T
T
H
H
È
È
S
S
E
E
En vue de l'obtention du
D
DO
OC
C
TO
T
OR
RA
AT
T
D
DE
E
L
L’
’U
UN
NI
IV
VE
E
RS
R
SI
IT
TÉ
É
D
DE
E
T
T
OU
O
UL
L
OU
O
US
SE
E
Délivré par l'Université Toulouse III - Paul Sabatier Discipline ou spécialité : Biochimie
& of the Doctorate of Philosophy of the
N
NA
AT
TI
I
ON
O
NA
AL
L
C
C
HE
H
EN
N
G
G
K
KU
UN
N
G
G
U
UN
NI
IV
V
ER
E
RS
SI
IT
TY
Y
Délivré par IInnssttiittuutteeooffBBaassiiccMMeeddiiccaallSScciieennccee of the National Cheng Kung University Discipline ou spécialité : Immunology
JURY
Mr. Wen-Tsan CHANG, Professor of National Cheng Kung University, Tainan, Taiwan Mr. Eric CLOTTES, Professor of Paul Sabatier University, Toulouse, France
Mr. Gilles MARCHAL, Professor of Pasteur Institute, Paris, France Mr. Germain PUZO, Director of researcher of CNRS, Toulouse, France
Mr. Michel RIVIERE, Senior researcher of CNRS, Toulouse, France
Mr. Jiu-Yao WANG, Professor of National Cheng Kung University, Tainan, Taiwan Mr. Hua-Lin WU, Professor of National Cheng Kung University, Tainan, Taiwan Mr. Trai-Ming YEH, Professor of National Cheng Kung University, Tainan, Taiwan
Présentée et soutenue par Chia-Fang, LIU Le 23/09/2008
Titre : The role of surfactant protein D in the Der p allergen-induced inflammation in the innate immunity of lung
Abstract
Allergic asthma is a disease of chronic airway inflammation, which is characterized by the infiltration of T helper 2 (Th2) cells and eosinophils in the airways, airway hyperresponsiveness, and excessive mucus secretion.
Dermatophagoides pteronyssinus (Der p) is among the most prominent and important allergens that cause allergic
asthma around the world. However, the mechanism of Der p-induced inflammation in the innate immunity of lung is not fully understood. Surfactant protein D (SP-D) is a member of the collectin family that binds to sugar motifs of microorganisms as well as airborne allergens. It plays an important role in the first-line defense of the lung. Recent investigations have highlighted that SP-D not only augments innate immune responses against invading microorganisms but also acts on the adaptive immune response, such as dendritic cells maturation and T cells proliferation. SP-D has been shown (1) to regulate airway functions and allergic inflammation through modulation of macrophage function, and (2) to inhibit histamine release from basophils and allergen-induced lymphocyte proliferation, two essential steps in the pathogenesis of asthma. Although the protective effect of SP-D in the lung has been proposed, the molecular mechanism by which SP-D reduces lung inflammation is still unclear. Our previous results demonstrate that intra-nasal instillations of recombinant SP-D to Der p-sensitized mice 6h after allergen challenge, but not at 24 h before allergen challenge, significantly reduces eosinophils infiltration, decreases levels of IL-4, IL-5, eotaxin, and TNF-α, but elevates levels of IFN-α in BALF (Broncholaveolar lavage fluid) in treated mice. Further, we have also found that Der p may directly induce inflammatory cytokines production in alveolar macrophages through TLR 4/CD14. However, very little is known about the putative receptors of Der p on the cell surface of alveolar macrophages which mediate and determine the outcome of the allergen-induced immunological responses. In this study, we used different TLR-transfected (TLR2, 3, 4, 5, 6, 7, 8 and 9) human embryonic kidney cells (HEK) to verify whether Der p signal activate NF-κB through TLR4/MD-2/CD14. The results suggested that Der p triggered not only TLR4/CD14/MD-2 but also TLR2-dependent NF-κB activation. In addition, Der p-induced NF-κB activation and IRAK and MKK3/6 proteins phosphorylations observed respectively by the fluorescence microscope assay and western blot are both inhibited by SP-D pretreatment. Furthermore, alveolar macrophage cell line (MH-S) treated with MKK/ERK and P38 pathway inhibitor compounds, UO126 and SB203580, respectively, successfully inhibited Der p-induced inflammatory response. These results suggest that Der p activates both MKK/ERK and p38 pathway which are dependent on the common adaptor of myeloid differentiation protein 88 (MyD88) activation. It is well known that LPS activates Th1 cytokine response and induced Th1 cytokine, IL-12, from macrophages. Interestingly, we found that pretreatment with Der p inhibited LPS-induced IL-12 production and T-bet expression in MH-S cell, while Der p alone triggered Th2 cell recruiting chemokine, MDC (macrophage-derived chemokine, CCL22), in MH-S cells. On the other hand, pretreatment with SP-D in MH-S cells could inhibit Der p-induced NO and TNF-α production, as well as Der-p-induced MKK3/6 and P38 pathways activation and NF-κB translocation. Therefore, to explore the inhibitory mechanisms of SP-D on
results revealed that SP-D can directly bind to CD14 molecule and prevent the binding of Der p to CD14 and inhibit Der p-induced TLR4/CD14/MD2 signal activation. Moreover, SP-D can also regulate Der p-induced down-regulation of DC-SIGN expression, which may also act as an inhibitory signal to inhibit Der p-induced inflammatory response. In summary, we found that (1) SP-D had a therapeutic effect on allergen-induced bronchial inflammation in the murine model of asthma. (2) Mite allergen-induced alveolar macrophage activation was mediated by CD14 /TLR4 and TLR2 and could be inhibited by SP-D pretreatment. (3) Der p allergen induced NF-κB-dependent pro-inflammatory mediators production, and prevented endotoxin-induced IL-12 and T-bet production through TLR2/4 co-activation in mouse alveolar macrophage cell line, and (4) SP-D inhibited Der p-induced inflammatory signaling pathway and inflammatory mediators production through regulating DC-SIGN expression in acting as an inhibitory signal to inhibit Der p-induced inflammatory response. In conclusion, SP-D, as an important molecule of innate immunity of lung, can regulate allergen–induced pulmonary inflammation. Our finding suggested that molecular mechanisms of the regulatory role of SP-D are through the direct interaction with cellular surface CD14 that prevent Der p-induced macrophage activation and indirectly regulate DC-SIGN expression that inhibit Der p-induced inflammatory response. These results may have major implications in the way of exploring new anti-asthmatic pharmaceuticals agents that target allergen-binding receptors and allergen-induced inflammatory signals, as well as enhancing endogenous SP-D production.
