•
•
Université de Sherbrooke
Culture of dimethylhydrazine induced murine angiosarcoma and an evaluation of anti-angiogenic therapy of primary tumor
and of tumor metastasis.
by
Radha NaikDépartement d'anatomie et biologie cellulaire
Thesis presented to the Faculté de médecine with the goal of obtaining the degree of
Masters of Science (M.Sc.)
•
•
Do not follow where the path may lead. Go, instead, where there is no path and leave a trail.
•
TABLE OF CONTENTSTitle . . . 1
Table of contents . . . m List of figures and tables Vll List of abbreviations 1X Summary X1 Resumé I. INTRODUCTION . . . 1
1. Kaposi's sarcoma . . . 1
1.1. Classical Kaposi's sarcoma . . . 2
1.2. African Kaposi's sarcoma . . . 2
1.3. Iatrogenic Kaposi's sarcoma . . . 3
1.4. AIDS associated Kaposi's sarcoma 3 1.4.1. Treatment . . . 5
1.4.2. Models for the study of AIDS associated K apos1 s sarcoma . . . ., 5
2. .A.ngiosarcomas 7 2.1. Comparison between angiosarcomas and Kaposi's sarcoma . . . 7
2.2. Mouse angiosarcomas 10 3. Dimethylhydrazine . . . 10
4. Neoplastic endothelial cell lines 13 4.1. Methods of endothelial cell culture . . . 14
•
•
•
4.2. Identification . . . 15
4.3. Angiosarcoma cell lines . . . 16
4.4. AIDS associated Kaposi's sarcoma cell lines 16 5. Angiogenesis . . . 17
5.1. Angiogenic factors . . . 17
5.1.1. Fibroblast growth factor . . . 19
5.1.2. Transforrning growth factor . . . 19
5.1.3. Tumor necrosis factor . . . 20
5.1.4. Platelet derived growth factor . . . 21
5.2. Tumor angiogenesis . . . 21
5.2.1. Necessity of tumor angiogenesis . . . 22
5.2.2. Tumor growth vs. angiogenesis . . . 22
5.2.3. Metastasis . . . 25
6. Anti-angiogenesis . . . 25
6.1. Heparin and cortisone . . . 26
6.2. Maltose tetrapalmitate 7. Objectives II. MATERIAL AND METHODS 1. Animais 27
28
29 29 1.1. Dimethylhydrazine treatment . . . 291.2. Cortisone and maltose tetrapalrnitate treatment . . . 30
1.3. Angiosarcoma transplants . . . 30
•
•
2. Cell culture . . . 31
2.1. Media . . . 31
2.2. Angiosarcoma cell lines . . . 32
2.3 Cell proliferation . . . 32
2.4. Cloning . . . 33
2.5. Identification . . . 33
2.6. Tumorigenicity studies . . . 34
2.7. In vitro maltose tetrapalmitate studies . . . 34
2.7.1. Complement fixation . . . 34
2.7.2. Endothelial cell proliferation . . . 34
3. Histology and electron microscopy . . . 35
3.1. Preparation of tissues for routine histology . . . 35
3.2. Preparation of blacks and staining . . . 36
3.3. Scanning electron microscopy ... . ID. RESULTS 1. Dimethylhydrazine treatment ... . 1.1. Dimethylhydrazine toxicity ... . 1.2. Effect of dimethylhydrazine on weight ... . 1.3. Angiosarcoma treatment 1.3.1. Angiosarcoma histology ... . 1.3.2. Angiosarcoma transplants ... . 1.4. Other tumors induced by dimethy!hydrazine ... . 2. Angiosarcoma cell culture 36 37 37 37 37 37 40 53 53 56 2.1. Establishment of angiosarcoma cell lines . . . 56
•
•
2.2. Growth characteristics of NAIK AS . . . 60
2.3. Morphology of NAIK AS . . . 65
2.4. Identification of NAIK AS . . . 65
2.5. Electron microscopy of NAIK AS . . . 80
2.6. Tumor induction by NAIK AS . . . 80
2.7. Maltose tetrapalmitate treatment of NAIK AS . . . 85
3. Cortisone and maltose tetrapalmitate treatment . . . 88
3.1. Complement fixation studies . . . 88
3.2. Angiosarcoma treatment . . . 88
3.2.1. Toxicity of cortisone and maltose tetrapalmitate 88 3.2.2. Effect of cortisone and maltose tetrapalmitate on weight . . . 88
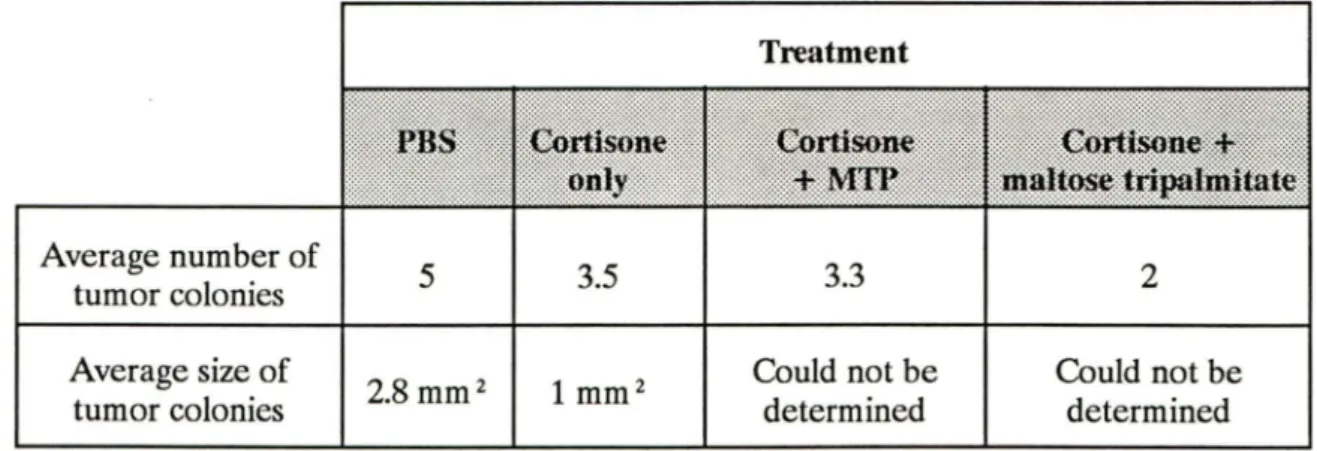
3.2.3. Effect of cortisone and maltose tetrapalmitate on angiosarcoma development . . . 92
3.2.4. Histology of cortisone and maltose tetrapalmitate treated C57Bl/ 6 mice . . . 92
3.3. Cortisone and MTP treatments of C3HBA metastases in C3H/NeN . . . 93 IV. DISCUSSION . . . 96 V. EPILOGUE . . . 108 ACKNOWLEDGEMENTS ... . ... . ... 111 REFERENCES . . . 112 APPENDIX ... . . . ... 126
•
•
Fi~res Fig. 1 Fig. 2 Fig. 3 Fig. 4 Fig. 5 Fig. 6 Fig. 7 Fig. 8 Fig. 9 Fig. 10 Fig. 11 Fig. 12 Fig. 13 Fig. 14 Fig. 15 Fig. 16 Fig. 17LIST OF FIGURES AND TABLES
Average weight ratio of DMH-treated mice vs. EDTA-treated mice. Examples of angiosarcoma presentation in the paratesticular tissue. An example of angiosarcoma presentation in periuretbral tissue.
Example of an angiosarcoma in the fat surrounding a kidney which strongly resembles a Kaposi's sarcoma lesion.
Retroperitoneal angiosarcoma whicb demonstrates the third major site of angiosarcoma presentation.
Examples of predominantly cystic and solid angiosarcomas. . A very early angiosarcoma lesion.
Primary angiosarcoma transplant.
Photograph of the first tumor explant whicb survived in culture.
The effect of various types of media on the establishment of NAIK AS. Growth of NAIK AS cells in conditioned media.
The arrangement of NAIK AS cells in monolayers and multiple layers. Diagrams of round and irregularly shaped lumen formed by NAIK AS. Diagrams of NAIK AS large and small vacuoles bordering the interstitial spaces.
Diagram of NAIK AS cells forming a lumen-like structure in a region of multiple layers.
