Study on the associations between physical activity,
inflammatory markers and two breast cancer risk
factors
Thèse
Mirette Hanna
Doctorat en médecine expérimentale
Philosophiae doctor (Ph.D.)
Québec, Canada
III
Résumé
L'activité physique peut diminuer le risque de cancer du sein par un effet anti-inflammatoire. Nous avons examiné: 1. L'association de l'activité physique avec l'expression protéinique des marqueurs pro-inflammatoires (IL-6, TNF-α, CRP, COX-2, leptine, SAA1, IL-8 et STAT3) et anti-inflammatoires (TGF-β, IL-10 et lactoferrine) dans le tissu mammaire normal; 2. L'association de différents niveaux d'expression protéinique des marqueurs inflammatoires avec deux facteurs de risque de cancer du sein: la densité mammaire et l’involution lobulaire liée à l'âge; 3. L'association de l'activité physique avec les deux facteurs de risque de cancer du sein. L'activité physique totale (de domicile, travail et loisir) a été évaluée par un questionnaire validé chez 164 femmes ayant un cancer du sein. L'expression protéinique des marqueurs inflammatoires a été évaluée par immunohistochimie dans du tissu mammaire normal situé à distance de la tumeur. La densité mammaire a été évaluée par une méthode assistée par ordinateur sur le sein non-affecté. L’involution lobulaire liée à l’âge a été évaluée visuellement sur le tissu mammaire normal éloigné de la tumeur. Les associations ont été évaluées par des modèles de régression linéaires multivariés. Nous avons observé une association entre des niveaux élevés de l'activité physique totale et des niveaux d'expression faibles du marqueur pro-inflammatoire TNF-α et des niveaux d'expression élevés du marqueur anti-pro-inflammatoire IL-10 dans le tissu mammaire normal. En outre, les niveaux d'expression plus élevés de certains marqueurs pro-inflammatoires (IL-6, TNF-α, et SAA1) ont été associés à une densité mammaire plus élevée alors que, les niveaux d'expression plus élevés du marqueur anti-inflammatoire TGF-β ont été associés à une densité mammaire reduite. Les niveaux d'expression plus élevés de certains marqueurs pro-inflammatoires (IL-6, TNF-α, CRP, COX-2, leptine, SAA1 et IL-8) et le marqueur anti-inflammatoire IL-10 ont été associés avec des seins moins involués. Nous n’avons observé aucune association significative entre l'activité physique totale et la densité mammaire ou le degré d'involution lobulaire. Bien que l'expression des marqueurs inflammatoires semble être associée à des facteurs de risque de cancer du sein, l'activité physique peut diminuer le risque de cancer du sein pas uniquement par un effet anti-inflammatoire.
V
Abstract
Physical activity may decrease breast cancer risk through an anti-inflammatory effect. We aimed to examine: 1. The association of physical activity with the protein expression levels of pro-inflammatory (IL-6, TNF-α, CRP, COX-2, leptin, SAA-1, IL-8 and STAT3) and anti-inflammatory markers (TGF-β, IL-10 and lactoferrin) within normal breast tissue; 2. The association of the protein expression levels of these inflammatory markers with two breast cancer risk factors: mammographic density and age-related lobular involution; 3. The association of physical activity with the two breast cancer risk factors. Total physical activity (household, occupational and recreational) performed during a one-year period was evaluated by a validated questionnaire among 164 women having breast cancer. The protein expression of inflammatory markers was evaluated by immunohistochemistry in normal breast distant from the tumor. Mammographic density was evaluated by a computer-assisted method on the non-affected breast. Age-related lobular involution was visually evaluated on normal breast tissue distant from the tumor. Associations were evaluated by multivariate linear regression models. We observed association between increasing levels of total physical activity and lower expression levels of the pro-inflammatory marker TNF-α and higher expression levels of the anti-pro-inflammatory marker IL-10 in normal breast tissue. Moreover, higher expression levels of some pro-inflammatory markers (IL-6, TNF-α and SAA1) were associated with higher mammographic density whereas, higher expression levels of the anti-inflammatory marker TGF-β were associated with lower percent mammographic density. Higher expression levels of some pro-inflammatory markers (IL-6, TNF-α, CRP, COX-2, leptin, SAA1 and IL-8) and the anti-inflammatory marker IL-10 were associated with less involuted breasts. We observed no significant association between total physical activity and mammographic density or the degree of lobular involution. Although the expression of inflammatory markers seems to be associated with breast risk factors, physical activity may not decrease
VII
Table of contents
Résumé ... III Abstract ... V List of tables ... XI List of figures ... XIII List of abbreviations ... XV Dedication ... XVII Acknowledgement ... XIX Foreword ... XXI Chapter 1. Introduction ... 1 1. Introduction ... 2 1.1 Breast cancer ... 2
1.2 Local inflammation and breast cancer development ... 3
1.3 Physical activity ... 5
1.3.1 Definition and assessment ... 5
1.3.2 Physical activity and reduced breast cancer risk ... 6
1.3.3 Physical activity and inflammatory markers ... 7
1.4 Intermediate breast cancer risk factors ... 17
1.4.1 Mammographic density ... 17
1.4.2 Age-related lobular involution ... 22
1.5 Inflammatory markers and intermediate breast cancer risk factors ... 24
1.6 Physical activity and breast cancer risk factors ... 26
1.7 References ... 27
Chapter 2. Does mammographic density reflect the expression of breast cancer markers? ... 37
2.1 Abstract ... 39
2.2 Introduction ... 40
2.3 Associations of known breast cancer risk factors with mammographic density ... 40
2.4 Associations of expression levels of proteins within the breast tissue with mammographic density ... 41
2.5 Associations of growth factors with mammographic density ... 42
2.5.1 Insulin-like growth factor-1 ... 42
2.5.2 Epidermal growth factors ... 42
2.6 Associations of hormone receptors with mammographic density ... 43
2.7 Associations of enzymes with mammographic density ... 43
2.7.1 Aromatase ... 43
2.7.2 Cyclooxygenase-2 ... 44
2.7.3 Matrix metalloproteinases ... 44
2.8 Associations of proteoglycans with mammographic density ... 45
2.8.1 Heparan sulphate proteoglycan ... 45
2.8.2 Leucine-rich proteoglycans ... 45
2.9 Associations of pro-inflammatory markers with mammographic density ... 46
2.10 Differential genes expression in breast tissue according to mammographic density ... 46
2.11 Conclusion ... 48
2.12 Acknowledgments ... 48
2.13 Conflict of interest ... 48
VIII
2.15 Update-Association of CD36 with mammographic density ... 58
2.16 References ... 58
Chapter 3. Hypotheses and objectives ... 59
Chapter 4. Association between physical activity and the expression of mediators of inflammation in normal breast tissue among premenopausal and postmenopausal women ... 61
4.1 Abstract ... 63
4.2 Introduction ... 64
4.3 Materials and methods ... 65
4.3.1 Study population and data collection ... 65
4.3.2 Physical activity assessment ... 66
4.3.3 Assessment of inflammatory markers ... 67
4.3.3a Tissue microarray construction ... 67
4.3.3b Immunohistochemistry staining ... 68 4.3.3c Interpretation of immunostaining ... 68 4.3.4 Statistical analyses ... 69 4.4 Results ... 70 4.5 Discussion ... 73 4.6 Conclusion ... 77 4.7 Author Contributions ... 77 4.8 References ... 77
Chapter 5. Association between expression of inflammatory markers in normal breast tissue and mammographic density among premenopausal and postmenopausal women ... 97
5.1 Abstract ... 99
5.2 Introduction ... 100
5.3 Materials and methods ... 101
5.3.1 Study population ... 101