中文摘要
過敏性氣喘是一個慢性呼吸道發炎反應,它的特徵包含了呼吸道中,家塵蹣過敏原引發的 Th2
細胞和嗜酸性白血球滲透、呼吸道呈現高度敏感性、以及分泌過多黏液。Dermatophagoides
pteronyssinus(Der p)歐洲塵蹣是和過敏性氣喘相關最常見和最重要的一種家塵蹣。然而,Der p
在先天性免疫中的機制至今還不清楚。肺泡表面活性蛋白質 D,surfactant protein D(SP-D),是 collectin 家族中的一位成員,它可以利用 carbohydrate recognition domain(CRD) 和微生物、灰塵及 過敏原上許多不同醣蛋白 motif 結合,對於我們肺部的第一道防線,SP-D 扮演一個非常重要的角 色。近年來的研究中,更強調 SP-D 不僅加強先天性免疫系統對入侵微生物的反應,而且也可以作 用在後天性免疫系統的功能,例如,樹突狀結胞的成熟與 T 細胞的增生。過去有報告指出 SP-D 可 以經由調節吞噬細胞的功能去控制呼吸道過敏的發炎反應,也可以抑制組織胺的釋放和過敏原所引 發淋巴球的增生;而這兩者正是氣喘致病機轉的主要原因。SP-D 雖然具有保護細胞降低發炎反應 功能,但其分子機轉仍舊有待釐清。過去實驗結果證明小鼠經由 Der p 刺激後六小時,再給予重組 SP-D 蛋白(rSP-D)可以降低肺泡沖洗液中嗜酸性球的滲透,且同時降低細胞激素 IL-4、IL-5、eotaxin 和 TNF-α 的產生量(治療性的),但是在 Der p 刺激老鼠二十四小時前給予 rSP-D 並沒有發現顯著 的改變(預防性的)(A 部分)。另外,我們也發現 Der p 有可能會經由 TLR 和 CD14 這兩個接受器 去活化下游反應(B 部分)。可是到目前為止,關於 Der p 在細胞表面的接受器以及所傳遞的下游 訊息尚不清楚。因此,我們利用不同的 TLR(TLR2、3、4、5、7、8 和 9)基因改造的人類 HEK 細胞,接上 NF-κB 的啟動子,來證明 Der p 是否經由 TLR4/MD-2-CD14 來活化下游 NF-κB 活性, 並進一步瞭此機制是否和脂多醣 Lipopolysaccharide(LPS)所引發的 TLR4 訊息傳遞機制不同。結 果顯示 Der p 可以活化 TLR4/CD14/MD-2 和 TLR2 為主的 NF-κB 訊息傳遞;同時在螢光顯微鏡的 觀察下,我們發現 Der p 可以直接活化 NF-κB;此外,利用西方墨點法,我們也發現 Der p 可以磷 酸化 IRAK 和 MKK 蛋白質;而上述兩種訊息傳遞都會受到 SP-D 的抑制。更進一步,我們利用 MKK/ERK 和 p38 訊息傳導的抑制劑 UO126 和 SB203580 可成功的抑制 Der p 所引起的發炎反應。 以此結果似乎顯示 Der p 會活化 myeloid differentiation protein 88 (MyD88)為主的訊息傳遞路徑。另 一方面,過去的研究對於 LPS 會引起 Th1 反應,並且產生 Th1 細胞激素(IL-12)已清楚瞭解。令 人覺得有趣的是,在 MH-S 細胞株中,先給予 Der p 刺激之後再給予 LPS,會降低原本 LPS 活化 T-bet 的表現及 IL-12 的產生,且 Der p 可以引起 Th2 反應的 chemokine MDC(macrophage-derived
言之,關於 SP-D 如何抑制 Der p 所引起的發炎反應及發炎媒介物同時,我們提出三種假說,(一) SP-D 可以和 Der p 接觸並防止 SP-D 結合到 TLR4/CD14/MD-2 上,經由競爭反應 Der p 結合到 CD14 上; (二) SP-D 可以直接結合到 CD14 或 TLR4 上,進而抑制 Der p 結合到 TLR4/CD14/MD2 上;(三) SP-D 本身存在一種抑制的功能,它可以經由另一條負調控機制進而抑制 Der p 引發的發炎反應,例如: DC-SIGN 接受器,藉此去調控 Der p 引發的 Th2 免疫反應及吞噬細胞的活化。經由我們的實驗証 實,SP-D 可以經由直接和 CD14 結合來抑制 Der p 和 CD14 的結合,同時,也能調控 DC-SIGN 使 其表現降低,並經由一個負調控訊號抑制 Der p 所引起的發炎反應。最後,綜合研究結果,我們結 論,在老鼠肺泡細胞的細胞株中,塵蹣過敏原 Der p 可以經由 TLR4 和 TLR2 共同活化,進而活化 NF- 為主的 NO 和 TNF-α發炎性媒介物,同時也可以抑制 LPS 引起的 IL-12 和 T-bet 的產生;B 另外,SP-D 可以抑制 Der p 所引起的發炎反應訊息傳遞及發炎媒介物。而對於先天性免疫來講, Der p 所引發的發炎反應,SP-D 扮演一個非常重要的角色。藉由這些發現探討過敏疾病的機制,針 對那些接受器及相關的訊息傳遞加上釐清 SP-D 在抑制 Der p 引發肺部的發炎反應及可能的訊息傳 遞所扮演的角色可進而幫助發展新的製藥方式,另外也可以提供治療過敏氣喘疾病在臨床上的應 用。
Résumé
L’asthme allergique est une maladie inflammatoire chronique caractérisée par un essoufflement, une constriction bronchique, une hyperréactivité des voies respiratoires et une sécrétion excessive de mucus. Les caractéristiques immunologiques de cette maladie sont associées à une infiltration de cellules Th2, d’éosinophiles, de neutrophiles et une production des médiateurs de la réponse immunitaire adaptative Th2 tels que l’IL-4, l’IL-5, l’IL-13, CCL17 et CCL22 qui sont induits après inhalation d’allergènes. Cependant les mécanismes et la régulation de la réponse immunitaire innée induite par les allergènes ne sont pas complètement connus. La protéine D du surfactant pulmonaire (SP-D) est une collectine capable de lier divers motifs sucrés de micro-organismes ainsi que des allergènes aériens et elle joue un rôle important dans la défense des poumons. Des travaux récents ont montrés que SP-D non seulement augmente la réponse immunitaire innée face aux micro-organismes, mais elle agit également sur la réponse immunitaire adaptative en intervenant dans la maturation des cellules dendritiques et la prolifération des cellules T. La SP-D régule les fonctions respiratoires et l’inflammation allergique en modulant les fonctions macrophagiques et en inhibant deux étapes essentielles dans la pathogénie de l’asthme, la sécrétion d’histamine par les basophiles d’une part, et, d’autre part, la prolifération lymphocytaire induite par les allergènes. Les mécanismes moléculaires de cet effet protecteur de la SP-D qui permettent la réduction de l’inflammation pulmonaire restent à caractériser clairement. Dans la première partie de nos travaux, nous démontrons que l’administration de la protéine SP-D recombinante (rSP-D) à des souris, préalablement sensibilisées 6 heures avant, par une stimulation allergénique, réduisait de façon évidente l’infiltration des éosinophiles, diminuait les niveaux d’IL-4, d’IL-5, d’éotaxine et de TNF-α et augmentait les niveaux d’IFN-α dans le fluide de lavage broncho-alvéolaire (BALF). Par contre quand l’injection de rSP-D a lieu 24 H avant la stimulation allergénique aucun effet n’est observé, ce qui suggère que la SP-D a un effet thérapeutique et non préventif. Dans le but de définir plus précisément les mécanismes cellulaires par lesquels la SP-D est capable de moduler et contrôler une réponse inflammatoire modèle de l’asthme allergique sur un modèle de lignée (MH-S) de cellules dérivées de macrophages alvéolaires murins, nous nous sommes attachés, dans un premier temps, à décrypter la réponse cellulaire induite par l’allergène Der p : un allergène majeur issu d’une espèce d’acariens rencontrés dans les poussières domestiques: Dermatophagoides pteronyssinus, responsable en grande partie des manifestations d’asthme allergique chez l’enfant
Nous avons ainsi pu montrer ces cellules stimulées par Der p répondent de façon spécifique par la production de NO et de TNF-a : deux médiateurs majeurs de la réponse inflammatoire. Nous avons également trouvé que l’induction par Der p de la production de cytokines inflammatoires dans les macrophages alvéolaires semblait impliquer le récepteur TLR4/CD14 des lipopolysaccharides (LPS).
Peu de choses étant connues sur les récepteurs putatifs de Der p présents à la surface des macrophages alvéolaires, nous avons donc entrepris une étude exhaustive de l’implication potentielle des
seulement induire l’activation de NF-κB par TLR4/CD14/MD-2 mais aussi via le TLR2. L’activation de NF-κB par Der p a été montrée par de la microscopie à fluorescence. De plus il a aussi été montré, par des expériences de western blot, que la stimulation par Der p active logiquement la phosphorylation des protéines IRAK et MKKK3/6 impliquées dans la voie de signalisation associée aux récepteurs TLR. Afin de confirmer ces observations, la lignée de macrophages alvéolaires MH-S a été traitée avec des inhibiteurs des voies MKKK/ERK et p38, respectivement UO126 et SB203580, et la réponse inflammatoire induite par Der p a été inhibée. Ceci confirme que Der p peut activer les deux voies MKK/ERK et p38 qui dépendent d’un adaptateur commun de la différenciation myéloïde activée par la protéine 88 (MyD88). Toutefois, bien que partageant certains éléments avec la voie de signalisation activée par le LPS au niveau du macrophage, la réponse inflammatoire allergique induite par l’allergène Der p se distingue radicalement du point de vue immuno-physiopathologique de la réaction inflammatoire au LPS par la mobilisation de populations lymphocytaires différentes productrices de cytokines Th2 spécifiques (versus Th1). Ceci nous a donc conduits à rechercher au niveau du macrophage s’il existait une différence dans la nature de la réponse innée induite par Der p versus LPS pouvant refléter la dichotomie de la réponse immune adaptative spécifiquement activée par ces 2 stimuli. Ainsi nous avons pu observer que contrairement au LPS qui active la réponse cytokinique Th1, Derp n’induit pas la sécrétion d’IL-12 par les macrophages. De plus et de façon intéressante, nous avons trouvé que dans les cellules MH-S le prétraitement par Der p peut inhiber la production d’IL-12 induite par le LPS ainsi que l’expression du facteur de transcription T-bet. En revanche Der p seul induit la sécrétion de MDC (macrophage derived chemokine, CCL22) mobilisatrice de cellules Th2. Nous proposons donc que ces réponses différentielles du macrophage pourraient rendre compte de la polarisation de la réponse immunitaire adaptative vers une réaction inflammatoire allergique.
