Carbon and nitrogen isotopic ratios of the seagrass
Posidonia oceanica : depth-related variations.
Gilles Lepoint*, Patrick Dauby, Michaël Fontaine, Jean-Marie Bouquegneau and Sylvie Gobert
Centre MARE, Laboratoire d’Océanologie, Département des Sciences de la Vie, B6, Institut de Chimie, Université de Liège, 4000 Liège, Belgium
corresponding author: G.Lepoint@ulg.ac.be; fax number: ++32 4 366 33 25
Abstract:
The nitrogen (15N) and carbon (13C) isotopic compositions of Posidonia
oceanica were determined during three seasons along a bathymetric gradient (4-38 m depth). The 15N values are low (2.2 0.9 ‰) and variable. They do not
show any relation to depth or sampling dates. There is a significant difference between the 15N values of the youngest and the oldest leaves, probably as a
result of N resorption and senescing during leaf ageing.
The 13C values of young Posidonia leaves show a clear relation to depth,
expressing the relation of the 13C values to the primary productivity rate and to
the use of a bicarbonate / CO2 mixture as an inorganic carbon source. The 13C
values of the oldest P. oceanica leaves are depleted in 13C compared to those
of young leaves. This modification of the 13C signatures in relation to leaf age is
particularly important between 20 and 29 m depth. This modification could be related to photosynthetic rate change during ageing, but also to change in carbohydrate composition and content.
Key words: stable isotopes, seagrass, Posidonia, NW Mediterranean
Introduction
The measurement of the natural abundance ratios of carbon and nitrogen stable isotopes (13C/12C and 15N/14N) has numerous applications in ecological studies,
particularly in the delineation of food sources in trophic webs. This approach was often applied to seagrass ecosystems where plants can display different
isotopic signatures allowing the distinction between potential food sources for consumers (e.g. Kitting et al., 1984; Fry, 1988; Dauby, 1989; Jennings et al., 1997; Marguillier et al., 1997; Lepoint et al., 2000; Moncreiff and Sullivan, 2001; Vizzini and Mazzola, 2002). Stable isotope ratios of carbon and nitrogen are presented as values (respectively, 13C and 15N), expressed in ‰ relative to
an international standard. The 13C/12C values of organic matter are depleted in 13C compared to the international standard and, therefore, 13C values of
organic matter are generally negative. On the contrary, 15N/14N values of organic
matter are often enriched in 15N compared to the international standard, and
therefore, 15N values of organic matter are generally positive.
The 13C values of seagrasses range among the highest values (i.e. the least
negative) reported for aquatic and terrestrial primary producers (see the review of Hemminga and Mateo, 1996). This could be related to the capacity of
seagrasses to use bicarbonate as an inorganic carbon source for photosynthesis (Beer et al., 2002), i.e., a source less 13C-depleted than
dissolved CO2 (0 vs. - 9 ‰) (Raven et al., 2002). However, there are many
inter- and intra-specific variations of the 13C values related to the variability of
environmental factors (i.e., irradiance, hydrodynamic, temperature,… ) and of primary productivity rates (e.g. Cooper and DeNiro, 1989; Grice et al., 1996, Hemminga and Mateo, 1996, Vizzini et al., 2003).
The 15N values of plants vary according to species, site location or nitrogen
sources or uptake rates and are used to trace ground and waste water impact on the nitrogen cycle in estuarine and coastal ecosystems (e.g., Fourqurean et al., 1997).
Posidonia oceanica (L.) Delile, a large and long-living seagrass, is a common, but endangered, species of the Mediterranean littoral. This species is distributed between 0 and 45 m depth and, therefore, P. oceanica experiences an
extended range of depth-related environmental conditions (e.g., light, water motion, nutrient availability,…) determining the large variability of biometric parameters (e.g., Gobert et al., 2003), demography (e.g., Olesen et al., 2002) and depth-related ecological processes (Mazzella et al., 1992) in the P. oceanica meadow. Because the isotopic composition of a plant is in part determined by ecological processes which are affected by this depth gradient (e.g. photosynthesis), we guess that stable isotope ratios of P. oceanica should be partly depth-related.
