To cite this version :
Kergoat, Mickaël
and Gibilaro, Mathieu
and
Massot, Laurent
and Chamelot, Pierre
Generalized method for
determining fluoroacidity by electrochemical diffusion coefficient
measurement (application to HfF4). (2015) Electrochimica Acta, 176.
265-269. ISSN 0013-4686
O
pen
A
rchive
T
OULOUSE
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
This is an author-deposited version published in :
http://oatao.univ-toulouse.fr/
Eprints ID : 20336
To link to this article: Doi :
10.1016/j.electacta.2015.06.124
URL :
http://doi.org/10.1016/j.electacta.2015.06.124
Any correspondence concerning this service should be sent to the repository
Generalized
method
for
determining
fluoroacidity
by
electrochemical
diffusion
coefficient
measurement
(application
to
HfF
4
)
M.
Kergoat,
M.
Gibilaro
*
,
L.
Massot,
P.
Chamelot
UniversitédeToulouse,UPS,CNRS,LaboratoiredeGénieChimique,118RoutedeNarbonne,F-31062Toulouse,France
Keywords: Fluoroacidity hafniumfluoride diffusioncoefficient masstransport ABSTRACT
Auniversalmethodforfluoroacidityevaluationwasdevelopedandbasedonamasstransportapproach:
itsimplyconsistsinmeasuringthediffusioncoefficientofanelectroactivespeciesinvariousmolten
media. Thereductionbehaviour ofHf(IV) ions was investigated inmolten fluoridesand diffusion
coefficientsofHf(IV)ions weremeasured. Resultsshowed thatdiffusioncoefficientsdecreasewith
fluoroacidity,due totheeffectof solventviscosity(whichis linked tobridgedfluorines). Aglobal
approachofmasstransportinsolutionwasthenproposed,takingintoaccountbothsoluteandsolvent.
TheSchmidtnumber(Sc)definedastheratiobetweensolventviscosityandsolutediffusivitywas
calculatedinordertotakeintoaccountthesetwoparameters.ResultsshowedthatScincreaseswith
fluoroacidity, in amuch more sensitive way than D. This universal method can extended to all
electroactivespeciesandtoallbathfluoroacidity.
1.Introduction
Byanalogywithaqueoussolutions,notionofacidityinmolten fluoridesolventsisdefinedbyfluoroacidity(pF)andbasedonthe freefluoridesexchangeinmoltenmixturesasdescribedinEq.(1):
fluorobaseÐfluoroacid +nF" (1)
pF="log(aF") (2)
Theactivityoffreefluorides(aF"),which quantifies
fluoroa-cidity, depends on the nature of the cations constituting the solvent,thecompositionandtemperature,andinfluences solva-tion,ionstabilityandprocessesreaction.Itthereforeneedstobe studied and quantified for a better understanding of physico-chemicalpropertiesofsolventsandsolutes.
Usual molten salts solvents are mixtures of alkaline and alkaline-earth fluorides (LiF, NaF, KF, MgF2, CaF2...) and the
amountoffreeF"ionsdependsontheirnatureandcomposition:
themoreamoltensaltcontainsfreefluorides(i.e.fluorodonor),the higheritsbasicityis.Asinaqueousmedia,theacidityisessentialas it influences so many parameters; however pF values are not availableyetsincetheF"activitycan'tbemeasured:onlyarelative
fluoroacidityscalewasestablishedbyindirectevaluations[1–3].
Oneelectrochemicalmethodtosortmoltenfluoridesmixtures inregardoftheirfluoroaciditywasproposedinourlaboratoryby Bieberetal.[4] andKergoat etal.[5].Basedonsilicon[4] and completedwithboron[5],itconsistsinstudyingtheequilibrium betweenadissolvedspeciesandagaseouscompoundsgivenby Eq.(3)forSiandEq.(4)forB:
SiF4+xx"(bulk)ÐSiF4(g)+xF" (3)
BF3+xx"(bulk)ÐBF3(g)+xF" (4)
Thus,bydefinition,afluorobasicbath(high[F"]
free)stabilizesa
speciesinsolution,whileafluoroacidbath(low[F"]
free)promotes
thereactionofgaseousspeciesrelease.
The study of these equilibria, moved by the free fluorides concentration, is then an indicator of fluoroacidity. Indeed the releaseofSiF4(g)orBF3(g)leadstoadecreaseofSi(IV)andB(III)ions
concentrationsrespectively,controlledbyin-situelectrochemical titrations.