Par ailleurs, le prétraitement des cellules MH-S par la SP-D peut inhiber la production de NO et de TNF-α, l’activation des voies MKKK3/6 et P38 ainsi que la translocation de NF-κB induites par Der p. Pour explorer les mécanismes inhibiteurs de la SP-D sur l’activation des macrophages alvéolaires et la sécrétion de cytokines induites par Der p nous avons émis hypothèse que soit la SP-D peut interagir avec Der p pour empêcher ainsi sa liaison aux récepteurs cellulaires TLR4/CD14/MD2, soit que la SP-D lie directement le CD14 ou TLR4, rendant la liaison de Der p aux récepteurs TLR4/CD14/MD2 impossible. La troisième possibilité serait un effet inhibiteur de la SP-D qui passerait par la modulation d’une voie de signalisation inhibitrice telle que celle activée par DC-SIGN, et qui régulerait l’activation des macrophages et la réponse immunitaire Th2 induite par Der p. Nos résultats montrent que la SP-D se lie au CD14 et bloque alors directement la liaison de Der p au CD14, inhibant ainsi l’activation du signal TLR4/CD14/MD2. De plus, la SP-D régule la diminution de l’expression de DC-SIGN par Der p ce qui conduit à inhiber la réponse inflammatoire induite par Der p. En résumé nous avons trouvé que (1) la SP-D a des effets thérapeutiques sur une inflammation bronchique due aux allergènes dans le modèle murin de l’asthme. (2) L’activation des macrophages alvéolaires par des allergènes d’acariens est médiée par CD14 et TRL4 et peut être inhibée par un prétraitement à la SP-D. (3) L’allergène Der p active les
inhibiteur de la réponse inflammatoire induite par Der p. En conclusion, SP-D est une molécule importante de l’immunité innée des poumons et elle peut réguler l‘inflammation pulmonaire induite par des allergènes. Nos résultats suggèrent que les mécanismes moléculaires du rôle régulateur de SP-D sont soit directs à travers son interaction avec CD14 à la surface cellulaire, liaison qui prévient l’activation du macrophage par der p ; soit indirects en régulant l’expression de DC-SIGN qui inhibe les réponses inflammatoires induites par Der p. Ces résultats peuvent contribuer de façon significative à la découverte de nouveaux composés pharmaceutiques contre l’asthme qui pourraient augmenter la production de SP-D endogène, ou bien qui cibleraient les récepteurs liés par les allergènes et ainsi que les voies de signalisation qui leurs sont associées pour traiter les maladies allergiques.
Contents
English abstract………...I Chinese abstract……….. III French abstract………..V Contents………...IX Figure Illustrations……….. XI Table Illustrations………...XV Abbreviations………...XVII Chapter 1-Introductions………...1 I. Introductions……….3
I.1 The prevalence of asthma………...3
II. Pathology of allergic asthma………...5
II.1 The characteristics of asthma………5
II.2 The pathogenesis of asthma………..7
II.3 Immune cells in allergic asthma………...7
II.4 Immune mediators in allergic asthma………...9
II.5 Allergic asthma treatment………....11
II.6 House dust mite is the major allergen in allergic asthma………11
III. The role of innate immunity in allergic asthma………...12
III.1 Alveolar macrophage……….13
III.2 Toll- like receptors……….14
III. 3 Pulmonary surfactants………...19
III.3.1 Lung surfactant specific hydrophobic SP-B and SP-C……….21
III.3.2 Lung surfactant specific hydrophobic SP-A and SP-D……….21
III.3.2.1 Collectin structure………21
III.3.2.2 Collectin functions………...23
III.3.3 Lung surfactant SP-A and SP-D components and functions………23
III.3.4 Receptors for lung surfactant SP-A and SP-D………..25
III.3.5 Surfactant proteins link innate and adaptive immunity………26
III.3.6 Surfactant protein D and allergic asthma………...27
Chapter 2- Specific aims………..37
Chapter 3- Results………....43
Part A. To verify the potential immunomodulating role of SP-D on the allergic response in mice, and its effects on alveolar macrophages (AMs) during allergic inflammatory respons……….45
p-induced inflammatory response………93 Chapter 4- Discussions………..107 Part A………...109 Part B………...113 Part C………...117 Part D………...121
Chapter 5- Materials and methods……….131
References………..143
Publications………165
I. Therapeutic effect of surfactant protein D in allergic inflammation of mite-sensitized mice………167
II. Mite allergen induces nitric oxide production in alveolar macrophage cell lines via CD14/toll-like receptor 4, and is inhibited by surfactant protein D……….175
III. House dust mite allergen activates NO and TNF-α and prevents IL-12 and T-bet production through TLR2/4 co-activation in alveolar macrophages………...185
Figure Illustrations
Fig. 1.1 The association between westernized lifestyle and asthma………...4
Fig. 1.2 Immunological pathways result in the inflammatory changes that occur in the airways of individuals with asthma……….6
Fig. 1.3 Compare the airway in asthma patient with normal airway………..8
Fig. 1.4. Interactions between Th1 and Th2 cells in asthma………..10
Fig. 1.5. Pathogen recognition by TLRs………16
Fig. 1.6. TLR signaling pathways………..18
Fig. 1.7. Lung host-defence mechanisms………...20
Fig. 1.8. Collectin and C1q structure……….22
Fig. 1.9. Gene and protein structure of human SP-A and SP-D………...24
Fig. 1.10. Functions of SP-A and SP-D as collectins………...28
Fig. 1.11. SP-A and SP-D receptors……….30
Fig. 1.12. SP-A and SP-D link innate and adaptive immunity to regulate host defense………..31
Fig. 1.13. Model for dual effects of lung surfactant proteins………...32
Fig. 2.1. The specific aim 1, 2, 3 and 4 of this study……….41
Fig. 3.1. The therapeutic effect of recombinant 60 kDa fragment of human surfactant protein D (rfh SP-D), Survanta and budesonide on Der p-induced airway inflammation in sensitized mice when administrated 6 h after allergen challenge (AC)………46
Fig. 3.2. The preventive effect of recombinant 60 kDa fragment of human surfactant protein D (rfh SP-D), Survanta, budesonide, or saline control in Der p-sensitized mice when administrated at 24 h before allergen challenge (AC)……….48
Fig. 3.3. Recombinant 60 kDa fragment of human surfactant protein D (rfh SP-D), but not Survanta or budesonide, decreases Der p-specific IgG and IgE antibodies of sensitized mice……….50
Fig. 3.4. Recombinant 60 kDa fragment of human surfactant protein D (rfh SP-D) augments T-helper type 1 and diminishes T-helper type 2 cytokines of Der p-sensitized mice. ……….52
Fig. 3.5. Recombinant 60 kDa fragment of human surfactant protein D (rfh SP-D) suppresses lipopolysaccharide- (LPS-) or Der p-induced inducible NO synthase (iNOS) expression of alveolar macrophages (AMs)………...53 Fig. 3.6. Concentration of nitrate in the supernatants of lipopolysaccharide- (LPS-), Der p-,
recombinant 60 kDa fragment of human surfactant protein D- (rfh SP-D-) treated alveolar macrophages (AMs), which were collected from naı¨ve and sensitized mice compared with the concentration of nitrite in the same AMs after pre-treatment of these AMs with rfh
AMJ2-C11 cell line………..58 Fig. 3.9. The production NO in LPS, and Der p- stimulated alveolar
macrophages isolated from bronchoalveolar lavage (BAL) fluids of C3H/HeN and C3H/HeJ mice………... ..60 Fig. 3.10. Western blot analysis of iNOs production from stimulated AMs cell lines………62 Fig. 3.11. The NF-κB (upper panel) and AP-1 (lower panel) activated by LPS and Der p were
determined by EMSA and inhibited by NF-κB inhibitor, PTDC, and AP-1 promoter inhibitor, curcumin………...64 Fig. 3.12. The production of inflammatory cytokines from LPS, and Der p-stimulated AMs cell
lines………...66 Fig. 3.13 The inhibitory effect of NO production on LPS-, or Der p-stimulated AMs cells by
SP-D………...68 Fig. 3.14. Dose-dependent nitric oxide production (a) and TNF-α production (b) from
Derp-stimulated macrophage cell line………...72 Fig. 3.15. IL-12 production from Der p-stimulated macrophage cell line………...74 Fig. 3.16. NO and TNF-α production from Der p-stimulated in human macrophage………...76 Fig. 3.17. Der p activated TLR-2 and -4 in TLRs-transfected HEK293 cell and is different from LPS signal pathway………...78 Fig. 3.18. Effect of the TLR4 antagonist Rhodobacter sphaeroides LPS on the LPS induced NO
production………...82 Fig. 3.19. Der p induced NO production involves the TLR2 and TLR4 associated
MyD88-dependent signaling pathways and is blocked by p38MAPK and MKK-1,-2 inhibitors………...84 Fig. 3.20. Translocation of NF-κB to the nucleus of MH-S cells stimulated by Der p………..86 Fig.3.21. Der p prevents LPS-induced Th1 response factors expression in MH-S cells……...88 Fig. 3.22. Pam3CSK4 (TLR2/TLR1 ligand) and LTA (TLR2/TLR6 ligand) blocks LPS-induced Th1
