THE PROTON FORMATION FROM METHANE BY DISSOCIATIVE
IONIZATION IN THE ELECTRON ENERGY RANGE OF 25-40 eV
R. LOCHT and J. MOMIGNYInstitut de Chimie, Département de Chimie Générale et Chimie Physique, Université de Liège, Sart-Tilman, B-4000 Liège I, Belgium
Abstract
The proton formation by dissociative electroionization of methane has been investigated in the energy range of 25-40 eV. The kinetic energy-versus-appearance energy shows five different H+ producing processes respectively at 26.3 ± 0.2 eV, 26.9 ± 0.2 eV, 29.4 ± 0.3 eV, 32.7 ± 0.2 eV and 35.7 ± 0.5 eV. These critical energies are discussed in terms of different dissociation channels probably opened through predissociation of doubly excited states of CH4
+
. On the high energy side of the electron energy range investigated in the present work, the proton would appear through the dissociation of the CH+ ion as an intermediate.
1. Introduction
In a recent paper [1] we published the results obtained for the first two processes producing protons and deuterons from methane and methane-d4 under the impact of low energy electrons in the range of 20-23 eV. The
comparison of our data with previous work on the dissociative ionization as well as the dissociative excitation of methane by electron and photon impact was extensively discussed. The appearance mechanisms involving thermal and 2.3 eV kinetic energy protons were both related to dissociative autoionization. The strong parallelism between the results obtained either by electroionization or by dissociative excitation were discussed.
The present paper is concerned with the investigation of the proton formation at higher electron energies, i.e. in the range of 25-40 eV. In this energy range the most detailed dissociative electroionization work so far has been published by Appell [2]. Both kinetic energy and appearance energy of the protons have been measured. Concerning the existence of electronically excited states of CH+4 in the energy range of interest,
Backx et al. [3] and van der Wiel et al. [4], using electron energy loss spectroscopy of high-energy primary electrons and (e, 2e) experiment, found two "photoelectron bands" corresponding to two doubly excited states of CH4
+
.
2. Experimental results
The operating conditions maintained in the course of this work are identical with those described in ref. [1]. An electronical improvement of the detection system enabled to increase the sensitivity for the onset energy measurements of highly energetic protons.
Figs. 1 and 2, respectively, show the kinetic energy distribution of H+/CH4 obtained with 50 eV
electrons and the ionization efficiency curve of the proton for the retarding potential setting VR = 0 V.
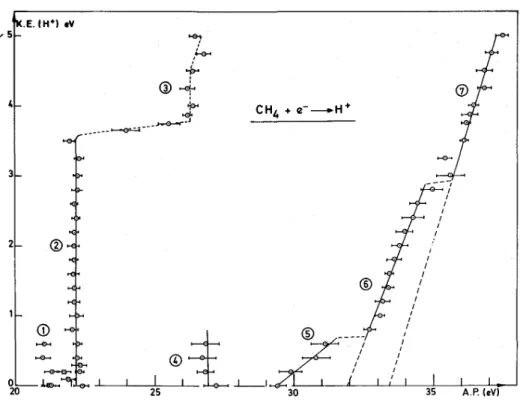
Fig. 3 shows the final result obtained by recording the ionization efficiency curve of H+ at retarding potential settings increased by 0.2 V between 0-5.0 V. Each data point thus obtained is the averaged value of at least five independent measurements. The error bars measure the largest deviation from the mean value. For higher retarding potentials, i.e. over 5.0 V, the signal is of too low intensity to allow valuable onset energy measurements. The processes numbered by (1) and (2) being extensively discussed in ref. [1], only the diagram portions (3)-(7) will be discussed in the following sections.
Fig. 1. Energy distribution of the protons from CH4 obtained with 50 eV electrons. Maxima measured at 0.0 eV,
0.5 eV, 0.9 eV, 2.3 eV and 3.9 eV (ref. [1]).
Fig. 2. First (di+/dEe ) and second (d2i+/dEe2) derivatives of the electroionization efficiency curve of H+/CH4
Fig. 3. Kinetic energy (KE)-versus-appearance energy (AP) plot for H+/CH4. For diagram portion numbering,
see text.
3. Discussion
In the energy range of interest, the data obtained in earlier dissociative electroionization and dissociative excitation work have been gathered in table 1 and the dissociation limits which could be involved in the same energy range are listed in table 2. For the sake of clarity each diagram portion (3)-(7) will be discussed separately.
