JSCS–4984 Original scientific paper
Antimicrobial, antioxidant, cytotoxic and anticholinesterase
activities of water-soluble polysaccharides extracted from
microalgae Isochrysis galbana and Nannochloropsis oculata
MHAMMED BEN HAFSA1,2*, MANEL BEN ISMAIL3, MARIEM GARRAB3, RAIES ALY1, JONATHAN GAGNON2 and KARIM NAGHMOUCHI1
1Laboratoire des Microorganismes et Biomolécules Actives (LMBA), Faculté des Sciences de
Tunis, Université El-Manar II 2092 El-Manar-II, Tunis, Tunisia, 2Département de biologie,
chimie et géographie, Université du Québec à Rimouski, 300 allée des Ursulines, Rimouski, Québec, G5L 3A1, Canada and 3Laboratoire de Microbiologie, Faculté de Médecine,
Université de Monastir, Monastir 5000, Tunisia
(Received 16 November 2016, revised 7 February, accepted 14 March 2017) Abstract: The present work is carried out to evaluate potential applications of aqueous extracts of two microalgae Isochrysis galbana (PEA) and Nanno-chloropsis oculata (PEB) containing mainly polysaccharides. The monosac-charide composition of microalgal extracts was determined. GC–MS analyses after derivatization show that glucose is the major compound in both mic-roalgae PEA (56.88 %) and PEB (68.23 %). Mannitol (38.8 %) and inositol (20.32 %) are respectively the second major compounds in PEA and PEB. Silylation of monosaccharides allows the determination of sorbitol that attained 3.38 % in PEB. The determination of antioxidant, antimicrobial and cytotoxic properties were also analyzed. Antioxidant activity was evaluated from the DPPH scavenging activity. PEA and PEB show a concentration dependent DPPH·radical scavenging activity. At concentration of 10 mg/mL, both PEA and PEB exhibit an antioxidant activity of 41.45 and 59.07 %, respectively. PEB and PEA are able to inhibit the growth of negative bacteria, Gram-positive bacteria and three Candida species. Cytotoxic activity was evaluated on human HeLa cervical cancer cells. HeLa cell proliferation was totally inhibited after treatment with PEA and PEB (1 mg/mL) and the inhibition was dose dependent (from 0.031 to 1 mg/mL). Their anticholinesterase activity was also investigated against butyrylcholinesterase enzymes. These polysac-charides possess interesting antimicrobial, anticancer and anticholinesterase activities that could represent an additional value for these microalgal products. Keywords: algae; DPPH; cytotoxic activity; antimicrobial activity; polysac-charides; GC-MS.
* Corresponding author. E-mail: mhammedbenhafsa@gmail.com https://doi.org/10.2298/JSC161016036B
INTRODUCTION
Algae represent a large diversity of species that are estimated from 40 000 to
10 million where the majority are microalgae.
1Microalgae are eucaryotic
photo-synthetic organisms that play a key role in aquatic ecosystems and account for
approximately 40 % of the global photosynthesis.
2They possess some different
morphological, physiological, and genetic traits that confer the ability to produce
several biologically active metabolites.
3Microalgae can yield a large pool of
biomolecules with biological activities, such as carotenoids, phycobilins,
polyun-saturated fatty acids, proteins, polysaccharides, vitamins, and sterols among other
chemicals.
4These microalgal molecules can possess several health benefits and
therefore be used in many sectors such as nutraceutical, pharmaceutical and
functional foods.
Besides, polysaccharides are polymeric carbohydrates, formed by repeating
units joined together by glycosidic bonds. Recently, they have widely been
investigated due to their chemical and biological activities.
5Polysaccharides
present a large diversity of structures attributable to their variety in composition,
substitutions and glycosidic bonds. Polysaccharides isolated from plants, fungi,
yeasts and algae have attracted considerable attention for their biological
acti-vities in biochemistry and medicine.
6They exhibit a wide range of biological
activities such as inflammatory, antioxidant, antitumor, anticoagulant,
anti-thrombotic, antimetastic, antiviral, antimicrobial and immunostimulatory.
7–10Laminarin and fucoidan are polysaccharides isolated from cell walls of brown
seaweeds that possess immunomodulatory, antitumor, antiviral and antioxidant
activities.
11Isochrysis galbana and Nannochloropsis oculata are two marine microalgae
that are produced industrially for aquaculture. They are important food source
and feed additive that were widely used especially in the aquaculture industry.
12N. oculata has been reported to reduce blood pressure on hypertensive rats.
13I.
galbana is well-known for its nutritional quality and to be a good source of lipids
that can be used as a substitution of fish oils in a healthy human diet.
14,15Some
promising curative effects were also reported including weight loss and reduction
of glucose, triacylglycerol and cholesterol levels in diabetic rats.
16Moreover
compounds from these microalgae exhibit interesting bioactivities like
antibac-terial, anti-inflammatory, anti-algal, antifungal, analgesic, and antioxidant
acti-vities.
17–19Herein, we report the extraction of water-soluble polysaccharides in two
microalgae pastes, I. galbana (PEA) and N. oculata (PEB), their composition in
monosaccharides, the evaluation of their cytotoxicity against a cancer cell line
and the antimicrobial activities against Gram-positive bacteria, Gram-negative
bacteria and Candida strains. Finally, this study also presents the antioxidant and
anticholinesterase activities of these polysaccharidic extracts.