IL-12 cytokine production and T-bet transcription factor expression………...90 Fig. 3.23. Der p induces macrophage-derived chemokine (MDC) CCL-22 production in MH-S
cells………..91 Fig.3.24. Dose-dependent nitrite production and TNF-α production are inhibited by SP-D from
Derp-stimulated macrophage cell line………...94 Fig.3.25. Translocation of NF-κB to the nucleus of MH-S cells stimulated by Der p is inhibited by
SP-D pre-incubation………...96 Fig.3.26. SP-D inhibits Der p induced NO production involves the TLR2 and TLR4 associated
MyD88-dependent signaling pathways………98 Fig 3.27. SP-D does not influence the expression of CD14 and TLR4 on the MH-S cell line and
from allergic asthmatics (AS) and non-allergic controls (NC)………..104 Fig. 4.1. Instillation of rSP-D to sensitized mice 6h after allergen challenge (therapeutic) obviously
reduced infiltration of eosinophils, and also reduced levels of IL-4, IL-5, eotaxin, and TNF-α but elevated levels of IFN-α in the BALF……….111 Fig. 4.2. Pretreatment of macrophages with SP-D, could inhibit NO production from Der p or
LPS-stimulated alveolar macrophages………...116 Fig. 4.3. Der p allergen activates NF-κB dependent NO and TNF-α pro-inflammatory mediators,
but prevent endotoxin induced IL-12 and T-bet production through TLR2/4 co-activation. Der p activates NO and TNF-α production in MH-S cells by through NF-κB signaling pathway………...120 Fig. 4.4. SP-D can inhibit Der p-induced alveolar macrophages activation through TLR signaling
pathways and influencing DC-SIGN expression………...125 Fig. 4.5 rCD14 binds to Der p in a dose-dependent manner………129
Table Illustrations
Table 1.1. Proposed diverse functions of SP-A and SP-D………33 Table 1.2. Receptors and binding proteins for SP-A and SP-D………34 Table 3.1. The inhibitory effect of NO production on LPS- or Der p-stimulated MH-S cell lines by
Abbreviations
2-ME 2-mercaptoethonal
AC After allergen challenge
AHR Airway hyperresponsiveness
AM Alveolar macrophage
AP-1 Activator protein 1
B6 C57BL/6NCrj
BALB/c BALB/cByJ
BALF Broncholaveolar lavage fluid
BMDC Bone marrow-derived dendritic cell
BSA Bovine serum albumin
BPB Bromphenil
C3 C3H/HeJ
DAPI 6-diamidino-2-phenylindole
DC-SIGN dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin
DTT Dithiothreitol
Der p Dermatophagoides pteronyassinus
DMEM Dulbecco’s modified Eagles medium
DMSO Dimethyl sulfoxide
EDTA Ethylenediamine teraacetic acid
ELISA Enzyme-linked immunosorbent assay
ERK Extracellular signal-regulated kinase
EMSA Electrophoretic mobility shift assay
FBS Fetal bovine serum
FITC Fluorescein isothiocyanate
GAPDH Glycerol-3-phosphate dehydrogenase
HEK293 Human embryonic kidney 293
IFN Interferon
IL Interleukin
iNOS Inducible NO synthase
i.p. Intraperitoneal
i.t. Intratracheal
IRAK IL-1 receptor-associated kinase
JNK c-jun NH2-terminal kinase
MyD88 Myeloid differentiation primary response protein 88
NADPH Nicotinamide adenine dinucleotide phosphate (reduced form)
NF-κB Nuclear factor-kappaB
NO Nitric oxide
O.D. Optical density
PAGE Polyacrylamide gel electrophoresis
PAR Protease activated receptor
PBMC Peripheral-mononuclear cell
PBS Phosphate buffered saline
PCR Polymerase chain reaction
PVDF Polyvinylidene difluoride
RGDS Arg-Gly-Glu-Ser
RT-PCR Reverse transcriptase polymerase chaine reaction
SDS Sodium dodecyl sulfate
siRNA Short interfering ribonucleic acid
SNP Single-nucleotide polymorphism
SP Surfactant protein
TARC Thymus and activation-regulated chemokine
Th T helper
TLR Toll-like receptor
TNF Tumor necrosis factor
Chapter 1.
Introduction
I. Introduction
The lung is constantly exposed to the environmental microbial components and the host develops the innate and adaptive immunity to protect the lung from external pathogen. Infections of the respiratory tract, especially with various viruses and bacteria, are classical triggers of asthma exacerbations (Renz et al., 2002; Beisswenger et al., 2006) but during the past twenty years more and more epidemiologic and clinical studies have provided indirect evidence that infections may prevent the development of allergy and asthma. This is referred to the “hygiene hypothesis”. The hygiene hypothesis was first proposed by Strachan in 1989, which suggested that infections and unhygienic contact may confer protection from the development of allergic illnesses. It was then proposed that viral and bacterial infections could inhibit the T-helper (Th)-2 immune response associated with allergic reaction by stimulating a Th-1 response involved in defense of viral and bacterial infection (von Mutius, 2007; Schroder et al., 2007). Although hygiene hypothesis offers an explanation for increasing rates of allergic diseases such as asthma in modern westernized societies (Sigsgaard et al., 2008), still there are unanswered questions regarding how airborne allergens interact with the innate and adaptive immunity of lung and mechanisms of allergen-induced airway inflammation in asthma are largely unclear.
I.1 The prevalence of asthma
Asthma is a chronic respiratory inflammatory disease characterized by difficulty in breathing that affects people of all ages. Now it’s a major public health burden worldwide. The latest WHO statistics (2007) estimate that 300 million people worldwide have asthma and each year, 250,000 people die of asthma (Peter J. et al., 2008). Since about 1960, the prevalence of asthma and allergic disease has increased sufficiently to become a major public-health concern. It is generally accepted that asthma and allergy are associated with industrialization, a westernized lifestyle, and their prevalence is higher in developed countries (Fig. 1.1). There are four features concerning the westernized lifestyle suggested as possible causes of the increased prevalence of asthma and allergy. (1) Exposure to the house dust mite (an important perennial allergen) has increased, because modern housing, in general,
Fig. 1.1. The association between a westernized lifestyle and asthma.
(Graham Devereux, 2006)
The variation in the global prevalence of asthma is shown, as determined using standardized methodology. Numbers indicate the percentage of individuals in each country who have asthma.
that has been processed, modified, stored and transported great distances. This is in contrast to the traditional diet that food was produced locally and was eaten shortly after harvesting (Devereux et al., 2006). In addition to the external factors, some studies indicate that the individuals with asthma also present an exaggerated tendency to mount IgE response to a wide variety of common environmental allergens (Hammad et al., 2008). This state is called atopy and seems to be influenced by several genetic loci. Atopy is the propensity of an individual to develop allergic disease, such as asthma or atopic dermatitis. It is defined operationally by elevation in serum levels of IgE reactive with allergens or by skin-test reactivity to allergens. There is 30% ~ 50% of the population with atopy (Hammad et al., 2008), but only 10-12% of the population actually suffers from asthma. This mplies that allergic Th2-cell sensitization and the presence of an IgE response to an inhaled allergen are only risk factors rather than the causative factors of asthma. Asthma is most common in childhood but is being diagnosed more and more in adults. In Taiwan, there are about 5% of school children having allergic asthma (Wang et al., 2007). From 1974 to 1994, four asthma mass screening studies were conducted among middle school students in Taiwan. The data showed that the rate of asthma prevalence increased rapidly from 1.3% among the middle students in Taipei city in 1974 to 5.08% in 1985, 5.80% in 1991 and up to 10.79% in 1994. During the past 20 years, it has increased more than 8 folds. The report also emphasizes that asthma in city region (Taipei and suburbs) and industrial region are higher than agriculture region (Wu et al., 1998). These evidences reveal that social civilization progression and industrialization influence asthma increasing as we mention previously. For the patients with atopic asthma, the prophylaxis is to avoid exposure allergen. However, with industrialization and westernized lifestyle, it is a difficult task.