5.3.2 Data collection ... 102
5.3.3 Inflammatory markers measurement ... 102
5.3.3a Tissue microarray construction ... 102
5.3.3b Immunohistochemistry staining ... 103
5.3.3c Interpretation of the immunohistochemistry staining ... 104
5.3.4 Mammographic density measurement ... 105
5.3.5 Statistical analyses ... 106 5.4 Results ... 107 5.5 Discussion ... 109 5.6 Authors’ Contributions ... 114 5.7 Financial support ... 114 5.8 References ... 114
Chapter 6. Association between Local Inflammation and Breast Tissue Age-Related Lobular Involution ... 129
6.1 Abstract ... 131
6.2 Introduction ... 132
6.3 Results ... 133
6.4 Discussion ... 135
6.5 Materials and methods ... 141
6.5.1 Study population and data collection ... 141
IX
6.5.2a Tissue microarray construction ... 142
6.5.2b Immunohistochemistry staining ... 142
6.5.2c Immunostaining interpretation ... 143
6.5.3 Age-related lobular involution assessment ... 144
6.5.4 Statistical analyses ... 145
6.6 Acknowledgment ... 146
6.7 Authors contribution ... 146
6.8 References ... 147
Chapter 7. Physical activity, mammographic density and age-related lobular involution among premenopausal and postmenopausal women ... 163
7.1 Abstract ... 165
7.2 Introduction ... 166
7.3 Material and Methods ... 167
7.3.1 Study population and data collection ... 167
7.3.2 Physical activity assessment ... 169
7.3.3 Mammographic density assessment ... 169
7.3.4 Age-related lobular involution assessment ... 169
7.3.5 Statistical analysis ... 170 7.4 Results ... 172 7.5 Discussion ... 174 7.6 Conclusions ... 177 7.7 Acknowledgements ... 177 7.8 References ... 177
Chapter 8. General discussion, conclusions and perspectives ... 191
8.1 Discussion ... 191
8.2 Conclusions and perspectives ... 205
8.3 References ... 207
Chapter 9. Bibliography ... 211
XI
List of tables
Chapter 1 P
Table 1 Association between physical activity and circulating levels of inflammatory markers among longitudinal studies
9
Table 2 Association between physical activity and circulating levels of inflammatory markers among randomized control trials
12
Chapter 2
Table 1 Main characteristics of cross-sectional studies examining the associations of expression levels of proteins within normal breast tissue with mammographic density
55
Chapter 4
Table 1 Characteristics of the study population by quartiles of total physical activity
81 Table 2 Association between total physical activity and the
expression of mediators of inflammation in normal breast tissue
83
Supplemental Table S1
Association between household physical activity and the expression of mediators of inflammation in normal breast tissue
87
Supplemental Table S2
Association between occupational physical activity and the expression of mediators of inflammation in normal breast tissue
90
Supplemental
Table S3 Association between recreational physical activity and the expression of mediators of inflammation in normal breast tissue
93
Chapter 5
Table 1 Primary antibody specifications 118
Table 2 Characteristics of the study participants 120
Table 3 Expression of inflammatory markers in normal breast
tissue and the percent mammographic density 122
Chapter 6
Table 1 Characteristics of the study population 150
Table 2 Association between the expression of inflammatory
markers in normal breast tissue and the degree of lobular involution
152
Table 3 Association between the expression of inflammatory
markers in normal breast tissue and the predominant lobule type
156
Chapter 7
XII
Table 2 Association between different types of physical activity (PA) in METs-h/week and the percent mammographic density (PMD)
183
Table 3 Association between different types of physical activity (PA) in METs-h/week and the degree of age-related lobular involution (ARLI)
185
Table 4 Association between different types of physical activity (PA) in METs-h/week and the predominant lobule type
186 Table 5 Association of different types of physical activity (PA) in
METs-h/week with the combination of the percent mammographic density (PMD) and the degree of age-related lobular involution (ARLI)
XIII
List of figures
Chapter 1 P
Figure 1 Hypothesised protective role of physical activity against breast cancer
2
Figure 2 Representative mammographic image of mammographic density 18
Figure 3 Evaluation of percent mammographic density by a computer-assisted method on digital mammographic image
20 Figure 4 Relative risk of breast cancer by percentage of the breast
showing mammographic densities 21
Figure 5 Different stages of development of mammary gland. 22
Figure 6 Hematoxylin-eosin staining of age-related lobular involution of
the breast 23
Chapter 4
Figure 1 Representative immunohistochemistry staining in tissue microarray (TMA) of normal breast tissue for the COX-2
86 Chapter 5
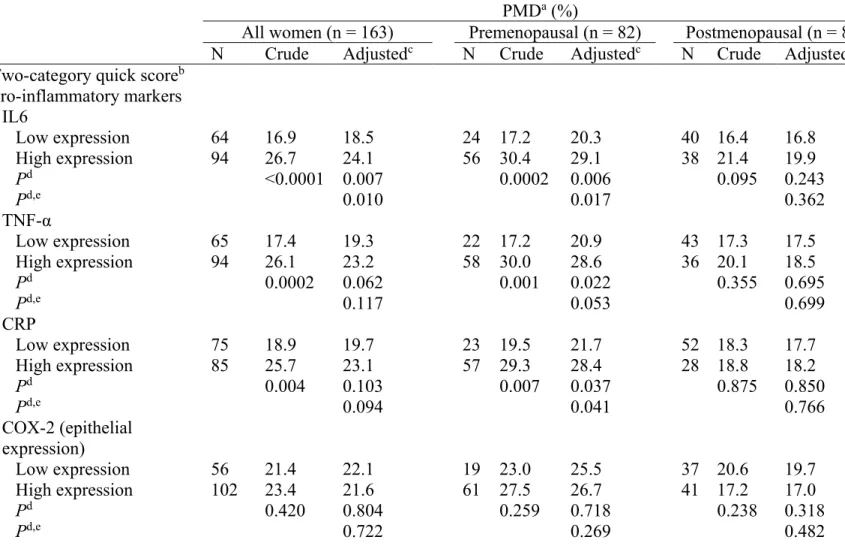
Figure 1 Examples of immunohistochemistry staining in tissue microarray (TMA) of normal breast tissue for the IL-6
126 Figure 2 Means of the percent mammographic density by the
two-category quick score of inflammatory markers for all women in the age- and waist circumference-adjusted model
127
Chapter 6
Figure 1A Representative immunohistochemistry staining for the intensity of the expression of TNF-α in tissue microarray (TMA) of normal breast tissue
160
Figure 1B Representative immunohistochemistry staining for the extent of the expression of TNF-α in tissue microarray (TMA) of normal breast tissue
161
Figure 2A Representative hematoxylin-eosin staining of different categories of degree of lobular involution in normal breast tissue
162 Figure 2B Representative hematoxylin-eosin staining of different lobule
types 162
Chapter 7
Figure 1A Representative hematoxylin and eosin staining of different degrees of lobular involution in normal breast tissue
189 Figure 1B Representative hematoxylin and eosin staining of different lobule
types
XV
List of abbreviations
ARLI age-related lobular involution
BC breast cancer
BI-RADS Breast Imaging Reporting and Data System
BMI body mass index
CI confidence interval
COX-2 cyclooxygenase-2
CRP C-reactive protein
DAB diaminobenzidine
ER estrogen receptors
HER-2 human epidermal growth fatror-2
HIER heat induced epitope antigen retrieval
H&E hematoxylin and eosin
IHC immunohistochemistry
IL-6 interleukin 6
IL-8 interleukin 8
IL-10 interleukin 10
IGF-1 insulin-like growth factor-1
mAb monoclonal antibody
MD mammographic density
METs-h/week metabolic equivalent of task-hour/week
MMPs matrix metalloproteinases
PA physical activity
pAb polyclonal antibody
PAI-1 plasminogen activator inhibitor-1
PMD percent mammographic density
PgR progesterone receptor
PR prevalence ratio
PYTPAQ Past Year Total Physical Activity Questionnaire
Q-PCR quantitative-polymerase chain reaction
RNA ribonucleic acid
SAA1 serum amyloid A1
SEER Iowa Surveillance, Epidemiology, and End Results
SNP single nucleotide polymorphism
STAT3 signal transducers and activators of transcription 3
TDLU terminal ductal lobular unit
TGF-α transforming growth factor-α
TGF-β transforming growth factor-β
TIMP-3 tissue inhibitor of metalloproteinases
TMA tissue microarray
TNF-α tumor necrosis factor-α
XVII
Dedication
XIX
Acknowledgement
I wish to express my profound appreciation for the assistance I have received from many who have generously provided help in this thesis. I would like to thank my supervisor Dr Caroline Diorio for her help during the whole PhD journey. I am also very appreciative of the assistance provided by the pathology staff at ‘Hôpital Saint-Sacrement’ for their invaluable help in preparing this material. I would like to make special thanks for Dr Bernard Têtu and Mrs Michèle Orain for their endless support. I would also like to thank Dr Simon Jacob and Mrs Isabelle Dumas for their help.