In this study, we assess this depth-related variability of 13C and 15N values in a
P. oceanica meadow during three seasons and we discuss the consequence of such a variability for trophic web studies using stable isotopes.
Material and methods
This study was carried out in Revellata Bay (Calvi, NW Corsica, France)
(42°35'N, 8°43'E) in front of the oceanographic station of STARESO (University of Liège). This meadow has been the object of several isotopic studies
concerning the food web (e.g., Dauby, 1989; Havelange et al., 1997 ; Lepoint et al., 2000 ; Pinnegar et al., 2000), the characterisation of particulate matter (Dauby et al., 1995) and the variability of the stable carbon isotope ratios of P. oceanica (Cooper and DeNiro, 1989).
Samplings were performed in October 1997 and in February and June 1998, from 4 to 38 m depth, which are, respectively, the upper and lower limits of this meadow. P. oceanica shoots (i.e. one bundle of leaves with a piece of rhizome and some roots) were collected along the same general direction for the three seasons, but not along a permanent transect. Each sample is composed of two shoots collected every two metres from 4 to 20 m depth, and every five metres from 20 to 38 m depth.
The leaves were scraped using a razor blade to remove epiphytes and were classified in three categories according to Giraud (1979): juveniles (length 5 cm), intermediates (length 5 cm and no ligule) and adult leaves (length 5 cm and presence of a ligule). On each shoot, three leaves were selected: the youngest intermediate, the youngest adult and the oldest adult leaves. These leaves were arbitrarily numbered leaf 1, 2 and 3, respectively.
Leaves, roots and rhizomes were dried for 48 h at 60°C, and ground for isotopic measurements performed with a mass spectrometer (VG Optima, Micromass, UK) coupled to an elemental analyser (Carlo Erba, Italy). Ratios are presented as value (‰), expressed relative to the vPDB (Vienna Peedee Belemnite) standard and to atmospheric N2 for carbon and nitrogen, respectively.
Experimental precision (based on the standard deviation of replicated measurements of P. oceanica leaf sample) was 0.3 ‰ for both carbon and nitrogen.
For statistical analysis, data were classified in four classes according to their sampling depth: 0-9, 10-19, 20-29, 30-39. Isotopic data have been analysed using a two-way ANOVA test with leaf type and depth as independent factors.
Post hoc Tukey test was used to assess pairwise differences when ANOVA revealed statistically significant effects.
A non parametric Kruskal-Wallis test for multiple comparison was used for seasonal comparison as normality conditions were not encountered in this case. All test results were considered as significant when p was 0.05.
Results
