T
T
H
H
È
È
S
S
E
E
En vue de l'obtention du
D
D
O
O
C
C
T
T
O
O
R
R
A
A
T
T
D
D
E
E
L
L
’
’
U
U
N
N
I
I
V
V
E
E
R
R
S
S
I
I
T
T
É
É
D
D
E
E
T
T
O
O
U
U
L
L
O
O
U
U
S
S
E
E
Délivré par l'Université Toulouse III - Paul SabatierDiscipline ou spécialité : Genes, Cellules et Développement
JURY
Prof. Elisabeth Christians, Président Prof. Claudia Bagni, rapporteur Dr. Sophie Pison-Rousseaux, rapporteur
Prof. Véronique Kruys, rapporteur Dr. Martino Donnini, invité
Prof. Sergio Capaccioli DR Dominique Morello
Ecole doctorale : Biologie-Santé-Biotechnologie Unité de recherche : UMR 5547
Présentée et soutenue par Mai NGUYEN CHI Le 9 décembre 2008
Titre : Post-transcriptional regulation during spermatogenesis:
Acknowledgement
It is a pleasure to thank the many people who made this thesis possible.
I wish to thank the members of examination board, Prof. Claudia Bagni, Dr. Sophie Pison-Rousseaux, Prof. Véronique Kruys, Prof. Elisabeth Christians and Dr. Martino Donnini, who kindly accepted to correct my thesis. It’s a pleasure for me to have an international jury composed by great quality researchers.
Il m’est difficile d’exprimer l’étendue de ma gratitude envers ma directrice de thèse, le Dr. Dominique Morello. Avec son enthousiasme, son soutien tout au long de ma thèse, et sa confiance, elle m’a aidée à m’épanouir dans la recherche. Dominique est une scientifique que j’estime beaucoup, mais aussi une personne avec une grande humanité. J’ai particulièrement apprécié les « brain storming » en sa compagnie.
Grazie mille al’ professore Sergio Capaccioli, mio direttore di dottorato. He gave me the possibility to work in his laboratory in Florence in a friendly atmosphere and to discover a very nice country and culture. I would like to express my gratitude to Dr. Martino Donnini, who taught me the work of researcher, many experiments and gave me a work fashion that I hope to conserve in the future. I thank also all the laboratory of Florence, Matteo, Andrea, Laura, Nicola and Eva for their sympathy and to have make biology “fun” for me.
Je tiens à exprimer ma reconnaissance aux membres de l’équipe « Morello » pour leur sympathie et leur aide. Jacques (qui m’a appris ma première PCR !) pour son aide indispensable concernant les souris mais aussi pour sa bonne humeur permanente et les cafés partagés. Nathalie pour son aide précieuse, ses conseils pointilleux et son enthousiasme vis à vis de mon projet. Mohammad pour son expertise concernant les « gradients », sa disponibilité, mais surtout pour le spectacle permanent qu’il nous offre au laboratoire. Fatima pour ses conseils et sa gentillesse.
J’aimerai remercier particulièrement mon tuteur, le Dr. Eric Agius, pour avoir toujours été disponible pour les discussions, m’avoir donné des conseils très pertinents et avoir dissipé mes doutes. Et surtout pour sa franchise….
Je remercie le Dr. Marc Haenlin, Directeur du Centre de Biologie du Développement, pour m’avoir accueillie dans son laboratoire et m’avoir permise de réaliser ce travail dans de bonnes conditions. Je remercie également les membres du CBD,
I am indebted to my collaborators, Khalid Khabar, James Turner, Dimitris kontoyiannis for their help, and their nice work.
Particulièrement Bernard Jégou pour ses encouragements et sa confiance en notre projet, Virginie Vallet pour son expertise dans le sujet de la spermatogénèse et Frédéric Chalmel pour sa sympathie et son enthousiasme et bien sûr son aide indispensable en bio-informatique.
Je remercie mes parents, Françoise et Loc, mon frère Ken et ma sœur Laure pour leur amour et leur soutien depuis toujours.
Mes amis (de Paris, d’Italie, de Grenoble et de Toulouse, la « colloc » du canon d’Arcole et tous les gens qu’elle a impliqués) : Anna, Adadia, Toto, Edith, Christophe, Greg, Coco, Carine, Céline, Manue, Yannick, Kevin, Xav, et tant d’autres, pour leur amitié, leur présence et pour leur compréhension.
Je tiens particulièrement à exprimer ma gratitude à mon compagnon Julien pour sa compréhension et son soutien, pour ses encouragements et pour avoir toujours cru en moi, mais surtout pour son amour.
Ce projet a été financé par l’Université Franco-Italienne, l’Université Paul Sabatier, le ministère de la recherche et de l’enseignement supérieur, la fondation pour la recherche médicale et le CNRS.
TABLE OF CONTENTS
INTRODUCTION 17
PART I : ARE-MEDIATED POST-TRANSCRIPTIONAL CONTROLS 19
A. GENERAL STEPS OF RNA METABOLISM 19
1/ MATURATION 19 2/ TRANSLATION 19 3/ RNA STABILITY AND DEGRADATION 22
B. ARE-MEDIATED POST-TRANSCRIPTIONAL CONTROLS 24
1/ CIS-ACTING ELEMENT 24 2/ AU-BINDING PROTEINS 26 3/ CONSERVATION OF ARES AND AU-BPS 30 4/ AU-BP POST-TRANSLATIONAL MODIFICATIONS, LOCALISATIONS AND FUNCTIONS 30 5/ SMALL NON CODING RNAS 37 6/ MICRORNA AND ARE-MEDIATED CONTROL 40 7/ ARE-MEDIATED CONTROL AND DISEASES 40
PART II: SPERMATOGENESIS 44
A. SPERMATOGENESIS: A MALE GERM CELL DIFFERENTIATION PROCESS 44 1/ MITOTIC PHASE 44 2/ MEIOSIS PHASE 44 3/ HAPLOID PHASE 46 4/ SPERMATOGENESIS WAVE AND GERM CELL ORGANIZATION 50
B. POST-TRANSCRIPTIONAL REGULATION DURING SPERMIOGENESIS 54 1/ TRANSCRIPTION IN SPERMATOGENIC CELLS 54 2/ TEMPORAL TRANSLATION CONTROL 56 3/ ROLE OF POLY(A) TAIL 60 4/ RNA-BINDING PROTEINS IN TESTIS 60 5/ SMALL NON-CODING RNAS-MEDIATED GENE REGULATION IN SPERMATOGENESIS 61 6/ PIWI-INTERACTING RNAS: GERM CELL-SPECIFIC SMALL RNAS 62
C. THE CHROMATOID BODY 65
1/ MORPHOLOGY AND MOVEMENT OF THE CHROMATOID BODY 65 2/ COMPONENTS OF THE CHROMATOID BODY 67 3/ A GERM-CELL-SPECIFIC RNA PROCESSING CENTRE 69 4/ MIWI AND PIRNAS 70 5/ OTHERS TYPES OF NUAGE IN MAMMALIAN GERM CELLS 70
PART III : PROJECT 72
RESULTS 73
CHAPTER I : RELEVANCE OF ARE FOR SPERMATOGENESIS 74
1/ NUMEROUS ARE-CONTAINING TRANSCRIPTS ARE PREFERENTIALLY EXPRESSED WITHIN MOUSE TESTIS 74 2/ EXPRESSION PROFILE OF ARE-CONTAINING TRANSCRIPTS IS CONSERVED IN HUMAN
DIFFERENTIATING GERM CELLS. 76 3/ ARE-CONTAINING TRANSCRIPTS ARE INVOLVED IN DIVERSE ASPECTS OF SPERMATOGENESIS.