XXI
Foreword
Mirette Hanna participated in the conception of the study design and the preselection of the slides containing normal breast tissue. She performed all laboratory analyses (tissue microarray (TMA) construction, immunohistochemistry staining, analysis of the immunostaining for the expression of inflammatory markers in normal breast tissue, histological examination of normal breast tissue and the evaluation of the age-related lobular involution), interpreted results, performed all statistical analyses and wrote the manuscripts.
Co-authors Isabelle Dumas
Axe Oncologie, Centre de recherche du CHU de Québec, Québec, QC, Canada Michèle Orain
Axe Oncologie, Centre de recherche du CHU de Québec, Québec, QC, Canada Simon Jacob
Axe Oncologie, Centre de recherche du CHU de Québec, Service de pathologie, Hôpital du Saint-Sacrement,
Centre des Maladies du Sein Deschênes-Fabia, Hôpital du Saint-Sacrement, Centre de recherche sur le cancer de l’Université Laval,
Département de biologie moléculaire, biochimie médicale et pathologie, Faculté de médecine, Université Laval, Québec, QC, Canada
Bernard Têtu
Axe Oncologie, Centre de recherche du CHU de Québec, Service de pathologie, Hôpital du Saint-Sacrement,
Centre des Maladies du Sein Deschênes-Fabia, Hôpital du Saint-Sacrement, Centre de recherche sur le cancer de l’Université Laval,
Département de biologie moléculaire, biochimie médicale et pathologie, Faculté de médecine, Université Laval, Québec, QC, Canada
François Sanschagrin
Axe Oncologie, Centre de recherche du CHU de Québec,
XXII
Centre de recherche sur le cancer de l’Université Laval,
Département de Médecine sociale et préventive, Faculté de médecine, Université Laval, Québec, QC, Canada
Alexandre Bureau
Centre de Recherche de l’Institut Universitaire en Santé Mentale de Québec,
Département de Médecine sociale et préventive, Faculté de médecine, Université Laval, Québec, QC, Canada
Brigitte Poirier
Axe Oncologie, Centre de recherche du CHU de Québec,
Centre des Maladies du Sein Deschênes-Fabia, Hôpital du Saint-Sacrement, Centre de recherche sur le cancer de l’Université Laval,
Département de chirurgie, Faculté de médecine, Université Laval, Québec, QC, Canada Caroline Diorio
Axe Oncologie, Centre de recherche du CHU de Québec,
Centre des Maladies du Sein Deschênes-Fabia, Hôpital du Saint-Sacrement, Centre de recherche sur le cancer de l’Université Laval,
Département de Médecine sociale et préventive, Faculté de médecine, Université Laval, Québec, QC, Canada
Publications related to this work
- Mirette Hanna and Caroline Diorio. Is mammographic density a biomarker to study the molecular causes of breast cancer? InTec Mammography Book 2, chapter 9, p173-198, 2012. Mammography -Recent Advances, ISBN: 978-953-51-0285-4.
- Mirette Hanna and Caroline Diorio. Does mammographic density reflect the expression of breast cancer markers?” Climacteric 2013;16(4):407-16.
- Mirette Hanna, Isabelle Dumas, Michèle Orain, Bernard Têtu, Simon Jacob, François Sanschagrin, Alexandre Bureau, Brigitte Poirier and Caroline Diorio. Association between local inflammation in breast tissue and breast age-related lobular involution. Submitted to Cancer Discovery.
- Mirette Hanna, Isabelle Dumas, Michèle Orain, Bernard Têtu, Simon Jacob, François Sanschagrin, Alexandre Bureau, Brigitte Poirier and Caroline Diorio. Association between the expression of inflammatory markers in normal breast tissue and the mammographic density among premenopausal and postmenopausal women. Submitted to Cancer Research.
XXIII - Mirette Hanna, Isabelle Dumas, Michèle Orain, Bernard Têtu, Simon Jacob and Caroline Diorio. Physical activity, mammographic density and age-related lobular involution among premenopausal and postmenopausal women. Accepted for publication in Menopause. The Journal of the North American Menopause Society.
- Mirette Hanna, Isabelle Dumas, Michèle Orain, Bernard Têtu, Simon Jacob, François Sanschagrin, Alexandre Bureau, Brigitte Poirier and Caroline Diorio. Association between physical activity and the expression of mediators of inflammation in normal breast tissue among premenopausal and postmenopausal women. To be submitted to Cancer Research, Biomarkers and Prevention.
This work was presented in parts at:
- The 51st Annual Scientific Day of Pathology (Carlton-Auger) 2014, October 18,
2014, Quebec City, Canada.
- 3rd international conference on Clinical and Cellular Immunology 2014, September
29-October 1st, 2014, Baltimore, USA.
- Conférences scientifiques, Centre des maladies du sein, September 17, 2014, Quebec City, Canada.
- Scientific Day of Cancer Research Center (CRC)/ Oncology Axis, August 22nd,
2014, Centre de Recherche de l'Hôtel-Dieu de Québec (CRHDQ), Quebec City, Canada. Winning the best presentation award.
- 50th Annual Scientific Day of Pathology (Carlton-Auger) 2013, October 26, 2013,
Quebec City, Canada. Winning the best presentation award.
- 65th Annual Meeting of the Canadian Association of Pathologists (CAP-ACP 2014),
July 12-15, Toronto, Canada.
- 17th Research day of Faculty of Medicine of Laval University, May 29, 2014, Laval
University, Quebec City, Canada.
- 7th Annual Canadian Cancer Immunotherapy Consortium Symposium, May 21-23,
2014, Quebec City, Canada.
- 82nd Congress of the “Association Francophone pour le Savoir” (Acfas), May
12-16, 2014, Montreal, Canada.
- Canadian Cancer Research Conference-2013 (CCRC), November 3-6, 2013, Toronto, Canada.
XXIV
- 55th Annual meeting of the Clinical Researches Club in Quebec (CRCQ), September
26-28, 2013, Manoir du Lac Delage, Canada.
- Scientific Day of Cancer Research Center (CRC)/ Oncology Axis-2013, Centre de Recherche de l'Hôtel-Dieu de Québec (CRHDQ), August 22, 2013, Quebec City, Canada
- 64th Annual Meeting of the Canadian Association of Pathologists held jointly with
the association des pathologistes de Québec and the 27th World Congress of the
World Association of Societies of Pathology and Laboratory Medicine (CAP-ACP 2013), June 8-11, 2013, Quebec City, Canada. Winning the best presentation award. - 16th Research day of faculty of Medicine, Laval University, May 30, 2013, Laval
University, Quebec City, Canada.
- 24th University-hospital day, May 16, 2013, Laval University, Quebec City, Canada
- 81st Congress of the “Association Francophone pour le Savoir” (Acfas), May 6-10,
2013, Quebec City, Canada. Other communications:
- Danielle Larouche, Mirette Hanna, Caroline Diorio. Étude de l’association entre l’apport alimentaire en antioxydants et le niveau d’expression de marqueurs d’inflammation dans le tissu mammaire normal de femmes atteintes du cancer du sein. Scientific Day of Cancer Research Center (CRC)/ Oncology Axis, August 22nd, 2014, Centre de Recherche de l'Hôtel-Dieu de Québec (CRHDQ), Quebec
City, Canada.
