Schizophrenia, Sleep and
Antipsychotics
Michel Floris
1, William Pitchot
2, Daniel Souery
3, Luc
Staner
4, Nicolas Zdanowicz
51 Psychiatric Unit, Notre-Dame Clinic, Tournai
2 Psychiatric Unit, Sart Tilman University Hospital, Liège 3 Psychiatric Unit, Erasme University Hospital, Bruxelles
4 Forenap and Secteur VIII, Rouffach Hospital, Rouffach, France
Abstract
Sleep disturbances are common in schizophrenic patients. They are never been systematically studied, unlike all the others symptoms (positive, negative, cognitive, aggressive, …). In the present paper the literature about these facts is reviewed and the possible mechanisms involved in the effects of antipsychotic drugs on sleep as well as the clinical implications are discussed. Most common sleep disturbances in schizophrenia are sleep initiation and maintenance difficulties characterizsed by a fragmented sleep. A slow wave sleep deficit is often observed and has been related to structural brain abnormalities, negative symptoms and a poor clinical outcome. Most antipsychotic drugs improve the sleep disturbances encountered in
schizophrenia. Drugs antagonising 5 HT2 receptors, such as atypical antipsychotics
have been shown to increase slow wave sleep. In this regard, studies in healthy volunteers or in schizophrenic patients have shown that risperidone, olanzapine and ziprasidone both improve sleep continuity and increase slow wave sleep. This suggests that antipsychotic drugs acting on the 5 HT2 receptors are particularly well
suited for the treatment of schizophrenic patients with sleep disturbances.
1. Introduction
The long-held belief that troubled sleep reflects a trouble mind, as well as the similarity between psychotic phenomena and dreams, has stimulated interest in sleep electroencephalographic (EEG) studies of schizophrenia.
Some research has shown that schizophrenia is related to changes in the
architecture of sleep evaluated with polysomnography. Specifically, lack of slow wave sleep (SWS) has been described, as well as changes in the amount of rapid eye movement (REM) sleep and a decrease in REM latency. At a structural level, SWS changes have been related to lateral ventricle dilatation, establishing a relationship with constant alterations, especially with those affecting cortico-thalamic neuronal nets. However, at the clinical level SWS changes seem to have some connection with cognitive deterioration, with a worse long-term prognosis and with generally negative symptoms (Royuela et al., 2002). According to this study it can be
suggested that alteration of sleep quality could be a marker of illness severity, but the effect of antipsychotics must be clarified adequately. The relationship between sleep and clinical outcome in schizophrenia is also exemplified by the study of Chemerinski et al. (2002) who showedn that schizophrenic patients with sleep disturbances are at a greater risk for worsening of positive symptoms after antipsychotic discontinuation.
In this regard, a partial improvement of some but not all sleep EEG measures in schizophrenic patients has been demonstrated through the course of antipsychotic treatment. It has been suggested that shortened REM latency and disturbed sleep continuity might represent reversible state abnormalities, while reduced SWS may represent a more persistent trait abnormality in schizophrenia (Maixner et al., 1998). It has also been shown that some antipsychotics, such as haloperidol, can induce as
a side effect some circadian rhythm sleep disorders (Dagan et al., 2002). Some investigations suggest as well that long-term antipsychotic treatment may promote sleep disturbances by increasing the risk of a nocturnal myoclonus related syndrome insomnia, mainly in elderly schizophrenics (Staedt et al., 2000).
Another point is that poor quality of life (QOL) reported by schizophrenic patients is substantially associated with poor sleep quality. This association appears both independently and synergistically with depression, distress and side effects of medication (Ritsner et al., 2004). Finally, some studies realized by Keshavan et al. (1994) and Lewis et al. (1996) suggest the possibility that poor sleep is a stressor that may independently increase the risk of suicide, perhaps by impairing judgement or impulse control, or by fatigue that favours a sense of hopelessness or futility. In that way, the relationship between serotonergic function and sleep changes in psychiatric disorders needs to be systematically studied, because one mechanism responsible for this possible association between suicide and sleep could be the role of serotonin (5HT). Indeed, 5-HT disturbances have been observed in patients who attempted and/or completed suicide, particularly those who used violent methods and 5-HT plays an important role in onset and maintenance of SWS and in REM sleep (Singareddy and Balon, 2001).
Thus, the above mentioned evidences suggest a close relationship between sleep and some clinical aspects of schizophrenia including cognitive disturbances and long-term prognosis. The present review successively discuss the different kind of sleep alteration observed in schizophrenia with a special emphasis on SWS, the effects of antipsychotic drugs on sleep and the mechanisms that can underlie these disturbances.