RESUME DU CHAPITRE II : ETUDE DES FONCTIONS D’AUF1 ET D’HUR AU
COURS DE LA SPERMATOGENESE 81
1/ INTRODUCTION 81 2/ RESULTATS ET METHODOLOGIE 81 3/ CONCLUSION 83
CHAPTER II : STUDY OF AUF1 AND HUR FUNCTIONS DURING MOUSE
SPERMATOGENESIS 84
1/ DIFFERENTIAL EXPRESSION OF AUF1 AND HUR DURING SPERMATOGENESIS 84 2/ OVEREXPRESSION OF BOTH TRANSGENES IN THE EARLY SPERMATIDS 86 3/ EARLY IMPAIRMENT OF SPERMATOGENESIS UPON HUR OVEREXPRESSION 89 4/ INCREASED APOPTOSIS IN HUR TRANSGENIC TESTIS 93 5/ HUR OVEREXPRESSION INCREASES ARE-CONTAINING MRNA EXPRESSION 95 6/ COMMON AND DISTINCT MRNA TARGETS FOR HUR AND AUF1 98 7/ BINDING OF SEVERAL HUR TARGET MRNAS TO HUR PROTEIN 98 8/ COMMON MRNA TARGETS FOR TRANSGENIC AND ENDOGENOUS HUR 101 9/ HUR OVEREXPRESSION INCREASES THE STABILITY OF ITS TARGET MRNAS 101
RESUME DU CHAPITRE III: LE TRANSIT D’HUR PAR LE CORPS CHROMATOÏDE CONTROLE LA BALANCE ENTRE STOCKAGE ET
TRADUCTION DES ARNM AU COURS DE LA SPERMATOGENESE. 105
1/ INTRODUCTION 105 2/ RESULTATS ET METHODOLOGIE 106 3/ CONCLUSION 106
CHAPTER III : TEMPORAL SWITCH OF HUR FROM CHROMATOID BODY TO POLYSOMES CONTROLS MRNA TRANSLATION DURING SPERMATOGENESIS
108 A.SUMMARY 108 1/ BACKGROUND 108 2/ METHODOLOGY/PRINCIPAL FINDINGS 109 3/ CONCLUSIONS/SIGNIFICANCE 109 B.ARTICLE 110
C.ADDITIONAL DATA:MVH INTERACTS WITH EIF3, A TRANSLATIONAL INITIATION
FACTOR 135
CHAPTER IV : HUR GENE INACTIVATION SPECIFIC IN MOUSE MALE GERM
CELLS 136
1/ CONSEQUENCES OF HUR INACTIVATION AT THE HETEROZYGOUS STAGE IN MALE GERM CELLS 136 2/ SPERMATOCYTE-SPECIFIC INACTIVATION OF HUR 138 3/ PRIMORDIAL GERM CELL-SPECIFIC INACTIVATION OF HUR 144
DISCUSSION 149
PART I : DIFFERENT OUTCOMES FOR AUF1 AND HUR IN SPERMATOGENESIS 151
1/ AUF1 AND HUR ARE DIFFERENTIALLY EXPRESSED DURING SPERMATOGENESIS 151 2/ DIFFERENT EFFECTS OF AUF1 AND HUR OVEREXPRESSION IN SPERMATOGENESIS 152 3/ AUF1 AND HUR REGULATE BOTH COMMON AND DISTINCT ARE-CONTAINING MRNAS 153 4/ GENERATION OF GERM CELL-SPECIFIC HUR-KO MICE TO UNDERSTAND HUR PHYSIOLOGICAL
FUNCTION 154
PART II : DYNAMIC LOCALIZATION OF HUR DURING SPERMATOGENESIS 156
1/ IS HUR INVOLVED IN ARE-MRNA EXPORT FROM NUCLEUS TO CHROMATOID BODY IN
SPERMATIDS? 156 2/ HUR TRAFFICKING BETWEEN CHROMATOID BODY AND POLYSOMES 157 3/ HUR FUNCTION IN MRNA STABILIZATION DURING SPERMATOGENESIS 158 4/ IS THERE AN INTERPLAY BETWEEN HUR AND MIRNA-MEDIATED SILENCING? 161 5/ HUR TRAFFICKING THROUGH CYTOPLASMIC BRIDGES 162
PART III : ARE-MEDIATED REGULATION IN TESTIS 163
1/ ROLE OF ARE-MRNAS IN SPERMATOGENESIS 163 2/ IS HUR A SPECIFIC OR WIDE POST-TRANSCRIPTIONAL REGULATOR? 164 3/ A LINK BETWEEN HUR TARGETS AND ALTERED SPERMATOGENESIS 166 CONCLUSION 169
MATERIALS AND METHODS 171
I. BIOLOGICAL MATERIAL 172
A.HUR AND P37AUF1 TRANSGENIC MICE 172
B.HUR+/-AND HURFL/FL MICE 172
C.CRE EXPRESSING MICE 172
II. METHODS 173
A.DNA EXTRACTION AND GENOTYPING 173
B.METHODS RELATIVE TO MRNA EXPRESSION 173
1/ RNA EXTRACTION, MICROARRAYS AND TRANSCRIPTOME ANALYSIS 173 2/ REVERSE TRANSCRIPTION QUANTITATIVE POLYMERASE CHAIN REACTION (R- SEMI-QPCR) AND REVERSE TRANSCRIPTION REAL -TIME POLYMERASE CHAIN REACTION
(RT-QPCR) 174
C. METHODS RELATIVE TO SPERMATOGENESIS ANALYSIS 175
1/ COLLECTION OF TISSUES, HISTOLOGICAL ANALYSIS, SPERM COUNTS AND STATISTICAL
ANALYSIS 175
3/ FLOW CYTOMETRY 176 4/ TERMINAL DEOXYNUCLEOTIDYLTRANSFERASE-MEDIATED DUTP-FLUORESCEIN NICK END LABELING (TUNEL) ASSAYS AND CELL DEATH COUNT 176
D.IMMUNOPRECIPITATIONS ASSAYS 177
1/ PROTEINS AND MRNAS COIMMUNOPRECIPITATION AND SEMI-QUANTITATIVE RT-PCR 177 2/ PROTEINS-PROTEIN COIMMUNOPRECIPITATION 178
E.ANALYSIS OF PROTEIN EXPRESSION LEVELS 178
1/PROTEIN EXTRACTION AND WESTERN BLOT ANALYSIS 178 2/ NUCLEO-CYTOPLASMIC FRACTIONATIONATION 178
F.IMAGERY 179
1/ TESTIS SECTIONS, TUBULE SQUASHES AND DRYING DOWN PREPARATIONS 179 2/ IMMUNODETECTION 180 3/ IN SITU HYBRIDIZATION TO DETECT POLYADENYLATED MRNAS 180 4/ IN SITU HYBRIDIZATION TO SPECIFIC MRNAS 180
G.ACTINOMYCIN D PULSE-CHASE ASSAY 182
APPENDIX 183 BIBLIOGRAPHY 197
FIGURES
1/ Introduction
Figure 1: mRNP life cycle
Figure 2: A stereotype of coding messenger RNA
Figure 3: Schematic presentation of the initiation of protein synthesis
Figure 4: Diversity of ARE sequences and AU-BPs with which they interact Figure 5: Schematic representation of the two AU-BPs HuR and AUF1 Figure 6: Different ARE-mRNA fate when bound by HuR or AUF1
Figure 7: Different signalling pathways control HuR nucleo-cytoplasmic shuttling and
function
Figure 8: Ephemera localization of mRNAs in different cytoplasmic granules Figure 9: Post-transcriptional regulation by microRNAs and small interfering RNAs Figure 10: Meiotic division
Figure 11: Spermatid differentiation
Figure 12: Spermatogenic cell types in germinative epithelium
Figure 13: Schematic representation of spermatogenic stages and germ cell
organisation in mouse seminiferous tubules
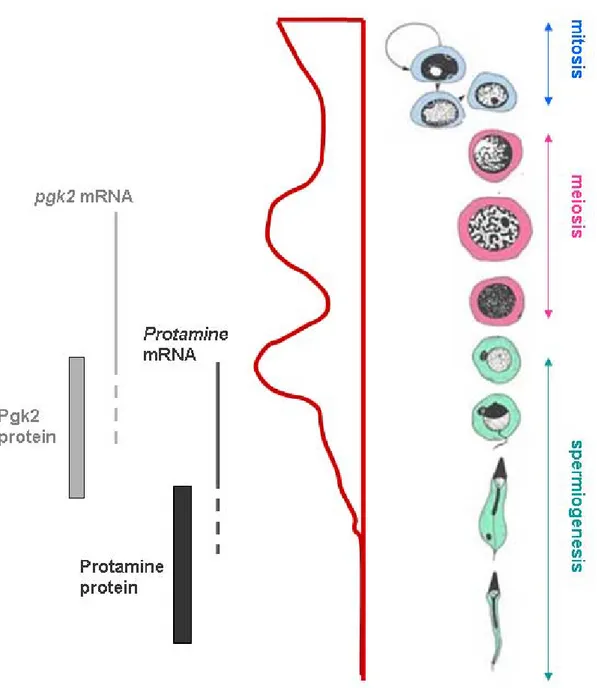
Figure 14: Temporal regulation of protamine 1 and Pgk2 mRNA translation during
spermatogenesis
Figure 15: The CB is a dense, fibrous, peri-nuclear structure found in the cytoplasm of
haploid round spermatids
Figure 16: Schematic representation of transcriptional activity and appearance of the
CB during spermatogenesis
2/ Results
Figure 17: ARE-mRNAs are expressed in testis
Figure 18: HuR and AUF1 expression during spermatogenesis
Figure 19: Immunofluorescence analysis of endogenous AUF1 and HuR expression
Figure 20: Expression of HuR and AUF1 transgenes (mRNAs and proteins) in adult
testes
Figure 21: Consequences of HuR overexpression at different stages of testis
ontogenesis
Figure 22: Spermatogenesis is altered at various degrees in HuR transgenic mice
Figure 23: Cell death in P40 HuRtg
testes
Figure 24: Comparative analysis of WT and HuRtg
ARE-transcriptome
Figure 25: Gene ontology (GO) of up-regulated mRNAs in HuRtg
testis relative to GO of ARE-transcriptome
Figure 26: Specific binding of HuR or AUF1 to its target mRNAs
Figure 27: Increased half life of HuR traget mRNAs in HuRtg
testis
Figure 28: HuR and MVH colocalize within the chromatoid body Figure 29: HuR associates with polysomes in late spermatids
Figure 30: Analyzis of testis ARE-transcriptome and selection of two HuR-target
mRNAs
Figure 31: ARE-mRNAs transiently localized within the CB of spermatids Figure 32: Correlation between HuR accumulation in CBs/mRNPs of transgenic
spermatids and spermatid differentiation delay
Figure 33: Weakened translation of HuR-target mRNAs in HuRtg
testis
Figure 34: Model for HuR role during spermiogenesis
Figure 35: HuR
display normal spermatogenesis
Figure 36: Sycp1-Cre mRNA expression in testis
Figure 37: Measure of Sycp1-Cre recombinase efficiency in spermatocytes Figure 38: Measure of Vav-Cre recombinase efficiency in spermatocytes Figure 39: HuR inactivation in male germ cells
Figure 40: Analysis of putative germ cell-specific HuR
mice
Figure 41: Identification of putative Cancer/testis genes targeted by HuR.
