5
Probiotics as a Source of Aromas in
Functional Food: Selected Examples
and Analytical Methodology
THIERRY KENNE,1 GEORGES LOGNAY,2 AND MARIE-LAUREFAUCONNIER1*ABSTRACT
Probiotics play an important role in functional foods by generating natural aromas that are fundamental for the acceptability of such products by consumers. Selected strains are added to conventional functional foods, not just for health properties but also for their capability to improve flavor and overall taste. Beyond their applications to functional dairy products, the use of microorganisms for the aroma in-situ generation is now expanded to meat and plant-based products. However, aromas produced by probiotics are often complex and all odor compounds are not always suitable to all foodstuff types. Therefore, it is also valuable to overview, in the present chapter, the specific analytical methodology currently used for characterizing and selecting interesting aroma compounds.
Key words: Probiotics, Natural aroma, Functional food, Sampling
methods, Gas chromatography
1 Chimie Générale et Organique, ULg-Gembloux AgroBioTech,
2 Chimie Analytique ULg-Gembloux AgroBioTech, Passage des Déportés 2, B-5030
Gembloux, Belgium
1. INTRODUCTION
Probiotic foods and beverages are part of an expanding industry with a global current estimated value of $35 billion (Global Market, 2017; Jankovic, 2010). Probiotics are accurately defined as live microorganisms which, when administered in adequate amounts confer a health benefit on the host (FAO/WHO, 2002). However, the acceptance of a new probiotic food is not only determined by its health capabilities (Granato et al., 2010).
Although consumers are increasingly demanding natural products with new functional properties, the consumption patterns of new foodstuffs are strongly determined by their sensorial characteristics (Breslin et al., 2001; Cheung et al., 2016). This consumer appeal for probiotic containing foodstuffs has opened the way to investigate probiotic new aspects: their role in flavor generation.
Flavor is the most important aspect of food (Weerawatanakorn et al., 2015). Flavor is defined as a combination of taste, aroma and trigeminal perception (Tournier et al., 2007). Aroma gives a clearly distinguishable character to foodstuffs. It generally consists of hundreds of volatile organic compounds with various chemical structures (acids, alcohols, ketones, esters, etc.) in a well-defined proportion (Schwarb et al., 2008). Individual aroma compounds are found at very low concentrations in food, typically parts-per-million (ppm) or part-per-billion (ppb) level (Mc Gorrin, 2002; Qian et al., 2006). On that point, the relationship between aroma concentration, obtained by analytical methods and their perception is not obvious. The human nose’s perception of volatile organic compounds can vary in a large extend depending on the compound, e.g., from 10–8 ppm for 1-p-Menthene-8-thiol “citrus” to 102 ppm for ethanol (Schieberle et al., 2009).
Aroma compounds can originate from various sources. They may come from the ingredients itself (e.g., lemon zest), added flavoring agents (natural or not) or technological transformation processes (heating, drying, smoking, frying, baking, etc.). On other side, aroma compounds can also derive from enzymatic reactions, including processing aids for the liberation of flavor compounds from nonvolatile precursors (e.g., cysteine conjugates), and the conversion of a precursor to a desired flavoring (Winterhalter et Skouroumounis, 1997; Sakho et al., 1998) or by fermentation through microbial activities.
Historically, probiotics are non-starter microorganisms but some strains have shown their capability to go along fermentation and generate volatile compounds contributing to food aroma (Salmeron et al., 2009). Therefore, probiotic cultures have been developed to specifically bring out the desired flavors in the products in which they are used (Saarela et al., 2000).
Based on these three categories of foodstuffs (dairy products, meat products and plant-based products), this chapter will provide an overview of probiotic strains which beyond their health benefit, significantly contribute to the food aroma. As analytical methods are clearly the bottleneck of the study of aroma compounds derived from probiotic strains, the second part of the chapter will focus on analytical aspects.
2. FUNCTIONAL FOODS WITH PROBIOTICS AS SOURCE OF AROMAS
2.1 Dairy Products
The substrates play a significant role in probiotic growth and viability. Dairy products such fermented milk, yogurt, and cheese are current food probiotic carriers. The physic-chemical composition of milk-based products, which is rich in carbohydrates, proteins and lipids, acts as a nutriment sources and protective matrix for the fermentation (Vijaya Kumar et al., 2015). The main actions of probiotics in dairy fermentations are preservation of the milk by generating antimicrobial compounds, production of flavor compounds and other metabolites that will provide consumers desired organoleptic properties (Parvez et al., 2006). Particularly, lactic acid bacteria are widely used for the fermentation of cheeses, yogurts and milks. These microorganisms have a variety of enzymes that enable them to turn milk carbohydrates and proteins, firstly into citrate and peptides and, secondary into aroma compounds (Matar et al., 1996). In this case, Table 1 presents the summarized form of key volatile organic compounds (VOCs) from probiotic dairy products.
a. Yogurt aroma compounds derived from probiotic activities
Yogurt is a basic dairy product that has been consumed for centuries as a part of the diet and its beneficial effect has well-known and scientifically proven. Typical yogurt results of the mixture of milk (whole, low-fat, or nonfat) or cream fermented with a culture of lactic acid-producing
Table 1: Key VOCs from probiotic dairy products
Product Main Character CAS Amount Descr Refere names microorganism impact number (ppm) iptors nces
compound
Soft cheese L. plantarum I91 Diacetyl 431-03-8 n.q Buttery Milesi et al.
L. casei I90 Acetoin 513-86-0 n.q Buttery (2010) Semi-hard L. reuteri INIA P5 3-methyl-1- 123-51-3 n.q Sweatyc
Gómez-ewe-milk butanol Torres et
Castellano 2-butanol 78-92-2 n.q Fruityc al. (2016)
cheeses 2-propen-1-ol 107-18-6 n.q Alliaceousc
2-butanone 78-93-3 n.q Fruityc 3-methyl- 108-99-6 n.q Greenc pyridineb 2,3-pentane- 600-14-6 n.q Butteryc dioneb propionic 79-09-4 n.q Vinegarc acidb
white- L. acidophilus diacetyl 431-03-8 n.q Buttery Ozer et al.
brined LA-5 (2009)
cheese B. bifidum BB- 75-07-0 7.2 - 27.8 Fresh 12 acetaldehyde
Cheddar L. casei LC2W heptanoic 111-14-8 n.q Cheesyc Hong-Xin
cheese acid et al. (2015)
nonanoic acid 112-05-0 n.q Cheesyc
undecanoic 112-37-8 n.q Creamyc acid
myristoleic 544-64-9 n.q Fattyc
acid
E. faecium PR88 diacetyl 431-03-8 n.q Buttery Gardiner et
al. (1999)
pentan-2-one 107-87-9 n.q Protein-aceous 3-methyl- 123-51-3 n.q Sweaty butanol
butyric acid 107-92-6 n.q Buttery 3-methyl- 503-74-2 n.q Cheesy butyric acid
Yogurt* L. bulgaricus, Diacetyld 431-03-8 0.2-3.0 Buttery Tamime et S. thermophilus, acetaldehyded75-07-0 2.0-41.0 Fruity Robinson L. rhamnosus acetoind 513-86-0 2.2-5.7 Buttery (2007); DSA LR1 2-butanone 78-93-3 n.q Fruity Innocente
2-heptanone 110-43-0 n.q Fruity et al. (2016)
2-nonanone 821-55-6 n.q Fruity Acetoned 67-64-1 1.3-4.0 Fruity
Table 1: (Contd...)