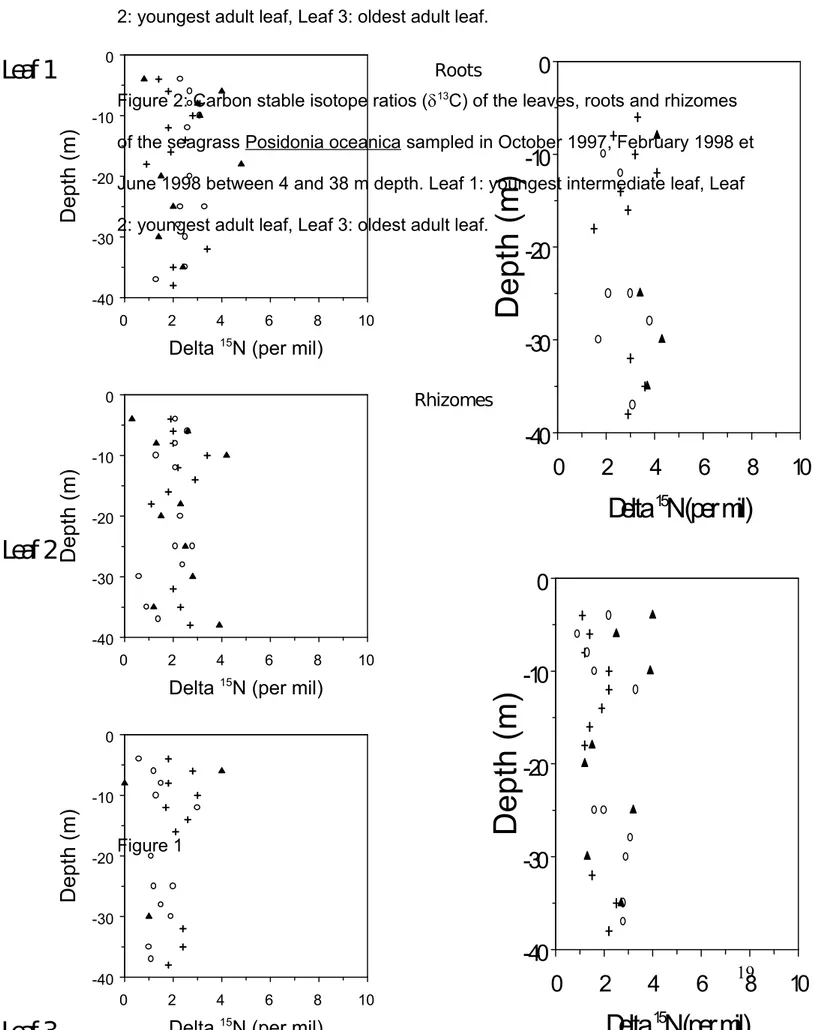
The 15N values of P. oceanica organs range from 0.6 to 4.8 ‰ for the leaves,
from 1.5 to 4.3 ‰ for the roots and from 0.9 to 3.9 ‰ for the rhizomes (fig 1.). There are significant differences between the 15N values of the different leaf
types (Table I). But, these differences are only significant when the youngest and the oldest leaves are compared (Tukey test, p < 0.05). The 15N values of
the organs do not show any significant differences in relation to the depth (Table I) or the sampling dates (Kruskal-Wallis test, p > 0.1).
The 13C values of P. oceanica organs range from –10.8 to – 19.7 ‰ for the
leaves, from - 11.3 to –17.1 for the roots and from – 13.3 to –17.5 ‰ for the rhizomes (fig 2.). There are significant differences between 13C values of the
three types of leaves (Table I). Post hoc comparison shows these differences are significant between all the leaf types (Tukey test, p < 0.05). The oldest leaves seem often depleted in 13C relative to leaves 1 and 2 (up to 5.5 and 4.0
‰ more negative than leaves 1 and 2, respectively).
There are highly significant differences in the 13C values of leaves according to
depth (Table I). The pairwise comparison shows that there are significant differences between all the depth classes (Tukey test, all p 0.04), except
between the 10 - 19 m and the 30 - 39 m classes. The 13C values of leaves are
generally the least negative (i.e., the least depleted in 13C) between 4 and 8 m
depth (fig 2.). The 13C values of leaves 2 and 3, but not of leaves 1, are the
most negative (i.e., the most depleted in 13C) in the zone comprised between 20
and 29 m depth. The 13C values of Posidonia rhizomes and roots show a
relation to depth like leaves 1. The two-way ANOVA shows that there are no significant interactions between the depth and the leaf type (Table I). The 13C
values of Posidonia organs do not show any significant difference in relation with the sampling dates (Kruskal-Wallis test, all p > 0.1).
Discussion and Conclusion
The 15N values of primary producers are mainly determined by the 15N values
of their N sources and the isotopic discrimination (i.e., the preferential use of one isotope against the other) during N uptake and assimilation (McClelland et al., 1997). P. oceanica and other Mediterranean primary producers generally have low 15N values compared to primary producers of the North Atlantic (e.g.,
Jennings et al., 1997 ; Lepoint et al., 2000 vs. Créach et al., 1998; McClelland et al., 1997). This difference is partly due to the significantly lower 15N signal of
inorganic nitrogen in the Mediterranean Sea than that of inorganic nitrogen in the North Atlantic, resulting from the greater influence of N2 fixation on the
nitrogen cycle in the Mediterranean (Pantoja at al., 2002).