Photographs of lumen formation in regions of multiple layers. Cloning of NAIK AS and formation of cobblestone pattern .
•
•
Fig. 18 Fig. 19 Fig. 20 Fig. 21 Fig. 22 Tables Table 1 Table II Table ID Table IV Table V Table VIFVIIIR Ag staining of NAIK AS. UEA 1 staining of NAIK AS.
Scanning electron microscope study of NAIK AS. MTP treatment of NAIK AS and CP A cells.
Average weight ratio of cortisone and MTP treated mice vs. DMH only treated mice.
Macroscopic and microscopie comparison of some of the characteristics of angiosarcomas and Kaposi's sarcoma.
Tumors present within various organs in each treatment group. MTP and lipid A inhibition of complement fixation via the classical pathway.
Cortisone and maltose palmitate treatment of C3HBA tumor m C3H/HeN mice: group 1.
Cortisone and maltose palmitate treatment of C3HBA tumor m C3H/HeN mice: group II.
•
•
AIDS AIDS KS AS BM bvCAM
CEC
CPMDMEM
DMH
EC
EDTA EGFFBS
FGF FVIIIR Ag HIV 1.p. i.v. KS LPS LIST OF ABBREVIATIONSacquired immune deficiency syndrome AIDS associated Kaposi's sarcoma angiosarcoma( s)
basement membrane blood vessel(s)
chorioallantoic membrane of the chick capillary endothelial cell(s)
counts per minute
Dulbecco's modified Eagle's medium dimethylhydrazine
endothelial cell(s)
( ethylenedinitrilo) tetraacetate epidermal growth factor
fetal bovine serum
fibroblast growth factor (may be a= acidic or b= basic) factor VIII related antigen
human immune deficiency virus intraperitoneal
intravenous Kaposi's sarcoma lipopolysaccharide
•
•
MAIDS MTP PDGF p.o. TAF TGFTNF
UEAImouse acquired immune deficiency syndrome caused by LP-BM5 VlfUS
maltose tetrapalmitate. Maltose palmitate will be used when referring to both maltose tripalmitate and MTP
platelet derived growtb factor porta oral
tumor angiogenesis factor transforming growtb factor tumor necrosis factor
•
•
angiogenic therapy of primary tumor and of tumor metastasis.
Radha Naik, Département d'anatomie et biologie cellulaire, Faculté de médicine,
Université de Sherbrooke. January 1992
This thesis consists of two parts; one deals with several aspects of dimethylhydrazine (DMH) induced carcinogenesis including the culture of DMH-induced endothelial cell (EC) tumors and the second, with anti-angiogenic therapy of primary tumor and tumor metastasis.
Part I DMH induced carcinogenesis and culture of endothelial cell tumors
Research on the treatment and study of AIDS associated Kaposi's sarcoma (AIDS KS), suspected to be of EC origin, has been retarded due to a lack of suitable animal models. The human tumor does not grow when transplanted into nude mice and spontaneous tumors equivalent to AIDS KS are as yet unknown in animais. Current experimental research centers on the induction and study of Kaposi's sarcoma (KS) like tumors and cell lines. In the laboratory of Dr. V.N. Nigam, it was noted that DMH induced mainly retroperitoneal angiosarcomas (AS) in male C57Bl/6 mice. This was consistent with the fact that DMH is metabolized primarily in the liver and that activated DMH, the ultimate carcinogen, accumulates in the retroperitoneal region. The first objective of this thesis was to study the early AS lesions in mice and compare them to the histology and growth characteristics of early KS lesions as described in published reports. Surprisingly, the early AS tumors resembled the late KS Jesions. The second objective was to transplant primary AS tumors in nude and syngeneic C57Bl/6 mice. It was found that the nude mice developed an angiogenic response even though the tumors failed to grow. In both the nude mouse and the C57Bl/6 mouse, the AS transplants were subsequently rejected. The third objective of this project was the development of an AS cell line from the primary AS tumors induced by DMH. In the development of this line, many growth promoting products and culture techniques were used. It was found that the expiant culture technique and a combination of DMEM and HAM's F-12 (1:1) containing 10% FBS and 48 µg/ml garamycin were best. The AS cell line ( designated as NAIK AS) which resulted had several aspects in common with AIDS KS cell lines. The NAIK AS line and KS cell lines have similar growth rates; however, the nutritional requirements seem to be different. Long-term survival of KS cell lines requires the addition of growth factors or the use of conditioned media; whereas the NAIK AS line Jacks this requirement. Both cell lines had similar morphologies and staining patterns to Ulex europaeus agglutinin 1 and factor VIII related antigen. Both cell lines also demonstrated an inability to form tumors when injected into mice. Based on the similarities between the two cell lines and the culture characteristics of the NAIK AS line, this cell line can be considered as a murine equivalent to cultured AIDS KS cells. Unlike AIDS KS cells in culture, the NAIK AS cell line has a long culture life and grows well under stressful culture conditions; thus, it may be useful for the screening of drugs against AIDS KS.
Part II Anti-angiogenic treatment of primary tumor and tumor metastasis
The treatment of advanced AIDS KS lesions is a problem since radiotherapy or debulking is usually not possible due to the multifocal nature of the neoplasm. Since maltose tetrapalmitate (MTP) and a combination of MTP and cortisone inhibit angiogenesis, it was hoped that MTP or the MTP-cortisone combination would have an inhibitory effect on primary AS due to their EC origin. The first objective, in part II, therefore, was to treat the C57Bl/6 mice bearing primary AS tumors with MTP and cortisone either singly or in combination. However, the results were not promising since C57Bl/6 H-2b mice were immunologically non-responders to
MTP. However, the lack of antitumor response observed in vivo was not reproduced in vitro since MTP suppressed the growth of cultured AS cells. lt is possible that two independent mechanisms are involved in MTP mediated anti-angiogenic activity: a direct effect on proliferating cells in vitro and another involving humoral MTP inactivating factor(s) in vivo, expressed in the MTP non-responders. Since the efficacy of anti-angiogenic therapy on tumor metastasis had not yet been studied, the second objective was to evaluate the effectiveness of the MTP-cortisone combination in comparison to MTP, cortisone or phosphate buffered saline (PBS) alone for tumor metastasis in MTP responder C3H/HeN H-2k mice. Metastasis studies were conducted
with the C3HBA H-2k mammary tumor line. It was found that MTP alone reduced the size of the lesions and
that MTP-cortisone had a similar effect. This demonstrated that anti-angiogenic therapy may retard the growth of metastatic colonies by limiting blood vessel extension into the tumor; thus limiting the supply of nutrients and elimination of metabolic waste. This result bas a direct bearing on the treatment of multifocal tumors where surgery and/ or radiotherapy are not applicable.
•
•
La mise en culture d'angiosarcomes murins induits par le diméthylhydrazine et l'évaluation du traitement anti-angiogénique de tumeurs primaires et métastasiques Radha Naik, Département d'anatomie et biologie cellulaire, Faculté de médecine, Université de Sherbrooke.
Ce mémoire comporte deux parties. La prerrùère se rapporte à l'étude de l'induction de la cancérogénèse par le diméthylhydrazine (DMH) et à la rrùse en culture des cellules endothéliales originant de ces tumeurs. La seconde partie se rapporte à la thérapie anti-angiogénique des tumeurs primaires et métastasiques.
1
Partie 1. Cancérogénèse induite par le DMH et culture de cellules endothéliales d'origine tumorale
La recherche sur le traitement et l'étiologie du sarcome de Kaposi associé au SIDA (SK SIDA) a peu évolué jusqu'à maintenant à cause de l'absence d'un modèle animal satisfaisant. La tumeur humaine ne prolifère pas lorsque transplantée chez la souris nue et des tumeurs spontanées semblables au SK SIDA n'ont pas encore été identifiées chez les animaux. Pour cette raison, les efforts de recherche se concentrent sur l'induction et l'étude de tumeurs semblables au sarcome de Kaposi (SK) ainsi que sur l'isolation de lignées cellulaires.