Product Main Character CAS Amount Descr Refere names microorganism impact number (ppm) iptors nces
compound
Kefir S. cerevisiae A13 ethanol 64-17-5 4800.0 Alcoholicc Beshkova L. helveticus MP1 2-butanone 78–93-3 0.04 Fruity et al. (2003) S. thermophilus diacetyl 431-03-8 1.62 Buttery
T15
L. delbrueckii acetaldehyde 75-07-0 18.3 Fruity subsp. bulgaricus
HP1
S. thermophilus Acetone 67-64-1 0.3 Fruity T15
L. lactis subsp. ethyl acetate 141-78-6 0.06 Fruity
lactis C15
Ice-cream L. reuteri, n.i n.i n.q Sour Salem et
B. bifidum al. (2005)
Pudding L. rhamnosus diacetyl 431-03-8 n.q Buttery Helland
et al. 2004 ayogurt makes with cow milk, bCheese formulation with specific adjuncts of glycol, cData
from http://www.thegoodscentscompany.com, dMix culture L. delbrueckii subsp. bulgaricus and S. thermophiles ni: not identify, nq: not quantify
bacteria, L. delbrueckii subsp. bulgaricus and S. thermophilus (Routray and Mishra, 2011). There are first potent probiotic strains that have well-researched health benefits (Mater et al., 2005). Whereas, L. rhamnosus DSA LR1 is a novel probiotic which has been recently experimented for its possible aroma contribution (Routray and Mishra, 2011). The most aroma compounds usually found in yogurt are acetaldehyde, acetone, acetoin, diacetyl, 2-butanone, 2-heptanone and 2-nonanone (Innocente et al., 2016; Routray and Mishra, 2011).
Indeed, acetaldehyde is the most impact flavor compound of the yogurt which concentration ranges between 2.0 and 41.0 ppm (Tamime et al., 2007). The common level reported is 10–15 ppm (Bottazzi, 1973). Consumer acceptability is generally associated with amount of acetaldehyde, it is responsible of the freshness taste and “fruity” note of the yogurt. The quality of yogurt aroma is quietly depending on its relationship with other volatile compounds; for instance, an aldehyde-to-acetone and aldehyde-to-diacetyl (Accolas, 1980). Overall good flavor of yogurt starts around 8.0 ppm of acetaldehyde, whereas concentration lower than 4.0 ppm give a product which flavor is weak and atypical (Routray and Mishra, 2011). Compounds such as glucose, catechol,
glyceraldehyde, and amino acids such as threonine and glycine, can act as the precursors of acetaldehyde. However, the breakdown of threonine to acetaldehyde and glycine by L. delbrueckii subsp. bulgaricus threonine aldolase has been identified as a main biosynthesis pathway (Mirjana Hruškar et al., 1995; Routray and Mishra, 2011).
Diacetyl is the second major aroma compound of the yogurt. It is highly exhibited buttery smell, allowed as an impact character of yogurt. Its amount varies from 0.3 to 3.0 ppm, and lowest concentration is observed in mix culture starters. Diacetyl is a secondary metabolite derived from the fermentation of citrate and lactose contained in the milk (Tamime et al., 2007; Nilsson et al., 2008; Cheung et al., 2016; Innocente et al., 2016). Microorganisms involved in its production are S. thermophilusis, L. bulgaricus and L. rhamnosus (Rasic and Kurmann, 1978; Beshkova et al., 1998; Innocente et al., 2016). Acetoin is derived from enzymatic degradation of diacetyl and imparts buttery notes similar to those of its precursor. The amount (2.2–5.7 ppm) of acetoin is increased during the storage and remains significant at the limit of expired date (Tamime et al., 2007; Innocente et al., 2016; Routray and Mishra, 2011).
Besides aldehydes and diacetyl, several ketones such as 2-butanone, 2-heptanone, 2-nonanone and acetone act in the yogurt aroma. Ketones are characterized by a “fruity” note that makes yogurt very pleasant. Among them, acetone derives from acetaldehyde, whereas 2-heptanone and 2-nonanone derive respectively from -oxidation of octanoic acid and decarboxylation of decanoic acid. Lactobacillus rhamnosus DSA LR1 has a strong activity of these two methyl ketones, while other ketones are common to lactic bacteria (McSweeney et al., 2000; Routray and Mishra, 2011; Innocente et al., 2016).
b. Cheeses aromas derived from probiotic activities
Freshly made curds of various cheeses have in similar bland flavors. Characteristic aroma compounds of each variety of cheese are formed during the ripening period (McSweeney et al., 2000). However, common cheeses produced are not probiotics. Indeed, ripening conditions induce highly decrease of cell viability under 8 log CFU, required for the labelling of products as probiotics. Soft cheese, semi-hard ewe-milk castellano cheese, white-brined cheese and cheddar cheese are some which have been successfully ripening with probiotic strains in order to highlight
aroma character of the final product (Gardiner, 1999; Özer et al., 2009; Milesi et al., 2010; Gomez-Torres et al., 2016; Hong-Xin et al., 2015).
In this context, Milesi et al. (2009) have studied production of flavor compounds in soft cheeses and reported that cheeses inoculated with non-starter probiotic strains are impacted by Diacetyl and acetoin. These volatiles are characterized by “buttery to cheesy” note and often produced though various starter Lactobacilli pathway. However, authors assume that cheeses inoculated with non-starters probiotic strains L. plantarum I91 or L. casei I90 are characterized by a significantly higher concentration of diacetyl and acetoin.
Besides, Gomez-Torres et al. (2016) have investigated the effect of reuterin-producing L. reuteri coupled with glycerol on the volatile fraction and aroma of semi-hard ewe milk cheese. They demonstrated that the addition of probiotic strain L. reuteri INIA P5 coupled with 30 mM glycerol enhanced the production 3-methyl-1-butanol “sweaty”, 2-butanol “fruity”, 2-propen-1-ol “alliaceous”, 2-butanone “fruity”, 3-methyl pyridine “green”, 2,3-pentanedione “buttery” and propionic acid “vinegar” of the cheese. Probiotic semi-hard ewe milk cheese is characterized by “cheesy” aroma due to the individual contribution of those aromatic compounds to the overall flavor.
Another relevant study was carried out in the viability of B. bifidum BB-12 and L. acidophilus LA-5 in model white-brined cheese by microencapsulation (Özer et al., 2009). The study is also compared the amount of acetaldehyde and diacetyl produced by probiotic cheese and a control. The results show that concentrations of medium and long-chain acids have been increased in experimental cheese. Similarly the acetaldehyde (7.2–27.8 ppm) and diacetyl levels were considerably higher than the control. The model cheese was highly scored in “aroma and flavor” attribute and for overall acceptability.
As regards Cheddar cheese, it has been proven to be a good carrier of probiotics. It contains a complex combination of microorganisms, which change with time, alongside the non-starter lactic acid bacteria (Gardiner et al., 1999; McSweeney et al., 2000; Özer et al., 2009; Phillips et al., 2006; Hong-Xin et al., 2015). In this sense, L. casei LC2W was used as an adjunct in the production of probiotic Cheddar cheese ripened. Analysis of aroma compounds showed that probiotic cheese is higher in aroma compounds than control cheese. Heptanoic acid “cheesy”, nonanoic acid “cheesy”, undecanoic acid “creamy” and myristoleic acid “fatty” were
only detected in probiotic cheese. Acids have a greater contribution to the flavor profile of cheese, and furthermore, they are not only aroma compounds by themselves but also precursors of other compounds, such esters, aldehydes and ketones (Delgado et al., 2011; Hong-Xin et al., 2015). The effect of a probiotic adjunct culture of E. faecium on the quality of Cheddar cheese was also reported by Gardiner et al. (1999). They found that E. faecium PR88 increases proteolysis and higher levels of acid compounds such as butyric acid. This compound has a strong impact on the cheese flavor due to its typical sweaty and cheesy odor.
c. Kefir aromas derived from probiotic activities
Kefir is a fermented milk beverage produced by combined action of bacteria and yeasts that exist in a symbiotic association in kefir grains. The large number of microorganisms present in kefir and their microbial interactions produce active compounds that confer to this beverage a status of natural probiotics (De Oliveira et al., 2013). Starter-kefir grains are the main source of volatile compounds that confers to traditional kefir its unique flavor and aroma. References providing qualitative and quantitative data about flavor production by kefir probiotic lactic acid bacteria and yeasts taking separately are quite rare (Güzel-Seydim et al., 2000; Simova et al., 2002). One of the few studies on this topic has been reported by Beshkova et al. (2003) who have followed the production of volatile aroma compounds by single-strain cultures of kefir. The results show that the highest amount of acetaldehyde (18.3 ppm), diacetyl (1.62 ppm), acetone (0.3 ppm), 2-butanone (0.04 ppm) and ethyl acetate (0.06 ppm) and ethanol (4800 ppm) were recorded with L. delbrueckii subsp. bulgaricus HP1, S. thermophilus T15, L. helveticus MP12, L. lactis subsp. lactis C15 and S. cerevisiae A13, respectively. The amount of ethanol combined with CO2 (1.98 ppm) provide to kefir their distinctive alcoholic aroma. Other volatile compounds are yogurt-like aroma commonly found in fermented dairy products.