Sleep basics
Electrophysiological recordings of human brain reveal three distinct state of existence: wakefulness, REM sleep and non-REM (NREM) sleep. The distinction between sleep versus wakefulness is attributed to the synchronization and
desynchronization of thalamocortical circuits (Steriade, 1996; Pace-Schott and Hobson, 2002). Wake-like or “desynchronised” (low amplitude and high frequency) EEG activity with clusters of rapid eye movement and very low levels of muscle tone characterize REM sleep. NREM sleep includes all sleep except REM sleep and is by convention divided in four stages that correspond to increase depth of sleep as indicated by the progressive dominance of “synchronized” EEG activity (also known as low voltage high amplitude delta or slow wave activity); in this respect, sleep stages 3 and 4 are collectively labelled as delta sleep or SWS. Recurrent cycles of NREM and REM sleep of about 90 minutes characterise normal human sleep. In the successive cycles of the night, the duration of stages 3 and 4 decrease, and the proportion of the cycle occupied by REM sleep tends to increase with REM episodes occurring late in the night having more eye movement bursts than REM episodes occurring early in the night (Lesch and Spire, 1990).
2. Sleep disturbances associated with
schizophrenia
Following the development of polysomnography, sleep researchers have repeatedly attempted to characterize sleep in schizophrenic patients. However, controversy still exists about which sleep EEG disturbances are observed in schizophrenia (Monti and Monti, 2004). Discrepancies may be related to:
the differing definitions of schizophrenia.
the inclusion of patients over a wide range of ages, in different phases of their illness (acute vs. chronic), and with varied subtypes of schizophrenia. the lack of screening for obstructive sleep apnea and periodic limb
movement disorder, which are prevalent in older people.
the inclusion of patients who had been taking antipsychotic drugs. Insomnia is a common feature in schizophrenia. However, it seldom is rarely the predominant complaint. Nevertheless, severe insomnia is often seen during
exacerbations of schizophrenia, and may actually precede the appearance of other symptoms of relapse. Less frequently, severe sleep disruption may complicate schizophrenia to the degree that patients can become suicidal. As described below and shown on table 1, most reports have found sleep disturbances in either never-medicated or previously treated schizophrenia patients that are characterized by a sleep-onset and maintenance insomnia. In addition, duration of stage 4 sleep, SWS, and of NREM sleep as well as REM latency are decreased (Monti and Monti, 2004).
2.1 Never-medicated schizophrenic patients
.In a recent study conducted by Julie Poulin at the Hôpital Louis-H. Lafontaine of Montréal, compared to controls, patients with schizophrenia had difficulty initiating sleep, decreased stage 4 duration, reduced REM sleep latency, and normal sleep spindles and REM’s densities. Positive symptoms correlated negatively with REM sleep latency. The Brief Psychiatric Rating Scale (BPRS) total score correlated negatively with REM sleep duration and REM’s density. These results indicate that first episode and neuroleptic-naïve patients with schizophrenia have difficulties initiating, but not maintaining sleep. These results also confirm other previous reports (see below) showing that the duration of stage 4 and REM sleep latency are reduced in first episode and neuroleptic-naïve patients with schizophrenia. The fact that measures of REM sleep correlate with clinical scales of schizophrenia suggests that REM sleep physiology shares common substrates with symptoms of this disease (Poulin et al., 2003).
In a review of 5 older studies (Jus et al.,1973; Ganguli et al.,1987; Tandon et al.,1992; Lauer et al., 1997; Lauer and Krieg, 1998) comparing never-medicated schizophrenic patients to controls it can be found that:
Stage 2 sleep latency is increased in all five studies (mean increase: 36.5 min {range:26.9 – 43.2}).
Wake time after sleep onset (min or % of total recording time) is increased in four studies (one missing value).
The number of nocturnal awakenings is increased in three studies (two missing values).
Values corresponding to total sleep time are found to be reduced in all five studies (mean decrease: 54.4 min {range:13 – 79 min}).
Sleep efficiency is reduced in four studies (mean decrease: 15.4 % {range:13.5 – 16.1 %}, one missing value). Stage 2 sleep is reduced in four studies (mean
reduction: 4.6 % {1.6 – 8.1 %}, one missing value.
Stage 4 amounts to very low values in both schizophrenics and controls
REM latency is moderately decreased in three studies, similar to controls in two studies.
REM sleep in minutes is moderately decreased in two studies.
These results have also to be compared with those obtained by Keshavan et al. (2004) using non linear analysis of the sleep EEG. In a series of antipsychotic naïve patients with first episode of schizophrenia compared to age-matched healthy
controls studied during awake period, stage ½, SWS (stage ¾) and REM sleep, they found significantly lower nonlinearity scores during awake stage in patients compared to controls suggesting that there may be a diminished interplay between various parameters for the genesis of waking EEG. Symbolic dynamics and the largest Lyapunov exponent (LLE) were significantly lower in patients during REM compared to healthy controls, suggesting decreased nonlinear complexity of the EEG time series and diminished chaos in schizophrenia. Decreased nonlinear complexity was also correlated with neurocognitive deficits as assessed by the Wisconsin card sorting test. Diminished complexity of EEG time series during awake and REM sleep in patients with schizophrenia may underlie the impaired ability to process
information in psychotic disorders such as schizophrenia.
