0 50000 100000 150000 200000 In te n si ty ( m A U )
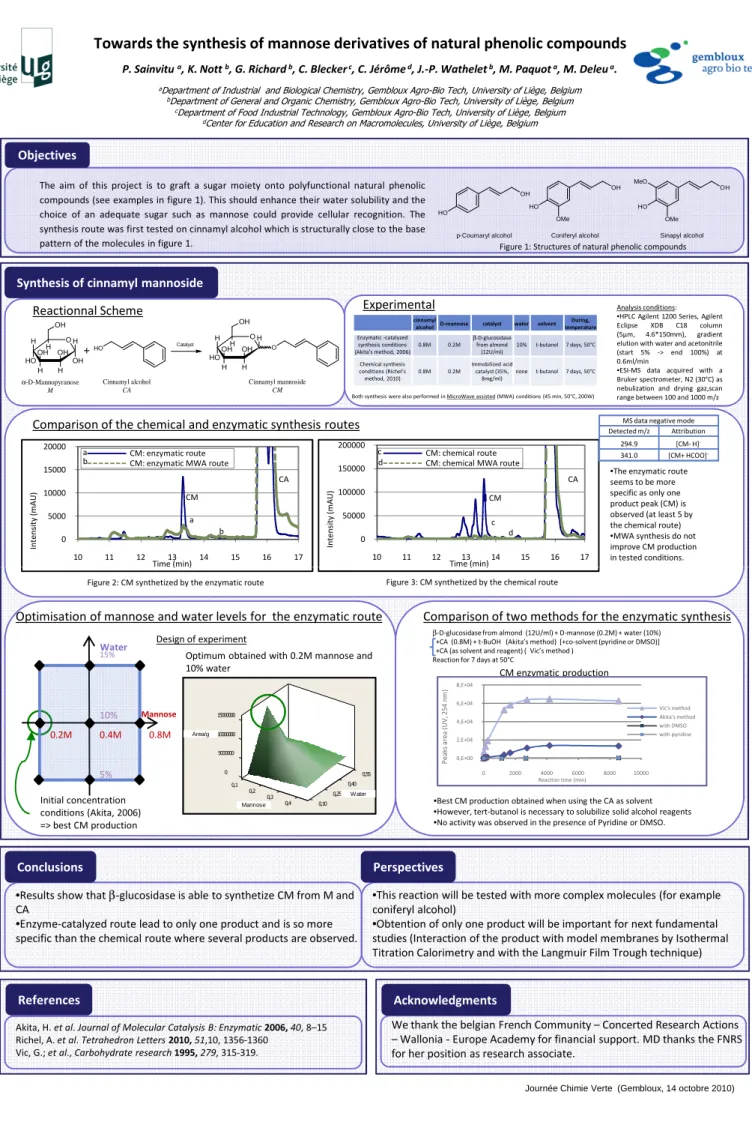
CM synthetized by the chemical route CM: chemical route CM: chemical MWA route
CM
CA
Towards the synthesis of mannose derivatives of natural phenolic compounds .
P. Sainvitu
a, K. Nott
b, G. Richard
b, C. Blecker
c, C. Jérôme
d, J.-P. Wathelet
b, M. Paquot
a, M. Deleu
a.
aDepartment of Industrial and Biological Chemistry, Gembloux Agro-Bio Tech, University of Liège, Belgium bDepartment of General and Organic Chemistry, Gembloux Agro-Bio Tech, University of Liège, Belgium
cDepartment of Food Industrial Technology, Gembloux Agro-Bio Tech, University of Liège, Belgium dCenter for Education and Research on Macromolecules, University of Liège, Belgium
The aim of this project is to graft a sugar moiety onto polyfunctional natural phenolic
compounds (see examples in figure 1). This should enhance their water solubility and the
choice of an adequate sugar such as mannose could provide cellular recognition. The
synthesis route was first tested on cinnamyl alcohol which is structurally close to the base
pattern of the molecules in figure 1.