- Kaoutar Ennour-Idrissi, Mirette Hanna, Caroline Diorio. Effet de l’activité physique sur les hormones sexuelles chez la femme : Revue systématique et méta-analyse des essais randomisés controlés. Scientific Day of Cancer Research Center (CRC)/ Oncology Axis, August 22nd, 2014, Centre de Recherche de l'Hôtel-Dieu de Québec
1
Chapter 1. Introduction
2
1. Introduction
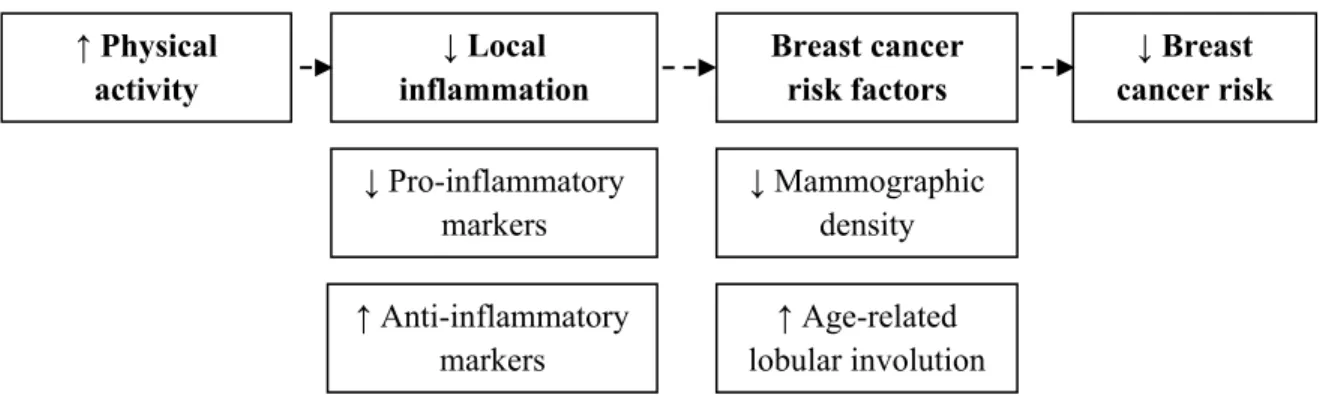
There is consistent evidence from ample previous studies that physical activity (household, occupational, recreational and total) reduces the incidence of breast cancer among pre- and postmenopausal women of diverse race and ethnicities [1, 2]. Moreover, physical activity delays the age of onset of breast cancer among women known to have genetic susceptibility such as women carriers of BRCA1 and BRCA2 mutations [3] and improves survival of women diagnosed with breast cancer[4]. However, mechanisms by which physical activity exerts its protective effect against breast cancer remain poorly determined. It is possible that physical activity affects the expression of various inflammatory markers in the breast tissue. Inflammatory markers are suspected to play a role in breast cancer etiology. The effect of these inflammatory markers could ultimately be reflected on a mammogram (mammographic density) or seen on histological examination (lobular involution). These radiological and histological changes are independently associated with breast cancer risk and can be viewed as intermediate markers of the disease [5]. The hypothesized role of physical activity against breast cancer could be schematized as follow; .
Figure 1. Hypothesised protective role of physical activity against breast cancer.
A better understanding of the biological mechanisms by which physical activity affects breast cancer risk could provide novel data for planning future breast cancer prevention interventions.
1.1 Breast cancer
Despite enormous effort made to defeat breast cancer, it remains a major health problem. Till date, breast cancer is so far the most common cancer affecting women
↑ Anti-inflammatory markers ↓ Mammographic density ↑ Age-related lobular involution lobular involution ↓ Breast cancer risk ↓ Pro-inflammatory markers ↓ Local inflammation Breast cancer risk factors ↑ Physical activity
3 worldwide. One-on-nine woman is expected to develop breast cancer during her lifetime. According to recent statistics, it represents the second cause of cancer deaths after lung cancer [6]. In Canada, an estimated 24,400 women were diagnosed with breast cancer during 2014 [7]. Only 5-10% of breast cancer cases are attributed to inherited genetic predisposition (such as BRCA1 and BRCA2 mutations) [8]. Yet, many of cancer cases may be linked to environmental (such as the exposure to radiation, industrial compounds, pollutants, and other chemicals) [9] and lifestyle factors (such as physical inactivity, smoking and alcohol consumption) [10]. Prolonged exposure to these factors may induce breast tissue injury creating some sort of local inflammation that is favourable for cancer development.
1.2 Local inflammation and breast cancer development
It is now widely accepted that chronic low-grade local inflammation is an important initiator of site-specific cancer development. Persistent exposure to various external or internal irritants may end by causing breast tissue injury. Healing process of the damaged tissue involves a local inflammatory response. Local inflammation results from the altered expression of pro- and anti-inflammatory markers in the breast tissue. Persistence of the offending agent results in a state of chronic low-grade local inflammation. Prolonged disruption of the balance between the expression of pro-inflammatory markers such as interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), C-reactive protein (CRP), cyclooxygenase 2 (COX-2), leptin, interleukin 8 (IL-8), serum amyloid A1 (SAA1) and signal transducer and activator of transcription 3 (STAT3) and anti-inflammatory markers such as transforming growth factor-β (TGF-β), interleukin 10 (IL-10) and lactoferrin with predominance of pro-inflammatory markers in the breast tissue may have a role in breast tumorigenesis [11-15]. Based on exhaustive review of literature, we confined our review to these eleven inflammatory markers, as indicators of local inflammation in the breast tissue, due to their expression in normal breast tissue.
Inflammatory markers are not only secreted by leukocytes but also by normal and malignant mammary cells [11-13]. It is suggested that pro- and anti-inflammatory markers exert opposing effects on mammary cells and that they can alter mammary cell function in an autocrine and paracrine fashion [12, 15]. Pro-inflammatory markers form a network to
4
promote sustained cellular proliferation, DNA damage, angiogenesis and to suppress anti-tumor adaptive immune response providing an ideal environment for cancer development [11, 12, 14, 16-18]. Moreover, pro-inflammatory markers can increase estrogen level in the breast tissue by stimulating aromatase enzyme [19, 20]. Aromatase enzyme is a key regulatory enzyme in the estrogen synthesis pathway. Conversely, anti-inflammatory markers exert an inhibitory activity on cellular proliferation [21, 22].
The inflammation-breast cancer link hypothesis is further supported by the observed increased expression of pro-inflammatory markers such as IL-6 [23], TNF-α [23], IL-8 [23], COX-2 [19], leptin [24] and phosphorylated (activated) form of STAT3 [25] as well as reduced expression of anti-inflammatory markers such as TGF-β [26] and lactoferrin [27] in malignant breast tissue compared to normal breast tissue. Similarly, levels of IL-6, TNF-α and IL-8 were found to be significantly higher in the nipple aspirate fluid of pre- and postmenopausal women with no breast cancer (n = 16) compared to that of women diagnosed with breast cancer (n = 19) [28]. Nipple aspirate fluid provides a somewhat accurate reflection of the breast tissue by a non-invasive procedure. Moreover, STAT3 gene was found to be highly expressed in biopsies obtained from breast cancer patients (n = 26) compared to that obtained from healthy women (n = 43) [29]. Nevertheless, the observed reduced mammary tumorigenesis associated with TNF-α deficiency in mice provides additional support to the hypothesized role of this marker in breast tumorigenesis [30]. The causal link between pro-inflammatory markers and breast cancer is further supported by the observed reduction in breast cancer risk associated with regular long-term use of non-steroidal anti-inflammatory drugs [31, 32]. Taken together, these findings suggest that increased expression of pro-inflammatory markers and decreased expression of anti-inflammatory markers in the breast tissue may be indicative of breast cancer risk.
Studies that considered the association between circulating levels of inflammatory markers, such as IL-6, TNF-α, CRP and leptin, and breast cancer risk provided inconsistent results [33-47]. However, several studies reported that higher circulating levels of CRP [41-44] and leptin [44-47] were associated with increased breast cancer risk. Additionally, many other studies observed an association between certain polymorphisms located in IL-6 [48, 49], TNF-α [50], IL-8 [49, 51, 52], COX-2 [53], STAT3 [54], TGF-β [55, 56] or IL-10
5 [49, 57, 58] genes and breast cancer risk. However, more research work is needed to determine the functional alleles. The association between circulating levels of SAA1, lactoferrin or their polymorphisms and breast cancer risk has not been explored yet.
