OATAO is an open access repository that collects the work of Toulouse
researchers and makes it freely available over the web where possible
Any correspondence concerning this service should be sent
to the repository administrator:
tech-oatao@listes-diff.inp-toulouse.fr
This is an author’s version published in:
http://oatao.univ-toulouse.fr/22930
To cite this version:
Dounia, Omar and Vermorel, Olivier and Poinsot, Thierry
Theoretical analysis and simulation of methane/air flame
inhibition by sodium bicarbonate particles. (2018) Combustion
and Flame, 193. 313-326. ISSN 0010-2180
Official URL:
https://doi.org/10.1016/j.combustflame.2018.03.033
Open Archive Toulouse Archive Ouverte
Theoretical
analysis
and
simulation
of
methane/air
flame
inhibition
by
sodium
bicarbonate
particles
Omar
Dounia
a,∗,
Olivier
Vermorel
a,
Thierry
Poinsot
ba Centre Européen de Recherche et de Formation Avancée en Calcul Scientifique (C.E.R.F.A.C.S), France b Institut de Mécanique des Fluides de Toulouse (I.M.F.T), France
Keywords:
Heterogeneous flame inhibition Sodium bicarbonate
DPS DNS
a
b
s
t
r
a
c
t
Thecapacityofsodiumbicarbonate(NaHCO3)s powdertochemicallyreduceflamespeedsandmitigate
theeffectsofaccidentalexplosionsiswellestablished.Theinhibitionofpremixedhydrocarbon/airflames bymonodisperse(NaHCO3)ssolidparticlesisinvestigated,here,usingtheoryandnumericalsimulations.
First,an analyticalsolution forthetemperature historyofasolid (NaHCO3)s particlecrossingaflame
showsthatthesizeofthelargest(NaHCO3)sparticlewhichcandecomposeinsidetheflamefront,and
actonchemicalreactionsefficiently,stronglydependsontheflamespeed.Forvariousfuelsandawide
rangeofequivalenceratios,particleswithastrongpotentialforflameinhibitionareidentified:hencea criterion,onthemaximumparticlesize,forefficientinhibitionisproposed.Thereafter,aone-dimensional
methane/airflame traveling ina premixed gas loaded with sodium bicarbonateis simulated usinga
chemicalmechanism basedonGRI-Mech,extendedtoincludeinhibitionchemistryand reducedto 20
specieswithaDRGEPmethod(Pepiot-DesjardinsandPitsch,2008).Inhibitorparticlesizeandmass load-ingarevariedtostudytheflameresponsetoinhibitionby(NaHCO3)s powders.Finally,two-dimensional
simulationsofaplanarflametravelinginaflowwithanon-uniforminhibitormassloadingdistribution areanalyzed.Inthecaseofstrongparticlestratification,anaccelerationoftheflameisobserved,instead ofamitigation.Thisfundamentalmechanismmaylimittheactualpotentialofinhibitionpowdersinreal configurations.
1. Introduction
Until the 1970s, industrial safety processes relied heavily on chlorofluorocarbon gasesas fire suppressantsin ground, sea and air systems [2]. Since then, the threat to Earth’s ozone of such gases has been established andtheir production prohibited. Fol-lowingthisban,intensiveresearchhasbeenconductedtofind effi-cienthalonreplacements.Alkalimetalcompounds,suchassodium bicarbonate (NaHCO3)s have received considerable attention be-cause of their higher effectiveness per mass basis compared to the halon 1301(CF3Br)[3]. Extensive experimental investigations on flame inhibition (reduction of flame speed) and flame sup-pression abilities of (NaHCO3)s demonstrate the effectiveness of thesepowdersonpremixedflames[4–7] andcounter-flowflames
[8–11].Morerecentlaboratoryscaleandmediumscale(50m3) ex-periments [12] show the capacity of alkali metal compounds to mitigatethepotentialaftermathofgaseousexplosions.
∗ Corresponding author.
E-mail address: dounia@cerfacs.fr (O. Dounia).
The first systematic investigation of the mechanism of flame inhibition/suppression by (NaHCO3)s particles was performed by Rosseretal.[4].The overallmechanismincludestwomajorsteps illustratedinFig.1:1)thermaldecompositionofsodium bicarbon-ateparticlesand2)gas-phasechemicalinteractionwiththeflame.
1.1. Particlethermaldecomposition
Exposed to a temperature gradient inside the flame front, (NaHCO3)ssolidparticlesundergothermaldecomposition.Iyaetal.
[7]demonstratedthatparticlesof(NaHCO3)sbelow10µm decom-posecompletelyinsidethemethane/airflamefront.Chelliahetal.
[11] also showed a monotonic behavior of suppression effective-ness with particle diameter for methane/air flames (contrary to the results of Trees and Seshadri [2] for heptane flames). These studies indicated that the particle size influences the position at which(NaHCO3)s decompositioniscomplete.Largeparticles, typ-ically larger than 60µm, will decompose too far downstream of the flamefront leadingto unsuccessful flame inhibition. The gas temperaturedecreaseduringthisdecompositionphaseisgenerally negligibleanddoesnotmodifytheflamespeedsignificantly.
Fig. 1. Schematic of the flame inhibition mechanism by sodium bicarbonate. Path S-G refers to the path from the Solid bicarbonate particle to the Gaseous agent NaOH. R • stands for a radical species and M denotes a group of stable species.
1.2.Chemicalinteractionwiththeflame
The strongpotential ofsodium bicarbonatepowdersforflame mitigation is attributedto a chemical ratherthan thermal (cool-ing)interactionwiththeflame.Iya etal.[7]founda good corre-lationbetweentheconcentrationofNaevaporated fromsaltsand thedegreeofflameinhibition.Theyconcludedthatthemechanism behindflameinhibitionbysaltsischemicalandhomogeneous,i.e. takingplace in the gas phase asa resultof the presence of va-porizedinhibitor.MorerecentlyChelliahetal.[11]conducted ex-perimentsontheextinctionconditionofcounterflowmethane/air flames.Multiple particle size classeswere considered. This study demonstratedthedominantchemicalnatureofflameinhibitionby fine (NaHCO3)s particles. Since flame inhibition by (NaHCO3)s is dueprimarily tochemical effects, manyauthors[7,11,13–15] have proposedkinetic models basedon NaOH beingthemain gaseous agent, able to act on chemical reactions in the flame. Gaseous sodium hydroxide (NaOH) is formed when the (NaHCO3)s parti-clesareexposed tohightemperatures insidetheflamefront.The particlesundergothermaldecompositionfollowinga seriesof re-actionpathways (path S-G in Fig.1) leadingto the formation of NaOH. The interaction of NaOH with the flame is homogeneous (gas-phasechemistry) andinvolvesthecatalytic recombinationof theradical species responsible forthe flame consumption speed, namelyH,OHandO.
Whilethemainproductofthedecompositionofthesodium bi-carbonateisagreedupon,thepathfromthe(NaHCO3)sparticleto thegaseousspeciesNaOH(pathS-GinFig.1)isstilluncertain.Hu etal.[16]andWuetal.[17]proposed atemperaturerange(370– 543K) andkinetic ratesforthefirststage ofthethermal decom-position:
(
NaHCO3)
s−→0.5(
Na2CO3)
s+0.5CO2+0.5H2O,However,thefollowingstepslackdetails andrates.Inadditionto the uncertainties surrounding (NaHCO3)s decomposition kinetics, theinfluenceofthe powderinjectionprocedure onthe effective-ness ofthe inhibitor hasnot beenaddressed yet.In practice the methodsusedtodeliverthepowdertothegascanhardly guaran-teeauniformparticlesdistribution.Astratifiedorsegregated pow-dercanperturbtheflamefrontandincreaseitssurface,suggesting apossibleoppositeeffect(increaseinflamespeed).Thisbehavior wasmentionedbyRosseretal.[4]:‘Intheabsenceofpowderthe flames were approximately hemispherical in shape; ... The
pres-enceof powder resulted at times inirregular flame shapes ... In some casestheflames werevery longandhighlytilted,probably asaresultofnon-uniformpowderdistribution’.Morerecently, var-iousinhibitors,gaseousandliquid,failedthequalificationtest per-formedby theFederal AviationAdministration(FAA).The Aerosol CanTest(ACT)report[18]issuedbytheFAAshowedthatinsome cases the overpressure increased in presence of inhibitor agents compared to thecase withno agent. Evenif theexact causefor thesedisappointing resultsisnot identified, thepowder segrega-tionmechanismmayexplain it:inthecontextofvaporcloud ex-plosions(VCE),theinhibitorinjectionwouldbeperformedthrough adiscretenumberofhighpressuremanifolds.Powdersegregation is thereforeunavoidable andmaypotentially lead tothe amplifi-cationofVCEdamages. Thispossibilityclearlyrequiresadditional studiesifsuchmitigationsystemsareusedinthefuture.