II. Pathology of allergic asthma
II.1 The characteristics of asthma
There are kinds of asthma defined clinically as allergic (extrinsic) and non-allergic (intrinsic) asthma. Generally, most patients belonging to allergic asthma are sensitive to environmental allergens and containing specific IgE antibodies against these allergens. Non-allergic asthmatics, in contrast, are
Fig. 1.2. Immunological pathways that result in the inflammatory changes that occur in the airways of individuals with asthma. (Graham Devereux, 2006)
Genetic, environmental, lifestyle, social and allergen-associated factors combine, resulting in CD4+ T helper (Th)-cell responses to allergens being biased towards Th2-cell responses. TH2 cells release the cytokines interleukin-4 (IL-4), IL-5, IL-9 and IL-13, and these cytokines induce and perpetuate inflammation mediated by eosinophils and IgE-induced mast-cell degranulation. In the airways of individuals with asthma, this inflammatory response results in airway narrowing because of mucosal oedema, smooth-muscle constriction, mucus hypersecretion and epithelial-cell shedding into the airways. Chronic inflammation in the airways of individuals with asthma might lead to permanent structural changes, such as smooth-muscle hypertrophy and subepithelial fibrosis. APC, antigen-presenting cell.
characterized by:
1. Reversible form of airflow obstruction (Spahn et al, 2002 and US DHHS, 1991), 2. Infiltration of eosinophil and T helper (Th) lymphocyte,
3. Airway hyperresponsiveness (AHR) (Djukanovic et al., 1990).
Although the process is incompletely understood, it is well established that several cytokines are involved in the recruitment of inflammatory cells such as T lymphocytes, eosinophils, and mast cells into the airways of patients with asthma (Fig. 1.2).
II.2 The pathogenesis of asthma & early phase reaction
Inhaled allergen challenge in allergic asthma patients leads to an early-phase, immediate type of hypersensitivity reaction which is followed by a late-phase inflammatory reaction. The early-phase reaction is initiated by the binding of the allergen to the cells bearing allergen-specific IgE. It is characterized by the rapid activation of airway mast cells and macrophages. The mast cells rapidly release pro-inflammatory mediators such as histamine (Jarjour et al., 1997), leukotriene C4, D4, and E4
and prostaglandin D2 (Murray et al., 1985; Liu et al., 1991) and macrophages release reactive oxygen
species (ROS) (Tonnel et al., 1983; Calhoun et al., 1992). The release of these mediators induces contraction of airways smooth muscle, mucous secretion, and vasodilatation. Also the inflammatory mediators induce microvascular leakage with exudation of plasma into the airways. Acute plasma protein leakage induces a thickened, engorged and edematous airway wall and results in the narrowing of the airway lumen (Fig. 1.3). Plasma exudation may compromise epithelial integrity, and its presence in the lumen may reduce clearance of mucus (Wanneret al., 1996). Plasma proteins may also promote the formation of viscid luminal plugs of exudate mixed with mucus, and inflammatory and epithelial cells. Together, these effects contribute to airflow obstruction. It’s the most important reason for allergic asthma patients to breathe difficultly.
II. 3 Immune cells in allergic asthma & late phase reaction
As we mentioned, the early-phase reaction may be followed by late-phase reaction in some cases. The late-phase inflammatory reaction occurs between 6 to 9 h after allergen stimulation and involves
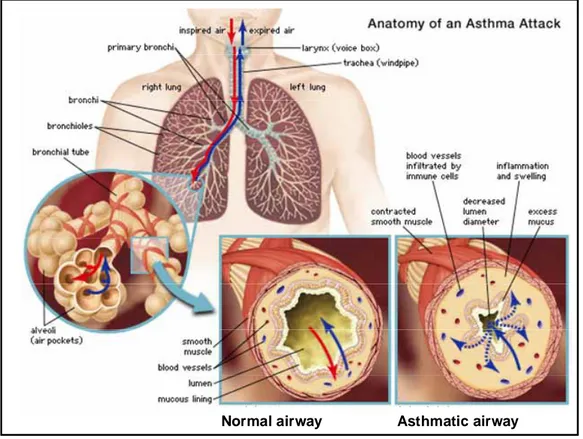
Normal airway Asthmatic airway Normal airway Asthmatic airway
Fig. 1.3. Comparison of the airway in asthma patient with normal airway. (Encyclopædia
Britannica, Inc. 2001)
During normal breathing, inhaled air travels through two main channels (primary bronchi) that branch within each lung into smaller, narrower passages (bronchioles) and finally into the tiny, terminal bronchial tubes. During an asthma attack, smooth muscles that surround the airways spasm; this results in tightening of the airways, swelling and inflammation of the inner airway space (lumen) due to fluid buildup and infiltration by immune cells, and excessive secretion of mucus into the airways. Consequently, air is obstructed from circulating freely in the lungs and cannot be expired.
Twenty-four hours after allergen challenge, an increase of activated interleukin-2 (IL-2)-positive T cells and of interleukin-5 (IL-5) or granulocyte–macrophage conony–stimulating factor (GM-CSF) messenger RNA (mRNA) expression are observed (Bentley et al., 1993). It suggests that T cells are involved possibly in the more chronic phase of the response. The long-term effect of airway inflammation results in chronic inflammation. Although allergic asthma is initially driven by a response to a specific allergen, the subsequent chronic inflammation seems to be perpetuated even in the apparent absence of further exposure to allergen. An important feature of chronic airways inflammation of asthma is characterized by the continued presence of increased numbers of Th2 lymphocyte, eosinophils, neutrophils, and other leukocytes.
II. 4 Immune mediators in allergic asthma
The inflammatory mediators which are released from immune cell are a key mechanism for allergic asthma. In asthmatic patients, there is an increase in the number of Th2 cell in the airways. Th2 cells secret the cytokines IL-4 and IL-13, which drive IgE production by B cells, IL-5, which is responsible for eosinophil differentiation in the bone marrow, and IL-9, which attracts and drives the differentiation of mast cell. These mediators have a central role in allergic inflammation. Moreover, the transcription factor GATA-3 (GATA-binding protein 3) expression is increased in asthmatic subjects compared with normal subjects. GATA-3 is crucial for the differentiation of naïve T cell into Th2 cell and also regulates the secretion of Th2 type cytokines. Also, GATA3 expression in T cells is regulated by transcription factor STAT6 (signal transducer and activator of transcription 6), which is in turn regulated by IL-4 (Maneechotesuwan et al., 2007). For Th1-cell differentiation and secretion of the Th1 type cytokine interferon-γ (IFN- γ), the crucial transcription factor, is T-bet. T-bet is reduced in the T cell of the asthmatic patients compared with non-asthmatic subjects, and T-bet-deficient mice spontaneously develops multiple physiological and inflammatory features resembling to human asthma. T-bet inhibits the function of GATA-3 and T-bet-deficient mice show increased expression of GATA-3 and production of Th2 cytokines, confirming that T-bet is a regulator of GATA-3 (Finotto et al., 2002). GATA3 expression is also down-regulated by IL-27, identified as a member of the IL-12 family, favoring the production of Th1 cytokine. In contrast, GATA-3 inhibits the production
Fig. 1.4. Interactions between Th1 and Th2 cells in asthma. (Peter et al., 2008)
The transcription factor GATA3 (GATA-binding protein 3) is regulated by interleukin-4 (IL-4) via STAT6 (signal transducer and activator of transcription 6) and regulates the expression of IL-4, IL-5, IL-9 and IL-13 from T helper 2 (TH2) cells and also inhibits the expression of T-bet via inhibition of STAT4. IL-33 enhances the actions of GATA-3. T-bet regulates T helper 1 (TH1)-cell secretion of IL-2 and interferon-γ (IFNγ) and also has an inhibitory action on GATA3. T-bet is regulated by IL-12 via STAT4 and by IL-27 via STAT1. This demonstrates the complex interplay of cytokines and transcription factors in asthma.