EXPERIMENTAL Culture conditions and samples
Microalgae pastes, I. galbana (clone T-iso) and N. oculata CCMP-1325, were obtained from NutrOcean (Rimouski, Canada). Briefly, microalgae came from NCMA-CCMP cultures and they were produced semi-continuous during two months (partial harvests and dilutions at 24 or 48 h). Microalgae were grown in airlift cylindrical photobioreactors. The temperature and salinity were 22±2 °C and 28±3 ‰, respectively. Irradiance was 140±20 µE (µmol m-2 s-1) on the reactor surface. The photoperiod was always light (24 h light and 0 h dark). Culture medium (f/2 without silicate) was sterilized (UV and ultrafiltration) before to be used. Extraction of water soluble polysaccharides
Each freeze-dried microalgae paste (20 g each) was extracted three times with 200 mL of methanol, the first time during 48 h and the two latter extractions during 24 h. The resulting microalgae pastes were then extracted twice with 200 mL of deionized water during 72 h and 24 h. Microalgae aqueous extracts were combined and were freeze-dried. The yields were determined.
Total sugar, proteins and sulfate measurement
Total sugar content in the aqueous extracts was determined by a modified phenol– –sulfuric acid method based on literature.20 Briefly, a mixture of 0.5 mL of sample and 0.5 mL of 5 % aqueous phenol solution was treated with 2.5 mL of concentrated sulfuric acid. The mixture was stirred during 30 min. The absorption was measured at 490 nm and glucose was used as external standard. Sulfate content was determined using barium chloride/gelatin method with some modifications.21 Concisely, the aqueous extracts (0.2 mL) were treated with trichloroacetic acid (3.8 mL) followed by the addition of 1.0 mL of barium chloride/gelatin. The mixture was stirred during 20 min. The absorbance was read at 360 nm and potassium sulfate was used as external standard.
Protein contents were measured from nitrogen percentage obtained by combustion elemental analysis. The percentage of crude protein (CP) in samples was calculated by multiplying the nitrogen percentage (N) by a conversion factor using the following equation:22 CP= N×6.25
Hydrolysis and silylation of polysacharidic extracts
Hydrolysis of extracts was carried out according to Yang et al. with some minor modifications.23 Freeze-dried aqueous extracts (10 mg) were hydrolyzed with 10 mL of aqueous trifluoroacetic acid (2 M, TFA) at 120 °C during 8 h. The solution was evaporated to dryness with a nitrogen flow. Samples were reacted with 0.2 mL of N,O-bis(trimethyl-silyl)trifluoroacetamide containing 1% of chlorotrimethylsilane in anhydrous pyridine (0.2 mL) during 3 h at 70 °C. The resulting solution was evaporated with a nitrogen flow. The solid was then extracted with n-hexane (2.00 mL) prior to monosaccharide analysis.24 Monosaccharide analysis
The silylated monosaccharide samples were analyzed using a Hewlett-Packard 6890 gas chromatograph (GC) equipped with a DB-5 capillary column (30 m×0.25 mm×0.25 µm film thickness) coupled to a mass spectrometer (MS, Micromass Platform II) operated to the electron impact mode (70 eV). The temperature of the injector was 300 °C. The column temperature was set at 80 °C during 5 min, then increased at a rate of 4 °C min-1 to 290 °C, and was then maintained isothermally for 20 min. The carrier gas was helium at a constant flow rate of 1.2 mL min-1. Arabinose was used as internal standard. A volume of 1 µL of
sample was splitless injected. Chromatograms were analyzed with the MSD ChemStation E.02.02.1431 software. Assignation of chromatographic peaks was achieved with the NIST mass spectra search program (version 2.0d).24
DPPH assay
The 2,2-diphenyl-1-picrylhydrazyl (DPPH, Sigma) radical scavenging activity was measured according to the literature.25 Microalgae polysaccharidic extracts were dissolved in 10 mL of distilled water to a final concentration of 100 µg/mL. Two milliliters of 0.2 mM DPPH in ethanol were added to 1 mL of each microalgal polysaccharidic solutions (PEA and PEB). The absorbance was measured at 517 nm after 20 min of incubation at 25 °C. Distilled water was used as the control. Percentage of inhibition was determined according to the following formula: DPPH radical scavenging activity (%) = 100(Ac–As)/Ac, where Ac is the absorbance value of the control group and As is the absorbance value of the group treated with the extract.
Vitamin E (Sigma Aldrich) was used as positive control and all measurements were performed in triplicate. The percentage inhibition of free radical activity was plotted against the concentration of polysaccharidic extracts and the concentration for 50% of inhibition (IC50) was determined.
Antimicrobial activity
Microorganisms and culture conditions. PEA or PEB were tested against Gram-positive cocci (Enterococcus faecalis ATCC 29212 and Staphylococcus aureus ATCC 25923) and Gram-negative bacilli (Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853). The antifungal effects of polysaccharidic extracts from I. galbana or N. oculata were also tested against a range of pathogenic reference yeasts (Candida albicans ATCC 90028, C. glabrata ATCC 90030, C. kreusei ATCC 6258 and C. parapsilosis ATCC 22019). Bacteria or Candida species were grown in nutrient brothand incubated aerobically without shaking for 24 h at 37 °C and sub-cultures were realized at least three times at 24 h intervals prior to tests. All microorganisms tested were provided from the laboratory of Parasitology–Mycology and the laboratory of Bacteriology of Monastir (Tunisia).
MIC determination. The minimum inhibitory concentration (MIC) of PEA and PEB was determined from a microdilution assay as described in literature.26 PEA and PEB stock solutions were prepared by dissolution of 10 mg of PEA or PEB in 2 mL of 10 % dimethyl sulfoxide (DMSO, Sigma-Aldrich). After an overnight incubation, broth cultures were adjusted to yield approximately to 1×106 CFU/mL of bacteria or fungus. A sample of each extract (200 μL) was added to four wells of the first column of each plate and then diluted with DMSO (10 %) solution (dilution factor (1:1)) up to the well number eight of first column. Each well was then inoculated with 50 µL of bacteria or Candida species and microplates were incubated during 24 h at 37 °C. Controls (wells inoculated with the tested culture without polysaccharides extracts) and blanks (wells containing uninoculated broth with polysaccharide extracts) were run on each microplate. Imipenem and vancomycin were used as positive control for bacteria strains and fluconazole was used for Candida species. All antibiotics and antimycotic (Sigma–Aldrich) were tested at a final concentration of about 1 mg/mL. The MIC was the lowest concentration of tested agent giving the complete inhibition of growth (i.e., optical density equal to OD of the blank). The microplate assays were repeated at least three times for each polysaccharide extract and the MIC was the average of three independent experiments.