The presentwork describesa theoretical andnumericalstudy oftheinfluenceofsodiumbicarbonatepowdersonflame propaga-tion.Thefirstobjectiveistoderiveamodel(HetMIS)forthe Het-erogeneous methane/air inhibition by Sodium bicarbonate suited for numerical simulations in large and complex configurations. HetMISis composed of:1) a simplified 1-stepmodel forparticle decomposition(Section 2.1);2) an analytically reducedchemistry (ARC) for flame-NaOH interaction (Section 2.2). The introduction ofcomplexchemistryusingARCschemes[19–22]ismandatoryto capturethechemicaleffectsofNaOHontheflameatareasonable cost. An analytical expression for particle temperatureis derived inSection 3to predict whereparticlesdecompose in apremixed flame,henceto proposecriteriaon particlesizeforcomplete de-composition inside theflame front.Based on thesecriteria, a se-lectionof particlesizesis used tostudythe effect ofsodium bi-carbonatepowder on thepropagation of a 1D flame (Section 4). Finally,theresponseofa planarflameto anon-uniform distribu-tionofsodiumbicarbonateparticlesisexaminedinSection5using two-dimensional simulations to show that the local flame speed reductioncanbe offsetbyaflamesurfaceincreasewhenthe dis-tributionofinhibitorisnothomogeneous.
2. ModelforHeterogeneousmethane/airflameInhibitionby Sodiumbicarbonate(HetMIS)
TheHetMISmodelincludesasimplifieddescriptionofthe par-ticle evolution from solid (NaHCO3)s particles to gaseous NaOH
(Section 2.1) and the homogeneous interaction of this gaseous agentwiththeflamechemistry(Section2.2).
2.1. Thermaldecompositionofsodiumbicarbonateparticles
(NaHCO3)s first decomposes to form sodium carbonate (Na2CO3)s attemperatures intherange370–543K[8]depending on the particle size (R1). At 1123 K [11], the sodium carbonate formsliquidsodiumoxide(Na2O)liq(R2):
(
NaHCO3)
s 370−543 K −→ 0.5(
Na2CO3)
s+0.5CO2+0.5H2O (R1)(
Na2CO3)
s 1123K −→(
Na2O)liq+CO2 (R2) Toproduce NaOH, thesodium oxide(Na2O)liq canfollowan het-erogeneous reaction(R′3) orgothrough phasechangefollowedby anhomogeneousreaction(R′′3).(
Na2O)liq+H2O−→2NaOH (R′3)!
!
!
!
Na(
Na2O2O)+liqH2−→O−→Na22ONaOH (R ′′ 3) Major uncertainties surround the thermal decomposition of (NaHCO3)s particles. From the formation of sodium carbonate to the production of sodium hydroxide, the only relevant informa-tion is the temperatureat which (Na2CO3)s decomposes [11] (ie.Tdecomp=1123K).Therefore,a simplifiedmodelis proposedbased
on a single step approach (Rglob) as suggested by Mitani et al.
[23,24]andChelliahetal.[11]:
(
NaHCO3)
s−→NaOH+CO2 (Rglob)This reduced decomposition kinetics involves phase change and requires additional equations for the solid particle mass mp and
its temperatureTp.Here,an isolated(NaHCO3)s particle,assumed spherical,isdescribedusingaLagrangianformalism.The tempera-tureanddensityaresupposeduniforminsidetheparticle.
NaOH cannot be producedbefore thesolid (NaHCO3)s particle hasreachedTdecomp.Therefore,themassoftheparticleiskept
con-stantaslongasTp≤ TdecompEq.(1).Theonlysourcetermleftinthe
particletemperatureequationisrelatedtothegaseousconductive heatflux(
!
c g): ForTp≤ Tdecomp: dmp dt =0 (1) dCp,pTp dt =−!
c g mp where!
cg=π
dpNuλ
Cp(
Cp(
Tp)
Tp− Cp(
T∞)
T∞)
(2)wheredpistheparticlediameter,T∞isthefarfieldtemperature,
λ
is thethermalconductivityandCp thegas calorificcapacity.Cp,pis the particle calorific capacity.To derive Eq. (2), the term
λ
/Cpisassumedconstant andequaltoits valueatthe particlesurface
[25,26].Forallpresentcases,theslipvelocityofthesolidparticles issmallinthefreshgasesandincreasesslowlythroughtheflame so thattheparticleReynoldsnumberremains smallandtheflow aroundtheparticleislaminar.Thus,theNusseltnumberisNu=2.
OnceTdecompisreached,theparticleundergoesfast
decomposi-tionEq.(3).Duringthisphase,thetemperatureoftheparticleTp
isassumedconstantEq.(4).t⋆
pis,foreachparticle,thetimewhen
the decomposition temperature is reached: Tp
(
t⋆p)
=Tdecomp. Thedecomposition duration isfixed by the parameter
τ
decomp=10,µsandkeptsmalltoensureanalmostinstantaneousliberationofthe gaseousagent. ForTp=Tdecomp: dmp dt =A
ρ
pdpexp(−(
t− t ⋆ p)
/τ
decomp)
(3) dCp,pTp dt =0 (4)where
ρ
p istheparticledensityandAisaconstantsetto1m2/s.Thismodel can be summarizedin the following equations using theHeavisidefunctionH:
dmp dt =A
ρ
pdpH(
t− t ⋆ p)
exp(
−(
t− t⋆p)
/τ
decomp)
(5) dCp,pTp dt =−!
c g mp H(
t⋆ p− t)
(6)Suchamodelistoosimpletoaccountforallthedetailsofthe in-hibitionprocess.Inpractice,theparticlesarefarfrombeing spher-icalandcanexhibitcomplexcrystalline likestructureswith vary-ingaspectratios.Moreover,themodelpredictsanabruptstepwise behavioroftheinhibitor:iftheparticlereachesTdecomp insidethe
reaction region,it will decompose completely in the flame zone. Otherwise,itwillvaporize withoutimpactingthe flame,whichis certainly far from being true. Taking into account the tempera-turedistributioninsidetheparticleshould improvethisaspectof the model by reproducing the release of certain amount of Na-containingspeciesinsidethereactionzone forlarge particlesdue to surface decomposition. However, this should result in a pro-hibitiveincrease ofthe computational cost ofthe model.The set ofEqs.(5)and(6)areusefultostudytheoreticallyandnumerically firstordereffectsofsodiumbicarbonateparticlesonflame propa-gationaslong astheir decompositionspeed islargecompared to allotherphenomena.
2.2.Kineticmodelforhomogeneousmethane/airflameinhibition OnceNaOH isformed,itsinteractionwiththeflamechemistry mustbemodeled.Hynesetal.[27]andWilliamsandFleming[13], proposedreactions(R4)–(R6):
NaOH+H! H2O+Na (R4)
Na+OH+M! NaOH+M (R5)
NaOH+OH! H2O+NaO (R6)
whereNaOHdepletestheHandOHradicalpools, explainingwhy theflame speed is reduced.This setof reactions andthe associ-ated species(NaOH,NaO andNa) are incorporated intothe GRI-Mech 3.0 mechanism [28] chosen to model methane oxydation chemistry. The resulting full mechanism, referred to as GRI-Inh, includes 56 species and 328 reactions. The YARC reduction tool
[29] is then used to derive an Analytically Reduced Chemistry (ARC)withreasonablecomputationalcost.FirsttheDRGEPmethod
[1]isused to eliminateirrelevant speciesand reactions.Second,a Quasi-SteadyStateAssumption(QSSA)isappliedonhighlyreactive species.Withthisapproximation,solving atransportequation for the considered speciesis no longer required: their concentration maybesimplycomputedusinganalyticalexpressions.This proce-dureallowstoreduceboththestiffnessandthecostofthe chem-ical scheme.The resulting mechanism contains156 reactions, 20 transportedspecies(i.e.speciesforwhich atransport equation is solved)and8speciesinQSS.It isreferredto asHomMISfor Ho-mogeneousmethane/airflameInhibitionbySodiumbicarbonate.