Dans le laboratoire du Dr. V.N. Nigam, il a été montré que le DMH induit des angiosarcomes (AS) dans la région rétropéritonéale chez la souris mâle C57Bl/6. Ceci était cohérent avec l'idée que le DMH est métabolisé principalement au niveau du foie et que le DMH activé, l'agent cancérigène, s'accumule dans la région rétropéritonéale. Le premier objectif de ce travail fut d' étudier les lésions précocement induites chez la souris et de comparer l'histologie et la croissance des
•
•
surprenante, les lésions précoces associées aux AS ressemblaient aux lésions tardives de type KS.
La deuxième objectif fut de transplanter des tumeurs AS primaires chez les souris nues et syngéniques C57Bl/6. Nous avons observé une réponse angiogénique même en absence de croissance tumorale. Chez les deux souches, les transplants AS furent rejetés.
Le troisième objectif de ce projet fut de développer une lignée cellulaire provenant de tumeurs AS primaires induites par'le DMH. Plusieurs promoteurs de croissance et techniques de culture furent testés. Nous avons constaté que la technique de culture d'explants et une combinaison de DMEM et HAM F-12 (1:1) contenant 10% FBS et 48
µg/ml
de garamycine furent les conditions les plus avantageuses. La lignée cellulaire AS (désignée NAIK AS) résultante avait plusieurs caractéristiques communes avec les lignées cellulaires SK SIDA dont le taux de croissance. Cependant, leurs besoins nutritionnels différaient. La survie à long terme des lignées SK requirent l'addition de facteurs de croissance ou l'utilisation de niilieux conditionnés. Les deux lignées cellulaires présentaient des morphologies similaires et des patrons de localisation comparables pour l'agglutinine 1 de Ulex europaeus et l'antigène relié au facteurvm.
Elles ont aussi démontré leur incapacité à former des tumeurs lorsqu'injectées chez la souris. Considérant les similarités évidentes entre ces lignées et les caractéristiques de culture de la lignée NAIK AS, cette dernière peut être considérée comme l'équivalent murin des lignées SK SIDA A la différence des cellules SK SIDA, la lignée NAIK AS exhibe une durée de survie relativement longue et croît bien sous des conditions de culture défavorables.•
•
Le traitement des lésions associées au SK SIDA représente un problème puisque la radiothérapie ou l'ablation tumorale sont difficilement applicables en raison de la nature multifocale des néoplasmes. Etant donné que le tétrapalmitate de maltose (MTP) et une combinaison de MTP
+
cortisone inhibe l'angiogénèse, nous avons supposé que ces régulateurs auraient un effet inhibiteur sur les AS primaires d'origine endothéliale. Le premier objectif de cette seconde partie fut donc de traiter des souris C57Bl/6 porteuses de tumeurs AS primaires au MTP ou MTP-cortisone. Malheureusement, les résultats n'étaient pas prometteurs à cause de l'insensibilité immunologique des souris C57Bl/6 au MTP. Cependant, l'absence de réponse antitumorale observée in vivo n'était pas reproduite in vitro car le MTP bloquait la croissance des cellules AS en culture. Il est possible que deux mécanismes indépendants coordonnent l'activité anti-angiogénique du MTP: un effet direct sur les cellules prolifératives in vitro et un effet indirect utilisant des facteurs humoraux qui inactivent le MTP in vivo (exprimé(s) chez les animaux insensibles au MTP).Comme l'efficacité de la thérapie anti-angiogénique sur des tumeurs métastasiques n'a pas encore été étudiée, le second objectif était d'évaluer les effets de la combinaison MTP + cortisone comparativement au MTP, à la cortisone et au PBS seules sur des tumeurs métastasiques chez les souris C3H/HeN H-2k MTP
sensibles. Les études furent réalisées au moyen de la lignée mammaire tumorale C3HBA H-2k . Nous avons trouvé que le MTP seul réduit la dimensions des lésions,
la combinaison MTP
+
cortisone exerçant un effet similaire. Ceci a démontré que la thérapie anti-angiogénique réduit possiblement le nombre de cellules•
•
prolifération des cellules endothéliales; en conséquence, l'apport de nutriments et l'élimination des déchets métaboliques sont insuffisants. Ce résultat indique que le traitement anti-angiogénique a des effets directs sur les tumeurs multifocales que la radiothérapie et/ ou la chirurgie ne peuvent éliminer.
•
•
1 - INTRODUCTION 1. Kaposi's sarcoma
In the early 1980's, Kaposi's sarcoma (KS) became one of the most discussed neoplasms. The rise in interest was due to an increased incidence of KS among the male homosexual population in certain American urban centers especially New York and the high frequency of KS and Pneumocystis carinii pneumonia in the San Francisco homosexual/bisexual community. The two diseases were accompanied by a severe decline in the patient's immunity and death occured within a short time after diagnosis. This disease was named acquired immune deficiency syndrome (AIDS) (FRIEDMAN-KIEN, 1981; MMWR, 1981; HA VERKOS, 1985; SAFAI, 1985; KRIGEL and FRIEDMAN-KIEN, 1988). Although AIDS research bas advanced rapidly over the past decade, the agent which causes AIDS associated KS remains unknown.
KS is a malignant tumor composed primarily of transformed endothelial cells (EC) of vascular or lymphatic origin. There is a spectrum of histological changes which occur during the development of AIDS KS. Sorne of them are caused by the human immunodeficiency virus (HIV) and others by the unknown KS agent (ENZINGER and WIESS, 1983; BARNES, 1988).
Historically, KS was first described by Moritz Kaposi in 1872 and was called multiple benign pigmented idiopathie hemorrhagic sarcoma due to the nature of the neoplasm. Since then, its name has changed to KS reflecting the pathologist who first described it and the tumor which encompassed a large variety of vascular
•
•
neoplasms. KS is now divided into four classes: 1) classical, 2) African, 3) immunosuppression-related (iatrogenic ), and 4) AIDS associated (TAYLOR ET AL.,
1971; KRIGEL and FRIEDMAN-KIEN, 1988; VOLBERDING, 1989; ARNOLD ET AL., 1990).
1.1. Classical Kaposi's sarcoma
Classical KS is the one originally described by Moritz Kaposi. It affects mostly elderly men of Mediterranean or Jewish ancestry and is characterized as a slowly progressive disease primarily affecting the extremities. The early lesions are reddish or bluish colored macules and patches on the skin which later form nodules. Treatment of classical KS usually involves excision of the tumor or local irradiation. The survival is long-term with most deaths occuring due to causes other than the neoplasm (KRIGEL and FRIEDMAN-KIEN, 1988; ARNOLD ET AL., 1990).
1.2. African Kaposi's sarcoma
African KS affects the young and elderly of both sexes in equatorial Africa. African KS is often subdivided into two subclasses: 1) KS in adults and 2) KS in the young. KS in adults is similar to classical KS since it has an indolent course and it mainly affects the extremities. In this sub-class long-term survival is noted. There is also the aggressive form, which has a poor prognosis and affects mostly the young population. Unlike indolent adult KS, KS in young adults is not confined to the extremities and mainly affects the lymph nodes. It resembles AIDS associated KS to a certain degree in its aggressivity. Chemotherapy of the aggressive form of KS prolongs life in the short term, but patients finally succumb to progressive disease .
•
•
Death usually occurs one to two years after the onset of the neoplasm. The indolent form of African KS is treated in much the same manner as classical KS whereas the aggressive form requires extensive treatments applicable to fast growing tumors (TAYLOR ET AL., 1971; KRIGEL and FRIEDMAN-KIEN, 1988; ARNOLD ET AL., 1990).
1.3. Iatrogenic Kaposi's sarcoma
The third type of KS, immunosuppression-related KS, affects both sexes at any age. It occurs commonly in renal transplant recipients, but is also present in persans undergoing immunosuppressive therapy. Morphologically, the lesions resemble those of classical KS, but have no regional preference for the initial presentation. Sometimes, removal of the immunosuppressive agent results in the disappearance of KS without the assistance of therapy; however, when left uncontrolled, it can cause death in some cases (KRIGEL and FRIEDMAN-KIEN, 1988; VOLBERDING, 1989; ARNOLD ET AL., 1990).