Cytotoxic activity. The human HeLa cervical cancer cell line was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in a humidified atmosphere at 37 °C in 5 % CO2. RPMI 1640 (Sigma-Aldrich) supplemented with 10 % fetal calf serum, 1 % (w/v) glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin was used for HeLa cell cultures. Cell viability cytotoxicity was measured using an MTT (3- -(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay with slight modific-ations.27 HeLa cells (5
×103) were seeded into wells with 100 μL of growth medium and incubated at 37 °C for 24 h. Cells were treated with polysaccharide extracts (0.031 to 1 mg/mL) and incubated for 48 h at 37 °C. After that, 10 μL of MTT (5 mg/mL) was added to each well and microplates were incubated for an additional 2 h. Then, the medium was dissolved with 100 μL of DMSO and the absorbance (A) was measured at 550 nm by a BioTek microplate reader. This assay was conducted in triplicate as a cell viability index. The percentages of cell growth were calculated as follow: Cell proliferation (%) = 100(As–Ac)/Ac, where Ac is the absorbance value of the control group and As is the absorbance value of the group treated with sample.
Anticholinesterase activity. The anticholinesterase activity was determined by color-imetry using a Cholinesterase Kit (Chronolab, Spain).28 PEA and PEB stock solutions were prepared by dissolution 20 mg of PEA or PEB in 2 mL of 10 % DMSO (Sigma–Aldrich). Human Plasma was provided from the Biochemistry–Toxicology Laboratory, University Hospital “Fattouma Bourguiba” of Monastir (Tunisia) and used as a source of butyr-ylcholinesterase (BChE). PEA or PEB (500 µL, 10 mg/mL) was added to 500 µL of plasma and the mixture was incubated at 37 °C during 5, 10, 15, 20 and 30 min. BChE activity was measured by COBAS INTEGRA® 400 (Roche diagnostics). The control (plasma and distilled water) was treated under the same conditions. The anticholinesterase activity was calculated by the same formula as for DPPH radical scavenging activity. All assays were carried out in triplicate.
RESULTS AND DISCUSSION
Total sugar, proteins, and sulfate composition
Figure 1 shows the composition in sugars, proteins and sulfate of the
micro-algae aqueous extracts. Total sugar content in aqueous extracts of I. galbana and
N. oculata were 86.9±0.8 % (22.8 % of total dried matter) and 59±0.1 % (4.1 %
of total dried matter), respectively. The sulfate groups represented respectively
7.9±1.2 % and 6.2±0.1 % of I. galbana and N. oculata extracts. Sulfate bands
were confirmed by infrared spectroscopy (data not shown). The percentage of
proteins in the microalgae aqueous extracts were respectively of 5.2±0.17 % and
21.0±0.2 % for I. galbana and N. oculata. According to literature, carbohydrates
represented around 13 % of dry matter of I. galbana.
29Brown
showed that N.
oculata is composed by 35 % of proteins and 7.8 % of carbohydrates.
30However,
Picardo et al. reported that carbohydrates represent 29.4 % when grown at 25 °C
(22 °C in our study).
31Many studies reported that chemical composition as
car-bohydrates, proteins and lipids in N. oculata and I. galbana was dependent of the
environmental growing conditions like salinity, light intensity, nitrogen content,
photoperiod, and stage of harvest.
26,27,32Fig. 1. Total sugar, proteins and sulfate content in water-soluble polysaccharidic extracts of Isochrysis galbana and Nannochloropsis oculata. All assays were carried out in triplicate.
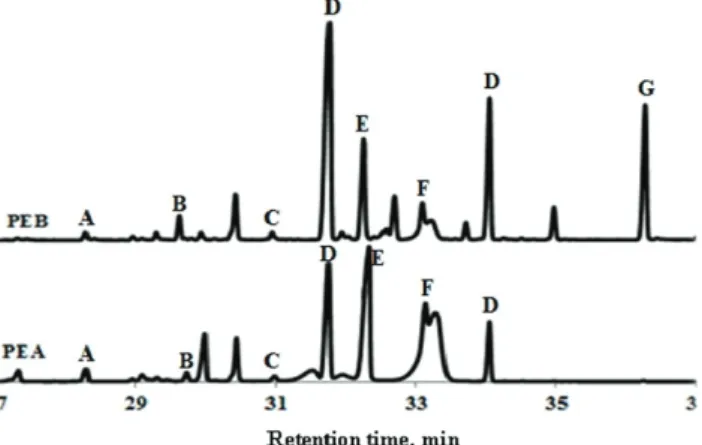
Monosaccharide analysis
Polysaccharides from microalgae were hydrolyzed with TFA into
monosac-charides, which were further trimethylsilylated to obtain volatile compounds for
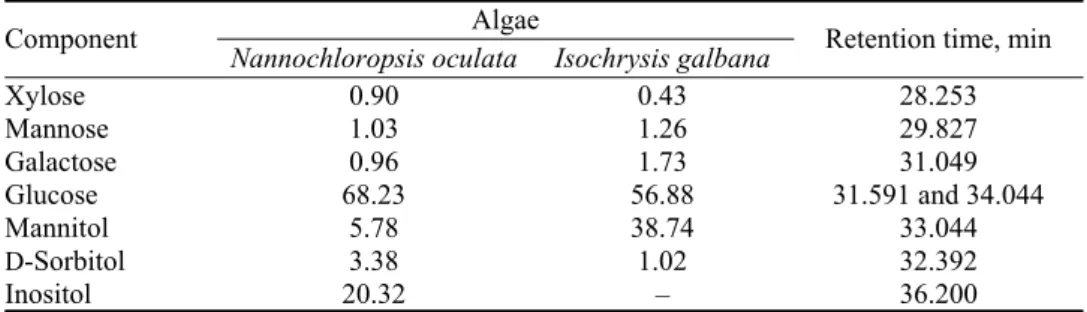
GC–MS analyses. Table I presents the monosaccharide composition in
microal-gal polysaccharidic extracts. Glucose was the major component in I. microal-galbana and
N. oculata extracts with 56.9 and 68.3 %, respectively. Mannitol (38.8 %) and
inositol (20.32 %) were respectively the second major compounds in PEA and
PEB (Fig. 2).