Figure 42: Model of HuR-mediated mRNA storage and translation during
spermiogenesis
TABLES
1/ Introduction
Table I: Localization, expression and functions of the most studied AU-Binding
Proteins (AU-BPs)
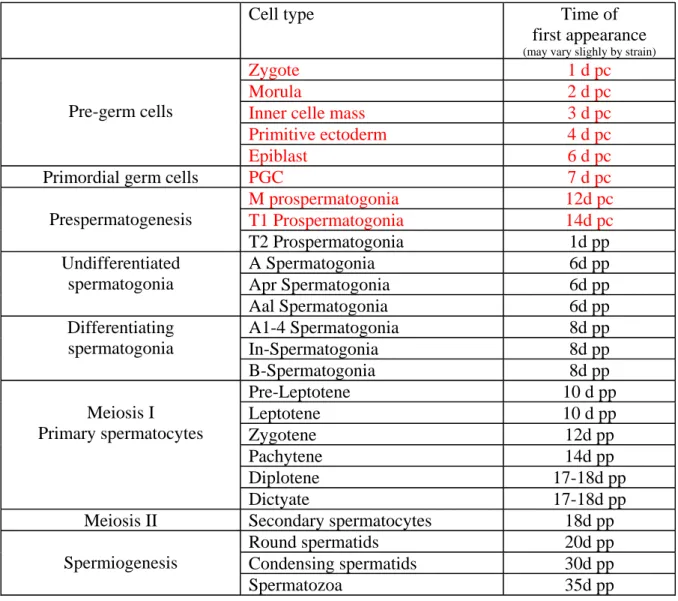
Table II: Time of germ cell first appearance in seminiferous tubules during mouse
testis ontogenesis
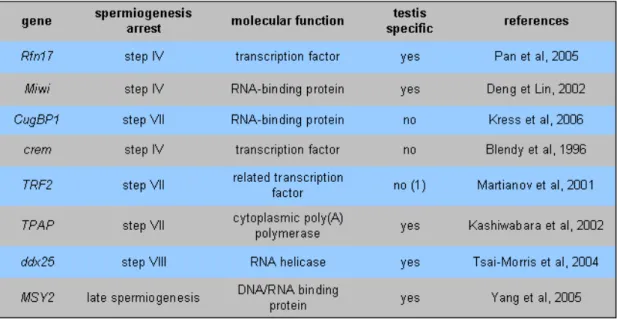
Table III: gene mutations leading to spermiogenesis arrest
2/ Results
Table IV: Mouse ARE-containing transcripts specifically expressed in the testis Table V: Mouse ARE-containing transcripts differentially expressed during
spermatogenesis with a peak of expression in meiotic stages (M) and bearing a conserved ARE between mouse and human
Table VI: Mouse ARE-containing transcripts differentially expressed during
spermatogenesis with a peak of expression in post-meiotic (PM) stages and bearing a conserved ARE between mouse and human
Table VII: Body and reproductive organ weights and sperm reserves of HuR transgenic
mice
Table VIII: Increased expression of mRNAs upon HuR transgene expression Table IX: Comparative analysis of transcriptomes from P28 HuR and AUF1 testes
Appendix
Appendix 1: human ARE-cDNA microarrays are useful to analyze mouse
Appendix 2: Reproducible microarray results for HuR transgene Appendix 3: Reproducible microarray results for AUF1 transgene
Appendix 4: HuR expression during the first wave of spermatogenesis and localization
in the chromatoid body
Appendix 5: Association of HuR and MVH with polysomes Appendix 6: Choice of HuR ARE-mRNA targets
Appendix 7: Consequences of HuR overexpression at different stages of testis
ontogenesis
Appendix 8: Numerous human ARE-containing transcripts are expressed in testis Appendix 9: Expression profile of mouse ARE-containing mRNAs
Appendix 10: eIF3 associates with MVH and localizes closed to the chromatoid body Appendix 11: GCNF mRNA regulatory sequences
Appendix 12: Primer table Appendix 13: Antibody table
Appendix 14: Different stages of squash preparations
Appendix 15: Contribution de la régulation post-transcriptionnelle à l’émergence de
maladies : L’enfance de l’ARE (review)
ARE AU-rich element
AU Adenine-Uridine
AU-BP AU-binding protein
CB chromatoid body
CRD coding region domain
IRE iron responsive element
IRES intern ribosomal entry sequence IRP iron regulatory proteins
KO knock out
miRNA micro RNA
nt nucleotid
P body processing body
PCR Polymerase chaine reaction PGC primordial germ cell pi RNA piwi interacting RNA poly(A) polyadénylated
RBP RNA binding protein
RNP ribo-nucleo particule
RRM RNA recognition motif
UTR untranslated region
RNA Ribonucleic-acid
EJC Exon Junction Complex
GM-CSF Granulocyte Macrophage Colony Stimulating Factor
DNA Desoxyribonucleic Acid
BRF1 Butyrate Response Factor 1
DCP1 RNA Decapping 1
eIF eukaryotic translation Initiation Factor GCNF Germ Cell Nuclear Factor
HSP Heat Shock Protein
IL11 Interleukin 11
IRES Internal Ribosome Entry Site
KH hnRNP-K Homology
LPS Lipopolysaccharide
m7G 7-methylguanosine
MAPK Mitogen Activated Protein Kinase
MVH Mouse Vasa Homolog
PABP Poly(A) Binding protein
PB Processing Body
SG Stress Granule
RISC RNA Induced Silencing Complex TNFα Tumor Necrosis Factor a
Gene expression is finely regulated in all organisms. Complex networks of genes drive development of embryos, homeostasis in adult, and determine tissue or cell specific response in a given situation. Understanding gene expression is difficult owing to a high variety of regulatory processes. Three types of regulation determine the level of gene expression: 1/ transcriptional control, mediated by transcription factors, cis-elements located on DNA and chromatin structure, which controls RNA molecule synthesis; 2/ post-transcriptional controls that determine the fate of previously transcribed messenger RNA (mRNA) and thus dictate whether mRNA is or not translated into protein; 3/ post-translational controls which affect the function or half-life of proteins. Although transcriptional and post-translational regulations are much more documented than post-transcriptional ones, it is now well established that the contribution of mechanisms that affect mRNA fate, such as control of its nucleocytoplasmic transport, degradation or translation is important and sometimes indispensable to guarantee completion of many biological processes.
PART I : ARE-mediated post-transcriptional controls
A. General steps of RNA metabolism
1/ Maturation
In eukaryotes, once transcribed in the nucleus, RNA associates with protein complexes that ensure its maturation. Some remain bound to the mRNA and form a ribonucleoparticle (RNP) (Moore, 2005). The immature RNA undergoes three main modifications: 1/ the splicing allows non-coding intron excision and thus restores the continuous coding sequence of RNA, 2/ the cap, a 5’-5’ phosphodiester link occurring at the 5’ end of the RNA, protects mRNAs from exonuclease activity and 3/ poly(A) tail is polymerized at the 3’end. All these modifications are indispensable for mRNA competence and ensure its stability, by protecting it from degradation (Jacobson and Peltz, 1996); (Sachs et al., 1997) (Figure 1 and 2). Before mRNA export to the cytoplasm, proteins of the Exon Junction Complex (EJC) bind the junction sites between exons (Tange et al., 2004) (Figure 2). Usually, these proteins promote translation. However, when mRNA contains a premature STOP codon (non sense codon), EJC induces mRNA degradation due to its localization close to this STOP codon (Le Hir and Seraphin, 2008; Maquat, 1995). mRNA export to the cytoplasm involves specific adaptator proteins (see below) or relies on localization signals present in RNP proteins (Kohler and Hurt, 2007).
2/ Translation
In the cytoplasm, translation is initiated by the binding of the initiation factor eIF4E to the mRNA cap together with the RNA helicase eIF4A and eIF4G. This complex recruits the 40S ribosomal subunit via its interaction with eIF3. This results in
Figure 1: mRNP life cycle
From their synthesis in the nucleus to their degradation in the cytoplasm, mRNAs associate with a large variety of proteins, forming ribonucleoprotein complexes (mRNPs). mRNA biogenesis and function require the concerted efforts of RNA-binding proteins (RBPs) that escort the mRNA transcript through its capping at its 5’ end, splicing, polyadenylation at its 3’ end, nuclear export, association with ribosomes and ultimate decay. Degradation might be accelerated when errors such as premature STOP codons, inducing non-sense-mediated decay are detected. From (Hieronymus and Silver, 2004).
Figure 2: A stereotype of coding messenger RNA
Different regions and sequences indicated on the figure are usually carried by all messenger RNAs. m7G is the cap modification at the 5’end, EJC (Exon Junction Complex) binds to exon-exon junctions and poly(A) tail is polymerized at the 3’end.
eIF1A and the ternary complex eIF2-GDP-Met-tRNA (Figure 2 and 3). AUG codons upstream of the main open reading frame can induce formation of a translation-competent ribosome. Indeed, upon recognition of the start codon, eIF5B stimulates GTP hydrolysis, resulting in the release of eIF2-GDP and probably of other 40 S-bound initiation factors. eIF5 catalyses the joining of the 60 S subunit to form 80 S ribosome and elongation can start. When the ribosome encounters a termination codon, the string of coupled amino acid residues is released, and the ribosome dissociates into its subunits and disconnects from the mRNA. Then, the ribosome re-initiates a round of translation (Figure 3). Usually, several ribosomes bind the mRNA forming thus a polysome (Moore, 2005). Interestingly, interaction between proteins associated with both the cap and the poly(A) tail bring closer the 3’ and 5’ ends, resulting in a circular mRNA. This phenomenon favours translation process and protects mRNA from degradation (Moore, 2005).