1.4. AIDS associated Kaposi's sarcoma
AIDS associated KS (AIDS KS) heralded the dawn of an entirely new form of KS that had the nature of an epidemic, previously unheard of for a human cancer. AIDS KS affected mainly the homosexual population, but as AIDS progressed to the heterosexual population, so had AIDS KS. A 1985 Center for Disease Control (CDC) study indicated that among the AIDS population, the majority of KS patients were in the male homosexual group (36% ). This group was followed by Haitians (10.4%), intravenous drug abusers (4.3%), transfusion recipients (1.9%), and
•
•
hemophiliacs (1.6%) (KRIGEL and FRIEDMAN-KIEN, 1988; HARA Wl and O'HARA, 1989; VOLBERDING, 1989). These percentages indicated that although AIDS KS affected all risk groups, the one which suffered most was the male homosexual/bisexual community. While AIDS KS was on the uprise among the non-gay risk groups, it was on a decline in the male homosexual/bisexual population. In 1981, 63% of San Francisco homosexual men with AIDS had KS; whereas in 1985, it reduced to 24%. The decline was attributed to a change in sexual practices among homosexual/bisexual men (KRIGEL and FRIEDMAN-KIEN, 1988; HA W ARI and O'HARA, 1989).
There are several theories about the agent which causes AIDS KS. One of the theories is based on a viral etiology of KS. It proposes cytomegalovirus, the Epstein Barr virus or herpes simplex III as the cause since these infections are common amongst the male homosexual population and are present, although less frequently, in the other AIDS risk groups (KRIGEL and FRIEDMAN-KIEN, 1988; HARA WI and O'HARA, 1989). The other theory is based on KS induction by organic nitrite inhalants used as recreational drugs by the male homosexual/bisexual population. The interest in nitrite inhalants (poppers) as the agent which causes KS bas been generated by the observation that oral and nasal lesions of AIDS KS are present in
a majority of homosexual KS cases. Nasal inhalation and oral intake are the routes of nitrite entry into the body (SAFAI, 1985; HARAWI and O'HARA, 1989).
AIDS KS is a disseminated, rapidly progressive neoplastic disorder with a high mortality rate. Its sites of presentation are the head, neck, trunk and mucous membranes. In this respect, it differs form classical KS and indolent African KS. It is similar to African KS of the young since it does infiltrate or occur directly
•
•
within the lymph nodes. If there is interna! involvement in AIDS KS, it occurs in the lungs, liver, spleen and gastro-intestinal tract. Interna! involvement of KS has a poor prognosis (SAFAI, 1985; BARNES, 1988; HARAWI and O'HARA, 1989; ARNOLD ET AL., 1990).
1.4.1. Treatment
The treatment of AIDS KS is rather difficult since the lesions are not localized; often, the lesions cover a large area. Another point which renders treatment of AIDS KS difficult is the pre-existing immune deficiency which can be worsened by chemotherapy or radiotherapy. The presentation of the lesions, when distributed over a large part of the body, renders radiotherapy difficult. Radiotherapy has been used with some success on early skin and oral lesions when their numbers are limited. Due to the immune deficiency, chemotherapeutic agents such as vincristine, vinblastine and etoposide are used for short periods. In addition to conventional therapies for cancer, interferon a has shown some promise in early KS. However, P.A.
VOLBERDING (1989) and KRIGEL and FRIEDMAN-KIEN (1988) are in agreement that there isn't any effective treatment for AIDS KS since current therapies do not prevent the development of new lesions, increase survival or ameliorate the depressed immune state (KRIGEL and FRIEDMAN-KIEN, 1988; VOLBERDING, 1989).
1.4.2. Models for the study of AJDS associated Kaposi's sarcoma
There are very few models which have been proposed for the study of AIDS KS. One of the models proposed is derived from transgenic mice .
•
•
VOGEL ET AL. (1988) have demonstrated that insertion of a 2-kilobase fragment of DNA containing the HIV LTR and Tat 3 genes into fertilized eggs from superovulated CDl mice produced male off-spring which later developed skin lesions resembling those of AIDS KS. The rational for using LTR-Tat 3 was that the Tat gene can up-regulate viral gene expression in cultured cells by transcriptional and post-transcriptional mechanisms. VOGEL ET AL. believed that the Tat gene product may regulate cellular gene expression which would result in the elaboration of specific KS-like disease. Thus, one would relate KS evolution to HIV infection. Although this model did look very promising, its usage is limited by the fact that only 15% of the male off-spring developed skin tumors and that micro-injection of DNA requires specialized technical staff (VOGEL ET AL., 1988).
Another model is based on the subcutaneous (s.c.) injection of cultured human AIDS KS cells in nude mice. This approach by Salahuddin and co-workers resulted in tumors which regressed 6 days after injection of the tumor cells (SALAHUDDIN ET AL., 1988).
The last model of great interest is currently under development in the laboratory of Dr. V.N. Nigam. It is based on the works of M. Dubé and O. Benrezzak. In 1987, BENREZZAK ET AL. found that 1,2-dimethylhydrazine (DMH) treated CDl male mice given difluromethylornithine had a high incidence (25%) of AS (BENREZZAK ET AL., 1987). Later, M. Dubé found that tbere was a greater incidence of angiosarcoma induction in male CDl mice and that tumor induction was accompanied by a decline in the immune response of treated mice. Dubé also found that there was a much
•
•
higher incidence (80%) of AS in DMH treated male C57Bl/6 mice (DUBÉ, 1990). The rational for using C57Bl/6 mice was not only due to the high incidence of AS, but also due to the macroscopical and histological resemblance between AS and AIDS KS lesions. Another point in favour of this model was that only C57Bl/6 mice could be infected with a murine AIDS-like virus (MAIDS); thus rendering the infected mice as immunosuppressed as AIDS patients (MOSIER ET AL., 1987).
2. Angiosarcomas
Angiosarcomas (AS) are rare, soft tissue neoplasms of vascular origin, usually derived from cappillary EC. Upon macroscopic and microscopie observation, AS can often be mistaken for KS. AS mostly affect elderly males and have a predilection for the skin and soft tissue of the head and neck. Its sites of presentation are in common with AIDS KS since it can occur in any part of the body. Unlike AIDS KS (where metastasis is rare), metastasis of AS occurs to the lungs and liver. AS may also occur in the pectoral tissues of women who have undergone mastectomy for breast cancer. Multiple AS lesions are present in approximately 50% of the cases and if the growth is persistent, it can cover large areas of skin (ENZINGER and WEISS, 1983; HOLDEN, 1989; CONRAD and ENNEKING, 1990).
2.1. Comparison between angiosarcomas and Kaposi's sarcoma
Histologically, both tumors (AS and KS) show spindle-shaped cells and abnormal proliferation of EC resulting in irregular, thin walled · vessels which anastomose. In early or well differentiated AS lesions the EC intertwine and wrap
•
•
around collagen bundles which results in "empty, angulated, vascular spaces." In the early patch stage lesions of AIDS KS, the EC behave in the same manner as the EC of AS and form similar "empty, angulated, vascular spaces." The AIDS KS lesions also have dispersed inflammatory cells. The late AIDS KS lesions are hemorrhagic nodules composed of a solid component of spindle shaped cells and blood filled spaces. AS are also hemorrhagic and have a sponge-like quality due to blood filled spaces which dissect the solid component (ENZINGER and WEISS, 1983; ALLES and BOSSLET, 1988; BARNES, 1988; HOLDEN, 1989; CONRAD and ENNEKING, 1990). The similarity between both tumors does not end with their macroscop1c or gross histological appearance as they have similar markers (see table I).
