Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus
COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company's public news and information website.
Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre - including this
research content - immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means
with acknowledgement of the original source. These permissions are granted for free by Elsevier for as long as the COVID-19 resource centre
Journal Pre-proof
Diffuse alveolar hemorrhage in infants: Report of five cases
E. Gkogkou, I. Broux, C. Kempeneers, H. Boboli, R. Viellevoye, A. Janssen, M.-C. Seghaye, M. Mastouri
PII: S2213-0071(20)30113-1
DOI: https://doi.org/10.1016/j.rmcr.2020.101121
Reference: RMCR 101121
To appear in: Respiratory Medicine Case Reports
Received Date: 6 April 2020 Revised Date: 6 June 2020 Accepted Date: 7 June 2020
Please cite this article as: Gkogkou E, Broux I, Kempeneers C, Boboli H, Viellevoye R, Janssen A, Seghaye M-C, Mastouri M, Diffuse alveolar hemorrhage in infants: Report of five cases, Respiratory
Medicine Case Reports (2020), doi: https://doi.org/10.1016/j.rmcr.2020.101121.
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Diffuse Alveolar Hemorrhage in Infants: Report of Five Cases 1
2
Gkogkou E 1, Broux I2 , Kempeneers C1, Boboli H1, Viellevoye R2, Janssen A1, 3 Seghaye M-C1 , Mastouri M1 4 5 Authors: 6 1
Department of Pediatrics, University Hospital of Liege, Belgium 7
2
Department of Neonatology, University Hospital of Liege, Belgium 8
9
Corresponding Author: 10
Gkogkou E , Department of Pediatrics, University Hospital of Liege, Belgium. 11 Email: Efthymia.Gkogkou@chrcitadelle.be 12 Tel: 00 32 472097297 13 14 Abstract: 15
Diffuse alveolar hemorrhage (DAH) is a rare life-threatening condition in 16
children. In this entity, the bleeding originates from the pulmonary 17
microvasculature as a result of microvascular damage leading to blood leakage 18
into the alveolar spaces. DAH can occur as an isolated medical entity or may be 19
associated with other organ system injury or dysfunction. The classic triad of 20
symptoms includes hemoptysis, anemia and diffuse pulmonary infiltrates. 21
Hemoptysis is the usual presenting symptom but is not constant. A variety of 22
diseases is associated with the development of DAH. Current classification 23
organize the etiologies of diffuse alveolar hemorrhage based on the presence of 24
severe immune disorders (such as systemic vasculitis and collagenosis) or non-25
immunodeficiency disorders (with an identified cardiac or non-cardiac origin, or 26
idiopathic). 27
The five cases of DAH presented in this study were all diagnosed in full-term 28
infants, four males and one female, with normal neonatal adaptation and without 29
family history of notable diseases. In all cases the diagnosis was made between 30
the age of three and eighteen weeks-old. Moreover, all five patients, at the time 31
of diagnosis, presented with hemoptysis, mild or severe dyspnea, anemia and 32
abnormal chest X-rays. Consequently, the diagnosis of DAH was strongly 33
suspected and, eventually, confirmed by bronchoscopy. Additional laboratory 34
tests, as well as selected serologic and radiographic studies were performed in 35
order to identify a specific etiology. The final diagnoses reflect a variety of 36
causes: infections, idiopathic pulmonary hemosiderosis, accidental suffocation 37
and Heiner syndrome. Treatment included oral corticosteroids except from one 38
patient that received antimicrobial therapy. 39
Keywords: 40
Diffuse Alveolar Hemorrhage, Bronchoscopy, BAL, Golde Score, Hemoptysis, 41 Anemia. 42 43 Background: 44
DAH is a rare but potentially life-threatening condition in infants. In this entity, 45
the bleeding originates from the pulmonary microvasculature (pulmonary 46
arterioles, alveolar capillaries, and pulmonary venules) as a result of 47
microvascular damage leading to blood leakage into the alveolar spaces [19, 23]. 48
Due to the lack of reported cases and cohorts described in the literature, the 49
epidemiology and the incidence of the different causes of DAH in pediatric 50
population remain imprecise. 51
A variety of diseases is associated with the development of the DAH. Current 52
classification schemes organize the etiologies of DAH according to the 53
association with severe immune disorders (such as systemic vasculitis and 54
collagenosis [19]), the association with non-immune disorders, which may be of 55
cardiac or non-cardiac origin, or idiopathic disorders [1,2 ]. In children, the most 56
frequent non-immune causes of DAH are infections [22] and cardiovascular 57
diseases. 58
Interestingly, a plethora of cases of DAH in children have been identified as 59
idiopathic pulmonary hemosiderosis (IPH). IPH is a diagnosis of exclusion, and 60
its pathogenesis remains controversial [10, 23]. Various hypotheses have been 61
proposed to explain the pathophysiology of IPH; allergic, environmental, 62
genetic and autoimmune [9, 10]. The allergic theory is based on the frequent 63