3/ RNA stability and degradation
RNA stability represents the mRNA life time and thus determines its availability for protein synthesis. mRNA stability relies on its protection from degradation and thus relies on the composition of RNPs. RNA degradation is mediated by non specific or specific pathways. The first step of degradation of all mRNAs (from yeast to mammalian cells) is the deadenylation process exerted by the CCR4-NOT complex at the 3' end of the mRNA (Ford et al., 1997; Parker and Sheth, 2007). This event results in a block of translation and allows the accessibility of mRNA to exonucleases. At the 5' end, the 5’-3’ degradation begins with cap excision by the decapping complex, containing the catalytic enzyme DCP2 and many other cofactors, including DCP1 (reviewed in (Simon et al., 2006)). The deccaped mRNA is then degraded by the exonuclease XRN1 from the 5’ extremity to the 3’ one. Alternatively or simultaneously, mRNAs can be degraded by the 3’-5’ degradation pathway. A complex of 9 proteins forms the exosome that degrades mRNA from 3’ extremity (Parker and Sheth, 2007). Different RNA-binding proteins (RBPs) either recruit these enzymes or by contrast protect mRNAs from their action (see below) (Figure 1 and 7).
Figure 3: Schematic presentation of the initiation of protein synthesis
Protein synthesis requires the assembly of 80S initiation complex on the mRNA. This complex consists of the two ribosomal subunits, the mRNA, and the ternary complex (eIF2 ± GTP ± Met-tRNA). According to the ribosomal scanning model, the 40S ribosomal subunit lands next to the 5’ cap structure and scans the 5’ UTR until an
B. ARE-mediated
post-transcriptional controls
1/ Cis-acting element
Messenger RNAs are regulated during all their life. Regulation acts at every single step: transport, stability and efficient translation. Regulatory mechanisms vary considerably from one mRNA species to another or according to the cellular context and are mediated by sequences within the RNA molecule. Some of these sequences are present in all mRNAs (Figure 2), others are more specific. As previously described, the cap and poly(A) tail are crucial for post-transcriptional regulation of all mRNAs. For instance, shortening of poly(A) tail (<30nt) of most mRNAs, except histone mRNAs which are not polyadenylated (Marzluff et al., 2008), usually induces translation inhibition and degradation, whereas long poly(A) tail (> 30nt) is sufficient to stabilize RNA in vitro (Beelmen and Parker, 1995). Post-transcriptional events could also be mediated by sequences present in the 5’ UTR or coding region of an mRNA. For example, the IRES (Internal Ribosome Entry Sequence), a sequence forming secondary RNA structure, found in the 5’ UTR of some mRNAs (such as c-myc and Igf-IR), directs internal binding of ribosomes and promotes the formation of a cap-independent translation initiation complex (Meng et al., 2005; Stoneley et al., 1998). Other motifs can be found in the coding region of mRNAs, such as the coding region domain (CRD) in c-myc mRNA which is recognized by the CRD-binding protein that protects c-myc mRNA from endonuclease action ((Sparanese and Lee, 2007) and reviewed in (Nguyen-Chi and Morello, 2008) and Appendix 15). Finally, 3’ UTRs are involved in post-transcriptional control of many mRNAs. For instance, the 3’ UTR of transferrin
receptor mRNA contains an instability determinant closed to a hairpin structure called
IRE (Iron responsive element). This later one is bound by IRP (Iron-regulatory proteins) when the iron level increases in the cell, resulting in accelerated mRNA degradation (Hentze and Kuhn, 1996).
One of the most studied cis-acting elements involved in mRNA degradation in mammalian cells is the adenylated and uridylated-rich (AU-rich) element (ARE) found in the 3’UTR of many mRNAs (Figure 4). ARE represents thus far the most widespread
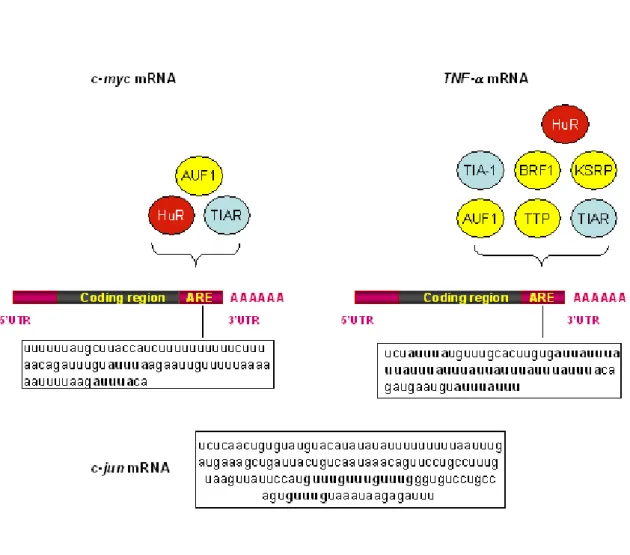
Figure 4: Diversity of ARE sequences and AU-BPs with which they interact
Initially classified into three different groups (classes I-III) (Chen and Shyu, 1995), AU-rich elements (ARE) belonging to the class II were recently divided into 4 sub-groups according to the number of AUUUA iteration (Bakheet et al., 2006). The ARE of c-myc (nt 2061-2141 NM-002467) and TNFα (nt 1311-1385 NM-000594) mRNAs illustrate the group I and group II, sub-group 3, respectively. By contrast, ARE of c-jun mRNA (nt 2401-2542 J04115), an example of group III does not contain any AUUUA pentamer but a U-rich region and several GUUUG motifs. Two scattered AUUUA in a U-rich context are present in c-myc ARE, whereas the pentamer is repeated 8 times, 6 being clustered, in TNFα ARE. The AU-Binding Proteins (AU-BPs) interacting with
c-myc and TNFα AREs are indicated (destabilizing AU-BPs are represented in yellow, translational regulators in blue and the only ubiquitously expressed stabilizing factor in red). miR16 contains the UAAAUAUU sequence and thus can hybridize with several sites of TNFα ARE (Jing et al., 2005). From (Nguyen-Chi and Morello, 2008).
and efficient determinant of RNA (in)stability in mammalian cells. The ARE was discovered by Show and Kamen, twenty years ago, on the growth factor, GM-CSF (Granulocyte Macrophage Colony Stimulating Factor) mRNA and was shown to induce its degradation (Shaw and Kamen, 1986). So far, it has been found by bioinformatic approach on approximatively 8% of mammalian coding mRNAs (Bakheet et al., 2006). A 13 base pairs ARE motif (WWWUAUUUAUWW) was first computationally derived from functional AREs, such as those of GM-CSF, TNFα, c-fos 3’ UTR and was
identified in more than 4000 mRNAs mapped in the entire human genome (http://brp.kfshrc.edu.sa/ARED) (Bakheet et al., 2001; Bakheet et al., 2006). As different AREs differ considerably in length, AU-content and the number of the characteristic AUUUA pentamer they contain, it is not clear which features represent the functionally critical element. However, AREs were divided into subclasses according to 1/ the presence of AUUUA motif, 2/ the number of AUUUA iterations and 3/ the motif organisation (scattered or clustered) (Bakheet et al., 2006; Chen et al., 1995) (Figure 4). ARE sequence alone is not sufficient to control the life of the RNA, but its activity depends on the recruitment of proteins called AU-BPs (AU-Binding Proteins) (Table I).