2.2 Previously treated schizophrenic patients.
In a review of 13 studies (Caldwell and Domino,1967; Feinberg et al.,1969; Stern et al., 1969; Hiatt et al., 1985; Zarcone et al.,1987; Kempenaers et al.,1988; Benson et al., 1991; Tandon et al., 1992; Benson and Zarcone,1993; Hudson et al., 1993; Benson et al.,1996; Keshavan et al.,1998; Hoffmann et al., 2000 ) comparing schizophrenic patients previously treated with antipsychotics to controls, it can be found that:
Stage 2 latency is increased in 12 studies (mean increase: 46.6 min. {range 11.7 – 87 min.}, one missing value).
Wake time after sleep onset is increased in three studies (mean increase: 19.5 min. {range: 8 – 33 min}, seven missing values).
Total sleep time is reduced in 12 studies (mean decrease: 61.7 min. {range: 20 – 112 min}).
Sleep efficiency is reduced in seven studies (mean decrease: 14.2 % {range: 7 – 23 %}, six missing values).
Stage 4 is reduced in eight studies (mean decrease: 28.4 min {range: 4 – 46.6 min}, five missing values) : period amplitude analysis (PAA) shows significant reduction of delta wave (0.5 – 3 Hz) counts during NREM sleep, especially during the first NREM period while power spectral analysis (PSA) shows significant reduction of power in the delta and theta ranges. REM latency is decreased in 10 studies (mean reduction: 27.7 min {range:
8 – 52 min}, one value missing).
REM sleep in min was is reduced in 10 studies (mean decrease: 18.6 min {range: 2 – 41 min}, three missing values).
REM sleep percentage of total sleep time is slightly reduced in five studies and slightly increased in three studies, five missing values).
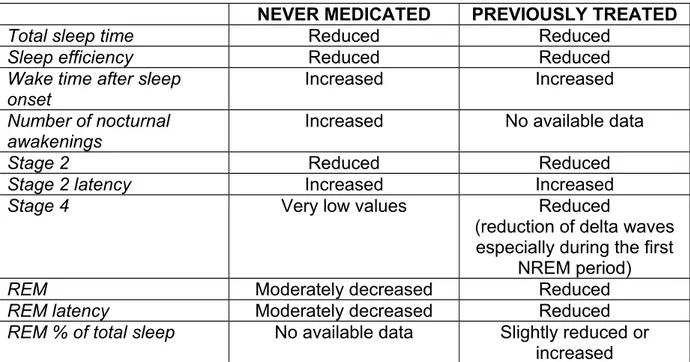
Table 1 : Data from never-medicated and/or previously treated schizophrenic patients.
NEVER MEDICATED PREVIOUSLY TREATED
Total sleep time Reduced Reduced
Sleep efficiency Reduced Reduced
Wake time after sleep onset
Increased Increased
Number of nocturnal awakenings
Increased No available data
Stage 2 Reduced Reduced
Stage 2 latency Increased Increased
Stage 4 Very low values Reduced
(reduction of delta waves especially during the first
NREM period)
REM Moderately decreased Reduced
REM latency Moderately decreased Reduced
REM % of total sleep No available data Slightly reduced or increased
2. 3 Delta sleep and negative symptoms of schizophrenia.
Most polysomnographic studies have detected a reduction of visually scored stage 4 sleep and SWS in schizophrenic patients. Schizophrenic patients also showed reduced sleep time and sleep continuity. However, observations of reduced average delta counts in the first NREM period, reduced delta wave accumulation in NREM sleep, and reduced delta power in power spectral analysis (PSA) suggest that sleep discontinuity is unlikely to explain the observed delta sleep (DS) deficits. Reductions in delta power in the neuroleptic naïve, first episode schizophrenic patients as well as the lack of a relationship between DS and illness duration or with the length of the medication wash-out period prior to sleep EEG recordings, suggest that these
findings are primary to schizophrenia and not simply epiphenomena of medication or illness chronicity.
Follow-up sleep studies of a subset of these patients have shown that DS deficits, unlike REM sleep changes, are persistent, suggesting that the former may be trait
related. Deficits in DS have been found to be associated with enduring traits of schizophrenia, such as negative symptoms, ventriculomegaly, attentional
impairment, poor outcome and impaired frontal lobe metabolism. These observations and the presence of DS deficits in the first episode, treatment naïve patients are consistent with the neurodevelopmental model of the schizophrenic illness
(Keshavan et al., 1998). In the same study, these authors showed that, according to Period Amplitude Analysis (PAA), significant reductions in delta wave counts but not in REM counts exist. They showed that total REM sleep amounts and delta ratios were not altered in schizophrenia, unlike in depression in which studies showed that REM amounts, especially in the first REM period, are increased and delta ratios decreased. The duration of the first REM period was shorter, but PAA disclosed no difference in REM counts, suggesting a deficit in tonic but not phasic REM activity. On the other hand, PSA showed reductions in delta as well in theta power. Delta deficits were more pronounced in the 2 to 4 Hz frequency range. This finding has to be discussed in the light of studies showing that slow delta waves (less than 1 Hz) are generated in the neocortex, while faster delta waves result from thalamocortical oscillations. The fact that schizophrenic patients have a deficit of faster delta could relate to observations of reduced thalamic volume, synaptic density and metabolism in schizophrenia.
Other studies showed that there is an inverse correlation between half-wave counts of higher amplitude delta waves and negative symptoms (Monti and Monti, 2004).