Table 1 Comparison of experimental results with previous studies. Onset energies (eV) and corresponding kinetic energy range in parentheses (eV)
Appell [2] Schiavone [5] Finn [6] Present work
process (3) 28 (4.7) 24.5 ± 0.5 (4.7-8.4) 25.5 ± 0.6 (<7.5) 26.3 ± 0.2 (4.0) process (4) 26.5 (0-1.2) 26.4 ± 0.5 (0.3-2.6) - 26.9 ± 0.2 (0-0.6) process (5) 31.0 (3-4.7) - - 29.4 ± 0.3 (0-0.6) process (6) - - - 32.7 ± 0.2 (0.6-2.8) process (7) 33.0 (3-4.7) 34.8 ± 0.5 (3-15) 36.7 ± 0.6 (>3.2) 35.7 ± 0.5 (3.0-5.0) 3.1. Process (3)
The vertical portion of process (3) is measured at an average value of 26.3 ± 0.2 eV (see fig. 3). Between processes (2) and (3) a sudden and step-like change in appearance energy is observed. Both processes are separated by ∆(AP) = 4.2 ± 0.3 eV for a variation of about 0.2 eV kinetic energy. With a monoenergetic electron beam and a better kinetic energy resolution, the broken line between processes (2) and (3) would tend to be parallel to the AP-axis. This feature in the diagram indicates that the energy of 4.2 eV is entirely converted into internal energy of the fragments.
Table 2 Thermochemically calculated dissociation limits leading to proton formation from methane in the energy range of 23-37 eV. Dissociation energies are taken from ref. [8], ionization and excitation energies of atoms from ref. [9], ionization and excitation energies of diatomic and poly-atomic radicals from refs. [10] and [11], respectively
Appell [2] observed the same jump of about 4.0 eV for the threshold energy in the interval of 24-28 eV. This author measured onset energies for protons carrying up to 7 eV total kinetic energy, and above 4.7 eV the translational energy is linearly dependent upon the electron energy in the range of 28-30 eV. By extrapolation to zero kinetic energy, Appell [2] obtained a dissociation limit of 23.1 ± 0.5 eV and ascribed the production of these ions to the processes
calculated as lying respectively at 23.78 eV, 22.95 eV and 22.85 eV. The difference in onset energy, i.e. 28 eV in ref. [2] and 26.3 eV measured in the present work, is unexplained.
Schiavone et al. [5] and Finn et al. [6] measured an onset energy of 24.5 ± 0.5 eV and 25.5 ± 0.5 eV for high-Rydberg H* atoms from CH4 carrying 4.7-8.4 eV kinetic energy. Oertel [7] measured the second onset for
Lyman α, β and γ emission from H*/CH4 at 25.3 ± 1.2 eV, 25.8 ± 1.2 eVand 25.8 ± 1.2 eV. None of these
authors give an interpretation of the processes observed between 24.5-25.8 eV.
Van der Wiel et al. [4] obtained an (e, 2e) spectrum with high-energy primary electrons on CH4. A first
"band" having its adiabatic ionization energy near 26 eV and the vertical ionization energy at about 28 eV, is ascribed to the simultaneous ionization and excitation of the CH4 molecule. Appell [2] and Backx et al. [3]
proposed the (1t2)-2 (3a1)1 configuration for the CH+4 molecular ion. This configuration gives rise to a manifold
of electronic states, i.e. 4 F1, 2
A1, 2
E, 2 F1 and 2
F2 in the tetrahedral symmetry Td [11].