association between IPH and cow's milk hypersensitivity (Heiner syndrome). 64
Published data associating pulmonary hemosiderosis with the exposure to a 65
toxigenic fungus provides some evidence that environmental factors may play a 66
role in DAH [11]. IPH has also been described in a small number of familial 67
cases, leading to the discussion of a genetic theory; however, no gene has been 68
identified yet [12]. Finally, considering the frequent association with 69
autoimmune diseases, the autoimmune theory is recognized as the most 70
probable. It is important to mention that alveolar hemorrhage may be the first 71
manifestation occurring well before (months to a year) the development of an 72
immunological disorders [14, 19,24]. Table 1 demonstrates the current 73
classification scheme for the causes of DAH. 74
75
Table 1: Classification of Diffuse Alveolar Hemorrhage in Young Infants 76
Classification Disorders 77
Immune Disorders
78 Idiopathic pulmonary capillaritis
(Not Common) 79 80 81 82 83
Non-immune Disorders (More Common) 84 Non-cardiovascular Origins: 85 86 87 88 89 Cardiovascular Causes: 90 91 92 93
Clinical manifestations, laboratory findings and imaging: 94
The clinical presentation of DAH can vary from acute respiratory distress 95
syndrome to a more insidious presentation with minimal symptoms such as 96
cough. The classic triad of symptoms includes hemoptysis, anemia and diffuse 97
Idiopathic pulmonary hemosiderosis Heiner syndrome
Celiac disease (Lane-Hamilton syndrome) Infections
Coagulation disorders Infanticide
Drugs and toxines
Mitral stenosis
Pulmonary veno-occlusive disease Arteriovenous malformations Pulmonary hypertension
Pulmonary capillary hemangiomatosis Chronic right heart failure
pulmonary infiltrates [21]. Hemoptysis is the usual presenting symptom, but is 98
not constant, as young children may not expectorate [2, 10]. 99
Diagnostic approach: 100
The most useful investigation to confirm the diagnosis of DAH consists of 101
bronchial fibroscopy and BAL [1, 21]. Bronchoscopy is the most direct way to 102
evaluate hemoptysis and determine the site of bleeding (if there is an active 103
bleeding) or another obvious cause, such as inhalation of a foreign body. A 104
macroscopically hemorrhagic BAL fluid, especially with increase blood content 105
on successive aliquots, is considered as a diagnostic of acute alveolar 106
hemorrhage (AH). However, the demonstration of hemosiderin-laden 107
macrophages (HLMs) is a good evidence of sub-acute AH. After a bleeding 108
episode into the lungs, hemoglobin is converted to hemosiderin by alveolar 109
macrophages. In a mice model, Epstein et al. [14] showed that HLMs and 110
hemosiderin appear within 48-72h after the initial bleeding, reach a maximal 111
concentration at day 6 and then remains within the lungs for 4-8 weeks. The 112
Golde score is a semi-quantitative assessment of HLMs, after a Prussian blue 113
stain, which evaluates both the percentage of macrophages containing 114
hemosiderin (evaluating 100 macrophages), and the intensity of staining on a 115
scale between 0 and 4. The score can therefore vary from 0 to 400. An AH is 116
confirmed for a score above 100 [3]. 117
Further investigations of DAH include a search for infectious agents and for 118
both precipitating antibodies to cow's milk proteins and milk-specific 119
immunoglobulin E (IgE). Complete work-up should also include evaluation for 120
coagulation disorders, screening for a cardiac etiology as well as for possible 121
pulmonary-renal syndromes [17,18], including urine analysis and renal function, 122
as well as specific serologic testing for antinuclear antibodies (ANA) and their 123
components, anti-glomerular basement membrane (GBM) antibodies, and 124
antineutrophil cytoplasmic antibodies (ANCA) to detect immune-mediated lung 125
disease [18, 23]. As the diagnosis of DAH can be performed by less invasive 126
procedures, lung biopsy is not justified [21]. 127
128
Presentation of the cases: 129
Case 1: 130
A seven-week-old male was admitted to the Pediatric Emergency Department 131
after an episode of hemoptysis and epistaxis. He was born full term, eutrophic, 132
with normal neonatal adaptation. He was fed with cow milk (CM) formula from 133
birth on. No other symptoms preceding the hemorrhage were reported by the 134
parents. At the admission, the patient presented a bloody nasal discharge. 135
Clothes were stained with blood. He was pale and hypotonic. He presented a 136
moderate tachypnea varying from 40 to 50 bpm but without associated 137
hypoxemia (SpO2 95% on room air). There was no fever, no central cyanosis, 138
the capillary refill time was normal, and there were no actively hemorrhagic skin 139
or mucous lesions. The infant was dyspneic with chest retractions and presented 140
a grunting. The pulmonary auscultation was normal, there were neither crackles 141
nor wheezes. The cardiovascular exam and the systemic examination were 142
unremarkable. Family and patient history was not contributive. Exposure to 143