Fig. 2. Monosaccharide chromatogram of polysaccharidic extracts of Isochrysis galbana (PEA) and Nannochloropsis oculata (PEB) determined by trimethylsilylation method. A: xylose; B: mannose; C: galactose; D: glucose; E: sorbitol; F: mannitol; G: inositol.
Mannitol represented a percentage of 5.8 % of the N. oculata extract and
inositol was absent in the I. galbana extract. Chu et al., using trimethylsilylation
method, observed 1.35 % of mannitol in I. galbana.
33Xylose, mannose and
gal-actose were minor constituents of the both PEA and PEB microalgae extracts
(< 2 %). Glucose, galactose, mannose and xylose have been reported in various
proportions in I. galbana extracts.
34Brown
reported that glucose was the major
sugar in N. oculata and I. galbana, which corresponds to 68.2 and 70.3 %,
res-pectively.
30During exponential and stationary growth phases of I. galbana, the
percentage of glucose reached 60 and 80 %, respectively.
33In our study, the
percentages of sorbitol in I. galbana and N. oculata were respectively of 1.02 and
3.38 %. Sadovskaya et al.
and Brown
reported that sorbitol was not observed in I.
galbana and N. oculata using the alditol acetate method.
30,34Templeton et al.
mentioned that it was impossible to distinguish between neutral sugar (glucose)
and reduced sugar (sorbitol) in the original mixture with the alditol acetate
deri-vatization method.
35TABLE I. Monosaccharides composition (%) of Isochrysis galbana and Nannochloropsis oculata aqueous extracts determined by the trimethylsilylation method
Retention time, min Algae Component Isochrysis galbana Nannochloropsis oculata 28.253 0.43 0.90 Xylose 29.827 1.26 1.03 Mannose 31.049 1.73 0.96 Galactose 31.591 and 34.044 56.88 68.23 Glucose 33.044 38.74 5.78 Mannitol 32.392 1.02 3.38 D-Sorbitol 36.200 – 20.32 Inositol
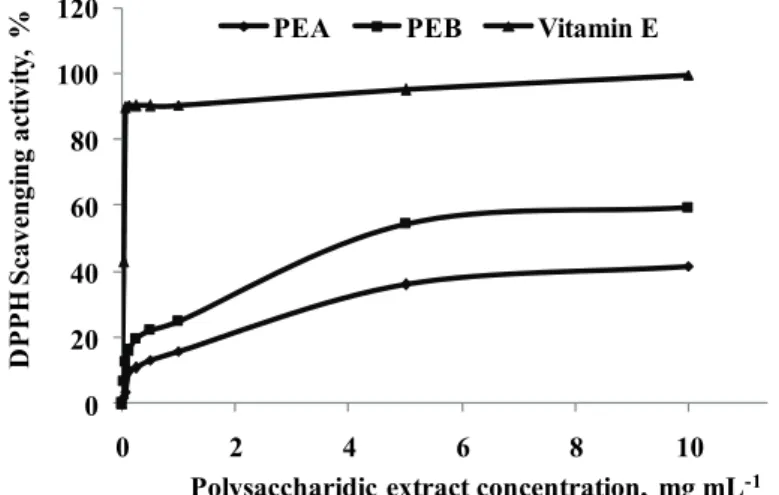
DPPH radical scavenging activity
DPPH is a free radical compound that has been widely used to evaluate the
ability of antioxidant to scavenge radicals.
36Fig. 3 shows the scavenging power
of DPPH radicals by PEA and PEB. The antioxidant capacities of both PEA and
PEB were dose dependent. The inhibition percentages of PEB and PEA (at 1
mg/mL) were 24.79±0.05 % and 15.71±0.03 %, respectively. At the maximal
concentration of 10 mg/mL, PEB (59.28±0.04 %; IC
50= 4.2 mg/mL) showed a
higher antioxidant activity compared to PEA (41.45±0.03 %, IC
50> 10 mg/mL).
This difference of activity could be explained by the presence of proteins and
highly branched polymers. Vitamin E (0.12 mg/mL) had a significant higher
anti-oxidant capacity of 90.312±0.005 % (IC
50= 0.040 mg/mL), compared to PEA
(9.74±0.003 %) and PEB (16.14±0.005 %). Custódio et al. indicated that organic
extracts from N. oculata had also antioxidant properties with IC
50values between
4.93 and 7.31 %.
37Moreover, Balavigneswaran et al.
reported that an ethanol
soluble polysaccharidic extract from I. galbana was active against DPPH (almost
40 %) at 10 mg/mL.
38The reducing properties are generally associated with the presence of
reduc-tones which have been shown to exert antioxidant action by breaking the free
radical chain by donating a hydrogen atom. Reductones are reported to react with
some precursors of peroxide, thus preventing peroxide formation.
39Carboxyl
groups may play an important role in scavenging radicals, possibly due to the
higher hydrogen donation ability of carboxyl groups than hydroxyl groups,
proteins and sulfate groups.