Bycalculatingkineticconstantsofsilicon(kSi)andboron(kB)
releases, ranking of various eutectic mixtures was established (Fig.1a) and a fluoroacidity scale of fluoride compounds was proposed(Fig.1b)[5].
Howeverseverallimitationswerepointedout:
- thechoiceofthesoluteiscriticalasithastoformagaseous speciesandbeinequilibriumwithit,
- thesolutemustnotreactwiththesolvent, * correspondingauthor:GibilaroMathieuTel.:+33561557219fax:+3356155
6139
- hastobemoreacidthanthesolventtocapturefreefluorides, - forthemostacidicbaths,thegaseousspeciesreleaseistoofast
todeterminethekineticconstants.
To avoid these drastic conditions, a universal method for fluoroacidity evaluation was developed and based on a mass transportapproach:itsimplyconsistsinmeasuringthediffusion coefficientofaspeciesinvariousmoltenmedia[5].However,small differenceswereobservedbetweenthediffusioncoefficientsand thediscriminationwascomplicatedbyalackofselectivity.Aglobal approachofmasstransportinsolutionwasthenproposed,taking intoaccountbothsoluteandsolvent.
Severalauthorsalreadyshowedthatanincreaseoffluoroacidity directlyimpactsthechemical structureofthemoltensalt[6–8] and leads to a decrease of the free F" which are involved in
coordinationofonecomplex.Forthemostacidicbaths,complexes shares fluorinesbyforming bridges leading toa structure asa network-likeliquid[9–11].Thischemicalbehaviour(coordination and bridging) affects physico-chemical parameters as solute solubility,vaporpressureandviscosity[12].
BymeasuringthediffusioncoefficientsofSi(IV)andB(III)ions and calculating theSchmidt number(Sc),defined asthe ration between solvent kinematic viscosity
y
(in m2s"1) and solutediffusivityD(inm2s"1)invariousmolten
fluoridesmixtures,itwas shown thatDdecreasesandSc (Sc=
y
/D)increaseswithacidityvalidatingthepreviousscaleobtained.
This novel approach is easier to set up than kinetic rates determination,asanaccuratemeasurementcouldbeperformed by electrochemical techniques; moreover, it could be certainly extendedtoallelectroactivespeciesandtoallbathfluoroacidity. Inordertoconfirmthisassumption,hafniumtetrafluorideHfF4
solutewas selected:itis stablein solution,inerttothesolvent constitutants,anddonotformgaseousspeciesinourexperimental conditions.
OnlyfewstudiesoftheelectrochemicalbehaviourofHf(IV)ions are presented in the bibliography. The available results were mainlyacquiredinmoltenchloridesorchloro-fluoridesmedia.In moltenchlorides,Poinsoetal.[13,14]andAdhoumetal.[15]did notagreeontheonHf(IV)ionreductionmechanisminNaCl-KCl mixture.Spinketal.[16]inCsCl,showedthatHf(IV)ionsreduction is a one step process exchanging 4 electrons leading to the formationofHfmetal.Quitethecontrary,Guang-Senetal.[17]and Serrano [18] demonstrated inNaCl-KClthat reduction ofHf(IV) ionstakesplaceinatwostagesprocess,Hf(IV)toHf(II)toHf(0). However, all theseauthors agree that fluorideions addition in moltenchloridesstabilizes Hf(IV)ionstoformhafniumfluoride complexHfF62"(Eq.(5))inmoltenchloro-fluoridesmedia,asthe
reductionofHf(IV)ionstoHf(0)isperformedinasingle4electrons reversiblestepunderdiffusioncontrol(Eq.(6)):
HfCl62"+6F"ÐHfF62"+6Cl" (5)
HfF62"+4e"ÐHf+ 6F" (6)
Inthefirstpartofthispaper,thereductionbehaviourofHf(IV) ionswasinvestigatedinmolteneutecticLiF-NaF(61–39mol.%)at 750#C by cyclic voltammetry, square wave voltammetry and
chronopotentiometricmethods.Then,diffusioncoefficientsofHf (IV) ionsweredeterminedindifferentconditionsvalidatingthe previousobtainedfluoroacidityscale.