The most specific markers which are used in the histological characterization of tumors believed to be of EC origin are factor VIII related antigen (FVIIIR Ag) and Ulex europaeus agglutinin I (UEA I). FVIIIR Ag is a protein synthesized primarily by endothelium. It has been seen in association with the pericellular matrix fibers of both EC and fibroblasts. FVIIIR Ag is considered a marker of choice for AS and KS since good labeling results have been obtained for EC in culture and in normal and benign neoplastic EC lesions. However, AS and KS lesions may be positive or negative for FVIIIR Ag. In KS lesions, the cells lining the vascular spaces are generally FVIIIR Ag positive whereas the spindle cells are negative. In AS lesions, the solid undifferentiated areas are negative while the other areas stain variably for FVIIIR Ag (BURGDORF ET AL., 1981; HORMIA, 1982;
ENZINGER and WEISS, 1983; MIETTINEN ET AL., 1983; RUTGERS ET AL.,
•
•
TABLE 1
MACROSCOPIC AND MICROSCOPIC COMPARISON OF SOME OF THE CHARACTERISTICS OF ANGIOSARCOMAS AND KAPOSl'S SARCOMA
ANGIOSARCOMA KAPOSl'S SARCOMA Macroscopic · purple nodules · reddish-purple plaques or
· affects skin and soft tissues of nodules
head and neck · mostly present on skin of head, neck and trunk · also affects m ucous · multicentric in 50% of the membranes
cases · mostly multicentric
Microscopie · composed of spindle-shaped · composed of spindle-shaped and epithelial-like cells and epithelial-like cells · abnormal proliferation of · abnormal proliferation of
endothelial cells endothelial cells
· irregular, thin-walled vessels · irregular, thin-walled vessels which anastomose which anastomose
· UEA 1 positive · UEA 1 positive
· patches of F VIII R Ag · patches of F VIII R Ag positivity within the tumor positivity within the tumor
•
•
UEA 1 is a lectin derived from Ulex europaeus. It binds non-covalently to a-L-fucose residues of cell surface glycoproteins and glycolipids of EC, epithelial cells and erythrocytes of blood group O; cells of mesenchymal origin. It is a marker of choice for tumors of EC origin since it is specific and more sensitive than FVIlIR Ag in benign vascular lesions. Furthermore, in neoplastic EC sarcomas which generally stain negative for FVIIIR Ag, UEA 1 stains positive. In the solid areas of KS lesions where FVIIIR Ag usually stains negative, UEA 1 stains positive. UEA 1 also stains positive in the solid, undifferentiated areas of AS where FVIlIR Ag is negative (LEATHEM and ATKINS, 1983; MIETTINEN ET AL., 1983; ORDONEZ and BATSAKIS, 1984; ALLES and BOSSLET, 1988). According to MIETTINEN ET AL. (1983), in both KS and AS lesions which were negative for FVIIIR Ag, they were found to be positive for UEA 1. These results indicated that UEA 1 is a reliable marker for both AS and KS lesions.
2.2. Mouse angiosarcomas
Mouse AS resemble human AS in all macroscopic and histological aspects; thus, the mouse AS model seems to be a good tumor model for the study of human AS or KS. The only difference between mouse AS and human AS is that the agent which causes human AS is unknown. As mentioned previously, in the laboratory of Dr. V.N. Nigam, it was found that DMH induced AS in both CDl and C57Bl/6 mice (MADARNAS ET AL., 1990; NIGAM ET AL., 1990).
3. Dimethylhydrazine
In 1963, LAQUER and co-workers established the base for DMH research. They observed neoplasms in rats fed untreated flour from nuts of~ circinalis .
•
•
The neoplasms comprised vascular tumors in the liver . and benign epithelial and mesenchymal lesions of the kidney (SUNTER and SENIOR, 1983). The nuts were shown to contain a derivative of hydrazine.
In 1967, H. DRUCKREY and co-workers were the first to study DMH and azoxymethane which are bath related to cycasin, the carcinogen implied in the work of LAQUEUR ET AL. (1963). Druckrey and colleagues postulated that DMH underwent several steps of chemical transformation in vivo before being conveÎted into the ultimate carcinogen, the methyldiazonium ion (FIALA, 1977; GRUNBERGER and GOFF, 1997).
Since Druckrey's work, there have been three very important discoveries about the carcinogenicity of DMH. Firstly, B. TOTH ET AL. (1976) reported incidences of vascular tumors (besides colorectal tumors) in male Swiss mice which had been treated with 10 weekly (10 µg/g of body weight) injections of DMH. TOTH ET AL. did not pay attention to the vascular tumors. Later, however, R.C. MOON and C.M. FRICKS (1977) and E. ODAGIRI ET AL. (1985) found that susceptibility to DMH induced colon cancer in BD-IX rats was higher in males. They suggested that androgens may play a role in DMH carcinogenicity (CAMPBELL ET AL., 1975; TOTH ET AL., 1976; R.C. MOON and C.M. FRICKS (1977); ODAGIRI ET AL., 1985).
The second major finding was based on two observations by Toth, one of which was that the routes of DMH administration (either oral [p.o.] or s.c.) may be important since the chemical may be metabolized by different methods (TOTH and WILSON, 1971). The other observation was based on the work of TOTH ET AL. (1976). They found that the dose of DMH may play an important role in
•
•
carcinogenesis. For example, frequent low dose administration of DMH (20 µg/g
of body weight) s.c. resulted in a higher incidence of vascular tumors in the livers of male Swiss mice (TOTH ET AL., 1976). In 1977, E.S. FIALA had made similar statements; thus, supporting Toth's work (FIAIA, 1977).
The third major fin.ding is based on the work of M.A BEDELL ET AL.
(1988) who found that there was a strong correlation between the inability of the rat to repair DNA damage caused by the conversion of DNA guanine to
Cf'-methylguanine and tumor induction. They exposed male F-344 rats to DMH p.o. and isolated the liver cells at various time periods. In the liver, hepatocytes (parenchymal cells) make up approximately 90% of the liver mass. The remaining mass is made up of nonparenchymal cells, the majority being EC and Kupfer cells. BEDDEL ET AL. noted that between 1 to 3 days of exposure to DMH, the hepatocytes had a rapid decline in Cf'-methylguanine concentration. The nonparenchymal cells accumulated Cf'-methylguanine during the first eight days of exposure. Furthermore, the nonparenchymal cells had a 30 fold greater concentration of 06 methylguanine than the corresponding hepatocytes. The tumors which developed in the liver were AS derived from non-parenchymal cells (BEDELL ET AL., 1988).
The latter three findings by Toth, Bedell and other workers demonstrated that the location and development of vascular tumors is not only dependent upon the sex and species of the animal, but also on the dose, route of administration and, most importantly, the ability of EC to repair defects in the DNA produced by the activated DMH metabolite .
•
•
Since AS induction by DMH takes approximately 29 to 39 weeks using low, s.c. doses of the chemical (20 mg/kg body weight) in male C57Bl/6 mice and most of the animals have damaged livers as well as cola-rectal tumors, it may be beneficial to develop an AS cell line which could produce the tumor upon transplantation and allow the study (in vitro and in vivo) of potential anti-KS drugs.
4. Neoplastic Endothelial cell lines
AS cell lines have rarely been reported in the literature and are not available from the repositories. It is possible that this is a result of the low frequency of spontaneous AS tumors. Also, the availability or simplicity of deriving EC cultures from human umbilical vein, bovine aorta, and bovine pulmonary vein and artery may have reduced interest in EC cell lines from other EC sources. Despite the availability of normal EC cell lines, no attempts on their neoplastic transformation in vitro have been described.
The first published attempt to grow normal EC in vitro was made by W.C. WOODWARD and C.M. POMEERAT in 1953. Interestingly, both groups attempted to grow capillary EC (CEC) from tissue explants. Neither of the groups was given recognition for the first EC culture since there wasn't any proof of the endothelial origin of the cultures which were quickly taken over by fibroblasts. In 1973, E.A. JAFFE and co-workers were the first to culture identifiable EC. Their EC were derived from the human umbilical vein. In 1974 aortic EC were successfully cultured by M.A. GIMBRONE ET AL.
P.J. DEL VECCHIO ET AL. (1977) were the first group to maintain a short-term culture of known CEC. These CEC were isolated from rat adrenal cortex .
•
•
The CEC did proliferate a little, but they could not be subcultured (DEL VECCHIO ET AL., 1977).
4.1. Methods of endothelial cell culture
There are two methods for the isolation of EC. The EC of large vessels are obtained by collagenase digestion of the interior of the vessel. Collagenase digests the basement membrane (BM) and yields aggregates of endothelium. The second method which is commonly used for the isolation of CEC consists mostly of mechanical separation of the capillaries into small fragments. The fragments may be treated with enzymes to obtain a single cell suspension which is grown as a monolayer (FRESHNEY and FRAME, 1983). The best results are obtained when the capillary fragments themselves are plated and the EC permitted to grow out of the explants (MAKARSKI, 1982; KIRKPATRICK ET AL., 1990; SPANEL-BOROWSKI and V AN DER BOSCH, 1990).