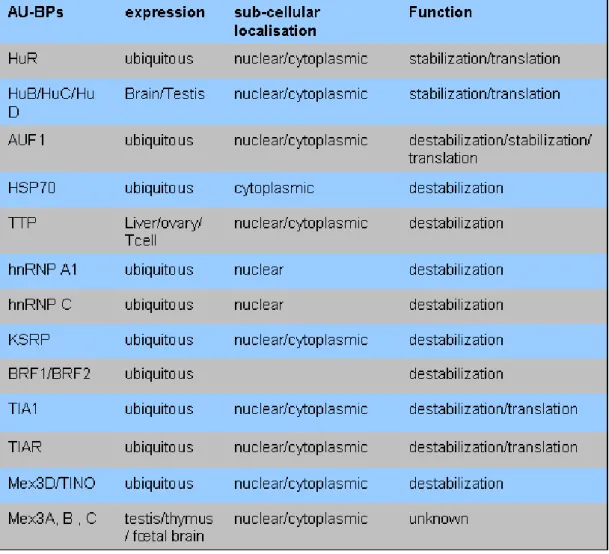
2/ AU-Binding proteins
AU-BPs participate to different steps of mRNA life. They were first studied for their role in mRNA stability control. Proteins of the ELAV/Hu family stabilize ARE-containing mRNAs by preserving them from degradation (Bevilacqua et al., 2003; Ma et al., 1996). The family consists of four highly conserved members that include the ubiquitously expressed HuR/HuA/Elavl1 and the neuronal and testis specific Hel-N1/HuB/Elavl2, HuC/Elavl3 and HuD/Elavl4 proteins (Brennan and Steitz, 2001; Keene, 1999). All family members contain 3 RNA recognition motifs (RRM) with high affinity for ARE (Figure 5). Other AU-BPs, like KSRP, tristetraprolin (TTP), the related Butyrate Response factor (BRF) proteins and TINO/RKHD1/MEX3D induce degradation (Bevilacqua et al., 2003; Donnini et al., 2004). Finally, some of them as AUF1/hnRNPD which comprises 4 different isoforms (Figure 5) play a double role, stabilizing or destabilizing ARE-mRNAs, according to either the isoform considered or the cellular context (Figure 5). Numerous studies have shown that in addition to their
Figure 5: Schematic representation of the two AU-BPs HuR and AUF1
HuR protein contains 3 RNA recognition motifs (RRM) by which it binds RNA sequences (Bevilacqua et al., 2003). HuR shuttling function between nucleus and cytoplasm relies on HuR Nucleo-cytoplasmic Shuttling sequence (HNS) (Fan and Steitz, 1998). The different sites of potential phosphorylation by the indicated kinases are shown. The phosphorylation state of HuR also influences its sub-cellular localisation (see also Figure 7) (S=Serine; T=Threonine; aa=amino acid). Four isoform of AUF1 exist resulting from alternative splicing of the same pre-mRNA. The shorter one, p37, is believed to harbour the strongest affinity for RNA. The two indicated Serines (83-87) upstream of RRM1 are subjected to phosphorylation that modifies AUF1 binding affinity for its target mRNAs (Wilson et al., 2003).
(de)stabilizing properties, several AU-BPs, as TIA/TIAR and ELAVL1/HuR, also control mRNA translation or storage, depending on the cellular context (Table I) (Kawai et al., 2006).
Generally, the more AUUUA iterations an mRNA contains the more efficient is the interaction between AU-BPs and AREs (Bevilacqua et al., 2003). ARE sequences might also form secondary structures that are also recognized by AU-BPs (Lopez de Silanes et al., 2004); (Bevilacqua et al., 2003). For instance, RNA sequence targets for the AU-BP HuR were used in computational analyses to identify and characterize HuR motifs, based on both primary RNA sequences and secondary structures. The identified motif comprising 17–20 nucleotides was suggested to form a stem loop secondary structure (Lopez de Silanes et al., 2004), which might contribute to mRNA translation, degradation and localization (Mignone et al., 2002).
Generally speaking, different AU-BPs are supposed to bind the same ARE and, owing to their stabilizing or destabilizing activity, can either synergize or compete. Such interplays have been studied using both cell cultures and in vivo mouse models. For example, wide scale analysis using microarray in HeLa cells, reveals that AUF1 and HuR bind to many common ARE-target mRNAs while exerting opposite influence on the stability of their target mRNA. Indeed AUF1 and HuR both bind p21 and cyclin D transcripts simultaneously on non-overlapping or common sites in the nucleus and the cytoplasm of HeLa cells, respectively, in a competitive fashion (Figure 6) (Lal et al., 2004). In addition, functional interplay between HuR and TIA-1 was recognized through their ability to control synergistically the translation of cytokine mRNAs in macrophages of HuR overexpressing transgenic mice (Katsanou et al., 2005). Conversely, translation rate of cytochrome c mRNA in HeLa cells is determined by the opposite influence of HuR and TIA-1 since 1/ both bind the 3’UTR of cytochrome c mRNA on different binding sites and 2/ HuR and TIA-1 are required for activation and inhibition of cytochrome c mRNA translation, respectively. Under unstressed condition, both AU-BPs seem to be required to maintain a proper translation of cytochrome c, but in response to endoplasmic reticulum stress, HuR binding to cytochrome c mRNA decreases, resulting in the decreased translation of its target mRNA (Kawai et al., 2006). Thus, the balance between different AU-BPs present in the cell determines ARE-mRNA fate.
Table I: Localization, expression and functions of the most studied AU-Binding Proteins (AU-BPs)
An intriguing observation in HeLa cells is that AU-BP expression is influenced post-transcriptionally by AU-BPs themselves. The autoregulation is exemplified by the fact that most AU-BPs (AUF1, HuR, TIA-1 and TIAR) might associate to their own mRNA and thus influence their own expression. For instance, HuR silencing leads to the decrease of HuR protein level (Pullmann et al., 2007) and AUF1 overexpression leads to accelerated AUF1 mRNA decay (Banihashemi et al., 2006). However, this AU-BP-mediated posttranscriptional regulation is extended to other AU-BPs, since most AU-BP mRNAs contain in their 3’UTR an ARE and are thus likely to be regulated by many AU-BPs. The cross-regulation is illustrated by the case of HuR and TIA-1 mRNAs. HuR expression up-regulates TIA-1 protein expression (Pullmann et al., 2007). These results reveal the complexity of the relationships between AU-BPs and point to the importance of their regulated expression, a field that is still poorly documented.
3/ Conservation of AREs and AU-BPs
Several studies performed in Yeast and Drosophila suggest that ARE-mediated post-transcriptional regulation is conserved during evolution. Indeed, in Saccharomyces
cerevisiae, the AU-BP Cth2, homolog of TTP, binds specifically AREs of mRNAs
encoding proteins that participate in iron-dependent processes in response to iron deficiency (Puig et al., 2005). Similarly, using reporter RNA containing an ARE from mammalian unstable transcripts, Jing and colleagues demonstrate that ARE promotes mRNA decay in Drosophila S2 in vitro cultured cells. Their decay requires dTIS11, the
Drosophila homolog of the mammalian TTP (Jing et al., 2005). In addition, many
conserved AU-BPs were described in the Drosophila (Campos et al., 1985; Gamberi et al., 2006) and the Drosophila Melanogaster genome contains many putative ARE-transcripts, as revealed recently by our laboratory through the computational search of the mammalian ARE motif (Cairrao et al, submitted).
4/ AU-BP post-translational modifications, localisations and functions
Most of AU-BPs are nuclear but shuttle between nucleus and cytoplasm, and numerous reports show the physiological relevance of AU-BP sub-cellular localisation
Figure 6: Different ARE-mRNA fate when bound by HuR or AUF1
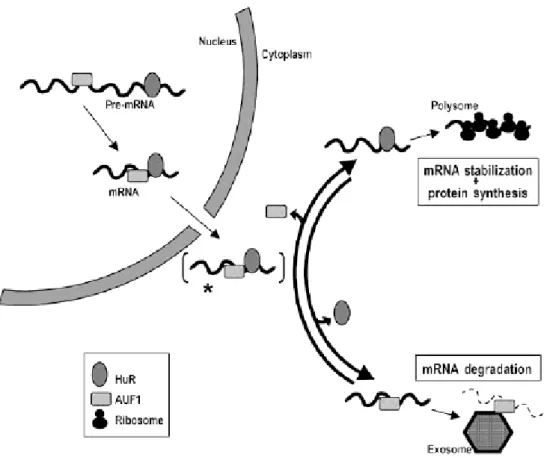
In this model, HuR and AUF1 can simultaneously bind to the same target mRNA, likely in its pre-mRNA state, in the form of stable nuclear RNP complexes. In the cytoplasm, a given mRNA will be preferentially found in association with HuR and/or AUF1 depending on a number of factors, including its nucleotidic sequence, the relative abundance of HuR and AUF1, the activity of different signalling pathways, and the state of the cells under analysis (unstressed or stressed). A dynamic interplay among these elements will ultimately dictate the cytoplasmic fate of the mRNA: HuR-bound mRNAs will likely be translated, whereas AUF1-bound mRNAs will undergo rapid exosome-mediated decay. From (Lal et al., 2004).
to fulfil their activity. For instance, HuR distribution between nucleus and cytoplasm, which depends on various signalling pathway (see below), has been proposed to regulate cellular growth and differentiation ((Gallouzi et al., 2001) and reviewed in (Doller et al., 2008)). Indeed, HuR is predominantly (>90%) localised in the nucleus of unstimulated cells, except nucleolus, where it binds to SETα, SETβ, pp32, and APRIL proteins. Using cell-permeable peptides, HuR export was shown to involve pp32 and APRIL which contain leucine-rich nuclear export signals that are recognized by the export receptor chromosome maintenance region 1 (CRM1) protein (Gallouzi et al., 2001; Gallouzi et al., 2003). Treatment with the CRM1 inhibitor leptomycin B causes nuclear accumulation of pp32 and APRIL, as well as that of HuR (Gallouzi et al., 2003). Alternative pathways of nuclear export have been proposed to involve a shuttling sequence within HuR, the HuR nucleocytoplasmic shuttling sequence (HNS), which bears similarities with the M9 shuttling signal of hnRNP A1 (Figure 5) (Fan and Steitz, 1998).
Furthermore, increased function of AMP-activated protein Kinase (AMPK), an enzyme involved in stress metabolic response, leads to reduce cytoplasmic level of HuR. Wang and colleagues showed that AMPK signalling pathway modifies post-translationally importin α, an adaptator protein involved in nuclear import, contributing thus to HuR nuclear import. Since the decay of ARE-bearing mRNAs is thought to occur in the cytoplasm (see below), HuR has been proposed to elicit its mRNA-stabilizing function by binding its target mRNAs in the nucleus, exporting and protecting them during cytoplasmic transit, and facilitating their recruitment to the translational machinery (Fan and Steitz, 1998; Keene, 1999). Thus AMPK activation results in the decrease of level and half-life of HuR-regulated mRNAs, most of which encode prominent cell growth actors, including P21, Cyclin A and Cyclin B (Wang et al., 2004).