Inasecondpart,measurementsofdiffusioncoefficientsofHf (IV) ionswereperformedintwoothersmoltenmedia:LiF-NaF-KF andLiF-CaF2selectedinregardsoftheirfluoroacidity(thefirstone
is more basic and thesecond one more acid than LiF-NaF:cf. Fig.1a),inthe800–900#Ctemperaturerange. Resultsobtained
werecorrelatedtosolventviscositythankstotheSchmidtnumber previouslydefined.RelationshipsbetweenD,Scandfluoroacidity previouslydemonstratedwereconfirmedwiththis electrochemi-calspecies.
Thisglobalmasstransportapproachisvalidatedwithasimple determinationofadiffusioncoefficient,andisapowerfultoolin ordertodeterminefluoroacidity.
2. Experimental
The cell consisted in a vitreous carbon crucible placed in a cylindricalvesselmadeofrefractorysteelandclosedbyastainless steellidcooleddownbycirculatingwater.Theinnerpartofthewall wasprotectedagainstfluoridevapoursbyagraphiteliner.Thiscell hasalreadybeendescribedinpreviouswork[19].Theexperiments wereperformed underaninert argonatmosphere. Thecellwas heatedusingaprogrammablefurnaceandthetemperatureswere measuredusingachromel-alumelthermocouple.
Threeeutecticmixtures(CarloErbaReagents99.99%)wereused as electrolyteand selectedaccording totheir fluoroacidityand relativeeasyhandling:
- a basic bath: LiF-NaF-KF (46.5-11.5-42 mol%, melting point 452#C),
- anacidbath:LiF-CaF2(79.2-20.8mol%,meltingpoint767#C),
- an intermediate bath: LiF-NaF (61-39 mol%, melting point 652#C).
Allthe solvents were initially dehydrated by heating under vacuum from ambient temperature up to their melting point during4days.Hafniumionswereintroducedintothebathinthe formofhafniumtetrafluorideHfF4powder(SigmaAldrich99.9%).
Silverwires(1mmdiameter,Goodfellow)wereusedasworking electrode.Thesurfaceareaoftheworkingelectrodewasdetermined aftereachexperimentbymeasuringtheimmersiondepthinthe bath.Theauxiliaryelectrodewasavitreouscarbonrod(V25,3mm diameter)withalargesurfacearea(2.5cm2).Thepotentialswere
referredtoaplatinumwire(0.5mmdiameter,Goodfellow)actingas aquasi-referenceelectrodePt/PtOx/O2"[20].
AlltheelectrochemicalstudieswereperformedwithanAutolab PGSTAT30potentiostat/galvanostatcontrolledbyacomputerusing theresearchsoftwareGPES4.9.
3. Resultsanddiscussion 3.1.Hf(IV)reductionmechanism 3.1.1.Cyclicvoltammetry
Hf(IV)ionsreductionbehaviourwasinvestigatedinmolten LiF-NaFinthe750-900#Ctemperaturerange.Ashafniumandsilver
Fig.1.Fluoroacidity scaleof various eutectic mixtures. Fluoroacidity scaleof fluoridecompounds.
arenotmiscibleatoperatingtemperature,silverwasselectedasan inertworkingelectrode[21].
Fig. 2 shows typical cyclic voltammogram of LiF-NaF-HfF4
(0.69molkg"1) on silver at 750#C and 100mVs"1. Only one
reduction peak and its corresponding reoxidation peak are observed at -1.43 and -1.25V vs. Pt respectively. The signal crossingbetweenforwardand backwardscansis typicalofthe formationofasolidphaseattheelectrode(crossover).Inaddition, theasymmetricalshapeofthereoxidationpeakischaracteristicsof ametaldepositeddissolutioninthecathodicrun(strippingpeak). AspresentedintheinsetofFig.2,novariationsofpeakpotentialat differentscan rateprovedthatHf(IV)electrochemicalreduction processisreversible[22].Thus,accordingtoequationsofcyclic voltammetryforareversiblesoluble/insolublesystem,thenumber of exchanged electrons can be calculated by measuring the differencebetweenthepotentialpeakandthehalfpeakpotential [22]: jEp" Ep 2j¼ 0:77 RT nF (7)
whereEpisthepeakpotential(V)andEp/2isthehalfpeakpotential
(V),Rtheidealgasconstant(8.314Jmol"1K"1),Tastheabsolute
temperature(K),nthenumberofexchangedelectronsandFthe Faradayconstant(Cmol"1).