In order to obtain cultures, the media used for the culture of EC were just as important as the method of EC isolation. Several types of media have been used in the literature, but the most common types are Dulbecco's Modified Eagle's medium (DMEM), M199 and HAM's. The DMEM, M199 and HAM's may be used singly or in combination (MAKARSKI, 1982; FRESHNEY and FRAME, 1983; SATO ET AL., 1986; JACKSON ET AL., 1990). The serum which is commonly used is fetal bovine serum (FBS); however, some groups bave used new barn calf serum or human serum (SATO ET AL., 1986; FRIEDMAN-KIEN, 1988; RUBIN and FARBER, 1988; JACKSON ET AL., 1990). Although the type of media and serum used were important, the use of growth factors such as fibroblast growth
•
•
factor (FGF), epidermal growth factor (EGF), and tumor angiogenesis factor (TAF) were paramount in permitting serial propagation beyond a few generations (FOLKMAN ET AL., 1979; MMWR, 1981). Sorne researchers have found it necessary to use substrates such as collagen, fibronectin, laminin or gelatin; many have been able to maintain successful cultures without their use (FOLKMAN ET AL., 1979; FRESHNEY and FRAME, 1983; MADRI ET AL., 1983; SATO ET AL., ·1986; FURUYA ET AL., 1990).
One major problem associated with the culture of EC is contamination by other cells. The methods and media used reduce this problem; however, other methods of purification are used once the cells have been placed in culture (POLLAK, 1969; FOLKMAN and HAUSENCHILD, 1982; FRESHNEY and FRAME, 1983). Sorne methods involve physical destruction of the contaminating cell or dilution of contaminating cells by repeated sub-culture (FRESHENY and FRAME, 1983; SATO ET AL., 1986). These methods rely on differentiation between the EC and the contaminating cells.
4.2. Identification
The markers which are considered most sensitive for EC identification are FVIIIR Ag and UEA I. Other markers such as oil red 0 and angiotensin converting enzyme are also used. EC generally stain positive with the markers mentioned above. Although markers are available for the identification of EC, the most frequently used method of identification is microscopie observation. Cultured EC are generally fiat, polygonal cells which form a monolayer (JAFFE, 1977; MAKARSKI, 1982). Upon confluence, the polygonal shape gives the illusion of a
•
•
cobblestone surface. It is generally considered that observation of the cobblestone pattern is an indication of an EC culture (SPANEL-BOROWKSI and VAN DER BOSCH, 1990). EC also form tubular structures in vitro (FOLKMAN ET AL., 1979).
4.3. Angiosarcoma cell lines
There seems to be only one report about a culture of DMH induced AS. It is unknown whether the cell lines (DlO and D14) are still in existence or whether there have been any previous AS cell lines produced from DMH induced tumors. The DlO and D14 cell lines were established by N. SATO ET AL. in 1986. AS were induced in specific pathogen free, male Balb/c mice by s.c. injections of DMH for 30 weeks. Twenty seven primary tumors were used in order to establish cell lines; however only two of the cell lines, DlO and D14, could be maintained in culture. DlO and D14 have doubling times of 24-36 hours. The cells of both lines are spindle shaped without any characteristics of epithelial cells. Except for morphological observations and attempts to reproduce tumors in syngeneic mice, no other methods of characterization were used. According to SATO ET AL., the DlO and D14 lines did produce tumors in syngeneic mice when lxlà cells were injected s.c. The tumors appeared after two weeks but regressed during the following 3-4 weeks (SATO ET AL., 1986).
4.4. AIDS associated Kaposi's sarcoma cell lines
There have been several publications about the successful culture of AIDS KS cell lines as compared to AS cell lines. The cultures tend to have a short
life-•
•
time in vitro and bave a doubling time of approximately 24 bours, wbicb is similar to normal EC. KS cells in culture also express EC markers. They stain negative for FVIIIR Ag and positive for UEA 1. A point of interest, as previously mentioned, is that they do not produce tumors in nude mice; bowever they do induce a biological response (BOVI ET AL., 1986, SALAHUDDIN ET AL., 1988). SALAHUDDIN ET AL. described the type of response elicited after injection of tbeir AIDS KS cell line in nude mice as an angiogenic response. An angiogenic response is a proliferation of blood vessels (SALAHUDDIN ET AL., 1988). Since the growtb of solid tumors including AS depend upon angiogenesis and anti-angiogenic tberapy may be useful for the treatment of KS and AS, a detailed review of angiogenesis and anti-angiogenesis is in order.
5. Angiogenesis
Angiogenesis is the term which was coined in 1935 to describe the formation of new blood vessels (bv) in the placenta (FOLKMAN and KLAGSBRUN, 1987). Just as normal angiogenesis is provoked by growth factors, so is tumor angiogenesis. Tumor angiogenesis is stimulated by factors secreted by the tumor cells themselves. E. Goldman hypotbesized tbat tbere was an angiogenic agent involved in neoplastic neovascularization; however, there wasn't any proof until 1968 (GREENBLATT and SHUBIK, 1968; SIMPSON ET AL., 1983).
5.1. Angiogenic factors
In 1968, M. Greenblatt and P. Shubik were the first to demonstrate that tumors secrete angiogenic factors (GREENBLATT and SHUBIK, 1968; MACIAG,
•
•
1984; FOLKMAN and KLAGSBRUN, 1987). They demonstrated that tumors implanted in Millipore filters could induce the formation of new bv. This finding was supported by FOLKMAN ET AL. (1971) who demonstrated that a partially purified tumor extract form the Walker 256 tumor induced neovascularization in the rat dorsal air sac. In 1972, T. CA V ALLO ET AL. indicated that neovascularization was associated with rapid CEC division. Although these experiments demonstrated that there was some factor involved in tumor angiogenesis, they did not chemically characterize it until much later.
Several compounds have demonstrated angiogenic activity in vivo. In general, they are divided into two groups. One group is the polypeptide factors and the other, the non-peptide factors. Unfortunately, the factors in the nonpeptide group have not been fully characterized (KLAGSBRUN and FOLKMAN, 1990). There have been a couple of problems associated with factors of the non-peptide group. Firstly, many of the factors are impure. Secondly, it is unknown whether these nonpeptide factors are cofactors or nutrients which "enhance" angiogenesis rather than "induce" angiogenesis. Part of the confusion arises from the classification of prostaglandins and nicotinamide, which are metabolites, within this group (KLAGSBRUN and FOLKMAN, 1990).
The peptide angiogenic factors include principally 3-4 growth factors which are known to stimulate normal EC proliferation. This group is comprised of fibroblast growth factor (FGF), transforming growth factor (TGF), tumor necrosis factor (TNF), and platelet-derived growth factor (PDGF) (KLAGSBRUN and FOLKMAN, 1990) .
•
•
5.1.1. Fibroblast growth factor
There are two types of FGF, acidic and basic. Basic FGF (bFGF) was discovered first; it was isolated from bovine pituitary. Basic FGF is also found in skeletal tissue, reproductive tissue, white blood cells and tumors. It has been found in cell cultures of EC and of hepatoma cells. Basic FGF synthesized by EC has also been found in the subendothelial extracellular matrix. It is both mitogenic and chemotactic for EC. Basic FGF has 53% homology with acidic FGF (aFGF) (KLAGSBRUN and FOLKMAN, 1990). Acidic FGF was found in the brain, eye and bone. Like bFGF, aFGF is chemotactic and mitogenic for EC. Both FGF's are implicated in angiogenesis, but their method of inducing angiogenesis is unknown. It has been hypothesized that it is necessary to "release" and not "secrete" FGF in order to induce neovascularization. The release occurs either through cell lysis or injury which would precipitate a disturbance in the extracellular matrix (VLODA VSKY ET AL., 1987; KLAGSBRUN and FOLKMAN, 1990).
5.1.2. Transforming growth factor
TGF is subdivided into two factors, a and B (KLAGSBRUN and FOLKMAN, 1990). TGF-a is secreted by many tumor cells and macrophages (ALLISON and KOWALSKI, 1989). It was first discovered as a mitogen secreted by transformed fibroblasts. Its most important property is its ability to reversibly transform fibroblasts in culture. TGF-a also stimulates the proliferation of epithelial cells and EC. TGF-a bas been demonstrated to induce angiogenesis in vivo (FOLKMAN and KLAGSBRUN, 1987). It bas been suggested that TGF-a stimulates the autocrine growth of tumor cells
•
•
(KLAGSBRUN and FOLKMAN, 1990) .
