The 3-D
structure
of
a
zinc
metallo-1-lactamase
from
Bacillus
cereus
reveals
a new
type
of protein fold
A.Carfi,
S.Pares, E.Duee, M.Galleni',
C.Duez1, J.M.Frere1
and
O.Dideberg2
Institut deBiologie Structurale Jean-Pierre Ebel(CEA-CNRS), Laboratoire deCristallographieMacromoleculaire,41 Avenuedes Martyrs,F-38027 Grenoble Cedex 1, France and 'Universit6de Liege, Institut deChimie (B6),Centre d'IngenieriedesProteines,
B-4000Sart-Tilman, Liege 1, Belgium 2Correspondingauthor
The 3-D structure of Bacillus cereus (569/H/9)
1-lactamase
(EC
3.5.2.6),
whichcatalyses
thehydrolysis
of nearly all
1-lactams,
has been solved at 2.5 Aresolution by the multiple isomorphous replacement method, withdensity modificationandphase combina-tion, from crystals of the native protein and of a specially designedmutant
(T97C).
The currentmodelincludes 212 ofthe 227 amino acid residues, the zinc ion and 10water molecules. The
protein
isfolded into a 11 sandwich with helices on each external face. To our knowledge, this fold hasnever beenobserved. Anapproximate internal molecular symmetry is
found,
witha2-fold axispassingroughly through the zinc ion and suggestinga possiblegene
duplication.
Theactive site is located at one edge of the13
sandwich and near the N-terminal end of a helix. The zinc ion is coordinated by three histidine residues (86, 88 and149) anda watermolecule. A sequencecomparison of the relevant
metallo-13-lactamases,
basedonthisprotein
structure, highlights a few well-conserved amino acid residues. The structure shows that most of these residues are in the active site. Among these,aspartic
acid 90 and histidine 210
participate
in aproposed
catalytic mechanism for
P-lactam
hydrolysis.Keywords: antibiotic/,B-lactamase/metalloenzyme/penicillin resistance/X-ray structure
Introduction
Only a few years ago,
metallo-1-lactamases
werecon-sidered as mere biochemical curiosities, compared with their widespread active-site serine counterparts which
are responsible for the resistance of a large number of pathogenic bacterial strains to penicillins and related antibiotics. Atthattime,only innocuous strains of Bacillus
cereus wereknown toproduce two verysimilar monomeric
metallo-1-lactamases.
Thediscovery of a thirdmetallo-13-lactamase (Saino et al., 1982; Bicknell et al., 1985) produced byXanthomonas maltophilia, composed of four subunits but devoid of allosteric properties, did not seem
to raise major concerns in the medical community.
However, the more recent finding that the resistance to
carbapenemsofanincreasingnumber ofclinicallynoxious
strains is caused by the synthesis of
Zn2+-containing
,B-lactamases is a cause of concern (Payne, 1993).Twomajor factors areresponsible for the re-evaluation of the clinical importance of these enzymes. Firstly,they
generally hydrolyse carbapenems (e.g. imipenem) with a high efficiency, while these compounds generally escape the activity of the active-site serine enzymes. Secondly,
an identical or similar gene has been found in various strains of Serratia, Pseudomonas and Enterobacter (Yang etal., 1990). Because this gene isprobably plasmid-borne, and on the basis ofprevious experience, its spread to an
ever increasing population of pathogenic species is now considered as a distinct threat to our ability to fight a
variety of infectious bacteria.
Allthepresently sequencedZn>2+ 3-lactamases (includ-ing theX.maltophiliamonomer) exhibit sequence similari-ties(seebelow), and it is likely thattheyare homologous and share a common catalytic mechanism. They appear to form a unique family, distinct from the other metallo-peptidases [e.g. thermolysin and carboxypeptidase A (CPA) families].
Enzymesproducedbytwo strains ofB.cereus, 569/H/9 and 5/B/6, often referred to as
P-lactamase
II (BCII),have already been studied extensively. The zinc BCII is considered as 'broad spectrum' because it hydrolyses a
large number ofpenicillins and cephalosporins (Crompton
andWaley, 1986; Felici etal., 1993), and three histidine residues have been identifiedastheZn2+ ligands (Baldwin etal., 1978, 1979; Galdes etal., 1980) with the possible participation ofa cysteineresidue (Davies and Abraham,
1974).
Preliminarycrystallographicstudiesofthe
Cd2+
deriva-tive(Suttonetal., 1987)haveconfirmedthesehypotheses,
but the rather low resolution (3.5 A) was not sufficient for proposing a complete chain tracing of the protein structure. Kinetic studies, sometimes performed at sub-zero temperatures and mainly with the Co2+ derivative, suggested the presence of various intermediates on the reaction pathway, none of them covalent (Bicknelletal.,
1986) but involving changes in the coordination sphere of the metalduring catalysis (Waley, 1992).
Here, we describe the crystal structure of the B.cereus
569/H/9enzymeat2.5A resolution and proposeapossible
catalytic mechanism.
Results and
discussion
Crystallization
Despite the recent progress in protein crystallography,
some protein structures remain difficult to solve. Zn 1-lactamases seem to belong to this group. For BCII,
problems arise mainly from the lack of isomorphism of the heavy-atom derivatives to the native protein (Sutton
DNAtechnology was used. A mutantprotein(T97C) was designed based on structural information from the
low-resolutionX-rayanalysis(Sutton etal., 1987).Inprinciple,
one or two sites for Hg binding are potentially accessible in the mutant protein depending on the size of the heavy-atom compound(the zinc site and regions near the newly introduced sulfydryl group).
Both proteins, the native and the T97C mutant, were crystallized in the same crystal form, close to that already
published (Sutton etal., 1987).
Quality of the model
The structure was solved by the multiple isomorphous replacement method, with density modification and phase combination, using X-ray data collected from the native
protein and the T97C mutant derivative crystals.