1.3 Physical activity
1.3.1 Definition and assessment
Physical activity is defined as any skeletal movement accompanied by energy expenditure. Physical activity main domains are household, occupational and recreational physical activity. Many methods exist for the assessment of physical activity including direct methods (such as motion sensors, direct calorimetry and in-person observation) and indirect methods (such as retrospective questionnaires, metabolic and cardiovascular fitness markers measurement). However, each method has its own limitations and no gold standard method exists [59].
Ideally, accurate evaluation of physical activity necessitates the assessment of physical activity three components; frequency (number of episodes per unit time; months/year, weeks/month and days/week), duration (number of hours/episode) and intensity (the degree of effort spent while performing this activity as perceived by heart rate acceleration and sweating: 1 = activities performed mainly while sitting; 2 = light activities not accompanied by heart rate changes nor sweating; 3 = moderate activities accompanied by slight increase in heart rate and light sweating; 4 = heavy activities causing marked increase in heart rate and heavy sweating) spend while performing each type of physical activity. The combination of the three components provides a measure of the total amount of energy spend while performing a particular physical activity. Therefore, physical activity is commonly expressed as metabolic equivalent task-hours/week (Mets-h/week), a physical activity quantitative index. The Mets-h/week is obtained by a stepwise equation. The first step is multiplying the recorded frequency by the duration. The next step is the multiplication of the net result of the first multiplication by an intensity code recommended by the Compendium of Physical Activities [60, 61]. The intensity code may be adjusted according to the individual perceived intensity.The use of Mets-h/week as an evaluation of physical activity has the advantage to ensure the comparability of results between studies.
6
1.3.2 Physical activity and reduced breast cancer risk
Data from epidemiological studies provide sufficient evidence that physical activity is associated with a 20-30% reduction in breast cancer risk [62]. A dose-response relationship between increased levels of performed physical activity and reduced breast cancer risk is constant throughout household, occupational, recreational and total physical activity. The current Canadian’s physical activity guidelines recommendation are to practice at least 30 min/day of moderate to vigorous physical activity on most days of the week to achieve the greatest breast cancer risk reduction [62].
The exact mechanisms by which physical activity exerts its protective effect against breast cancer development remain poorly determined. Though, a long list of biological mechanisms has been proposed to explain the link between physical activity and reduced breast cancer risk [63-66]. It is likely that physical activity induced-weight reduction is of particular importance among physical activity protective mechanisms. Body weight is constantly shown to be associated with increased breast cancer risk especially among postmenopausal women [67, 68]. Moreover, adipose tissue is an important source of endogenous estrogen notably among postmenopausal women. Estrogen is a well-established breast cancer risk factor for pre- and postmenopausal women [69, 70]. Besides, physical activity may alter the lifetime breast tissue exposure to endogenous estrogen independently of weight loss. For instance, physical activity is known to be associated with delayed menarche as well as menstrual dysfunction due to absence of mid-cycle estradiol surge [71]. Next to the weight loss and estrogen levels reduction, comes the physical activity-insulin resistance link. There is convincing evidence that physical activity may improve insulin sensitivity especially among those suffering from impaired glucose tolerance [65]. Insulin resistance is positively associated with breast cancer risk. This association is somewhat more evident among postmenopausal women [72]. Moreover, physical activity may alter the expression of several proteins and genes, such as insulin-like growth factor (IGF-1), involved in the regulation of many cellular functions; proliferation, differentiation and apoptosis. The mitogenic effect of IGF-1 on mammary epithelial cells is well documented. It can stimulate the proliferation and inhibit apoptosis of mammary epithelial cells [62]. In particular, higher circulating levels of IGF-1 are associated with increased breast cancer risk among premenopausal women [73]. Another mechanism by
7 which physical activity may reduce breast cancer risk is through the reduction of accumulation of genetic lesions in the cells. Physical activity may decrease reactive oxygen species accumulation inside the cell reducing thus the oxidative stress-induced DNA damage. It is also suggested that physical activity plays a role in the enhancement and activation of the anti-tumorigenic immune system function. It is suggested that physical activity may have an anti-inflammatory effect[74].
1.3.3 Physical activity and inflammatory markers
One of the proposed mechanisms by which physical activity decreases breast cancer risk is through its anti–inflammatory effect [65, 75]. Therefore, it is conceivable that physical activity exerts its anti–inflammatory effect through altering the expression of various inflammatory makers. Some of these inflammatory markers are suspected to play a role in breast cancer etiology.
Several longitudinal and cross-sectional studies investigated the effect of physical activity on circulating levels of various inflammatory markers, whether pro- and anti-inflammatory markers. Knowing that the greatest impact on some anti-inflammatory markers would be achieved by long-term physical activity [65, 76] and to avoid the consideration of acute effect of exercised physical activity, we confined our review on longitudinal studies [77-87] and randomized control trials [88-108] that assessed the effect of long-term exercise training; 12 weeks [79, 82, 83, 85, 97, 98, 105, 106], 13 weeks up to 6 months [77, 80, 84, 86-89, 91, 93, 96, 100, 102, 107, 108] and 7 months up to one year [78, 81, 90, 92, 94, 95, 99, 101, 103, 104] on the circulating levels of pro- and anti-inflammatory markers suspected to have a role in breast cancer development (description of included studies with their observed results is provided in Table 1 and 2). Several studies, but not all, observed that long-term exercise training reduced circulating levels of IL-6 [84, 96, 99, 100, 104, 108], TNF-α [81, 85, 86, 105, 106, 108], CRP [81, 82, 87, 88, 92, 93, 95, 96, 99, 101, 104, 105], leptin [77, 78, 80, 81, 83, 84, 94, 96, 105, 106], IL-8 [106] and SAA1 [82]. Moreover, circulating levels of IL-6 [109-111], CRP [96, 109, 110] and leptin [96, 111-113] were significantly reduced after long-term exercise training combined with hypocaloric diet among premenopausal [109, 111, 112] and postmenopausal women [96, 110, 113]. Conversely, serum IL-10 mRNA was increased among obese postmenopausal women who
8
underwent resistance exercise training for 12 weeks duration [98]. To our knowledge, the association between physical activity and the protein or gene expression levels of IL-6, TNF-α, CRP, COX-2, leptin, IL-8, STAT3, SAA1, TGF-β, IL-10 and lactoferrin in normal breast tissue has not been examined yet. However, it is important to mention that it is previously shown that circulating levels of two inflammatory markers (leptin and TGF-β) did not reflect their protein expression in the breast tissue [114, 115]. Therefore, it is not known whether physical activity can have the same effect on the protein expression levels of inflammatory markers in the breast tissue. In other words, it is not known if the physical activity anti-inflammatory effect observed in the serum extends to the breast tissue.