In this study, the difference was found to be 18&2mV, correspondingtoavalueof3.8&0.3exchangedelectrons.
ThelinearrelationshipbetweenHf(IV)reductionpeakcurrent density at -1.43V vs. Pt and the square root of the scan rate presented in the inset of Fig. 2, proved that electrochemical reductionprocessiscontrolledbyHf(IV)diffusion[22].Diffusion coefficients were determined using Berzins-Delahay equation (Eq.(8))forareversiblesoluble/insolublesystem[23]:
Jp¼0:61nFCðnFDv RT Þ
1=2 (8)
where Jp is the peak current density (Am"2), C the solute
concentration (mol m"3), D the diffusion
coefficient (m2 s"1)
and
y
thepotentialscanrate(Vs"1).At750#C,avalueof(3.5&0.3)x10"9m2s"1forDwasfound.
Serrano [18] and Poinso [13] found a Hf(IV) ions diffusion coefficientinNaCl-KCl-NaFat750#Cequalto2.2and4.9)10"9
m2s"1respectively.
DiffusioncoefficientsweredeterminedinLiF-NaFinthe800– 900#C temperaturerangeand are presentedinTable 1. Results
showedthatDandTfollowanArrheniuslawtype,therelationship isexpressedasfollows:
InD¼"EA
RTþInDo¼" 7863:4
T "11:8 (9)
FromEq.(9),theactivationenergyisfoundtobe65.4&0.7kJ mol"1.Thisvalueisbythesameorderofmagnitudewithprevious
studies,asforinstance213kJmol"1forSi(IV)ions[4]or48.9kJ
mol"1forNd(III)ions[24].
3.1.2.Squarewavevoltammetry
The square wave voltammetry was used to confirm more accuratelythenumberofexchangedelectronsofHf(IV)reduction thancyclicvoltammetry.
Fig.3showsasquarewavevoltammogramoftheLiF-NaF-HfF4
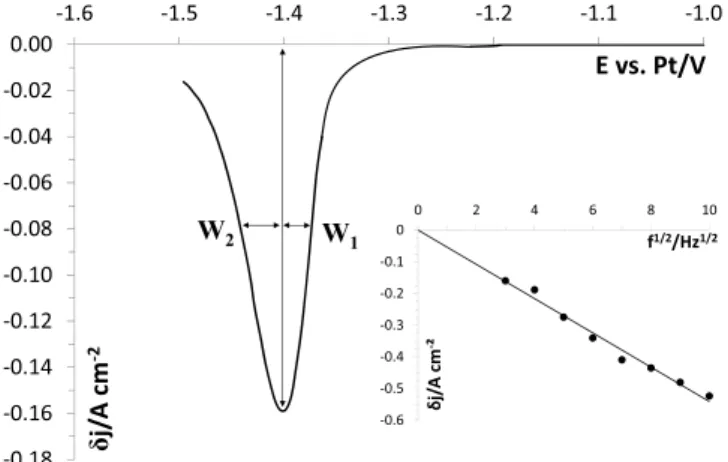
(0.69molkg"1)systemonsilverelectrode at750#C and9Hz.A
singlepeakaround-1.40Vvs.Ptisobserved,inagreementwith cyclicvoltammetryandpresentinganasymmetricGaussianshape characteristicofasoluble/insolublesystem[25].
Theasymmetryofthepeakisduetothecurrentlessnucleation overvoltage needed for the formation of the first crystals of metallichafniumattheelectrodesurface.Thisphenomenondelays theappearanceofthefaradiccurrent,leadingtoasignaldistortion [26]. The nucleation overvoltage
h
(V) can be determined bymeasuringthewidthatmid-height ofthetwohalfpeaksusing Eq.(10):
h
=2(10)(W2-W1) (10)The obtained value,
h
=32&2mV, is in agreement with previous overvoltage values for metals deposition available in theliterature[27].After checking in the frequency domain that the reduction reactionofHf(IV)hasareversiblebehavior(linearvariationofthe peakdifferentialcurrentdensitywiththesquarerootoffrequency (cf. inset Fig. 3)), the number of exchanged electrons was determinedfrom themeasurement of thewidthat mid-height W1/2(V)carriedoutonanisolatedpeak.