3. Effects of typical and atypical antipsychotics on
polysomnographic measures of sleep.
3.1 Classical antipsychotics.
In 1996, Obermeyer and Benca had summarized the effects of various psychotropic drugs on sleep and the effects of typical antipsychotics are described in table 2. Classical antipsychotics appear to increase light and “instable” NREM sleep,
increasing amount of stage 1 sleep. Regarding REM sleep, classical antipsychotics do not change the total or relative amounts of REM sleep (Keshavan et al.,1990), nor the duration of REM latency; however, their effects on REM density remain
controversial (Wetter et al., 1996)
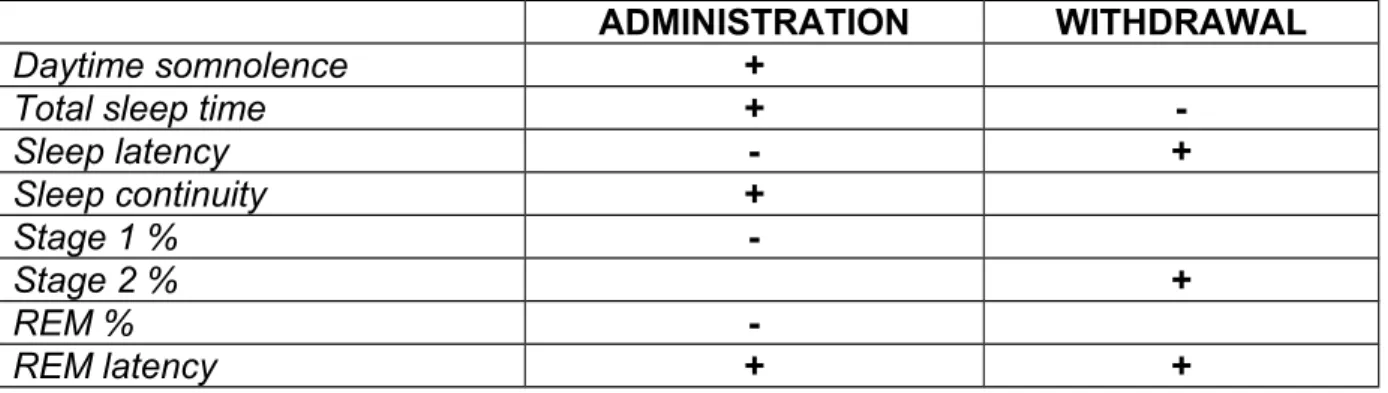
Table 2: Changes in sleep due to typical antipsychotics (haloperidol or chlorpromazine)
ADMINISTRATION WITHDRAWAL
Daytime somnolence +
Total sleep time +
-Sleep latency - + Sleep continuity + Stage 1 % -Stage 2 % + REM % -REM latency + +
3.2 Atypical antipsychotics
The objective of the Yamashita’s et al. (2004) study was to investigate the effects of atypical antipsychotic drugs on the subjective quality of sleep in patients with
schizophrenia. The results demonstrated that atypical antipsychotic drugs improved subjective quality of sleep in schizophrenic patients compared with conventional antipsychotic drugs, suggesting that the marked potency of serotonin 2 receptor blockade in atypical antipsychotic drugs may be involved in the mechanism of this improvement and that these improvements were correlated with improvement of negative symptoms.
On the other hand, not all antipsychotic medications have the same sedative effect, which is related to dosage and affinity for histamine H1 receptors (see table 3).
Table 3: Comparison of Potency, Dose and Sedation in Antipsychotic Medications.
potency (mg) range (mg/d) H1 Conventional antipsychotics Chlorpromazine 100 100-600 Moderate Mesoridazine 50 50-150 Moderate Fluphenazine 1-2 2-20 Mild Haloperidol 2 5-20 0.38 Mild Atypical antipsychotics Clozapine 50 250-500 32 Marked Quetiapine 80 300-800 5.2 Moderate Olanzapine 4 15-30 1149 Moderate Ziprasidone 20 80-160 22 Mild Risperidone 1 2-6 19 Mild Studies have shown that, compared with conventional antipsychotics, atypical
antipsychotics such as risperidone, olanzapine, quetiapine and ziprasidone generally cause less sedation and are as effective in controlling psychosis and agitation (Miller, 2004). Sedation can be troublesome to patients who are trying to become
reintegrated into society and interfere with their treatment regimen.
In a longitudinal study of schizophrenic patients (Hinze-Selch et al., 1997) clozapine significantly improved sleep continuity, increasing NREM sleep, in particular stage 2 sleep, while the amounts of stage 4 and SWS sleep decreased significantly. On the other hand, clozapine increased significantly REM density, but it did not affect the amount of REM sleep. In a risperidone-treated group of schizophrenic patients, the SWS period was significantly longer than in a haloperidol-treated group, without other significant differences in sleep variables between these groups (Yamashita et al., 2002).