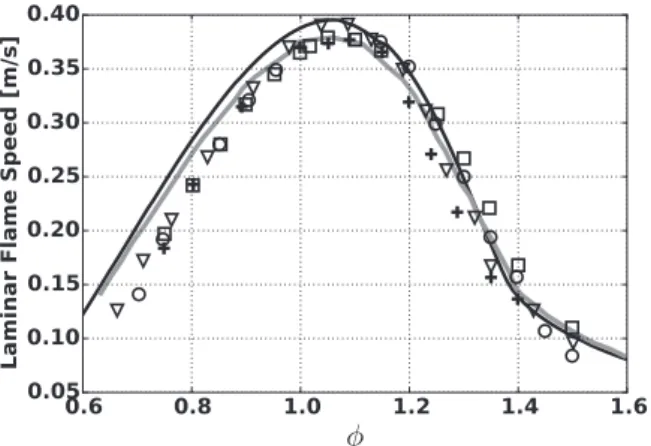
TheHomMISmechanismisvalidatedagainstexperimentaldata, withoutinhibitor, interms oflaminar flame speed inFig. 2. The
Fig. 2. Laminar premixed methane/air flame speeds at atmospheric conditions without inhibitor. (Black line) HomMIS mechanism. (Grey line) GRI-Inh mecha- nism. Experimental data: ( !) Dyakov et al. [42] ; ( ◦) Van Maaren et al. [43] ; (+) Vagelopoulos and Egolfopoulos [44] ; ( ▽ ) Gu et al. [45] .
reducedscheme fits the full scheme GRI-Inh within 5% accuracy between
φ
=0.6andφ
=1.6.Afirstvalidationexercisefortheinhibitionchemistry(R4)–(R6) istoassumethat(NaHCO3)sparticlesaresmallenoughto decom-posefaraheadoftheflamefrontandstudytheeffectsofthe ad-ditionofgaseousNaOH tothepremixedreactants.A stoichiomet-ric methane/air flame is diluted with variable amounts of NaOH (YNaOH=[0,...,0.02]) tomeasuretheinfluenceofthisgasonthe
flamevelocity, neglecting the solid/gas transformation of the ac-tual inhibition process. The experiments of Rosser et al. [4] can beusedasvalidationdataforthehomogeneous inhibition mech-anism. Indeed,the small size ofthe particles used in the Rosser etal.experiments(mean diameterd¯p=2.3µm) ensures fastand
completedecompositioninsidetheflamefront.Theeffectsofsuch particles can therefore be attributedto an homogeneous mecha-nismandcomparedtotheHomMISmodelresults.
Figure3showsagoodagreementwiththeexperimentaldataof Rosseretal.[4]andthenumericalresultsofBabushoketal.[15]in which the kinetic submodel for the interaction between NaOH and the flame is more detailed than the set of reactions (R4)–
(R6). The flame speed nonlinear response to NaOH is attributed to the rateof consumption of radical species by NaOH (Fig. 3b). Forsmall quantities ofthe gaseous agent (YNaOH≤ 0.005) a dras-ticdecreasein theradical speciespeak concentration and conse-quentlyinheatreleaseisobserved.However,forNaOH mass fac-tionabove 0.005,the flame inhibition process reaches a plateau. Thisphenomenoniscorrelatedwiththeradicalspecies concentra-tions:forYNaOH≥ 0.005,the maximum radical speciesmass frac-tionisclosetotheburntgasequilibriumvalueandthescavenging effectof thegaseous agent diminishes. Thissaturation effect has beenaddressedindetailsin[30,31].Finally,theadiabatic tempera-tureisquasi-insensitivetoNaOHconcentration.Forthesmall con-centrationsof NaOH considered here(YNaOH≤ 0.02),the chemical mechanismof sodium bicarbonate inhibition dominates over the thermalone.
TheHetMISmodeldescribedinSection2isusedinthe follow-ingsectionsforaphenomenologicalstudyoftheflameinhibition bysodiumbicarbonateparticlesassummarizedinFig.4.The par-ticlessizedistributionissupposedmonodisperseinthispaper.
3. Influenceof(NaHCO3)s particlesizeonthedecomposition
position
Section 2.2 has shown that the chemical description of the gaseousNaOHinteractionwithflamechemistrywascorrectwhen theparticles evaporate before entering the flame front. This
sec-tionfocusesonthegeneralcasewhereparticles arenotinfinitely smallandproposesan analytical modelableto providethe loca-tion,relativetotheflamefront,wherethe(NaHCO3)sparticles de-compose.Indeed,iftheparticlesarelargerthanacriticalsize,they canevencrosstheflameanddecomposedownstreamofthe reac-tionzonewheretheywillhavenoeffect.Theobjectiveofthis sec-tionistoevaluatethecriticaldiameterdc
pabovewhichthe
inhibi-tioneffectcannotbeobserved.dc
pcanbedefinedasthediameter
ofthe largest particlewhich can decompose inside theregion of radicalspeciesproduction(
δ
RinFig.5).dcpcanbeestimatedusingananalyticalsolutionforthetemperatureofanisolated(NaHCO3)s particle passing through a premixed stationary flame. Since this section focuses on the particle decomposition position, only the part Tp<Tdecomp is considered and the chemical interaction with
theflameisnottreated:themassandthediameteroftheparticle areassumedconstant.
3.1. Analyticalsolutionforthetemperatureofanisolated(NaHCO3)s
particlecrossingaflame
Consider a sodium bicarbonate particle entering a stationary flame(seeFig.5).Theparticleissmallenoughtoassumethatitis initiallyatequilibriumwiththegas:u0
p=s0L andTp0=T0 wheres0L
andT0 arethelaminarflamespeedandthefreshgastemperature respectively. The equation for the particle temperature Eq.(2) is furthersimplifiedassumingconstantheatcapacityfortheparticle Cp,p: dTp dt =
π λ
Nudp mpCp,p(
Tf− Tp)
(7) = 6λ
Nuρ
pd2pCp,p(
Tf− Tp)
(8)whereTfisthegastemperatureacrosstheflame.Furthermore,the
particle velocity is assumedequal to the gas velocity: up=uf=
s0
LTf/T0.Thisassumptionisgenerallytoostrongtobevalidforall particles.However,theparticlesconsidered inthissectionare rel-atively small (<50µm) andinitially at equilibriumwith the gas sothat thisequalityisatleasttrueinthepreheatingzoneofthe flame.The error onthe particletemperature induced by this hy-pothesisisthereforeacceptableasshowninSection3.2.2.Via vari-ablesubstitutiononecanthenobtain:
dTp dx = 1 uf dTp dt = 6
λ
Nuρ
pd2pCp,p"
1−TTp f#"
T0 s0 L#
(9) or dTp dx =)
T(
dp,s 0 L)
"
1−TTp f#
(10)where
)
T is a temperature gradient coefficient. The gas thermaldiffusivity
λ
isevaluated at T=Tdecomp. To integrate Eq. (10), anexpression for the gas temperature Tf is needed. To provide a
fullyanalyticalexpressionforTp,an analyticalexpressionforTfis
needed.Here,themodelofEchekkiandFerziger(EF)[32]isused toexpressthegastemperatureTf:
Forx≤
δ
EF: Tf=T0+(
Tad− T0)
(1
− 1/β
)
e− 1(
exp(
x/δ
EF)
− 1)
=C1+C2exp$
xδ
EF%
andforx>δ
EF: Tf =T0+(
Tad− T0)
"
1− exp&
(1
−β
)(
x−δ
EF)
δ
EF'
/β
#
=D1+D2exp&
(1
−β
)
xδ
EF'
Table 1
Particle temperature profile parameters. T p,EF is defined by Tp (x = δEF), and Tf, EF is equal to T0 + (Tad − T0 )(1 − 1/ β). 0 ≤ x ≤δEF x > δEF "T(dp , s 0L , T 0 , T ad , δEF) δEF)T / C 1 δEF)T / [ D 1(1 −β)] v exp(−x δEF) exp(− x(1 −β) δEF ) G C1 / C 2 D1 / D 2 Y 2 F 1 (1 , 1 −!T ; 2 −!T ; 1 + G ) 2 F 1 (1 , 1 ;1 + !T ; −exp(1 −β) /G )
ET T0 1 −!T(1 −κϒ ) (Tp,EF − T f,EFϒ ) / (exp(β− 1) T f,EF)!T
icalkineticsanddeducedfromtheradical speciesnetproduction rateprofile(seeFig.5).
δ
Risusuallyhigherthantheflamethermalthickness
δ
0L =
(
Tad− T0)
/max(
|
dTfdx
|
)
butlowerthantheflametotalthicknessdefinedbythedistancefromfreshtoburnedgases.The nondimensionalvariable
δ
⋆=χ
⋆/δ
Risthereforeusedto
character-izeparticleinhibitionproperties.If
δ
⋆<1,areductionoftheflame burningvelocity is possible since the particle decomposesinside orbeforethereactionzone;otherwise,NaOHisliberatedafterthe reactionzone,withnointeractionwiththeflamechemistry. 3.2.Validationoftheanalyticalsolution3.2.1. Numericalmethods
Section 3.1gives an analytical expression for the temperature Tp ofparticles crossinga premixedflameandtheposition where
particlesreachthedecompositiontemperatureTdecomp.Tovalidate
theory,simulations of the configuration considered in Fig. 5 are performed using the DNS/LES solver for the fully compress-ible multispecies Navier–Stokes equations AVBP, co-developped by CERFACS and IFP-EN. A centered continuous Taylor–Galerkin scheme third-order in space and time (TTGC [33]) is used. Gas-phase boundaries are described by Navier–Stokes characteristic boundary conditions [34]. The HomMIS mechanism described in
Section 2.2(Fig.4) is used to modelflame andinhibition chem-istry.