However,togetridofthenucleationprocess,thewidthat mid-heightW1/2measurementhasbeendeterminedbydoublingthe
half-widthatmid-heightW2(V)oftheGaussiancurve(Eq.(11)).
Thismethodologyhasalreadybeenvalidatedinmoltenfluorides fordifferentmetalsstudies[24].
W1=2¼2W2¼3:52 RT nF (11) -0.2 -0.1 0.0 0.1 0.2 0.3 -1.5 -1 -0.5 0 0.5 1 1.5
J/
A
cm
-2E vs. Pt/V
-0.3 -0.25 -0.2 -0.15 -0.1 -0.05 0 0 0.2 0.4 0.6 0.8 Jp /A cm -2 υ1/2/V1/2s-1/2 -1.45 -1.44 -1.43 -1.42 -1.41 -1.4 -1.5 -1 -0.5 0 Ep v s. P t/ V log υ/V s-1Fig.2.CyclicvoltammogramofLiF-NaF-HfF4(0.69molkg"1)at100mVs"1and T=750#CWorkingEl.:Ag;AuxiliaryEl.:vitreouscarbon;ReferenceEl.:PtInsets: LinearrelationshipofHf(IV)reductionpeakcurrentdensityversusthesquareroot ofthescanningpotentialrate.Hf(IV)reductionpeakpotentialversusthelogarithm ofthescanningpotentialrate.
Table1
Diffusioncoefficient(D),kinematicviscosity(y)andSchmidtnumber(Sc)ofHf(IV) ionsfordifferentfluoridemediaatdifferenttemperature.
T(#C) LiF-NaF-KF LiF-NaF LiF-CaF 2 Molarcomp. 46.5–11.5–42 61–39 79.2–20.8 109D(m2s"1) 800 5.34 4.96 1.49 850 9.25 6.80 1.94 900 16.66 9.46 2.48 107y(m2s"1) 800 7.8 12.1 30.2 850 6.5 10.8 29.6 900 5.6 9.7 29.2 Sc=y/D 800 145 245 2028 850 71 158 1528 900 34 102 1178
82&4mVwasfoundforW1/2,correspondingtoanumberof
exchangedelectronsof3.8&0.2.
Theresultsobtainedbycyclicandsquarewavevoltammetries allowconcludingthatHf(IV)ionsreductioninmoltenfluoridesisa one-stepprocessexchanging4electronsunderdiffusioncontrol. 3.1.3.Chronopotentiometry
To confirm the diffusion-controlled process of the Hf(IV) electrochemicalreduction,chronopotentiogramswereperformed. Fig.4showsthevariationofthechronopotentiogramsofHfF4with
theappliedcurrentonsilverelectrodeat750#C inLiF-NaF-HfF
4
(0.05mol kg"1). These curves exhibit a single wave, obviously
associatedtothereductionofhafniumionsinthepotentialrange (+-1.4Vvs.Pt)alreadyobservedonvoltammograms.
According to the data plotted in the inset of Fig. 4, Sand's equation(Eq.(12))wasverified[22]:
I
t
1=2C ¼ nFSD1=2
p
1=22 ¼constant
¼0:160&0:004Acm3s"1=2mol"1 (12)
whereIistheappliedcurrent(A),
t
thetransitiontime(s),andStheimmergedelectrodesurfacearea(m2).
Thushafniumreductionprocesswasconfirmedtobelimitedby the diffusionof Hf(IV) ionsin solution. Its diffusioncoefficient calculated using Eq. (12) and assumingthat n=4 is:(3.3&0.2)
x10"9m2s"1at750#C,inaccordancewithourpreviousresultby
cyclicvoltammetry((3.5&0.3)x10"9m2
s"1).
The reversal chronopotentiogram presentedin Fig. 5 proves thataninsolublecompoundisformedonsilverelectrodeduring thecathodicrun,asthetransitiontimeofthereductionwaveis veryclosetotheoxidationtime(
t
red+t
ox+0.6s)[28].ThisresultconfirmsonemoretimethatthereactionleadstoHfmetalonthe workingelectrode.
Allthese electrochemical techniques allow to concludethat reductionofHf(IV)ionsaddedasHfF4inmoltenfluorides:
- isaone-stepprocessexchanging4electrons, - iscontrolledbydiffusionofHf(IV)ions, - andleadstotheformationofmetallichafnium.