The 15N values can be used as a signal to trace waste and ground water inputs
in the environment. Inorganic nitrogen derived from anthropogenically modified or natural ground waters are enriched in 15N against marine inorganic nitrogen
and this enrichment is detectable in the coastal primary producers (Fourqurean et al., 1997; McClelland et al., 1997; Kamermans et al., 2002, Yamamuro et al., 2003). Fourqurean et al. (1997) have recorded 15N values for Zostera marina in
a site influenced by urbanisation (Tomales Bay, California) that are significantly higher than those of P. oceanica (9.7 0.3‰ vs. 2.2 0.9). McClelland et al. (1997) report that 15N values of Z. marina in Waquoit Bay vary between – 2.0
and 6 ‰, according to the increase of wastewater N loads. Although our samples from the shallowest station were collected near the STARESO harbour, P. oceanica appears little enriched in 15N, and the 15N values do not
show any clear variation in relation to depth. This could reflect the small
influence of this potential pollution on these shoots. However, P. oceanica has a complex nitrogen budget where the inorganic N incorporated from the water column by leaves and roots represents respectively 25 and 35 % of the N annual requirement of the plant (Lepoint et al., 2002). Moreover, nutrients diffusing from the sediment probably constitute an important source of N for leaf uptake (Gobert et al., 2002). Therefore, the 15N of P. oceanica could be less
sensitive to an inorganic N groundwater or wastewater load than Z. marina, which generally depends more on the water column nitrogen, or than
macroalgae from rocks which depend completely on this N source. Moreover, our data show that the 15N values slightly decrease with leaf ageing. This
change is probably related to the N resorption and recycling process which occur in P. oceanica and represent about 40 % of the N plant needs (Lepoint et al., 2002).
The range of 13C values is quite large (fig 2), and the lowest values are close to
(i.e. macroalgae and phytoplankton) (Lepoint et al., 2000; Pinnegar et al., 2000). As a result, the distinction between the different food sources is potentially difficult. It is therefore very important in trophic web studies to
compare the isotopic values of plants and animals coming from a same location and a same depth to reduce the 13C range of primary producers and to make
the isotopic signatures more distinguishable (Vizzini et al., 2003). This also means that the distinction between the different food sources is not possible for all depths of the Posidonia meadow.
The 13C values of the youngest intermediate leaves are clearly related to
depth, as reported by Cooper and DeNiro (1989) for this meadow, and,
therefore, to light supply and productivity rate. Grice et al. (1996) showed that the 13C values of seagrasses grown in aquaria increases when the light
irradiance increased. The 13C values are significantly correlated to the
productivity rate of seagrasses (Cooper and DeNiro, 1989; Hemminga and Mateo, 1996). Indeed, the productivity rate affects the extent of the isotopic discrimination (i.e. the preferential use of one isotope relative to the other). However, this correlation is not observed in the shallowest station for P. oceanica (Mateo et al., 2000). These authors suggest that the carbon isotopic discrimination in seagrasses saturates earlier than production at increasing light irradiance. This would explain that, for the shallowest sampling depth, the 13C
values of Posidonia leaves show smaller changes than the productivity rate. Recent works have shown that bicarbonate use by seagrasses, among them by P. oceanica, is more important than previously recognised (Beer et al., 2002; Invers et al., 2001). Bicarbonate has a less negative 13C than CO
2 (0 vs. –9 ‰)
and, consequently, a variable 13C which sometimes shows very high value
(Raven et al., 2002). Bicarbonate use is clearly an adaptation of seagrasses to marine life (Beer et al., 2002). Indeed, in the aquatic environment, CO2 diffusion
rate and availability can be a limiting factors for inorganic carbon supply and, therefore, for primary production. The highest 13C values (i.e. – 10.8 ‰) which
was recorded in the shallowest location of this study could imply that, at this depth, P. oceanica photosynthesis relies mainly on bicarbonate as an inorganic carbon source (Raven et al., 1995, 2002). For the other depths, the
interpretation is more difficult, but, the observed variation could signify that the Posidonia leaves rely, in fact, on a varying mixture of CO2 and bicarbonate to
meet their inorganic C demand (Raven et al., 2002), although the contributions of these two sources are not calculable by using the 13C values (Raven et al.,
1995).