The particles are describedusing aLagrangian formalism. The smallparticlesize(1− 30µm)allowstoapplythepointsource ap-proximation.Two-waycouplingbetweensolidandgaseousphases isaccountedforbutcollisionsareneglectedduetothelowvolume fractionsconsidered here(
α
s<0.1%). Stokes drag force isappliedontheparticles.Inallsimulations,theslipvelocityoftheparticles remainssmallsothattheparticleReynoldsnumberislowerthan 5. Thedrag coefficient then readsCd=24/Rep
(
1+3/16Rep)
[35].Thedecompositionofsodiumbicarbonateparticlesisdescribed us-ingthemodelofSection2.1.
3.2.2. Validation
Asodiumbicarbonateparticleisplacedinfrontofastationary methane/airflame inAVBP.The particleisinitially atequilibrium withthegas(u0
p=s0L andTp0=T0).
Figure 6 (Left)compares the gastemperature profile across a stoichiometric premixedmethane/air flame givenby the EF sim-plifiedone-stepmodel andby theDNSusingtheHomMIS mech-anism presented in Section 2.2. It shows that the EF analytical modelforTfworksreasonablywell.Figure6(Right)showsthe par-ticletemperaturehistoryextractedfromthesimulationand com-paredto theanalyticalsolutionEq.(11).Agood predictionofthe particle decomposition position is observed. For all sizes, parti-clesdecompose atlocations where the gas temperatureis much higherthanthe decompositiontemperatureTdecomp,showingthat
thethermalinertiaoftheparticlesplayaroleandthatthe analyt-icalsolutionEq.(11)isuseful.Themodelalsoconfirmsthat,asthe sizeoftheparticlesincreases,theydecomposedownstreamofthe flamefrontasseeninthecasedp=20µmforexample.
Figure 7 displays profiles of gas velocity uf and particle ve-locity up obtained numerically. Even though the slip velocity is
not nulland increaseswith increasing particlesize, Fig. 7shows that the particle velocity remains relatively close to the gas ve-locityevenforlargeparticles(dp=20
µ
mforexample).Therefore,the assumption (up=uf), used to derive the analytical solution
Eq.(11),is acceptable for the particle diameterrange considered inthispaper.
The decomposition position is extracted from the profiles shown in Fig. 6(Right) formethane/air flames at various equiva-lence ratios
φ
=0.8,1.0,1.2and confronted to simulationresults inFig.8.The influenceoftheparticlediameterdpontheparticledecompositionpositionisinvestigated.Therangeofparticlesizes is chosen so that the critical value
δ
⋆=1 is reachedwithin the limits. A good agreement betweentheory andsimulations is ob-served.AsalreadysuggestedbyFig.6(Right),thepositionatwhich thesodiumbicarbonateparticledecomposes,shiftsdownstreamas the particle size is increased. Beyond 22µm, no inhibition effect isexpectedforthemethane/airflamesintherangeφ
=[0.8..1.2]. Theshapeoftheδ
⋆vs. dp curvesisattributedtothe1/d2p
depen-danceofthetemperaturegradientcoefficient
)
TinEq.(9).For a given particle size, the nondimensional decomposition position
δ
⋆ is the highest at stoichiometry:δ
⋆(
φ
=1.)
>δ
⋆(
φ
= 1.2)
>δ
⋆(
φ
=0.8)
. Thestoechiometric flame isthe mostdifficult toinhibitforagivenparticlesize.Theflameburningvelocity fol-lowsthesameorder.Thisobservationsuggestsadependanceofδ
⋆ on s0L henceu0p since the particleis initiallyin a thermodynamic
equilibriumwiththegas.Foragivendiameter,theparticleswith highervelocitydecomposefartherfromtheinitialposition explain-ingtherelativepositionofthethreecurves.Notethat
δ
R alsode-pends on the equivalenceratio. In thiscase,
δ
R(
φ
=1.)
<δ
R(
φ
=1.2
)
<δ
R(
φ
=0.8)
,contributingtotheresultpresentedinFig.8.An interesting value dc
p, corresponding to
δ
⋆=1, can beex-tractedfromFig.8.dc
pisthelargestparticlediameterthatcanlead
to successful inhibition. This value is computed forvarious fuels andequivalenceratiosinthenextsection.
3.2.3. Extensionoftheanalysistootherfuels.
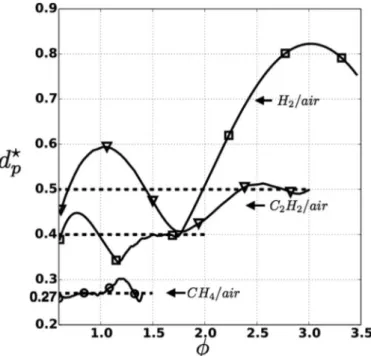
Three fuels with increasing burning velocities (methane/air, acetylene/air and hydrogen/air) at various equivalence ratios are considered in Fig. 9. To evaluate dc
p, the parameters Tad, s0L and
δ
R are extracted from1D laminar premixedflames:the HomMISmechanismisusedformethane/airflames,theWangetal.[36] de-tailed mechanism is used for acetylene/air flames andthe UCSD
[37]detailedmechanismisusedforhydrogen/airflames. The critical powder size for inhibition dc
p depends strongly
on the fuel and on the equivalence ratio. The lowest dc p value
corresponds to the point of maximum flame speed (
φ
≈ 1.1 for methane/air flames for example). dcp,max≈ 16 µm is the largest
size that can lead to successful inhibition for all atmospheric methane/airflames.Acetylene/airandhydrogen/airflames,dueto their higherflamespeed andlower thickness, needsmaller parti-clestobeinhibited:dc
p,maxisreducedto9µmand3µmfor
acety-lene/airandhydrogen/airflames,respectively.
These results are in good agreement with experimental re-sults found in the literature (Fig. 9). Iya et al. [7] studied pre-mixedmethane/airflames(
φ
=1.2)inhibitedbysodium bicarbon-ateparticles.TheyconcludedthatthecriticaldiameterwasintheFig. 6. Left: Stoichiometric laminar premixed methane/air flame at atmospheric conditions using EF simplified one-step model and HomMIS detailed chemistry description. Right: Particle temperature history given by the analytical solution Eq. (11) (dashed lines) is compared to the numerical results obtained with AVBP: ( ▽ ) dp = 10 µm; ( ◦) d p = 20 µm. T f is the gas temperature across the flame. The + symbol indicates the position where the particle reaches T decomp according to theory.
Fig. 7. Gas velocity u f and particle velocity u p history across the flame front ob-
tained with AVBP for two particle diameters: ( ▽ ) dp = 10 µm; ( ◦) dp = 20 µm.
range [12–28]µm which is coherent with the value dc
p≈ 20 µm
predictedby the HetMISmodel (simulation andtheory).Chelliah et al. [11], when studying the effect of sodium bicarbonate par-ticles on the extinction conditions of non-premixed counterflow methane/airflame,usedthreeclassesofparticles.Theyfoundthat particlesintheclasses[0,...,10]µmand[10,...,20]µmare effec-tive particles.A dramaticreduction ineffectivenesswasobserved when switching to the class [20−]µm. They concluded that the
Fig. 8. Influence of particle diameter on the relative decomposition position. Methane/air flames at three equivalence ratios are considered. (solid line) theory. (Symbols) simulations: ( !) φ= 0 . 8 ; ( ◦) φ= 1 . and ( ▽ ) φ= 1 . 2 .
criticalsizemustbelocatedinthesecondclasswhichisagain co-herentwiththeresultsgivenbythemodel.
An analytical expression for the inhibitor decomposition has also been obtained by Mitani [23]. It predicts that for a hydro-gen/airflameat
φ
≈ 0.5(φ
≈ 1.4resp.)witha burningvelocity of 50cm/s(300cm/s resp.),onlyparticleswithsize <17µm(<3µm resp.)willbeabletofullydecomposeinthereactionzone,which isclosetothevaluedcp≈ 13µm (4µmresp.)givenbythemodel.