36The low percentage of sulfate in PEA and PEB (7.9
and 6.21 %, respectively) could explain the moderate activity against DPPH
radicals. The antioxidant activities depend on polysaccharides molecular weight,
degree of ramification, monosaccharide composition, sulfate content and
config-uration.
40–42The influence of sulfate content on the antioxidant activity depends
rather on the origin of polysaccharides. For example, the polysaccharides from
Ulva fasciata and other macro and microalgae with low sulfate content
demon-strated a strong antioxidative power, while the antioxidant activity observed in
polysaccharides from Enteromorpha linza and other seaweeds showed to be
dep-endent of sulfate content. Furthermore, highly sulfated polysaccharides were
shown to have an enhanced scavenging power, this property being also
depen-dent on the sulfate distribution pattern.
430 20 40 60 80 100 120 0 2 4 6 8 10 D P P H S cave ngi n g ac ti vi ty , %
Polysaccharidic extract concentration, mg mL-1
PEA PEB Vitamin E
Fig. 3. DPPH scavenging power of polysaccharidic extracts of Isochrysis galbana (PEA) and Nannochloropsis oculata (PEB). Vitamin E was tested as positive control. All assays were
carried out in triplicate.
PEA and PEB antimicrobial activity
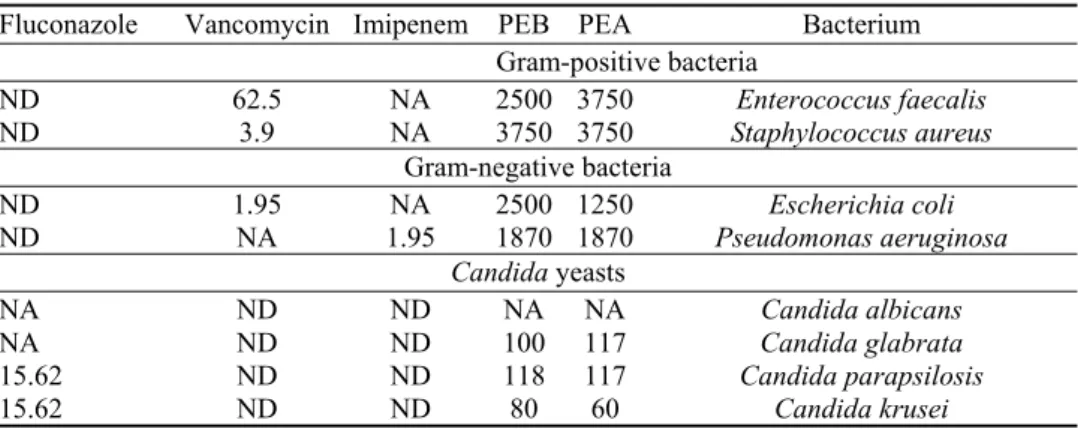
Table II shows the MIC of polysaccharidic extracts (PEA and PEB) from
microalgae I. galbana and N. oculata. All tested bacterial strains were sensitive
to PEA and PEB. Results show that Gram-negative bacteria were more sensitive
to PEA than Gram-positive bacteria. MICs of PEA against Escherichia coli (E.
coli), Pseudomonas aeruginosa (P. aeruginosa) and Enterococcus faecalis were
1250, 1870 and 3750 µg/mL, respectively. MICs of PEB against E. coli and P.
aeruginosa were 2500 and 1870 µg/mL, respectively. On the other hand, the
same author showed that methanolic extract is not active against multiresistant
Gram-positive (Staphylococcus aureus and Bacillus subtilis) and Gram-negative
(P. aeruginosa and Klebsiella pneumomiae) pathogens. Bruce et al.
reported that
acetone extract of I. galbana was active against S. aureus and Micrococcus sp.
with corresponding inhibition zone diameters of 10 and 15 mm, respectively.
44TABLE II. Antimicrobial activity of polysaccharidic extracts of Isochrysis galbana (PEA) and Nannochloropsis oculata (PEB) against Gram-positive bacteria, Gram-negative bacteria and Candida strains. Imipenem, vancomycin and fluconazole were used as positive controls. Minimum inhibitory concentration, µg mL-1, was the average of three independent replic-ations; NA: no activity; ND: not determined
Bacterium PEA PEB Imipenem Vancomycin Fluconazole Gram-positive bacteria Enterococcus faecalis 3750 2500 NA 62.5 ND Staphylococcus aureus 3750 3750 NA 3.9 ND Gram-negative bacteria Escherichia coli 1250 2500 NA 1.95 ND Pseudomonas aeruginosa 1870 1870 1.95 NA ND Candida yeasts Candida albicans NA NA ND ND NA Candida glabrata 117 100 ND ND NA Candida parapsilosis 117 118 ND ND 15.62 Candida krusei 60 80 ND ND 15.62
For the antifungal activity, Candida krusei shows a higher sensitivity to PEA
(60 µg/mL) and PEB (80 µg/mL) than other Candida species (Table II). C.
para-psilosis was inhibited by PEB and fluconazole with MIC values of 118 and 15.62
µg/mL, respectively. No inhibitory activity against C. albicans for both PEA and
PEB was detected. C. glabrata was resistant to fluconazole (1 mg/mL) and
appeared to be sensitive to PEA or PEB, with respective MICs of 117 and 100
µg/mL. The mechanisms involved in antimicrobial activity of polysaccharides
extracts are worthy of further investigations.
45Polysaccharides influence the
cytoplasm permeability, the DNA decomposition after a polysaccharide/DNA
binding, and the denaturation of essential bacterial proteins.
46On the other hand,
the activity against microorganisms can be related to the bacterial membrane
composition, resistance capacity of yeasts, polysaccharides structure, degree of
ramification and degree of sulfation. Goy et al.
reported that polysaccharides
inhibited the fungi growth by reacting with enzymes in hyphae.