tobacco smoke or abnormal humidity at home were denied. 144
Initial routine laboratory results revealed a hemoglobin level of 10.5 g/dL, a 145
hematocrit value of 22%, a white blood cell count (WBC) of 13.9 x 10*9/L, a 146
platelet count of 360 x 10*9/L, with normal coagulation profile (prothrombin 147
time: 16.6 seconds, activated partial thromboplastin time: 39 seconds, 148
fibrinogen: 2.2 g/L and D-dimers: 760ng/mL). C-reactive protein level, renal 149
function, electrolytes, and aminotransferases were normal. Central venous blood 150
gases showed a moderate respiratory acidosis (pH of 7.17, partial pressure of 151
carbon dioxide of 68 mm Hg). Toxicology screen was negative, and urinalysis 152
was normal. 153
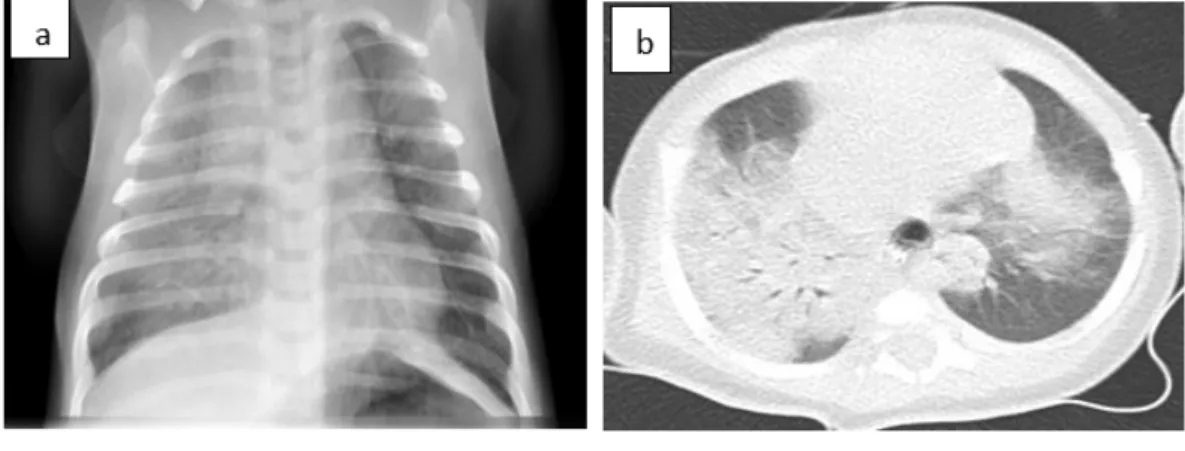
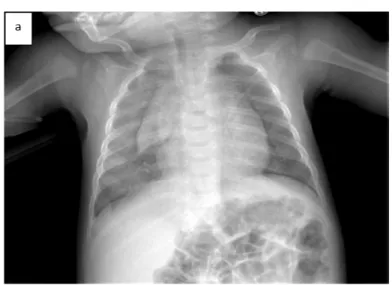
The chest radiograph showed diffuse alveolar infiltrates mostly in the right lung 154
(Fig. 1a). Chest computed tomography (CT) showed consolidated opacities 155
mainly in the right lung (Fig. 1b). 156
Within the first hour of hospitalization, the oxygen saturation decreased (SpO2 157
75%), and the anemia worsened(hemoglobin of 8,5 g/dL); therefore, a 158
supportive therapy including volume expansion, ventilatory support, and 159
transfusion of packed red blood cells at a dose of 20ml/kg was necessary. The 160
infant was intubated and transported to our Pediatric Intensive Care Unit (PICU) 161
under initial antibiotic coverage. 162
The infant underwent emergency bronchial fibroscopy, and diffuse active 163
bleeding [diffuse alveolar hemorrhage (DAH)] was spotted. There were large 164
amounts of blood and clots present in the whole airway. Unfortunately, it was 165
not possible to perform a bronchoalveolar lavage (BAL) because of the 166
hemodynamic instability of the patient. Full etiologic assessment was done and 167
was not contributive (see Table 1). The diagnosis of idiopathic hemosiderosis 168
was proposed. 169
The immunosuppressive therapy was prescribed using 2 mg/kg of prednisolone 170
daily. The infant was extubated on day 2, and discharged 7 days after admission. 171
The infant returned home on steroid therapy (2mg/kg/day during one month and 172
followed by a gradual dose reduction), with hypoallergenic amino acid-based 173
milk and under home cardio-respiratory monitoring. The patient is regularly 174
followed in the respiratory outpatient clinic, and he is doing very well. At 3 175
months of age, the chest radiograph and the CT were normalized. 176
177
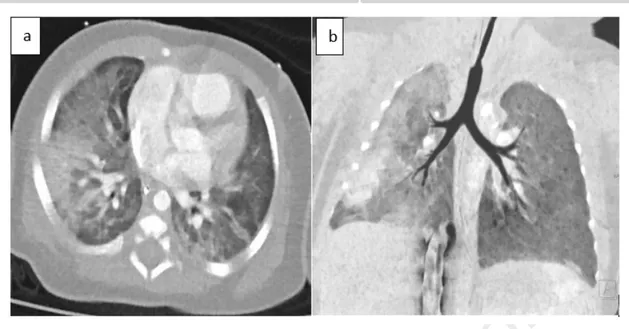
Fig. 1: (a) Chest radiography showed diffuse alveolar infiltrates mostly in the right pulmonary 178
hemi-field. (b) Chest computed tomography scan showed, within the right upper lobe, 179
complete consolidation in the posterior segment and nearly complete consolidation in the 180 anterior segment. 181 182 Case 2: 183
A 3-month-old male infant, born at full term, eutrophic, with normal neonatal 184
adaptation, presented at the emergency department for cough followed by 185
several episodes of hemoptysis. 186
History included hospitalization at the age of two and a half months for 187
tachypnea, cough and upper airways congestion accompanied by oral bloody 188
secretions. The initial diagnosis was gastroesophageal reflux and the patient was 189
discharged from hospital with a proton pump inhibitor and a strict cow’s milk 190
elimination. 191
On admission, the infant was well perfused, and not hypoxic (SpO2 98% on 192
room air). Physical examination showed nasal congestion and bilateral lung 193
crackles on pulmonary auscultation. No dyspnea was noted. Central venous 194
blood gases showed a pH of 7.3 and partial pressure of carbon dioxide of 54 mm 195
Hg. Laboratory studies showed anemia (hemoglobin level: 8.1 g/dL, hematocrit 196
value: 24%). WBCs and platelet count, coagulation profile, quantitative 197
immunoglobulins, and complement studies were normal. Urine analysis was 198
normal. 199
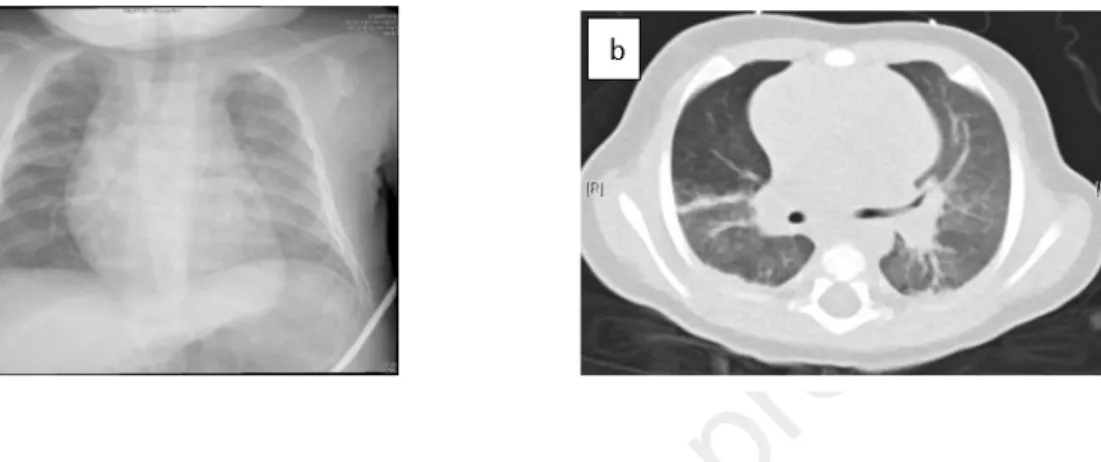
Chest x-ray revealed bilateral alveolo-interstitial opacities and left lower lobe 200
parenchymal consolidation (Fig. 2a). Chest CT showed diffuse bilateral alveolar 201
and ground-glass opacities (Fig. 2b). 202
The infant received packed red cells but no ventilatory support was necessary. A 203
bronchial fibroscopy was performed early after admission that active diffuse 204
bleeding was objectified. Due to the precocity of BAL, hemosiderin-laden 205
macrophages were not demonstrated in the cytological analysis. Etiologic 206
assessment was done and was not contributive except the presence of Chlamydia 207
Trachomatis on BAL culture (see Table 1). The diagnosis of an interstitial 208
pneumonitis with subsequent diffuse alveolar hemorrhage was made. 209
Antimicrobial therapy using oral clarithromycin 15mg/kg/day for 21 days was 210
given, while the systemic corticotherapy initiated at admission was stopped. The 211
patient's symptoms completely resolved shortly after the start of treatment and 212
he was discharged from the hospital on the 8th day on hypoallergenic amino 213