d. Probiotics contribution in overall aroma of ice cream and pudding
Ice cream and pudding are two dairy products that have been recently investigated as innovative matrices for probiotic growth and viability. Ice cream is a frozen product and pudding is characterized by high thickness and more hydrocolloidal environment than the normal yogurt. Some lactic bacteria strains have displayed their ability to survive in
these restrictive environments (Alamprese et al., 2002; Helland et al., 2004; Moussa Salem et al., 2005; Cruz et al., 2009). Alongside the health benefit, additional flavor functionalities of probiotics in such matrices were reported by the same authors. Salem et al. (2005) reveal that ice cream containing L. reuteri has been judged to be sourer and attained a higher score for probiotic flavor. But their studies do not provide more qualitative and quantitative data regarding volatile organic compound contributions. After study the growth and the metabolism of probiotic strain in milk-based pudding, Helland et al. (2004) found that high concentration of diacetyl (18 ppm) were detected in puddings inoculated with L. rhamnosus GG. They assume that due to a smaller amount of acetaldehyde produced, diacetyl has a significant impact to the overall pudding aroma.
2.2 Meat Products
The market of probiotics is much expanded in dairy industry, but the concept of probiotic functional food has not obtained similar development in the meat industry (Arihara and Ohata, 2010). Manufacturing of most meat products include thermal treatment, and this process stage is very critical for the survival of probiotic microorganisms. Main probiotic meat products target dry sausages and, to a lesser extent, hams due to their convenient thermal process. Several studies have been recently reported in the topics related with the possibility of functional meat including probiotics (Leroy et al., 2006; Ammor and Mayo, 2007; Arihara and Ohata, 2010). However, most of them are focused in the strain survivability and health benefit. The fewer studies that correlated probiotic adjuncts and aroma improvement are presented in Table 2.
a. Aroma contribution of probiotic strains in fermented sausages
Dry-fermented sausage is a food usually made from meat that is ground, fermented, and then dry cured. During the fermentation period, lactic bacteria add in the sausage formula proliferate and produce volatile organic compounds. Among the compounds produced, some of them have aromatic properties that impart the overall flavor of sausage. Ruiz-Moyano et al. (2011) have investigated the volatile compounds change of Iberian dry fermented sausages by adding probiotic L. reuteri PL519 during the manufacturing. The products were characterized by significant amount of 2-butanone, 3-methylbutanal and benzene
acetaldehyde which concentrations represent 16.3, 0.93 and 2.1 per cent of total volatile compounds, respectively. The authors emphasize that benzene acetaldehyde and 3-Methyl butanal are associated with floral and nutty notes, respectively, and correlated with the flavor of cured meat products, whereas 2-butanone has a moderate effect with an aceton-like flavor. These aroma compounds make dry sausage fermented with L. reuteri PL519 fairly acid, and globally more flavored.
b. Aroma contribution of probiotic strains in heat treated sausages
Volatiles of heat treated probiotic sausages were studied with Gas chromatography analytical method by Sidira et al., (2015). These authors reported that immobilized L. casei cells resulted in a higher content of ethyl esters and alcohols. Main ethyl esters produced in that case, are ethyl lactate (0.01 ppm), ethyl decanoate (0.03 ppm) and ethyl butanoate (0.1–1 ppm) which are all fruity-like notes. Ethyl-esters contribute to proper sausage aroma (Flores et al., 2004).
c. Aroma contribution of probiotics strains in fermented hams
Functional properties and volatile compounds of fermented hams have Table 2: VOCs from probiotic functional meats
Product Main Character CAS Amount Descr Refere names microorganism impact number (ppm) iptors nces
compound
Dry- L. reuteri PL519 2-Butanone 78–93-3 n.q Aceton- Santiago
fermented 3-Methyl- like
Ruiz-sausages butanal 590-86-3 n.q Nutty Moyano
Benzene 122-78-1 n.q Floral et al.
acetaldehyde (2011)
Heat trea- L. casei ATCC Ethyl 105-54-4 0.1-1 Fruityb Sidira et
ted probiotic 393 butanoate al. (2015)
sausages Ethyl 110-38-3 0.03 Fruityb
decanoate
Ethyl lactate 97-64-3 0.01 Fruity, butteryb
Fermented L. plantarum Ethanola 64-17-5 2.08 Alcoholicb Kim et al.
hams L155, L. plant- Ethyl 141-78-6 0.70 Fruity (2016)
arum L167, P. acetatea
damnosus L12 2-Furfurala 98-01-1 0.03 Bready
aAfter 21 days ripening, bData from http://www.thegoodscentscompany.com, nq: not
been evaluated by a mixed probiotic starter cultures isolated from Kimchi which is the most popular dish Korea. In the same context, Kim et al. (2016) have noticed that hams fermented with Kimchi, which is composed mostly by L. plantarum L155, L. plantarum L167 and P. damnosus L12 (LLP), give similar aroma profile to those obtained from the commercial starter. Ethanol, ethyl acetate and 2-furfural, representing approximately 78 per cent of total VOCs, were very close in experimental and control harms. Thus, they suggested that probiotic (LLP) isolated from kimchi, could be used as starter culture due to their similarity in composition of volatile compounds produced.
2.3 Plant-based Products
Plants are generally rich in carbohydrates and have long been used as fermentation materials. The trend to apply probiotic microbial strains for the fermentation of cereals, fruits and legumes is a rational approach for the development of functional foods (Mishra and Mishra, 2013). Furthermore, the manufacturing of probiotic food with non-dairy food matrices can overcome limitations imposed by milk-based products. Lactose and high content in cholesterol of milk prevent consumption of dairy probiotic foods by the segment of people suffering from lactose intolerance or exposed to hypercholesterolemia related diseases (Granato et al., 2010). Except fruits, the quality of the aroma produced is a permanent challenge in other vegetal materials. Indeed, raw cereals and legumes are often characterized by intense green and beany notes, respectively. Using of suitable probiotic strains can produce volatile compounds that help to mask the negative notes at the same time developing quality aroma compounds. Table 3 presents probiotic strains which have already beneficially used to develop aroma active compounds in plant-based functional foods and their aroma contribution.
a. Aroma compounds derived from probiotics in cereal-based beverages
Cereal-based probiotics are largely dominated by beverages. Salmeron et al. (2014) have studied the volatile compounds and flavor characteristics of a malt fermented beverage formulated with B. breve NCIMB 702257. Twelve key aroma compounds were produced and most of them (acetic acid, butanoic acid, propanoic acid, acetoin, ethanol 2-butoxy and 2-pentanone 4-hydroxy-4-methyl) were made through the
Table 3: VOCs from probiotic functional foods with plant materials
Product Main Character CAS Amount Descr Refere names micro- impact number (ppm) iptors nces
organism compound
Fermented B. breve Acetic acid 64-19-7 n.q Vinegare Salmerón
malt NCIMB Butanoic acid 107-92-6 n.q Sour, rancid et al. beverage 702257 Propanoic acid 79-09-4 n.q Slightly sour (2014)
Acetoin 513-86-0 n.q Flowery, wet Ethanol 2-butoxy 111-76-2 n.q Sweet, fruity 2-pentanone 68113- n.q Butter, 4-hydroxy-4-methyl 55-3 caramel
Probiotic L. plant- Acetic acida,b 64-19-7 n.q Acidic, Salmeron
cereal arum Isoamyl vinegary et al.