An analytical expression for solid inhibitors decomposition was alsoderived byRosseretal.[4],butwasappliedtoNaFandNaCl in the paper. It is worth noting that the analytical expressions of Mitani and Rosser et al. assume a constant particle velocity throughthe flamefront (Eq.(A1)in[23] andEq.(6) in[4]) con-trarytotheHetMISmodelwhichaccountsforits evolutioninside the flame. Moreover, the derivations of Mitani and Rosser et al. do not take into account the relative position of radical species productioninsidethe flamefrontandits dependenceonthe fuel (whichisdoneinHetMISvia
δ
R).Forthesereasons,HetMISshouldFig. 9. Critical particle diameter d c
p (defined by δ⋆(dcp) = 1 ) and laminar flame speed s 0L for CH 4 /air, C 2 H 2 /air and H 2 /air flames at atmospheric conditions and various
equivalence ratios. The gray dashed bars [7,11] and the star symbols [23] represent values extracted from the literature.
Fig. 10. Non-dimensional critical particle diameter d ⋆ p = d cp /δEF as a function of the
equivalence ratio φfor various fuels. δEF = D th /s 0 L is the flame preheat zone thick-
ness.
improvethedependanceofdc
p onthecombustible(fuel and
com-position),comparedtootherapproachesfoundintheliterature.
Eq. (11) also showsthat, fora given particlesize, Tp depends
onthe reduced parameter x/
δ
EF whereδ
EF=Dth/s0L. Therefore, itispossible todefine a reducedcritical particlesize forinhibition d⋆
p=dcp/
δ
EF.Figure10displaysd⋆pasafunctionoftheequivalenceratioforthe three fuels considered in thissection. It showsthat for methane/air flames, the theoretical scaling d⋆
p=0.27 is
per-fectforallequivalenceratioswithinflammabilitylimits.For acety-lene/airflames,thescalingd⋆
p=0.5isalsosatisfactory.Finally
hy-drogen, asoften,exhibitsa specific behavior witha d⋆
p=0.4law
valid over a limited rangeof equivalence ratios but not for very richflames (
φ
>1.8).Tofirstorder,however,Fig.10confirmsthat a scaling law based on the reduced critical diameter for inhibi-tion d⋆p=dcps0L/Dth=
η
whereη
is a constant dependingonly onthefuelisreasonablyaccurate.Notethat,d⋆
pmaydependonother
parameters inpractice.Nevertheless,theseresults canbe usedto obtaina firstorder estimationoftherangeofsizesto useforan efficientsodiumbicarbonatepowder.
4. Transientbehaviorofthe1Dpremixedmethane/airflame speedwithadditionof(NaHCO3)sparticles
Theprevioussectionshavedescribedhowparticlesdecompose in the flame (path S-G in Fig. 1) and how NaOH homogeneous chemicalinhibition effectmaybe modeled.However,thedetailed chemicalinteractionbetweenNaOHandtheflamereactionzoneat thelocationofparticledecompositionhasnot beendiscussedyet. Inthis section,the flameresponse toinhibition isstudied. Based ontheresultsofthetheoreticalanalysis,aselectionofparticle di-ameters around thecritical value dc
p is usedto discussthe
influ-ence of sodium bicarbonate on the flame consumption speed in
Section 4.1. Theimpact oftheinhibitormass loadingm⋆
Inh1 is
as-sessedinSection4.2.
Sodium bicarbonate particles are placed in front of a moving 1Dpremixedmethane/airflameatstoichiometry.Thecoldmixture isatatmosphericconditions.Theflameisfirstinitialized without particles to stabilize the flame speed. Then, particles are initial-ized in thefresh gases, atthe fresh gasspeed, avoiding any im-pactof theinitialization process on the results.The particles are initially in thermodynamic equilibrium withthe gas (Fig. 11). In the following, the consumption speed (sc,het) of the propagating
1 The particles mass loading is m ⋆
Inh = N pρp V p / (mg + N pρp V p ), where N p is the to-
tal number of particles, V p is the volume of each particle and m g is the mass of
the gas. The inhibitor mass loading m ⋆ Inh is linked to NaOH mass fraction after total
decomposition by: Y NaOH = W (NaHCO3)s m ⋆ Inh /W NaOH , where W k denotes the molecular
Fig. 11. A 1D premixed methane/air flame at stoichiometry propagating in a mixture filled with sodium bicarbonate particles. L = 0 . 1 m . The particles are initially in a thermodynamic equilibrium with the gas.
Fig. 12. Temporal evolution of the flame consumption speed s c,het for various in-
hibitor particle diameters. (Solid line) d p = 5 µm. ( ◦) d p = 10 µm. ( !) d p = 15 µm.
( ▽ ) d p = 20 µm. (x) d p = 30 µm.
flamewillbecomparedtothelaminarflamespeedssε
L,hom shown
in Fig. 3. sεL,hom is the laminar speed of a methane/airflame in-hibited homogeneously by gaseous NaOHatYNaOH=
ε
.Finally, as mentioned before, radical species reach a peak concentration in-side theflamefront beforebeingrelaxedtotheirburntgas equi-libriumvalue.Hence,inthefollowingYmaxk denotesthemaximum
valuereachedbyaspecieskinsidetheflamefront(seeFig.5). 4.1. Effectofparticlesizeonflamepropagation
The mass loading of inhibitor introduced is constant and set to m⋆
Inh=0.84%. Once fully decomposed,this mass of (NaHCO3)s is equivalent to a mass fraction of NaOH:YNaOH=0.004. There-fore the value s0.004
L,hom is used as a reference. The temporal
evolu-tionoftheflameconsumption speedsc,hetispresentedinFig.12. From the results presented inSection 3.2.3, particles with diam-eter lower than 16µm should lead to successful inhibition. The presentsimulationsconfirmthisconclusion:adrasticdecayinthe flame consumption speedis observedfor particleswithdiameter dp=[5,10,15] µm.Immediatelyafter thefirstparticles reachthe
decompositiontemperature, sc,het startsdecreasingduetothe in-teraction betweenthe gaseous agentNaOH and theflame chem-istry. sc,het eventually stabilizes atthe predicted value s0L,hom.004.On
the other hand, larger particles (dp≥ 20
µ
m) decomposedown-stream of theflame. The latterthen remains quasiinsensitive to the liberation ofNaOH inthe burntgases. Figure 13displaysthe effects oftheliberated NaOH byparticle decompositiononflame temperature, heat release andmajor speciesprofiles forthe case dp=10
µ
m.Atthelocationwhere(NaHCO3)sparticlesdecompose, theproductionofgaseousNaOHleadstoradicalspecies consump-tion andheat release reduction which causes the flame to slowdown.TheconsumptionofNaOHdependsontheflamespeedand thelocation of(NaHCO3)s decompositioninsidethereactionzone. Therefore, for the case dp=10
µ
m, only a fraction of theliber-atedNaOHfinally interactswiththeflamereactions.The remain-ingNaOHisleftbehindtheflame.
AsseeninFig.12,theflame burningvelocity inthecasedp=
20
µ
m is not equal to the unhibited flame speed, suggesting a partial effect ofthe inhibitor inthis case. This can be explained by considering NaOH back diffusion. Indeed, even though NaOH isliberated pastthereactionzone (ata distance2
=χ
⋆−δ
R> 0
fromthe latter), it can diffuse back to reach the exothermic re-gion provided that NaOH mass diffusion velocity (estimated by sNaOH=DNaOH/
2
,withDNaOH NaOHthe NaOHmassdiffusion co-efficient)isgreater thanthe flameconvectionspeed (ρ
u/ρ
b)sL.Inthepresentcase,
2
≈ 150µmandtheratio(ρ
u/ρ
b)sL/sNaOH≈ 1.1is closetounity.Therefore,apartialreductionoftheflamespeedcan beobservedforparticlesizesslightlyhigherthandcp.
Before stabilizingat s0.004
L,hom,a transient phase can be observed
inFig. 12 fordp≤ 15
µ
m. Thistransient phaseis stronger asthesize ofthe particles increases.Indeed, smallparticles, dp≤ 5
µ
mforexample,decompose far ahead ofthe flamefront in a region wherethegaseousNaOH efficientlyconsumestheradicalspecies. Subsequently,sc,hetdecreasessmoothlytoreachs0L,hom.004.Inthecase
dp=[10,15]
µ
m however, the particles decompose further awayfromtheflametip.
4.2.Effectofinhibitormassloadingonflamepropagation
Theparticlesize isnow fixed(dp=5
µ
m),andthetotal massloadingoftheinjectedinhibitorisvaried(m⋆
Inh=[0.21,0.84,1.6]%
corresponding, respectively, to YNaOH=[0.001,0.004,0.008]).