Refine-ment statistics are summarized in Table I. The current model consists of 212 of the 227 residues, one
Zn2+
ion and 10 water molecules. The model has been refined to an R-factorof22% using 6683 reflections [IFI > 2a(F)]in the resolution range 8.0-2.5
A.
Two disordered chainTable I. Refinement data
Number ofreflections R-factora
IF1>2I (%)
Resolution range8.0-2.5A 6683 22.0
Proteinatoms 1646
Water molecules 10
R.m.s.deviation from ideality
Bondlengths(A) 0.016
Bond angles(0) 1.47
aR-factor= £o--F 0'/7lFOl,whereFoandF,areobserved and
calculated structurefactor amplitude, respectively.
segments couldnotbemodelled: six residues (Serl-Lys6)
atthe N-terminus andeightresidues(Gly32-Val39) located between two n-strands.
All main-chain dihedral angles fall within the allowed
regions of a Ramachandran plot (Ramachandran et al., 1963), except those for Thrl3 and Asp56 which were
modelledwith unfavourable dihedralangles of
0
= 63.9°, v= 140.8' and0
= 72.8°,xv = 139.7°, respectively. The first of these residues (Thrl3) is located ina loop region weakly defined in the electron density map; the second (Asp56) is buried in the protein, making a salt bridge with Arg9l in the groove of theenzymatic cavity.Overallstructureof theZnmetallo-fi-lactamase
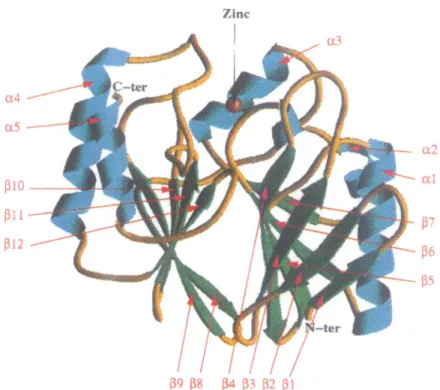
A ribbon view of the 3-D structure of BCII is shown in Figure 1. BCII is a compact
cx3p3x
protein ofapproximatedimensions 32X30X28 A. The polypeptide chain is divided into twodomains. These domainscomprise strands andhelices in thefollowingorder:
PIP2P3P4J35a5I6a2I37a3
andP P8Ji9OP1i11a4t122a5
for the N- andC-terminaldomains, respectively (Figure 2). a2 has a kink caused by glycineresidues 93 and 94. The core of the molecule is formed
by twotightly packed f-sheets and is surrounded by five helices. Both ,-sheets have the usual twist commonly observed in many proteins. Large loop regions connect
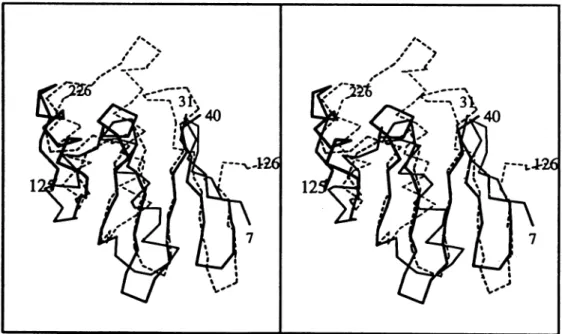
somesecondary structure elements. The two ,B-sheetscan
be superimposed,
(IP2
to 6) correspondingto (i38to0312),
by a 2-fold rotation. The result of the superposition is shown inFigure 3. The best fit of the two parts involves a rotation of exactly 1800 without translation. For 58 equivalent Ca positions, we observed an r.m.s. deviation of 1.29 A.Figure 3 shows manyinsertionsand substantial deviations in helix positions. However, each domain contains the same set oftopologically equivalentsecondary structureelements,namely a,8,jc4kzxmotif. No signific-antsequencehomologywasfound betweenthe two motifs./ijic
Fig.1. RibbondiagramofthemolecularstructureofB.cereus1-lactamaseviewedroughlyalongthetwon-sheets.Theconcavesurfaces of thetwo
The BCII structure has a 1343 sandwich fold which is reminiscent of DNase I (Suck et al., 1984; Oefner and
Suck, 1986),the N-domainofglutamine 5-phosphoribosyl-1-pyrophosphate aminotransferase (Smith et al., 1994), the multifunctional DNA repair enzyme exonuclease III (Mol et al., 1995) and the proteasome subunits (Lowe
etal., 1995). However, the secondary structure elements
formingthe 13-sheet arein adifferent order inBCII. The observed a1313a fold has no similarity with any knownmetalloprotein structure, including a
D-alanine-D-alanine carboxypeptidase (Dideberg et al., 1982) which
cleaves the peptide bond between twoD-alanine residues
(the D-ala-D-alamoiety and penicillin are often considered isosteric).
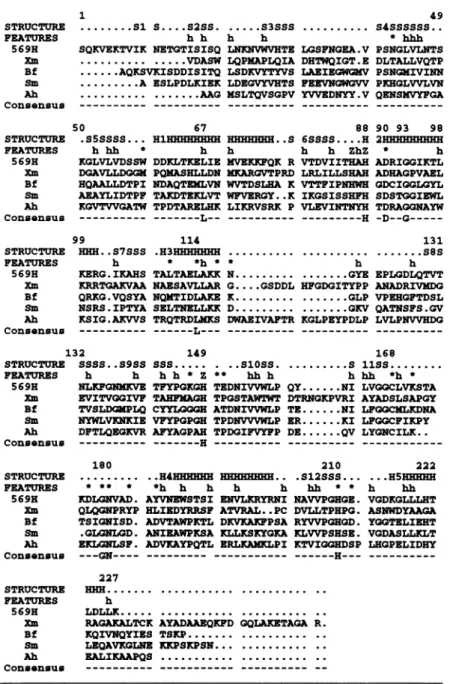
Comparison with otherZn1-lactamases
All sequences were searched for in the EMBL Data Bank
using FASTA (Lipman and Pearson, 1985) and aligned
,,otAAW 5 Argl99 U5 3
Leu225
51 4 10 1 9 8
Lys21 Vail6
His2IO 0i167 1114Tr44T2h129bl
Fig.2. BCIIpolypeptide topology of thetwo f-sheets.Then-strands
aredepictedasarrows,with thearrowheadsindicatingthedirection of the chain.ax-Helicesareshownasrectangles.