Other signalling kinases that regulate HuR sub-cellular abundance and/or association with specific target mRNAs have been reported. Activation of MAPK (mitogen-activated protein kinase)-(mitogen-activated protein kinase 2 (MK2), a downstream target of MAPK p38 involved in inflammatory stimuli response, elevates cytoplasmic HuR levels and increases its ability to bind its target mRNAs encoding Cox2 (Jin et al., 2007),
Figure 7: Different signalling pathways control HuR nucleo-cytoplasmic shuttling and function
Different post-translational modifications of HuR itself or of its interacting proteins influence HuR shuttling between nucleus and cytoplasm and HuR stabilizing activity towards its target mRNAs, some of which are indicated. (1) (Wang et al., 2004), (2) (Subbaramaiah et al., 2003), (3) (Doller et al., 2007), (4) (Kim et al., 2008).
TNFα (Dean et al., 2001), urokinase-type plasminogen activator and its receptor (Tran
et al., 2003). However, HuR does not contain putative phosphorylation sites for MAPKs and therefore, a direct modulation of HuR shuttling or HuR RNA-binding affinity via MAPK-induced phosphorylation seems unlikely (Doller et al., 2008).
Doller and his colleagues reported that PKCα, a member of the serine/treonine kinase family activated following increased ATP level, phosphorylates HuR. This post-translational modification thus induces HuR export in cytoplasm of human mesangial cells (MC). Indeed ATP-dependent HuR shuttling in human MC is strongly impaired by specific PKCα inhibitors as well as small interference (si) RNA inducing PKCα silencing (Doller et al., 2007).
Finally, a recent study demonstrates that in HeLa cells, CDK1, the G2-phase kinase (also known as cell division cycle 2, Cdc2) phosphorylates HuR during G2-phase at S202, near the HNS, preventing cytoplasmic accumulation of HuR. This phosphorylation leads to HuR association with nuclear 14-3-3 protein. Accordingly, the nonphosphorylatable mutant HuR (S202A) has a decreased association with 14-3-3 and a more pronounced cytoplasmic accumulation leading to the increased expression of HuR target mRNAs implicated in cell proliferation and cell survival. These results suggest that CDK1 S202 phosphorylation of HuR retains the AU-BP in the nucleus in association with 14-3-3 during G2, preventing HuR post-transcriptional function (Kim et al., 2008). The various signalling pathways controlling HuR shuttling between the nucleus and the cytoplasm are summarized in Figure 7.
The case of AUF1 is more complex due to the fact that it encodes a family of four isoforms generated by alternative splicing denoted from their apparent molecular weights as p37AUF1, p40AUF1, p42AUF1, p45AUF1 (Bevilacqua et al., 2003), Figure 5). All isoforms primarily localize in the nucleus. However, heterokaryon analysis revealed that p40 and p37 exhibit a continuous shuttling between the nucleus and cytoplasm. Conversely, p42 and p45 appear exclusively in the nucleus and have been shown to interact physically to the scaffold attachment factor B (SAF-B), a nuclear matrix-associated protein (Arao et al., 2000). Function and nucleocytoplasmic localization of AUF1 is thus isoform-specific. Moreover, two serines present upstream of RRM1 are
subjected to phosphorylation upon conditions which mimic inflammatory response, resulting to a decreased binding to their ARE-target mRNAs ((Wilson et al., 2003)and Figure 5). Post-translational modifications of other AU-BPs, such as TTP (Sandler and Stoecklin, 2008), KSRP (Briata et al., 2005) or Mex3B (Courchet et al., 2008) have also been described. They might modify AU-BP ability to bind its target, similarly to AUF1. Alternatively, they might induce AU-BP association with proteins of the 14-3-3 family, leading to their trapping in cytoplasmic compartments where AU-BPs are no more available for degradation (He and Schneider, 2006).
AU-BPs do not bear intrinsic enzymatic activity but might be considered as adaptative proteins that recruit different cellular machineries involved in mRNA translation, degradation or selective storage (see above). Actors of these machineries are concentrated with mRNAs in cytoplasmic foci called granules or bodies. The exosome, a complex of 3’-5’ exoribonuleases, has been considered for a long time as the only actor of ARE containing mRNA decay (Chen et al., 2001). Small granules concentrating ARE-mRNAs and exosome components were recently described (Lin et al., 2007). However, ARE-containing transcripts are also localized in cytoplasmic foci called processing bodies (P bodies or PBs). These foci correspond to aggregates of translationally repressed mRNAs associated with translation repression and mRNA decay machinery. The complete protein composition of P bodies is not yet determined, but they include decapping enzymes (DCP1/DCP2) that cleave the mRNA cap in its 5’ end, XRN1, a 5’-3’ activity exoribonulease, GW182 and the RISC complex (RNA Induced Silencing Complex), a microRNA associated degradation machinery (Eulalio et al., 2007; Liu et al., 2005; Pillai et al., 2007; Rehwinkel et al., 2005). P bodies associated mRNAs might exit the PBs and reenter translation, as shown with the case of
Cat-1 mRNA whose localisation in PBs involves the miR122 (See below and
(Bhattacharyya et al., 2006)).
In addition to P bodies, ARE-containing mRNAs can be targeted to another type of RNA granule termed stress granule (SG). This cytoplasmic structure is formed under stress condition by aggregates of untranslating mRNAs in conjunction with a subset of translation initiation factors (eIF4E, eIF4G, eIF4A, eIF3, eIF2), the 40S ribosomal
Figure 8: Ephemera localization of mRNAs in different cytoplasmic granules
mRNAs are localized in different cytoplasmic structures where they are either stored (stress granules and P-bodies) or degraded (exosome bodies and P-bodies). Following activation of a given signalling pathway, mRNAs can be released from these granules and actively translated in polysomes. mRNA localizations are very dynamic and rely on the composition of proteins and small RNAs associated to a given mRNA at e precise moment. (Illustration modified from (Parker and Sheth, 2007)).
formation (reviewed in (Parker and Sheth, 2007). Stress granules and P bodies share several actors and recent results showed that in mammalian cells treated with sodium arsenite which mimics an oxidative stress, they could physically interact. The destabilizing AU-BP TTP, could favour their association. Microscopy on living cells shows that stress granules, P bodies and their components are dynamic (Kedersha et al., 2005). The functional significance of stress granules is still unclear. One possibility is that under stress conditions, sequestration of mRNAs into stress granules represents a form of translational repression, allowing only the translation of mRNAs that are not trapped into SGs such as those encoding stress proteins. SGs have been also proposed to act as triage centers, where mRNAs are stored before being reoriented towards polysomes or P bodies. A model of ARE-mRNA cycling between different cytoplasmic compartments is shown in Figure 8.
Actively translating mRNAs are covered by several ribosomes (polysomes) in the cytoplasm. Translation factors and ribosomes are enriched in regions where translation is favoured, such as around endoplasmic reticulum, or where newly synthesized proteins are required. Localized translation is an important and energy-efficient mean of accumulating proteins in a specific site. mRNAs associate to cytoskeleton and traffic from their translationally repressed sites to their translation sites. Examples of this mode of localization can be found in oligodendrocytes, where mRNAs such as those encoding myelin basic protein are transported in granules to the dendritic synapse, in fibroblasts, where β-actin mRNA is localized to the leading edge
of the cell during migration, or in Drosophila embryos, where mRNAs such as bicoid are transported to the appropriate pole (reviewed in (Hoyle and Ashe, 2008). So far, this type of regulation has not been yet described for ARE-containing mRNAs.
5/ Small non coding RNAs
In the past 15 years, the discovery of RNA-silencing phenomena that are mediated by expending assortment of small, non-coding RNAs, has revealed an unanticipating mechanism of post-transcriptional modulation of gene expression (Lee et
Figure 9: Post-transcriptional regulation by microRNAs and small interfering RNAs
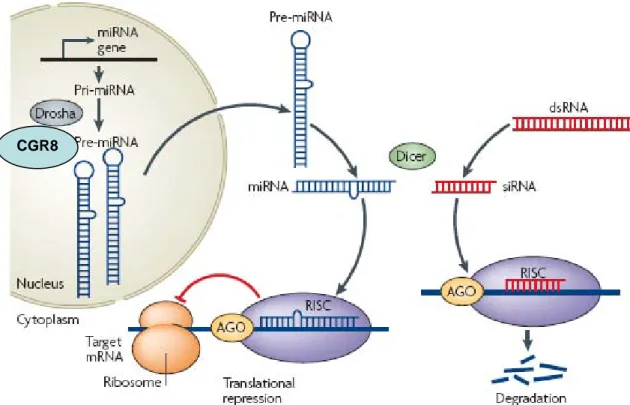
RNA interference (RNAi) and microRNA (miRNA) pathways are evolutionarily conserved control mechanisms that use RNA molecules to inhibit gene expression at the level of mRNA degradation, translational repression, or chromatin modification and silencing. Two classes of small RNAs, small interfering (si)RNAs and miRNAs, function as sequence-specific regulators of gene expression. miRNA precursors that are encoded by endogenous genes are folded into double-stranded (ds) RNA-like hairpins. The precursor molecules (pre-miRNAs) are first processed from primary miRNA transcripts (pri-miRNAs) by the nuclear enzyme Drosha and its co-factor CGR8 and the pre-miRNAs are then transported to the cytoplasm. siRNAs are generated from longer dsRNA precursors formed in cells by DNA- or RNA dependent synthesis of complementary strands, or introduced into cells by viral infection or artificial expression. Both siRNA and miRNA precursors are processed to mature small RNAs in the cytoplasm of cells by the large endonuclease Dicer. Mature miRNAs and siRNAs are assembled into an RNA-induced silencing complex (RISC), which subsequently functions on targets by translational repression or mRNA cleavage. Members of the Argonaute/PIWI family of proteins, which have PAZ and PIWI domains, are essential components of the RISC. siRNAs mediate cleavage of mRNAs that have sequences fully complementary to their sequence, whereas miRNAs are proposed to regulate gene expression by inhibiting protein synthesis or affecting the stability of mRNAs through imperfect base-pairing to the 3′ untranslated regions of target mRNAs. From (Kotaja and Sassone-Corsi, 2007).