Process (2) (see fig. 3) having its onset at 22.2 eV [1] has been ascribed to the protons producing mechanism [1]
where the vibrationally excited radical is formed by the dissociative autoionization of a superexcited CH4 *
state and carries translational and internal energy. The lowest dissociation limit for this process is calculated at 18.1 eV and the maximum total translational energy measured on H+ is of 3.5 eV. The internal energy carried by
Dissociation products Energy
(eV) CH4 + e~ →H+ + CH3(B~2A'1) + 2e- (1) 23.78 (C~2E") (2) 26.30 (D~ ²A'1) (3) 26.33 → H+ + H + CH2(X 3 B1) + 2e -(4) 22.95 (1B1) (5) 24.75 (1A'1) (6) 27.35 → H+ + H2 + CH(X 2 Π) + 2e- (7) 22.85 (A2∆) (8) 25.70 (B2 Σ -) (9) 26.08 (C2Σ+) (10) 26.79 → H+ + 2 H + CH(X 2 Π) + 2e- (11) 27.32 (A2∆) (12) 30.17 (B2Σ -) (13) 30.55 (C2Σ+) (14) 31.26 → H+ + H2 + H + C( 3 P) + 2e- (15) 26.28 (1D) (16) 27.54 (1S) (17) 28.96 → H+ + 3 H + C(3P) + 2e- (18) 30.75 (1D) (19) 32.01 (1S) (20) 33.43 →H+ + CH3 + (X1A'1) + 3e -(21) 27.89 a) (3E") (22) 32.81 a) (1E") (23) 34.15 a) →H+ + H + CH2 + (X~2A1) + 3e -(24) 33.35 →H+ + H+ + CH2(X~ 3 B1) + 3e -(25) 36.55 → H+ + H2 + CH + (X1 Σ +) + 3e- (26) 33.49 (A1Π) (27) 36.41 (1∆) (28) 37.03
CH3 is 0.6 eV. The 4.2 ± 0.3 eV energy difference between processes (2) and (3) is entirely converted into
internal energy (see fig. 3).
A first interpretation of the proton formation at 26.3 eV would be that the total internal energy, i.e. 4.8 ± 0.3 eV, is stored in the CH3 radical. This quantity has to be compared with the value of the dissociation energy
D(CH2-H) = 4.9 eV [8]. The minimum observed kinetic energy involved in this process is 4.1 ± 0.2 eV. The tail
rising up from 4.2-5.0 eV kinetic energy indicates the occurrence of the same process where the proton carries an increasing amount of translational energy. However, the accuracy of the present measurements does not allow us to determine the slope of the straight line fitting the data between 4.5-5.0 eV. The slope of about 1.0 drawn in fig. 3 would lie on the assumption that H+ only carries the total excess translational energy. In this case a two-step mechanism would give rise to protons, i.e.
where step (b) is kinetically slower than step (a). Such a mechanism could take place through predissociation of the first doubly excited state of CH+4 corresponding to the configuration (1t2)-2 (3a1)1 with an adiabatic ionization
energy of about 26 eV [4]. This state, probably the 4F1 state, is correlated with a 4
A' in the Cs symmetry [11] for
which the energy has been calculated by Leclerc and Guissard-Galloy [14]. This could lead to the dissociation limit CH2 (X~
3
B1) + H + H +
as schematically shown in fig. 4. These products would give rise to 2A" and 4A" states which would predissociate the 4A' state of CH4. Accounting for the error on the measurements, a lower
limit of 0.5 for a slope could be estimated and would account for a straight dissociation of CH+4 by
An alternative dissociation path, having its thermochemical threshold at about the same energy, would be process (7) in table 2. The same two-step mechanism could be involved.
3.2. Process (4)
The vertical line in fig. 3 at 26.9 ± 0.2 eV corresponds to the threshold of a process observed in the kinetic energy range of 0-0.6 eV. Being very difficult to observe, the onset of this process could not be measured for higher values of the translational energy (see fig. 5 in ref. [1]).
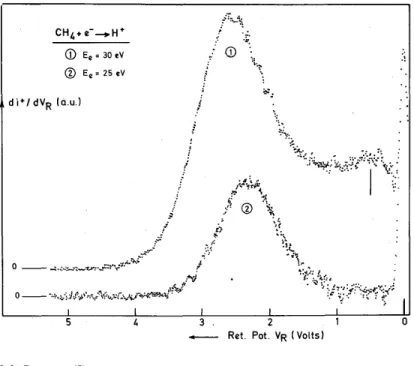
As shown in fig. 5, a suddenly rising contribution of low energy protons is observed when the kinetic energy distribution of H+/CH4 is recorded at 25 eV and 30 eV. This noticeable change when the electron energy
rises from 25 to 30 eV, indicates that the proton energy distribution corresponding to process (4) is probably given by the maximum observed at 0.5 eV (see figs. 1 and 5).