As it is stated in thelitterature that cations coordinancyis stronglyinfluenced byfluoroacidity,reductionmechanismofHf (IV) ionsinmoltenfluoridescanbewrittenasinEq.(13): HfF4+xx"+4e"ÐHf+(4+x)F" (13)
3.2.Diffusioncoefficientsandmasstransportapproach
The effect of fluoroacidity on Si(IV) and B(III) ions mass transportswaspreviouslydemonstrated[5].Resultsshowedthat anincrease ofthefluoroacidityleadstoa decreaseof diffusion coefficientsduetocoordinancyandbridgingsolventphenomena. Thesephysico-chemicalsparametersleadtoanincreaseofsolvent viscosity with fluoroacidity. As a consequence solutes mass transportbecomemoredifficult.
In order to confirm these resultson a simple electroactive species,investigationonhafniumsolutewasperformedinthree moltensystemswithdifferentfluoroacidities:
- abasicbath:LiF-NaF-KF, - anacidbath:LiF-CaF2,
- andanintermediatebath:LiF-NaF.
Diffusion coefficients of Hf(IV) ions were determined by calculating the average of cyclic voltammetry (Eq. (8)) and chronopotentiommetry (Eq. (11))values. Results are presented inTable1wheresolventsaresortedfromthemorebasictotheleft tothemoreacidtotheright.
ResultsonHf(IV)ionsshowthatdiffusioncoefficientsdecrease with fluoroacidity whatever the temperature.However for the lowesttemperature,theminordifferencesbetweenvaluesandthe -0.18 -0.16 -0.14 -0.12 -0.10 -0.08 -0.06 -0.04 -0.02 0.00 -1.6 -1.5 -1.4 -1.3 -1.2 -1.1 -1.0
δ
j/
A
cm
-2E vs. Pt/V
W
2W
1 -0.6 -0.5 -0.4 -0.3 -0.2 -0.1 0 0 2 4 6 8 10 δ j/ A cm -² f1/2/Hz1/2Fig.3.SquarewavevoltammogramofLiF-NaF-HfF4(0.69molkg"1)atfrequency= 9HzandT=750#CWorkingEl.:Ag;AuxiliaryEl.:vitreouscarbon;ReferenceEl.:Pt Inset:LinearrelationshipofHf(IV)reductionpeakcurrentdensityversusthesquare rootofthefrequency.
Fig.4.TypicalchronopotentiogramswiththeintensityofthesystemLiF-NaF-HfF4 (0.05molkg"1) at T=750#C Working El.: Ag; Auxiliary El.: vitreous carbon; ReferenceEl.:Pt. -1.75 -1.5 -1.25 -1 -0.75 -0.5 0 0.5 1 1.5 2
E
v
s.
P
t/
V
Time/s
τred= 0,591s τox= 0,610sFig.5.ReversalchronopotentiogramofHfF4(0.083molkg"1)inLiF-NaF,J=&207
mAcm"2atT=750#CWorkingEl.:Ag;AuxiliaryEl.:vitreouscarbon;ReferenceEl.:
uncertaintyofmeasurement(&3)10"10m2s"1)donotallowthe
discriminationofsolventsfluoroacidity.
Itisobviousthattheionicradiusoftheelementinsolutionand itscoordinancyaffecttheabilityofthespeciestomovethroughthe solution(representedbyD).Inhighlyacidbaths,wherethecontent offreeF"islow,hafniumcomplexeshavetoshareoneormore
fluorinebybridging,andthenform,bypolymerization,largersizes species,whichslowsdownthemasstransportinsolution.
AsthehaniumHfF4+xx"coordinancyisafunctionof
fluoroa-cidityand cannot bedeterminebyelectrochemistry, thesolute transportresultshavetotakeinaccountthesolventcontribution throughitsviscosity:aviscosityincreaseleadstoamoredifficult solutemasstransport.
Kinematicviscosities
y
(inm2s"1)definedastheratiobetweendynamicviscosity
m
(inkgm"1s"1)anddensityr
(inkgm"3)werecalculatedfromtheMoltenSaltsHandbookvalues[29].Notethat data for LiF-CaF2 viscosity at operating temperatures are not
availableinliterature.Then,viscositywasextrapolatedfromthe work of Robelin et al. as thesum of the fluoridescompounds viscosities balanced by their molar fractions in the eutectic mixture[30].ThesevaluesaregatheredinTable1.