The effect of a single dose of olanzapine on sleep in healthy females and males has been shown in several studies (Sharpley et al., 2000; Lindberg et al. 2002; Staner et al., 2002; ). The increase in both actual sleep time and SWS in females (same tendency in men, but not so significant) correlated with the increase in theta power, while delta power was not significantly elevated, suggesting that theta power may be a sensitive indicator of changes in sleep. Olanzapine seems to preserve the normal structure of sleep and to increase the amount of SWS, which might be of additional benefit in treatment of schizophrenia.
An acute administration of olanzapine in schizophrenic patients increases sleep stage 2 and delta sleep, and so the sleep efficiency (Salin-Pascual et al., 1999). In a study comparing to baseline the subchronic effects of olanzapine on sleep EEG, there was a significant improvement of parameters of sleep efficiency and an
increase of delta REM as well as REM sleep (Miller et al., 2004).
Sleep promoting properties of quetiapine are shown in healthy subjects (Cohrs et al., 2004). Quetiapine 25 mg and 100 mg significantly improved sleep induction and continuity under standard and acoustic stress conditions. Increases in total sleep time, sleep efficiency, percentage sleep stage 2 and subjective sleep quality were
seen. A significant increase in periodic leg movements during sleep was observed with quetiapine 100 mg. Ziprasidone 40 mg has been shown to improve sleep continuity, to increase SWS and to suppress REM sleep in healthy subjects under both standard routine sleep EEG and during acoustic stress conditions. The authors suggested that these effects are most likely related to ziprasidone's 5-HT(2C)
antagonism, 5-HT(1A) agonism, and serotonin and norepinephrine reuptake inhibition (Cohrs et al., 2005).
We tried to summarize the existent studies concerning atypical antipsychotics as follow (see table 4 and 5). Data about aripiprazole are until now missing.
Table 4: Effects of atypical antipsychotics on sleep parameters in schizophrenia (1).
MEDICATIONS STUDIES
Olanzapine Salin-Pascual et al., 1999 Sharpley et al., 2000 Sharpley et al., 2001
Risperidone Dursun et al., 1999 Haffmans et al., 2001 Yamashita et al., 2002
Clozapine Wetter et al., 1996 Hinze-Selch et al., 1997 Touyz et al., 1978
Table 5: Effects of atypical antipsychotics on sleep parameters in schizophrenia (2).
OLANZAPINE RISPERIDONE CLOZAPINE
Dosage (mg) 5-10 3-10 150-350
SLEEP INDUCTION
Stage 2 sleep latency Decreased No available data Decreased
SLEEP MAINTENANCE
Wake time after sleep onset Decreased Decreased Decreased
Total sleep time Increased Increased Increased
Sleep efficiency Increased Higher movement index
Increased
SLEEP ARCHITECTURE
Stage 1 sleep Decreased No available data Decreased Stage 2 sleep Increased No available data Increased
SWS Increased Increased Decreased
REM latency Increased No available data No available data
REM sleep Decreased No available data Decreased
REM density Increased No available data Increased
4. Mechanisms involved in the disruption of sleep in
schizophrenic patients and in the effects of antipsychotic
drugs on sleep.
4.1 Schizophrenic patients
The dopamine hypothesis of schizophrenia formulated in the sixties states that the symptoms of schizophrenia depend upon the overactivity of the dopaminergic system (Carlsson et al., 1963). The latter would be related to an increased density of
dopamine D2 receptors. Nevertheless, recent post-mortem and in vivo imaging
studies indicate that about 30% of patients show striatal D2 receptor densities that do
not differ significantly from matched control subjects. These results remain significant irrespective of analyzing drug naïve and drug treated patients separately (Monti and Monti, 2004). More recent studies have refined the dopamine hypothesis of
schizophrenia by stressing that in addition to the increased density of D2 receptors,
there is an enhanced sensitivity of dopaminergic neurotransmission.
On the base (or basis) of these findings it could be proposed that the sleep disturbance of schizophrenic patients might be related to an overactivity of the dopaminergic system. Indirect evidence derived from pharmacological studies tends to support this contention. Accordingly, the dopamine D2 receptor preferring agonists
apomorphine, bromocriptine, and pergolide have been shown to mimics sleep disturbances encountered in schizophrenic patients, i.e., it enhances wakefulness and reduces SWS and REM sleep when administered to laboratory animals at doses that interact with postsynaptic D2 receptors (Monti et al., 1988). In contrast, a
substituted benzamide that selectively blocks D2 receptors, increases light sleep
(Monti et al., 1989). The possibility remains that insomnia in schizophrenic patients is not exclusively dopamine dependent but that other neurotransmitter systems are also involved in the disruption of this behavioural state.
4.2 Antipsychotic drugs.
The blockade of D2 receptors has been proposed to be responsible for the
improvement of positive symptoms. Moreover, the blockade of 5-HT2a receptors
contribute to the improvement of negative symptoms and of cognitive functions. On the other hand, the blockade of alpha1, H1, and cholinergic (muscarinic) receptors
induces a number of side effects. (Monti and Monti, 2004). The blockade of D2
receptors could be partly responsible for the improvement of sleep in schizophrenic patients. However, the improvement of sleep has been related almost exclusively to the blockade of alpha1, H1, and cholinergic (muscarinic) receptors. This is based on
the premise that drugs antagonising alpha1 receptor (prazosin and related drugs), fH1
receptor (diphenhydramine), or cholinergic (muscarinic) receptor (scopolamine) produce somnolence, an increased likelihood of falling asleep and reduced concentration ( Monti and Monti, 2000).