and piwi-interacting RNAs (piRNAs) are implicated in various aspects of animal development (reviewed in (Stefani and Slack, 2008).
miRNAs constitute a class of short (19-25 nucleotides), single-stranded RNAs that are present in plants and animals. miRNAs can be encoded in independent transcription units, in polycistronic clusters or within the introns of protein coding genes (Bartel, 2004). They are transcribed mostly by RNA polymerase II, as capped and polyadenylated primary miRNAs (pri-miRNAs) that contain extended hairpin structures. Pri-miRNAs are cleaved in the nucleus by the RNAse III enzyme, Drosha, and its associated factor DGCR8, releasing the precursor miRNA (pre-miRNA) hairpin structure. Independently of Drosha, a subset of pre-miRNA hairpins can also be generated from introns by the combined actions of the spliceosome and the lariat-debranching enzyme (LDBR) (reviewed in (Bartel, 2004)). On export into the cytoplasm by Exportin-5, the pre-miRNA is further processed by a second RNase III, Dicer, which excises a 19-25 nt double-stranded duplex. This short duplex is incorporated into the functional miRNA induced silencing complex (miRISC), where the mature miRNA strand is preferentially retained. The RISC contains miRNA, Argonaute proteins and other factors and is the effector complex of the miRNA pathway (Figure 9). However, the totality of factors involved in miRNA biogenesis is still to be discovered.
The miRISC is directed to mRNAs that are complementary to its miRNA component. miRISC inhibits the expression of mRNA in one of the two following mechanisms, according to the degree of complementarity between miRNA and the target. When complementarity is perfect, as is mostly the case in plants, the target mRNA is cleaved and degraded. By contrast, the complementarity between miRNAs and their target in animals is frequently imperfect, such that in most cases it is not the stability of the target mRNA that is affected but its translation. Various mechanisms have been proposed such as translation inhibition at initiation or elongation step, rapid degradation of nascent peptide, mRNA sequestration into P bodies and mRNA degradation (reviewed in (Pillai et al., 2007)). The impact of miRNA mediated biological regulation is expected to be wide: hundreds of miRNAs have been cloned
that overexpression of a single miRNA can result in decreased expression of more than one hundred mRNAs, strongly suggesting that a large fraction of protein-coding genes are regulated by miRNAs (Lim et al., 2005).
6/ MicroRNA and ARE-mediated control
Different recent studies describe a functional interplay between ARE-mediated control and microRNA pathway. Components of the microRNA machinery have been shown to accumulate in P bodies (reviewed in (Pillai et al., 2007). In human hepatocarcinoma cells, without stress, the cationic amino acid transporter (Cat-1) mRNA normally localizes in the P bodies because of its association with miR122. In response to different stresses, HuR is mobilized from the nucleus to the cytoplasm. HuR relocalization enables Cat-1 mRNA recruitment to polysomes by preventing its association with the microRNA miR122 and subsequent storage in PBs, leading to translational increase of Cat-1 mRNA (Bhattacharyya et al., 2006). Another example of a link between AU-BPs and miRNAs is given by the work of Jing et al. They showed that, in Drosophila cultured cells expressing a reporter mRNA containing the mammalian TNFα ARE, dTIS11 recruits the microRNA, miR16, on the ARE sequence of TNFα mRNA by interacting with a key component of miRNA machinery, the AGO2 protein. This association leads to the degradation of the ARE-containing reporter mRNA (Jing et al., 2005). More recently our laboratory has shown that Dicer silencing induces stabilization of Drosophila endogenous ARE-mRNAs, such as cecropin mRNA, in Drosophila cells (Vanzo, unpublished data).
7/ ARE-mediated control and diseases
Even though deletion of genes encoding for AU-BPs have not yet been reported in human pathology, AU-BPs-null mice have been experimentally generated. Mice deficient in the TTP-family member BRF1 die around midgestation due to placenta failure (Stumpo et al., 2004). This phenotype is reminiscent of the observation that HuR is required for embryonic development since HuR-KO mice die between 10.5 to 14.5 dcp due to placenta defects (Katsanou et al, submitted). However, AREs and AU-BPs
are not only required for embryonic developmental processes but play also a fundamental role in regulating inflammatory response gene expression in adult. Most of cytokines and chemokines produced during immune response are encoded by unstable ARE-containing RNAs, such as TNFα, Il-1β, Il-6, Il-8 and GM-CSF. Experimental
Knock-In deletion of the ARE of the proinflammatory cytokines TNFα in mice led to
TNFα mRNA stabilization and spontaneous production of TNFα protein (Kontoyiannis
et al., 1999). Mice bearing this mutation develop an inflammatory chronic arthritis and intestinal inflammation. Interestingly, the Knock-Out mice which do not express TTP exhibit similar symptoms (Taylor et al., 1996). Ex vivo analyses revealed that TTP destabilizes TNFα mRNA. In accordance with these results, macrophages from TTP -/-mice express more TNFα than WT macrophage (Carballo et al., 1997). Furthermore,
TIA-1 targeted deletion does not lead to any apparent defect (Piecyk et al., 2000).
However, in response to LPS injection which induces septic-shock-like responses (endotoxemia), mice develop inflammatory chronic arthritis with high level of TNFα, that is not due to alteration in TNFα mRNA stability, but de-repression of TNFα mRNA translation. Combining TTP- and TIA-1-KO leads to more severe arthritis than single mutant (Phillips et al., 2004). Finally, the increased susceptibility of TIA-1-/- mice to endotoxin challenge is reminiscent of the phenotype of mice lacking the auf1 gene (Lu et al., 2006). After LPS stimulation, AUF1-/- mice are acutely susceptible to endotoxin, since a typically sub-lethal dose of endotoxin leads to a fivefold lower survival rate of AUF1 knockout mice. This treatment induces overexpression of the TNFα and IL-1β cytokines which was shown to result from abnormal stabilization of
TNFα and IL-1β mRNAs in macrophages. These experiments show that AUF1 and TIA-1 control TNFα by different mechanisms and demonstrate that a precise control of cytokine expression relies on regulation at multiple levels ((Lu et al., 2006; Nguyen-Chi and Morello, 2008; Phillips et al., 2004) and Appendix 15).
ARE-mediated post-transcriptional regulation is emerging as a particularly important regulation process in cancer. Alteration of ARE sequences in the 3’UTR of critical mRNAs or modification of AU-BP expression level, localization or capacity to bind AREs can lead to cancer development. In this manner, a mutation or deletion of the
been observed in several cancers. As an example, c-Myc is a proto-oncogene whose expression is largely controlled at the level of mRNA stability. Different translocations leading to ARE-containing 3’UTR deletion have been described in human malignant hemopathies (Figure 4 and reviewed in (Lopez de Silanes et al., 2007)). In addition AU-BP overexpression is observed in malignant tumours and numerous studies have focused on HuR. Subcutaneous injection of HuR overexpressing RKO colon cancer cells into nude mice produced significantly larger tumours than those arising from control populations, whereas RKO cells expressing reduced HuR gave rise to significantly smaller and slower-growing tumours. Another AU-BP whose mis-regulation could lead to cancer development is AUF1. Transgenic mice overexpressing one isoform of AUF1 (p37) exhibit altered levels of expression of several target mRNAs, such as c-myc, c-jun, c-fos, GM-CSF. Moreover the transgenic line with the highest amount of p37 developed sarcomas (Gouble et al., 2002).
Apart from embryonic development and cancer, AU-BPs have been shown to be involved in various processes of differentiation, such as myogenesis (Briata et al., 2005) and gametogenesis. Germ cell targeted HuR overexpression in transgenic mice leads to impaired transgene transmission to the offspring because of spermatogenesis alteration ((Levadoux-Martin et al., 2003) and see below). Moreover, TIAR-KO mice, when they survive, are sterile because TIAR is required for primordial germ cell proliferation in male gonads (Beck et al., 1998). Numbers of genetic mouse models have been generated to facilitate the study of AU-BP function in vivo. However, several AU-BP mutant mice are embryonic lethal, such as HuR-KO (Katsanou et al, submitted), TIAR-KO (Beck et al., 1998) and members of TTP family (Stumpo et al., 2004). Appropriate genetic tools based on floxed alleles and mice that express cell-specific Cre recombinase are thus required to bypass embryonic lethality and study involvment of a given AU-BP in post-natal processes.