TGF-B is unrelated to TGF-a. TGF-B was purified from placenta, platelets, kidney, cartilage, bone, tumor cells and other types of tissue. It is secreted in an inactive form which is activated by heat, acidification and proteases. TGF-B both inhibits and stimulates cell growth (GOUSTIN ET AL., 1986). It induces angiogenesis in the chorioallantoic membrane assay. In vitro, it is an inhibitor of CEC and aortic EC growth regardless of the presence or absence of substrate. It bas been suggested that TGF-B may not induce neovascularization, but promote it (KLAGSBRUN and FOLKMAN,
1990).
5.1.3. Tumor necrosis factor
TNF like FGF and TGF is divided into two factors, TNP.a and
TNF-B. TNF-a is mostly derived from macrophages and lymphocytes. TNF-B is a product of activated lymphocytes. TNF can also be released from other cells. It bas pleiotropic functions. One of its interesting properties is that of tumor necrosis. TNF's ability to kill tumor cells was observed before 1893; however it was not a published observation until W.B. Coley wrote about it almost 100 years ago. Coley noticed that patients with streptococcal infection could have partial remission. It was later demonstrated by M.J. Shear in 1944 that bacterial lipopolysaccharide (LPS) was responsible for inducing the production of a serum factor. This factor induced tumor necrosis without causing shock (SEMENZA TO, 1990). The second interesting function of TNF is its ability to induce angiogenesis. It is believed that following injury
•
•
or wounding, macrophages migrate into the affected area, become activated and release more TNF and other macrophage derived factors; thus stimulating neovascularization. In vivo, TNF-a bas demonstrated its angiogenic ability. In vitro, it stimulates EC chemotaxis and formation of capillary tubes from CEC on type 1 collagen. Surprisingly, TNF inhibits EC proliferation in vivo. The latter suggests that TNF may be involved in EC differentiation and/ or indirect stimulation of EC proliferation (KLAGSBRUN and FOLKMAN, 1990).
5.1.4. Platelet derived growth factor
There are many types of PDGF, but the one of interest is platelet-derived EC growth factor (PD-ECGF). It is the only EC growth factor produced by platelets and may be the only "specific" EC mitogen. It is also angiogenic, but its mechanism of angiogenesis is unknown (KLAGSBRUN and FOLKMAN, 1990).
5.2. Tumor angiogenesis
All of the angiogenic factors described above, except TGF B, have the property of stimulating and inducing the proliferation of EC by some mechanism. TGF B alone is inhibitory to EC proliferation but acts in synchrony with TGF a: in
renal development. The factors released by the tumor cells themselves or by factors from tumor infiltrating macrophages, stimulate the CEC to degrade the BM (PLIVERINI and LEIBOVICH, 1984; FOLKMAN, 1985). Degradation of the BM causes CEC migration toward the angiogenic stimulus and later, their proliferation .
•
•
Proliferation is followed by lumen formation. Subsequently, there is loop formation as the capillary sprouts anastomose. Blood flow begins after loop formation. The process is repeated as the tumor mass and requirement for nutrients increase (FOLKMAN, 1985).
Most solid, malignant tumors are capable of inducing angiogenesis, but the ability to induce angiogenesis does not signify a malignant or solid tumor. For example, adrenal adenomas are benign tumors which may induce neovascularization (FOLKMAN and KLAGSBRUN, 1987). Also, some ascites tumors are capable of inducing angiogenesis in the chorioallantoic membrane of the chick (CAM). In general, however, ascites tumors don't require a vascular supply in vivo; therefore, they do not induce angiogenesis (CARIOU and TOBLEM, 1989).
5.2.1. Necessity of tumor angiogenesis
Angiogenesis is necessary for tumor growth and metastasis. In 1976, J. Folkman found that tumor fragments implanted in isolated, perfused organs do not grow larger than one to two rnm3
• When the same tumor fragments
are transplanted in mice, vascularization occurs and the size becomes one to two cm3 (FOLKMAN, 1976). When tumor vascularization does not take
place, the lack of oxygen and nutrients as well as the inability to eliminate metabolic waste limits tumor survival (JAIN, 1988).
5.2.2. Tumor growth vs. angiogenesis
Tumor neovascularization and the arrangement of vessels in and around the tumor is dependent upon the type of tumor, its location in the
•
•
hast and its growth rate. If the tumor is one to two mm away from the host's vasculature, the tumor can grow towards the vessel and incorporate it. If the tumor is distant from the host's vasculature, it must elicit angiogenesis to further its growth. In this particular case, microvessels sprout from the host's vasculature and prolif erate towards the tumor. As the new vessels approach the tumor, the tumor itself can proliferate towards the vessels and incorporate them into the tumor mass (THOMPSON ET AL., 1986). These growth patterns indicate that there are at least two types of vessel origin in tumor masses, those which pre-exist and serve a purpose for the hast and those which are induced by angiogenic factors derived from the tumor cells (V AUPEL ET AL., 1989). It is important to note that not all tumor cells within a tumor mass are capable of inducing angiogenesis (ZICHE ET AL.,
1985).
The EC compnsmg tumor vasculature corne from normal bv. However, the bv structures developed by tumor associated EC have some abnormal characteristics. For example, tumor bv are not always lined by a continuous endothelium. Sometimes the tumor cells are found between the EC. Sorne tumors such as melanomas and sarcomas contain blood channels. These channels are lined solely by the tumor cells so that the blood is forced to move between and around the tumor cells. The channels are supplied and drained by either sinusoids or other types of bv (JAIN, 1988). Another difference between normal bv and tumor bv is the presence of capillary sprouts which are present only in tumors. Structurally, tumor bv are more fragile (due to dilation of the bv) and are irregular in length and width (JAIN,
•
•
1988; V AUPEL ET AL., 1989) .
Regardless of the type of bv associated with a solid tumor, an increase in the number of tumor cells corresponds to an increase in the number of bv within and around the tumor mass. W.D. THOMPSON ET AL. (1987) studied the development of the initial circulatory system of tumors. They transplanted fragments of mammary adenocarcinoma in C3H-Hmg mice. Histological studies of these tumors which were necropsied from day 0 to 12 indicated that the proportion of bv to tissue increased until the density of bv was 1.5% of the tumor volume. The density of bv in the surrounding normal tissue was 0.36% of the tissue volume. These results indicated that there was a 400% increase in bv density in tumor tissue as compared to normal tissue. Thompson's work demonstrated that although the number of bv and the area of the tumor increased, the proportion of bv to tumor tissue did not change after they had reached their ideal proportion. THOMPSON ET AL. showed that the number of normal bv in the host which were associated with the tumor did not increase. This implied that neovascularization occured within and around the tumor (THOMPSON ET AL., 1987).
ln 1974, D.E. HILMAS and E.L. GILLETTE studied the microcirculation of tumors. Their results supported the work of THOMPSON ET AL. as well as other investigators. HILMAS AND GILLETTE used a mammary adenocarcinoma transplanted in the right rear leg of C3H/Bl mice. They found that as tumor volume increased, the vascular volume remained constant; however, the vessel length, within the tumor, decreased. It was also noted that the volume of necrotic tissue increased. HILMAS AND
•
•
GILLETTE demonstrated that as the tumor grew the vessel surface area decreased and the vessel diameter increased. Ali morphological changes in the bv contributed to reduced blood flow which in turn increased tumor necrosis (JAIN, 1988).
5.2.3. Metastasis
Tumor vasculature is not only important for tumor growth and survival, but for metastasis as well. Metastasis is thought to involve three steps. First, the tumor cells are thought to bind to a matrix component in the BM. After binding, the tumor cells secrete enzymes which digest the BM. Third, tumor cells migrate through the space created and into the bv. The tumor cells remain in the blood stream until they either reach their organ of preference or are lodged in the microvasculature. Many tumor cells are killed in the blood stream. Once the tumor cells have reached their metastatic site, they either attach to nude BM or the EC themselves. Entry into the new site probably occurs by degradation of the BM and migration into the interstitial space of the tissue (RUBIN and FARBER, 1988).
