one strand of the duplex is stably associated with a miRISC complex—the miRNA strand is usually more strongly favored than the miRISC* strand, although there are exceptions (Carthew and Sontheimer, 2009).
I.2.3. Trans-acting factors:
The above-mentioned cis-regulatory elements are often binding sites of one or more trans-acting factors and regulate mRNA stability.
I.2.3.1. sncRNAs:
sncRNAs are part of the non coding RNAs family which are RNA molecules that function directly as structural, catalytic or regulatory RNAs, by contrast with mRNAs that encode proteins. The ncRNAs family is divided into 2 categories; (a) housekeeping ncRNAs which include ribosomal, transfer, small nuclear and small nucleolar RNAs and, (b) regulatory ncRNAs which include microRNAs (miRNAs), small interfering RNAs (siRNAs), Piwi-associated RNAs and long non-coding RNAs (Carthew and Sontheimer, 2009; Malone and Hannon, 2009; Ponting et al., 2009). To date only miRNAs have been found to play an essential role in human cancer (Zhang et al., 2007), thus we will be focusing on them.
miRNAs are 20-30 nucleotide (nt) long with a hairpin secondary structure that regulate gene expression by directing cleavage of their targeted mRNAs or inhibiting their translation through perfect or nearly perfect complementarities, respectively, to targeted mRNA. Double-stranded RNA precursors are first processed by Drosha in the nucleus, then by Dicer in the cytoplasm into short 20-30 nt fragments. The double stranded products of Dicer enter into a RNA-Induced Silencing Complex (RISC) assembly pathway that involves duplex unwinding, culminating in the stable association of only one of the two strands with an Argonaute (Ago) protein, and therefore enabling target RNA recognition through Watson-Crick base pairing (Fig. 13). The recruitment of the RISC complex on a complementary target mRNA will result in its translational repression or degradation through various possible mechanisms shown and discussed in Fig. 14(Carthew and Sontheimer, 2009; Filipowicz et al., 2008; Valencia-Sanchez et al., 2006). Bioinformatics analysis indicates that more than 30% of protein-coding genes may be targeted by miRNAs (Lewis et al., 2005), emphasizing the role of these miRNAs in different biological processes. Although it is still unclear how miRNAs regulate the
Table 3: The major ARE-binding proteins and their effect on their target mRNAs. This
table has been constructed by compiling data from (Bevilacqua et al., 2003; Lopez de Silanes et al., 2007; Zhang et al., 2002) reviews and updated. RRM: RNA recognition motif; KH domain: heterogeneous nuclear ribonucleoprotein K homology domain; CS-RBD: consensus RNA-binding domain; ds-RBM: double-stranded RNA-RNA-binding motif; ND: Not determined.