II. 5 Allergic asthma treatment
In allergic individuals, allergen stimulation is fundamental for the development of allergic asthma. Therefore, the most important prophylaxis for the allergic asthma is to avoid allergen exposure. On the other hand, after allergen sensitization, corticosteroids and β2-adrenoceptor agonists are used as main preventive treatment of asthma. Corticosteroid suppresses inflammation in asthmatic airway by inhibiting the production of Th2 cytokine and chemokine and decreasing the expression of adhesion molecules. While inhaled corticosteroid is highly effective in suppressing airway inflammation, they do not influence the original history, even when treatment is started in early childhood. β2-adrenoceptor agonist is an effective bronchodilators and it can reduce the symptoms of
asthma rapidly. Upon β2-adrenoceptor agonist binding to β2-adrenoceptor, GS protein stimulates
adenylate cyclase to increase cyclic adenosine 3’5’-monophosphate (cAMP) which in turn activates protein kinase A. It mediates smooth muscle relaxation through the phosphorylation of myosin light-chain kinase and by opening Ca2+-dependent K+ (KCa) channels, which relieves airway constriction in asthma. In addition to these drugs, other therapeutic approaches may be used or are currently under study: such as allergen-specific immunotherapy, IgE therapy, inhibitors of mast cells and cytokine-based immunotherapies. Allergen-specific immunotherapy is now used for mild asthma and allergic disorder, and other methods are still under study or in clinical trials. Despite these drugs and efficient therapeutic approaches, there are still 250000 people worldwide die from asthma each year currently.
II.6 House dust mite is the major allergen in allergic asthma
Allergens from the house dust mite (HDM) are major environmental trigger factors for allergic disorders, such as asthma, atopic dermatitis, or rhinitis (Arlian et al., 1991; Platts-Mills et al., 1992). House dust mite can be found everywhere in human habitation and are fed on organic detritus such as flakes of shed human skin. A typical house dust mite is measured 0.42mm in length (almost 0.5 mm) and 0.2~0.3mm in width. The European house dust mite (Dermatophagoides pteronyssinus, Der p) and the American house dust mite (Dermatophagoides farinae, Der f) are the two major species causing allergic asthma (Huss et al., 2001) (Salo et al., 2008) (Wu et al., 2007), but are not necessarily
allergen of mites is known to be a cyseine protease (Chapman et al., 1980), while the second group (Der p 2) belongs to the NPC2 (Niemann-Pick disease type 2) protein family (Heymann et al., 1989). These two groups are considered as the most important allergens because of high-frequency binding of IgE (Chua et al., 1992). The following group are: group III (Der p 3): trypsin-like activity (Stewart et al., 1992), group IV (Der p 4): Alpha amylase activity (Lake et al., 1991), group VI (Der p 6): chymotrypsin-like activity (Yasueda et al., 1993), group VIII (Der p 8): Glutathione S-transferase (GST) activity (O'Neill et al., 1994), group IX (Der p 9): Collagenolytic serine protease activity (King et al., 1996), group X (Der p 10): tropomyosin (Asturias et al., 1998), group XI (Der p 11): paramyosin (Lee et al., 2004), group XIV (Der p 14): apolipophorin (Epton et al., 2001) and group XX (Der p 20): arginine kinase activity (Hales et al., 2006). The characteristic property of allergens is their ability to induce Th2 response leading to IgE production and allergic disease (Ford et al., 1990; Illi et al., 2003; O’Brien et al., 1997). Recently, some studies highlighted the potential role of the proteolytic activity of Der p in the activation of an allergic inflammatory reaction. For instance, the property of Der p 1 to elicit IgE is due to its ability to cleave proteolytically cell surface molecules: CD40 on dendritic cells, CD23 (Fcε RII) (Shakib et al., 1998) on B cells, (leading to an increase of soluble CD23 which prevent IgE ligation to membrane CD23 to deliver a negative IgE regulatory signal on B cell (Haczku et al., 2000) (Hewitt et al., 1995)), and CD25 on T cell (IL-2 receptor α subunit) [resulting in low level of IFN-γ and high level of IL-4 (Schulz et al., 1998) (Holtzman et al., 1996)]. The proteolytic activity of Der p makes the proliferation of Th1 cells diminished and a bias of immune response towards Th2 cells. Although increasing number of studies suggest that the ability of Der p to induce Th2 immune response may depend on its proteolytic activity of Der p, many allergic components of Der p are devoid of such proteolytic properties. For example, Der p 2, 5 and 7, have not yet been associated with any specific enzymatic activity (Lynch et al., 1994; Stewart et al., 1996).
III. The role of innate immunity in allergic asthma
Although adaptive immune responses are well known to play essential roles in allergic asthma, the role of innate immunity is not yet defined, and the pathogenesis and functional consequences of initiating allergic inflammation remain to be clarified. As the innate immune system is an important
of lung innate immune system components which were proposed to be potentially involved in allergic asthma.
III.1 Alveolar macrophage
Allergic asthma is an immune mediated disease initiated by definite allergens which activates converging mediators of airway inflammation through multiple interactions with the immune system cellular network. From the immunological point of view, the hallmark of allergic asthma is the central role played by the CD4+ Th2 T lymphocytes which modulate the production of the key Type II cytokines IL-4, IL-5 and IL-13 involved in many of the clinical and immunological asthma features such as AHR, activation of specific IgE producing B lymphocytes, recruitment of inflammatory cells or airways remodeling (Cohn et al., 2004). It is now widely admitted that the induced biased differentiation of naïve T cells into allergy specific Th2 cytokines (IL-4, IL-5, IL-13) producing T cells vs classical inflammatory mediators (IL-12, IFN-γ) associated Th1 cells occurs under the tutoring of local antigen presenting cells (APCs). Among these the pulmonary dendritic cells (DCs) have been shown to act as sentinels capable to alert and to instruct naïve T cells by carrying and presenting them foreign antigens in draining lymph nodes. However, although the mechanism by which DCs instruct naive CD4+ T cells to undergo Th1 differentiation in response to TLRs recognized microbe antigens is becoming clear, the events and molecular determinants which conduct the DCs to trigger a characteristic Th2 allergic immune response to allergens remains quite obscure. Related to these DCs, the pulmonary alveolar macrophages (AMs) are the most abundant innate immune effector cells of normal non inflamed lungs (Martin et al., 2005). They represent up to 90% of the hematopoietic cell (including myeloid (monocytes and macrophages, neutrophils, basophils, eosinophils, erythrocytes, megakaryocytes/platelets, dendritic cells), and lymphoid lineages (T-cells, B-cells, NK-cells)) content of the alveolar space though their functions in the initiation and progression of allergic asthma are fairly unknown. Hence pulmonary AMs are among the first cells capable to detect incoming inhaled allergens and to trigger an adaptive immune response through the secretion of cytokines and chemokines obviously associated to inflammation. Paradoxically, at the opposite they are found to suppress allergen induced lung inflammation clearly contributing to the control and containment of
lung of experimental asthma sensitive rats (Careau et al., 2004).
Moreover, AMs from asthmatic patients are more “activated” than those from normal subjects in
that they express elevated levels of ICAM-1 and LFA-1 (Chanez et al., 1993). Stimulated AM from asthmatics produce more GM-CSF, TNF-α, IL-8, and leukotrienes than AMs from non-asthmatics (Hallsworth et al., 1994; Damon et al., 1987), and it has also been shown that AM influence the production of IL-5 by CD4+ T cells (Tang et al., 1998). In addition, AMs from allergic asthmatics exhibit an increased expression of the costimulatory molecule CD80 and are more efficient in antigen (Ag) presentation than AMs from normal subjects (Burastero et al., 1999). In animal model, Chen and his colleagues demonstrate that dust mite allergens from Dermatophagoides farinnae, Der f, could directly induce AMs production of inflammatory cytokines and direct naïve T cells to become Th2-cytokine producer (Chen et al., 2003). In addition, allergen up-regulate AMs inducible NO synthase (iNOS) mRNA and subsequent release of NO and pro-inflammatory cytokines (Tylor-Robinson et al., 1994; Currie et al., 2000). Although these evidences demonstrate that AM has the potential to play a significant role in initiating and regulating airway inflammation following exposure to allergens, the receptors of allergen on AMs and the mechanism of allergen-induced inflammatory response are not well identified and need further exploration.