using the hydrophobic cluster analysis program (Gaboriaud etal., 1987). Sixsequences wereconsidered first: B.cereus
(569/H/9 and 5/B/6), Xmaltophilia, Bacteroides fragilis, Serratia marcescens and Aeromonas hydrophila. Because the two B.cereussequences differby only 24 amino acid substitutions, only 569/H/9 was used for further analysis.
When B.cereus (569/H/9) was compared with the other four sequences, sequence similarities ranged from 41 (B.fragilis) to 21% (X.maltophilia). While the other enzymes are monomeric, the Xmaltophilia enzyme is a tetramerin the native state.
For alignment, the lengths of all secondary structure elements of the BCII structure were retained for the four other sequences. As seen inFigure 4, onlyafewdeletions or insertions are required to align all the sequences. As expected, the main differences appear forX.maltophilia,
with two large insertions between secondary structures:
a3 and 18 (16 amino acids), and
1l3
andPI,
(six aminoacids). Finally, only nine amino acid residuesare strictly
conserved in the five sequences: Leu67, His88, Asp9O, Gly93, Leu114, His149, Gly179, Asnl80 and His210. Structure of the active site
The active site is located at the bottom of a groove running between the two
13-sheets
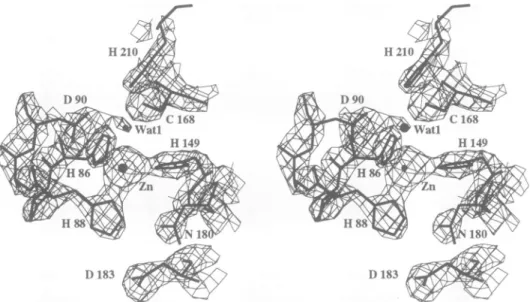
(Figure 1). The zinc ion is bound (Zn2+--..N or 6, -2.2 A) to three protein ligands (His86,His88 and His 149). In theligandsphere, adensitywasobservedin a
(IF01
-IFcI)
mapwhich,asfor all known zinc metalloproteases, could be attributed to a water molecule (Watl)orahydroxyl ion. The fourZn2+
ligands are arranged in a distorted tetrahedral shape. The region ofthe(21F01
-IFcI)
electron density map around the zincion is shown in Figure 5. Seven out of the nine strictly
conserved residues are located in the active site: His88, Asp9O, Leull4, Hisl49, Glyl79, Asnl80 and His210. Clearly, a hydrogen-bond networkinvolvingZn2+, Watl,
Asp9O and Cys168 is important for enzymatic activity.
Fig.3. Stereoview of the superposition oftheCaatoms of the two domains of the B.cereusI-lactamase.The N-terminal domain (7-105) and the
C-terminaldomain(126-226)arerepresented by continuousand dashed lines betweenCaatoms, respectively.
Comparison of relevant sequences of metallo-i-lactamases with the secondary structure of B.cereus (569/H/9)
STRUCTURE FEATURES 569H Xm Bf Sm Ah Consensus STRUCTURE FEATURES 569H Xm Bf Sm Ah Consensus STRUCTURE FEATURES 569H Xm Bf Sm Ah Consensus .... Sis....sass. .. s3sss. h h h h
SQKVEKTVIK NETGTISISQ LNRNVWVHTE LGSFNGEA.V
... ...VDASW LQPNAPLQIA DHTWQIGT.E
...AQKSVKISDDISITQ LSDKVYTYVS LAEIEGWGKV
...A ESLPDLXIEK LDEGV!VHTS FEEVNGWGVV
... ...AAG NSLTQVSGPV YVVEDNYY.V
50 67 88
.S5SSSS... HlHHHHHHHH HHHHHHH..S 6SSSS....H h hh * h h h h ZhZ
KGLVLVDSSW DDKLTKELIE MVEKIFQK R VTDVIITHAH
DGAVLLDGGM PQKASHLLDN KKARGVTPRD LRLILLSHAH
HQAALLDTPI NDAQTENLVN WVTDSLHA K VTTFIPNHWH
AEAYLIDTPF TAKDTEKLVT WFVERGY..K IKGSISSHFH KGVTVVGATW TPDTARELHK LIKRVSRK P VLEVINTNYH
__________ ---L-- --- ---H
99 114
HHHN. S7SSS .H3HHHHHHH...
h * *h * * h
KERG.IKAHS TALTAELAKK N... ...GYE
KRRTGAKVAA NAESAVLLAR G....GSDDL HFGDGITYPP
QRKG.VQSYA NQMTIDLAKE K... ...GLP
NSRS.IPTYA SELTNELLKK D... ...GKV
KSIG.AKVVS TRQTRDLMKS DWAEIVAFTR KGLPEYPDLP
- _________ ---L--- ---49 S4SSSSSS.. * hhh PSNGLVLNTS DLTALLVQTP PSNG4IVINN PKHGLVVLVN QENSIVYFGA 90 93 98 2HHEHEHHHH * h ADRIGGIKTL ADHAGPVAEL GDCIGGLGYL SDSTGGIEWL TDRAGGNAYW -D--G ---131 ...8S h EPLGDLQTVT ANADRIVMDG VPEHGFTDSL QATNSFS.GV LVLPNVVHDG 132 149 168
STRUCTURE SSSS ..S9SS SSS... . . SlOSS... S liSS.