drinks NCIMB alcoholb 123-51-3 n.q Pungent (2009)
(malt, barley 8826 2-butanola 78-92-2 n.q Leaves,
and wheat) grasses
2-methoxyet- 3558-60-9 n.q Floral,
chry-hylbenzenebc santhemum
Cereal-mix P. kudri- Tetradecan- 544-63-8 n.q Creamy, Ogunremi slurry (Pearl avzevii oic acid cheesy et al.
millet, red OG32d 9, 12-octadec- 112-63-0 n.q Bland (2015)
sorghum, adienoic acid white sorgh- methyl ester
um, wheat) Benzyl alcohol 100-51-6 n.q Floral, rose
Probiotic L. acido- Acetic acid 64-19-7 n.q Vinegare Lee et al.
coconut philus Diacetyl 431-03-8 n.q Butterye (2013)
L10 2,3-Heptanedione 96-04-8 n.q Butterye
2-Nonanone 821-55-6 n.q Fruitye
Benzaldehyde 100-52-7 n.q Bittere almond
2-Heptanone 110-43-0 n.q Fruitye
2-Heptanol 543-49-7 n.q Citruse 2-Nonanol 628-99-9 n.q Citruse
ä-octalactone 698-76-0 n.q Coconute
ä-dodecalactone 705-86-2 n.q Coconute
L. casei Acetic acid 64-19-7 n.q Vinegare
L26 Diacetyl 431-03-8 n.q Butterye Acetoin 513-86-0 n.q Butterye ä-Decalactone 705-86-2 n.q Coconute 3-Methyl-3- 4516-90-9 n.q Herbe buten-1-ol 1-Octanol 111-87-5 n.q Orangee P-tolualdehyde 104-87-0 n.q Fruitye
Ethyl 2-hydro- 97-64-3 n.q Fruitye
xypropanoate
Linalool 78-70-6 n.q Citrus, florale
aBarley, bMalt, cWheat, dProbiotic yeast cells, eData from http://www.thegoodscentscom pany.com, nq: not quantify
metabolic activity of the B. breve NCIMB 702257. The sensory evaluation exhibited that the fermented malt beverage had a sour flavor. The authors have concluded that volatile acid compounds identified can be appointed as significant character aroma compounds of the novel fermented cereal beverage.
Salmeron et al. (2009) had previously issued a study in the same topic. They have formulated various cereal-based media (oat, barley, wheat and malt) with probiotic strain L. plantarum NCIMB 8826 in order to obtain functional drink. About sixty volatile compounds have been identified after Gas chromatography analysis of each fermented drink. Significant differences have been noticed in the amount of acetic acid (vinegary), isoamyl alcohol (pungent), 2-butanol (leaves, grasses) and 2-methoxyethylbenzene (chrysanthemum) produced. Eventually, this study emphasizes the contribution of probiotic strains in the production of flavor compounds and provides useful information to develop probiotic drinks.
b. Aroma compounds derived from probiotics in cereal-mix slurry
The effect of probiotic yeast strains in quality attributes and functional value on traditional fermented cereal-based food has been proved. Cereal-mix slurry (pearl millet, red sorghum, white sorghum, wheat) has inoculated with P. kudriavzevii OG32 in selected parameters of the fermentation process. Volatile organic compounds generated during the fermentation have been compared with a control. Forty volatile compounds were identified in the fermented product, while acids (32.21%) and esters (32.37%) accounted for the largest proportions (Ogunremi et al., 2015). In particular, levels of 9, 12-octadecadienoic acid (cheesy), benzyl alcohol (floral) and tetradecanoic acid (creamy) acid were increased after fermentation.
c. Aroma compounds derived from probiotics in fermented coconut water
Another novel probiotic beverage has been assessed with coconut water. Lee et al. (2013) have studied the fermentation of coconut water by probiotic strains L. acidophilus L10 and L. casei L26 and have found significant variations between the two cultures in their ability to produce aroma compounds. L. acidophilus L10 generated higher amounts of
2-heptanone (fruity), 2-nonanone (fruity), benzaldehyde (bitter almond), 2-heptanol (citrus), 2-nonanol (citrus), octalactone (coconut) and -dodecalactone (coconut), whereas L. casei L26 produced higher amounts of acetic acid (vinegar), diacetyl (buttery), acetoin (buttery), -decalactone (coconut), 3-methyl-3-buten-1-ol (herb), linalool (citrus), 1-octanol (orange), p-tolualdehyde (fruity) and ethyl 2-hydro-xypropanoate (fruity). Although this notice able qualitative and quantitative differences before and after fermentation, authors emphasize that sensory analysis is required to evaluate the relative contribution of these volatile to the organoleptic characteristics of the fermented coconut water.
To sum up, milk-based substrates are well known and widespread matrices for probiotic foods and beverages. However, fruit juices, vegetables and cereals, constitute an emerging segment of non-dairy matrices for the design of probiotic foods. Several studies presented in this paper have shown the ability of some probiotic strains to produce aroma compounds, and also highlighted their contribution to the overall flavor of functional foods. Nevertheless reported studies are primarily focused in qualitative data of volatile; therefore, the establishment of these substrates will require quantitative data for quality control of aromas and further product development. Sensory activity of the individual components of the probiotic food aromas and the dependence between the odor and the chemical composition of these functional foods will, no doubt, lead to a development of specific gas chromatography methods.
3. PROBIOTIC AROMAS: ANALYTICAL ASPECTS
3.1 Introduction
Flavor perception is one of the most important human factors in consumer’s food preference. It is tightly linked to specific chemical senses of smell and taste which are of great importance in everyday life. As concerns smell, the “odor” perception depends upon volatile substances liberated by rough or elaborated food products. Nowadays, the terms “Aroma” and “Off-flavor” are used to characterize positive or negative smell properties respectively. Aroma stability of foods and drinks – like probiotic preparations – as well as the study of what produces aroma changes are overriding concerns for commercial purposes and research. As aroma components are complex, their analysis needs powerful tools
allowing both separation performances and sensitivity. Indeed in many cases some molecules with high odor threshold are present in extremely low amounts. Thanks to gas chromatography (GC and comprehensive GC x GC) and hyphenated techniques (gas chromatography coupled to mass spectrometry GCMS) probiotic aroma characterization is easily achievable for quality assessment. Nevertheless beside instrumental techniques, sensory analysis broadens the performances of the undertaken instrumental investigations. The data supplied by sensory panels is of great help to guide aroma development as well as shelf-life monitoring. This “hybrid” approach (instrumental and sensory) is mandatory (Goodner and Roussef, 2011). In what follows some methodology aspects of aroma analysis are summarized with special attention to probiotic aroma characterization.
3.2 Techniques of Odor Analysis
The main goal of the “odor” analytical chemist is the qualitative and quantitative characterization of aromas. The first challenge he meets is the selection of sampling procedures which must be relevant and of optimal accuracy. Therefore, the choice of extraction method – adapted to the probiotics of interest – is so crucial that it is the keystone for performant determination. Several criteria have to be taken into account when sampling approaches are considered:
a. The “aroma sample” has to be representative; that is, constituents may not be altered during extraction and overheating of the sample should be strictly limited to avoid matrix modification and by-products formation;
b. Reproducible, rapid and possibly automated (whenever possible) methods have to be preferred;
c. When “equilibrium procedures” (see below, § 3.2.1) are used, extraction parameters like temperature, time, pH, ionic strength and salting out of the medium… have to be firstly optimized and systematically applied in routine analyses;
d. While qualitative profiling (comparative studies) is generally the rule in aroma analysis, quantitative measurements of “key molecules” are also required. For that purpose, the establishment of recoveries of the targeted components, as well as the use of internal standard (allowing quantitation by comparison of GC peak areas), are strongly recommended.