9 Table 1. Association between physical activity and circulating levels of inflammatory markers among longitudinal studies
Study Study participants Intervention Duration of
intervention Inflammatory marker measured Detected change in circulating levels of inflammatory marker Baylor et al., [77] 21 premenopausal women aged 20.0±0.8 years old; exercise (n = 17) and control (n = 4) groups
Intense exercise (rowing, running, weight lifting) daily
20 weeks Leptin Decrease in circulating levels
of leptin (P < 0.05)
Botero et al., [78]
23 postmenopausal women aged 63.0±4.4 years old
Periodized
resistance training twice /week
1 year Leptin Decrease in circulating levels
of leptin (P < 0.05) Devries et al.,
[79]
24 women aged 20-50 years old; obese (n = 12) and lean (n = 12) Endurance training (biking) for 15-60 min three times/week 12 weeks IL-6 CRP Leptin No significant changes in circulating levels of IL-6, CRP or leptin
Di Blasio et al.,[80]
34 postmenopausal women aged 55.9±3.6 years old
Exercise (walking at moderate intensity) 40-50 min four times/week
13 weeks Leptin Decrease in circulating levels
of leptin (P < 0.001)
Kondo et al.,
[81] 16 obese women aged 18-23 years old; exercise intervention (n= 8) or
control (n = 8) group
Exercise training (fast slope walking, slope jogging, dumbbells, stretching, leg cycling and jumping) 30-60 min five times/week 7 months TNF-α CRP Leptin
Decrease in circulating levels of TNF-α (P < 0.01), CRP (P < 0.05) and leptin (P < 0.05)
Ogawa et al., [82]
21 elderly women aged 85.0±4.5 years old
Low intensity resistance exercise training for 40 min
12 weeks IL-6 TNF-α CRP - Decrease in circulating levels of CRP (P < 0.05) and SAA1 (P < 0.05)
10
SAA1 - No significant change in
circulating levels of IL-6 and TNF-α
Polak et al.,
[83] 25 obese premenopausal women aged 40.4±6.7 years old
Aerobic exercise for 45 min twice a week for five days/week 12 weeks IL-6 TNF-α Leptin - Decrease in circulating levels of leptin (P < 0.001) - Non-significant decrease in circulating levels of IL-6 and TNF-α (P = 0.08)
Prestes et al., [84]
35 postmenopausal women aged 63.2±4.8 years old
Periodized resistance exercise for 50 min 16 weeks IL-6 TNF-α Leptin - Decrease in circulating levels of IL-6 (P = 0.01) and leptin (P = 0.04)
- No significant change in circulating levels of TNF-α Simonavice et
al.,[87] 12 postmenopausal breast cancer survivors aged 64±5 years old
Resistance training
twice/week 6 months CRP Non-significant decrease in circulating levels of CRP (P = 0.29)
Traczkowski
et al., [85] 24 obese pre- and perimenopausal women aged 30-50 years old divided into either exercise intervention with normal glucose tolerance (n = 8), exercise intervention with impaired glucose tolerance (n = 8) or control (n = 8) group
Exercise training (bicycle ergometer) for 30 min five times/week
12 weeks TNF-α Decrease in circulating levels
of TNF-α among the two intervention groups (P < 0.05)
Tsukui et al.,
[86] 27 women aged 41-69 years old Moderate intensity exercise (brisk walking and/or swimming) for two months followed by
5 months TNF-α Decrease in circulating levels
11 home-based
program for 30-45 min 4-5 times/week
12
Table 2. Association between physical activity and circulating levels of inflammatory markers among randomized control trials
Study Study participants Intervention Duration of
intervention Inflammatory marker measured Detected change in circulating levels of inflammatory marker Arikawa et al., [88] 319 premenopausal women aged 18-30 years old randomized into either
exercise intervention (n =166) or no exercise group (n =153)
Aerobic exercise training for 45 min for five times/week
16 weeks CRP Leptin SAA1 - Decrease in circulating levels of CRP (P = 0.040) - No significant change in circulating levels of leptin (P = 0.804) or SAA1 (P = 0.759)
Arsenault et al., [89]
349 obese postmenopausal women aged 45-75 years old randomized into either
exercise intervention (n = 267) or control group (n = 82)
Exercise training for 3-4 times/week
6 months IL-6
TNF-α CRP
No significant change in circulating levels of any of the examined markers
Bergstrom et al., [90]
92 overweight
postmenopausal women with osteoporosis aged 45-65 years old, randomized into either exercise intervention (n = 48) or control group (n = 44)
Brisk walk three times/week and aerobic exercise 1-2 times/week
1 year CRP No significant change in
circulating levels of CRP (P = 0.36)
Bijeh et al., [91] 19 women randomized into either exercise intervention (aged 41.3±3.7, n = 11) or control group (aged 43.2±2.9, n = 8)
Aerobic exercise for 60 min three times/week 6 months CRP Leptin No significant change in circulating levels of CRP (P = 0.25) or leptin (P = 0.51) Campbell et al., [92] 115 overweight/obese
postmenopausal women aged 50-75 years old, randomized into either exercise
Aerobic moderate-vigorous intensity exercise 1 year IL-6 CRP SAA1 - Decrease in circulating levels of CRP among all (P = 0.01) and obese (BMI ≥ 30 kg/m2) women (P = 0.002)
13 intervention (n = 53) or
control group (n = 62)
- Decrease in circulating levels of SAA1 among obese women (P = 0.08)
- No significant change in circulating levels of IL-6 (P = 0.45)
Fairey et al.,[93] 53 postmenopausal breast cancer survivors aged 50-69 years old, randomized into either exercise intervention (n = 25) or control group (n = 28)
Exercise (cycle ergometer) for 15-35 min three times/week
15 weeks CRP Decrease in circulating levels
of CRP (P = 0.066)
Freidenreich et al.,[94]
320 postmenopausal women aged 50-74 years old, randomized into either
exercise intervention (n = 160) or control group (n = 160)
Aerobic exercise for 45 min five
times/week
1 year Leptin Decrease in circulating levels
of leptin (P < 0.001)
Friedenreich et
al., [95] 310 postmenopausal women aged 50-74 years old, randomized into either
exercise intervention (n = 154) or control group (n = 156)
Moderate to vigorous aerobic exercise for 45 min five times/week 1 year IL-6 TNF-α CRP - Decrease in circulating levels of CRP (P = 0.005) - No significant changes in circulating levels of IL-6 (P = 0.785) or TNF-α (P = 0.912) Giannopoulou et
al., [96]
33 Postmenopausal women aged 30-70 years old, randomized into either diet alone (n = 11), exercise alone (n = 11) or diet and exercise group (n = 11)
Walk for 60 min 3-4 times/week 14 weeks IL-6 TNF-α CRP Leptin -Decrease in circulating levels of IL-6 (P = 0.07), CRP (P < 0.01 ) and leptin (P = 0.06) in exercise alone group
-No significant change in circulating levels of TNF-α in exercise alone group
Hammett et al.,
[97] 88 women aged 18-65 years old, randomized into exercise intervention (n = 48) or health
Exercise (treadmill, cycle or rowing ergometer) for 45
12 weeks CRP No significant change in
circulating levels of CRP (P = 0.71)
14
education group (n = 40) min three times/week Henagan et al.,
[98]
23 obese women aged 65.6±2.8 years old, randomized into either
exercise intervention (n = 12) or control group (n = 11) Resistance exercise three times/week 12 weeks TNF-α RNA IL-10 RNA - No significant change in TNF-α RNA - Non-significant increase in IL-10 RNA (P = 0.249) Imayama et al.,
[99] 438 overweight/obese postmenopausal women aged 50-75 years old, randomized into either exercise
intervention (n = 117), caloric restriction diet intervention (n = 118), combined diet and exercise intervention (n = 116) or control group (n = 87) Moderate to vigorous exercise for 225 min/week 1 year IL-6 CRP SAA1 - No significant change in circulating levels of IL-6 (P = 0.485), CRP (P = 0.367) or SAA1 (P = 0.149) in exercise intervention group compared to control group
- Non-significant decrease in circulating levels of IL-6 (P = 0.077) and CRP (P = 0.088) among women with ≥ 5% weight loss
Jones et al., [100]
65 postmenopausal breast cancer survivors aged 34-79 years old, randomized into either exercise intervention (n = 36) or control group (n = 32)
Moderate intensity exercise (brisk walking) for 30 min five times/week
6 months IL-6
TNF-α CRP
- No significant change in circulating levels of IL-6 (P = 0.91), TNF-α (P = 0.54) or CRP (P = 0.73)
- Significant decrease in circulating levels of IL-6 among women who increased exercise by 120 min/week (P < 0.01)
Kemmler et al.,
[101] 65 postmenopausal women aged ≥65 years old, randomized into either
exercise intervention (n = 33) or control group (n = 32)
High intensity aerobic and
resistance exercise for 60 min four times/week
1 year CRP Non-significant decrease in
circulating levels of CRP (P = 0.36)
15 Ligibel et al.,
[102]