acid-based milk and with a cardio-respiratory monitor. 214
215
216
Fig 2: (a) Chest radiograph demonstrated bilateral alveolo-interstitial opacities. (b) Chest 217
computed tomography scan showed diffuse bilateral alveolar and ground-glass opacities. 218
219
Case 3: 220
A two-month-old female infant, born at 37 weeks of gestation from 221
consanguineous parents, eutrophic, needed prolonged hospitalization in the 222
Neonatal Intensive Care Unit (NICU) for polymalformative syndrome including 223
a common atrium, rectovulvar fistula, alopecia, and dysmorphic features. Initial 224
adaptation at birth was marginal but she quickly stabilize after birth, and a non-225
invasive ventilatory support was needed for mild apneic syndrome. She was 226
found unconscious, apneic, bradycardic with a heart rate of 60-70 beats per 227
minute and cyanotic (SpO2 40% on room air), with foamy bloody secretions in 228
her mouth and nose. No history of trauma, fever or bleeding from any other site 229
was noted. 230
The acute situation required emergency tracheal intubation and fresh blood was 231
aspirated from the endotracheal tube. Heart rate and oxygen saturation quickly 232
improved with conventional mechanical ventilation. High ventilatory pressures 233
were needed to achieve adequate tidal volumes. Physical examination showed 234
normal skin, bilateral lung crackles and a systolic murmur already known. 235
Chest radiograph showed cardiomegaly with diffuse alveolar infiltrates mostly 236
in the right lung. Complete blood count showed normal WBCs count 237
(8500/mm3 with 22% neutrophils, 69% lymphocytes), a hemoglobin level of 10 238
g/dl, a hematocrit value of 30.4%, a platelet count of 247,000/mm3, and a 239
normal coagulation profile. Central venous blood gases showed mild respiratory 240
acidosis and elevated lactate level (50mg/dL). Screening for common causes of 241
DAH (see Table 1) was performed but was not contributive. 242
Two days later, packed red blood cells were transfused for anemia and 243
intravenous antibiotics were administered for suspected infection (fever and 244
raised inflammatory markers). Blood culture and nasopharyngeal aspiration 245
were obtained for viral and bacterial screening. Thoracic angiography scan 246
showed consolidated opacities mainly within the right lung, and arteriovenous 247
malformation were excluded (Fig. 3a, 3b, 3c). The echocardiography confirmed 248
large inter-atrial left-to-right shunt. A bronchial fibroscopy was performed 6 249
days after admission, excluding active bleeding. BAL was not performed 250
because of the patient’s respiratory instability. 251
The first extubation attempt was done on day 3, but reintubation was necessary 252
due to the recurrent bleeding episode and respiratory distress. 253
The patient initially continued to present fresh blood and clots aspirated from 254
her endotracheal tube and then slowly improved from day 4 after the first 255
bleeding episode. Her ventilatory support was weaned and she was successfully 256
extubated on day 6. Her chest radiograph showed significant clearance of the 257
initial haziness. Respiratory virus PCR panel from nasopharyngeal aspiration 258
and tracheal secretions was positive for coronavirus NL63. Bacterial cultures 259
from respiratory secretions and blood were negative. Antimicrobial therapy was 260
stopped after 48 hours and the infant received oral corticosteroid treatment. 261
262
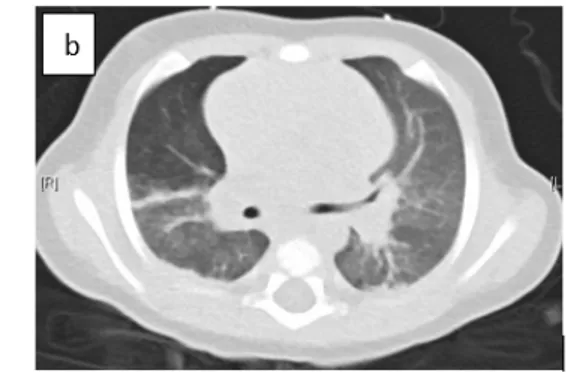
Fig 3: (a,b) Thoracic angiography scan showed consolidated opacities mainly within the right 264 lung. 265 Case 4: 266
A three-week-old male, born at full term, eutrophic with normal neonatal 267
adaptation, was admitted to the emergency department for a severe episode of 268
loss of consciousness accompanied by hypotonia and cyanosis. The infant, was 269
found unconscious in bed one hour after feeding on his stomach, surrounded by 270
soft toys and pillows. His mother noticed some foamy red secretions on his lips. 271
At admission, he was pale, capillary refill time was prolonged (4 seconds), 272
central temperature was 35.5 ̊C. He showed nasal flaring and retractions, a 273
respiratory rate of 40-50 bpm, and SpO2 at room air of 85%. The pulmonary 274
auscultation revealed bilateral crackles. A venous blood gas showed a severe 275
metabolic acidosis (pH: 7.08, partial pressure of carbon dioxide: 38 mmHg, 276
bicarbonate: 10 mmol/L and base excess: 18 mmol/L). Hemoglobin level was 277
normal (16 g/d). Further routine laboratory results including markers of 278
inflammation and infection, of hemostasis, of renal and hepatic function were 279
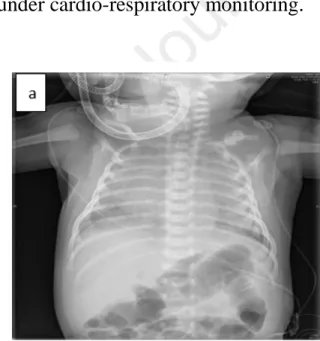
normal. Urinalysis was also normal. Chest x-ray revealed diffuse and bilateral 280
opacities (Fig.4a). The cardiac evaluation, including echocardiogram, revealed a 281
physiologic patent foramen ovale. 282
The infant was admitted to the Intensive Care Unit under initial triple 283
intravenous antibiotic coverage, non-invasive ventilatory support and 284
supplemental oxygen. The first 48 hours of hospitalization, he presented a 285