Aroma analysis (AA) involves complementary stepwise procedures which are schematically summarized as follows:
The principles of sampling modes for volatile organic compounds (aroma major constituents) studies are exhaustively described by Bazemore (2011) and important criteria are outlined in a white paper: “The Challenges of Flavour Analysis Comparison and Choices of Extraction Techniques” by Ridgway K. (https://www.rssl.com/~/ media/rssl/en/files/documents/white-paper/challenges-of-flavour-analysis.pdf). They can be broadly divided into three main categories: the distillation methods, the equilibrium methods and “purge and trap” (P&T) techniques (both descriptions and some typical examples in the field of probiotics are given below).
a. Sampling of volatile constituents of aromas
Distillation methods
Combined steam distillation-extraction (SDE) allows isolation of volatiles while gentle heating the matrix for a fixed time. This method can lead to some artefacts therefore a modified version named “Solvent Assisted Flavor Evaporation” (SAFE) which combines: vacuum distillation, cold trapping with liquid nitrogen and possibly solvent extraction has been developed by Engel et al. (1999). This technique keeps sample at low temperature that minimizes alteration processes and avoid artefacts. In the field of probiotics “classical” SDE has been used to extensively investigate cheese (Hong-Xin et al., 2015), industrial culture medium (Ono et al., 2015) and white salsify (Tragopogon porrifolius L.) probiotics (Riu-Aumatell et al., 2011).
Equilibrium methods (head space sampling)
Head space analysis is of special interest for the investigation of volatile compounds liberated by complex matrices and need a reproducible
partition between sample and gaseous phase. Volatile products can be sampled directly (Static Head Space, HS) or after ad(b)sorption on selected polymer phases (Solid Phase MicroExtraction, SPME or Stir Bar Sorptive Extraction, SBSE, See also Goodner and Rouseff, 2011 for general application rules). HS has been applied to the determination of the aroma impact compounds of yogurt flavor (Ott et al., 1997).
SPME has been developed in the early 1990 by Pawliszyn and co-workers. This solvent-free technique is widely used for volatile profiling of food and environmental samples. Due to its flexibility, its broad range of applications and its easy handling, the technique has a widespread acceptance in flavor analytical chemistry. Moreover, in some cases, with SPME, limits of detection approaching the nanogram level can be reached. For long time, the technique has been considerably improved for many and diversified applications see Spietelun et al., 2013) and Ouyang and Jiang, (2016).
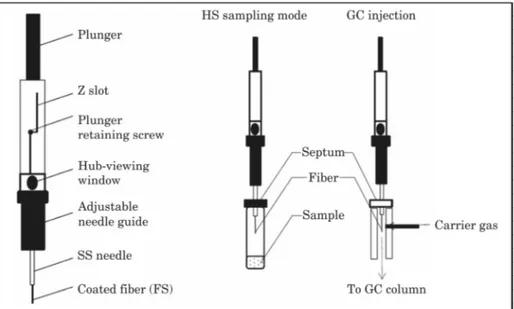
SPME design is shown in Fig. 1 whereas the principle of sampling (SPME diagrams) is presented in Fig. 2. The latter is simple and can be briefly presented as follows:
1. The fused silica coated fiber is exposed to the gaseous phase in equilibrium with the probiotic sample in a sealed vial
2. Volatile compound partition between gas phase and the fiber polymer coating is established during optimized sampling time and temperature afterward the fiber is withdrawn and removed into the stainless steel needle
3. Finally the needle is inserted into the GC injection port and the fiber is exposed to rapid thermodesorption leading to the transfer of the analytes to the GC column.
Fiber coating nature and dimensions, as well as intrinsic molecular properties (nature, volatility, etc.), are important factors that influence the interactions. Before (manual or automated) routine analyses, preliminary trials are required to optimize analytical conditions because phase-retention kinetics are strongly influenced by several factors like T°, sampling time, pH, salt effect and so on (Pawliszyn, 1999, 2011; Marsili, 2011, Lin et al., 2007). These particular aspects and developments of SPME have been extensively reviewed by Vas and Vékey, (2004); Merkle et al. (2015), Jelen et al. (2012); Heaven and Nash (2012); Ouyang and Jiang, (2016). This easy-to-use technique leads to reproducible aroma-profiles nevertheless robust quantification requires careful calibration and method validation (Ouyang and Pawliszyn, 2008; Begnaud and Chaintreau, 2015).
SPME automation is easily undertaken and allows optimal sample throughput. Moreover the use of cooled septum free injection port with narrow –bore inlet liners lead to well resolved chromatographic peaks with good shapes. In order to speed-up the micro-extraction process, the samples can be thoroughly agitated with small magnetic bars or salted at controlled temperature. Head space SPME sampling conditions limit analyte breakdown and therefore warrants sample representativeness.
Purge and Trap (P&T) extraction
P&T is a dynamic process in which volatile organic compounds are continuously displaced from the matrix (purge) or swept from the head space by a continuous flow of inert gas like Helium. The flow passes through a selected trap which adsorbs the molecules of interest which are then desorbed and introduced into a GC column. Cryocooling allows
condensation of very volatile molecules. Typical example of P&T has been described for the study of yogurt flavor by Ott et al., 1997.
b. Chromatographic techniques and molecular identification
Capillary GC is far the most employed technique for aroma analysis. It is easily hyphenated with mass spectrometry (GCMS). The separated molecules are identified on the basis of their typical mass fragmentations in the electron impact mode that are compared with computed data bases (NIST, WILEY, etc.). The identifications are confirmed by comparing their linear retention indices obtained under thermal gradient conditions with those of pure references or from the literature (Goodner and Rouseff, 2011).
c. Gas chromatography/olfactometry (GC/O) and sensory analysis
According to Delahunty et al. (2006) “GC/O refers to the use of human assessors as a sensible and selective detector for odor-active compounds”. In GC/O this odor-activity of the molecules separated by capillary GC and splitted to a “sniffing-port” are noted and described by human selected and trained “sniffers”. Their role is to identify and characterize (with typical sensory vocabulary) potent aroma constituents. GC/O also allows off-flavor detection in food products. Different approaches underpin GC/O analysis: dilution analysis, detection frequency, time intensity, etc. They are described in details in Goodner and Rouseff (2011) (see this reference for further details). The method has been reviewed extensively (Brattoli et al. 2013; Van Ruth, 2001). Finally, several studies focusing on GC/O are reported within the literature for dairy products (Friederich et al., 1998), fermented sausages (Olivares et al., 2015; Marco et al., 2007) and fruits (Hongley et al., 2014). Beside instrumental techniques a lot of sensory analysis protocols have been described. They are not taken into account in the present overview. Nevertheless, very interesting and systematic descriptions can also be found in Goodner and Rouseff (2011), Borsa et al. (2015), Karimi et al. (2012), Cruz et al. (2010).
d. Literature survey
Among the various available methods and according to the three last decades of scientific literature, SDE and headspace methods have been
used for probiotic flavor analysis. Nevertheless as part of the different head space configuration modes, SPME is the most frequently reported for probiotic aroma profiling. In what follows, typical applications are given.
3.3 Probiotic Aroma Analysis – Some Indicative Examples
To the Authors best knowledge and according to an advanced bibliographic insight, it is confirmed that the majority of the published papers report SPME as sampling procedure for volatile organic compounds. The survey indicates SPME-GCMS as a very powerful method employed for qualifying probiotics. Indeed this could be linked to some observations coming also from the author’s experience:
(a) The main drawbacks of simultaneous distillation-extraction (SDE) methods are the potential poor recovery of very volatile components as well as the disappearance of thermolabile molecules associated with possible artefact formation. When solvent evaporation is required, risk of losses of more or less compounds has to be suspected. It seems therefore that these methods are hardly used anymore in recent investigations;
(b) When carefully validated, HS-SPME-GCMS is a fast and reproducible (automated) approach of volatile compound determination even for the detection and quantification of minor compounds in traces.