Thus, two linked processes are involved in the variation of the young leaves 13C: the ability to use a varying mixture of CO
2 and bicarbonate (i.e., two
sources with different isotopic signatures and incorporation ways) and the primary production rate (i.e., a rate which influences the extend of the isotopic discrimination) which is related to light supply, and, therefore, to depth.
The 13C values of the different leaves of P. oceanica show significant
differences, particularly when the youngest and the oldest leaves are compared (fig 2.). The general trend is a decrease of the 13C values with the increasing
leaf age, except in October 1997. This is a significant difference, considerably above the measurement precision ( 0.3 ‰), already observed by Vizzini et al. (2003). A second trend is that this difference is more pronounced between 20 and 30 m depth than at the shallowest and the deepest sampling stations. It is
worth to notice that the structure of the P. oceanica meadow of the Revellata Bay changes drastically between 20 and 30 m depth (i.e. shoot density and biomass decrease more quickly than between 0 and 20 m depth or between 30 and 40 m depth) (Gobert et al., 2003). Although changes of 13C values are
relatively confusing, they show that some processes concerning the carbon budget of the plant occur differently at the different depths and, probably, seasons. The following processes could be involved in the differentiation of the change of 13C values with the increasing leaf age: firstly, change of the
photosynthesis rate (and thus of isotopic discrimination), and, secondly, change of the carbohydrate leaf composition and content (for example addition of structural carbohydrate or re-mobilisation of stored carbohydrates). Concerning the photosynthetic capacities, Modigh et al. (1998) show that the leaf age is a major factor determining the leaf capacity for carbon assimilation. Decrease of photosynthetic rate and change of photosynthetic parameters with ageing are well documented for P. oceanica (e.g. Alcoverro et al., 1998; Modigh et al., 1998) and, as for depth variation of 13C values, a lower photosynthetic rate
could be related to a lower 13C. The photosynthetic rate is also related to
seasonal variations of irradiance and carbon demand, and, although in this study no seasonal pattern of the 13C values is observed, this seasonal cycle
probably influences the 13C values of the plant (Vizzini et al., 2003).
On the other hand, leaf carbohydrate composition and contents also change with leaf age and season (Pirc, 1985) due to variations of photosynthesis rate, storage / reclamation processes (Alcoverro et al., 2001), transfer processes between leaves and ramets (Libes and Boudouresque, 1987; Marba et al. 2002) or additions of structural components (e.g. cellulose, lignin) (Klap et al., 2000).
These processes, which are often related to depth (e.g. Olesen et al., 2002) and season (e.g. Pirc, 1985; Alcoverro et al., 1998, 2001), could modify the isotopic signature of a leaf during its life. Indeed, every process involving chemical reactions may be theoretically responsible for isotopic discriminations. Because structural and soluble carbohydrates may have different isotopic signatures, change in carbohydrates composition and content should be related to 13C
change in the leaf. Senescence, which implies a resorption of soluble carbon and nitrogen contents, is probably also involved in the modification of the 13C
and 15N.
Acknowledgements
We wish to thank the staff of the oceanographic research station STARESO for their hospitality and for scientific facilities. We are grateful to Prof. J Raven and one anonymous referee who improved the quality of the manuscript with their comments and suggestions. G.L. received grants from the "Fonds de la
Recherche pour l'Agriculture et l'Industrie" (F.R.I.A.) and is a postdoc reseacher of the “Fonds National pour la Recherche Scientifique” (FNRS). This study was funded by the French Community of Belgium (ARC 97/02-212) and the Belgian National Fund for Scientific Research (FRFC 2.4570.97). This paper has the MARE publication number 023 (MARE023).