sudden drop of 4 g/dL of the hemoglobin levels. A bronchial fibroscopy and a 286
BAL were performed on day 4. No active bleeding was noted. Hemosiderin-287
laden macrophages (siderophages) at 68%, and a positive Golde score at 129% 288
were demonstrated in the BAL confirming the DAH. Etiologic assessment for 289
DAH was done and was not contributive (see Table 1). During hospitalization, 290
he received prednisone 2mg/kg/day and he was discharged after one week with 291
the diagnosis of AH probably due to accidental suffocation, as he was found 292
lying with his face against the mattress. The patient was on steroid treatment 293
with gradual dose reduction, with hypoallergenic amino acid-based milk and 294
under cardio-respiratory monitoring. 295
296
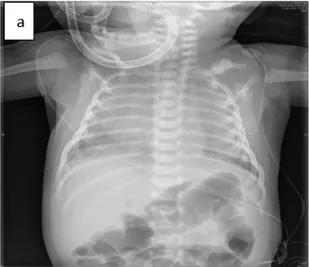
Fig 4: (a) Chest x-ray showed diffuse alveolar infiltrates. 297
Case 5 298
A 3-month-old male infant, born at full term, eutrophic, with a history of 299
transient tachypnea of the newborn after birth, was admitted at the emergency 300
department for brutal cough and hemoptysis while sleeping. He was described 301
hypotonic during this episode. No symptoms were reported by parents preceding 302
the hemorrhage. He was fed with cow milk (CM) formula from birth on. 303
On admission, the infant was eupneic and normoxic (SpO2 98% on room air). 304
Physical examination and in particular the pulmonary auscultation was normal. 305
One hour later, he developed dyspnea with moderate chest retractions. Central 306
venous blood gases showed a respiratory acidosis (pH of 7.08 and partial 307
pressure of carbon dioxide of 71 mm Hg). Initial laboratory study showed 308
anemia (hemoglobin level: 9.4 g/dL, hematocrit value: 29.3%), thrombocytosis 309
(platelet count: 595.000/mm³), hyperleukocytosis (WBC count: 18.530/mm3, 310
54% lymphocytes, 39% neutrophils) but normal C-reactive protein levels. 311
Further routine laboratory results including septic, hemostatic, renal, and hepatic 312
profile returned normal. Urinalysis was also normal. 313
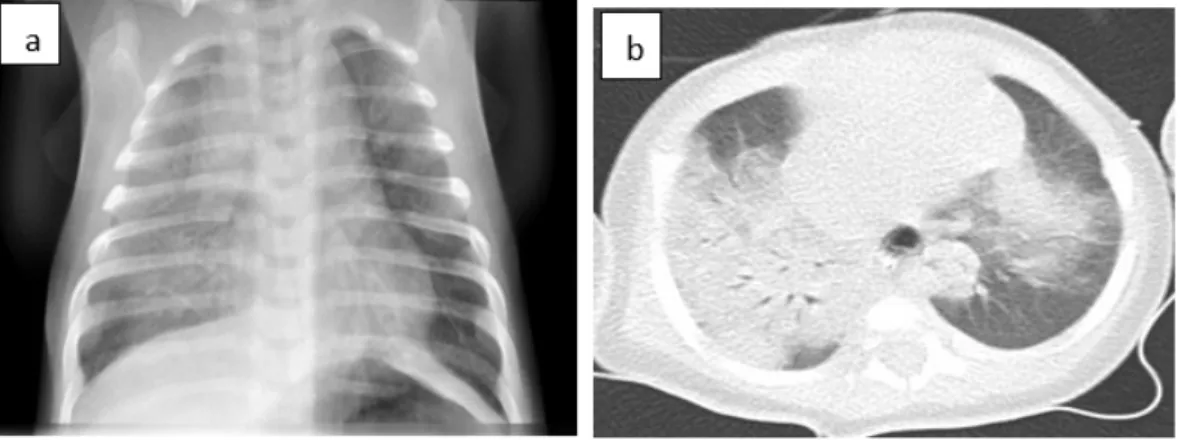
Chest x-ray revealed diffuse interstitial opacities in the right lung (Fig. 5a), 314
Thoracic angiography scan confirmed diffuse interstitial opacities in the right 315
lung and excluded vascular malformation (Fig. 5b). 316
A bronchial fibroscopy was performed on day 4 after the episode of bleeding 317
and did not demonstrate any active bleeding. Hemosiderin-laden macrophages 318
(siderophages) at 89%, and a positive Golde score at 212% were demonstrated 319
in the BAL, confirming DAH. The infant received prednisone 2mg/kg/day. A 320
complete etiologic assessment for DAH (see Table 1) was done and was not 321
contributive except for the positivity of precipitins against cow’s milk proteins 322
(see Table 2). The diagnosis of Heiner syndrome was made. Hypoallergenic 323
amino acid-based milk diet was proposed. 324
The patient’s evolution during the hospitalization was favorable and no 325
subsequent hemoptysis was noted. The patient was discharged from hospital 326
with amino acid-based milk and systemic corticosteroid. During regular 327
outpatient visits, the patient presented a favorable respiratory evolution without 328
any recurrent hemoptysis. The corticosteroid treatment was stopped after three 329
months. 330
332
Figure 5: (a) Chest radiography showed diffuse alveolar infiltrates at the right pulmonary 333
hemifield. 334
Discussion: 335
Five cases of DAH are described in this report. Our five patients presented 336
episodes of hemoptysis, anemia of varying degrees, and their chest radiographs 337
showed diffuse bilateral interstitial infiltrates consistent with pulmonary 338
hemorrhage. Consequently a diagnosis of DAH was strongly suspected. In three 339
of the five cases, the severity of the initial pulmonary hemorrhage was sufficient 340
to cause acute pulmonary distress, requiring adequate ventilatory support and to 341
cause severe anemia requiring packed red blood cell transfusion. In all our cases, 342
due to young age, systemic vasculitis and collagen vascular disorders were 343
unlikely. 344
In all cases, pediatric pulmonologists performed a bronchial fibroscopy in order 345
to look for active bleeding in the airways. In the absence of obvious cause of the 346
bleeding, BAL was performed. Hemosiderin-laden macrophages were found in 347
BAL only in 2 of our 5 patients, either due to early performance of the BAL 348
within 24 hours from hemoptysis or due to limitations of the bronchoscopy 349
procedure because of hemodynamic instability of the patients. As previously 350
mentioned, HLMs were not demonstrated in the cytological analysis until 48-72 351
hours after the acute phase. However, the visualization of areas of active 352
bleeding, and the aspiration of fresh blood confirmed the pulmonary hemorrhage 353
in these cases. 354
Table 2 presents bronchoalveolar lavage results for these five cases. 355