100 pre- and postmenopausal women aged 53 years old, randomized into either
exercise intervention (n = 51) or control group (n = 49)
strength exercise for 50 min twice/week
16 weeks Leptin No significant change in
circulating levels of leptin (P = 0.76)
Mediano et al., [103]
54 women aged 25-45 years old, randomized into either exercise intervention (n = 26) or control group (n = 28)
Low to moderate intensity exercise for 40 min ≥three times/week
1 year CRP No significant change in
circulating levels of CRP (P = 0.37)
Olson et al.,
[104] 28 premenopausal women aged 25-44 years old, randomized into either
exercise intervention (n = 16) or control (n = 12)
Resistance training
twice/week 1 year IL-6 CRP - Decrease in circulating levels of CRP (P < 0.01) - Non-significant decrease in circulating levels of IL-6 Phillips et al.,
[105]
23 obese postmenopausal women aged 65.6±2.6 years old, randomized into either exercise intervention (n = 11) or control group (n = 12) Resistance exercise three times/week 12 weeks IL-6 TNF-α CRP Leptin - Decrease in circulating levels of TNF-α (P < 0.05), CRP (P < 0.05) and leptin (P = 0.047)
- Increase in circulating levels of IL-6
Rogers et al., [106]
Pre- and postmenopausal women aged 18-70 years old, randomized into either
exercise intervention (n = 12) or control group (n = 10)
Moderate intensity aerobic (150 min/ week) and resistance exercise (twice/week) 3 months IL-6 TNF-α Leptin IL-8 IL-10
- Decrease circulating levels of leptin (P = 0.031) - Non-significant decrease in circulating levels of TNF-α (P = 0.690), IL-8 (P = 0.758) and IL-10 (P = 0.518) - Non-significant increase in circulating levels of IL-6 (P = 0.121)
Stewart et al.,
[107] 421 overweight/obese postmenopausal women aged 45-75 years old, randomized
Aerobic exercise (semi-recumbent cycle ergometer and
6 months CRP No significant change in
circulating levels of CRP between any of the groups (P
16
into either exercise intervention with 4 kcal/kg/week energy expenditure (n = 143), exercise intervention with 8 kcal/kg/week energy
expenditure (n = 143), exercise intervention with 12 kcal/kg/week energy expenditure (n = 95) or control group (n = 96) treadmill) 3-4 times/week = 0.6) Tartibian et al., [108] 79 postmenopausal women aged 58-78 years old, randomized into either exercise and supplement (n=21), exercise only(n=20), supplement only (n=20) or control group (n=18)
Aerobic exercise (walk and jogging on a treadmill 25-30 min/day for 3-4 days/week for the first 12 weeks than 40-45 min/day for 4-6 days/week
24 weeks IL-6
TNF-α
Decrease in circulating levels of IL-6 (P = 0.001) and TNF-α (P = 0.001) in exercise only group
IL-6, interleukin 6; TNF-α, tumor necrosis factor-α; CRP, C-reactive protein; IL-8, interleukin 8; SAA1, serum amyloid A1; IL-10, interleukin 10; BMI, body mass index.
17
1.4 Intermediate breast cancer risk factors
If it is true that local inflammation increases the risk of developing breast cancer, altered expression of inflammatory markers should be detected on normal breast tissue. Therefore, emerges the need for an intermediate marker of breast cancer. Using intermediate markers as endpoint in studies permit the relatively quick exploration of the effect of several mitogens on breast tissue. This has the potential to eliminate the long waiting period of time elapsing between the exposure to mitogens and the development of breast cancer. Moreover, longitudinal studies are time consuming and expensive. Both mammographic density and age-related lobular involution are independently associated with breast cancer risk [5]. The two can be considered as intermediate markers of breast cancer.
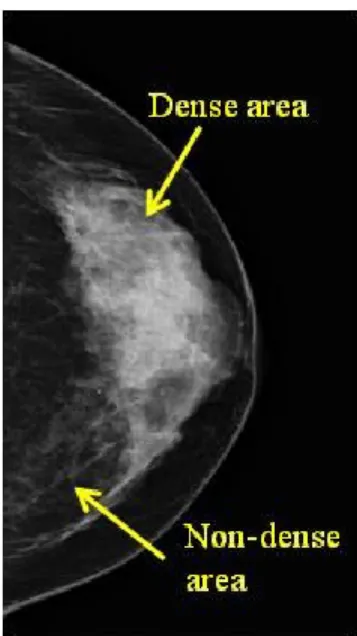
1.4.1 Mammographic density
Mammographic density is now described as the variation in the radiological appearance of the breast depending on the amount of fibroglandular tissue (epithelium and stroma) relative to adipose tissue. In other words, it reflects the proportion of the breast occupied by radiologically dense fibroglandular tissue. Fibroglandular tissue attenuates more X-ray and thus appears white on a mammogram whereas, radiolucent adipose tissue appears black [116] (Figure 1). Greater nuclear area, epithelial and nonepithelial cells, and higher amount of collagen in the breast tissue are positively associated with the mammographic density, while adipose tissue is inversely associated with the mammographic density [116-118]. Collagen was most strongly associated with the mammographic density. It accounts for 29% of the variance in mammographic density [116, 118]. Nuclear area and glandular area explain 4% and 7% of the variance in mammographic density, respectively [118].
18
Figure 2. Representative mammographic image of mammographic density.
Several metrics were proposed to classify variations in the mammographic density.
Wolfe was the first to describe a method for classifying the mammographic density in 1976 [119]. Wolfe visually classified mammographic density into four categories based on the extent of dense breast tissue on the mammogram as well as on the characteristics of observed densities (prominent ducts and dysplasia). Wolfe’s four-parenchymal patterns are: N1, breast mainly fatty with small amount of dysplasia; P1, breast mainly fatty with prominent ducts occupying less than one fourth of the breast; P2, prominent duct pattern occupying more than one fourth of the breast and DY, dysplastic breast. Wolfe’s four categories correlate with breast cancer risk in different populations. Breasts with the densest patterns were associated with the highest breast cancer risk [119]. Tabar’s five patterns (I-V) were based on the anatomic-mammographic correlation with 3-dimentional; subgross (thick slice) technique [120]. Tabar’s five patterns are: I, breast with scalloped contour, some scattered lucent areas of adipose tissue and 1-2 mm of scattered nodularities; II, breast is completely lucent corresponding to adipose tissue replacement; III, breast containing prominent duct pattern in the retroareolar area corresponding to periductal elastosis and involution; IV, nodular and linear densities throughout the breast and V, breast with convex contour with homogenous ground glass like appearance. Later, the Breast Imaging Reporting and Data System (BI-RADS) classification of the American College of Radiology largely replaced previous classifications. The BI-RADS four
19
categories are: 1, almost entirely fatty breast; 2, breast containing scattered densities; 3, heterogeneously dense breast and 4, extremely dense breast [121]. The BI-RADS categories are still widely applied. Thereafter, several methods were developed seeking to provide a quantitative and continuous measure of the mammographic density. In 1995, Boyd proposed a six-category scale based on the proportion of the breast occupied by radiologically dense tissue as visually estimated by a radiologist. Boyd six categories are: 0%, <10%, 10%-<25%, 25%-<50%, 50%-<75% and ≥75% [122]. All these methods depend on the visual assessment and are thus subjected to bias [123].
Later, emerges more objective semi-automated methods based on the interactive thresholding techniques; the planimetry and the computer-assisted method. The planimetry, a two-dimensional method, measures areas of dense tissue present on the mammogram using a planimeter [124]. More recently, mammographic density is quantitatively measured by a computer-assisted method (such as the Cumulus software) on digitized or digital mammographic images. The computer-assisted method is known to be highly reliable and reproducible [116, 122]. In either method, the reader uses the planimeter or the software to trace the outlines of the entire breast and that of dense breast areas to compute total breast area and dense breast area. Herein, results are expressed as absolute dense area in square centimeters or percent mammographic density [116] (illustrated figure of the computer-assisted measurement of mammographic density is presented in Figure 2). The percent mammographic density is the ratio of dense breast area to the total breast area multiplied by 100. However, the main limitation of these methods is that they do not take into account the gradual transition from dense to non-dense breast tissue. Overall, quantitative methods have the advantage over qualitative categorical methods in providing a continuous measure of mammographic density. Moreover, quantitative approaches give a more consistent estimate of the associated breast cancer risk and this risk persists after adjustment for other risk factors.