FEATURES h h h h *Z ** hh h hhh *h * 569H NLKFGNMKVE TFYPGKGH TEDNIVVWLP QY...NI LVGGCLVKSTA
Xm EVITVGGIVF TANFEAGE TPGSTAWTWT DTRNGKPVRI AYADSLSAPGY
Bf TVSLDM4[PLQ CYIQGGEH ATDNIVVWLP TE...NI LFGGCNLRDNA
Sm NYWLVKNKIE VFYPGPGH TPDNVVVWLP ER...KI LFGGCFIKPY Ah DFTLQEQKVR AFYAGPAH TPDGIFVYFP DE...QV LYGNCILK..
Consensus --- ---H --- --- ---STRUCTURE FEATURES 569E Xm Bf Sm Ah Consensus STRUCTURE FEATURES 569H Xm Bf Sm Ah Consensus 180 * ** * KDLGNVAD. QLQGNPRYP TSIGNISD. .GLGNLGD. EKLNLSF. -- -GN----210 222 ..H4EHHHEH . . .S12SSS... ...HSHHHH *h h h h h hh * * h hh
AYVNEISTSI ENVLIRYRNI NAVVPGHGE. VGDKGLLLHT HLIEDYRRSF ATVRAL..PC DVLLTPHPG. ASNWDYAAGA
ADVTAWPKITL DKVKAKFPSA RYVVPGEGD. YGGTELIEHT
ANIEAWPKSA KLLKSKYOKA KLVVPSESE. VGDASLLKLT ADVKAYPQTL ERLKANKLPI KTVIQQEDSP LEGPELIDHY
- ___ -_ -- ---H--- ---227 Hm ... ... .. h LDLLK... ... ..
RAGAKALTCK AYADAAEQKFD GQLAKETAGA R.
KQIVNQYIES TSKP... ..
LEQAVKGLNE XKPSKPSN... ..
EALIKAAPQS ... ... ..
__________ --- ---
--Fig.4.Comparison of relevantsequencesofmetallo-13-lactamaseswiththesecondary structuresofB.cereus(569/H/9).Residuesinthe activesite, zinc ionbinding residues and conservedhydrophobic residuesaremarked withan '*',a'Z' andan'h',respectively.Structure codes: S, J-strand;
H,a-helix. Sequencesare:569H, B.cereus (569/H/9) (Ambleretal., 1985;Hussainetal., 1985;Kato etal., 1985); Xm, X.maltophilia (Walshetal., 1994); Bf,B.fragilis(Rasmussenetal., 1990; Thompson and Malamy, 1990); Sm,Smarcescens(Osanoetal., 1994); Ah, A.hydrophila(Massida etal., 1991).
NMR studies have suggested Cysl68, which is replaced by a serine in X.maltophilia, as a potential fourth Zn2+ ligand (Galdes etal., 1980), but the
Zn2+-S
distance is 4.4A,
too long for metal ion ligation. However,Cys168
interacts with Watl, which is held in a correct position by three strong interactions: Zn2+--Watl (3.0 A), Cysl68SG-..Watl (2.9 A) andAsp9OOD2---Watl (2.6
A).
Leul 14 makes vander WaalscontactswithAla87,located
near twozincligands(His86andHis88).Finally, Gly
179,
Asn180
and His210 are too far from the active site toparticipate directly inthe
enzymatic mechanism,
butthey
may be responsible for
specific
interactions with the3-lactam substrate (see below).
Mechanismof catalysis
The catalytic mechanism of zinc
peptidases
has been understood forsometime.However,theexactrole attribu-ted to theZn2+
ion has been alteredslightly
after the determination of the high-resolution structures of nativeproteins or tightly bound complexes. Today, after the pioneering work of Matthews (1988) on
thermolysin
and ofLipscomb(1983)onCPA,twosuperfamilies
have been studied extensively: thethermolysin-metzincin
family
(Stocker etal.,
1993,
1995; Hooper,
1994;
references to the original workcan befoundinthese threepapers)
and the carboxypeptidasefamily
(Hooper,1994). However,
1) 183 1)183
Fig. 5. View of the final (2F' -Fc)electrondensitymap aroundtheZn2+ ion, computedwithcalculatedphasesandcontouredat2c above the
mean.
diverse chemical reactions are also mediated by zinc catalysis (e.g. carbonic anhydrase, various
dehydro-genases, adenosine deaminase).
In zinc peptidases, the metal ion has a dual role in catalysis. Firstly, the
Zn2+-bound
water molecule is activated to performa nucleophilic attack on the peptide carbonyl. Secondly, theZn2+
binds and polarizes thiscarbonyl group. A negatively charged group, Glu143 in thermolysin and Glu270 in CPA, participates in the
activation ofthe watermolecule. After the water
attack,
atetrahedral intermediateisformed, followed bythe back
delivery of a proton to the peptide nitrogen and the
cleavage of the peptidebond.
Similar partners are present in BCII. A schematic
diagramof the interactionwithasubstrate
(Figure
7) wasobtained by docking a cephalosporin molecule in the active sitesothat eachpartnerwasinanadequateposition
to act as mentioned above: Zn2+>.O9 (3.1 A), Watl-..C8 (2.9
A).