47Cytotoxic activity
Figure 4 shows that cell proliferation decreases with increasing of PEA and
PEB content. The proliferating cells reached 42.7 and 13.8 % at PEA concentrations
of 31.25 and 500 µg/mL, respectively. After a treatment with PEB (125 and 250
µg/mL), the cell proliferation percentages were 51.5 and 38.36 %, respectively.
Both PEA and PEB inhibited HeLa cell proliferation at a final concentration of 1
mg/mL. Remarkably, HeLa cell proliferations were more abundant with N.
ocul-ata aqueous extract (PEB) than with I. galbana extract (PEA) at different
con-centrations of extracts. The cell proliferation percentages of PEA and PEB at the
minimal studied concentration (31 µg/mL) were respectively of 44 and 59 %.
Sadovskaya et al.
showed that the polysaccharide extracts from I. galbana
inhibited U937 human leukemic monocyte lymphoma cell proliferation (30 % at
100 µg/mL) and consequently have potential antitumor activity.
34Atasever-Arslan et al.
reported that essential oils from N. oculata extract (at 500 µg/mL)
caused K562 cell lines cytotoxicity (human chronic myeloid leukemia cell line)
of 45.64 %.
48Polysaccharides, especially sulfate polysaccharides, could affect
the proliferation, differentiation, apoptosis and metastasis of tumor cells.
49They
bound the proteins like growth factors and inhibit the growth of tumors.
4,50Inhibition of the cell proliferation may be mediated by the chemical properties of
sulfated polysaccharides and the species of tumor cells.
49Another mechanism of
antiproliferative effect is to block the G1 phase.
49Sulfated polysaccharides
iso-lated from the filtrate of marine Pseudomonas sp. culture induced the apoptosis
of human leukaemic cells.
50Fucoidan induced apoptosis in human lymphoma
HS-Sultan cell lines, which is accompanied by the activation of caspase-3 and
down-regulation of extracellular signal-regulated kinase pathway.
51The sulfated
heteropolysaccharides isolated from red alga Schizymenia dubyi can induce the
terminal maturation of non-small bronchopulmonary carcinoma cells and arrest
cells in the G1 phase.
49Fig. 4. Percentage of cell proliferation in presence of polysaccharidic extracts of Isochrysis galbana (PEA) and Nannochloropsis oculata (PEB). Error bars represent the standard
deviation calculated from duplicate experiments.
Anticholinesterase activity
Alzheimer’s disease is a deadly neurodegenerative disease with progressive
character and has become a major health problem especially in industrialized
countries where the life expectancy is higher. It is also a common form of
dem-entia especially among the elder population in which irreversible neuronal loss and
abnormal behavioral changes are evident in this disease.
52Antioxidant extracts
from plants play an important role in the prevention of Alzheimer’s disease.
53In
addition, reports indicated a correlation between antioxidant power and the
anticholinesterase activity.
54The use of antioxidants may reduce the Alzheimer’s
disease progression and minimize the neuronal degeneration by inhibition of the
acetylcholinesterase enzyme.
55Treatments of the Alzheimer’s disease include
disease-modifying treatments, psychotropic agents and especially the
cholinesterase inhibitors, which block the hydrolysis of two chemical
neurotransmitters, i.e., acetylcholine and butyrylcholine (by butylcholineesterase,
BChE).
52However, most of these drugs have side effects such as liver damage
and bradycardia. Synthetic antioxidants also caused liver damage and
carcino-genesis in rats, that stimulated scientists to find new natural and harmless
anti-oxidants, as well as anticholinesterase compounds.
55Figure 5 shows the effects
of PEA or PEB (10 mg/mL) on anticholinesterase activity at different incubation
times (5 to 30 min). Remarkably, time-dependent inhibition of
butyrylcholine-sterase was observed after PEA and PEB treatments. PEB was more active than
PEA (Fig. 5). For PEA, the percentage of BChE inhibition after 5 and 30 min
were respectively of 1.25±0.25 % and 7.30±0.48 %. After 30 min of PEB
treat-ment, the BChE inhibition reached a maximum of 11.53±0.12 %.
Anticholine-sterase activities of polysaccharides were not well studied. No significant
evid-ence has been proven that they were specifically active toward the Alzheimer’s
disease. But many polysaccharides could have regenerative properties and
func-tions as memory and learning enhancers.
56Asker et al. suggested that
polysac-charides isolated from Bacillus sp. may be a good natural source for Alzheimer’s
disease therapy.
56On the other hand, Custódio et al. evaluated the BChE activity
of N. oculata organics and water extracts.
37Maximum inhibition (21 %) was
Fig. 5. Effect of polysaccharidic extracts from Isochrysis galbana (PEA) and Nannochloropsis oculata (PEB) on anticholinesterase activity. The results were expressed as
butyrylcholine-sterase inhibition percentage (%). Error bars represent the standard deviation calculated from triplicate experiments.
observed after treatment with N. oculata aqueous extract at the maximal
concen-tration of 0.5 mg/mL. Custódio et al. indicated that aqueous extract from
Iso-chrysis galbana possessed an anticholinesterase activity (IC
50of 0.11 mg/mL).
57CONCLUSIONS
I. galbana and N. oculata are used widely in aquaculture for feeding and
pathogens prevention. PEA and PEB possessed important functional properties
such as antioxidant, antimicrobial, anticholinesterase and antiproliferation
acti-vities, demonstrating the important value of these microalgae. I. galbana and N.
oculata can be further tested for their nutritional and medical human applications.
The mode of action of polysaccharidic extracts on pathogenic bacteria or fungi
constitutes also an important field of study for future works.