Figure 14displaysthe temporalevolution ofthe flame consump-tionspeedforthethreecases.Asexpected,aftertheflamecrosses theparticles path,a drastic decrease insc,het isobserved due to
thereduction ofthe radicalspeciesconcentrations,thus theheat release.sc,hetreachestheexpectedsteadystatevalue correspond-ingtotheinhibitor loadingconsidered.Thelattervaluedecreases withincreasinginhibitormassloading.
5. Effectofanon-uniformparticlespatialdistributiononthe propagationofa2Dplanarflame
In the previous sections, the flame inhibition problem has been discussed in its one-dimensional form. Moving to a two-dimensional configuration allows to assess the effect ofparticles spatialdistributionontheflamepropagation.Inpractice,injection manifoldsusedtodisperseinhibitionpowdersinagascanhardly guaranteeanhomogeneousparticledistribution.Flamespeed vari-ationsalongtheflamesurface, inthecaseofinhomogeneous dis-tribution,mustbeexpectedandthey caninduceflamesurface in-creasewhichmayoffsettheinhibitioneffectsasalreadyobserved experimentally by Rosser et al. [4]. Similarly to turbulent flames
Fig. 13. The effect of NaOH liberation inside the reaction zone on: (left) flame temperature T f (solid lines) and heat release rate H RR (dotted lines); (right) major species
profiles for the case d p = 10 µm. In both figures, lines without symbols refer to the flame prior to inhibition, lines with ▽ symbol refer to the inhibited flame and lines with
◦ symbol represent the particle trajectory. X f is the position of the leading point of the flame.
Fig. 14. Temporal evolution of the flame consumption speed s c,het for various in-
hibitor mass loadings. ( ◦) m ⋆ Inh = 0 . 075 mg . ( !) m ⋆ Inh = 0 . 3 mg . ( ▽ ) m ⋆ Inh = 1 . 2 mg .
wherethetotalheatrelease
3
istheproductoftheaveraged con-sumptionspeed <sc> andtheflamesurfaceAf,itispossiblethata decrease in <sc> dueto the inhibitors could be offset by an
increase inAf leadingto an overall increase of
3
˙. It is thereforeimportanttoinvestigatepossiblecounter-inhibitioneffectsrelated toanon-uniformagentdistribution.
5.1.Numericalsetup
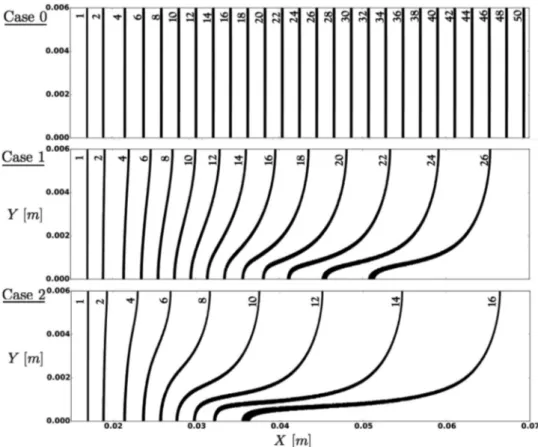
The 6mm wide and 70mm long 2D channel is illustrated in
Fig. 15. A symmetry condition is used for the top and bottom boundaries.Ano-slipconditionisappliedattheleftboundaryand
therightoneisan outlettreated withtheNSCBCformalism [34]. Initially,theflameisplanarandlocatedneartheleftendofthe do-main.Amethane/airflameatstoichiometryandatmospheric con-ditionsisconsideredhere.
Theparticlesareplacedontherightsideoftheflamefront.To ensureacompletedecompositionoftheparticlesinsidetheflame front,theparticlediameterissettodp=5
µ
m(thecriticaldiame-terdc
pforthiscaseis16µm).Thetotalmassloadingoftheinjected
inhibitorism⋆
Inh≈ 1%correspondingtoYNaOH=0.0047andan
in-hibitedflamespeeds0.0047
L,hom ≈ 0.15m/s.Alinearstratificationis
ap-pliedin thevertical direction.Three casesare considered. Case0 correspondstoanhomogeneousdistributionof(NaHCO3)s.The in-hibitormass loadinggradientisincreasedforcases1and2.Case 2corresponds to themostinhomogeneous distribution whereno particleispresentatthetopboundary.
5.2. Results
The flameshape atdifferenttimesispresented inFig.16.For thehomogeneouscase(case0),theflameremainsplanar andthe flame slows down similarly to the 1D simulations in Section 4. The resulting displacementspeed is approximately 1.1m/s andis closetothepredictedvalueTadsL,hom0.0047/T0.Asexpected,an increase offlamesurfaceisobservedforcases1and2asaresultofthe in-homogeneousdistributionof(NaHCO3)sparticles.Thefingershape exhibited by theflame in cases1 and2 isdue tothe strong lo-calflamespeedvariationsalongtheflamesurface,whichcreatesa strongflamestretchandleads, after5ms,to aglobalacceleration oftheflame.
Theremarkabledifferenceintotalflamelengthsbetweencases 1and2isattributedtotheflamestretchcausedbythevariations ofthelocalflamespeedalong theflamesurface. Thesevariations are due to the non-linear response of the flame speed to NaOH concentration associatedwiththe”idealinhibitor model” (Fig.3).
Figure17displaystheNaOHmassfractionalongtheflamesurface averaged over theprogress variable c andthe distributionof the local flame consumption speed sc at t=2.5ms. Theses curves
Fig. 15. Particle distribution in the vicinity of a planar methane/air flame at stoichiometry and atmospheric conditions. The channel is 70 mm long and 6 mm wide. Right: Mass density distributions over the vertical axis for the three cases considered. Case 0 corresponds to an homogeneous distribution and Case 2 to the most inhomogeneous one where no particles are present at the top boundary.
Fig. 16. Propagation of a 2D initially planar methane/air flame in a inhomogeneous distribution of (NaHCO 3 ) s particles. The flame shape (isosurface of progress variable c ) and the corresponding time in milliseconds are shown for cases 0, 1 and 2.
changewithtimeandareplottedinFig.17whentheflamestarts interacting with particles. As expected, the local consumption speeds are all smaller than the uninhibited value s0
L≈ 0.39m/s
but they are also changing strongly from bottom to top for cases 1 and 2 explaining the flame surface increase observed in
Fig.16.
Figure 18displaysthetemporal evolutionoftheflame surface Afandof themeanheat release
3
inthe channel.The flamere-sponse to an homogeneous particle field (case0) is asexpected: the flame remains planar, its surfaceis constant (Af/A0f=1) and
themeanheat releaseinthechamber dropssignificantlyasa re-sult ofinhibition. Fora stratified distribution however,the curve exhibits a parabolic shape. For case 2, the inhibition effect is dominant before ≈ 4.2ms and a negative slope is observed in the
3
˙ curve. However, since theinhibition effectis not homoge-neousalongtheflamefront,theflameareaincreases.Att≈ 4.2ms (marked by ∗),the flamesurfaceeffectdominatesthelocalflame speedreductionsothatthetotalheatreleasestartsincreasing.By the time the flame reaches the endof the domain, the heatre-leased inside thechamber hasmore than doubled asa resultof theflamesurfaceincrease.Forbothcases1and2,
3
˙ attheendof thesimulationexceedsitsinitialvalue.Figure18underlinespossiblecounter-effectstoflameinhibition related to non-uniform particle distributions. This phenomenon might also explain overpressure increase observed in the FAA-ACT experiments [18] where the introduction of inhibition pow-dersactuallyled tofaster flamesandhigheroverpressures. Many authors [38–41] have proposed an explanation for the observed overpressure increase based on thermodynamic equilibrium cal-culationsandperfectlystirredreactorsimulations. Anon-uniform inhibitor distribution prior to ignition,and the subsequent flame surface increase, may also explain the increase in pressure dur-ing these experiments. This is particularly true since agent dis-tribution was not characterized in the aerosol can test experi-ments. In real explosions, the presence of obstacles in the path of a flame might add up to the above effects and lead to dam-ages amplification instead of the desired mitigation ofexplosion hazards.
Fig. 17. Inhomogeneous flame displacement speed along the flame surface as a result of stratified particle distribution (cases 0, 1 and 2) at t = 2 . 5 ms . Left: NaOH mass fraction along the flame surface averaged over the progress variable c . Right: Flame consumption speed along the flame surface.
Fig. 18. Flame response to (NaHCO 3 ) s particles distribution. Left: temporal evolution of the flame surface normalized by its initial value. Right: Temporal evolution of the mean heat release in the channel. The symbol ∗ indicates the slope change in the heat release evolution for case 2 related to the transition from planar to finger shaped flame.