Idzikowski et al. (1986, 1987) have demonstrated that the 5-HT2a/c receptor
antagonist ritanserin selectively increases SWS in normal volunteers. A same effect has been described in poor sleepers and in depressive patients (Adam and Oswald, 1989; Staner et al., 1992). The finding by Katsuda et al. (1993) that the 5-HT2c
receptor agonist metachlorophenylpiperazine (mCPP) reduces SWS in normal subjects prompted Sharpley et al. (2000) to propose that the ritanserin- or
Dugovic et al. in 1994 showed that during deep SWS, ritanserin induced a dose-dependent increase of the power density in the low frequency range (delta and theta bands) and a decrease of the EEG power in the higher frequency range (alpha and beta bands) and she called it “state specific modulation of EEG spectral activity mediated by 5-HT2 receptors in the rat”. Later, Imeri et al. (1999) showed that
blockade of 5-HT2 receptors alters interleukin-1-induced changes in rat sleep. These
data suggest that interactions between the serotonergic system and interleukin-1 may be important in regulating sleep-wake behaviour.
5. CONCLUSION
The sleep disturbances of either never medicated or previously treated schizophrenic patients are characterized by a sleep onset and maintenance insomnia. In addition, duration of stage 4 sleep, SWS (stages 3 and 4), NREM sleep and of REM latency are decreased. The typical antipsychotics significantly reduce stage 2 sleep latency and increase sleep efficiency. The atypical antipsychotics significantly increase total sleep time and stage 2 sleep. Moreover, Olanzapine, risperidone and ziprasidone
moreover enhance SWS.
We need others studies including:
sleep patterns in subtypes of schizophrenic patients.
role of neurotransmitters in the disruption of sleep in schizophrenia.
brain imaging during NREM and REM sleep of the functional alterations in CNS areas related to the pathophysiology of schizophrenia.
short-, intermediate- and long-term effects of atypical antipsychotics on sleep variables.
RESUME
Schizophrénie, sommeil et antipsychotiques
Les troubles du sommeil se rencontrent fréquemment dans la schizophrénie mais n’ont jamais fait l’objet d’études systématiques comme les autres symptômes de cette maladie (symptômes productifs ou déficitaires, symptômes cognitifs,
agressivité, …). Cet article est une revue de la littérature sur le sujet et sur les mécanismes impliqués dans l’effet des antipsychotiques sur le sommeil ainsi que sur les implications cliniques qui en découlent. Les perturbations du sommeil les plus fréquentes concernent des difficultés dans l’initiation ou le maintien du sommeil, caractérisées par un sommeil fragmenté. Un déficit du sommeil à ondes lentes a été observé et mis en relation avec des anomalies structurelles du cerveau, une
symptomatologie déficitaire et un mauvais pronostic. La plupart des antipsychotiques améliorent les troubles du sommeil rencontré dans la maladie. Des études ont montré que les molécules qui antagonisent les récepteurs 5 HT2, comme les
antispychotiques atypiques, augmentent le sommeil à ondes lentes. A cet égard, la risperidone, l’olanzapine and la ziprasidone améliorent la continuité du sommeil et augmentent le sommeil profond chez des volontaires sains ou des patients
schizophrènes. Ces études suggèrent que les antipsychotiques antagonisant les récepteurs 5 HT2 sont particulièrement indiqués pour le traitement des patients
7. REFERENCES
Adam K, Oswald I. Effects of repeated ritanserin on middle-aged poor sleepers.
Psychopharmacology 99:219-21 (1989)
Benson K., Sullivan E., Lim K. Slow wave sleep and computed tomographic
measures of brain morphology in schizophrenia. Psychiatry Res 60: 125-134 (1996) Benson K., Faull K., Zarcone V. Evidence for the role of serotonin in the regulation of slow wave sleep in schizophrenia. Sleep 14 : 133-139 (1991)
Benson K., Zarcone V. Rapid eye movement sleep in schizophrenia and depression. Arch Gen Psychiatry 50: 474-482 (1993)
Caldwell D., Domino E. Electroencephalographic and eyes movement patterns during sleep in chronic schizophrenic patients. Electroenceph Clin Neurophysiol 22: 414-420 (1967)
Carlsson A., Lindquist M. Effects of chlorpromazine or haloperidol on formation of 3-methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol 20: 140-144 (1963)
Chemerinski E., Ho B., Flaum M., Arndt S., Fleming F., Andreasen N. Insomnia as a Predictor for Symptom Worsening Following Antipsychotic Withdrawal in
Schizophrenia. Comprehensive Psychiatry 43: 393-396 (2002)
Cohrs S., Rodenbeck A., Guan Z., Pohlmann K., Jordan W., Meier A., Rüther E. Sleep-promoting properties of quetiapine in healthjy subjects. Psychopharmacology 174: 421-429 (2004)
Cohrs S, Meier A, Neumann AC, Jordan W, Ruther E, Rodenbeck A. Improved sleep
continuity and increased slow wave sleep and REM latency during ziprasidone treatment: a randomized, controlled, crossover trial of 12 healthy male subjects. J Clin Psychiatry. 66:989-96 (2005).