6. Anti-angiogenesis
Since solid tumor growth and metastasis depend upon the bv, it seems that destruction of existing tumor vasculature or inhibition of tumor angiogenesis would be ideal for tumor treatment and management. The idea of anti-angiogenesis or the inhibition of vascular growth was first proposed by FOLKMAN in 1972. However, the evidence that anti-angiogenic substances do exist was not put forward
•
•
until 1973 when R. EISENSTEIN ET AL. discovered that cartilage resisted vascular invasion. The resistance was lost after cartilage was extracted with guanidine. In 1975, H. BREM AND FOLKMAN demonstrated that the placement of a cartilage fragment adjacent to a tumor in the rabbit comea inhibited the growth of new bv toward the tumor. lt was found by N. SORGENTE ET AL. (1975) that protease inbibitor was responsible for the anti-angiogenic effect of cartilage.
6.1. Heparin and cortisone
Protamine sulfate was the first chemically defined, effective angiogenesis inbibitor when given systemically. This discovery was made by S. TAYLOR and FOLKMAN in 1982. TAYLOR AND FOLKMAN had also found that heparin increased angiogenesis in the CAM while they were working on protamine (TAYLOR and FOLKMAN, 1982). In 1983, FOLKMAN ET AL. studied angiogenesis in the CAM by using heparin. They thought that the addition of cortisone would reduce background inflammation which was created by eggshell dust falling on the CAM. They found that heparin alone increased vasculature while cortisone alone had very little or no effect. However, a combination of cortisone and heparin inhibited angiogenesis. FOLKMAN ET AL. also demonstrated that heparin and cortisone had an inhibitory effect on tumor angiogenesis. They transplanted various tumors (reticulum cell sarcoma, Lewis lung carcinoma, B-16 melanoma and a bladder carcinoma) s.c. into 5-6 week old male C57Bl/6 mice. They found that hydrocortisone could be substituted for cortisone. Interestingly, FOLKMAN ET AL. also found that heparin preparations other than Panbeparin (Abbott) had a reduced effect or no effect on tumor angiogenesis when combined with cortisone (FOLKMAN ET AL., 1983) .
•
•
The major problem with Folkman's work on heparin-cortisone treatment of tumors and the CAM is that the effect of heparin-cortisone was not reproducible. M. ZICHE ET AL. (1985) tried to reproduce Folkman's results in C57Bl/6 mice which had been injected intramuscularly with different types of B16 melanomas. They also tried fibrosarcoma (M4) and Lewis lung carcinoma. Their results demonstrated that the melanomas did respond to cortisone treatment alone and in combination with heparin; however, the difference between the cortisone only group and the cortisone with heparin group was insignificant unlike Folkman's results. There was very little response if any to cortisone alone or in combination with heparin in the mice with fibrosarcoma or Lewis lung carcinoma tumors (ZICHE ET AL., 1985). Folkman had also noticed a complete regression of tumors treated with cortisone and heparin (FOLKMAN ET AL., 1983). ZICHE ET AL. reported that the tumors did not increase in size, but did not regress either (ZICHE ET AL., 1985).
6.2. Maltose tetrapalmitate
In the laboratory of Dr. V.N. Nigam, interest in anti-angiogenic therapy developed due to difficulties in explaining the antitumor activity of maltose tetrapalmitate (MTP). MTP is a synthetic glycolipid which was developed in the laboratory of Dr. Nigam. NIGAM ET AL. were interested in synthesizing a lipid A analog since, as previously mentioned, LPS was shown to have an antitumor effect. The problem with LPS is its endotoxic activity. Lipid A is considered to be the active component of LPS responsible for the antitumor activity. This idea made lipid A the best component for the modeling of synthetic analogs. MTP bas no endotoxic activity. Its antitumor activities include the ability to enhance the host's
•
•
capacity to reject many tumor cells, retardation of tumor growth and induction of hemorrhagic necrosis in certain tumors (NIGAM ET AL., 1978). MTP also had no effect on ascites tumors. An interesting difference between MTP and LPS is that MTP is a poorer immunoadjuvant but a better antitumor agent. lt was suspected that the immunoadjuvant property of MTP was not responsible for its antitumor effect. Due to the latter fact and some of the previous properties which support the basis of tumor angiogenesis, it was determined that MTP may be anti-angiogenic (MADARNAS ET AL., 1989).
In 1989, P. MADARNAS ET AL. demonstrated that a combination of cortisone and MTP could prevent tumor growth in C3H/He~ mice containing
implants of C3HBA tumor. It was also demonstrated that tumor vascularization and proliferation could occur when the cortisone-MTP treatment was discontinued. This work demonstrated that cortisone-MTP could be used as a prophylactic treatment in an in vivo experimental system (MADARNAS ET AL., 1989).
P. MADARNAS ET AL. (1989) were able to reproduce the results of FOLK.MAN ET AL. who had demonstrated that heparin and cortisone had an anti-angiogenic effect. MADARNAS ET AL. used Fisher rats which received transplants of bladder tumor. Unlike other scientists who had used commercial preparations of heparin, MADARNAS ET AL. used Haplean heparin (Organon) which was supplied without preservatives. The results not only demonstrated that heparin and cortisone treatment was indeed anti-angiogenic, but also that the MTP and cortisone combination was just as effective. MADARNAS ET AL. thought that the heparin results obtained by Folkman were due to an endotoxin-like contaminant in the Panheparin (Abbott) (MADARNAS ET AL., 1989). lt is important to note that heparin preparations may be contaminated with products of gram negative bacteria
•
•
since heparin is manufactured from porcine intestine (BENREZZAK ET AL., 1988) . Thus, it seems that MTP is an excellent substitute for heparin and LPS in antitumor studies. If MTP is indeed an anti-angiogenic drug, it may be of use in the treatment of AIDS KS.
7. Objectives
The objectives of the present studies were first, to study the development of angiosarcomas in C57Bl/6 mice through the study of early lesions. Second, to transplant primary AS tumors, induced by DMH, in nude and syngeneic C57Bl/6 mice in order to determine the tumorigenicity of the early, primary AS lesions. The third objective was to develop a cell line derived either from the primary AS itself or the transplanted tumor so that in vitro studies on AS and their treatment could be conducted. Fourth, to conduct studies on the treatment of mice containing the early, primary AS tumors. The latter study consisted of cortisone and MTP treatment to determine if MTP alone and in combination with cortisone had any effect on AS and possibly EC in vivo. All of these studies (objectives 1-4) were evaluated with the goal of obtaining a mouse model for the study and treatment of AIDS KS.
Lastly, the fifth objective was to evaluate the efficacy of MTP and cortisone treatment of i.v. transplanted C3HBA tumor. The goal of this objective was to determine whether anti-angiogenic therapy could prevent or reduce metastasis of tumor cells already present within the blood stream since anti-angiogenic therapy has not previously been evaluated in this manner .
•
•
II -
MATERIAL AND METHODS
1. Ani mals
Ali animais were obtained from Charles River (St. Constant, Québec). Male C57Bl/6N rnice of approximately 10 grams in weight and 21 days of age were used for studies involving DMH and MTP and cortisone. Sorne male and female (retired breeder) C57Bl/6N mice were used for AS transplants. Male CDl homozygous nude rnice of 28-42 days were used for AS transplants while female retired breeder C3H/HeN rnice were used for metastasis studies.
1.1. DMH treatment
The protocol used for DMH treatment was similar to the one used by M. DUBÉ (1990). DMH (Sigma) dissolved in 0.001 M disodium (ethylenedinitrilo) tetraacetate (EDTA) was administered at a dose of 20 mg/kg s.c. once a week to male C57Bl/6 mice for 29 weeks. The dose of DMH was divided into two, one half was injected on the anatomical right side while the other was injected on the left. Five control rnice received 0.3 ml EDTA. Since the DMH-EDTA combination was very acidic, the pH was adjusted to 6.5 using SN NaOH. Forty seven of the mice treated with DMH were used for studies on the development of AS. The rest (67) were used for cortisone and MTP treatment. The mice were randomly divided into four groups as follows: 1) 17 mice received PBS, 2) 17 mice received cortisone only, 3) 16 mice received MTP only and 4) 17 mice received combined cortisone and MTP treatment. The cortisone and MTP treatment lasted for 10 days and was administered according to the schedule in appendix A.