References
1. Alcoverro, T., M. Manzanera and J. Romero. 1998. Seasonal and age-dependent variability of Posidonia oceanica (L.) Delile photosynthetic parameters. J. Exp. Mar. Biol. Ecol. 230: 1-13.
2. Alcoverro, T., M. Manzanera, and J. Romero. 2000. Annual metabolic carbon balance of the seagrass Posidonia oceanica: the importance of carbohydrate reserves. Mar. Ecol. Prog. Ser. 211: 105-116.
3. Cooper, L.W. and M. J. De Niro. 1989. Stable carbon isotope variability in the seagrass Posidonia oceanica: evidence for light intensity effects. Mar. Ecol. Prog. Ser. 50: 225-229.
4. Créach, V., M.T. Schricke, G. Bertru and A. Mariotti. 1997. Stable isotopes and gut analyses to determine feeding relationships in saltmarsh
macroconsumers. Est. Coast. Shelf Sci. 44: 599-611.
5. Dauby, P. 1989. The stable carbon isotope ratios in benthic food webs of the gulf of Calvi, Corsica. Cont. Shelf Res. 9: 181-195.
6. Dauby, P., A.J. Bale, N. Bloomer, C. Canon, R.D. Ling, A. Norro, J.E. Robertson, A. Simon, J.M. Théate, A. Watson and M. Frankignoulle. 1995. Particle fluxes over a Mediterranean seagrass bed: a one year case study. Mar. Ecol. Prog. Ser. 126 : 233-246.
7. Fourqurean, J.W., T.O. Moore, B. Fry and J.T. Hollibaugh. 1997. Spatial and temporal variation in C:N:P ratios, 15N, and 13C of eelgrass Zostera marina as
indicators of ecosystem processes, Tomales Bay, California, USA. Mar. Ecol. Prog. Ser. 157: 147-157.
8. Fry, B. 1988. Foodweb structure on Georges bank from stable C, N, and S isotopic compositions. Limnol. Oceanogr. 33: 1182-1190.
9. Giraud, G. 1979. Sur une méthode de mesure et de comptage des structures foliaires de Posidonia oceanica (Linnaeus) Delile. Bull. Mus. Hist. Nat. Marseille 39: 33-39.
10. Gobert, S., M. Kyramarios, G. Lepoint, C. Pergent-Martini and J.M.
Bouquegneau. 2003. Variations à différentes échelles spatiales de l'herbier à Posidonia oceanica (L.) Delile ; effets sur les paramètres physico-chimiques du sédiment. Oceanologica Acta 26: 199-207.
11. Gobert, S., N. Laumont and J.M. Bouquegneau. 2002. Posidonia oceanica meadow: a low nutrient high chlorophyll (LNHC) system? BMC Ecology 2: 9 12. Grice, A.M., N.R. Loneragan and W.C. Denison. 1996. Light intensity and
the interactions between physiology, morphology and isotope ratios in five species of seagrass. J. Exp. Mar. Biol. Ecol. 195: 91-110.
13. Havelange, S., G. Lepoint, P. Dauby and J.M. Bouquegneau. 1997. Feeding of the sparid fish Sarpa salpa in a seagrass ecosystem: diet and carbon flux. PSZNI Mar. Ecol. 18: 289-297.
14. Hemminga, M.A. and M.A. Mateo. 1996. Stable carbon isotopes in
seagrasses: variability in ratios and use in ecological studies. Mar. Ecol. Prog. Ser. 140: 285-298.
15. Jennings, S., O. Renones, B. Morales-Nin, N.V.C. Polunin, J. Moranta and J. Coll. 1997. Spatial variation in the 15N and 13C stable isotope composition of
plants, invertebrates and fishes on Mediterranean reefs: implications for the study of trophic pathways. Mar. Ecol. Prog. Ser. 146: 109-116.
16. Kamermans, P., M.A. Hemminga, J.F. Tack, M.A. Mateo, N. Marba, M. Mtolera, J. Stapel, A. Verheyden and T. Van Daele. 2002. Groundwaters effects on diversity and abundance of lagoonal seagrasses in Kenya and on Zanzibar Island (East Africa). Mar. Ecol. Prog. Ser. 231: 75-83.