Typical very indicative applications (studies) related to probiotic aroma are gathered in Table 4. They show the versatility and the flexibility of the HS-SPME-GCMS approaches for the study of complex matrices like dairy products, fermented sausages and other plant products. The method has been applied to the careful sampling of a lot of different compounds of various chemical classes (for some significant examples see related aroma contributing molecules derived from probiotics in Tables 1 to 3)
3.4 Concluding Remark Over Probiotic Characterization
As shown herein, nowadays, the analysis of probiotic assisted production of aroma is mainly guided by the development of head space sampling protocols with GCMS as identification-quantification tool. Nevertheless, other less frequently reported techniques like Proton-Transfer Reaction
Table 4: Typical examples of probiotic aroma analyses by HS-SPME (2007-2017)
Topics Authors
Probiotic dairy products
• Fermented dairy beverage Kefir Walsch et al., 2016 • Probiotic viability yogurts produced with wheat bran Terpou et al., 2017
• Probiotic bacterium in yogurt Settachaimongkon et al., 2014 • Plant derived lactococci-milk products Alemayehu et al., 2014 • Caprine Coalho Cheese aromas Bezzera et al., 2016 • Volatile flavors from yogurt Cheng, 2010
Sausages & meat products - probiotics
• Dry fermented sausages produced with probiotics Sidira et al., 2013 • Effect of L.casei on probiotic fermented sausages Sidira et al., 2015 • Probiotic cultures, effect on dry fermented sausages Sidira et al., 2016
• Enterococci- Slightly fermented sausages Latorre-Moratella et al., 2011 • Effect of mixed probiotics starter on fermented hams Kim et al., 2016
• Volatiles from slow dry fermented sausage Cano-Garcia et al., 2013 • Key aromas in Traditional fermented sausages Corral et al., 2014 • Odor active compounds of fermented sausages Marco et al., 2007
Other probiotic foods from plant products
• Lactobacillus plantarum in cereal based Salmeron et al., 200 substrates (aroma)
• Volatiles of sourdough breads Plessas et al., 2008 • Aromas from white salsify (Tragopogon porrifolius L.) Riu-Aumatell et al., 2011 • LAB starters – aroma from tomato juices Di Cagno et al., 2009
Mass Spectrometry (PTR-MS) and electronic nose technology (which implies odor “perception” by gas sensors) have undergone significant improvements for flavor characterization (Gallardo-Escamilla et al., 2005). Beside these two complementary techniques, and due to its higher peak capacity than mono-dimensional gas chromatography modern “omic” approach like GC x GC (using two columns of different chemistries – Principle of orthogonality) offers very powerful way to deliver comprehensive fingerprints of probiotic food aromas (Cordero et al., 2015; Gasior and Wojtyczak, 2016). Nevertheless the descriptive aroma evaluation requires human nose for a continuous improvement of the reliability of the information. Instrumental and sensory analyses are therefore strictly compatible and complementary.
REFERENCES
Alamprese, C., Foschino, R., Rossi, M., Pompei, C. and Savani, L. (2002). Survival of Lactobacillus johnsonii La1 and inûuence of its addition in retail-manufactured ice cream produced with different sugar and fat concentrations. Int Dairy J., 12: 201–8.
Alemayehu, D., Hannon, J.A., McAuliffe, O. and Ross, R.P. (2014). Characterization of plant-derived lactococci on the basis of their volatile compounds profile when grown in milk. Int J Food Microbiol., 172(Supplement C): 57–61.
Ammor, M.S. and Mayo, B. (2007). Selection criteria for lactic acid bacteria to be used as functional starter cultures in dry sausage production: An update. Meat
Sci., 76(1): 138–46.
Andreas Ott, L.B.F. and Alain Chaintreau (1997). Determination and origin of the aroma impact compounds of yogurt flavor. J Agric Food Chem., 45: 850–8. Arihara, K., Ohata, M. (2010). Func Meat Prod., pp. 423–39.
Aurora Marco Jln and Monica Flores (2007). Quantitation of selected odor active constituents in dry. J Agric Food Chem., 55: 3058–306.
Bazemore, R. (2011). Sample Preparation. Practical Analysis of Flavor and Fragrance Materials: John Wiley & Sons, Ltd; pp. 23–44.
Begnaud, F. and Chaintreau, A. (2016). Good quantification practices of flavours and fragrances by mass spectrometry. Philos T R Soc A., 374(2079).
Beshkova, D.M., Simova, E.D., Frengova, G.I., Simov, Z.I. and Dimitrov, Z.P. (2003). Production of volatile aroma compounds by kefir starter cultures. Int Dairy J., 13(7): 529–35.
Bezerra, T.K.A., Araujo, A.R.R., Arcanjo, N.M.D.O., Silva, F.L.H.D., Queiroga, R.D.C.R.D.E. and Madruga, M.S. (2016). Optimization of the HS-SPME-GC/ MS technique for the analysis of volatile compounds in caprine Coalho cheese using response surface methodology. Food Sci Tech., (Campinas). 36(1): 103–10.
Borºa, A., Muste, S., Vodnar, D.C., Salanþã, L.C. and Cuibus, L. (2015). Sensory analysis of a new generation of probiotic drinks with functional gastrointestinal health impact. Bull Univ Agr Sci Vet Med Cluj-Napoca Food Sci Tech., 72(2). Brattoli, M., Cisternino, E., Dambruoso, P.R., de Gennaro, G., Giungato, P., Mazzone,
A. et al. (2013). Gas chromatography analysis with olfactometric detection (GC-O) as a useful methodology for chemical characterization of odorous compounds.
Sensors, 13(12): 16759–800.
Breslin, P.A.S. (2001). Human gustation and flavour. Flavour Frag J., 16(6): 439–56.
Cano-García, L., Belloch, C. and Flores, M. (2014). Impact of Debaryomyces hansenii strains inoculation on the quality of slow dry-cured fermented sausages. Meat
Sci., 96(4): 1469–77.
Carole Tournier, C.S. and Elisabeth Guichard (2000). Flavour Perception Aroma Taste and Texture Interactions. Food (C) 2007 Global Science Books. 2: 246–57. Cheng, H. (2010). Volatile flavor compounds in yogurt: A review. Crit Rev Food Sci.,
50(10): 938–50.
Cheung, T.T., Junghans, A.F., Dijksterhuis, G.B., Kroese, F., Johansson, P., Hall, L.
et al. (2016). Consumers’ choice-blindness to ingredient information. Appetite,
106: 2–12.
Cordero, C., Kiefl, J., Schieberle, P., Reichenbach, S.E. and Bicchi, C. (2015). Comprehensive two-dimensional gas chromatography and food sensory properties: Potential and challenges. Anal Bioanal Chem., 407(1): 169–91. Corral, S., Salvador, A. and Flores, M. (2015). Elucidation of key aroma compounds
in traditional dry fermented sausages using different extraction techniques. J
Cruz, A.G., Antunes, A.E.C., Sousa, A.L.O.P., Faria, J.A.F. and Saad, S.M.I. (2009). Ice-cream as a probiotic food carrier. Food Res Int., 42(9): 1233–9.
Cruz, A.G., Cadena, R.S., Walter, E.H.M., Mortazavian, A.M., Granato, D., Faria, J.A.F. et al. (2010). Sensory Analysis: Relevance for prebiotic, probiotic, and synbiotic product development. Compr Rev Food Sci F., 9(4): 358–73.
Beshkova, D., Simova, E., Frengova, G. and Simov, Z. (1998). Production of flavour compounds by yogurt starter cultures. J Ind Microbiol Biot., (20).
De Oliveira, A.M. Leite, M.A.L.M., Peixoto, R.S., Rosado, A.S., Silva, J.T. and Flosi Paschoalin, V.M. (2013). Microbiological technological and therapeutic properties of kefir. Braz J Microbiol., 44(2): 341–9
Delahunty, C.M., Eyres, G. and Dufour, J.-P. (2006). Gas chromatography olfactometry. J Sep Sci., 29(14): 2107–25.
Delgado, F.J., Gonzalez-Crespo, J., Cava, R. and Ramirez, R. (2011). Formation of the aroma of a raw goat milk cheese during maturation analysed by SPME-GC-MS. Food Chem., 129(3): 1156–63.
Di Cagno, R., Surico, R.F., Paradiso, A., De Angelis, M., Salmon, J.-C., Buchin, S. et
al. (2009). Effect of autochthonous lactic acid bacteria starters on
health-promoting and sensory properties of tomato juices. Int J Food Microbiol., 128(3): 473–83.
Simova, E., Beshkova, D., Angelov, A., Hristozova, T.S., Frengova, G. and Spasov, Z. (2002). Lactic acid bacteria and yeasts in kefir grains and kefir made from them. J Ind Microbiol Biot., 28: 1–6.43.