20
Figure 3. Evaluation of percent mammographic density by a computer-assisted method on digital mammographic image. The red line identifies the total breast area. Green areas represent regions of elevated mammographic density and grey areas represent area of low mammographic density. The percent mammographic density is the ratio of number of pixels in dense breast area to that in the total breast area multiplied by 100.
Mammographic density constitutes one of the major risk factors for breast cancer. Women with the highest mammographic density (≥75% of the breast occupied by dense tissue) have 3-5 fold greater risk of developing breast cancer compared to women with the lowest mammographic density (<5% of the breast occupied by dense tissue) (Figure 3, [125]). This increased risk with higher mammographic density is constant no-matter the applied method for measuring mammographic density [126]. In the most recently published meta-analysis both the percent mammographic density and the absolute dense area were associated with increased breast cancer risk [127]. For one standard deviation of increase in the percent mammographic density or the absolute dense area, OR=1.52, 95% CI=1.39-1.66 and OR=1.37, 95% CI=1.29-1.47, respectively, among premenopausal women; and OR=1.53, 95% CI=1.44-1.64 and OR =1.38, 95% CI=1.31-1.44, respectively, among postmenopausal women after adjustment for age, parity and body mass index (BMI).
21
Figure 4. Relative risk (RR) of breast cancer by percentage of the breast showing mammographic densities. Each ● represents a RR for women in 18 categories of percent density (1–9, 10–14, . . ., 80–84, 85–89, and 90–100%) compared with women with no density. RRs were obtained by logistic regression adjusting for age and body weight. The dotted curve is a weighted cubic smoothing spline function that models the adjusted RRs. The vertical lines represent the 95% CIs around each RR predicted by the spline function [125].
In 2005, Tice and colleagues’ prospective study provided evidence of the possibility of using mammographic density as a predictor of developing breast cancer. Tice and colleagues observed that mammographic density-only model, adjusted for age and ethnicity, had the same predictive accuracy as the Gail model (concordance index 0.67, 95% CI = 0.65-0.68)[128]. Gail model is the first predictive model for the estimation of the probability of developing invasive or in situ breast cancer in a healthy woman proposed in 1989. It includes multiple risk factors such as age, age at menarche, age at first live birth, first-degree family history of breast cancer and the number of breast biopsy [129]. In Tice and colleagues study, 81,777 women of broad ethnic and age distributions were followed for about 5.1 years. Among these, 955 developed invasive breast cancer. Authors concluded that the mammographic density may be used to provide an easily obtainable predictor of breast cancer risk. Nevertheless, adding the mammographic density to the Gail model improved its predictive power for short term breast cancer risk. However, this
22
improvement was minimal (change of concordance index from 0.67, 95% CI = 0.65-0.68 to 0.68, 95% CI = 0.66-0.70, P <0.01) [128].
A review of mammographic density as an intermediate biomarker and its use to study molecular causes of breast cancer is provided in Chapter 2.
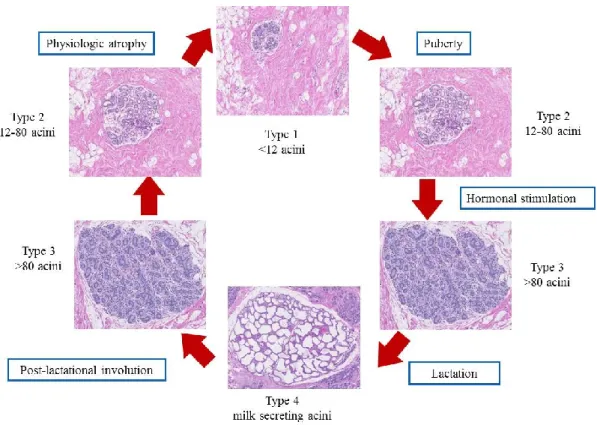
1.4.2 Age-related lobular involution
The mammary gland undergoes a unique cycle of maturation-involution throughtout a woman’s life [130]. Before menarche, the breast is mainly formed of small undifferentiated type 1 lobules that contain <12 acini. During the stage of lobular maturation, type 1 lobules evolve into type 2 lobules that contain 12-80 acini. Under hormonal stimulation or pregnancy, type 2 lobules develop into the most differentiated type 3 lobules (>80 acini). Type 4 lobules, composed of milk secreting acini, are only present during lactation. The age-related lobular involution represents the physiological breast atrophy. It is believed to begin by the age of 40 [131]. Illustrated figure of the different stages of development of mammary gland is provided in Figure 4.
23
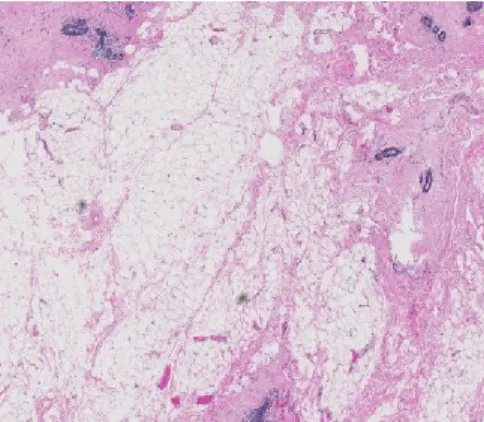
The age-related lobular involution is characterized by a progressive reduction in the size and number of acini per lobule contained in the terminal ductal lobular units (TDLUs), the functional unit of the breast (Figure 5). This is accompanied by a reduction in the epithelium within the TDLUs and the replacement of the intralobular stroma by dense collagen and eventually adipose tissue [131]. During this progressive process, both type 3 lobules (fully differentiated) and type 2 lobules (moderately differentiated) regress back to the least differentiated type 1 lobules [130]. The age-related lobular involution is shown to be a homogenous process throughout each breast and across both breasts [132]. It is expressed either as the degree of lobular involution or the predominant lobule type. The degree of lobular involution is categorized according to the proportion of involuted TDLUs present on a selected representative hematoxylin and eosin stained slide containing normal breast tissue: no (0%); partial (1-74%); or complete involution (≥75%) [133]. Involuted TDLUs are defined as those having few to several small acini and flattened inconspicuous acinar epithelium with fibrosed intralobular stroma [133]. The predominant lobule type (either lobule type 1, 2 or 3) must constitute ≥60% of the total number of lobules contained on the examined slide [134].
Figure 6. Hematoxylin-eosin staining of age-related lobular involution of the breast (magnification 5x). Involuted breast characterized by decreased fibroglandular tissue and increased adipose tissue.
The age-related lobular involution may be an important determinant of breast cancer risk since it is accompanied by a reduction in the epithelium where breast cancer develops [12, 133]. Accordingly, it is not surprising to know that the age-related lobular involution is inversely associated with breast cancer risk (RR = 1.88, 95% CI = 1.59-2.2; RR = 1.47,
24
95% CI = 1.33-1.61 and RR = 0.91, 95% CI = 0.75-1.10 for no, partial and complete involution, respectively; test for heterogeneity P <0.001) [133]. Similarly, women having greater proportion of type 1 lobules and no type 3 lobules, an indicator of complete involution, have reduced breast cancer risk compared to those with no type 1 lobules (OR = 0.63, 95% CI = 0.44-0.91) [135].
It is important to mention that the age-related lobular involution is inversely associated with the mammographic density among women having benign breast diseases after adjustment for BMI and other confounding factors (Ptrend <0.02) [135]. Interestingly,
combined together, increased mammographic density and no involution are associated with even higher breast cancer risk. Women having extremely dense breast (Wolfe P2, DY) and no involution had a higher breast cancer risk compared with those having non-dense breast (Wolfe N1, P1) and complete involution after adjustment for BMI and other confounding factors (HR = 4.08, 95% CI = 1.72-9.68) [5].