Onlyone orientation of thecephalosporincan fit into the groove without colliding with protein atoms. Figures 6 and 7 suggest a reaction pathwayin which the zinc ion activates Watl for nucleophilic attack with theparticipation of
Asp9O,
actingas ageneralbasetoremove aprotonfrom thewatermolecule. Both sidechains,Asp9Oand
Cys168,
maintain the water molecule in a positionwhich allows the nucleophilic attack. A water proton is back delivered to N5, and the C8-N5 bond is cleaved
by a concerted mechanism. The simulated binding of
cephalosporin (Figure 7) revealed two other interesting
potential interactions: (i) the
P-lactam
carboxylate group makes an electrostatic interaction with His210, and (ii) the1-lactam
side chain isneartheloop containingGly179and Asn180. These three aminoacids are strictly conserved inallZn,B-lactamasesequences.Insupport of theproposed
mechanism,the substitution ofresidues
Asp9O
(Lim et al.,1991),
Cysl68
and His210(Clark, 1993) by site-directed mutagenesis resulted in severely impaired enzymes.Apreliminarycomparison of the B.cereus Zn
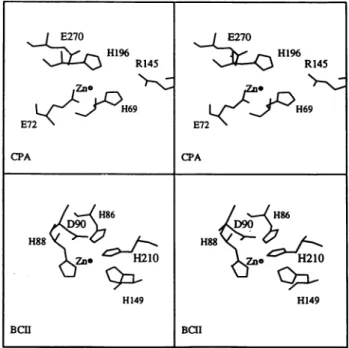
,3-lactam-ase with CPA was made by superposing the active sites
ofCPA andBCII (Figure 8). Both enzymes interact with
Fig. 6. View oftheactive site. TheZn2+ ion andthe watermolecule (Watl)arerepresentedbyanorangeandawhitesphere, respectively.
asubstrate bearinga carboxylate group. Because the two structureshavedifferentfolds,thesuperposition was made intwosteps: the two zincions were overlaid and then the three zinc ligands were superimposed. Among the three
possible superpositions, that which brings the Glu270 of CPA near the Asp9O of BCII was selected. With this
orientation,Argl45, which makes a salt bridge interaction with the carboxylate group of the substrate in CPA, is located nearHis210 of BCII. A more detailed comparison between B.cereus Zn ,B-lactamaseandother Znpeptidases
must await the results of the further refinement of the
BCII structure athigh resolution.
Materials and
methods
Site-directedmutagenesis
The procedures used in recombinant DNA techniques wereessentially
those described in Maniatis etal. (1982). The oligonucleotides were
purchased from Eurogentec (Belgium). The site-directed mutagenesis
was performed by inverse PCR (Clackson et al., 1991) using the following strategy. A SstII-PstI (-650 bp) fragment comprising the triplet tobe mutated was isolated from plasmid pRWHO12 (Hussain et al., 1985) and subcloned into plasmid pSL1 180 (Pharmacia, LKB Biotechnology). The pair of primerswasdesigned in inverted tail-to-tail
directions: a 23 base primer (AATTCGATCAGCGTGCGCATGTG)
annealing perfectly with the target sequence and a 28 base primer
(GGCGGAATAAAATGTTTGAAAGAAAGAG)introducingthree
con-tiguous mismatches for replacing the ACGtriplet in the codingsequence.
Theinverse PCR product wasanalysed byagarose gelelectrophoresis andpurifiedon anUltrafreeMC filter(Millipore). A fraction (one-sixth)
of the purified product was then phosphorylated in 20 ml of DNA ligation buffer (Boehringer) using 5 U of T4 polynucleotide kinase for 30 min at 37°C. After heat denaturation of the kinase, ligation was
performed by adding I U of T4 DNA ligase tothe mixture and the
product was used totransform RRI-competent cells. The presence of the desired mutationand the absence ofanyother unwanted mutationwere
Asrn180
R2 N
Fig. 7. Schematic diagram of the proposed interaction ofa
cephalosporin molecule priortocatalysis.
checked by DNA sequencing using the CTGCAGTTAATGCTGTAC
oligodeoxynucleotide (annealing 46 bases upstream of the mutated
triplet)as aprimer.
BCIIexpression in Escherichiacoil
The B.cereus 569/H/9 and T97C mutant metallo-,B-lactamases were
producedin EcoliusingthepRTWHO12plasmidcloned inEcoli DHI.
Purification of BCIIandBCII(T97C)
A total of20 1 of Luria-Bertani mediumwere inoculated with 300ml ofanovernightculture ofDHI/pRTWHO12. After10 h ofgrowthunder agitationat37°C, the Ecoli cellswereharvested by centrifugation. The pellet was resuspended in 10 mM Tris, pH 8.0, buffer containing lysozyme (0.1 mg/ml). The solutionwasincubated for 30min on ice.
Followingcelllysis bythreecycles of freezing and thawing, theextract wascentrifuged for 30 min at 30 000 g. The soluble fraction (which contains the1-lactamase)wassubmittedtoultrafiltration andanovernight
dialysis against 10 mM cacodylate sodium, pH 6.5, buffer containing 50 jMZnSO4 (bufferA). The fractionwasloadedontoaCM-Sepharose
(55X3 cm) column(Pharmacia, Uppsala, Sweden) equilibrated with the samecacodylate bufferat aflowrate of2.0ml/min. Theenzyme was eluted by a salt gradient (0.0-0.5 MNaCI). The active fractions were
Fig. 8. Stereoview of the catalytic sites for selected amino acid residues around theZn2+ ion for CPA (top) and BCII(bottom).
Table II.Crystallographic data
Datacollectionparameter Crystal
1 2 3 4 5
Dataset(protein) (native) HgAc2 (native) pHgAc (T97C) pHgAc (T97C) pCMBS(T97C)
Resolution (A) 2.5 2.3 2.5 2.5 2.5
No. of reflectionsmeasured 11 206 20 937 31 281 17070 15 819
Rmergea 0.030 0.036 0.047 0.052 0.040
No. ofunique reflections 6911 9503 7942 7547 7776
Completeness (%) 86.0 90.6 98.2 93.9 96.0 Riso - 0.18 0.17 0.16 0.16 Phasing powerb - 1.42 1.81 1.95 1.31 (3.0 A) Rculsisoc - 0.75 0.67 0.64 0.75 RcuIIisanoc - 0.75 - 0.91 -No. of sites I(Zn) 1 1 I
HgAc2, mercuricacetate;pHgAc, phenylmercuricacetate;pCMBS, para-chloro-mercuribenzene sulfonic acid.
aRmerge of isomorphous data= (XII -<I>I/X<I>), whereIisthe measuredintensityfor each reflection and<I>is themeanintensity averaged frommultiple observationsofsymmetry-related reflections.
bPhasingpower = r.m.s.(<FH>IE), whereFH is the heavyatom structurefactoramplitudeand E is the residual lack of closure.