Acknowledgment. We thank NutrOcean for the grateful donation of microalgae pastes. И З В О Д
АНТИМИКРОБНА, АНТИОКСИДАТИВНА, ЦИТОТОКСИЧНА И
АНТИХОЛИНЕСТЕРАЗНА АКТИВНОСТ ПОЛИСАХАРИДА МИКРОАЛГИ Isochrysis galbana И Nannochloropsis oculata РАСТВОРНИХ У ВОДИ
MHAMMED BEN HAFSA1,2, MANEL BEN ISMAIL3, MARIEM GARRAB3, RAIES ALY1,
JONATHAN GAGNON2 и KARIM NAGHMOUCHI1
1
Laboratoire des Microorganismes et Biomolécules Actives (LMBA), Faculté des Sciences de Tunis, Université El-Manar II 2092 El-Manar-II, Tunis, Tunisia, 2Département de biologie, chimie et géographie, Université du
Québec à Rimouski, 300 allée des Ursulines, Rimouski, Québec, G5L 3A1, Canada и 3Laboratoire de Microbiologie, Faculté de Médecine, Université de Monastir, Monastir 5000, Tunisia
У овом раду је испитана могућност примене водених екстраката микроалги Iso-chrysis galbana (PEA) и Nannochloropsis oculata (PEB), који садрже претежно полисаха-риде. Одређен је садржај моносахарида у екстрактима. GC–MS анализа након дерива-тизације је показала да је главни састојак обе микроалге глукоза: у PEA 56,88 % и у PEB 68,23 %. Манитол (38,80 %) и инозитол (20,32 %) су следећи по заступљености у PEA односно PEB. Силиловањем моносахарида је утврђено да сорбитола има 3,38 % у PEB. Даље су анализиране антиоксидативне, антимикробне и цитотоксичне особине екстраката. Антиоксидативна активност је утврђивана DPPH методом и зависила је од концентрације. При концентрацији екстракта од 10 mg/mL, антиоксидативна активност PEA и PEB је била 41,45 %, односно 59,07 %. Екстракти су били способни да инхибирају раст Грам негативних и Грам позитивних бактерија, као и три врсте гљиве Candida. Цитотоксична активност је процењена на хуманим HeLa ћелијама тумора грлића материце. Пролиферација HeLa ћелија је била потпуно инхибирана третманом PEA и PEB екстрактима у концентрацији 1 mg/mL, а инхибиција је зависила од дозе у опсегу 0,03 до 1 mg/mL. Антихолинестеразна активност је потврђена спрам бутирилхоли-нестеразе. Због својих активности, полисахариди наведених микроалги могу имати додатну примену осим нутритивне. (Примљено 16. новембра 2016, ревидирано 7. фебруара, прихваћено 14. марта 2017) REFERENCES
2. M. I. Garrido, Bioresour. Technol. 99 (2008) 3949
3. M. F. J. Raposo, A. M. B. Morais, R. M. S. C. Morais, Mar. Drugs 13 (2015) 2967 4. G. Markouk, E. Nerantzis, Biotechnol. Adv. 31 (2013) 1532
5. S. Rubavathi, M. Ramya, Int. J. Curr. Microbiol. Appl. Sci. 5 (2016) 253 6. T. Zhu, H. J. Heo, K. H. Row, Carbohydr. Polym. 82 (2010) 106 7. V. E. C. Ooi, F. Liu, Curr. Med. Chem 7 (2000) 715
8. S. Li, A. Gao, S. Dong, Y. Chen, S. Sun, Z. Lei, Z. Zhang, Int. J. Biol. Macromol. 96 (2017) 26
9. M. Meng, D. Cheng, L. Han, Y. Chen, C. Wang, Carbohydr. Polym. 157 (2017) 1134 10. C. Zhao, L. Gao, C. Wang, B. Liu, Y. Jin, Z. Xing, Carbohydr. Polym. 144 (2015) 382 11. M. Wang, P. Zhu, S. Zhao, C. Nie, N. Wang, X. Du, Y. Zhou, Int. J. Biol. Macromol. 95
(2017) 809
12. S. P. Wasser, Appl. Microbiol. Biotechnol. 60 (2002) 258
13. C. Shene, Y. Chisti, D. Vergara, C. Burgos-Díaz, M. Rubilar, M. Bustamante, J. Biotechnol. 239 (2016) 47
14. M. H. T. Nguyen, Z. J. Qianb, V. T. Nguyenb, I-W. Choi, S. J. Heo, C. H. Ohd, D. H. Kang, G. H. Kime, W. K. Jung, Process Biochem. (Oxford, U.K.) 48 (2013) 1387 15. C. P. Liu, L. P. Lin, Bot. Bull. Acad. Sin. 42 (2001) 207
16. C. C. Yu, H. W. Chen, M. J. Chen, Y. C. Chang, S. C. Chien, Y. H. Kuo, F. L. Yang, S. H. Wu, J. Chen, H. H. Yu, L. K. Chao, Nat. Prod. Commun. 5 (2010) 1941
17. K. Nuno, F. Lopez, A. F. Puebla, E. Velarde, A. G. Puebla-Mora, F. Ascencio, A. Cerón, A. Villaruel, A. M. Vidal-Perez, A. L. Rodríguez, J. Funct. Foods 5(2013) 106 18. D. Surendhiran, M. Vijay, A. R. Sirajunnisa, T. Subramaniyan, A. S. Shellomith, K.