Even though we learnt a lot on ARE/AU-BP interaction from cultured cells, mechanisms of ARE-mediated regulation and its contribution in gene expression in vivo remain poorly understood. During my PhD, I focused on two AU-BPs: HuR and AUF1 and studied their role in a highly regulated developmental process, the spermatogenesis. As gametes rely heavily on post-transcriptional mechanisms to regulate their differentiation, they constitute an excellent system to study the function of these two
proteins. Indeed, similarly to eggs, where maternal RNAs are stored and selectively activated or degraded during development, in male germ cells, transcription ceases during spermiogenesis, necessitating post-transcriptional regulation of paternal mRNAs to fulfil spermatozoa differentiation. In the next chapter, I will describe spermatogenesis and give some key elements to catch the complexity of post-transcriptional regulations in germ cells.
PART II: Spermatogenesis
A. Spermatogenesis: a male germ cell differentiation
process
In all species, reproductive function depends on the ability of the individual to produce functional differentiated gametes. Indeed, this event called gametogenesis is a critical process in transmission of genetic material to subsequent generations. Spermatogenesis is a cyclic and elaborated process by which diploid spermatogonia differentiate into mature spermatozoa. It is marked by dramatic cell proliferation and differentiation. Spermatogenesis can be divided into three phases: mitotic, meiotic and haploid.
1/ Mitotic phase
Spermatogenesis starts in the seminiferous tubules of testis with undifferentiated spermatogonia (stem cell). A part of them transforms into differentiate type A1 spermatogonia. Through a well controlled series of mitotic cell divisions, Type A1 spermatogonia gives rise to a high number of more advanced spermatogonia, in the mouse identified as A2, A3, A4 intermediates and Type B spermatogonia. Type B spermatogonia enter meiosis to become spermatocytes.
2/ Meiosis phase
Meiosis is a special process of reductional cell division by which a diploid spermatogonia (2N) gives rise to four gametes (1N) containing half the number of chromosomes. Meiosis starts in primary spermatocyte with DNA replication that duplicates each chromosome into two sister chromatids (4N). After replication, the paternal and maternal copies of each chromosome pair synapse and undergo
Figure 10: Meiotic division
Schematic illustration of meiosis. Two homologous chromosomes (maternal and paternal) are represented, each one containing a centromere and 2 genes. Each gene exists in 2 different alleles (A and a; B and b). Meiosis begins with duplication of chromosome (bichromatidian chromosome). The first meiotic division separates the two homologous chromosomes and the second one, the two sister chromatids. Crossing over allows the generation of four haploid cells carrying different genetic material.
recombination during the prophase of the first meiotic division, which is further divided into five stages: preleptotene, leptotene, zygotene, pachytene and diplotene. This first meiotic division separates homologous chromosomes resulting in two secondary spermatocytes (2N), which almost immediately enter the second meiotic division. During this second meiotic division, the sister chromatids separate resulting in four spermatids (Figure 10).
3/ Haploid phase
After meiosis, the haploid round spermatids do not divide further but differentiate into elongating spermatids and ultimately into spermatozoa. This complex differentiation and morphogenesis process is called spermiogenesis and is similar in all species. It involves the formation of the acrosome, nuclear changes, the development of the flagellum or sperm tail, the reorganisation of the cytoplasm and cell organelles and the process of release from the Sertoli cell termed spermiation (Figure 11).
The first significant cytological feature is the formation of the acrosome, a secretory vesicle attached to the nucleus of mammalian sperm. It contains hydrolytic enzymes that are believed to participate in the interaction with and penetration of the zona pellucida of the ovocyte (Barros et al., 1996; Ramalho-Santos et al., 2002). Acrosome biogenesis is a multi-step process: the first step, known as the Golgi-phase, is marked by the fusion of Golgi-derived pro-acrosomal granules into a single acrosomal granule that attaches to the cell nucleus. During the cap-phase, fusion of many (presumably Golgi-derived) vesicles takes place around the acrosomal granule, forming the acrosomal vesicle. This initially round vesicle spreads over the nucleus concomitant with Golgi apparatus migration to the opposite cell pole. During acrosome-phase, the nucleus of the spermatid begins to elongate and microtubule configurations change to form the manchette that determines the nuclear and acrosome shaping (Huang and Ho, 2006). The acrosome reaches its final shape at the end of the maturation phase.
The second major feature of spermiogenesis is the modification in chromatin organization occurring in spermatid nuclei. Indeed, the chromatin which in early spermiogenesis steps consists of a typical fibrillar material becomes an extremely
Figure 11: Spermatid differentiation
The morphological, nuclear and cytoplasmic changes occurring during human spermiogenesis which lead to the transformation of a round spermatid into a mature spermatozoon are shown. This complex differentiation and morphogenesis is consistent between all species. Drawing from de Kretser and Kerr (1994).
1 2 3 4 5 6 7
compact, dense, and stabilized structure in mature spermatid (Kimmins and Sassone-Corsi, 2005; Lewis et al., 2003). This nuclear condensation is accomplished by modifying histones and replacing somatic and testis-specific histones with transition proteins (TP1 and TP2) and, subsequently, protamines (PRM1 and PRM2) (Meistrich et al., 1978) leading to a high degree of DNA packaging inside the sperm head.
The next event is the formation of the flagellum (or tail), involved in spermatozoa motility. It begins early in spermiogenesis when a filamentous structure emerges from one of the pair of centrioles which lie close to the Golgi complex. The developing flagellum and the pair of centrioles become lodged in a fossa in the nucleus at the opposite pole to the acrosome. The central core of the axial filament, called the axoneme, consists of nine doublet microtubules surrounding two single central microtubules, which represents a common pattern found in cilia. This basic structure is modified at the region of its articulation with the nucleus through the formation of a complex structure known as the connecting piece (Figure 11). As spermiogenesis proceeds, the outer dense fibres and fibrous sheath, which characterize the regions of the sperm known as the mid and principle-piece, are developed. Mitochondria become concentrated into the mid-piece to provide energy to the flagellum (Figure 11). Finally, at the end of spermiogenesis, spermiation, the process by which spermatozoa are released from the seminiferous epithelium into the lumen of the tubule occurs. Spermatozoa acquire motility and fertilizing capacity in the epididymis.
Most of the "excess baggage" (cytoplasm and organelles) of the spermatid is discarded within the seminiferous epithelium in the form of a residual body which is phagocytised and digested by Sertoli cells (see below) (Kerr and de Kretser, 1974; Russell et al., 1990). A small amount of cytoplasmic material, the cytoplasmic droplet, remains attached within the neck region or around the middle piece as the spermatozoon makes its way into the epididymis. All together these data show that haploid phase is a critical step at which both structural and genetic defects can be introduced in the maturing male gamete.
Figure 12: Spermatogenic cell types in germinative epithelium
Germ cells differentiate from the distal part of the seminiferous tubule to the luminal part and arrange along the germinative epithelium. Note the cytoplasmic bridges between cells originally derived from the same undifferentiated spermatogonia.
Distal part
Luminal part Elongated Spermatid
Round Spermatid
Cytoplasmic bridge
Sertoli cell cytoplasm
Sertoli cell nucleus Spermatocyte
4/ Spermatogenesis wave and germ cell organization
Spermatogenesis starts at the birth of the individual and continues during all his life. Germ cells differentiate from the distal part of seminiferous tubule to the lumen (Figure 12). Although the number of stages varies between species, mouse seminiferous tubules are classified in 12 stages (I-XII), each one consists in a unique assortment of differentiating germ cells. The development of acrosome and nucleus shaping in spermatid are closely correlated with each stage of spermatogenesis (Figure 13). A spermatogenic wave is defined as a complete round of differentiation from spermatogonia to spermatozoa. In this manner, several waves occur asynchronously during adulthood. However during testis ontogenesis, initial appearance of spermatogenic cell types (first wave) occurs synchronously in the seminiferous epithelium soon after birth and the sequence of events can be followed as a function of increasing age in mice: at day 9 post-partum (9dpp or P9) spermatogonia first enters meiosis, by 14dpp the first pachytene spermatocytes are seen, by 22dpp haploid round spermatids appear and by 29dpp, elongating spermatids appear. In mice, the first wave of spermatogenesis takes end at 35dpp (Table II).
Testis is composed by mixed cell types belonging to two different cell lineages, i.e. the somatic and germinal lines. Male germ cell differentiation relies on this specific environment provided by the anatomical and cellular relationships that take place in the seminiferous tubules. Indeed the close association between Sertoli cells and germ cells at each stage of their development is clearly visible in the seminiferous epithelium. Extensive interactions and communications that take place between these cells coordinate the various events of spermatogenesis. Germ cells rely on Sertoli cells for structural and nutritional support (reviewed in (Jegou, 1993)). For instance, the entire process of germ cell development, except for the early phase of spermatogenesis from type B spermatogonia up to leptotene spermatocytes, is segregated from the systemic circulation because of the blood testis barrier created by tight junctions between Sertoli cells near the basal lumina. As such, germ cells and Sertoli cells develop an intimate and elaborate cellular network for cell-cell communications via paracrine factors and signalling molecules, so that Sertoli can provide developing germ cells with the needed nutriments and biological factors. Indeed, in vitro studies have shown bi-directional
Figure 13: Schematic representation of spermatogenic stages and germ cell organisation in mouse seminiferous tubules
Seminiferous tubules are divided into XII stages. Each stage is characterized by a stereotyped association of germ cells at different stages of differentiation. From (Russell et al, 1990).