Engel, W., Bahr, W. and Schieberle, P. (1999). Solvent assisted flavour evaporation – A new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur Food Res Technol., 209(3): 237–41. FAO/WHO (2002). Guidelines for the Evaluation of Probiotics.
Flores, M., Dura, M.A., Marco, A. and Toldra, F. (2004). Effect of Debaryomyces spp. on aroma formation and sensory quality of dry-fermented sausages. Meat Sci., 68(3): 439–46.
Friedrich, J.E. and Acree, T.E. (1998). Gas chromatography olfactometry (GC/O) of dairy products. Int Dairy J., 8(3): 235–41.
Gallardo-Escamilla, F.J., Kelly, A.L. and Delahunty, C.M. (2005). Sensory characteristics and related volatile flavor compound profiles of different types of whey. J Dairy Sci., 88(8): 2689–99.
Gangfeng Ouyang and Jiang, R. (2017). Solid Phase Microextraction Recent Developments and Applications. p. 313.
G¹sior, R. and Wojtycza, K. (2016). Sense of smell and volatile aroma compounds and their role in the evaluation of the quality of products of animal origin – A review. Ann Anim Sci., p. 3.
Gardiner Gillian, E.R.P.R., Wallace Jean, M., Scanlan Francis, P., Jagers Paul, P.J.M., Fitzgerald Gerald, F., Kevin Collins, J. and Catherine Stanton (1999). Influence of a probiotic adjunct culture of Enterococcus faecium on the quality of cheddar cheese. J Agric Food Chem., 47: 4907–16.
Gomez-Torres, N., Avila, M., Delgado, D. and Garde, S. (2016). Effect of reuterin-producing Lactobacillus reuteri coupled with glycerol on the volatile fraction, odour and aroma of semi-hard ewe milk cheese. Int J Food Microbiol., 232: 103–10.
Granato, D., Branco, G.F., Cruz, A.G., Faria, J.D.A.F. and Shah, N.P. (2010). Probiotic dairy products as functional foods. Compr Rev Food Sci F., 9(5): 455–70. Helland, M.H., Wicklund, T. and Narvhus, J.A. (2004). Growth and metabolism of
selected strains of probiotic bacteria in milk- and water-based cereal puddings.
Int Dairy J., 14(11): 957–65.
Hong-Xin, J., Mi-Ya, S. and Guang-Yu, G. (2015). Influence of Lactobacillus casei LC2W on the proteolysis and aroma compounds of Cheddar cheese during ripening period. CyTA - J Food., 13(3): 464–71.
Innocente, N., Biasutti, M., Rita, F., Brichese, R., Comi, G. and Iacumin, L. (2016). Effect of indigenous Lactobacillusrhamnosus isolated from bovine milk on microbiological characteristics and aromatic profile of traditional yogurt. LWT
- Food Sci Tech., 66: 158–64.
Insights, G.M. (2016). Probiotics Market Size By End Use (Human, Animal), By Application (Functional Foods & Beverages [Dairy, Non-dairy, Cereals, Baked Goods, Fermented Meat Products, Dry Foods], Dietary Supplements [Food, Nutritional, pecialty, Infant Formula], Animal Feed Probiotics), Industry Analysis Report, Regional Outlook (U.S., Germany, UK, China, Japan, India, Brazil), Application Potential, Price Trends, Competitive Market Share & Forecast, pp. 2016–2023.
Accolas J.P.D.H., Desmazaud D.M.J., Vassal, L., Bouillanne, C. and Veaux, M. (1980). Les levains. Le LaitINRA, pp. 487–524.
Jankovic, I., Sybesma, W., Phothirath, P., Ananta, E. and Mercenier, A. (2010). Application of probiotics in food products–challenges and new approaches. Curr
Opin Biotech.
Karimi, R., Sohrabvandi, S. and Mortazavian, A.M. (2012). Review Article: Sensory Characteristics of Probiotic Cheese. Compr Rev Food Sci F., 11(5): 437–52. Kim, Y.J., Park, S.Y., Lee, H.C., Yoo, S.S., Oh, S., Kim, K.H. et al. (2016). Evaluation
of mixed probiotic starter cultures isolated from kimchi on physicochemical and functional properties, and volatile compounds of fermented hams. Korean
J Food Sci An., 36(1): 122–30.
Latorre-Moratalla, M.L., Bover-Cid, S., Veciana-Nogués, M.T. and Vidal-Carou, M.C. (2012). Control of Biogenic Amines in Fermented Sausages: Role of Starter Cultures. Front Microbiol., 3: 169.
Lee, P.-R., Boo, C.X. and Liu, S.-Q. (2013). Fermentation of coconut water by probiotic strains Lactobacillus acidophilus L10 and Lactobacillus casei L26. Ann
Microbiol., 63(4): 1441–50.
Leroy, F., Verluyten, J. and De Vuyst, L. (2006). Functional meat starter cultures for improved sausage fermentation. Int J Food Microbiol., 106(3): 270–85. Lin, L.-Y., Peng, C.-H., Wang, H.-E., Wu, T.-H., Chen, C.-C., Yu, T.-H. et al. (2007).
Factors affecting solid phase microextraction (SPME) to concentrate the odorants of Chinese white salted noodles for GC–MS analysis. Flavour Frag J., 22(4): 274–9.
Sakho, D.C.M., Jaus, A., Chiarazzo, E. and Crouzet, J. (1998). Enzymatic maceration: Effects on volatile components of Mango Pulp. J Food Sci., 63(6): 975–8. Marsili, R. (2011). Analysis of musty microbial metabolites by stir bar sorptive
extraction. Flavor, Fragrance, and Odor Analysis, Second Edition: CRC Press. pp. 63–92.
Matar, C., Amiot, J., Savoie, L. and Goulet, J. (1996). The Effect of milk fermentation by Lactobacillus helveticus. J Dairy Sci., 79.
Mater, D.D., Bretigny, L., Firmesse, O., Flores, M.J., Mogenet, A., Bresson, J.L. et al. (2005). Streptococcus thermophilus and Lactobacillus delbrueckii subsp.
bulgaricus survive gastrointestinal transit of healthy volunteers consuming
yogurt. Fems Microbiol Lett., 250(2): 185–7.
McGorrin, R.J. (2002). Character Impact Compounds: Flavors and Off-Flavors in Foods. by Marcel Dekker, Inc.
Merkle, S., Kleeberg, K. and Fritsche, J. (2015). Recent developments and applications of solid phase microextraction (SPME) in food and environmental analysis—A Review. Chromatogr., 2(3): 293–381.
Milesi, M.M., Wolf, I.V., Bergamini, C.V. and Hynes, E.R. (2010). Two strains of nonstarter Lactobacilli increased the production of flavor compounds in soft cheeses. J Dairy Sci., 93(11): 5020–31.
Mirjana Hruškar, N.V.I.M.R. (1995). Aroma profiles and sensory evaluation of yogurt during storage. Mljekarstvo Milk and Dairy product, 45(3): 175–90. Mishra, S. and Mishra, H.N. (2013). Technological aspects of probiotic functional
food development. Nutrafoods., 11(4): 117–30.
Moussa, M.E., Salem, F.A.F. and Awad, R.A. (2005). Production of probiotic ice cream. Pol J Food Nutr Sci., 14(3): 267–71.
Nilsson, D. (2008). Metabolically engineered lactic acid bacteria and their use. Patent number US 7,465,575 B2.
Ogunremi, O.R., Agrawal, R. and Sanni, A.I. (2015). Development of cereal-based functional food using cerealmix substrate fermented with probiotic strain -Pichia kudriavzevii OG32. Food SciNut., 3(6): 486–94.
Ono, T., Usami, A., Nakaya, S., Maeba, K., Yonejima, Y., Toyoda, M. et al. (2015). Chemical compositions and aroma evaluation of volatile oil from the industrial cultivation medium of Enterococcus faecalis. J Oleo Sci., 64(10): 1125–33. Ouyang, G. and Pawliszyn, J. (2008). A critical review in calibration methods for
solid-phase microextraction. Anal Chim Acta., 627(2): 184–97.
