Prediction of isoelectric point of manganese and cobalt lamellar oxides : application to controlled synthesis of mixed oxides
Texte intégral
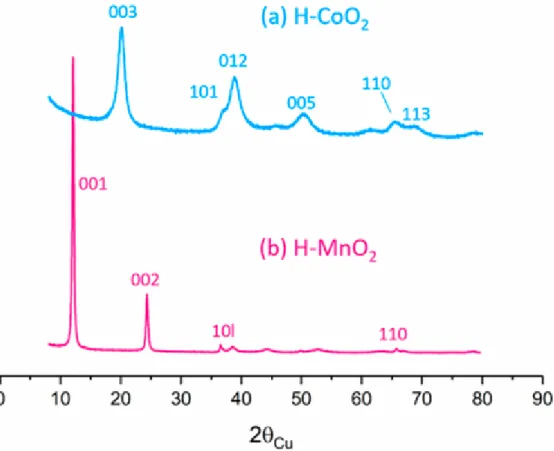
Figure



Documents relatifs
Pyridine adsorption FT-IR studies have shown that the surface acidity of the catalysts was due to the presence of Lewis acid sites and the appearance of strong Brønsted sites
Also independent of preparation mode, XRD analysis shows that samples containing 10 to 25mol % Mn exhibit two phases: a bixbyite type phase (Mn2O3) with iron inclusion, and a
Generally, the oversaturation is affected by the concentration of surfactants through the chemical potential as described above. Higher concentration of the surfactant
[27] Catalysis for sustainable energy conversion, such as PEC water splitting for the production of hydrogen, syngas (CO + CO 2 + H 2 ) conversion for the synthesis of high-
THE APPLICATION OF EXAFS SPECTROSCOPY TO THE STRUCTURAL DETERMINATION OF FLUORITE TYPE OXYGEN- EXCESS URANIUM OXIDES AND OF MIXED (U,Ce) OXIDES... THE APPLICATION OF
Of significance in the normalized EXAFS spectra is their overall shape which is nearly identical for lithiophorite and asbolane at both Co and Mn K-edges (Fig. Before any analysis
Three objectives were defined to answer this : (i) synthesize the Mn oxide and Co oxyhydroxides “building blocks” through exfoliation of layered metal oxides, (ii) restack
Nowadays the mechanism of POM is still controversial. In the literature two basic mechanisms of the partial oxidation of methane have been discussing [5-10]. The first involves the