It shows that molten fluorides viscosity increase with fluoroacidity, and could be correlated to a bridging phenomen by sharing available free fluorides, modifying the molten salt structure and forming a network-like liquid. This behaviour is particularlyimportantwiththepresenceofcalcium,which was demonstratedasoneofthemoreacidcompounds(Fig.1a).
InordertotakeintoaccounttheeffectoftheviscosityonHf(IV) diffusioncoefficients,theadimensionalSchmidtnumber(Sc=
y
/D)wascalculated tocharacterizethe soluteglobalmass transport throughitsenvironment.ThecalculatedScnumberaregatheredin Table1.Atagiventemperature,theresultsshowedthatScforHf (IV) ionsincreaseswithfluoroacidity, allowing tosort melts in regardoftheirfluoroacidity.
Thus relationships between fluoroacidity, bridging fluorines andviscositydirectlyimpactmasstransportofasimplespeciesin solution.Thedeterminationofdiffusioncoefficientsofaspecies, whichisdirectlyaffectedbyacumulativeeffectofviscosityand ionicradiusofthesolute,allowstodiscriminatemoltenfluorides mediaasa function of fluoroacidity.However, ifthe difference betweenD values is very low, the Schmidt number by taking accountviscosityincreasethisdifference,asforinstancebetween LiF-NaF-KFandLiF-NaFat800#Cwherethedifferenceisaround7%
onDand69%onScinthesameconditions. 4. Conclusion
TheHfF4electrochemicalbehaviourwasinvestigatedinmolten
fluorides. By cyclic voltammetry, square wave voltammetry, chronopotentiometry and reversal choronpotentiometry, Hf(IV) ionsreduction mechanism was demonstrated tobe a one step processexchanging4electronsunderHf(IV)ionsdiffusioncontrol leadingtotheformationofhafniummetal:HfF4+xx"+4e"ÐHf+
xF".
Diffusion coefficients of Hf(IV) were determinated in three moltenfluoridessolvents,withdifferentfluoroacidities,between 800 and 900#C. Hf(IV) diffusion coefficients decrease with
fluoroacidity:thisphenomenumisduetoacumulativeeffectof solventviscosity(whichislinkedtobridgedfluorines)andionic radiusofthesolutewithfluoroacidity. Asviscosityreferstothe solventanddiffusioncoefficienttothesolute,theSchmidtnumber wascalculatedinordertotakeintoaccountthesetwoparameters. Resultsshowed that Sc increases withfluoroacidity, in a much moresensitivewaythanD.
Thismasstransportapproachconsistinginthedetermination of thediffusion coefficient and the calculation of the Schmidt number is easier to set up than kinetic rates determination. Moreover,thisuniversalmethodcanextendedtoallelectroactive speciesandtoallbathfluoroacidity.
References
[1]D.Elwell,Electrowinningofsiliconfromsolutionsofsilicainalkalimetal fluoride/alkalineearthfluorideeutectics,Sol.Energ.Mater.5(1981)205. [2]E.W.Dewing,Theeffectsofadditivesonactivitiesincryolitemelts,Metall.
Trans.B20B(1989)657.
[3]B. Gilbert, E. Robert, E. Tixhon, J.E. Olsen, T. Østvold, Structure and ThermodynamicsofNaF-AlF3MeltswithAdditionofCaF2andMgF2,Inorg. Chem.35(1996)4198.
[4]A.L. Bieber, L. Massot, M. Gibilaro, L. Cassayre, P. Chamelot, P. Taxil, Fluoroacidityevaluationinmoltensalts,Electrochim.Acta56(2011)5022. [5]M.Kergoat,L.Massot,M.Gibilaro,P.Chamelot,Investigationonfluoroacidity
ofmoltenfluoridessolutionsinrelationwithmasstransport,Electrochim. Acta120(2014).
[6]C.Bessada,A.-L.Rollet,A.Rakhmatullin,I.Nuta,P.Florian,D.Massiot,Insitu NMRapproachofthelocalstructureofmoltenmaterialsathightemperature, C. R.Chim9(2006)374.
[7]A.-L.Rollet,S.Godier,C.Bessada,HightemperatureNMRstudyofthelocal structureofmoltenLaF3-AF(A=LiNa,KandRb)mixtures,PCCP10(2008) 3222.