Özer, B., Kirmaci, H.A., ªenel, E., Atamer, M. and Hayaloðlu, A. (2009). Improving the viability of Bifidobacterium bifidum BB-12 and Lactobacillus acidophilus LA-5 in white-brined cheese by microencapsulation. Int Dairy J., 19(1): 22–9. Parvez, S., Malik, K.A., Ah Kang, S. and Kim, H.Y. (2006). Probiotics and their
fermented food products are beneficial for health. J Appl Microbiol., 100(6): 1171–85.
Paul, L.H. and McSweeney, M.JS. (2000). Biochemical pathways for the production of flavour. Lait INRA, EDP Sciences. 80: 293–324.
Pawliszyn, J. (2011). Development of SPME Devices and Coatings. Handbook of Solid Phase Microextraction. Oxford: Elsevier; pp. 61–97.
Phillips, M., Kailasapathy, K. and Tran, L. (2006). Viability of commercial probiotic cultures (L. acidophilus, Bifidobacterium sp., L. casei, L. paracasei and L.
rhamnosus) in cheddar cheese. Int J Food Microbiol., 108(2): 276–80.
Plessas, S., Fisher, A., Koureta, K., Psarianos, C., Nigam, P. and Koutinas, A.A. (2008). Application of Kluyveromyces marxianus, Lactobacillus delbrueckii ssp.
bulgaricus and L. helveticus for sourdough bread making. Food Chem., 106(3):
Ramaswamy, H.S. and Basak, S. (1992). Pectin and Raspberry Concentrate Effects on the Rheology of Stirred Commercial Yogurt. J Food Sci., 57(2): 357–60. Riu-Aumatell, M., Vargas, L., Vichi, S., Guadayol, J.M., López-Tamames, E. and
Buxaderas, S. (2011). Characterisation of volatile composition of white salsify (Tragopogon porrifolius L.) by headspace solid-phase microextraction (HS-SPME) and simultaneous distillation–extraction (SDE) coupled to GC–MS. Food Chem., 129(2): 557–64.
Rouself, K.G.R. (2011). Index. Practical Analysis of Flavor and Fragrance Materials: John Wiley & Sons, Ltd; pp. 223–8.
Routray, W. and Mishra, H.N. (2011). Scientific and technical aspects of yogurt aroma and taste: A Review. Compr Rev Food Sci F., 10(4): 208–20.
Ruiz-Moyano, S., Martin, A., Benito, M.J., Aranda, E., Casquete, R. and Cordoba Mde, G. (2011). Implantation ability of the potential probiotic strain,
Lactobacillus reuteri PL519, in “Salchichon,” a traditional Iberian dry fermented
sausage. J Food Sci., 76(5): M268–75.
Ruth, S.M.V. (2001). Methods for gas chromatography-olfactometry a review. Biomol
Eng., 121–8.
Saarela, M.G., Fondén, M., Mätto, F. and Janna. Mattila-Sandholm, T. (2000). Probiotic bacteria safety functional and technological. J Biotechnol, 84. Epub 215.
Salmeron, I., Fuciños, P., Charalampopoulos, D. and Pandiella, S.S. (2009). Volatile compounds produced by the probiotic strain Lactobacillus plantarum NCIMB 8826 in cereal-based substrates. Food Chem., 117(2): 265–71.
Salmeron, I., Rozada, R., Thomas, K., Ortega-Rivas, E. and Pandiella, S.S. (2014). Sensory characteristics and volatile composition of a cereal beverage fermented with Bifidobacterium breve NCIMB 702257. Food Sci Technol Int= Ciencia y
Tecnologia de los Alimentos Internacional, 20(3): 205–13.
Schwab, W., Davidovich-Rikanati, R. and Lewinsohn, E. (). Biosynthesis of plant-derived flavor compounds. The Plant Journal: for Cell and Molecular Biology, 54(4): 712–32.
Settachaimongkon, S., Nout, M.J.R., Antunes Fernandes, E.C., van Hooijdonk, T.C.M., Zwietering, M.H., Smid, E.J. et al. (2014). The impact of selected strains of probiotic bacteria on metabolite formation in set yoghurt. Int Dairy J., 38(1): 1–10.
Sidira, M., Kandylis, P., Kanellaki, M. and Kourkoutas, Y. (2016). Effect of curing salts and probiotic cultures on the evolution of flavor compounds in dry-fermented sausages during ripening. Food Chem., 201(Supplement C): 334–8. Sidira, M., Kandylis, P., Kanellaki, M. and Kourkoutas, Y. (2015). Effect of immobilized Lactobacillus casei on volatile compounds of heat treated probiotic dry-fermented sausages. Food Chem., 178: 201–7.
Sidira, M., Kanellaki, M. and Kourkoutas, Y. (2013). Profile of aroma-related volatile compounds isolated from probiotic dry-fermented sausages produced with free or immobilized L. casei using SPME GC/MS Analysis. Nutrition, Functional and
Sensory Properties of Foods: The Royal Society of Chemistry, pp. 135–47.
Spietelun, A., Kloskowski, A., Chrzanowski, W. and Namiesnik, J. (2013). Understanding solid-phase microextraction: Key factors influencing the
extraction process and trends in improving the technique. Chem Rev., 113(3): 1667–85.
Tamime, R.K.R.A.Y. (2007). Biochemistry of fermentation. Yoghurt SciTech., pp. 535–607.
Terpou, A., Bekatorou, A., Kanellaki, M., Koutinas, A.A. and Nigam, P. (2017). Enhanced probiotic viability and aromatic profile of yogurts produced using wheat bran (Triticum aestivum) as cell immobilization carrier. Process Biochem., 55(Supplement C): 1–10.
Tian, H., Zhan, P., Deng, Z., Yan, H. and Zhu, X. (2014). Development of a flavour fingerprint by GC-MS and GC-O combined with chemometric methods for the quality control of Korla pear (Pyrus serotina Reld). Int J Food Sci Tech., 49(12): 2546–52.
Bottazzi, V.B.B. and Montescani, G. (1973). Influence des souches seules et associ ìees de L. bulgaricus. INRA Editions. 53(525–526): 295.
Vas, G. and Vekey, K. (2004). Solid-phase microextraction: A powerful sample preparation tool prior to mass spectrometric analysis. J Mass Spectrom., 39(3): 233–54.
Vijaya Kumar, B., Vijayendra, S.V. and Reddy, O.V. (2015). Trends in dairy and non-dairy probiotic products - A review. J Food Sci Tech., 52(10): 6112–24. Walsh, A.M., Crispie, F., Kilcawley, K., O’Sullivan, O., O’Sullivan, M.G., Claesson,
M.J. et al. (2016). Microbial succession and flavor production in the fermented dairy Beverage Kefir. mSystems, 1(5).
Wang, Y., Qian, M. and Burbank, H. (2006). Preseparation techniques in aroma analysis. Sensory-Directed Flavor Analysis. Food Sci Technol: CRC Press; pp. 111–54.
Weerawatanakorn, M., Wu, J.-C., Pan, M.-H. and Ho, C.-T. (2015). Reactivity and stability of selected flavor compounds. J Food Drug Anal., 23(2): 176–90. Winterhalter, P. and Skouroumounis, G.K. (1997). Glycoconjugated aroma
compounds: Occurrence, role and biotechnological transformation. In: Berger, R.G., Babel, W., Blanch, H.W., Cooney, C.L., Enfors, S.O., Eriksson, K.E.L. et al. (ed.), Biotechnology of Aroma Compounds. Berlin, Heidelberg: Springer Berlin Heidelberg; pp. 73–105.
Güzel-Seydim, Z.B.A.C.S., Greene, A.K. and Bodine, A.B. (2000). Determination of organic acids and volatile flavor substances. J Food Compos Anal., 13(1): 35– 43.
Zhang, J., Yang, Z., Yang, Y., Han, L., Yu, Q., Cao, H. et al. (2017). Development of a flavor fingerprint by gc-ms with chemometric method for volatile compounds of yak and yellow cattle bone soup. Food Anal Methods., 10(4): 943–54.