III.2 Toll- like receptors
Innate immunity, the first defense line of the host involves receptors able to detect a limited set of conserved molecule pattern that are unique to the microbial world. These conserved molecule patterns are shared by entire classes of pathogen such as gram-positive and negative bacteria, fungi and viruses and are called pathogen-associated molecular patterns (PAMP). These PAMPs are selectively detected by so called pattern recognition receptors (PRR) which are able to signal rapidly to the host in the presence of an infectious process. The Toll-like receptor (TLR) family is the best identified class of signaling PRR in mammalian species. Mammalian TLRs were evolutionarily conserved molecules and were originally identified in vertebrates on the basis of their homology with Toll, a molecule that stimulates the production of antimicrobial proteins in Drosophila melanogaster (Lemaitre, 2004 and Medzhitov et al., 1997). Flies do not have an adaptive immune system and dependent entirely on their innate immune system to fight against and contain microbial infection. The Toll protein is a type I
transmembrane proteins which consist of an extracellular domain comprising multiple copies of leucine-rich repeats (involved in ligand recognition), and a conserved region of about 200 amino acids in their cytoplasmic portion (Chaudhuri et al., 2005). Within the cytoplasmic component, a well characterized region shares sequence and functional homology with the IL-1R [IL (interleukin)-1 receptor], providing regions crucial to signaling, and is thus named the TIR (Toll/IL-1R) domain (Slack et al., 2000). TLRs are known to form a family of a minimum of 10 proteins (an 11th functional receptor has been identified in mice not in human) (Akira et al., 2003; Zhang et al., 2004) and they play an important role against environmental pathogen in regulating both innate and adaptive immunity (Heine et al., 2003).
TLR4 was the first found TLR and proved as an important factor for lipopolysaccharide (LPS) signal transduction (Medzhitov et al., 1997). In 1968, C3H/HeJ mouse known to be resistant to LPS was shown to harbor a mutant gene which was named for Lps (Sultzer, 1968). This gene was demonstrated as TLR4 gene and this inbred mouse strain was showed to harbor a point mutation (P712H) in the intracellular region of TLR4, resulting in defective signal transduction in response to LPS (Poltorak et al., 1998). Comparing with TLR4 wildtype mouse (C3H/HeN), C3H/HeJ is more resistant to gram-negative infection. Other TLRs, with the exception of TLR10, have been involved in the signaling of specific compounds. These include TLR1 (together with TLR2) involved in the response to lipoprotien, lipoteichoic acid (LTA), peptidoglycan and lipoglycan (Alexopoulou et al., 2002; Nigou et al., 2008). TLR3 for response to PolyI:C (double-stranded RNA) (Alexopoulou et al., 2001), TLR5 for responses to flagellin and flagellated bacteria (Hayashi t al., 2001), TLR6 (together with TLR2) for response to diacylated bacterial lipoproteins (Takeuchi et al., 2001) and soluble factor from group B streptococci (Henneke et al., 2001), TLR7 and TLR8 in mediating cellular stimulation induced by imidazoquinolones (small antiviral compounds) (Hemmi et al., 2002 and Jurk et al., 2002) and TLR9 as a main signaling molecule for CpG bacterial DNA (Hemmi et al., 2000) (Fig. 1.5). The signal transduction of TLRs begins at the intracellular Toll/Interleukin-1 receptor (TIR) domain which has the ability to bind and activate signaling molecules including MyD88 and TIR containing adaptor protein (TIRAP). This leads to the stimulation of several important signaling pathways such as mitogen-associated protein kinase (MAPK), signal transducer and activator of transcription
Fig. 1.5. Pathogen recognition by TLRs. (Akira et al., 2006)
TLRs recognize molecular patterns associated with a broad range of pathogens including bacteria, fungi, protozoa and viruses.
Most TLR-dependent responses in innate immune cells were found to be MyD88-dependent. However, the maturation of DCs in vitro induced by TLR3 and TLR4 agonists was not abolished in MyD88 knockout mice (Alexopoulou et al., 2001; Kaisho et al., 2001), revealing the existence of a MyD88-independent signaling pathway. Subsequently, Toshchakov and colleagues found that macrophages from MyD88-deficient mice could be stimulated with LPS to secret IFN-β (Toshchakov et al., 2002). Τhe data were consistently with the presence of the other adaptor molecules involved in TLR4 signaling, latter identified as TIR containing adaptor inducing IFN-β (TRIF) and TRIF related adaptor molecule (TRAM). Moreover, MyD88-independent pathway was found to lead to the activation of the transcription factors interferon regulatory factor (IRF)-3 and -7 and to the production of IFN-β (Fig. 1.6).
Increasing evidences from epidemiological studies and mouse models of allergic inflammation suggest that TLRs are intimately associated with allergic control. For example, TLR4 activation by LPS can favor both Th1- and Th2-type responses to allergen. Low doses of inhaled LPS were found to induce Th2 responses to inhaled antigen and eosinophilic inflammation (Rodriguez et al., 2003). In contrast, inhalation of high doses of LPS with antigen induced Th1 responses without eosinophilic inflammation (Eisenbarth et al., 2002). These results suggest that the level of inhaled LPS can determined the type of immune response generated. In addition, bone marrow-derived DC from MyD-88-deficient mice retains the capacity to up-regulate MHC class II and B7 co-stimulatory molecules, and to induce a Th2 response. Conversely, pulmonary DC from MyD88-deficient mice lost the ability to develop Th2 response when administrating intranasal antigen with a low dose of LPS (Piggott et al., 2005). Therefore, the type and localization of immune cell is also important for the role of TLR-induced regulation of Th2 immune response. Although the mechanisms by which they can exacerbate or in contrast, control the allergy clinical manifestation are still poorly understood.
Divergent effects are also found with TLR2. In mouse model of ovalbumin sensitization, TLR2 ligands, Pam3Cys or peptidoglycan, increase Th2 response when given at the time of sensitization (Redecke et al., 2004). In contrast, TLR2 ligands such as lipopreotein I from Pseudomonas
aeruginosa or Pam3CSK4 were found to enhance the abilities of mouse DC to induce the
Fig. 1.6. TLR signaling pathways. (Akira et al., 2006)
Each TLR family member has its specific signaling pathway. MyD88-dependent pathway possessed by all the TLR family members except for TLR3 is a common pathway to induce inflammatory cytokines. In addition, TLR3 and TLR4 possess TRIF-dependent pathway. TLR7, TLR8 and TLR9 also have a unique pathway to induce IFN-α in plasmacytoid DCs.
model (Kline et al., 1998; Serebrisky et al., 2000). Activation of TLR9 results in high levels of indoleamine 2, 3- dioxygenase (IDO) in the lung. IDO activity expressed by pulmonary epithelial cell suppresses lung inflammation and airway hyperreactivity (Hayashi et al., 2004). CpG-ODN inhibition of B cell IgE production may protect against allergic lung inflammation (Horner et al., 2001). Furthermore, CpG-activated DC can induce the production of regulatory T cell (Moseman et al., 2004). Moreover, CpG in vitro treatment of plasmacytoid DC from allergic patients reverses their capacity to favor the development of Th2 response and induces a Th1 response (Farkas et al., 2004). Thus, administration of CpG-ODN may directly inhibit allergic response and provide a potent mechanism of suppressing the atopic response. All these studies highlight the importance of TLR-mediated regulation of allergic asthma.
In addition, the TLR4-associated co-receptor CD14 is one of many genes that appear to contribute to the expression of the allergic asthma. It is localized on chromosome 5q31.1, a region linked to both asthma and total serum IgE concentration (Marsh et al. 1994; Meyers et al. 1994). CD14 is found in two distinct forms: a 55-kDa membrane molecule (mCD14) expressed primarily on the surface of monocytes/macrophages, dendritic cells and neutrophils (Haziot et al. 1993), and a soluble form (sCD14) in serum (Durieux et al. 1994). CD14 is responsible for environmental lipopolysaccharide (LPS) recognition and depends on TLR4 for signaling because it has no cytoplasmic tail to mediate intracellular signals. Recent studies suggested that promoter polymorphism of the CD14 gene is associated with serum IgE. The promoter region of the CD14 gene has been found to be associated with low levels of sCD14 expression and high levels of total serum IgE (Wang et al., 2005, O’Donnell et al., 2004 and Sharma et al., 2004) in the allergic asthma patient. In contrast, some independent studies indicated that promoter polymorphism of sCD14 gene is not associated with asthma (Heinzmann et al., 2003). The inconsistency of these results may be due to the importance of environmental exposure (Vercelli et al., 2004). Then, CD14 polymorphisms may play an important role in development of asthma and may determine the susceptibility to environmental allergen.