Dagan Y., Circadian Rhythm Sleep Disorders (CRSD) in Psychiatry – A Review. Isr J Psychiatry Relat Sci 39:19-27 (2002)
Dugovic C., Van Den Broeck W., Clincke G. State-specific modulation of EEG
spectral activity mediated by 5-HT2 receptors in the rat. J Sleep Res 3 (Suppl 1) S139
(1994)
Dursun S., Patel J., Burke J. Effects of typical antipsychotic drugs and risperidone on the quality of sleep in patients with schizophrenia: a pilot study. J Psychiatry Neurosci 24: 333-337 (1999)
Feinberg I., Braun M., Koresko R. Stage 4 sleep in schizophrenia. Arch Gen Psychiatry 21: 263-266 (1969)
Ganguli R., Reynolds C., Kupfer D. Electroencephalographic sleep in young never-medicated schizophrenics. Arch Gen Psychiatry 44: 36-44 (1987)
Haffmans P., Oolders J., Hoencamp E. The effect of risperidone versus haloperidol on sleep patterns of schizophrenic patients: results of a double-blind, randomised pilot trial. Eur Neuropsychopharm 11: S260 (2001)
Hiatt J., Floyd T., Katz P. Further evidence of abnormal non-rapid-eye-movement sleep in schizophrenia. Arch Gen Psychiatry 42: 797-802 (1985)
Hinze-Selch D., Mullington J., Orth A., Lauer C., Pollmächer T. Effects of Clozapine on Sleep: a longitudinal study. Biol Psychiatry 42: 260-266 (1997)
Hoffmann R., Hendrickse W., Rush A. Slow-wave activity during non-REM sleep in men with schizophrenia and major depressive disorders. Psychiatry Res 95: 215-225 (2000)
Hudson J., Lipinski J., Keck P. Polysomnographic characteristics of schizophrenia in comparison with mania and depression. Biol Psychiatry 34: 191-193 (1993)
Idzikowski C., Mills F., Glennard R. 5-Hydroxytryptamine-2-antagonist increases human slow wave sleep. Brain Res 378: 164-168 (1986)
Idzikowski C., Cowen P., Nutt D. The effects of chronic ritanserin treatment on sleep and the neuroendocrine response to L-Tryptophan. Psychopharmacology 93: 416-420 (1987)
Imeri L., Mancia M., Opp R. Blockade of 5-Hydroxytryptamine (serotonin)-2 receptors alters interleukin-1-induced changes in rat sleep. Neuroscience 92, 745-749 (1999) Jus K., Bouchard M., Jus A-K. Sleep EEG studies in untreated long-term
schizophrenic patients. Arch Gen Psychiatry 29: 386-390 (1973)
Katsuda Y., Walsh A., Ware C. meta-Chlorophenylpiperazine decreased slow wave sleep in humans. Biol Psychiatry 33: 49-51 (1993)
Kempenaers C., Kerkhofs M., Linkowski P. Sleep EEG variables in young schizophrenic and depressive patients. Biol Psychiatry 24: 833-838 (1988) Keshavan M., Cashmere D., Miewald J., Yeragani V. Decreased nonlinear complexity and chaos during sleep in first episode schizophrenia: a preliminary report. Schizophrenia Research 71: 263-272 (2004)
Keshavan M., Reynolds C., Miewald J. Delta sleep deficits in schizophrenia. Arch Gen Psychiatry 55: 443-448 (1998)
Keshavan MS, Reynolds CF, Kupfer DJ. Electroencephalographic sleep in
Lauer C., Schreiber W., Pollmächer T. Sleep in schizophrenia: a polysomnographic study on drug-naive patients. Neuropsychopharmacology 16: 51-60 (1997)
Lauer C., Krieg J. Slow-wave sleep and ventricular size: a comparative study in schizophrenia and major depression. Biol Psychiatry 44: 121-128 (1998)
Lesch DR, Spire JP. Clinical electroencephalography, In, Thorpy MJ (ed), Handbook of Sleep Disorders, Marcel Dekker Inc., New York, 1990, pp 1-31.
Lewis CF, Tandon R, Shipley JE, DeQuardo JR, Jibson M, Taylor SF, Goldman M.