17. Kitting, C.L., B. Fry and M.D. Morgan. 1984. Detection of inconspicuous epiphytic algae supporting food webs in seagrass meadows. Oecologia 62: 145-149.
18. Klap, V.A., M.A. Hemminga and J.J. Boon. 2000. Retention of lignin in seagrasses: angiosperm that returned to the sea. Mar. Ecol. Prog. Ser. 194: 1-11
19. Lepoint, G., F. Nyssen, S. Gobert, P. Dauby and J.M. Bouquegneau. 2000. Relative impact of a Posidonia seagrass bed and its adjacent epilithic algal community in consumers diet. Mar. Biol. 136: 513-518.
20. Lepoint, G., S. Millet, P. Dauby, S. Gobert and J.M. Bouquegneau. 2002. An annual nitrogen budget of the seagrass Posidonia oceanica as determined by in situ uptake experiments. Mar. Ecol. Prog. Ser. 237: 87-96.
21. Libes, M. and C.F. Boudouresque. 1987. Uptake and long-distance transport of carbon in the marine phanerogam Posidonia oceanica. Mar. Ecol. Prog. Ser. 38: 177-186.
22. Marba, N., M.A. Hemminga, M.A. Mateo, C.M. Duarte, Y.E. Mass, J. Terrados and E. Gacia. 2002. Carbon and nitrogen translocation between seagrass ramets. Mar. Ecol. Prog. Ser. 226: 287-300.
23. Marguillier, S., G. Van der Velde, F. Dehairs, M.A. Hemminga and S. Rajagopal. 1997. Trophic relationships in an inter-linked mangrove-seagrass ecosystem as traced by 13C and 15N. Mar. Ecol. Prog. Ser. 151: 115-121.
24. Mateo, M. A., M. A. Hemminga, J. Romero, M.M. Littler and D.S. Littler. 2000. Evidence of the coupling between light, 13C, and production in the
25. Mazzella, L., M.C. Buia, M.C. Gambi, M. Lorenti, G.F. Russo, M.B. Scipione and V. Zupo. 1992. Plant-animal trophic relationships in the Posidonia oceanica ecosystem of the Mediterranean Sea: a review. In: (John DM, Hawkins SJ, Price HJ, eds) Plant-animal interaction in the marine benthos. Clarendon Press, Oxford. pp. 165-187.
26. McClelland, J.W., I. Valiela and R.H. Michener. 1997. Nitrogen-stable isotope sigatures in estuarine food webs: a record of increasing urbanization in coastal watersheds. Limnol. Oceanogr. 42: 930-937.
27. Modigh, M., M. Lorenti and L. Mazzella. 1998. Carbon assimilation in Posidonia oceanica: biotic determinants. Bot. Mar. 41: 249-256.
28. Moncreiff, C.A. and M.J. Sullivan. 2001. Trophic importance of epiphytic algae in subtropical seagrass beds: evidence from multiple stable isotopes analysis. Mar. Ecol. Prog. Ser. 215: 93-106.
29. Olesen, B., S. Enriquez, C.M. Duarte and K. Sand-Jensen. 2002. Depth – acclimation of photosynthesis and demography of Posidonia oceanica and Cymodocea nodosa in the Spanish Mediterranean Sea. Mar. Ecol. Prog. Ser. 236: 89-97.
30. Pantoja, S., D.J. Repeta, J.P. Sachs and D.M. Sigman. 2002. Stable isotope constraints on the nitrogen cycle of the Mediterranean Sea water column. Deep-Sea Res. I 49: 1609-1621.
31. Pinnegar, J.K. and N.V.C. Polunin. 2000. Contribution of stable isotope data to elucidating food webs of Mediterrenean rocky-littoral fishes. Oecologia 122: 399-409.