The present onset energy measurement has to be compared with the threshold measured by Appell [2] at 26.5 eV for low-energy protons and by Schiavone et al. [5] at 26.4 ± 0.5 eV for low-energy high-Rydberg H* atoms. The latter authors observed a linear dependence of the kinetic energy with respect to the onset energy and suggested that the ionic dissociation limit would involve the CH3 radical excited in the C
~ 2
E" or D~2 A'1 state
[processes (2) and (3) in table 2]. However, as mentioned by Appell [2] many other dissociation channels are energetically open in the energy range of 26-27 eV (see table 2). One of these, leading to H+ + H + H2(
1
Σg +
) + C(3P), is represented schematically in fig. 4.
Fig. 4. Correlation diagram between CH4+ and its dissociation products in the energy range of 26 eV. For the
discussion, see text.
Fig. 5. Kinetic energy distribution of H+/ CH4 obtained with 30 eV (1) and 25 eV (2) electrons. Note the intensity
increase of low energy ions at 30 eV.
3.3. Process (5)
The last onset in the electroionization efficiency curve of H+ for the retarding potential setting VR = 0 V
is measured at 29.4 ± 0.3 eV. As shown in fig. 3, the appearance energy is linearly dependent upon the kinetic energy in the energy range of 0-0.6 eV. These protons contribute to an intensity increase of the thermal and nearly thermal ions in the kinetic energy distribution.
Considering the low scattering of the average values of the threshold energies, a least-squares fitted straight line has been attempted. This line extrapolates to 29.6 eV for KE(H+) = 0 eV and has a slope of 0.3.
For low energetic protons, Appell [2] measured an appearance energy of about 31 eV: no interpretation is proposed. Schiavone et al. [5] do not observe low-energy high-Rydberg H atoms at this energy.
The energy range covered by process (5) and the following protons producing dissociations overlap the second electron band ascribed by van der Wiel et al. [4] to a doubly excited configuration of CH+4. The adiabatic
and vertical energies are estimated at 28.5 eV and 31 eV, respectively.
The extrapolated onset energy of 29.6 ± 0.3 eV determined in the present experiment corresponds fairly well to the energy for the process
calculated at 30.2 eV. The slope of 0.3 could account for the equipartition of the excess kinetic energy on the lost proton and the two hydrogen atoms. The existence of an ionized state of CH4 in the energy range of 30 eV and
the absence of low energy high-Rydberg hydrogen atoms in the same energy range would favour a mechanism where the last process proceeds through direct dissociative or predissociative ionization.
However, other mechanisms like processes (13) and (18) in table 2 could not be definitely ruled out. 3.4. Process (6)
Well separated from process (5) the next proton producing process extends over an ion energy range of 0.8-2.8 eV. These ions could be responsible for the 0.9 eV maximum observed in the proton energy distribution observed at 50 eV electron energy (see fig. 1).
The kinetic energy-versus-appearance energy diagram shows a straightforward linear dependence (see fig. 3). The least-squares fit applied to the measured onset energies gives a slope of 1.0 and from the extrapolation to zero ion energy an appearance energy of 31.9 eV is obtained.
The extrapolated value of 31.9 eV for the appearance energy agrees well with the thermochemical on-set calculated for the mechanism
at 32.0 eV. In this one-step process, however, the theoretical slope of the straight line in the kinetic energy-versus-appearance energy diagram has to account for the loss of one proton and three hydrogen atoms. Assuming the equipartition of the excess translational energy on the four lightest particles, a slope of 1/4 x 12/13 = 0.19 is obtained. This value has to be compared with 1.0 determined from the experimental data.
To account for both the experimental slope and the extrapolated value of the appearance energy, a two-step mechanism has to be invoked for the appearance of H+, i.e.
The proton being produced by process (b), the slope of the curve giving the excess translational energy carried by H+, as a function of the appearance energy, is given by the ratio 12/13 = 0.92. The thermochemical threshold for process (a) is calculated at 24.36 eV, when implicitly assuming the formation of CH+ without excess internal and translational energy. This value has been fitted on the potential energy diagram of CH+ as calculated by Lorquet et al. [12] and partially reproduced in fig. 6. The experimental data and the proton energy distribution have been introduced in this diagram, where the energy of 31.9 eV corresponds fairly well to the dissociation process (b) and the kinetic energy distribution observed on H+ could arise from a transition to the continuum of the C1Σ+ and/or the 1Π state converging both to 32.0 eV.