Tamilselvam, J. Coastal Life Med. 2 (2014) 859
19. N. C. Moroney, M. N. O’Grady, S. Lordan, C. Stanton, J. P. Kerry, Mar. Drugs 13 (2015) 2447
20. M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers, F.A. Smith, Anal. Chem. 28 (1956) 350
21. K. S. Dodgson, A. G. Lloyd, Biochem. J. 78 (1961) 319
22. A. Alves, S. G. Caridade, J. F. Mano, R. A. Sousa, R. L. Reis, Carbohydr. Res. 345 (2010) 2194
23. B. Yang, J. Wang, M. Zhao, Y. Liu, Wang W, Y. Jiang, Carbohydr. Res. 341 (2006) 634 24. S. Ma, Z. Wang, X. Bi, G. Sheng, J. Fu, Chin. Sci. Bull. 54 (2009) 4500
25. B. Yang, M. Zhao, J. Shi, N. Yang, Y. Jiang, Food Chem. 106 (2008) 685
26. B. Marzouk, Z. Marzouk, R. Décor, H. Edziri, E. Haloui, N. Fenina, M. Aouni, J. Ethnopharmacol. 125 (2009) 344
27. R. Yan, Y. Yang, Y. Zeng, J. Ethnopharmacol. 121 (2009) 451
28. H. Teyeb, H. Mabrouk, M. Neffati, W. Douki, M. F. Najjar, J. Biol. Active Prod. Nat. 6 (2011) 344
29. A. P. Batista, L. Gouveia, N. M. Bandarra, J. M. Franco, A. Raymundo, Algal Res. 2 (2013) 164
30. M. R. Brown, J. Exp. Mar. Biol. 145 (1991) 79
31. M. C. Picardo, J. L. De Medeiros, O. Q. F. Araújo, R. M. Chaloub, Bioresour. Technol.
143 (2013) 242
32. K. Faidi, S. Hammami, A. Ben Salem, R. El Mokni, M. Garrab, M. Mastouri, M. Gorcii, M. Trabelsi Ayedi, O.Taglialatela-Scafati, Z. Mighri, J. Med. Plants Res. 8 (2014) 550 33. F. E. Chu, J. L.Dupuy, K. L. Webb, Aquaculture 29 (1982) 241
34. I. Sadovskaya, A. Souissi, S. Souissi, T. Grard, P. Lencel, C. M. Greene, S. Duin, P. S. Dmitrenok, A. O. Chizhov, A. S. Shashkov, A. I. Usov, Carbohydr. Polym. 111 (2014) 139
35. D. W. Templeton, M. Quinn, S. VanWychen, D. Hyman, L. M. L. Laurens, J. Chromatogr. 1270 (2012) 225
36. J. Zhang, X. Hou, H. Ahmad, H. Zhang, L. Zhang, T. Wang, Food Chem. 145 (2014) 57 37. L. Custódio, F. Soares, H. Pereira, M. J. Rodrigues, L. Barreira, A. P. Rauter, F.
Alberício, J. Varela, J. Appl. Phycol. 27 (2015) 839
38. C. K. Balavigneswaran, T. S. J. Kumar, R. Moses Packiaraj, A.Veeraraj, A. S. Prakash, Int. J. Biol. Macromol. 60 (2013) 100
39. H. Qi, Q. Zhang, T. Zhao, R. Chen, H. Zhang, X. Niu, Int. J. Biol. Macromol. 37 (2005) 195
40. T. Zhao, G. Mao, W. Feng, R. Mao, X. Gu, T. Li, Q. Li, Y. Bao, L. Yang, X. Wu, Carbohydr. Polym. 105 (2014) 26
41. Y. Chen, H. Zhang, Y. Wang, S. Nie, C. Li, M. Xie, Food Chem. 156 (2014) 279 42. H. Song, Q. Zhang, Z. Zhang, W. Jing, Carbohydr. Polym. 80 (2010) 1057
43. K. G. Ramawat, J. M. Mérillion, Polysacharides: Bioactivity and Biotechnology, Springer International Publishing: Cham, Switzerland, 2015, pp.1683-1727
44. D. L. Bruce, D. C. B. Duff, J. Gen. Microbiol. 48 (1967) 293
45. H. Cheng, S. Feng, S. Shen, L. Zhang, R. Yang, Y. Zhou, C. Ding, Carbohydr. Polym. 96 (2013) 101
46. H. Feng, Y. Ying, Y. Guang, Y. Longjiang, Food Control 2 (2010) 1257 47. C. R. Goy, D. de Britto. O. B. G. Assis, Polímeros 19 (2009) 241
48. B. Atasever-Arslan, K. Yilancioglu, K. Alkanz, A. C. Timucin, H. Gür, F. B. Isik, E. Deniz, B. Erman, S. Cetiner, Eur. J. Pharm. Sci. 83 (2016) 120
49. X. Z. Wu, D. Chen, West Indian Med. J. 55 (2006) 270 50. L. Desnoyers, Curr. Pharm. Des. 10 (2004) 3913
51. Y. Aisa, Y. Miyakawa, T. Nakazato, H. Shibata, K. Saito, Y. Ikeda, M. Kizaki, Am. J. Hematol. 78 (2005) 7
52. G. Rajakumar, T. Gomathi, M. Thiruvengadam,V. D. Rajeswari, V. N. Kalpana, I.-M. Chung, Microb. Pathog. 103 (2017) 123
53. N. Dorosti, F. Jamshidi, J. Appl. Biomed. 14 (2016) 235 54. I. Hacıbekiroglu, U. Kolak, Arab. J. Chem. 8 (2015) 264
55. A. Muhammad, G. Tel-Çayan, M. Öztürk, M. E. Duru, S. Nadeem, I. Anis, S. W. Ng, M. R. Shah, Pharm. Biol. (Abingdon, U.K.) 54 (2016) 1649
56. M. M. S. Asker, A. Y. Ibrahim, M. G. Mahmoud, S. S. Mohamed, Am. J. Biochem. Biotechnol. 11 (2015) 103
57. L. Custódio, F. Soares, H. Pereira, L. Barreira, C. Vizetto-Duarte, M. J. Rodrigues, A. P. Rauter, F. Alberício, J. Varela, J. Appl. Phycol. 26 (2014) 151.