CRcuiIis = 1IIFHI-(IFpHI-1Fpl)l/11FHI, for centric reflections, where FPHis theheavy-atomderivativestructurefactor.
E270 E270 H1 R145 R145 Zno 0 -|gRO H~~69 C 4 < H69 E72 E72 CPA CPA H86 H86 H88 H88<j ,J. . H149 H149 BCII BCII I Hi,
pooled and concentrated by ultrafiltration. The sample was filtered
through a SuperdexTm 75 10/30 molecular sieve column (Pharmacia) pre-equilibrated withbuffer A at a flowrateof 0.7 ml/min. Thepurity
of thesamplewasestimatedonthe basis of thespecific activity and by SDS-PAGE analysis andCoomassie Blue staining. The samples were
analysed by electrospraymassspectroscopy.For thispreparation, 60mg
of B.cereus 569H/9P-lactamasewereobtained. The enzyme(4mg/ml)
wasstored inbufferA at-20°C. Thepurification of the T97Cmutant
wasperformedby thesameprocedure, but dithiothreitol (DTT; 50gM)
was added to the different buffers to prevent oxidation of the newly
introduced cysteine.
Crystallization
Crystalsofthe wild-type protein (TableII, column 1)were grown by thevapourdiffusionhanging dropmethod.Theproteinsolution(9mg/ml
in 10 mM cacodylate buffer containing 100 tM ZnSO4 and 50 ,uM DTT)wasbroughtto25mMsodium citrateand18%(w/v) poly(ethylene glycol)8000(PEG 8K)(Sigma), pH5.6. For theT97Cmutantprotein, the DTTconcentrationwasincreasedto400,uM.For thepreparationof mercuric acetate (HgAc2) derivative (Table II, column 2), wild-type crystals were washed for 4 days in Zn2+-free solutions containing
1.5 mM EDTA and20%PEG8K, and then soaked for 12 h in a20% PEG and 300mMHg2+ solution. Forderivatives of the mutantT97C, crystals were transferred to a DTT-free solution with 100 ,uM Zn2,
20% PEG 8Kand either0.35 mMphenylmercuric acetate(pHgAc)for
12 hor0.5 mMpCMBSfor24h. The secondPMAcderivative(Table II, column4)waspreparedinsolutionbydialysingtheproteinsolution
for5 hagainstasolutioncontainingsaturatedpHgAcatpH8.0(50mM
Tris-HCIbuffer)and thencrystallizedasthewild-type protein. Data collection andprocessing
Crystals belonged to space group C2, with unit cell dimensions a =
53.79 A, b = 61.98 A, c = 69.97 A, i = 93.67°, onemolecule per
asymmetricunit andasolventcontent of35%.
X-raydataforcrystals reportedincolumns 1,2, 4and 5of Table II
werecollectedusingaFast/Enraf-Noniusareadetector withaCurotating
anode generator (FR5H) and a graphite monochromator, and were
processed usingtheMADNES program(Messerschmidtand Pflugrath, 1987).
Datafor the T97C mutant (Table II, column3) werecollected on a
Mar-Researchimage platedetectorusingaCurotatinganodegenerator
(RU 200), and were processed withthe XDSprogram(Kabsch, 1988, 1993).TheCCP4programsuite(1993)wasusedfor furtherprocessing. Structuresolutionandrefinement
The structure wassolvedby multiple isomorphous replacement (MIR).
The mercuryatomofthewild-typederivative(Table II,column2)was
foundtoreplacethezincatom. InthemutantT97Cderivatives, theHg
atomwasboundtothe -SHgroupin slightlydifferentpositionsfor the pCMBS derivative and the two pHgAc derivatives. Heavy-atom
para-meterrefinementandphasingwereperformed usingMLPHARE(CCP4, 1993).The MIR mapwasimproved byanelectrondensitymodification
involving solventflattening, histogram matchingand Sayre's equations options of the dm program (CCP4, 1993). The resulting map was
partially interpretable, and allowed -60% ofthe protein atoms to be
located. Successivecyclesofphasecombinationwith SIGMAA (Read, 1986)and modelbuildingresultedin the finalmodel,whichdefines the proteinstructureexceptfortheC-terminalLys227andtwostretchesof
the sequence: thefirst sixN-terminal residues and theGly32-Val39 loop
which appearstobe disordered.
Model buildingwasperformed using thegraphic 0program (Jones
et al., 1991) on an ESV station. Refinement was performed using X-PLOR (Bruinger, 1992) with 5% of the reflection data setused for
definingtheR-free factor.Thecorrectnessofthe chain trace was verified
byaninspectionof the electrondensityof(21FOI-IFcI),(1F01-
IFJI)
and GAweighted(IFOI-IFJI)omit mapscalculatedwith I10% of the residues omitted for thephasecalculation.The final model contains 212 amino acid residues (out of 227), one Znatomand 10watermolecules which wereclearly located in the map
and refined withaB-factor of <50A2.The final R-factorwas0.22for the 6683 reflections with IFI > 2a(F) (0.221 for all 6709reflections),
and theR-freefactorwas0.33in the resolution range 8.0-2.5A.
Structure comparison
The structures were taken from the Protein Data Bank (Abola etal., 1987). Figures were prepared using the following programs:
MOLSCRIPT (Kraulis, 1991), RIBBONS (Carson, 1991) and 0 (Jones etal., 1991).