Fig. 6. Potential energy diagram of CH+ taken from ref. [12]. For the sake of clarity only those curves used in the discussion (see text) have been reproduced in this figure.
3.5. Process (7)
In the proton energy range of 3.0-5.0 eV the data show a linear dependence upon the appearance energy and lead to a least-square-fitted straight line with a slope of 1.1 and an appearance energy of 33.4 eV as extrapolated to zero kinetic energy. The obtained slope is close to the theoretical one that one would obtain under the unlikely assumption that the total excess kinetic energy be carried away by the proton, leaving the three H atoms at rest.
The dissociation limit, i.e. 33.4 eV obtained by extrapolation of the least-square-fitted straight line, is in good agreement with the onset calculated for the process
which is at 33.4 eV. As in the foregoing section, in this process we should have to observe a slope of 0.19 the excess translational energy being shared by all the particles involved in the dissociation. The observed slope of 1.1 could only be interpreted by the two-step mechanism
for which the KE-versus-AP straight line would have a slope of 0.92 and the thermochemical threshold is at 33.4 eV.
The proton energy distribution as well as the experimental data have been introduced in the potential energy diagram reproduced in fig. 6. The formation of protons with 3-5 eV kinetic energy could be ascribed to transitions to the 3∆ state predissociated by the 1Σ+ state converging to the H+ + C(1 S) dissociation limit. The higher energetic protons could be produced by direct transitions to one or both continua of the 3∆ or 1Σ+ states.
The possible formation of the proton through transitions to unstable doubly ionized states of CH4 , such
as processes (24) and (26) listed in table 2 could probably be discarded because double ionization starts around 40 eV [13].
4. Conclusions
The careful investigation of the proton formation through dissociative ionization of methane (and methane-d4 in ref. [1]) shows that (i) at electron energies in the vicinity of the first threshold only dissociative
associated with doubly excited states of CH+4, give rise to protons. The interpretation of some of the
experimental data gathered in the present work would require the appearance of H+ from the dissociation of one intermediate fragment ion (CH+).
Acknowledgement
We thank the Belgian "Fonds National de la Recherche Scientifique" and the "Fonds de la Recherche Fondamentale Collective" for financial support.
References
[1] R. Locht, J. L. Olivier and J. Momigny, Chem. Phys. 43 (1979) 425. [2] J. Appell, These d'Etat, Université Paris-Sud, France (1972).
[3] C. Backx, G.R. Wight, R.R. Tol and M.J. van der Wiel, J. Phys. B8 (1975) 3007.
[4] M. J. van der Wiel, W. Stoll, A. Hamnett and C.E. Brion, Chem. Phys. Letters 37 (1976) 240. [5] J. A. Schiavone, D. E. Donohue and R.S. Freund, J.Chem. Phys. 67 (1977) 759.
[6] T. G. Finn, B. L. Carnahan, W. C. Wells and E.C. Zipf, J. Chem. Phys. 63 (1975) 1596. [7] H. Oertel, Diplomarbeit, Universität Hamburg, Germany (1977).
[8] B. de B. Darwent, Bond Dissociation Energies in Simple Molecules, NSRDS-NBS 31 (1970). [9] C.E. Moore, Atomic energy levels, Circ. NBS 467 (1949).
[10] B. Rosen, Selected constants. Spectroscopic data relative to diatomic molecules (Pergamon Press, New York, 1970).
[11] G. Herzberg, Molecular spectra and molecular structure,Vol. 3 (Van Nostrand, Princeton, 1967). [12] A. J. Lorquet, J.C. Lorquet, H. Wankenne and J. Momigny, J. Chem. Phys. 55 (1971) 4053. [13] C. Backx, M. J. van der Wiel, J. Phys. B8 (1975) 3020.
[14] J.C. Leclerc, C. Guissard-Galloy, Bull. Soc. Chim. Belg. 83 (1974) 327.


![Fig. 6. Potential energy diagram of CH + taken from ref. [12]. For the sake of clarity only those curves used in the discussion (see text) have been reproduced in this figure](https://thumb-eu.123doks.com/thumbv2/123doknet/6640855.181317/8.918.141.449.151.452/potential-energy-diagram-clarity-curves-discussion-reproduced-figure.webp)