Table 2: Bronchoalveolar lavage results. 356 Red blood cells, mm3 White blood cells, mm3 Neutrophils, % Lymphocytes, % Macrophages, % Hemosiderin-laden macrophages, %, Golde score Case 1 510 450 ND ND ND ND ND Case 2 1910 400 73 4 23 ND ND Case 3 1540 110 ND ND ND ND ND Case 4 280 450 1.3 1.9 96 69 129 Case 5 80 460 33 3 63 89 212 ND: not done 357
In all cases, the diagnosis of DAH was confirmed by the bronchoscopy. 358
Subsequently and in order to identify a specific etiology, we proceeded to 359
additional laboratory tests, as well as selected serologic and radiographic 360
In our first case, as no underlying pathology was found, the diagnosis of 362
idiopathic pulmonary hemosiderosis was retained. As previously mentioned 363
pulmonary hemosiderosis is a diagnosis of exclusion, based on an association of 364
anemia, chest X-ray pulmonary infiltrates and the presence of hemosiderin-laden 365
macrophages in the bronchoalveolar lavage. Further investigation was non 366
contributive for this infant. 367
The next two cases of DAH were due to infectious causes. In our second case, 368
culture analysis of tracheal aspirate samples identified the presence of 369
Chlamydia Trachomatis. Infants born vaginally from infected mothers are at risk 370
of acquiring Chlamydia trachomatis, which can lead to severe tracheobronchial 371
or pneumonic infection and DAH. In our third case, polymerase chain reaction 372
of tracheal aspirate samples identified the presence of Coronavirus NL63. 373
Coronavirus is a common cause of upper respiratory tract infection in children 374
and has also been occasionally associated with lower respiratory tract infections 375
(such as bronchiolitis and pneumonia) and with pulmonary hemorrhage [8]. At 376
present, there are seven coronaviruses recognized as human pathogens (HCoV): 377
HCoV-OC43, HCoV-299E, HCoV-HKU1, HCoV-NL63, SARS CoV, MERS-378
CoV and the newly identified SARS COV-2 [25]. The use of reverse 379
transcriptase polymerase chain reaction helps in the rapid and reliable detection 380
of these viruses. It is noteworthy, that pulmonary hemorrhage in children has 381
been mainly associated with bacterial infections, such as Staphylococcus aureus, 382
in relation to necrotizing pneumonia [6], by fungi, such as Stachybotrys 383
Chartarum [11] and occasionally by virus such as H1N1 influenza [7]. To our 384
knowledge, our patients are the second reported cases of pulmonary hemorrhage 385
in infants associated with chlamydia trachomatis [5] and coronavirus infection 386
[8], respectively. 387
In our fourth case, mainly according to detailed anamnestic data, accidental 388
suffocation and asphyxia were probably the causes of the DAH. It is worth 389
mentioning that the infant received an objective evaluation including 390
ophthalmologic examination and head imaging because of initial suspicion of 391
abuse. Suffocation and shaken baby syndrome are the most difficult 392
components to exclude in the differential diagnosis of pulmonary hemorrhage. 393
In our last case, serum immunoglobulin levels (IgG, IgA, IgM, IgE) and milk-394
specific serum IgE were normal but interestingly high precipitating cow’s milk 395
proteins antibodies titers was found and Heiner syndrome (HS) was diagnosed. 396
Heiner syndrome (HS) is a cow's milk (CM) hypersensitivity pulmonary disease 397
that affects primarily infants. Moissidis et al, recently reported a series of infants 398
with recurrent respiratory symptoms and iron deficiency anemia. All of these 399
children presented symptomatic and radiologic improvement with the eviction 400
of cow's milk proteins [2,13]. 401
The immunologic mechanism in milk-induced pulmonary disease is 402
type III) or cell-mediated reaction (Gell and Coombs' type IV) may have a role. 404
Patients with HS characteristically have high titers of milk-specific IgG 405
antibodies (which is not pathognomic of the disease) and some of them may 406
have high serum total IgE levels and milk-specific IgE antibodies. Although this 407
syndrome remains a controversial entity which has been rarely reported in the 408
medical literature, it should be particularly suspected in pediatric pulmonary 409
practices. Insufficient awareness about this disease is probably a major factor in 410
its misdiagnosis and its morbidity. 411
Table 3 presents data regarding assessments, diagnosis, and treatment for the 412
five cases presented. 413
Table 3: Demographic data, clinical presentation, etiology assessment and diagnosis. 414
415
Case 1 Case 2 Case 3 Case 4 Case 5
Sex M M F M M Age at onset 7-w 12-w 8-w 3-w 18-w Hemoptysis Acute hemoptysis 2-w history of intermittent hemoptysis Acute hemoptysis Acute hemoptysis Acute hemoptysis
Anemia + + + + + Venous blood gases RA RA RA MA RA Chest X-Ray AI AI AI AI AI CT scan CO ILD CO ND CO BAL + + ND + + HLMs - - ND + + Golde score ND ND ND + + Microbiologic culture in BAL N Chlamydia trachomatis N N N Respiratory virus PCR panel N N COV N N Immunologic abnormalities N N N N N Milk RAST Milk precipitin test N ND N ND N ND N ND N + Blood transfusion + + + - -