Biological predictors of suicidality in schizophrenia. Acta Psychiatr Scand 94:416-20 (1996)
Lindberg N., Virkkunen M., Tani P., Appelberg B., Virkkala J., Rimon R., Porkka-Heiskanen T. Effect of a single-dose of olanzapine on sleep in healthy females and males. Int Clin Psychopharmacology 17: 177-184 (2002)
Maixner S., Tandon R., Eiser A., Taylor S., DeQuardo J.R., Shipley J. Effects of Antipsychotic Treatment on Polysomnographic Measures in Schizophrenia: A Replication and Extension. Am J Psychiatry 155:1600-1602, (1998)
Miller D. Atypical antipsychotics: Sleep, Sedation and Efficacy. J Clin Psychiatry 6 (Suppl 2): 3-7 (2004)
Monti J., Monti D. Sleep in schizophrenia patients and the effects of antipsychotic drugs. Sleep Medicine Review 8: 133-148 (2004)
Monti J., Hawkins M., Jantos H. Biphasic effects of Dopamine D2 receptor agonists
on sleep and wakefulness in the rat. Psychopharmacology 95: 395-400 (1988) Monti J., Jantos H., Fernandez M. Effects of the dopamine D2 receptor agonist,
quinpirole on sleep and wakefulness in the rat. Eur J Pharmacol 169: 61-66 (1989) Monti J., Monti D. Histamine H1 receptor antagonists in the treatment of insomnia. Is there a rational basis for use ? CNS Drugs 13: 87-96 (2000)
Müller M., Rossbach W., Mann K., Röschke J., Benkert O. Subchronic effects of olanzapine on sleep EEG in schizophrenic patients with predominantly negative symptoms. Pharmacopsychiatry 37: 157-162 (2004)
Obermeyer W., Benca R. Effects of drugs on sleep. Neurologic Clinics 14: 827-840 (1996)
Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci 3:591-605 (2002).
Poulin J., Daoust A-M., Forest G., Stip E., Godbout R. Sleep architecture and its clinical correlates in first episode and neuroleptic-naïve patients with schizophrenia. Schizophrenia Research 62: 147-153 (2003)
Ritsner M., Kurs R., Ponizovsky A., Hadjez J. Perceived quality of live in
schizophrenia: Relationships to sleep quality. Quality of Life Research 13: 783-791 (2004)
Royuela A., Macias J.A., Pastor J.F., Maniega M.A., Alonso J., Román J.M., De Paz F., Barbosa M., Rami-Gonzalez L., Boget T. Sleep in schizophrenia: a preliminary study using the Pittsburg Sleep Quality Index. Neurobiology of Sleep-Wakefulness Cycle 2: 37-39 (2002)
Salin-Pascual R., Herrera-Estrella M., Galicia-Polo L., Laurrabaquio M-R. Olanzapine acute administration in schizophrenic patients increases delta sleep and sleep
efficiency. Biol Psychiatry 46: 141-143 (1999)
Sharpley A., Vassallo C., Cowen P. Olanzapine increases show-wave sleep:
evidence for blockade of central 5-HT2C receptors in vivo. Biol Psychiatry 47: 468-470
(2000)
Sharpley A., Vassallo C., Pooley E. Allelic variation in the 5-HT2C receptor (5HR2RC) and the increase of show wave sleep produced by olanzapine, Psychopharmacology 153: 271-272 (2001)
Singareddy R., Balon R. Sleep and Suicide in Psychiatric patients, Annals of Clinical Psychiatry 13: 93-101 (2001)
Staedt J., Dewes D., Danos P., Stoppe G. Can chronic neuroleptic treatment promote sleep disturbances in elderly schizophrenic patients ? Int. J. Geriat. Psychiatry 15: 170-176 (2000)
Staner L., Kempenaers C., Simonnet M.P., Fransolet L., Mendlewicz J. 5-HT2
receptor antagonism and slow wave sleep in major depression, Acta Psychiatrica Scandinavica, 86, 133-137, 1992.
Staner L., Granier L.A., Vandenhende R., Luthringer R., Macher J.P. Comparison of the effects of sleep EEG of morning and evening administration of olanzapine : a placebo controlled study in healthy volunteers. Eur Neuropsychopharmacol 12 (Suppl 3): S326 (2002).
Steriade M. Arousal: revisiting the reticular activating system. Science 272:225-6, (1996)
Stern M., Fram D., Wyatt R. All-nigth sleep studies of acute schizophrenics. Arch Gen Psychiatry 20: 470-477 (1969)
Tandon R., Shipley J., Taylor S. Electroencephalographic sleep abnormalities in schizophrenia. Arch Gen Psychiatry 49: 185-194 (1992)
Touyz S., Saayman G., Zabow T. A psychophysiological investigation of the long-term effects of clozapine upon sleep patterns of normal young adults.
Wetter T., Lauer C., Gillich G., Pollmächer T. The electroencephalographic sleep pattern in schizophrenic patients treated with clozapine or classical antipsychotic drugs. J Psychiat Res 30: 411-419 (1996)
Yamashita H., Morinobu S., Yamawaki S., Horiguchi J., Nagao M. Effect of risperidone on sleep in schizophrenia: a comparison with haloperidol. Psychiatry Research 109: 137-142 (2002)
Yamashita H., Mori K., Nagao M., Okamoto Y., Morinobu S., Yamawaki S. Effects of changing from typical to atypical antipsychotic drugs on subjective sleep quality in patients with schizophrenia in a Japanese population. J Clin Psychiatry 65: 1525-1530 (2004)
Zarcone V., Benson K., Berger P. Abnormal rapid eye movement latencies in schizophrenia, Arch Gen Psychiatry 44: 45-48 (1987)