O H O OH OH H H O H OH H H H OH α-D-Mannopyranose M
+
Catalyst O OH H H O H OH H H H OH O Cinnamyl alcohol CA Cinnamyl mannoside CM O H OH p-Coumaryl alcohol O H OH OMe Coniferyl alcohol O H OH OMe MeO Sinapyl alcoholObjectives
Figure 1: Structures of natural phenolic compounds
Synthesis of cinnamyl mannoside
Reactionnal Scheme
Comparison of the chemical and enzymatic synthesis routes
Experimental
Analysis conditions:•HPLC Agilent 1200 Series, Agilent Eclipse XDB C18 column (5µm, 4.6*150mm), gradient elution with water and acetonitrile (start 5% -> end 100%) at 0.6ml/min
•ESI-MS data acquired with a Bruker spectrometer, N2 (30°C) as nebulization and drying gaz,scan range between 100 and 1000 m/z
•The enzymatic route seems to be more specific as only one product peak (CM) is observed (at least 5 by the chemical route) •MWA synthesis do not improve CM production in tested conditions. 0 5000 10000 15000 20000 10 11 12 13 14 15 16 17 In te n si ty ( m A U ) Time (min) CM: enzymatic route CM: enzymatic MWA route
CA CM a b a b 0 50000 100000 150000 200000 10 11 12 13 14 15 16 17 In te n si ty ( m A U ) Time (min) CM: chemical route CM: chemical MWA route
CM CA c d d c
MS data negative mode Detected m/z Attribution
294.9 [CM- H]
-341.0 [CM+ HCOO]
-cinnamyl
alcohol D-mannose catalyst water solvent
During, temperature Enzymatic -catalyzed synthesis conditions (Akita’s method, 2006) 0.8M 0.2M β -D-glucosidase from almond (12U/ml) 10% t-butanol 7 days, 50°C Chemical synthesis conditions (Richel’s method, 2010) 0.8M 0.2M Immobilized acid catalyst (35%, 8mg/ml)
none t-butanol 7 days, 50°C
Both synthesis were also performed in MicroWave assisted (MWA) conditions (45 min, 50°C, 200W)
We thank the belgian French Community – Concerted Research Actions
– Wallonia - Europe Academy for financial support. MD thanks the FNRS
for her position as research associate.
10 11 12 13 14 15 16 17
Time (min)
Akita, H. et al. Journal of Molecular Catalysis B: Enzymatic 2006, 40, 8–15
Richel, A. et al. Tetrahedron Letters 2010, 51,10, 1356-1360
Vic, G.; et al., Carbohydrate research 1995, 279, 315-319.
•
Results show that
β
-glucosidase is able to synthetize CM from M and
CA
•
Enzyme-catalyzed route lead to only one product and is so more
specific than the chemical route where several products are observed.
•
This reaction will be tested with more complex molecules (for example
coniferyl alcohol)
•
Obtention of only one product will be important for next fundamental
studies (Interaction of the product with model membranes by Isothermal
Titration Calorimetry and with the Langmuir Film Trough technique)
Conclusions
•Best CM production obtained when using the CA as solvent •However, tert-butanol is necessary to solubilize solid alcohol reagents •No activity was observed in the presence of Pyridine or DMSO.
0 0,55 5000000 10000000 15000000 0,40 0,1 0,2 0,25 0,3 0,4 0,10 Aire/g 113h Eau Mannose
Comparison of two methods for the enzymatic synthesis
Initial concentration
conditions (Akita, 2006)
=> best CM production
Optimisation of mannose and water levels for the enzymatic route
0 0,55 5000000 10000000 15000000 0,40 0,1 0,2 0,25 0,3 0,4 0,10 Aire/g 113h Eau Mannose Area/g Mannose Water
Optimum obtained with 0.2M mannose and
10% water
β-D-glucosidase from almond (12U/ml) + D-mannose (0.2M) + water (10%) +CA (0.8M) + t-BuOH (Akita’s method) [+co-solvent (pyridine or DMSO)] +CA (as solvent and reagent) ( Vic’s method )
Reaction for 7 days at 50°C
Acknowledgments
Area/g Mannose WaterCM enzymatic production
Perspectives
References
Time (min) Time (min)
Mannose
Water
15%10%
5%
0.8M
0.4M
0.2M
Figure 2: CM synthetized by the enzymatic route Figure 3: CM synthetized by the chemical route
Journée Chimie Verte (Gembloux, 14 octobre 2010)
Design of experiment
0,E+00 2,E+04 4,E+04 6,E+04 8,E+04 0 2000 4000 6000 8000 10000 P e a k s a re a ( U V , 2 5 4 n m )Reaction time (min)
Vic's method Akita's method with DMSO with pyridine