[8]C.Bessada,A.Rakhmatullin,A.-L.Rollet,D.Zanghi,HightemperatureNMR approachofmixturesofrareearthandalkalifluorides:Aninsightintothelocal structure,J.FluorineChem.130(2009)45.
[9]C.F.BaesJr.,ApolymermodelforBeF2andSiO2melts,J.SolidStateChem.1 (1970)159.
[10]V.Dracopoulos,J.Vagelatos,G.N.Papatheodorou,Ramanspectroscopicstudies ofmoltenZrF4-KFmixturesandofA2ZrF6A3ZrF7(A=LiKorCs)compounds,J. Chem.Soc.DaltonTrans.(2001)1117.
[11]M.Salanne,C.Simon,P.Turq,P.A.Madden,Conductivity-Viscosity-Structure: UnpickingtheRelationshipinanIonicLiquidy,J.Phys.Chem.B111(2007) 4678.
[12]D.Williams,L.Toth,K.Clarno,AssessmentofCandidateMoltenSaltCoolants for the Advanced High-Temperature Reactor (AHTR), ORNL/TM-2006/12 (2006).
[13] J.Y.Poinso,Ph.DThesis,(1993).
[14]J.Y. Poinso, S.Bouvet, P. Ozil, J.C. Poignet, J. Bouteillon, Electrochemical ReductionofHafniumTetrachlorideinMoltenNaCl-KCl,J.Electrochem.Soc. 140(1993).
[15]N.Adhoum,L.Arurault,J.Bouteillon,A.Cotarta,J.C.Gabriel,J.C.Poignet, ElectrochemistryofRefractoryMetals:Hf,Mo,Cr,in:D.Kerridge,E.Polyakov (Eds.),RefractoryMetalsinMoltenSalts,vol.53,Springer,Netherlands,1998, pp.61–72.
[16]D.R.Spink,C.P.Vijayan,HafniumElectrowinningStudies,J.Electrochem.Soc. 121(1974).
[17]C.Guang-Sen,M.Okido,T.Oki,Electrochemicalstudiesofzirconiumand hafniuminalkalichlorideandalkalifluoride-chloridemoltensalts,J.Appl. Electrochem.20(1990).
[18] K.Serrano,Ph.DThesis,UniversitéToulouseIII-PaulSabatier,(1998). [19]L.Massot,P.Chamelot,F.Bouyer,P.Taxil,Electrodepositionofcarbonfilms
frommoltenalkalinefluoridemedia,Electrochim.Acta47(2002)1949. [20]A.D.Graves,D.Inman,Adsorptionandthedifferencialcapacitanceofthe
electricaldouble-layeratplatinum/halidemetalinterface,Nature208(1965) 481.
[21]H.Okamoto,Ag-Hf(silver-hafnium),J.PhaseEquilib17(1996).
[22]A.J. Bard, L.R. Faulkner, Electrochemical Methods: Fundamentals and Applications,2ed.,JohnWiley&Sons,NewYork,2001.
[23]T.Berzins,P.Delahay,OscillographicPolarographicWavesfortheReversible DepositionofMetalsonSolidElectrodes,J.Am.Chem.Soc.75(1953). [24]C.Hamel,P.Chamelot,P.Taxil,Neodymium(III)cathodicprocessesinmolten
fluorides,Electrochim.Acta49(2004).
[25]L.Ramaley,M.S.Krause,Theoryofsquarewavevoltammetry,Anal.Chem.41 (1969).
[26]G.J.Hills,D.J.Schiffrin,J.Thompson,Electrochemicalnucleationfrommolten salts—I.Diffusioncontrolledelectrodepositionofsilverfromalkalimolten nitrates,Electrochim.Acta19(1974).
[27]C.Nourry,L.Massot,P.Chamelot,P.Taxil,Dataacquisitioninthermodynamic andelectrochemicalreductioninaGd(III)/Gdsystem inLiF–CaF2media, Electrochim.Acta53(2008).
[28]H.B. Herman, A.J. Bard, CyclicChronopotentiometry.Diffusion Controlled ElectrodeReactionofaSingleComponentSystem,Anal.Chem35(1963). [29]G.J.Janz,MoltenSaltsHandbook,ElsevierScience, 2013.
[30]C.Robelin,P.Chartrand,Aviscositymodelforthe(NaF+AlF3+CaF2+Al2O3) electrolyte,TheJournalofChemicalThermodynamics43(2011).