Datadeposition
The atomiccoordinates have beendepositedin the Protein Data Bank (IBMC), Chemistry Department, Brookhaven National Laboratory,
Upton, NY 11973, with an embargo of 2 years afterpublication.
Acknowledgements
This ispublication No. 276 of the Institut deBiologie Structurale Jean-Pierre Ebel. The authorswould like to thank a numberofpeople for their assistance: E.Forest and Y.Petillot (LSMP, Grenoble, France) for mass spectra analysis and P.Charlier (Liege, Belgium) for early crystallizationtrials. This work wassupportedby an ECC grant Human
Capital and Mobility No. ERBCHRXCT930268, a French IMABIO program,an ActionConcertee (convention 89/94-130)and the Belgian Government aspart of aP6led'Attraction Interuniversitaire(PAI No. 19).
References
Abola,E.E., Bernstein,F.C., Bryant,S.H., Koetzle,T.F.andWeng,J.(1987) Proteindata base. In Allen,F.H., Bergerhoff,G.and Sievers,R. (eds), Crv.stallographicDatabases -InformationContent,SoftwareS'steins,
Scientific Applications. Data Commission of the International Union ofCrystallography, Bonn, Germany,pp. 107-132.
Ambler,R.P., Daniel,M., Fleming,J., Hermoso,J.M., Pang,C. and
Waley,S.G. (1985)The amino acid sequence of the zinc-requiring f-lactamase II from the bacterium Bacilluscereus569.FEBSLett., 189,
207-211.
Baldwin,G.S., Galdes,A., Hill,H.A.O., Smith,B.E., Waley,S.G. and
Abraham,E.P. (1978)Histidineresiduesaszincligandsin,B-lactamase II.Biochem. J., 175,441-447.
Baldwin,G.S., Waley,S.G. and Abraham,E.P. (1979) Identification of
histidine residues that act as zinc ligands in P-lactamase II by
differential tritiumexchange.Biochein.J., 179,459-463.
Bicknell,R., Emanuel,E., Gagnon,J. and Waley,S.G. (1985) The
production and molecular properties of the zinc f-lactamase of PseudomonasmaltophiliaII D 1275.Biochem.J.,229,791-797.
Bicknell,R., ShafferA., Waley,S.G. and Auld,D.S. (1986) Changes in the coordination geometry of the active-site metal during catalysis
of benzylpenicillin hydrolysis by Bacillus cereus ,B-lactamase II.
Biochemistry,25,7208-7215.
Brunger,A.T. (1992) A Systemfor X-ray Crystallography and NMR: XPLOR. Version3.0, YaleUniversity Press,NewHaven,CT.
Carson,M.C. (1991)RIBBONS 2.0. J.App!. Cryst., 24, 958-961.
CCP4(1993)CollaborativeComputing ProjectNo.4.ActaCrx'stallogr, D50,760-763.
Clackson,T., Gussow,D. and Jones,P.T. (1991) In McPherson,M.J., Anirke,P. and Taylor,G.R. (eds). PCR: A PracticalApproach. IRL
Press,Oxford, UK,pp.202-203.
Clark,S.D. (1993) Site directed mutagenesis of bacterial
metallo-,B-lactamase. PhDThesis,Texas TechnicalUniversity,TX.
Crompton,I.E. and Waley,S.G. (1986)The determination ofspecificity
constantsinenzyme-catalysedreactions. Biochem. J.,239,221-224.
Davies,R.B. andAbraham,E.P. (1974)Metal cofactorrequirement of -lactamaseLI. Biochem. J., 143, 129-135.
Dideberg,O.,Charlier,P.,Dive,G.,Joris,B., Frere,J.M.andGhuysen,J.M. (1982) Structure of a Zn2+-containing D-alanyl-D-alanine-cleaving carboxypeptidaseat2.5A resolution.Nature,299,469-470.
FeliciA., Amicosante,G., Oratore,A., Strom,R., Ledent,P., Joris,B.,
Fanuel,L.andFrere,J.M. (1993)Anoverview ofthekineticparameters of class B 3-lactamases.Biochem.J.,291, 151-155.
Gaboriaud,C., Bissery,V., Bencherit,T. and Mornon,J.P. (1987)
Hydrophobicclusteranalysis: anefficient new way to compare and
analyseaminoacid sequences. FEBSLett.,224, 149-155.
Galdes,A., Hill,H.A.O., Baldwin,G.S., Waley,S.G. and Abraham,E.P.
(1980)The ' Hnuclear-magnetic-resonancespectroscopyof
Cobalt(II)-f-lactamase II.Biochem. J., 187,789-795.
Hooper,N.M.(1994)Familiesofzincmetalloproteases.FEBS Lett., 354, 1-6.
Hussain,M., Carlino,A.,Madonna,M.J.andLampen,J.O.(1985) Cloning and sequencing of the metallothioprotein ,B-lactamase 11 gene of
Bacilluscereus569/H inEscherichiacoli. J.Bacteriol., 164,223-229. Jones,T.A., Zou,J.Y., Cowan,S.W.and Kjeldgaard,M. (1991) Improved
methods forbuilding proteinmodels inelectrondensitymaps and the
location oferrorsinthesemodels.ActaCrvstallogr, A47, 110-119. Kabsch,W. (1988) Evaluation of single X-ray diffraction data from a
positionsensitive detector.J.Appl. Crvst., 21,916-924.
Kabsch,W.(1993)Automaticprocessingofrotationdiffractiondata from
crystals of initially unknown symmery and cell constants. J. Appl. Crvst., 26, 795-800.