Ventilatory support + - + + - Corticosteroid therapy + - + + + Antimicrobial therapy - + - - - Diagnosis Idiopathic pulmonary hemosiderosis DAH due to Chlamydia trachomatis infection DAH due to coronavirus infection DAH due to accidental suffocation Heiner syndrome 416
BAL= bronchoscopic alveolar lavage, HLMs= hemosiderin laden macrophages, RA= 417
respiratory acidosis, MA= metabolic acidosis, AI=alveolo-interstitial pattern, ILD=interstitial 418
lung disease, CO=consolidative opacities, ND=not done, N=normal, COV= Coronavirus 419
NL63, DAH= Diffuse alveolar hemorrhage 420
Treatment and prognosis: 421
In our five cases, none had elevated milk-specific IgE antibodies and only one 422
had positive IgG antibodies against CM proteins. However, considering the 423
young age of our five patients and the immaturity of the infant’s immune 424
system, our policy was to remove cow's milk from the diet of infants and control 425
the serum immunoglobulins levels later in life. 426
In acute phase, the treatment was symptomatic (transfusion, oxygen therapy and, 427
if needed, ventilatory support) for all the patients. For the second case we 428
concluded to interstitial pneumonitis and a diffuse alveolar hemorrhage due to 429
an infectious agent for which the patient received antimicrobial therapy by oral 430
clarithromycin 15mg/kg/day for 21 days. For all the other cases, the first-line 431
curative treatment was oral corticosteroids. Corticosteroids have been reported 432
to be associated with decreased pulmonary bleeding relapses and pulmonary 433
fibrosis progression, as well as with higher survival rates [10]. Since the 434
recommended duration of corticosteroids treatment is variable among the 435
patients based on clinical, radiological, and biological evolution, our patients 436
received prolonged treatment for 3 to 6 months [23]. In small case series of 437
severe DAH, which does not improve with intravenous corticosteroids, the use 438
of intrapulmonary recombinant factor VII a, has been described to obtain good 439
results [20]. In chronic cases of pulmonary hemorrhage with poor response to 440
steroids, or in cases associated with systemic diseases, immunosuppressive 441
agents such as azathioprine, methotrexate, and cyclophosphamide have been 442
used with variable results [10, 16]. 443
All of our five cases are followed up closely in respiratory outpatient clinic and 444
none of them has had further episodes of pulmonary bleeding up to 12 months 445
after discharge. 446
Recent retrospective case studies have shown significantly better survival, with 447
five-year survival rates above 80%. Prolonged remission is possible; however, 448
later death from the disease remains a possibility [9]. The cause of death is 449
generally terminal chronic respiratory failure but may also be massive 450
hemoptysis. Sometimes DAH is immune-mediated and the prognosis of these 451
patients in terms of morbidity and mortality may be affected by the extent of 452
involvement in other organs [19]. 453
454
Figure 6: Algorithm for management of intra-alveolar haemorrhage 455
457
BAL : bronchoscopic alveolar lavage; RF : rheumatic factor ; ANCA : Antineutrophil 458
Cytoplasmic Antibodies; ANA: Antinuclear Antibodies; GBM : Glomerular Basement 459
Membrane 460
Conclusion: 461
This article focuses on diffuse alveolar hemorrhage in infancy. In childhood and 462
mostly in infancy, diffuse alveolar hemorrhage present as a rare but serious 463
medical emergency. The differential diagnosis in infants includes 464
cardiopulmonary vascular malformations, infection, coagulation disorders, food 465
hypersensitivity syndromes and idiopathic pulmonary hemosiderosis. Vasculitis 466
highlighted by these case reports, high index of suspicion, adequate etiologic 468
screening and appropriate treatment are mandatory to improve survival. 469
Abbreviations: 470
DAH: Diffuse Alveolar Hemorrhage, CM: Cow Milk, CT: Computed 471
tomography, WBC: White Blood Cells, BAL: Bronchoscopic Alveolar Lavage, 472
NICU: Neonatal intensive Care Unit, PDA: Patent Ductus Arteriosus, VSD: 473
Ventricular Septal Defect, SIDS: Sudden Infant Death Syndrome, IPH: 474
Idiopathic Pulmonary Hemosiderosis, AH: Alveolar Hemorrhage, HLMs: 475
Hemosiderin Laden Macrophages, ANA: Antinuclear Antibodies, GBM: 476
Glomerular Basement Membrane, ANCA: Antineutrophil Cytoplasmic 477
Antibodies, bpm: beats per minute. 478 Acknowledgements: 479 Not applicable. 480 Authors' contributions: 481
EG and MM were involved in writing, reading and editing the manuscript. All 482
authors read and approved the final manuscript. 483
Funding: 484
The authors thank the University of Liege for the support and funding of this 485
publication 486
Competing interests: 487
The authors declare that they have no competing interests. 488
References: 489
1. Parrot, A., Picard, C., Fartoukh, M., Vincent, F., & Mayaud, C. (2005). Hémorragies intra-490
alvéolaires. Diagnostic et traitement. Réanimation, 14(7), 614-620. 491
2. Godfrey, S. (2004). Pulmonary hemorrhage/hemoptysis in children. Pediatric 492
pulmonology, 37(6), 476-484.