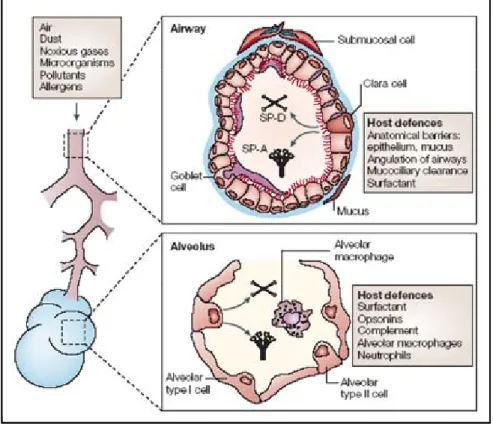
Fig. 1.7. Lung host-defense mechanisms. (Wright, 2005)
The lung is constantly challenged by inhaled pathogens, pollutants and particles. Several different defense mechanisms contribute to lung defense. These include filtration in the naso-oropharynx and conducting airways, sneezing, coughing and mucociliary clearance. Small particles might reach the alveolar gas-exchange regions of the lung. Host-defense functions in the peripheral air-spaces include surfactant, other opsonins (such as immunoglobulins) and innate immune cells (including alveolar macrophages and neutrophils). SP-A, surfactant protein A; SP-D, surfactant protein D.
bacterial, viruses, oxidants, pollutants and allergens. Thus, the presence of a local host defense system in the lung is critical for maintenance of normal lung function and defense against infection.
proteins which predominantly include the specific hydrophobic surfactant proteins (SP)-B and SP-C and hydrophilic proteins SP-A and SP-D (Weaver, 1991). Lung surfactant lipid and protein are synthesized and secreted by type II alveolar epithelial cells and are packaged together in a unique secretory organelle known as the lamellar body (Fig. 1.7) (Persson et al., 1988). Following appropriate stimulation, such as a deep breath (Wirtz et al., 1990 and Haller et al., 2001), lamellar-body is secreted into thin liquid hypophase that covers the alveolar epithelium.
III.3.1 Lung surfactant specific hydrophobic SP-B and SP-C
SP-B (14 kDa) and SP-C (6 kDa) are small proteins and have important roles in phospholipid packaging. They organize the surfactant and lower the surface tension at the air-liquid interface in the peripheral air space allowing expiration (Clement et al., 1957). SP-B specially has been considered to stabilize the phospholipid monolayer via its interaction with DPPC (Dipalmitoylphosphatidylcholine; the major lipid component of surfactant). SP-C may be involved in stabilizing the phospholipid layers that form during film compression at low lung volume. Some studies show that patients with low level of SP-B due to genetic mutations suffer from respiratory distress syndrome (RDS). Genetic mutation of SP-C is associated with interstitial lung disease (Nogee et al., 2004).
III.3.2 Lung surfactant specific hydrophobic SP-A and SP-D
SP-A and SP-D are complex oligomeric protein belonging to the collectin (collageneous lectin) family
III.3.2.1 Collectin structure
Collectin monomers are organized basically in four regions: (i) a cysteine-containing N-terminus (required for disulfide-dependent oligomerization) that is linked to (ii) collagen region composed of repeating Gly-X-Y triplets, where X means any amino acid and Y is often a hydroxyproline residue, (iii) an α-helical coiled-coil neck region involved in protein stabilization), and (iv) a globular structure at the C-terminus comprising CRD (carbohydrate recognition domain) belonging to the C-typecalcium-dependent lectin family (Fig. 1.8). The collectins are composed of trimeric subunits,
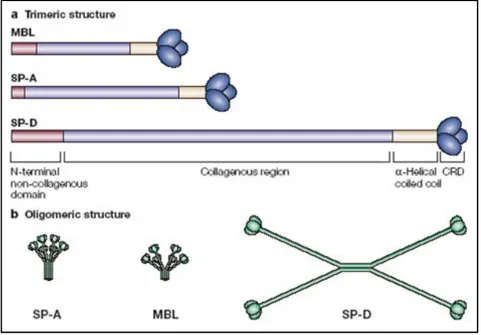
Fig. 1.8. Collectin and C1q structure. (Wright et al., 2004)
Surfactant protein A (SP-A) and SP-D are members of a family of proteins known as collectins. a. Collectins have collagen-like amino (N)-terminal regions and C-type (calcium dependent) carbohydrate-recognition domains (CRDs). Collectins consist of structural subunits that are composed of trimeric polypeptide chains, which are identical except for human SP-A. The trimers are assembled into oligomers. b. SP-A and mannose-binding lectin (MBL) are octadecamers (18-mers), consisting of six trimeric subunits. SP-D is a dodecamer (12-mer), consisting of four trimeric subunits. Although C1q is structurally homologous to SP-A and MBL, it is not a collectin as it does not have a lectin domain (CRD).
expressed in vascular endothelial cells (Ohtani et al., 2001).
(4) The bovine collectins: conglutinin, CL-43 and CL-46 (Hansen et al, 2002).
The structure of the trimeric clusters of C-type CRD has been first identified and defined by Drickamer (Drickamer, 1988). The lung collectins have distinct and common carbohydrate-binding activities. For instance, human SP-A prefers to bind to N-acetylmannosamine, L-fucose and mannose but SP-D binds preferentially to inositol, maltose, glucose and mannose. The trimeric clusters of C-type carbohydrate-recognition domain (CRD) has relatively low affinity for monosaccharides but with higher affinity for clustered oligosaccharides. Moreover, the high affinity of the collectin for clustered oligosaccharide is thought to be important to differentiate non-self from self, like lots of carbohydrates in animals are terminated by sugars, such as sialic acid and galatose, which are not recognized by the collectin (Haagsman et al., 1987 and Persson et al., 1990).
III.3.2.2 Collectin functions
The first collectin was identified more than 90 years ago by Bordet and Streng (Bordet et al, 1906). They demonstrated that the bovine serum protein conglutinin agglutinated erythrocytes coated with antibody and complement. Since then, more and more studies have demonstrated that the collectins bind Gram-positive and Gram-negative bacteria, virus, fungi and allergens (Crouch et al., 2001 and Shepherd et al., 2002).
III.3.3 Lung surfactant SP-A and SP-D components and functions
In addition to the originally described function of surfactant in reducing the surface tension at the air-liquid interface of the lung (Kishore et al., 2006), SP-A and SP-D are now recognized as a guard in lung immune host defense (Nogee, 2004). In humans, genes that encode SP-A, SP-D and Mannose- binding lectin (MBL) have been mapped as a cluster on the chromosome 10 (Bruns et al., 1987, Fish et al., 1987 and Criuch et al., 1993). The molecular weight of the monomer of SP-A and SP-D are 35 and 43 kDa respectively. SP-A has an octodecamer (18 chains) structure in which six structural trimeric subunits, of 105 kDa each, associate to yield a molecule of 630 kDa. Similarly, MBL is composed of six basic trimers, like SP-A, and resemble to the complement component, C1q except
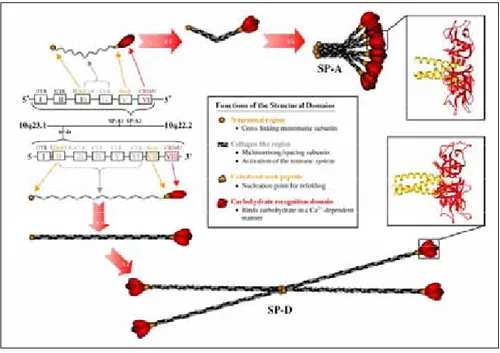
Fig. 1.9. Gene and protein structure of human SP-A and SP-D.
(Kishore et al., 2006)
Located on the long arm of chromosome 10 at 10q22.2–23.1, SP-A1 and A2 have six exons (four coding), while the more telomerically located SP-D has eight exons (seven coding). In SP-A, single polypeptide chains from SP-A1 and SP-A2 fold together to form either hetero- or homotrimers. Six of these units combine to give the SP-A its classical bouquet shaped 18-mer structures. In the case of SP-D, three polypeptide chains combine to form a trimeric structure that combines with another three similar structures to give SP-D its characteristic cruciform structure. Ribbon drawings of the X-ray crystal structures of trimeric recombinant fragments of human SP-D and rat SP-A show three-dimensional structure of the CRDs found in both of these surfactant proteins. The primary ligand-binding sites (one per CRD) are located at the CRD surface opposite the triple-helical collagen region. The SP-A and SP-D molecules are drawn approximately to scale.