Kato,C., Kudo,T., Watanabe,K. and Horikoshi,K. (1985) Nucleotide sequence of the ,-lactamase gene ofalkalophilic Bacillus sp. strain
170. J.Gen. Microbiol., 131,3317-3324.
Kraulis,P.J. (1991) MOLSCRIPT: a program to produce both detailed
andschematic plotsofprotein.J.Appl. Crvst.24. 946-950. Lim,H.M., Iyer,R.K. andPene,J.J. (1991) Site-directed mutagenesis of
dicarboxylic acids near the active site ofBacillus cereus 5/B1/6 -lactamase II. Biochem.J.,276,401-404.
Lipman,D.J. and Pearson,W.R. (1985) Rapid and sensitive protein similarity searches.Science,227. 1435-1441.
Lipscomb,W.N. (1983) Structure and catalysisofenzymes. Annul. Rex: Biochem.,52, 17-34.
Lowe,J.,Stock,D.,Jap,B., Zwickl,P., Baumeister,W.andHuber,R. (1995) Crystal structure of the 20S proteasome from the archaeon T acidophilumat3.4 A resolution. Science,268,533-539.
Maniatis,T., Fritsh,E.F. and Sambrook,J. (1982) Molecular Cloning:A
Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor,NY.
Massida,O., Rossolini,G.M. and Satta,G. (1991) The Aeromonas
hvdrophilaA gene: molecularheterogeneity among class B
metallo-P-lactamases.
J. Bacteriol., 173,4611-4617.Matthews,B.W.(1988)Structuralbasis of theaction ofthermolysinand relatedzincpeptidases.Acc.Chem. Res.,21,333-340.
Messerschmidt,A. andPflugrath,J.W. (1987) Crystalorientationand X-raypatternpredictionroutinesforarea-detector diffractometersystems inmacromolecularcrystallography.J.Appl. Crvst.,20,306-315. Mol,C.D., Kuo,C.-F., Thayer,M.M., Cunningham,R.P. and Tainer,J.A.
(1995) Structure and function of the multifunctional DNA-repair
enzymeexonuclease111.Nature,374,381-386.
Oefner,C.andSuck,D. (1986) Crystallographicrefinementandstructure
ofDNase Iat2 Aresolution. J.Mol.Biol., 192,605-632.
Osano,E., Arakawa,Y., Wacharotayankun,R., Ohta,M., Horii,T., Ito,H., Yoshimura,F. and Kato,N. (1994) Molecular characterization of an
enterobacterial metallo-p-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antitnicrob. Agents Chemother:, 38,71-78.
Payne,D.J. (1993)Metallo-f-lactamase: a new therapeutic challenge.J.
Med.Microbiol., 39,93-99.
Ramachandran,G.N., Ramakrishnan,C. and Sasisekharan,V. (1963)
Stereochemistryofpolypeptidechainconfigurations.J. Mol.Biol., 7, 95-99.
Rasmussen,B.A., Gluzman,Y. and Tally,F.P. (1990) Cloning and
sequencingof theclassB 3-lactamasegene(ccrA)from Bacteroides fragilisTAL3636. Antimicrob.Agents Chemother,34, 1590-1592. Read,R.J. (1986) Improved Fourier coefficients formaps using phases
from partialstructureswitherrors.Acta Crvstallogr,A42, 140-149.
Saino,Y., Kobayashi,F., Inoue,M. andMitsuhashi,S. (1982)Purification
and properties of inducible penicillin 3-lactamase isolated from Pseudomonas maltophilia. Antimicrob. Agents Chemnother, 22,
564-570.
Smith,J.L., Zaluzec,E.J., Wery,J.-P., Niu,L., Switzer,R.L., Zalkin,H.and
Satow,Y.(1994)Structure of theallostericregulatoryenzymeofpurine biosynthesis. Science, 264, 1427-1433.
Stocker,W., Gomis-RUth,F.-X., Bode,W. and Zwilling,R. (1993) Implications of the three-dimensional structure of astacin for the
structure and function of the astacinfamily ofzinc-endopeptidases. Eur J. Biochem.,214. 215-231.
Stocker,W., Grams,F., Baumann,U., Reinemer,P., Gomis-Ruth,F.-X., McKay,D.B.andBode,W. (1995)The metzincins-Topologicaland
sequential relations between the astacins, adamalysins, serralysins,
and matrixins(collagenases) defineasuperfamilyofzinc-peptidases.
ProteinSci., 4, 823-840.
Suck,D., Oefner,C. and Kabsch,W. (1984)Three-dimensional structure
of bovine pancreatic DNase I at 2.5 A resolution. EMBO J., 3, 2423-2430.
Sutton,B.J.,Artymiuk,P.J.,Cordero-Borboa,A.E., Little,C.,Phillips,D.C.
andWaley,S.G.(1987)AnX-ray-crystallographic studyof
P-lactamase
II from Bacillus cereus at 0.35 nm resolution. Biochem. 1., 248, 181-188.Thompson,J.S. and Malamy,M.H. (1990) Sequencing the gene of
imipenem-cefoxitin-hydrolyzing enzyme (CfiA) from Bacteroides fragilis TAL2480 reveals strong similarity betweenCfiAand Bacillus
cereus
P-lactamase
II.J.Bacteriol., 172, 2584-2593.Waley,S.J.(1992) f-lactamase: mechanism of action. In Page,M.(ed.),
The Chemistry of 1-Lactams. Blackie Academic and Professional,
Chapman andHall, London, UK,pp. 198-228.
Walsh,T.R., Hall,L., Assinder,S.J., Nichols,W.W., Cartwright,S.J., MacGowan,A.P. and Bennett,P.M. (1994) Sequence analysis of the LI metallo-,-lactamase from Xanthomonas maltophilia. Biochim. Biophys. Acta, 1218, 199-201.
Yang,Y., Wu,P. and Livermore,D.M. (1990) Biochemical characterization of a
P-lactamase
that hydrolyses penems and carbapenems from two Serratia marcescens isolates. Antimicrob. Agents Chemother, 34,755-758.