6. Conclusion
Inhibition of hydrocarbon/air flames by monodisperse solid sodiumbicarbonateparticleshasbeenstudiednumericallyand an-alytically. The higher effectiveness per mass basis of sodium bi-carbonate compared to other inhibitors motivated the choice of (NaHCO3)s. Thisefficiencyis attributedto thechemical nature of flame/(NaHCO3)sinteraction.(NaHCO3)s particlesundergothermal decompositionwhenexposed toflametemperature.As aresult,a gaseousagentNaOH isliberated andactsasradicalspecies scav-engerreducingheatrelease,henceflamespeed.
Toaccount forthe pathfrom(NaHCO3)s toNaOH,asimplified thermaldecompositionmodelisproposed based ona singlestep approach.Although it is too simple to account forthe details of particle heatingand decomposition, thismodel is able to repro-ducefirstordereffects.Thediameterofthelargestparticledc
pable
to decompose inside the flame front for different fuels is evalu-atedusingananalytical expressionofparticletemperatureandis consistentwiththeliterature.Theseresultsshow that,unlessthe samplesarefinelycrushed,usingthesamepowderwithoutaclear understandingofits limitsmayleadto unsuccessfulinhibition in the caseof highly reactive fuels forinstance. For safety reasons,
the particle size distribution inside the inhibitor injection tanks must be carefully characterized. A simple scaling law giving the maximum particlesize dc
p in a sodium bicarbonate powder used
toinhibit agivenflame isproposed fromtheory andsimulations andreadsdc
ps0L/Dth=
η
,whereη
isaconstantdependingonlyonthefuel:
η
=0.27formethane/airflames,η
=0.5foracetylene/air flamesandη
=0.4forhydrogenflames.ToreproduceNaOHinteractionwiththegasphasechemical re-actions, an Analytically Reduced Chemistry (ARC) is derived and validated against both Rosser et al [4] experimental data and Babushoketal. [15]detailedchemistry findings. Thismechanism iscoupledwiththesinglestepdecompositionmodeltoinvestigate theimpactofsodiumbicarbonateparticlesonthepropagationofa 1Dstoichiometricmethane/airflame.Thisallows todescribe tran-sienteffectsofflameinhibition.Eventually,theflameconsumption speed reachesasteady statevalue dependent onthe massof in-jectedinhibitor:thisspeediscoherentwiththehomogeneous in-hibitionresults.
Finally,theeffectofspatialinhibitordistributiononthe propa-gationofa2D stoichiometricmethane/airflameisdiscussed.This simulation highlights possible counter-inhibition effects: as a re-sponsetosodiumbicarbonatenon-uniformdistribution,theflame
is highlystretched leadingto a global flame accelerationinstead ofmitigation.Thisresultechoesexperimentalobservationswhere theintroductionofinhibitionpowdersactuallyincreasedthe over-allcombustionspeedandtheoverpressure[4,18].
Interms ofapplications,tolead toan efficientinhibition, two conditions must be met: (1) the powder size must be smaller that a limit value dc
p whichdepends on theflame thickness and
speed(becauseoftheirhigherflamespeedandlowerflame thick-ness, hydrogen/air flames require much smaller particles than methane/airflames forexample);(2) theinhibitor distributionin themixturemustbequasi-homogeneous,otherwiseflame acceler-ationcanbeobserved.Property(1)iseasytoensureandthe pro-posedsimplescalinglawdc
p=
η
Dth/s0L canbeusedtosetupanef-ficientpowder. Property(2),however,willdependonthepowder injectionsystem andismuch moredifficult toachieve. From the firesafetypointofview,thesideeffects,relatedtopowder stratifi-cation,maybeeasilycircumventedensuringthedeliveryofa min-imummassneededtosuppressa givenflame.However, fromthe perspectiveofgasexplosions,theobjectiveistoavoid extinguish-ing the flame since after flame suppression, the volume remains filledwithareactivemixturesusceptibletoigniteandtoexplode. Itisthenofcrucialimportancetoverifyproperty(2).
Acknowledgments
Thisworkissupported byTotalcompanyandtheassistanceof Dr.L.Hoorelbeke,P.RicouxandA.Dutertreisgratefully acknowl-edged.The authorsacknowledgeCINESofGENCIforgivingaccess to HPC resources under the allocation A0012B10157. The authors aregratefultoDr.GregoryT.LinterisfromtheNationalInstituteof StandardsandTechnology(NIST)forprovidingkineticratesforthe inhibitionsub-mechanism.
Appendix
TheequationforparticletemperatureEq.(10)reads: dTp dx =
)
T(
dp,s 0 L)
"
1−TTp f#
Afterintegration,theparticletemperatureequationbecomes: Tp=
)
T +ν
(
x)
dx+Cν
(
x)
(12) whereν
(
x)
=exp,
)
T + [1/Tf]dx -.The expression of the flame temperature Tf, given by (EF) model, dependsonthe position relativeto
δ
EF.However, thena-tureofTfexpressionforx≤
δ
EF isidenticaltothatofTfforx≥δ
EF.Therefore,inthefollowing,thederivationoftheanalytical expres-sionEq.(11)isdetailedforthecasex≤
δ
EFonly.Forx≤
δ
EF: . 1 Tf dx= . 1 C1+C2exp,
δxEF -dx =Cx 1−δ
EF C1 ln$
C1+C2exp$
xδ
EF%%
Therefore,ν
(
x)
=/
C1exp(−
xδ
EF)
+C20
−)TδEF/C1 (13)TheonlytermleftinEq.(12)is,
.
ν
(
x)
dx=−δ
EF . [C 1v
+C2]−)TδEF/C1v
dvwithv
=exp$
− xδ
EF%
(14) =−δ
EFC2−)TδEF/C1 . g−)TδEF/C1 g− 1 dgwithg=1+ C1 C2v
(15) Here, g=1+CC1 2v
g=1+"
T0(
e− 1)
(
Tad− T0)(1
− 1/β
)
− 1#
v
g=1+$
(
σ
e− 1 − 1)(1
− 1/β
)
− 1%
v
withσ
= Tad/T0 Sinceintypicalflamesσ
>3.andβ
>2,onecanprovethat:∀
x∈]0,δ
EF],|
g|
<1Therefore,the integral (15) is absolutelyconvergent.Using Taylor seriesexpansions, onecanlinkthisintegraltothegauss hyperge-ometricfunction: . g−)TδEF/C1 g− 1 dg=− . g3 1− gdg with
3
=−)
Tδ
EF/C1 =− . g31 j gjdg =−1 j gj+3+1 j+3
+1 =−g 3+13
+1 1 j3
+1 j+3
+1g j =−g 3+13
+1 1 j(1)
j(3
+ 1)
j(3
+ 2)
j gj/ j! =−g 3+13
+ 12F1(1
,1+3
; 2+3
; g)
(16)(a)nisthePochhammersymboldefinedas:
(
a)
0=1,∀
n>0,(
a)
n= n2
k=1
(
a+k− 1)
TheGaussHypergeometricfunctionisdefinedforanya∈C,b∈C andc∈C
\{
Z−∪{
0}}
bythefollowingTaylor-Seriesexpansion: 2F1(
a,b; c; z)
= ∞ 1 j=0(
a)
j(
b)
j(
c)
j zj j! if|
z|
<1CombiningEqs.(13)and(16)withEq.(12)leadsto theanalytical expressionEq.(11).
Forthecasex>
δ
EF,the stepsare very similar. Theintegral inEq.(14)becomes: .
ν
(
x)
dx=−1δ
EF −β
.)
D1v
′+D2*
−)TδEF/((1−β )D1)v
′ dv′ (17)where
v
′=exp(
−(1δ−EFβ )x)
.Toendupwithaconvergentintegral,the followingvariablesubstitutionisproposed:g′=DD2
1
v
′with
∀
x≥δ
EF,|
g′|
≤σ
− 1βσ
<1/β
OnecanstillretrievetheGausshypergeometricfunction,onlywith differentparametersEq.(11).
Finally, the Gauss hypergeometric series converges for |z|<1 butthe number ofterms needed to reach an accurate value de-pend on the value of |z|. This number is significantly reduced if |z|<1/2. Therefore,oneis advisedto usethefinal transformation formulaeafterthestep16:
g′=
3
g if0≤
|
g|
≤ 1/2 1− g if1/2<|
g|
<1References
[1] P. Pepiot-Desjardins , H. Pitsch , An efficient error-propagation-based reduction method for large chemical kinetic mechanisms, Combust. Flame 154 (2008) 67–81 .
[2] D. Trees , K. Seshadri , Experimental studies of flame extinction by sodium bi- carbonate (NaHCO 3 ) powder, Combust. Sci. Technol. 122 (1997) 215–230 . [3] A. Hamins , Flame extinction by sodium bicarbonate powder in a cupburner,
Symp. (Int.) Combust. 27 (1998) 2857–2864 .