32. Pirc, H. 1985. Growth dynamics in Posidonia oceanica (L.) Delile. 1.
Seasonal changes of soluble carbohydrates, starch, free amino-acids, nitrogen and organic anions in different parts of the plant. PSZNI Mar. Ecol. 6: 141-165. 33. Raven, J.A., A.M. Johnston, J.E. Kübler, R. Korb, S.G. McInroy, L. L.
Handley, C.M. Scrimgeour, D.I. Walker, J. Beardall, M. Vanderklift, S.
Fredriksen and K.H. Dunton. 2002. Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses. Funct. Plant Biol. 29: 355-378.
34. Raven, J.A., D.I. Walker, A.M. Johnston, L.L. Handley and J.E. Kübler. 1995. Implication of 13C natural abundance measurements for photosynthetic
performance by marine macrophytes on their natural environment. Mar. Ecol. Prog. Ser. 123: 193-205.
35. Vizzini, S. and A. Mazzola. 2002. Stable carbon and nitrogen ratios in the sand smelt from a Mediterranean coastal area: feeding habits and effect of season and size. J. Fish Biol. 60: 1498-1510.
36. Vizzini, S., G. Sarà, M.A. Mateo and A. Mazzola. 2003. 13C and 15N
variability in Posidonia oceanica associated with seasonality and plant fraction. Aquat. Bot. (in press)
37. Yamamuro, M., H. Kayanne and H. Yamano. 2003. 15N of seagrass leaves
for monitoring anthropogenic nutrient increases in coral reef ecosystems, Mar. Pollut. Bull. 46: 452-458.
Table I. Summary of ANOVA results. MS: mean square; F: Snedecor’s F; p: significance level; N.S.: non-significant difference (p>0.05).
Source of variation 15N (‰) 13C (‰)
MS F p MS F p
Leaf type 2.31 F2,78= 3.49 <0.05 20.45 F2,96= 9.29 <0.001
Depth 1.13 F3,78=1.46 NS 38.01 F3,96=20.28 <0.001
Leaf type X Depth 0.11 F6,78=0.15 NS 2.04 F6,96=1.09 NS
Figure 1: Nitrogen stable isotope ratios (15N) of the leaves, roots and rhizomes
of the seagrass Posidonia oceanica sampled in October 1997, February 1998 et June 1998 between 4 and 38 m depth. Leaf 1: youngest intermediate leaf, Leaf 2: youngest adult leaf, Leaf 3: oldest adult leaf.
Figure 2: Carbon stable isotope ratios (13C) of the leaves, roots and rhizomes
of the seagrass Posidonia oceanica sampled in October 1997, February 1998 et June 1998 between 4 and 38 m depth. Leaf 1: youngest intermediate leaf, Leaf 2: youngest adult leaf, Leaf 3: oldest adult leaf.
Figure 1
19
O
ctober 97F
ebruary 98June 98
Leaf 1
Leaf 2
Leaf 3
Roots Rhizomes 10 8 6 4 2 0 0 -10 -20 -30 -40Delta 15N (per mil)
D
ep
th
(
m
)
10 8 6 4 2 0 0 -10 -20 -30 -40Delta 15N (per mil)
D
ep
th
(
m
)
10 8 6 4 2 0 0 -10 -20 -30 -40Delta 15N (per mil)
D
ep
th
(
m
)
10
8
6
4
2
0
0
-10
-20
-30
-40
Delta
15N (per mil)
D
ep
th
(
m
)
10
8
6
4
2
0
0
-10
-20
-30
-40
Delta
15N (per mil)
D
e
pt
h
(m
)
Figure 2
21
O
ctober 97F
ebruary 98June 98
-10 -12 -14 -16 -18 -20 0 -10 -20 -30 -40
Delta 13C (per mil)
D ep th ( m ) -10 -12 -14 -16 -18 -20 0 -10 -20 -30 -40
Delta 13C (per mil)
D ep th ( m ) -10 -12 -14 -16 -18 -20 0 -10 -20 -30 -40
Delta 13C (per mil)
D ep th ( m ) Leaf 1 Leaf 2 Leaf 3