493
3. Sherman, J. M., Winnie, G., Thomassen, M. J., Abdul-Karim, F. W., & Boat, T. F. (1984). 494
Time course of hemosiderin production and clearance by human pulmonary 495
macrophages. Chest, 86(3), 409-411. 496
4. Epstein, C. E., Elidemir, O., Colasurdo, G. N., & Fan, L. L. (2001). Time course of 497
hemosiderin production by alveolar macrophages in a murine model. Chest, 120(6), 2013-498
2020. 499
5. Thompson, J. W., Nguyen, C. D., Schoumacher, R. A., Lazar, R. H., Hamdan, F., Stocks, 500
R. M., & Van Nguyen, K. (1996). Evaluation and Management of Hemoptysis in Infants and 501
Children a Report of Nine Cases. Annals of Otology, Rhinology & Laryngology, 105(7), 516-502
520. 503
6. Boussaud, V., Parrot, A., Mayaud, C., Wislez, M., Antoine, M., Picard, C., ... & Cadranel, 504
J. (2003). Life-threatening hemoptysis in adults with community-acquired pneumonia due to 505
Panton-Valentine leukocidin-secreting Staphylococcus aureus. Intensive care
506
medicine, 29(10), 1840-1843.
7. Gilbert, C. R., Vipul, K., & Baram, M. (2010). Novel H1N1 influenza A viral infection 508
complicated by alveolar hemorrhage. Respiratory care, 55(5), 623-625. 509
8. Agarwal, P., Arora, H., Abdulhamid, I., Asmar, B., Natarajan, G., & Chawla, S. (2015). 510
Pulmonary Hemorrhage in an Infant with Coronavirus Infection. J Neonatal Biol, 4(175), 511
2167-0987. 512
9. Le, L. C., Le, M. B., Fauroux, B., Forenza, N., Dommergues, J. P., Desbois, J. C., ... & Pin, 513
I. (2000). Long-term outcome of idiopathic pulmonary hemosiderosis in 514
children. Medicine, 79(5), 318-326. 515
10. Taytard, J., Nathan, N., De Blic, J., Fayon, M., Epaud, R., Deschildre, A., ... & Cros, P. 516
(2013). New insights into pediatric idiopathic pulmonary hemosiderosis: the French 517
RespiRare® cohort. Orphanet journal of rare diseases, 8(1), 161. 518
11. Dearborn, D. G., Smith, P. G., Dahms, B. B., Allan, T. M., Sorenson, W. G., Montana, E., 519
& Etzel, R. A. (2002). Clinical profile of 30 infants with acute pulmonary hemorrhage in 520
Cleveland. Pediatrics, 110(3), 627-637. 521
12. Gencer, M., Ceylan, E., Bitiren, M., & Koc, A. (2007). Two sisters with idiopathic 522
pulmonary hemosiderosis. Canadian respiratory journal, 14(8), 490-493. 523
13. Moissidis, I., Chaidaroon, D., Vichyanond, P., & Bahna, S. L. (2005). Milk‐induced 524
pulmonary disease in infants (Heiner syndrome). Pediatric allergy and immunology, 16(6), 525
545-552. 526
14. Jacanamijoy, A. B., Silva, D. C. G., Méndez, J. C. B., Roa, J. D., & Vásquez, P. (2016). 527
Acute idiopathic pulmonary hemorrhage in infants. Report of two cases and literature 528
review. Case reports, 2(2), 19-29. 529
15. Oehmichen, M., Gerling, I., & Meissner, C. (2000). Petechiae of the baby's skin as 530
differentiation symptom of infanticide versus SIDS. Journal of Forensic Science, 45(3), 602-531
607. 532
16. Fullmer, J. J., Langston, C., Dishop, M. K., & Fan, L. L. (2005). Pulmonary capillaritis in 533
children: a review of eight cases with comparison to other alveolar hemorrhage 534
syndromes. The Journal of pediatrics, 146(3), 376-381. 535
17. Von Vigier, R. O., Trummler, S. A., Laux‐End, R., Sauvain, M. J., Truttmann, A. C., & 536
Bianchetti, M. G. (2000). Pulmonary renal syndrome in childhood: A report of twenty‐one 537
cases and a review of the literature. Pediatric pulmonology, 29(5), 382-388. 538
18. Harris NLMD, McNeely WFMD, et al. Case records of the Massachusetts General 539
Hospital: Case 30-2002. N Engl J Med. 2002; 347(13):1009-1017.
540
19. Susarla, S. C., & Fan, L. L. (2007). Diffuse alveolar hemorrhage syndromes in 541
children. Current opinion in pediatrics, 19(3), 314-320. 542
20. Park JA, Kim BJ. Intrapulmonary recombinant factor VIIa for diffuse alveolar 543
hemorrhage in children. Pediatrics. 2015 ; 135 (1): 216-20
544
21. Lazor, R. (2011). Alveolar hemorrhage syndromes. Orphan Lung Diseases. Eur Respir 545
Monogr, 54, 15-31.
546
22. Coss-Bu, J. A., Sachdeva, R. C., Bricker, J. T., Harrison, G. M., & Jefferson, L. S. (1997). 547
Hemoptysis: a 10-year retrospective study. Pediatrics-English Edition, 100(3), e7. 548
23. Schwarz, M. I. (2015). The diffuse alveolar hemorrhage syndromes. Alphen aan den Rijn: 549
Wolters Kluwer.
24. Milman, N., & Pedersen, F. M. (2013). Idiopathic pulmonary hemosiderosis. UpToDate 551
Oct. 2017
552
25. Teramoto, S. (2014). Clinical significance of aspiration pneumonia and diffuse aspiration
553
bronchiolitis in the elderly. J Gerontol
Fig 2: (a) Chest radiograph demonstrated bilateral alveolo-interstitial opacities. (b) Chest computed tomography scan showed diffuse bilateral alveolar and ground-glass opacities.
Fig 3: (a,b) Thoracic angiography scan showed consolidated opacities mainly within the right lung.
Fig. 1: (a) Chest radiography showed diffuse alveolar infiltrates mostly in the right pulmonary hemi-field. (b) Chest computed tomography scan showed, within the right upper lobe, complete consolidation in the posterior segment and nearly complete consolidation in the anterior segment.
Figure 5: (a) Chest radiography showed diffuse alveolar infiltrates at the right pulmonary hemifield.
Figure 6: Algorithm for management of intra-alveolar haemorrhage
BAL : bronchoscopic alveolar lavage; RF : rheumatic factor ; ANCA : Antineutrophil Cytoplasmic Antibodies; ANA: Antinuclear Antibodies; GBM : Glomerular Basement Membrane
Declaration of competing interest