[4] W. Rosser , S. Inami , H. Wise , The effect of metal salts on premixed hydrocar- bon-air flames, Combust. Flame 7 (1963) 107–119 .
[5] M. Dewitte , J. Vrebosch , A. van Tiggelen , Inhibition and extinction of premixed flames by dust particles, Combust. Flame 8 (1964) 257–266 .
[6] P. Laffitte , R. Delbourgo , J. Combourieu , J. Dumont , The influence of particle di- ameter on the specificity of fine powders in the extinction of flames, Combust. Flame 9 (1965) 357–367 .
[7] K.S. Iya , S. Wollowitz , W.E. Kaskan , The mechanism of flame inhibition by sodium salts, Symp. (Int.) Combust. (1975) 329–336 .
[8] R. Friedman , J. Levy , Inhibition of opposed jet methane-air diffusion flames. the effects of alkali metal vapours and organic halides, Combust. Flame 7 (1963) 195–201 .
[9] M. Vanpee , P. Shirodkar , A study of flame inhibition by metal compounds, Symp. (Int.) Combust. 17 (1979) 787–795 .
[10] M. Vanpee , P.P. Shirodkar , Characterization of physical, thermal and chemi- cal contributions of sodium bicarbonate particles in extinguishing counterflow nonpremixed flames, 5th ASME/JSME Thermal Engineering Joint Conference (1999) .
[11]H. Chelliah , P. Wanigarathne , A. Lentati , R. Krauss , G. Fallon , Effect of sodium bicarbonate particle size on the extinction condition of non-premixed counter- flow flames, Combust. Flame 134 (2003) 261–272 .
[12] L. Hoorelbeke , Numerical and experimental study of vapour cloud explosions – mitigation by inhibitors, VUB Brussel, 2011 Ph.D. thesis .
[13] B.A. Williams , J.W. Fleming , Suppression mechanisms of alkali metal compounds, 1999 Halon Options Technical Working Conference (1999), pp. 157–169 .
[14] B.A. Williams , J.W. Fleming , CF3Br and other suppressants: Differences in ef- fects on flame structure, Proc. Combust. Inst. 29 (2002) 345–351 .
[15] V. Babushok , K. McNesby , A. Miziolek , R. Skaggs , Modeling of synergistic ef- fects in flame inhibition by 2-H heptafluoropropane blended with sodium bi- carbonate, Combust. Flame 133 (2003) 201–205 .
[16] W. Hu , J.M. Smith , T. Do ˘gu , G. Do ˘gu , Kinetics of sodium bicarbonate decompo- sition, AIChE J. 32 (1986) 1483–1490 .
[17]Y.-L. Wu , S.-M. Shih , Intrinsic kinetics of the thermal decomposition of sodium bicarbonate, Thermochim. Acta 223 (1993) 177–186 .
[18] J.W. Reinhardt , Behavior of bromotrifluoropropene and pentafluoroethane when subjected to a simulated aerosol can explosion, Technical Report, Fed- eral Aviation Administration, 2004 . DOT/FAA/AR-TN04/4
[19] W. Jones , V. Prasad , Large eddy simulation of the sandia flame series (d-f) using the eulerian stochastic field method, Combust. Flame 157 (2010) 1621–1636 .
[20]B. Franzelli , E. Riber , B. Cuenot , Impact of the chemical description on a large eddy simulation of a lean partially premixed swirled flame, C. R. Méc. 341 (2013) 247–256 .
[21]O. Schulz , T. Jaravel , T. Poinsot , B. Cuenot , N. Noiray , A criterion to distinguish autoignition and propagation applied to a lifted methane-air jet flame, Proc. Combust. Inst. 36 (2017) 1637–1644 .
[22]T. Jaravel , E. Riber , B. Cuenot , G. Bulat ,Large eddy simulation of an industrial gas turbine combustor using reduced chemistry with accurate pollutant pre- diction, Proc. Combust. Inst. 36 (2017) 3817–3825 .
[23]T. Mitani , Flame retardant effects of CF3Br and NaHCO 3 , Combust. Flame 50
(1983) 177–188 .
[24]T. Mitani , T. Niioka , Extinction phenomenon of premixed flames with alkali metal compounds, Combust. Flame 55 (1984) 13–21 .
[25]D. Spalding , The combustion of liquid fuels, Symp. (Int.) Combust. 4 (1953) 847–864 .
[26]K.K. Kuo , Principles of combustion, Second ed., John Wiley & Sons, Inc., Hobo- ken, New Jersey, 2005 .
[27]A.J. Hynes , M. Steinberg , K. Schofield , The chemical kinetics and thermody- namics of sodium species in oxygen-rich hydrogen flames, J. Chem. Phys. 80 (1984) 2585–2597 .
[28] G.P. Smith, D.M. Golden, M. Frenklach, N.W. Moriarty, B. Eiteneer, M. Golden- berg, C.T. Bowman, R.K. Hanson, S. Song, J. William C. Gardiner, V.V. Lissianski, Z. Qin, GRI-Mech 3.0 http://www.me.berkeley.edu/gri _ mech/ , 2017.
[29]P. Pepiot , Automatic strategies to model transportation fuel surrogates, Stan- ford University, 2008 Ph.D. thesis .
[30]T. Noto , V. Babushok , A. Hamins , W. Tsang , Inhibition effectiveness of halo- genated compounds, Combust. Flame 112 (1998) 147–160 .
[31]G. Dixon-Lewis , R. Simpson , Aspects of flame inhibition by halogen com- pounds, Symp. (Int.) Combust. 16 (1977) 1111–1119 .
[32]J.H. Ferziger , T. Echekki ,A simplified reaction rate model and its application to the analysis of premixed flames, Combust. Sci. Technol. 89 (1993) 293–315 . [33]O. Colin , M. Rudgyard , Development of high-order Taylor–Galerkin schemes for
unsteady calculations, J. Comput. Phys. 162 (20 0 0) 338–371 .
[34]T. Poinsot , S. Lele , Boundary conditions for direct simulations of compressible viscous flows, J. Comput. Phys. 101 (1992) 104–129 .
[35]C.W. Oseen , Hydrodynamik, Vol. 1, Akad. Verl.-Ges., 1927 .
[36]H. Wang , A. Laskin , Z. Djurisic , C. Law , S. Davis , D. Zhu , A comprehensive mech- anism of C 2 H x and C 3 H x fuel combustion, Fall Technical Meeting of the Eastern
States Section of the Combustion Institute, Raleigh (1999), pp. 129–132 . [37]P. Saxena , F.A. Williams , Testing a small detailed chemical-kinetic mechanism
for the combustion of hydrogen and carbon monoxide, Combust. Flame 145 (2006) 316–323 .
[38]G.T. Linteris , V.I. Babushok , P.B. Sunderland , F. Takahashi , V.R. Katta , O. Meier , Unwanted combustion enhancement by C 6 F 1 2O fire suppressant, Proc. Com-
bust. Inst. 34 (2013) 2683–2690 .
[39]V.I. Babushok , G.T. Linteris , D.R.B. Jr. , P.T. Baker , Hydrocarbon flame inhibition by C 3 H 2 F 3 Br (2-BTP), Combust. Flame 162 (2015) 1104–1112 .
[40]G.T. Linteris , V.I. Babushok , J.L. Pagliaro , J. Donald Raymond Burgess , J.A. Man- ion , F. Takahashi , V.R. Katta , P.T. Baker ,Understanding overpressure in the FAA aerosol can test by C 3 H 2 F 3 Br (2-BTP), Combust. Flame 167 (2016) 452–462 . [41]W. Xu , Y. Jiang , R. Qiu , X. Ren , Influence of halon replacements on laminar
flame speeds and extinction limits of hydrocarbon flames, Combust. Flame 182 (2017) 1–13 .
[42]I. Dyakov , A .A . Konnov , J.D. Ruyck , K.J. Bosschaart , E.C.M. Brock , L.P.H.D. Goey , Measurement of adiabatic burning velocity in methane–oxygen–nitrogen mix- tures, Combust. Sci. Technol. 172 (2001) 81–96 .
[43]A. Van Maaren , D. Thung , L.R.H. De Goey , Measurement of flame temperature and adiabatic burning velocity of methane/air mixtures, Combust. Sci. Technol. 96 (1994) 327–344 .
[44]C.M. Vagelopoulos , F.N. Egolfopoulos , Direct experimental determination of laminar flame speeds, Symp. (Int.) Combust. 27 (1998) 513–519 .
[45]X. Gu , M. Haq , M. Lawes , R. Woolley , Laminar burning velocity and markstein lengths of methane–air mixtures, Combust. Flame 121 (20 0 0) 41–58 .